ABSTRACT
This Phase I/IIa open-label, single-arm clinical trial addressing advanced, refractory, metastatic breast cancer was conducted at six medical centers in the United States. We repeated inoculations with irradiated SV-BR-1-GM, a breast cancer cell line with antigen-presenting activity engineered to release granulocyte-macrophage colony-stimulating factor (GM-CSF), with pre-dose low-dose cyclophosphamide and post-dose local interferon alpha. Twenty-six patients were enrolled; 23 (88.5%) were inoculated, receiving a total of 79 inoculations. There were six Grade 4 and one Grade 5 adverse events noted (judged unrelated to SV-BR-1-GM). Disease control (stable disease [SD]) occurred in 8 of 16 evaluable patients; 4 showed objective regression of metastases, including 1 patient with near-complete regressions in 20 of 20 pulmonary lesions. All patients with regressions had human leukocyte antigen (HLA) matches with SV-BR-1-GM; non-responders were equally divided between matching and nonmatching (p = .01, Chi-squared), and having ≥2 HLA matches with SV-BR-1-GM (n = 6) correlated with clinical benefit. Delayed-type hypersensitivity (DTH) testing to candida antigen and SV-BR-1-GM generated positive responses (≥5 mm) in 11 (42.3%) and 13 (50%) patients, respectively. Quantifying peripheral circulating tumor cells (CTCs) and cancer-associated macrophage-like cells (CAMLs) showed that a drop in CAMLs was significantly correlated with an improvement in progression-free survival (PFS; 4.1 months vs. 1.8 months, p = .0058). Eight of 10 patients significantly upregulated programmed cell death ligand 1 (PD-L1) on CTCs/CAMLs with treatment (p = .0012). These observations support the safety of the Bria-IMT regimen, demonstrate clinical regressions, imply a role for HLA matching, and identify a possible value for monitoring CAMLs in peripheral blood.
KEYWORDS: SV-BR-1-GM, breast cancer, immunotherapy, therapeutic cancer vaccine, histocompatibility markers, Trial registration, NCT03066947
Introduction
Inoculating cancer patients with replication incompetent whole tumor cells has been a popular treatment strategy for many investigators, fostering hundreds of clinical trials.1 These trials are based on the hypothesis that the injected tumor cells will elicit an immune response that will then be cytotoxic to the established cancer.2–4
Conventional opinion holds that this strategy will be most useful in those with a minimal tumor burden, i.e., subclinical disease, and most authorities predict that such inoculations will be ineffective in patients with more advanced metastatic presentations.5,6 In a careful review of the literature, it appears that this strategy in advanced patients does elicit responses, although responses are uncommon and often mixed.7 Insofar as such responses do indeed occur, however rare, there is justification for special attention to understand the mechanism of action and perhaps inform a wider application of the strategy.
The current investigation is based on a small pilot study in patients with metastatic breast cancer, inoculating patients with irradiated SV-BR-1-GM cells.8 The parental cell line, SV-BR-1, was derived from a chest-wall metastasis of a human epidermal growth factor receptor 2 (HER2)-positive, estrogen receptor/progesterone receptor (ER/PR)-negative breast cancer patient. SV-BR-1 cells were stably transfected with a colony-stimulating factor 2 (CSF2) plasmid to release granulocyte-macrophage colony-stimulating factor (GM-CSF) in situ and consequently augment local dendritic cell activity. The transfected and expanded cells are referred to as SV-BR-1-GM.7–9As part of the characterization of the SV-BR-1/SV-BR-1-GM, we documented the human leukocyte antigen (HLA) profile. In a previous trial, we observed a near-complete response in a patient at multiple metastatic sites and a secondary response after relapse and retreatment. We noted that the patient had HLA alleles that matched the SV-BR-1-GM cell line.8 While matching histocompatibility markers, together with immunosuppressive medications, reduces the likelihood of allograft rejection, we suspected that under the conditions of an appropriate clinical trial, HLA matching might actually facilitate an anti-tumor response.2,8 It can be argued that the immunizing tumor cells expressing matching HLA molecules could function as antigen-presenting cells, presenting tumor antigens to the patient’s immune system. Recognition by the T-cell receptor might then initiate an adaptive response with a cascade of tumor-specific cytotoxic T-cells.2 Of interest, we found the cell line not only expressed HLA Class I alleles but also class II alleles, an important finding here insofar as there has been increasing attention that Class II and CD4 responses play a pivotal role in host responses to cancer. 7, 8–11
We also found the cell line very strongly overexpressed the HER2/neu antigen,7,12 the target of a number of clinical trials. The development of immune responses to HER2 can allow for antigenic drift and immune responses to other breast cancer-associated antigens.5
This Phase I/IIa study was designed to evaluate the safety, tolerability, efficacy, and possible clinical benefit of the SV-BR-1-GM regimen, possibly correlated with a number of clinical and pathological features. Studies of humoral and cell-mediated immunity were also implemented, to be described in a separate publication.
Materials and methods
This investigation was an open-label, Phase I/IIa study that incorporated elements of Phase I design such as safety and tolerability. In addition, we implemented Phase II elements such as clinical efficacy. The exploratory portion of the study included evaluation of tumor response by the response evaluation criteria in solid tumors (RECIST)/iRECIST criteria, progression-free survival over 12 months, documentation of tumor grade, HLA histocompatibility subtyping of the patients, development of delayed-type hypersensitivity (DTH) to a control antigen and to the SV-BR-1 or SV-BR-1-GM cells, quality of life (QOL) evaluation, and changes in circulating tumor cells and cancer-associated macrophage-like cells (CTCs/CAMLs).13,14
Study design
The Bria-IMT administration cycle began with an intravenous (IV) infusion of low-dose cyclophosphamide (300 mg/m2) given 2–3 d before SV-BR-1-GM inoculation. Premedication with low-dose cyclophosphamide is performed because of its effect on regulatory T-cells15–18 and potential synergism with the vaccine process by fostering cytokine responses, induction of MHC antigens on tumor cells or other mechanisms not yet identified.19 Following the cyclophosphamide injection, an initial test dose of 1 million SV-BR-1 or SV-BR-1-GM cells in 0.1 mL Lactated Ringer’s solution was injected intradermally in the volar aspect of the forearm, monitored for 20 minutes for immediate hypersensitivity, and then 20 million irradiated, viable SV-BR-1-GM cells were injected, followed at 48 and 96 hrs with 10,000 IU interferon-α2b per inoculation site and time point (4 inoculation sites; 80000 IU total). The protocol specified inoculations to be performed every 2 weeks × 3, followed by a monthly maintenance phase that included Bria-IMT cycles for 6 months (eight total cycles).
Inclusion/exclusion criteria
Participants were included in the study if they were female, aged ≥18 y, had histologically confirmed breast cancer with recurrent and/or metastatic lesions, Eastern Cooperative Oncology Group (ECOG) status ≤2, received prior radiation therapy (XRT) for brain metastases if present, and showed signs of consistent, recurring, or progressive disease (PD) after failing at least one round of community-standard systemic chemotherapy. Participants were excluded from the current study if they had concurrent or recent chemotherapy, immunotherapy, general anesthesia, or major surgery within 3 weeks, or XRT within 1 week, had a history of clinical hypersensitivity to GM-CSF, interferon-alpha-2b (Merck), beef, or any components used in the preparation of the experimental therapy.
Before initiating the study, the required independent ethics committee and institutional review board approval were obtained. Each patient provided written informed consent prior to enrollment. The trial was registered at ClinicalTrials.gov (NCT03066947) and was consistent with standards established by the Declaration of Helsinki and compliant with the International Council for Harmonization (ICH) guidelines for Good Clinical Practice (GCP).
Procedures
Cell culture
The derivation of SV-BR-1-GM has been previously described,12 and a summary of cell-line development and manufacturing is shown in Figure S1. SV-BR-1-GM was cultured at the University of California, Davis’ Good Manufacturing Practice (GMP) facility in the Institute for Regenerative Cures (Sacramento, CA). The cells were grown in RPMI 1640 supplemented with Glutamax with 10% fetal bovine serum (FBS) and stored in cryopreservation media in liquid nitrogen. The master cell bank (MCB) was extensively tested for sterility, viral agents, identity, purity and potency and the cells produced GM-CSF at a rate of 50–60 ng/million cells/24 hours. Subsequent working cell bank (WCB) derived from the MCB was similarly tested and produced GM-CSF at a rate of 33 ng/million cells/24 hours. For use, cells were thawed and cultured for several days and then kept in serum-free medium for 24 hrs prior to harvest. Subsequently, the cells were harvested with Tryp-LE, centrifuged, washed, and irradiated (20,000 cGy, cesium source with cell cycle arrest shown by BrDU staining). The cells were tested for endotoxin, gram stain, sterility and mycoplasma prior to administration. The irradiated cells were shipped to the clinical site under established temperature conditions (2–8°C) and used within 24 hours of irradiation.
