ABSTRACT
Respiratory syncytial virus (RSV) is an important cause of respiratory illness. While most attention is paid to childhood infection, the RSV burden in adults ≥60 y should also be considered. In Brazil, this is generally underrecognized, where greater focus is toward other respiratory pathogens. This article presents insights from a multidisciplinary panel gathered to review epidemiologic data and current diagnostic approaches to RSV in Brazil (and their limitations) and develop communication strategies to improve knowledge and awareness. National surveillance data indicate a steady increase in cases of RSV-related severe acute respiratory illness (RSV-SARI) in those aged ≥60 y in recent years, with high fatality rates (>30%). Routine RSV testing in older individuals with respiratory symptoms is relatively low. Educational activities targeted toward health-care professionals and the general public are critical to raising awareness of the importance of RSV in older individuals, particularly as protective vaccines are now available.
KEYWORDS: Respiratory syncytial virus, RSV, disease burden, older adults, Brazil
Introduction
Respiratory syncytial virus (RSV) is a leading cause of morbidity and mortality in children <5 y of age, especially in younger infants.1–3 However, RSV is being increasingly recognized as an important cause of acute, and often severe respiratory illness in older adults ≥60 y of age, where hospitalization and mortality may be substantial.4–8 Prospective community-based studies from the United States (US) and Europe indicate that symptomatic RSV illness occurs in between 3–7% of older adults each year.9–11 As many as 25% of older adults with RSV infection will require hospitalization,7 and in high-income countries, RSV accounts for 4–12% of all acute respiratory infection (ARI) hospitalizations in adults ≥60 y of age (and broadly comparable to those reported for influenza).12–14 RSV disease severity is greater in individuals with comorbidities (cardiopulmonary disease, diabetes, immunocompromised), and hospitalization rates are 3–10-fold higher in such high-risk patients, and where RSV infection is associated with worsening of underlying cardiopulmonary disease.7,15,16 RSV-associated mortality is also substantial in older adults, both in the acute phase and over the longer term, and again is far greater in those with comorbidities.16–18 Furthermore, it is generally recognized that estimates for morbidity and mortality are likely to underestimate the true burden.19,20
Despite this, the role of RSV as an important respiratory illness and a frequent cause of severe acute respiratory infection (SARI) in older adults, and it remains relatively underrecognized, both by clinicians and by the broader lay public, with a general perception that RSV is a pediatric infection.21–23 For older adults presenting with any SARI in the clinical setting, more attention is paid toward influenza diagnosis (and obviously to coronavirus disease 2019 [COVID-19] disease in recent years) with the possibility of RSV considered less likely and infrequently investigated.24 This perception is generally apparent in most countries, including Brazil, and indeed across Latin America, where studies focusing on RSV in older adults are relatively sparse.25–27 At a broader public health level, influenza in older adults also gathers greater attention than RSV, as routine vaccination of older adults is embedded within national immunization policy, and where broader routine surveillance allows assessment of the impact and benefits of such policies.28–30 As we describe below, RSV case notifications in older adults are increasing within Brazil,31 within a broader pattern of substantial increases in RSV notifications in recent years (although in part, this also reflects expanded testing for viral respiratory pathogens initiated during the COVID-19 pandemic). Nevertheless, routine RSV testing in older adults is relatively low, although, to some extent this is a global pattern, rather than one specific to Brazil.21,22,24
Such considerations highlight the need to raise awareness of the significance of RSV infection in Brazilian older adults to health-care professionals and the general public. Indeed, this has been highlighted nationally, with a parliamentary health committee public hearing in October 2023 regarding RSV epidemiology and the need for greater awareness.32 This is particularly important given the recent development of effective RSV vaccines for use in individuals ≥60 y of age,33–35 and their recent approval for use in Brazil.36,37 To this aim, a multidisciplinary panel was formed to provide insights into the current status of RSV involving older adults within Brazil, including the potential benefits of protective vaccination. The present paper represents the major findings and insights from these discussions.
Methods
A multidisciplinary panel was established comprising individuals with experience and expertise across a range of specialties (infectious disease, pulmonologists, geriatrics, pediatrics, gynecology, epidemiology and immunization) from across Brazil. The panel convened for a one-day meeting in Sao Paulo on July 21, 2023, to review RSV pathophysiology and immunology, recent surveillance data, and the significance of comorbidities in RSV outcomes in older adults. The panel discussion was organized by GSK. As part of this activity, a specific retrospective analysis of RSV-SARI notifications within the Sistema de Informação da Vigilância Epidemiológica da Gripe (SIVEP-Gripe) database for 2020–2022 was performed, with some data subsequently presented in September 2023 at a national conference.38 The panel then discussed and provided perspectives on perceptions of RSV risk and diagnostic testing within Brazil and strategies that may help increase awareness of RSV disease among health-care professionals and the lay public.
This project was conducted through a review of publicly available literature and anonymized datasets and did not involve specific human participants or any identifiable data. Institutional review board approval and informed consent were not required.