Safety assessment
All adverse events (AEs) and serious adverse events (SAEs) were evaluated for safety assessment. Clinical laboratory results, electrocardiograms (ECGs), and vital signs were assessed. Adverse events of special interest (AESI), including new or worsening autoimmune disease, major cutaneous reactions at the inoculation sites (e.g., ulcers, necrosis), allergic reactions to SV-BR-1-GM, and cardiac events, were also to be reported. Toxicity responses were characterized and graded according to the National Institutes of Health’s (NIH) common terminology criteria for adverse events (CTCAE) version 4.03.
Efficacy assessment
Imaging
Efficacy was assessed by measurable and non-measurable disease and tumor responses. Measurable disease required lesions to be accurately measurable (±10%) in at least one dimension on computed tomography (CT; ≤1.0 cm cuts), magnetic resonance imaging (MRI), plain X-ray, or medical photographs and have a major axis of 2.0 cm or more. Tumor lesions seen on images obtained by spiral CT were to be 1.0 cm or greater to be considered measurable. Target lesions were defined as measurable lesions, up to five sites per patient and no more than two sites in any organ. The development of new lesions was documented. Bone lesions were not considered under these criteria. In addition, non-measurable disease included lesions smaller than 1.0 cm by radiological imaging, effusions, poorly defined lung infiltrates, and bone lesions. The tumor response was defined according to the RECIST/iRECIST criteria.20,21 The tumor response included objective response rate (ORR), disease control (non-progressive) rate, and durability of response. The ORR was assessed as complete response (CR) or partial response (PR), the disease control rate was assessed as CR, PR, or stable disease (SD), and the durability of response was evaluated by considering those patients who were eligible to finish the optional therapies between 9 and 12 months.
Exploratory assessments
Markers
In addition to the widely used assays for carcinoembryonic antigen (CEA), cancer antigen (CA) 27–29, and CA 15–3, the program involved a study of CTC and CAML in collaboration with Creatv MicroTech. For CTC and CAML analysis, 7.5 mL anonymized blood samples were collected in CellSave vacutainer (Menarini Silicon Biosystems), processed, and stained using a commercially available LifeTracDx test, as previously described.13,22,23 Briefly, 7.5 mL peripheral blood was collected and processed with a CellSieve Microfiltration Assay using a microfiltration system.13,22,23 CellSieve Microfiltration Assay collects circulating cells based on a size exclusion microfluidic system. Isolated cells are identified by a trained cytologist that identifies CTCs and CAMLs based on pre-established morphologic features, including phenotypic expression of CD14/CD45, PD-L1-Alexafluor555 (Creatv MicroTech clone q9nzq7), cytokeratin and DAPI.22,23 PD-L1 expression in isolated cells was quantified for by overall pixel intensity measured using an Olympus BX61 microscope, imaged using Carl Zeiss AxioCam with Zen2011 Blue software and scored as previously described as a binary scoring system (1 = low and 2/3 = high).22,23 In addition, patients had serial bleeds to archive sera and peripheral blood lymphocytes.
The CTCs and CAMLs were quantified and imaged using an Olympus BX51WI fluorescent microscope with a Carl Zeiss AxioCam monochrome.13,22,23
Delayed-type hypersensitivity skin test
Testing for anergy involved intradermal Candida antigen (Nielson Biosciences, Inc., San Diego, CA). Immediate and DTH immune responses to the vaccine were evaluated with intradermal inoculation of 106 SV-BR-1 or SV-BR-1-GM cells in the volar aspect of the forearm. The participants were monitored for 20 minutes prior to therapeutic inoculations to protect against the possibility of immediate hypersensitivity and anaphylaxis. The DTH response to the vaccine and the Candida antigen were recorded in the electronic data capture (EDC) system at the 2 d (±1 d) visit. The DTH response at the therapeutic inoculation sites was also recorded. Erythema or induration ≥5 mm in length or width was considered a positive DTH.
Quality of life
The SF-36 questionnaire was administered at the start of each cycle. A significant improvement in one or more questionnaire scales in 50% or more patients was considered an improvement in quality of life. Patients self-reported their status and lifestyle changes via the questionnaire provided at baseline and at each inoculation.
Major histocompatibility complex assessment
Considering the previous pilot study that found HLA allele matching with the SV-BR-1-GM cell line in a patient with an unusual, rapid PR,8 we documented histocompatibility profiles in all patients. Buccal swabs were analyzed by HLA typing, conducted via LabType R-SSO Kits (One Lambda) at Terasaki Research Institute (Los Angeles, CA).
Statistical analyses
The primary endpoint was safety, which did not require statistical analysis. However, for scientific data review, de-identified data were entered into case report forms (CRFs) and into a computer database for possible statistical analysis. Various statistical analyses assessed the relationship between clinical response, immunological response, and possible prognostic factors. Other parametric and nonparametric tests were used to evaluate relationships of interest.
Results
Patient disposition, demographic, and baseline characteristics
From 28 April 2017 through 28 December 2018, a total of 30 female patients with histological confirmation of breast cancer were screened (Table 1 and Figure 1). There were 4 screen failures, 26 patients (86.7%) were enrolled, and 23 patients (76.7%) were inoculated. A majority of the patients (15 [57.7%]) were taken off the study due to disease progression. Other reasons for discontinuation included voluntary withdrawal (5 patients [19.2%]), death (3 patients [11.5%]), adverse events (2 patients [7.7%]), and physician decision (1 patient [3.8%]).
Table 1.
Clinical characteristics.
| Characteristics | Number of Patients n (%) |
|---|---|
| Patients screened | 30 |
| Screen failures | 4 |
| Patients enrolled | 26 (86.7) |
| ECOG Performance Status | |
| 0 | 9 (34.6) |
| 1 | 15 (57.7) |
| 2 | 1 (3.8) |
| Missing | 1 (3.8) |
| Age, years | |
| Mean ± SD (Range) | 58.1 ± 10.0 (33.0-74.3) |
| Gender, n (%) | |
| Female | 26 (100.0) |
| Race, n (%) | |
| White | 22 (84.6) |
| Black or African American | 2 (7.7) |
| Other | 2 (7.7) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 6 (23.1) |
| Not Hispanic or Latino | 19 (73.1) |
| Unknown | 1 (3.8) |
| Reproductive status | |
| Females of child-bearing potential | 4 (15.4) |
| Serum beta HCG negative | 4 (15.4) |
| Females of non-child – bearing potential* | 18 (69.2) |
| Prior systemic therapy regimens | |
| Patients, n (%) | 26 (100.0) |
| Prior chemotherapy regimens | |
| Patients, n (%) | 24 (92.3) |
| Mean ± SD (Range) | 5.9 ± 3.3 (1-14) |
| Prior targeted therapy** | |
| Patients, n (%) | 16 (61.5) |
| Mean ± SD (Range) | 2.3 ± 1.3 (1-5) |
| Prior hormonal therapy** | |
| Patients, n (%) | 11 (42.3) |
| Mean ± SD (Range) | 2.7 ± 1.7 (1-6) |
ECOG = Eastern Cooperative Oncology Group; HCG = Human chorionic gonadotropin; n = Number of patients with observation; SD = standard deviation.
*Documented absence of menses for 2 y.
**Two patients received both targeted and hormonal therapies.
Figure 1.
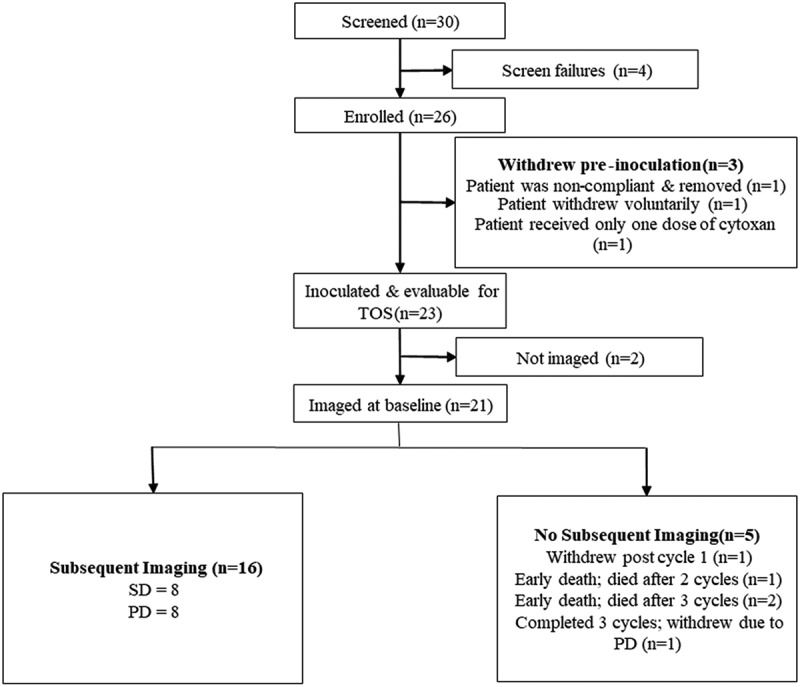
Patient disposition.
CR = Complete response; PD = Progressive disease; SD = Stable disease; TOS = Time on study.
The mean (± SD) age of the patients was 58.1 ± 10.0 y, ranging from 33.0 to 74.3 y. A majority of the patients were white (84.6%), not Hispanic or Latino (73.1%), and of non-child – bearing potential (69.2%; Table 1).
All enrolled patients had received prior systemic therapy regimens. A total of 24 (92.3%) patients received prior chemotherapy regimens (ranging from 1 to 14 [mean ± SD: 5.9 ± 3.3]), 16 (61.5%) patients received prior targeted therapies (ranging from 1 to 5 [2.3 ± 1.3]), and 11 (42.3%) patients received prior hormonal therapies (ranging from 1 to 6 [2.7 ± 1.7]).
Eastern Cooperative Oncology Group performance status
At baseline, the proportions of patients with ECOG performance statuses of 0, 1, and 2 were 57.7%, 34.6%, and 3.8%, respectively.
From baseline to the last reported visit, 46.2% patients did not have any change in ECOG performance status, and 30.8% patients had improved ECOG performance status. Only 11.5% of patients had deteriorated ECOG performance status.
Cancer history
Tumor grade data were available for 15 (62.5%) patients. Four (16.7%) patients had either Grade 1 or 2 tumors. Most of the patients (11 [45.8%]) had poorly differentiated Grade 3 histology.
The pathological characteristics of the patients’ metastatic breast cancer are presented in Table 2. Five patients (19.2%) were ER/PR+, while eight (30.8%) patients were ER/PR-. Nine (34.6%) patients had HER2/neu ≥1+ immunohistochemistry (IHC) graded. Six (23.1%) patients had triple negative breast cancer (IHC graded).
Table 2.
Pathological characteristics.
| Characteristics | Number of Patients N = 26 n (%) |
|---|---|
| Metastatic breast cancer | |
| ER/PR+ | 5 (19.2) |
| ER+ | 12 (46.2) |
| PR+ | 5 (19.2) |
| ER/PR- | 8 (30.8) |
| ER- | 8 (30.8) |
| PR- | 16 (61.5) |
| HER2/neu (IHC graded) | 20 (76.9) |
| 3+ | 1 (3.8) |
| 2+ | 3 (11.5) |
| 1+ | 5 (19.2) |
| 0 | 11 (42.3) |
| HER2/neu (FISH) | |
| 3+ | 0 |
| 2+ | 0 |
| 1+ | 1 (3.8) |
| 0 | 1 (3.8) |
| HER2/neu (DUALFISH) | |
| 3+ | 0 |
| 2+ | 0 |
| 1+ | 1 (3.8) |
| 0 | 0 |
| Triple negative* | 7 (26.9) |
ER = Estrogen receptor; HER2 = Human epidermal growth factor receptor 2; IHC = Immunohistochemistry; n = Number of patients with observation; PR = Progesterone receptor; SD = standard deviation.
*HER2/neu: IHC graded (n = 6), FISH (n = 1).
Exposure
A total of 23 patients received the protocol-specified administration of the study drug, SV-BR-1-GM. Measured from Treatment Day 1 (not enrollment), the mean treatment duration was 51 d, ranging from 3 to 178 d (Table 3). Of the 23 (88.5%) patients who completed Cycle 1, 21 (80.8%) patients completed Cycle 2, and 17 (65.4%) patients completed Cycle 3. A summary of the number of patients with multiple cycles is provided in Table S1. In the course of the study, 23 patients received 79 inoculations of SV-BR-1-GM (mean 3.4, range 1–8; Table S2).
Table 3.
Summary of exposure.
| Characteristics | Exposure |
|---|---|
| Treatment duration (days) | |
| n | 23 |
| Mean | 51.43 |
| SD | 33.00 |
| Median | 41.63 |
| Min, Max | 3, 178 |
| Total number of SV-BR-1-GM inoculations | |
| Mean | 3.4 |
| Median | 3.0 |
| Min, Max | 1, 8 |
n = Number of patients with observation; SD = standard deviation
Safety results
Of 271 treatment-emergent adverse events (TEAEs), a majority of the TEAEs (181 [66.8%]) were reported unrelated to the study drug; 41 (15.1%), 24 (8.9%), 15 (5.5%), and 10 (3.7%) TEAEs were definitely, possibly, probably, and unlikely related to the study drug, respectively.
There were eight (30.8%) patients with TEAEs leading to discontinuation and two (7.7%) patients with TEAEs leading to death. The adverse events leading to the two deaths were respiratory failure and restrictive cardiomyopathy, both considered unrelated to the regimen; there were no SV-BR-1-GM-related deaths in the study.
The adverse events leading to discontinuation included dehydration, cancer pain, respiratory failure, vomiting, gastroesophageal reflux disease (GERD), hypertension, urticaria, and pleural effusion. All these events were judged unrelated to SV-BR-1-GM except for cancer pain, which was judged probably related to SV-BR-1-GM, and GERD, which was also judged possibly related.
The adverse events seen in ≥20% of patients were pruritus (26.9%), nausea (23.1%), fatigue (23.1%), erythema (23.1%), and injection site erythema (23.1%; Table S3). There were 193 (71.2%) Grade 1, 60 (22.1%) Grade 2, 11 (4.1%) Grade 3, 6 (2.2%) Grade 4, and 1 (0.4%) Grade 5 TEAEs.
With regard to the anticipated adverse events of special interest, there were no new or worsening autoimmune disease, major cutaneous reactions at the inoculation sites (e.g., ulcers, necrosis), allergic reactions, or cardiac events attributable to inoculation with SV-BR-1-GM.
Quality of life
At baseline, the highest SF-36 mean ± SD score was reported for emotional wellbeing (71.30 ± 17.79), followed by social functioning (70.11 ± 26.58). The mean score for physical functioning (61.09 ± 28.08) was also higher compared to the other SF-36 parameters. The lowest SF-36 scores were reported in the case of role limitations due to physical health (43.48 ± 47.20) and energy/fatigue (43.04 ± 21.52). The mean ± SD mental component summary (MCS) score was higher when compared to the physical component summary (PCS) score (60.97 ± 21.27 vs. 51.79 ± 23.97).
For patients with stable disease, the largest impairments after being on the study occurred in social functioning, pain, and general health; changes in the other categories, while negative, were small. The mean change from baseline in the SF-36 scores is presented in Table 4.
Table 4.
SF-36 scores at baseline and mean change from baseline.
| SF-36 Parameter | All Inoculated Patients |
Patients with Stable Disease |
Patients with Progressive Disease |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Baseline Mean ± SD |
Change from Baseline Mean ± SD |
N | Baseline Mean ± SD |
Change from Baseline Mean ± SD |
N | Baseline Mean ± SD |
Change from Baseline Mean ± SD |
|
| Physical Functioning | 23 | 61.09 ± 28.08 | −5.53 ± 15.84 | 8 | 66.88 ± 18.89 | −4.02 ± 14.63 | 8 | 62.50 ± 33.59 | −4.08 ± 15.50 |
| Role Limitations due to Physical Health | 23 | 43.48 ± 47.20 | −5.59 ± 29.36 | 8 | 59.38 ± 49.89 | 3.05 ± 29.15 | 8 | 50.00 ± 53.45 | −11.2 ± 29.46 |
| Role Limitations due to Emotional Problems | 23 | 59.42 ± 41.39 | −5.32 ± 33.97 | 8 | 83.33 ± 30.86 | 0.00 ± 34.96 | 8 | 70.83 ± 41.55 | −15.8 ± 30.74 |
| Energy/Fatigue | 23 | 43.04 ± 21.52 | −3.19 ± 12.29 | 8 | 48.13 ± 23.29 | −1.34 ± 13.83 | 8 | 45.63 ± 20.43 | −6.32 ± 10.82 |
| Emotional Wellbeing | 23 | 71.30 ± 17.79 | −1.57 ± 15.46 | 8 | 75.00 ± 8.75 | 4.39 ± 13.97 | 8 | 69.00 ± 26.43 | −10.4 ± 13.33 |
| Social Functioning | 23 | 70.11 ± 26.58 | −13.6 ± 22.05 | 8 | 85.94 ± 10.43 | −17.1 ± 25.12 | 8 | 67.19 ± 30.57 | −9.54 ± 20.64 |
| Pain | 23 | 56.09 ± 25.59 | −9.92 ± 23.58 | 8 | 70.31 ± 26.44 | −14.4 ± 24.11 | 8 | 61.25 ± 22.32 | −11.9 ± 23.73 |
| General Health | 23 | 46.52 ± 20.42 | −3.67 ± 15.66 | 8 | 46.88 ± 19.63 | 0.98 ± 15.58 | 8 | 46.25 ± 23.11 | −9.61 ± 13.67 |
| Health Change | 23 | 45.65 ± 26.81 | −2.93 ± 27.89 | 8 | 62.50 ± 26.73 | −11.0 ± 31.65 | 8 | 46.88 ± 20.86 | 0.00 ± 20.13 |
| PCS | 23 | 51.79 ± 23.97 | −6.18 ± 13.98 | 8 | 60.86 ± 23.97 | −3.60 ± 14.33 | 8 | 55.00 ± 27.53 | −9.19 ± 14.78 |
| MCS | 23 | 60.97 ± 21.27 | −5.91 ± 13.69 | 8 | 73.10 ± 11.90 | −3.51 ± 13.47 | 8 | 63.16 ± 25.91 | −10.5 ± 14.17 |
MCS = Mental component summary; N = Number of patients in specific group; PCS = Physical component score; SD = Standard deviation.