Overview of RSV infection
RSV is a negative-sense single-stranded RNA capsulated enveloped pneumovirus belonging to the Paramyxoviridae family within the broader Mononegavirales order.39,40 Virulence is associated with two key capsular glycoproteins. The G protein facilitates attachment to respiratory epithelial cells, while the F protein mediates cell entry and also fusion of the virus envelope with the host cell membrane and adjacent cell–cell fusion of the virus envelope with the host cell membrane and adjacent cell–cell fusion, generating multinucleated syncytia, hence the virus name. The G protein shows genetic and antigenic diversity, which underlies the classification of RSV into RSV-A and RSV-B groups/subtypes. Both RSV-A and B can co-circulate, although in general, for most settings (continental, national or regional level), one subtype will generally predominate over an individual season.41 For example, national serological data for Brazil indicate that RSV-B predominated in 2016 and 2017, while in 2018, RSV-A was predominant.42 More recently, data indicate that RSV-A was the most frequent form identified within samples in Sao Paulo in 2021, whereas RSV-B predominated in 2022, with a further switch to RSV-A being predominant in 2023.43,44 Whether these subtypes are associated with differences in disease severity is open to debate, and while some studies indicate that RSV-A may cause more severe disease, this is not a consistent finding.42,45 In contrast to the G protein, the F protein is highly conserved (across A and B subtypes) and is the target antigen for RSV vaccines developed for older adults,34,35 and also for passive immunization treatments for infants.46
RSV infection shows a seasonal pattern, typically peaking in temperate countries in the winter months.47 In Brazil, which spans a vast geographical region and differing climates (from temperate to sub-tropical), this seasonal pattern is less pronounced, with some variations across the country. However, the general pattern is for RSV activity to begin from early March onwards, peaking from May to July42,47,48 (Figure 1). As we discuss below, these usual patterns, globally and within Brazil, were disrupted by declines in seasonal respiratory infections (both influenza and RSV cases) in the first year of the COVID-19 pandemic associated with nonpharmaceutical interventions (lockdowns and school/workplace closure, physical distancing, reduced movement and social mixing, mask policies, and increased hand hygiene) implemented to mitigate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission.49–53 Some elements of under-testing for other respiratory pathogens due to limited diagnostic capacity during the COVID-19 pandemic may also have contributed to the decline in RSV case numbers.54 As such measures relaxed, RSV infection resurged, with more atypical seasonal patterns. Similar changes were observed globally in both Northern and Southern temperate zones.51,52,55
Figure 1.
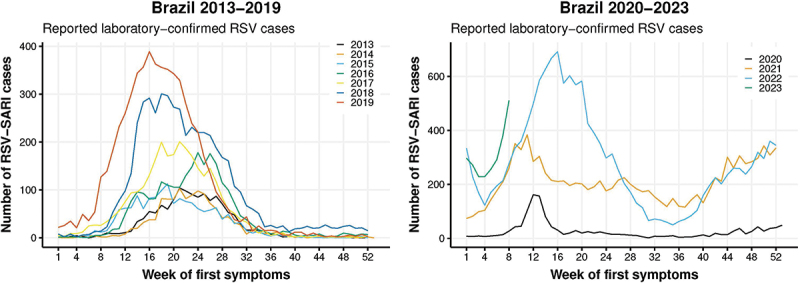
Overview of RSV-SARI notifications and seasonal patterns in Brazil since 2013.
Data reflect weekly number of RSV positive SARI cases reported in the SIVEP-Gripe database for 2013–2019 and 2020–2022 (and early 2023). The broad pattern is that of an increasing number of cases reported each year. Prior to COVID-19, RSV activity typically begins from March onwards, peaking through May to June. In 2020, case numbers fell substantially, with a resurgence in case numbers in 2021 and particularly 2022 onwards. RSV activity in these years shows a different pattern, with an initial peak in March/April followed by a subsequent increase in the latter part of the year.
Abbreviations: COVID-19; coronavirus disease 2019; RSV; respiratory syncytial virus; SARI: severe acute respiratory infection
The RSV virus is highly contagious, chiefly transmitted by aerosol/droplet infection, direct contact with infected individuals and indirect contact via contaminated objects and surfaces. Transmission between infected individuals, including children of different age-groups (siblings or at school) and between children and adults (at home or within the community) is poorly understood.56 Neither is transmission within the hospital setting (between and among patients and health-care workers), although nosocomial infection and hospital outbreaks are well reported, as are outbreaks within geriatric long-term care facilities.57,58
Following exposure, symptoms initially manifest after 3–4 d and are broadly similar to those seen with influenza or other influenza-like illnesses (ILIs); (cough, nasal congestion and rhinorrhea, wheezing and sore throat). In healthy younger adults, RSV typically manifests as a mild upper respiratory tract infection, resolving within 7–10 d and rarely causing severe disease.59 Clinical progress is quite different at both ends of the age-spectrum. In young children, and especially infants, lower respiratory tract infection (LRTI) with bronchiolitis and pneumonia are far more common, often with superimposed bacterial infection.59 Similarly, LRTI with pneumonia complications are also far more frequent in older individuals and, as discussed below, especially in those with comorbidities or otherwise frail, and where exacerbations of existing cardiopulmonary disease contribute to greater adverse outcomes.15,21,59 In older adults with RSV, cough and dyspnea are common. Dyspnea is often severe, particularly in high-risk patients, with over 20% of cases requiring ventilatory support and admission to intensive care units (ICU).7,16,60 In contrast, fever is generally less frequent than found with influenza or other ILIs, occurring in less than 50% of cases, and often mild (<38°C).21,59,60 This aspect has implications for testing where protocols may involve overt fever as a specific testing criterion.
Immune responses to RSV are complex and, in high-risk age-groups, are influenced by either immunological immaturity (in infants) or by age-associated immune decline ‘immunosenescence’ (older individuals).61 In severe disease, tissue inflammation is mediated through T-cell (Th2) pathways and often exaggerated pro-inflammatory cytokine/chemokine responses,40,61 and these may also contribute to acute cardiopulmonary exacerbations seen with RSV infection.16,62 RSV re-infection is common across the lifespan; in older individuals, while antibody responses against RSV infection can be robust, these are relatively short-lived, as are RSV-specific T cell responses.40,61
Burden of RSV in older adults in Brazil
In Brazil, epidemiologic studies reporting on burden in older adults are relatively sparse.25,26 Data for RSV-SARI reported within the SIVEP-Gripe database provide a useful indication of the overall burden of severe respiratory illness due to RSV within Brazil29,63 (Figure 1). This database records all notified reverse transcriptase polymerase chain reaction (RT-PCR) confirmed RSV-SARI cases, where the SARI case definition involves any patient hospitalized with fever (>37.5°C), cough and sore throat, presenting with either dyspnea, oxygen saturation <95%, respiratory distress,64 although it should be recognized that, as we later discuss, not all SARI cases are routinely tested for RSV.
One important feature of these data is the increasing number of cases reported annually from 2013 onwards. From a temporal perspective, a general trend between 2013 and 2019 was that RSV cases in older adults aged ≥65 y lagged behind those in children <2 y by approximately 2 weeks. As described above, a substantial decline in RSV cases was observed in 2020, the first year of the COVID-19 pandemic. As measures to contain COVID-19 relaxed, RSV infection shows a more off-season pattern, with 2021 and 2022 each showing an initial peak in March/April followed by a second peak in the latter part of the year, from September through December (Figure 1). Such disruption to more typical patterns is not unique, with similar changes observed in both Northern and Southern temperate zones.51,52,55 Perhaps of greater significance is the evidence of RSV resurgence since 2020. In part, this reflects an increase in surveillance activities and testing for a wider range of respiratory pathogens, particularly from 2020 onwards to monitor COVID-19, and also changes to surveillance guidelines (with the expansion of SARI definitions to include cases without fever, especially in the elderly),65,66 and greater adherence to SARI notification by health practitioners across the country. While this substantial increase in recent years highlights the importance of SARIs due to RSV, it complicates making direct comparisons of disease burden in these different periods. However, looking more specifically at these recent data does allow a fuller understanding of the present burden of RSV in Brazil, which is the focus of this article.
From January 2020 to December 2022, a total of 30,934 cases of RSV-SARI were reported to the SIVEP-Gripe database. In 2020, 1,681 cases were reported, peaking in March, after which a relatively low number of cases were reported for this year (when stringent COVID-19 mitigation strategies were in place). This was followed by RSV resurgence, and some disruption to typical seasonal patterns. In 2021, 12,478 cases were reported, with an initial peak in mid-March, followed by a gradual decline and then increasing case numbers, with a second peak in November maintained until the year-end. In 2022, 16,775 cases were reported, with a marked increase in cases from February onwards, peaking in April and then with a marked decline followed by a further increase in RSV-SARI case numbers, and then with a marked decline followed by a further increase in RSV-SARI case numbers, then subsequently increased from September onwards. This two-peak pattern was not observed in Brazil before COVID-19 introduction in 2020 and is a clear signature of the disruption of RSV-SARI seasonality in the country. Whether this scenario will be the new seasonal pattern or just a transition phase before re-establishing a stable one-peak season remains to be seen. It should be noted that most cases were reported in young children <5 y of age. However, in 2022, 934 RSV-SARI cases were reported in those aged ≥60 y (Figure 2 and Supplementary Table S1). More detailed temporal data from the SIVEP-Gripe database which may inform RSV transmission patterns between different age groups are currently being evaluated. A high proportion of these hospitalized older adults required ICU admission (approaching 30% in each of these years, Supplementary Table S2). RSV-SARI associated mortality was also substantial, especially in older adults (Figure 3). Between 2020 and 2022 a total of 852 deaths due to RSV-SARIs were reported in the SIVEP-Gripe database. While RSV-SARIs in those aged ≥60 y accounted for 5.7% of all such cases across this period, deaths in older adults accounted for 48% of all notified RSV-SARI deaths. Fatality rates were far more significant in those aged ≥60 y, with a case fatality rate (CFR) of 32.4% for hospitalized RSV-SARI cases in 2020 and 20.8% in 2022 (Figure 3). In contrast, CFRs for those aged ≤9 y were between 1–2%, a feature also reported in other studies evaluating pediatric RSV-SARI over this same period.31,67 Another recently reported study, evaluating RSV-confirmed ARI and hospitalized SARIs in the southeast of Brazil (Rio Grande do Sul) in 2021, also reported high fatality for RSV-SARI in hospitalized patients aged ≥60 y (31.2%), and where 73.6% of all deaths in patients hospitalized with RSV were in this older population.31
Figure 2.
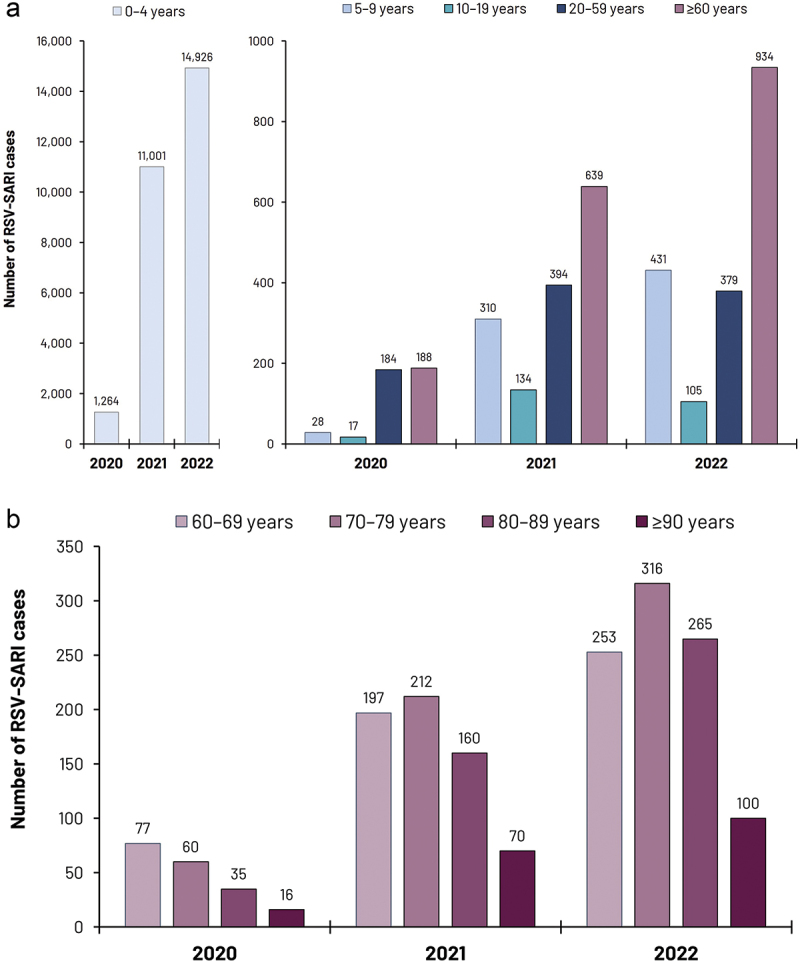
RSV-SARI case numbers reported within the SIVEP-Gripe database 2020–2022.