For patients with progressive disease, not only were there similar changes in the above categories but also deterioration in most of the others. However, as noted above, only 11% of the patients had a deterioration in their ECOG score.
A graphical representation of SF-36 scores over time is presented in Figure S2.
Efficacy results
Imaging and clinical benefit
Of 26 patients, imaging results were available for 22 (84.6%) patients; 4 (15.4%) patients had no imaging (one each with metastatic disease in the left medial arm, pleural cavity, chest wall, and lymph node which was not imaged) while another 3 (11.5%) had evaluable but not measurable disease (Table 5). Of 19 (73.1%) patients with measurable disease, 16 (61.5%) patients had at least one subsequent imaging event. Of patients with subsequent imaging, a similar proportion of patients were reported with SD or non-CR/non-PD (8 [30.8%]) and PD (8 [30.8%]; Table 5 and Figure 2(a)). No patient achieved a CR or PR in the study. In terms of histologic subtype, there were 25 patients with known histology: 17 were HR+ and HER2- or low (9 evaluable), 8 were triple negative (TNBC) (6 evaluable), one was HER2+ (no baseline imaging) and one lacked this histologic evaluation (1 evaluable). Of these, 4, 3, 0 and 1 patients achieved stable disease, respectively (Table 5).
Table 5.
Imaging analysis.
| Number of Patients n (%) |
|||||
|---|---|---|---|---|---|
| |
All Patients N = 26 |
ER+/HER2- or low N = 16 |
TNBC N = 8 |
HER2+ N = 1 |
No Histology N = 1 |
| Baseline Imaging | 22 (84.6) | 12 (46.2) | 8 (30.8) | 1 (3.8) | 1 (3.8) |
| No Imaging | 4 (15.4) | 4 (15.4) | 0 | 0 | 0 |
| Measurable disease | 19 (73.1) | 11 (42.3) | 6 (23.1) | 1 (3.8) | 1 (3.8) |
| Non-measurable/non-target disease | 3 (11.5) | 1 (3.8) | 2 (7.7) | 0 | 0 |
| Post baseline imaging data | 16 (61.5)1 | 9 (34.6) | 6 (23.1) | 0 | 1 (3.8) |
| Best overall response | |||||
| Complete response (CR) | 0 | 0 | 0 | NE | 0 |
| Partial response (PR) | 0 | 0 | 0 | NE | 0 |
| Stable disease (SD)2 | 8 (30.8) | 4 (44.4)3 | 3 (50)3 | NE | 1 (100)3 |
| Progressive disease (PD) | 8 (30.8) | 5 (55.6)3 | 3 (50)3 | NE | 0 |
N = Number of patients in specific group; n = Number of patients with observation; NE=not evaluable.
1Six (23.1%) patients did not have subsequent imaging. One patient completed one cycle then dropped out without further imaging. Another patient received one dose of cyclophosphamide, had a negative reaction, and dropped out prior to dosing with SV-BR-1-GM. Another patient developed restrictive cardiomyopathy and died shortly after the second inoculation. Another three patients completed three cycles then dropped because of PD, with no further imaging available.
2Includes one non-CR/non-PD.
3The percentages shown are for the evaluable patients with that histologic type.
Figure 2.
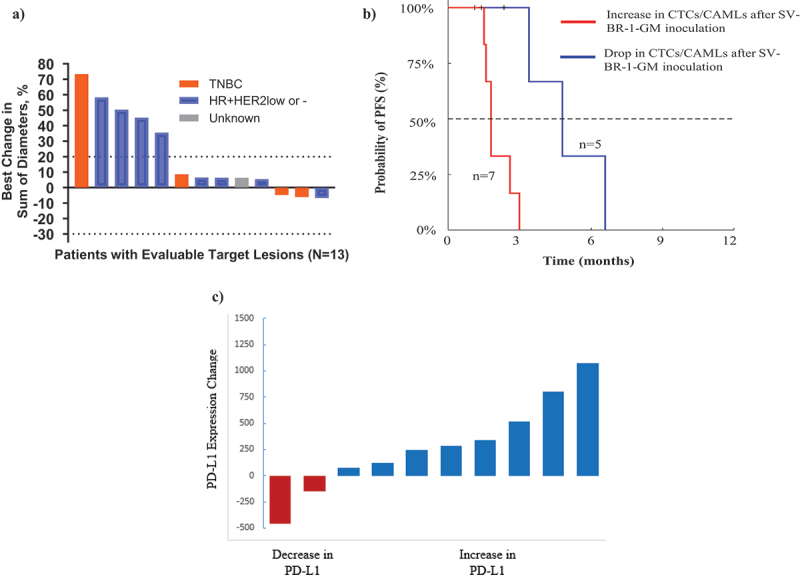
a) waterfall plot of all evaluable monotherapy patients, b) progression-free survival of monotherapy patients with CTC/CAML data, c) change in PD-L1 levels on CAMLs post-inoculation.
Percentage Change in the longest diameter of target lesions is shown as per RECIST criteria. In the data entry, histology can be entered as per the original diagnosis or metastatic lesions. For this analysis, the metastatic data is used whenever possible. One patient did not have tumor type but had measurements available. Two patients who had disease progression did not have measurements and, therefore, are not included in this graph.
CAMLs = Cancer-associated macrophage-like cells; CTC = Circulating tumor cells; PFS = Progression-free survival
CAMLs = Cancer-associated macrophage-like cells; PD-L1 = Programmed cell death ligand 1
Four patients showed objective tumor regression but in non-target lesions (Figure 2(a)). At baseline, one patient had 20 non-target lesions in the upper and lower lobes of each lung, measuring 3–10 mm. At follow-up, all lesions had either disappeared or had only residual scarring (Figure S2B). The other three patients each had measurable regression of cutaneous, cerebral, and mammary lesions (Table 6).
Table 6.
Characteristics of objective tumor regressions.
| Patient No. | Site | Baseline Size | Follow-up Size | DTH (Fore-arm) | DTH (Inocula-tion Site) | HLA Type (Matches with SV-BR-1-GM) |
|---|---|---|---|---|---|---|
| 01-002 | Lungs | Multiple 3-10 mm mets | Complete resolution or tiny residual scar 20/20 lung mets | Positive | Positive | A *24:02, DRB3 × 02:02 (2) |
| 01-005 | Cutaneous | 80% of breast | 30% | Positive | Positive | A *24:02 (1) |
| 02-003 | Brain (L parietal periventricular) | 5 mm | 2 mm | Negative | Positive | C * 04:01 (1) |
| Brain (L posterior parietal) | 2 mm | Undetectable | ||||
| 05-002 | Breast | 22.9 mm | 16.9 mm | Positive | Positive | DRB3 × 02:02, C * 04:01 (2) |
DTH = Delayed-type hypersensitivity; HLA = Human leukocyte antigen.
The HLA alleles of patients with tumor regressions matched SV-BR-1-GM at either one or two of three alleles: A * 24:02, C * 04:01, and DRB3 × 02:02.
For 23 patients inoculated, there seemed to be a trend for patients with DTH to have longer days on study, but the opposite was seen for HLA matches. The number of patients documented is small, and caution is necessary to draw conclusions. Of eight (30.7%) patients with SD, six (23.1%) patients generated immunological responses in DTH testing, and five (19.2%) patients had HLA matches with SV-BR-1-GM, while of eight (30.7%) patients with PD, four (15.4%) patients generated immunological responses in DTH testing, and seven (26.9%) patients had HLA matches with SV-BR-1-GM (Table 7).
Table 7.