Upper panel (a) reports the number of reported RSV-SARI cases in different age strata. Lower panel (b) reports RSV-SARI cases in specific age-strata in older adults.
Abbreviations: RSV; respiratory syncytial virus; SARI: severe acute respiratory infection
Figure 3.
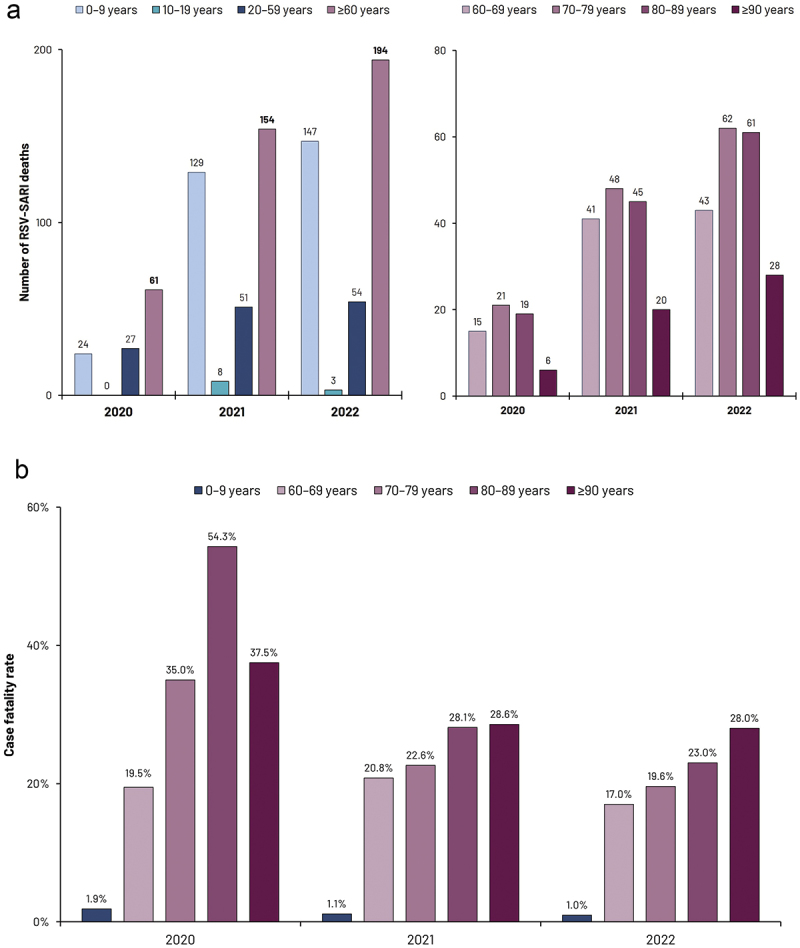
RSV-SARI mortality 2020–2022.
Upper panel (a) reports the number of reported RSV-SARI deaths in different age strata. Lower panel (b) illustrates case fatality rates
Abbreviations: RSV; respiratory syncytial virus; SARI: severe acute respiratory infection
These high fatality rates in older adults are similar to, if not greater than those reported for influenza SARI cases, with one recent study also utilizing SIVEP-Gripe data reporting CFRs of between 19–22% in those aged 60–79 y,68 while another reported a CFR of 24.6%.69 Based on population demographics,70 overall mortality rates in those aged 60–69 y across 2020–2022 ranged from 0.09 per 100,000 in 2021 to 0.24 per 100,000 (in 2021 and 2022), with greater rates seen with increasing age; up to 2.3 per 100,000 in those aged ≥90 y in 2021 and 3.1 per 100,000 in 2022 (Supplementary Table S3).
It is worth noting that these data relate to RSV-SARI notifications and chiefly represent short-term in-hospital mortality. It is generally recognized that hospital mortality alone underestimates RSV mortality, accounting for only 25% of the overall RSV mortality in younger infants, with as much as three times as many deaths occurring within the community setting.2 One might expect similar underestimations for older adult mortality due to RSV. In addition, subsequent re-hospitalization and mid- to longer-term mortality associated with RSV in the elderly is well-recognized,16 although at present such data are lacking for Brazil.
While the data we describe here relate to SARIs within SIVEP-Gripe, such data provide only a snapshot of the overall RSV burden within Brazil, and at a national level. Other data from SIVEP-Gripe and additional reporting systems, such as that from the Instituto Todos pela Saúde (ITpS) indicate that the greatest RSV burden is observed in the South and Southeast of the country.48 However, more data are required to fully understand regional differences in adult RSV disease in Brazil. It should also be understood that the number of cases of less severe disease within both the hospital and broader community setting (primary care and emergency department visits) will be far greater still; ITpS data, which also accounts for samples taken from outpatient care clinics, points toward this.71 A fuller analysis of such data can provide greater insights into the broader overall burden of RSV disease and the impact of healthcare resource use, which may be under considerable stress during peak seasonal months.