Characteristics of patients evaluable for disease control and progressive disease.
| Days on Study | Number of Patients N = 26 n (%) |
||
|---|---|---|---|
| DTH Positive | HLA Match | ||
| Disease Control* | 82 | 6 (23.1) | 5 (19.2) |
| Progressive Disease | 57 | 4 (15.4) | 7 (26.9) |
DTH = Delayed-type hypersensitivity; HLA = Human leukocyte antigen; N = Number of patients in specific group; n = Number of patients with observation.
*Disease control consisted of eight patients with SD.
By disease response, considering time on study (TOS) beginning with the first inoculation, the patients with SD remained on the study longer than those with PD (82 d vs 57 d).
Best Response by Tumor Grade: Clinical benefit and tumor grade were closely correlated; patients with the less differentiated grade 3 were more likely to have PD and shorter time on study. For 10 patients with grade 3 differentiation, 7 had PD. For patients with grades 1–2, 5/6 had SD or non-PD (p = .01, Chi-square). Patients with tumor grade 3 were on study for a mean time of 39.1 d (median 36 d), while for patients with grade 2 or grade 1 tumors, the mean time on study was 94.5 d (median 97 d).
Markers
From baseline to the off-treatment evaluation, CEA levels were out of the normal laboratory range in 6 (23.1%) patients, CA 27–29 levels in 3 (11.5%) patients, and CA 15–3 levels in 6 (23.1%) patients. From these, a clinically significant change in markers was reported for two patients, which could be due to disease progression.
Pre-inoculation blood draws for CTC/CAML analysis were possible for 21 patients, and post-inoculation blood draws were available for 12 patients. Baseline samples were taken on average 3.1 d before inoculation (median = 2 d). The on/post-treatment samples were taken on average 75.8 d after the first inoculation (median = 79.5 d). Pre-inoculation, CTCs were found in 29% (n = 6/21) of patients, and CAMLs were found in 100% (n = 21/21), with the presence of CTCs not being correlated to progression-free survival (PFS; HR = 0.8 [CI 95% 0.3–2.4], p = .9105). Post-inoculation, CTCs were found in 33% (n = 4/12) of patients, and CAMLs were found in 100% (n = 12/12), with the presence of CTCs not correlated to PFS (HR = 0.3 [CI 95% 0.1–1.7], p = .3638). However, a drop in CTCs and/or CAMLs after inoculation was observed in 42% of patients, which correlated with a significantly improved PFS (HR = 17.1 [CI 95% 3.0–97.1], p = .0058; Figure 2(b)). Overall, patients with a decrease in CTCs and/or CAMLs after inoculation with SV-BR-1-GM therapy had an improvement in median PFS (4.1 months vs. 1.8 months).
We also identified a statistically significant and frequent increase in programmed cell death ligand 1 (PD-L1) expression in circulating stromal cells after inoculation with SV-BR-1-GM (8 of 10 [80.0%] patients, p = .01180; Figure 2(c) and Figure S2C).
Immune responses
DTH skin tests in the forearm to SV-BR-1-GM: A total of 13 (50%) patients generated a positive immunological response of ≥5 mm in DTH skin testing with SV-BR-1-GM. Seven of eight patients with SD generated a positive immunological response, while only four of eight patients with PD generated a positive immunological response in DTH skin testing. Of nine patients who were candida anergic at Cycle 1, six patients developed a positive immunological response to DTH at post-Cycle 1 visits.
Seventeen (65.4%) patients generated a positive immunological response by DTH skin testing criteria at the inoculation site. Seven of eight patients with SD generated a positive immunological response, while only five of eight patients with PD generated a positive immunological response by DTH criteria at the inoculation site. Of nine patients who were candida anergic at Cycle 1, eight patients developed a positive immunological response to DTH at the inoculation site in post-Cycle 1 visits.
Eight of nineteen (42.1%) patients with poorly differentiated tumors (tumor grade 3) generated an immunological response. In contrast, 4 of 5 (80%) and 1 of 3 (33.3%) patients with tumor grades 2 and 1, respectively, generated a positive immunological response by DTH skin testing. In patients with a positive immunological response, the highest DTH response of 83 mm was observed in a patient with a well-differentiated tumor (tumor grade 1), whereas in patients with tumor grades 2 and 3, the highest DTH responses were 41.3 mm and 76.4 mm, respectively (Table 8 and Table S4).
Table 8.
Correlation of DTH (patients with immunological Response; ≥5 mm) and tumor grade.
| Tumor Grade* | No. of Patients with Tumor Grade N = 26 n (%) |
No. of Patients with DTH Response ≥5 mm N = 26 n (%) |
Highest DTH Response (mm) |
|---|---|---|---|
| I-Well-Differentiated | 3 (11.5) | 1 (3.8) | 83 |
| II-Moderate | 5 (19.2) | 4 (15.4) | 41.3 |
| III-Poorly | 19 (73.1) | 8 (30.8) | 76.4 |
DTH = Delayed-type hypersensitivity; N = Number of patients in specific group; n = Number of patients with observation.
*One patient was diagnosed with grade 2 original cancer and grade 1 metastatic breast cancer.
Human leukocyte antigen results
All of the four patients with objective regressions had allele matches with SV-BR-1-GM; HLA alleles: A * 24:02, DRB3 × 02:02, and C * 04:01 (Table 6). Two patients with objective regressions had one match, and the other two had two matches. Having an HLA match was possibly a necessary (but not sufficient) feature for regressions. However, among the remaining group, half had matches, half did not, and by a simple 2 × 2 Chi-square statistic, these values give a statistical significance of p = .01. While the number of patients is small, this interesting finding needs to be further evaluated with subsequent studies.
Of five patients who had two or more HLA matches with SV-BR-1-GM, four had disease control. As expected, immunocompetence was a favorable characteristic independent of matching alleles, and three of three patients with a positive immunological response to Candida who also had a double HLA match had disease control. All patients who had responding DTH and two matches had clinical benefit; for DTH-reactive patients who were unmatched or matched with only one HLA-allele, clinical benefit could still occur, but they did so less frequently (50%). The patient HLA types, HLA matching loci count with SV-BR-1-GM, and the best overall response are provided in Table S5.
Patients who had ≥2 HLA matches with SV-BR-1-GM had the highest rate of disease control (Table 9). The increase in disease control rate is even more pronounced in patients with immune responses measured by DTH reactions to SV-BR-1-GM.
Table 9.
Disease control in all patients according to HLA match and immune responses.
| Patient | HLA Match | *Disease Control | **Disease Control in Immune Responders |
|---|---|---|---|
| All Patients | |||
| N = 6 | ≥2 | 50% | 100% (N = 3) |
| N = 19 | ≥1 | 21% | 50% (N = 8) |
| N = 6 | 0 | 33% | 50% (N = 4) |
| Patients who received study treatment | |||
| N = 5 | ≥2 | 60% | 100% (N = 3) |
| N = 17 | ≥1 | 24% | 50% (N = 8) |
| N = 6 | 0 | 33% | 50% (N = 4) |
HLA = Human leukocyte antigen; N = Number of patients in specific group.
*Disease Control includes eight patients with SD (stable disease) vs. eight patients with progressive disease (PD).
**Immune response measured by delayed-type hypersensitivity (DTH) measured by either induration or erythema ≥5 mm at the forearm DTH test site.
Discussion
This study enrolled patients with far-advanced, refractory breast cancer. We present data about response, survival, safety, and possible biological mechanisms at play. The data presented here describing four patients with tumor regressions, together with the previously described responder,12 support investigating a role for treatment with the Bria-IMT regimen. In addition to falsifying the notion of non-responsiveness to cancer vaccines, the essential role of GM-CSF is confirmed here insofar as there were no regressions in the previous pilot study of 14 patients (not HLA typed) inoculated with the non-transfected parent cell line, SV-BR-1.24 We emphasize that in this study, responses occurred in very heavily treated patients; conventional wisdom holds (incorrectly) that patients heavily treated with chemotherapy will not be responsive to immunotherapy.
These pilot studies by our group were designed to assess safety, clinical benefit, and possible biological correlates. While positive studies of cancer vaccines have been rare, some recent work is more supportive. Notably, a randomized clinical trial in melanoma, KEYNOTE-942 has received widespread recognition.25 That study showed an improvement in survival after inoculation with a personalized peptide/RNA anti-cancer vaccine. Their randomized clinical trial of high-risk resected melanoma patients showed a 78.6% rate of recurrence-free survival (RFS) in a group of melanoma patients after intramuscular inoculation of the vaccine in combination with pembrolizumab compared with pembrolizumab alone, that is, a 44% better RFS than pembrolizumab alone. There is increasing interest in such nucleic acid vaccines, including RNA vaccines for melanoma, non-small cell lung cancer, renal cell carcinoma, and prostate adenocarcinoma.26 This technology continues to develop, for example, with circular RNA vaccines27 and DNA vaccination.28 Promising initial results have been noted in breast cancer with a DNA vaccine for HER2/ERBB2.28 However, such vaccines are limited to the antigens that are administered and the need to break tolerance if these are self-antigens.