RSV impact on patients with comorbidities
There is a wealth of evidence that comorbidities are associated with greater adverse outcomes and poorer prognosis with RSV infection.4,7,11,15,16,72–79 Although risk and that associated with specific comorbidities vary across studies, most common comorbidities frequently occurring in older adults confer a substantially greater risk of RSV-related hospital admission (3–10-fold higher), more severe disease, and higher short- and long-term RSV-associated mortality.7,15,16 A recent large surveillance study from the US reporting on older patients hospitalized with RSV found that 95% had at least one comorbidity, most commonly obesity (37.8%), chronic obstructive pulmonary disease (COPD, 33.7%), congestive heart failure (CHF, 33.2%), diabetes mellitus (32.6%) and chronic kidney disease (29.3%).80 Another study found that overall, older adults with any comorbidity were six times more likely to be hospitalized with RSV than otherwise healthy older individuals.4,15 The risk of hospitalization due to RSV disease in patients with specific comorbidities shows some variation (Figure 4). In their pooled analysis of US data (chiefly that reported by Branche et al.73), McLaughlin et al. reported that COPD was associated with the greatest risk of RSV hospitalization (an almost 9-fold higher risk in those aged ≥65 y, and 6-fold in those aged 50–64 y).15 Substantially greater RSV hospitalization rates in adults aged ≥65 y were also reported for those with CHF (7-fold), diabetes (almost 5-fold), coronary artery disease (CAD, almost 5-fold), and asthma (2.4 fold).15,73 Greater risk was also associated with obesity.15,73 Other US studies also indicate higher RSV hospitalization rates in older patients with chronic kidney disease and immunocompromised older adults (including patients with solid or hematologic cancers and with HIV infection).18 These patterns are evident across most regions and different ethnic populations,4,7,11,15,16,72–79,81 with one systematic review reporting that one-third of high-risk older adults with RSV infection will require hospitalization, with over 25% requiring ICU admission.7 While our focus here is on those older than 60 y, individuals aged ≥50 y with comorbidity are also at greater risk of hospitalization due to RSV infection compared to otherwise healthy individuals (Figure 4).15,73
Figure 4.
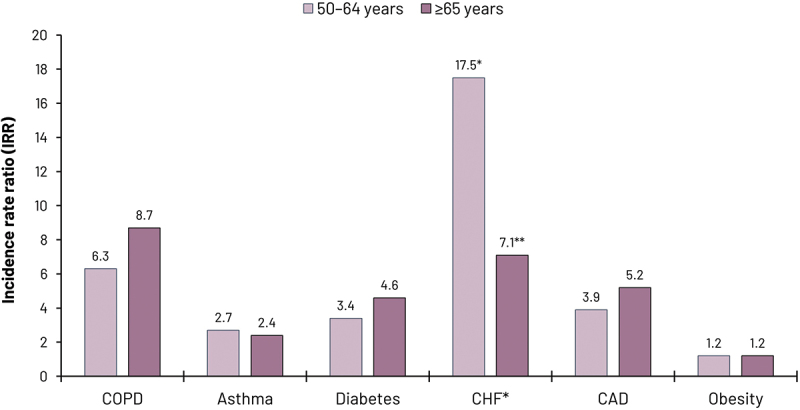
Risk of hospitalization due to RSV associated with comorbidities.
Hospitalization risk derived from incidence rates for hospitalization in patients with and without selected comorbidities and associated incidence rate ratios. Data represents pooled analysis of data for US adults reported by McLaughlin et al.,15 derived from data originally reported by Branche et al.73
* For patients with CHF, data represents individuals aged 40–59 y* and 60–79 y**
Abbreviations: CAD: coronary artery disease; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; RSV: respiratory syncytial virus; SARI: severe acute respiratory infection; US: United States.
RSV-associated pneumonia complications are more common in such high-risk older adults,7,16 and acute exacerbations of underlying cardiopulmonary disease (COPD, acute decompensation in CHF), contribute to greater mortality in this population.16,62 Some studies suggest higher viral loads in adults with more severe disease, although this is an inconsistent finding.82,83 While estimates vary, a systematic review of studies from industrialized and also developing countries reported a CFR in older adults with a comorbidity of 11%.17 These all point toward a substantial impact of comorbidity on outcomes following RSV infection, and we should note that while our focus here is on older adults, the patterns we describe are also apparent in younger adults with comorbidity).7 Furthermore, older adults hospitalized with RSV show an often prolonged functional decline, with a substantial impact on routine activities.9,84
Data for comorbidity status and impact in Brazilian patients with RSV are more limited, although one might expect a similar range of comorbidities to be equally prevalent, and some data indicate that adults with chronic heart are at risk of complications associated with RSV infection.85 This assumption is supported by one recent smaller study from Sao Paulo, reporting on RSV and influenza hospitalizations in older patients (mean age 59 y) in early 2022, with most due to RSV.60 This study reported comorbidities in 80%, including CHF (29%), diabetes (27%), COPD (10%) and renal disease (12%). In addition, high rates of ventilatory support (46%) and ICU admission (10%) were required, with a mortality of 33%.60
Perception of RSV risk in older adults and diagnostic gaps
The panel reflected on physicians’ general awareness of RSV as an important pathogen in older adults, which, on balance, may be relatively low. This is not necessarily unique to Brazil; this is also generally apparent in the wider Latin American region,27 and indeed in most developed countries.21–23 Many reasons may account for this, including a greater focus on reporting on younger children in RSV surveillance activities which could be understood to highlight RSV as solely a pediatric concern.3,42,48 As mentioned earlier, in the clinical setting, greater attention is paid toward influenza or SARS-CoV-2 infection as a cause of SARI in older adults, and RSV is infrequently considered. A lack of specific antiviral treatment for adult RSV infection may also contribute to RSV under testing.22,23 However, RSV confirmation may help identify patients at greater risk of adverse outcomes, including clinical deterioration associated with acute CHF and COPD exacerbations.16,62 Consequently, routine RSV testing of older adults presenting with respiratory symptoms is generally low, both in Brazil and indeed in most countries, e.g., even in hospitalized SARI cases in countries such as the US, and this represents the major limitation in establishing reliable rates for the overall RSV burden in the older population.20,24,86
In Brazil, specific testing policies and processes vary across institutions, and there remains a need to establish a more uniform approach. The panels’ view is that all adults presenting with acute respiratory symptoms with chronic cardiopulmonary comorbidities (e.g., CHF, CAD, COPD, asthma, uncontrolled hypertension), and those with diabetes or chronic renal disease should be tested for RSV, as should immunocompromised patients. In addition, patients presenting with acute comorbidity exacerbations (e.g., acute cardiac decompensation) should be questioned about recent respiratory symptoms, and RSV should be considered as a contributory factor. To this point, we would note that while historically, SARI case definitions have included fever as a key criterion, fever is often absent or mild in older patients with RSV, and while fever is important, the current guidance does not require fever as a core criterion for considering RSV infection or indeed any ILI.65 More broadly, we would advocate for greater routine RSV testing for all adults aged ≥60 y with respiratory symptoms, in particular, those within long-term care facilities or other institutional settings where acute ILI outbreaks are well recognized.87 While our focus is on adults aged ≥60 y, we would also advocate for routine RSV testing of younger adults, with comorbidities presenting with ILI-symptoms.
A wide range of antigenic and molecular tests can be used in the clinical setting, with results obtained directly at the point-of-care or following laboratory analysis.88–90 While testing is usually performed on specimens from nasal/nasopharyngeal swabs, aspirates or nasal wash specimens may yield greater sensitivity. Using sputum samples improves RSV detection and can be especially useful in older adults with more severe LRTI.15,86,91 Antigenic tests such as rapid antigen detection tests (RADTs), direct or indirect immunofluorescence (IF), and molecular methods, i.e., RT-PCR assays, each have a place in routine diagnosis, although with differing sensitivity, specificity and costs. Molecular RT-PCR methods have greater sensitivity and specificity and generally are considered the gold standard in routine diagnosis,90,92 with multiplex assays, e.g., quadruplex assay (for influenza A and B, SARS-CoV-2, and RSV detection) particularly useful for broader initial diagnosis, although these are more expensive, an important consideration in resource-poor settings.
While RADTs and immunofluorescence assays can provide rapid results and are especially useful in younger children,93,94 sensitivity is far lower in older individuals, where molecular RT-PCR tests are preferred.90,93 This said, RADTs are an important tool, especially at initial presentation, and can also contribute to surveillance activities by providing some approximation of circulating RSV within the older adult population.27
As such, although preferred and far more useful for older adults with RSV infection, routine RT-PCR use is low, partly due to associated costs, with antigenic testing being more frequently used, although far from routine. To this point, it must be recognized that test costs are relatively small when considering the overall costs of managing severe RSV disease. These aspects pose challenges to improving RSV testing and diagnosis in Brazil, and they may also differ between public and private health-care domains. Observations from the panel were that while RT-PCR testing is often covered through private insurance plans, routine molecular testing within private hospitals remains low, even in hospitalized patients, where antigenic tests may be more frequently used. In the public health-care setting, RSV diagnostic costs may contribute to the broader view (described earlier) that the diagnosis would not influence clinical care and so is unnecessary. Patient pathways may also influence diagnostic patterns. For many with acute respiratory symptoms, initial consultation will be within primary care, where routine RSV testing is generally unavailable, and if available, chiefly RADTs. There remains a need to develop and more widely deploy affordable diagnostic tests that are more suited to improve RSV diagnosis in older adults. In turn, this could also provide greater and more reliable estimates of the overall burden within Brazil.
Challenges in communicating RSV disease
The panel unanimously agreed that epidemiological data and their reporting mainly influence disease awareness and perception of RSV risk. In this respect, our data highlights RSV burden and its importance as an often-significant pathogen in older adults. However, this is just a start. There is, in fact, a wealth unpublished RSV data captured within reporting systems whether that be surveillance registries (SIVEP-Gripe, ITpS and others) or collected locally within hospitals. While such data are often available through periodic epidemiologic reports, these generally cover a range of respiratory pathogens.71 More focused analysis and formal publication of RSV disease burden and impact on health-care resources in medical journals are necessary to bring this to the attention of the wider medical community, particularly the impact of comorbidities on RSV disease outcomes in older adults. In turn, this can improve clinical suspicion and RSV testing. To date, most reports on RSV within Brazil are generally reported in journals with an infectious disease or epidemiologic perspective. Ideally, such articles should be readily available to the broad range of specialists involved in older adult care, and also toward a primary care audience. As such, publication in specialty journals is encouraged.