Another approach is the use of peptide vaccines, as in a dose-ranging study from Adotévi et al. in France.11 They described a potential universal vaccine, UCPvax, and reported findings in a study of 59 patients with stage IV refractory non-small cell lung cancer. Two highly selected peptides were administered to elicit CD4+ immune responses to telomerase, an enzyme highly overexpressed by cancer cells. One patient developed a complete response, and 39% had clinical benefit (stable disease), which clearly again falsifies conventional wisdom. They observed an 87% specific CD4+ response, which correlated with survival. The OS was 11.6 months in patients with tumor regression vs. 5.6 months in the non-responders.11 The authors take special note of these positive results insofar as most authorities consider the CD8+ T-cell as the more relevant class for cytotoxicity. Our data show CD4+ T-cells are activated by SV-BR-1-GM cells via peptide-loaded major histocompatibility complex (MHC) class II8 and function as antigen-presenting cells for CD4+ T cells. This functionality is likely critical to developing a strong tumor-directed immune response by SV-BR-1-GM.
A notable study by the Kandalaft group, studying refractory ovarian cancer patients treated with whole-tumor lysate-pulsed dendritic cell vaccine, bevacizumab, and cyclophosphamide, demonstrated improved overall survival (OS) in patients who developed vaccine-specific T-cell responses.29 Moreover, the investigators documented a dose–response curve between PFS and the quantity of immunoreactive T-lymphocytes, an analysis rarely if ever described in vaccine publications. A dose–response curve is an extremely powerful finding in establishing causality,30 and causality is an issue often overlooked in many publications.31 The dose–response curves were of particular interest insofar as there was a fairly strong correlation between PFS and the number of reactive T-cells, but the curve for OS was not so steep, possibly implying a role for other immune mechanisms.
Note that studies of therapeutic cancer vaccines, as discussed here, do not represent all potential cancer vaccines. Preventative vaccination for cancer is also possible as has been shown especially for cervical cancer.32 This is linked to the association of specific pathogens with the development of these cancers. As not all cancers arise from exposure to pathogens, preventative vaccination may be more difficult in identifying a particular target. Further research is clearly needed in this area.
In the analysis of the circulating cell markers (CAMLs and CTCs), the correlation with clinical activity shown in other cancer subtypes was also found in these MBC patients following SV-BR-1-GM inoculation. This was shown via the correlation between drops in CAMLs and significantly improved PFS.33 The SV-BR-1-GM cell line was developed from a woman with Grade 2 moderately differentiated breast cancer and has been shown to have cancer-testis antigens (especially PRAME), HER2/neu, and many others.7 The more favorable responses in Grades 1 and 2, as opposed to Grade 3, are consistent with the likely loss of antigenicity in the more poorly differentiated tumors.7,8 While the cell line expresses high levels of HER2, we did not have any evaluable HER2+ patients in the study. This will be an interesting group to evaluate in future studies. Patients with TNBC and ER+/HER2- or low had similar rates of stable disease. Future studies should further explore the effect of histologic subtype on patient responses.
Our data suggest an important element of developing clinical benefit is the matching of HLA alleles for the hypothetical strengthening of the immune response. We direct attention to the response of one patient, who had a match in both the Class I and Class II alleles. This patient enjoyed a durable subtotal regression of 20, up to 1 cm-sized, nodules in the lung, albeit with no significant reduction in rather massive liver metastasis. Also, we noted that this regression occurred rather rapidly (3 months) and occurred in a patient who had become refractory to seven regimens of community-standard chemotherapy; it is also remarkable that this responding patient with a positive HLA match had the highest increase of the group in PD-L1 levels.
Mechanistically, the injected irradiated cells would be expected to eventually die and provide antigens to local antigen presenting cells (APCs). The APCs should uptake the antigens, process them and generate peptides that can be potentially presented by the endogenous HLA alleles of the patient. The proposed role of GM-CSF in promoting the activity of the patient’s dendritic cells is consistent with this mechanism. However, we have previously shown that SV-BR-1-GM is also capable of stimulating an HLA-DR restricted T cell clone in an antigen-specific HLA-restricted manner.7 This suggests that SV-BR-1-GM may also function as an APC and present breast cancer antigens to the immune system. This may result in a boosting of the immune response in an HLA-dependent manner and could account for our clinical findings. Further study, including studies of patient immune responses, is needed to confirm these findings.
These data support the further evaluation of SV-BR-1-GM in the treatment of advanced breast cancer. The therapy appears very well tolerated, with the main side effect being local irritation at the inoculation sites which is transient. The upregulation of PD-L1 on CTCs and CAMLs suggests that this active, targeted immunotherapy vaccine approach may induce the expression of immune checkpoints in the tumor and/or tumor microenvironment. Thus, combination with immune checkpoint inhibitors is a logical next step in the development of SV-BR-1-GM. The observations on HLA matching and clinical benefit suggest that efficacy could be improved by engineering the expression of different HLA alleles in the cells. This would permit the cell lines to be personalized while maintaining the ability to pre-manufacture the cells and avoid individual manufacturing. This appears a promising avenue for further investigation.
Limitations: This study has numerous limitations, as would be expected from a small proof-of-concept study. There were relatively few patients treated. There was no randomization and no control arm. The patients had a variety of breast cancer types making comparisons with any historical data complicated, as breast cancer is usually treated based on hormone receptor and HER2 status. The patients had all failed numerous prior lines of therapy, and many could not mount a DTH response to the SV-BR-1-GM. In spite of these limitations, the initial data appear encouraging and warrant additional investigation of this therapeutic modality.
Conclusion
The findings from this cohort of 23 inoculated patients’ document safety, potential biological correlates, and, in several cases, rapid objective regression of metastases (within 3 months). The data also suggest a possible association of benefit with HLA-matching, especially if it is associated with the development of a positive immune response. Although preliminary, we found a fall in circulating CAMLs to be positively correlated with survival. A subsequent study, in combination with an immune checkpoint inhibitor, was initiated based on these results and is ongoing as of this writing. ClinicalTrials.gov Identifier: NCT03066947
Supplementary Material
Acknowledgments
The study was supported by BriaCell Therapeutics Corp. Employees of the sponsor had a role in study design, data analysis and manuscript preparation. Employees of the funder had no role in data collection.
Biographies
Dr. Charles L. Wiseman is the Co-Founder of BriaCell, and Principal Research Advisor. He was previously the principal investigator for immunotherapy treatment protocols at the St. Vincent Cancer Treatment Center and the Los Angeles Oncologic Institute. As the former Director of the Breast Cancer Basic Research Laboratory at the University of Texas MD Anderson Hospital in the 1970s, Dr. Wiseman was a pioneer in the field of cancer vaccine therapeutics. Dr. Wiseman has served as Clinical Professor of Medicine at the Division of Medical Oncology, Keck-USC School of Medicine, and Acting Chief of the Division of Oncology/Hematology at White Memorial Medical Center.
Dr. Jarrod P. Holmes completed his medical degree at Duke University School of Medicine and his Residency in Internal Medicine at the Naval Medical Center in San Diego, where he served as Chief Resident. He completed a fellowship in Hematology/Oncology with the National Capitol Consortium in Bethesda, Maryland, a combined program of the National Naval Medical Center and Walter Reed Army Medical Center. Dr. Holmes is a collaborating investigator with the Cancer Vaccine Development Program since 2006. His special area of interest has been the role of breast cancer vaccines in the prevention of breast cancer recurrence.
Dr. Carmen Calfa is an Associate Professor of Clinical Medicine at the University of Miami Miller School of Medicine, Associate Director of Community Outreach and Medical Co-Director of the Survivorship Cancer Program at Sylvester Comprehensive Cancer Center. She also serves as the Clinical Research Lead for the Breast Site Disease Group and Physician Leader of the Genetic Predisposition Syndrome Initiative at Sylvester Comprehensive Cancer Center. Dr. Calfa is the recipient of the Zubrod 2020 Outstanding Clinical Researcher of the Year Award and the 2020 Miami Dolphins Everyday Hero Award.
Dr. Shaker R. Dakhil obtained his medical degree in 1975 from St. Joseph University School of Medicine, Beirut, Lebanon. He completed his residency in Internal Medicine at Wayne State University, and fellowship in Hematology/Oncology at the University of Michigan. He is board certified in Internal Medicine and Medical Oncology. Dr. Dakhil joined the Cancer Center of Kansas in 1981 and has served as President since 2000. He is a clinical Professor of Medicine at the University of Kansas School of Medicine. He served as the Principal Investigator of the Wichita NCI Community Oncology Research Program, a National Cancer Institute sponsored research program.
Dr. Saveri Bhattacharya is an Assistant Professor of Clinical Medicine (Hematology-Oncology) at the Perelman School if Medicine of the University of Pennsylvania. She received her bachelor of arts degree from Cornell University in 2005. She went to medical school at Touro University-California graduating in 2011. She completed a residency and fellowship at the University of Pittsburgh Medical Center. She is a member of the American Society of Clinical Oncology and the American Association for Cancer Research. Dr. Bhattacharya has been active in research and has a number of peer reviewed publications including evaluation of novel therapeutics in breast cancer.
Dr. George E. Peoples is a graduate of the U.S. Military Academy and Johns Hopkins School of Medicine. He completed his surgical training at Harvard’s Brigham and Women’s Hospital and a surgical oncology fellowship at MD Anderson Cancer Center (MDACC). He served 30 years of active duty as a surgeon and research scientist in the U.S. military. He is the Founder and Director of the Cancer Vaccine Development Program. He founded LumaBridge, which is an oncology-focused CRO. Dr. Peoples serves as a Professor of Surgery at USUHS and Professor (adjunct) of Surgical Oncology at MDACC. He has over 350 peer-reviewed publications.
Markus D. Lacher earned his Ph.D. from the University of Bern, Switzerland and conducted cancer research at the University of California San Francisco. He worked in molecular cancer diagnostics at OncoCyte Corporation and critical limb ischemia at CESCA Therapeutics as Senior Clinical Scientist. Markus founded T cell Therapeutics, Inc., developing bispecific antibodies targeting prostate cancer. He led BriaCell’s R&D team as Senior Director, playing critical roles in conception and development of the Company’s pre-clinical and clinical immunotherapy pipeline. Markus joined Notable Labs in August 2020, initially serving as Head of Immuno-Oncology, and since August 2022 as Vice President of Research and Development.
Dr. Miguel Lopez-Lago received his Bachelor of Science and his doctorate in Molecular Biology from Santiago of Compostela University, Spain. Dr. Lopez-Lago worked as a cancer scientist at Memorial Sloan-Kettering Cancer Center, New York (MSKCC). He investigated various aspects of tumor biology, including the development of targeted therapies for Mesothelioma and the characterization of the biological mechanisms underlying cancer metastasis. Dr. Lopez-Lago has been interested in the study of the tumor immune-microenvironment and in the development of immunotherapies for thoracic cancers using chimeric antigen receptor (CAR) T cell technologies. He is currently the Chief Scientific Officer at BriaCell Therapeutics Corp.
Dr. Alex Kharazi earned his Ph.D. in immunology and medical degree in internal medicine and pathology in Kiev, Ukraine. His served as Chief Scientist of the Immunotherapy laboratory at St. Vincent Medical Center in Los Angeles and Chief Pathologist of a large animal study at the University of California, Los Angeles. He is a named inventor on eight U.S. patents and several foreign patents. Dr. Kharazi co-invented Bria-IMT™, BriaCell’s lead clinical candidate, in collaboration with Dr. Charles L. Wiseman. Dr. Kharazi currently serves as Chief Technology Officer at Stemedica Cell Technologies, Inc.
Dr. Giuseppe Del Priore completed his BA at City University of New York, MPH in Biostatistics and Epidemiology at University of Illinois Chicago School of Public Health, medical degree with Distinction from State University of New York, and additional training at Memorial Sloan Kettering Cancer Center, University of Chicago, Northwestern University, and University of Rochester. He served as a biotech Chief Medical Officer (CMO), a National Director at the Cancer Treatment Centers of America, and faculty at Indiana University School of Medicine, Weill Cornell Medicine, and New York University School of Medicine. He is currently the CMO at BriaCell Therapeutics Corp.
Dr. Mingjin Chang received a Bachelor’s of Science from National Taiwan University, Masters of Science in Cell Biology/Immunology, from University of Tübingen, Germany, and Doctorate of Philosophy in Biochemistry from Tulane University School of Medicine. She was a post-doctoral fellow in cancer pharmacology at Case Western University. She went on to be a Group Leader in Biologics production at ProtTech, Inc., a Senior Scientist and Group Leader in non-clinical bioanalysis at Janssen Diagnostics, and Scientist at Teva Pharmaceuticals. Dr. Chang served as a Clinical Scientist at BriaCell Therapeutics Corp. She is currently a Senior Lead Clinical Scientist at Rocket Pharma.
Daniel L. Adams is the Director of Clinical Research and Development at Creatv MicroTech, Inc. where he is the lead investigator in the isolation & identification of rare blood analytes, such as Circulating Tumor Cells (CTCs), Circulating Cancer-Associated Macrophage-Like Cells (CAMLs), Circulating Tumor DNA (ctDNA), and Circulating Tumor Proteins (CTPs) from cancer patients. He previously served as a research associate at SBH Sciences where he used various purification techniques from periplasmic, extracellular and intracellular proteins including bioactive cytokines and antibody development. Daniel earned his Bachelor of Science (BS) Biochemistry and Molecular Biology at UC Santa Barbara.
Dr. William V. Williams earned two Bachelors of Science degrees from MIT in Chemistry and Biotechnology and a Medical Doctorate from Tufts University School of Medicine. Board certified in Internal Medicine and Rheumatology, he did post-doctoral work at the University of Missouri, Columbia and the University of Pennsylvania School of Medicine. He served as Assistant Professor of Medicine and Head of Rheumatology Research at the University of Pennsylvania, VP of Clinical Pharmacology and Experimental Medicine at GlaxoSmithKline and VP of Exploratory Development at Incyte Corporation. Dr. Williams is currently BriaCell’s President & CEO.
Funding Statement
This study was fully funded by BriaCell Therapeutics Corp.
Disclosure statement
Charles L Wiseman is a paid scientific advisor and owns stock in BriaCell Therapeutics Corp. Makus D. Lacher is a paid scientific advisor and owns stock in BriaCell Therapeutics Corp. Miguel Lopez-Lago is a full time employee and owns stock in BriaCell Therapeutics Corp. Alex Kharazi is a paid scientific advisor and owns stock in BriaCell Therapeutics Corp. Giuseppe Del Priore is a full time employee and owns stock in BriaCell Therapeutics Corp. William V. Williams is a full time employee and owns stock in BriaCell Therapeutics Corp.
Author contributions
CW originated SV-BR-1-GM, designed the study, and was primarily responsible for developing the manuscript. JH aided in study design, patient treatment, and data acquisition. CC aided in study design, patient treatment, and data acquisition. SD aided in study design, patient treatment, and data acquisition. SB aided in study design, patient treatment, and data acquisition. GP aided in study design, patient recruitment, and data interpretation. ML aided in study design and data interpretation. ML aided in study design and data interpretation. AK developed SV-BR-1 and SV-BR-1-GM and aided in study design and data interpretation. GP aided in study design and data interpretation. MC aided in study design, data analysis, and data interpretation. DA aided in data analysis and data interpretation. WW supervised the study, aided in study design, data analysis, and data interpretation.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2379864
References
- 1.Tiwari A, Alcover KC, Carpenter EL, Thomas KK, Krum JM, Nissen AP, Van Decar S, Smolinsky TR, Valdera FA, Vreeland TJ, et al. Utility of cell-based vaccines as cancer therapy: systematic review and meta-analysis. Hum Vaccines Immunotherapeutics. 2024;20(1). doi: 10.1080/21645515.2024.2323256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leko V, Rosenberg SA.. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors. Cancer Cell. 2020;38(4):454–13. doi: 10.1016/j.ccell.2020.07.013. PMID: 32822573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soiffer RJ, Kooshesh KA, Ho V. Whole tumor cell vaccines engineered to secrete GM-CSF (GVAX). Immuno Med. 2021;1(1):e1025. doi: 10.1002/imed.1025. [DOI] [Google Scholar]
- 4.Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021. June. 21(6):360–378. doi: 10.1038/s41568-021-00346-0. Epub 2021 Apr 27. PMID: 33907315. [DOI] [PubMed] [Google Scholar]