Awareness of RSV in older adults within the general public in Brazil is also low, although again, this is not unique, with low awareness reported among the US public and elsewhere.95,96 There is a clear need for focused educational activities and disease awareness campaigns tailored toward both health-care professionals and to the lay public. Some insight into this can be gained from previous campaigns to increase awareness of other adult vaccine-preventable disease; obviously, education was a critical element of physician training during the COVID-19 pandemic.97
While the focus of this article relates to the burden and impact of RSV-infection, effective RSV vaccines for use in adults ≥60 y are now available. Two such vaccines, an adjuvanted RSVPreF3 vaccine (AREXVY, GSK) and the RSVpreF vaccine (Abrysvo, Pfizer), each targeting the F fusion protein, have demonstrated high efficacy against RSV infection, including severe LRTI disease.33–35 Inclusion of an adjuvant within AREXVY enhances antibody and T-cell responses to help overcome age-related immune decline, with sustained efficacy in older adults, including those with underlying cardiorespiratory comorbidity.98,99 Additional vaccines are in the later stages of clinical development and regulatory approval.100 Both AREXVY and Abrysvo are already included in national immunization recommendations for older adults in several countries, including the US,33 where the estimated public health impact due to disease reduction in older adults is substantial.101 Notably, such benefits are not only for RSV-SARI; reductions in milder RSV cases requiring medical attendance within primary care will far exceed RSV-SARI case numbers.101 In addition, specialist societies and expert initiatives such as the Global Initiative for Chronic Obstructive Disease (GOLD) and the Global Initiative for Asthma (GINA) also now recommend RSV vaccination for those aged ≥60 y with chronic heart or lung disease.102,103 In Brazil, both vaccines have now been approved (AREXVY in December 2023, and Abrysvo in March 2024) and available for use in adults aged ≥60 y, given as a single dose.36,37 One useful pragmatic aspect is the ability to co-administer either of these RSV vaccines along with the seasonal influenza vaccine. The Brazilian Society of Immunization (SBIm) recommends the use of RSV vaccination in all adults aged ≥60 y, with these endorsed by the Brazilian Society of Geriatrics and Gerontology, the Brazilian Thoracic Association (SBPT), the Brazilian Society of Cardiology and other specialist societies.104–108 Although their introduction is only at an early stage in Brazil and, at present, would be funded privately, RSV vaccines now provide an important tool in reducing RSV disease and resultant morbidity and mortality in older adults. Education on vaccine benefits (especially in high-risk older adults) can complement broader RSV awareness campaigns.
Conclusions
The significance of RSV as an important respiratory pathogen in older adults ≥60 y is generally under-recognized. Although there remains a need to better characterize the RSV burden within and across Brazil, available data indicates that RSV represents an important cause of SARI in older adults, with high impact (up to 50% of all RSV deaths in Brazil in recent years). The prognosis is poorest in patients with comorbidities. Furthermore, in part due to increased surveillance of respiratory pathogens in recent years, SARI case numbers are increasing, and although data are lacking, one may expect a similar pattern and with even greater numbers of less severe RSV cases requiring medical care. These patterns are broadly similar to those observed in other countries with more established data on the clinical burden of disease in older adults. Rates of RSV testing in the clinical setting are low, and this obscures diagnosis and hinders clinical epidemiology and broader RSV surveillance. While we provide some insight into the RSV burden, there are substantial knowledge/research gaps for Brazil, and we encourage greater data reporting and communication. Crucially, we advocate for improved testing while recognizing challenges facing clinicians and institutions in this respect. Educational initiatives and awareness campaigns will be important to improve understanding of the clinical significance of RSV in older adults. The recent availability of protective RSV vaccines offers a valuable tool for RSV prevention in this vulnerable older adult population.
Supplementary Material
Acknowledgments
The authors would like to thank Marcus Rodrigues, Rafael Pegoraro and Ana Carolina Ferreira (all from GSK, Brazil) for their support in developing and facilitating the advisory board meeting. They also thank the Brazilian national surveillance network of respiratory infections for publicly providing the underlying SIVEP-Gripe database, and Thatiana Pinto (GSK, Wavre, Belgium) for their support in the analysis of these data. The authors thank Business & Decision Life Sciences Medical Communication Service Center for editorial assistance, manuscript coordination, and design support, on behalf of GSK, and Iain O’Neill (freelance, funded by GSK) for providing medical writing support based on authors’ input and direction.
Biography
Prof. Lessandra Michelin is a Brazilian medical doctor and Infectious Diseases expert from the Federal University of Health Sciences of Porto Alegre, with a master’s and PhD from the Federal University of Health Sciences of Porto Alegre-UFCSPA, Healthcare Audit by Gama Filho University and Hospital Epidemiology & Infection Control by the Brazilian Medical Association. For over 25 y, she has been a Professor of Infectious Diseases, Vaccines, and Travel Medicine at the University of Caxias do Sul/Brazil (UCS), providing guidance and training for doctors, health-care professionals, and medical and postgraduate students. Former member (2014–2021) of the Advisory Board of the National Viral Hepatitis Program (2006–2010) and the National Immunization Program (CTAI/PNI) for the Brazil Ministry of Health, and Former Director of the Brazilian Society of Infectious Diseases (2018–2021). Dr Michelin has published books, book chapters and manuscripts on infectious diseases and vaccines. In addition to remaining a professor of Infectious Diseases and Pediatrics (UCS/RS), she currently works as a Vaccines Medical Manager at GSK Brazil.
Funding Statement
The initial advisory board meeting, related study/research and subsequent manuscript development were sponsored by GlaxoSmithKline Biologicals SA. Support for third-party writing assistance and the Rapid Service and open access fees for this article were also funded by GlaxoSmithKline Biologicals SA in accordance with Good Publication Practice (GPP 2022) guidelines.