- 5.Disis ML, Stanton SE. Can immunity to breast cancer eliminate residual micrometastases? Clin Cancer Res. 2013;19(23):6398–6403. doi: 10.1158/1078-0432.CCR-13-0734. PMID: 24298070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy PM, Clifton GT, Vreeland TJ, Adams AM, O’Shea AE, Peoples GE. AE37: a HER2-targeted vaccine for the prevention of breast cancer recurrence. Expert Opin Investig Drugs. 2021;30(1):5–11. doi: 10.1080/13543784.2021.1849140. PMID: 33191799. [DOI] [PubMed] [Google Scholar]
- 7.Lacher MD, Bauer G, Fury B, Graeve S, Fledderman EL, Petrie TD, Coleal-Bergum DP, Hackett T, Perotti NH, Kong YY, et al. SV-BR-1-gm, a clinically effective GM-CSF-Secreting breast cancer cell line, expresses an immune signature and directly activates CD4+ T lymphocytes. Front Immunol. 2018;9:776. doi: 10.3389/fimmu.2018.00776. PMID: 29867922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiseman CL, Kharazi A, Sunkari VG, Galeas JL, Dozio V, Hashwah H, Macúchová E, Williams WV, Lacher MD. Regression of breast cancer metastases following treatment with irradiated SV-BR-1-gm, a GM-CSF overexpressing breast cancer cell line: intellectual property and immune markers of response. Recent Pat Anticancer Drug Discov. 2022;18(2):224–240. doi: 10.2174/1574892817666220518123331. PMID: 35593340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JY, Cha H, Kim K, Sung C, An J, Bang H, Kim H, Yang JO, Chang S, Shin I, et al. MHC II immunogenicity shapes the neoepitope landscape in human tumors. Nat Genet. 2023;55(2):221–231. doi: 10.1038/s41588-022-01273-y. Epub 2023 Jan 9. PMID: 36624345. [DOI] [PubMed] [Google Scholar]
- 10.Kreiter S, Vormehr M, van de Roemer N, Diken M, Löwer M, Diekmann J, Boegel S, Schrörs B, Vascotto F, Castle JC, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015. Epub 2015 Apr 22. Erratum in: Nature. 2015 Jul 16;523(7560):370. PMID: 25901682. 520(7549):692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adotévi O, Vernerey D, Jacoulet P, Meurisse A, Laheurte C, Almotlak H, Jacquin M, Kaulek V, Boullerot L, Malfroy M, et al. Safety, immunogenicity, and 1-year efficacy of universal cancer peptide-based vaccine in patients with refractory advanced non-small-cell lung cancer: a phase Ib/phase IIa De-escalation study. J Clin Oncol. 2023;41(2):373–384. doi: 10.1200/JCO.22.00096. PMID: 36070539. [DOI] [PubMed] [Google Scholar]
- 12.Wiseman CL, Kharazi A. Objective clinical regression of metastatic breast cancer in disparate sites after use of whole-cell vaccine genetically modified to release sargramostim. Breast J. 2006;12(5):475–480. doi: 10.1111/j.1075-122X.2006.00319.x. PMID: 16958969. [DOI] [PubMed] [Google Scholar]
- 13.Adams DL, Martin SS, Alpaugh RK, Charpentier M, Tsai S, Bergan RC, Ogden IM, Catalona W, Chumsri S, Tang CM, et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc Natl Acad Sci USA. 2014;111(9):3514–3519. doi: 10.1073/pnas.1320198111. PMID: 24550495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang CM, Zhu P, Li S, Makarova OV, Amstutz PT, Adams DL. Blood-based biopsies-clinical utility beyond circulating tumor cells. Cytometry A. 2018;93(12):1246–1250. doi: 10.1002/cyto.a.23573. PMID: 30369050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mjh BD, Mastrangelo M, Mastrangelo MJ. Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res. 1986;46(5):2572–2577. [PubMed] [Google Scholar]
- 16.Kuroi K, Sato Y, Yamaguchi Y, Toge T. Modulation of suppressor cell activities by cyclophosphamide in breast cancer patients. J Clin Lab Anal. 1994;8(3):123–127. doi: 10.1002/jcla.1860080302. [DOI] [PubMed] [Google Scholar]
- 17.Proietti E, Bracci L, Puzelli S, Di Pucchio T, Sestili P, De Vincenzi E, Venditti M, Capone I, Seif I, De Maeyer E, et al. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J Immunol. 2002;169(1):375–383. doi: 10.4049/jimmunol.169.1.375. [DOI] [PubMed] [Google Scholar]
- 18.Wiseman C, Hood Y, Presant C, Kennedy P. OKT-3/cyclophosphamide up-regulation of peripheral blood killer-lymphocyte subsets in human cancer patients. Mol Biother. 1991;3:63–67. [PubMed] [Google Scholar]
- 19.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, Proietti E. Cyclophosphamide induces type I interferon and augments the number of CD44hi T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95(6):2024–2030. doi: 10.1182/blood.V95.6.2024. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. PMID: 27189322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et al. RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):143–152. doi: 10.1016/S1470-2045(17)30074-8. PMID: 28271869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran JA, Adams DL, Edelman MJ, Lopez P, He J, Qiao Y, Xu T, Liao Z, Gardner KP, Tang CM, et al. Monitoring PD-L1 expression on circulating tumor–associated cells in recurrent metastatic non–small-Cell lung carcinoma predicts response to immunotherapy with radiation therapy. JCO Precis Oncol. 2022;6(6):e2200457. doi: 10.1200/PO.22.00457. PMID: 36516370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghavakaimal A, Cristofanilli M, Tang CM, Alpaugh RK, Gardner KP, Chumsri S, Adams DL. CCR5 activation and endocytosis in circulating tumor-derived cells isolated from the blood of breast cancer patients provide information about clinical outcome. Breast Cancer Res. 2022;24(1):35. doi: 10.1186/s13058-022-01528-w. PMID: 35606863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiseman CL, Kharazi A. Phase I study with SV-BR-1 breast cancer cell line vaccine and GMCSF: clinical experience in 14 patients. The Open Breast Cancer J. 2010;2(1):4–11. doi: 10.2174/1876817201002010004. [DOI] [Google Scholar]
- 25.Khattak A, Carlino M, Meniawy T, Ansstas G, Medina T, Taylor MH, Kim KB, McKean M, Long GV, Sullivan RJ, et al. Abstract CT001: a personalized cancer vaccine, mRNA-4157, combined with pembrolizumab versus pembrolizumab in patients with resected high-risk melanoma: efficacy and safety results from the randomized, open-label phase 2 mRNA-4157-P201/Keynote-942 trial. Cancer Res. 2023;83(8_Supplement):CT001. doi: 10.1158/1538-7445.AM2023-CT001. [DOI] [Google Scholar]
- 26.Zhang TY, Xu H, Zheng XN, Xiong XY, Zhang SY, Yi XY, Li J, Wei Q, Ai JZ. Clinical benefit and safety associated with mRNA vaccines for advanced solid tumors: a meta-analysis. MedComm. 2020. [2023 Jul 18]. 4(4):e286. doi: 10.1002/mco2.286. PMID: 37470066; PMCID: PMC10353527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie J, Ye F, Deng X, Tang Y, Liang JY, Huang X, Sun Y, Tang H, Lei J, Zheng S, et al. Circular RNA: a promising new star of vaccine. J Transl Int Med. [2023 Dec 20]. 11(4):372–81. doi: 10.2478/jtim-2023-0122. PMID: 38130633; PMCID: PMC10732498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Disis MLN, Guthrie KA, Liu Y, Coveler AL, Higgins DM, Childs JS, Dang Y, Salazar LG. Safety and outcomes of a plasmid DNA vaccine encoding the ERBB2 intracellular domain in patients with advanced-stage ERBB2-positive breast cancer: a phase 1 nonrandomized clinical trial. JAMA Oncol. [2023 Jan 1]. 9(1):71–78. doi: 10.1001/jamaoncol.2022.5143. PMID: 36326756; PMCID: PMC9634596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanyi JL, Chiang CL, Chiffelle J, Thierry AC, Baumgartener P, Huber F, Goepfert C, Tarussio D, Tissot S, Torigian DA, et al. Personalized cancer vaccine strategy elicits polyfunctional T cells and demonstrates clinical benefits in ovarian cancer. NPJ Vaccines. 2021. Mar 15. 6(1):36. doi: 10.1038/s41541-021-00297-5. Erratum in: NPJ Vaccines. 2021 May 4;6(1):68. PMID: 33723260; PMCID: PMC7960755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S . Surgeon general’s advisory committee on smoking. Smoking and health: report of the advisory committee to the surgeon general of the public health service. US Department of Health, Education, and Welfare, Public Health Service; 1964. [Google Scholar]
- 31.Wiseman MDC. Questions from the fourth son: a clinician reflects on immunomonitoring, surrogate markers and systems biology. Math Biosci Eng. 2011. Apr. 8(2):279–87. doi: 10.3934/mbe.2011.8.279. PMID: 21631130. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Yu K, Zhu B, Mei S, Huo J, Zhao Z. Trends in research related to vaccine and cancer prevention from 1992 to 2022: a 30-years bibliometric analysis. Hum Vaccin Immunother. [2023 Dec 31]. 19(1):2207441. doi: 10.1080/21645515.2023.2207441. Epub 2023 May 9. PMID: 37158187; PMCID: PMC10294762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardner KP, Aldakkak M, Tang CM, Tsai S, Adams DL. Circulating stromal cells in resectable pancreatic cancer correlates to pathological stage and predicts for poor clinical outcomes. NPJ Precis Oncol. 2021;5(1):25. doi: 10.1038/s41698-021-00161-8. PMID: 33742084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