Disclosure statement
Otavio Cintra is employed by and holds shares in GSK. Bruna M G de Veras and Lessandra Michelin are employed by GSK. Nancy Bellei declares payment for lectures and advisory board from GSK. Marcelo Ferreira da Costa Gomes declares being an employee of Fiocruz, payement for lectures by SBIm (Sociedade Brasileira de Imunizações - Brazilian Society of Immunization), support for attending meetings by Butantan Instisute, ICTP-SAIFR and Brazilian Ministry of Health, being a member of Technical Advisory Board for covid, influenza and other respiratory viruses of the Brazilian Ministry of Health without any financial compensation and payment for advisory board and travel expenses from GSK. Sonia M Raboni declares participation in advisory boards for GSK, payment for producing scientific informative material from Sanofi-Pasteur, and support for attending meetings and/or travel from Sanofi-Pasteur, Takeda, and Pfizer. Maisa Kairalla declares grant for advisory board from GSK and Pfizer; payment for lectures from GSK, Sanofi, Pfizer and Adium/Moderna; support for attending meetings and/or travel from Pfizer and Adium/Moderna. Ricardo Amorim Correa declares consulting fees from Bayer and MSD; honoraria for lectures and speaker’s bureau from Bayer, Janssen and GSK; support for attending meetings from Bayer and being on education director and president elect (2025-2026) of SBPT. Monica Levi, declares grant for advisory board from GSK and Merck Shape & Dome; payment for lectures from GSK, Merck Shape & Dome and Sanofi; support for attending meetings and/or travel from Merck Shape & Dome, Adium/Moderna and Sanofi. Alberto Chebabo declares grant for advisory board from GSK; payment for lectures from Merck Shape & Dome, Pfizer and Takeda; support for attending meetings and/or travel from Pfizer, Takeda and Adium/Moderna and participation to advisory boards for GSK, Sanofi, Pfizer and Takeda. Isabela Ballalai declares Support from GSK for material and virtual meetings; consulting fees from GSK and support for attending meetings and/or travel from GSK, Pfizer and MSD. Sergio Cimerman declares grant for advisory board from GSK; payment for lectures from Merck Shape & Dome, Pfizer and Adium/Moderna; support for attending meetings and/or travel from Merck Shape & Dome and Adium/Moderna and participation to advisory boards for GSK. Cecilia M Roteli-Martins declares consulting fees from Pfizer, MSD, Sanofi-Pasteur and GSK; payment for lectures from Pfizer, Sanofi-Pasteur and GSK; support for attending meetings and/or travel from MSD and Pfizer and participation to the Comissão Nacional de vacinas da Febrasgo. Susana Aidé declares receiving study materials supplied by GSK, participation in advisory boards for GSK, being part of the National Vaccine Commission of the Brazilian Federation of Gynecology and Obstetrics (Febrasgo). Margareth P Dalcolmo declares nothing. Renato De Ávila Kfouri declares support for material and virtual meeting from GSK; consulting fees for participation in an advisory board from GSK and participation to advisory boards for Clover flu and COVID vaccines.
Author contributions
All authors were involved in the analysis and interpretation of the relevant literature and the development of the manuscript. All authors gave final approval before submission.
Data availability statement
All data generated or analyzed are included in this published article or in the original cited data sources.
Prior presentation
Some of the data in this manuscript were previously presented at the 23rd Brazilian Congress of Infectious Diseases in Salvador, Bahia, Brazil, from September 19–22, 2023.
Trademarks
AREXVY is a trademark owned by or licensed to GSK.
Abrysvo is a trademark of Pfizer.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2388943
References
- 1.Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, Albertson SB, Deshpande A, Farag T, Abebe Z, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. 2018;18(11):1191–13. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, Madhi SA, Omer SB, Simoes EAF, Campbell H, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–2064. doi: 10.1016/S0140-6736(22)00478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staadegaard L, Caini S, Wangchuk S, Thapa B, de Almeida WAF, de Carvalho FC, Njouom R, Fasce RA, Bustos P, Kyncl J, et al. The global epidemiology of RSV in community and hospitalized care: findings from 15 countries. Open Forum Infect Dis. 2021;8(7):ofab159. doi: 10.1093/ofid/ofab159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V.. Estimates of hospitalization attributable to influenza and RSV in the US during 1997-2009, by age and risk status. BMC Public Health. 2017;17(1):271. doi: 10.1186/s12889-017-4177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of mortality attributable to influenza and RSV in the United States during 1997-2009 by influenza type or subtype, age, cause of death, and risk status. Influenza Other Respir Viruses. 2014;8(5):507–515. doi: 10.1111/irv.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi T, Denouel A, Tietjen AK, Campbell I, Moran E, Li X, Campbell H, Demont C, Nyawanda BO, Chu HY, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2020;222(Suppl 7):577–583. doi: 10.1093/infdis/jiz059. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen-Van-Tam JS, O’Leary M, Martin ET, Heijnen E, Callendret B, Fleischhackl R, Comeaux C, Tran TMP, Weber K. Burden of respiratory syncytial virus infection in older and high-risk adults: a systematic review and meta-analysis of the evidence from developed countries. Eur Respir Rev. 2022;31(166): doi: 10.1183/16000617.0105-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savic M, Penders Y, Shi T, Branche A, Pircon JY. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: a systematic literature review and meta-analysis. Influenza Other Respir Viruses. 2023;17(1):e13031. doi: 10.1111/irv.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 10.Korsten K, Adriaenssens N, Coenen S, Butler C, Ravanfar B, Rutter H, Allen J, Falsey A, Pircon JY, Gruselle O, et al. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur Respir J. 2021;57(4): doi: 10.1183/13993003.02688-2020. [DOI] [PubMed] [Google Scholar]
- 11.Belongia EA, King JP, Kieke BA, Pluta J, Al-Hilli A, Meece JK, Shinde V. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥60 years old. Open Forum Infect Dis. 2018;5(12):ofy316. doi: 10.1093/ofid/ofy316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falsey AR, McElhaney JE, Beran J, van Essen GA, Duval X, Esen M, Galtier F, Gervais P, Hwang SJ, Kremsner P, et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis. 2014;209(12):1873–1881. doi: 10.1093/infdis/jit839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206(1):56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackerson B, Tseng HF, Sy LS, Solano Z, Slezak J, Luo Y, Fischetti CA, Shinde V. Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin Infect Dis. 2019;69(2):197–203. doi: 10.1093/cid/ciy991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis. 2022;9(7):ofac300. doi: 10.1093/ofid/ofac300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng HF, Sy LS, Ackerson B, Solano Z, Slezak J, Luo Y, Fischetti CA, Shinde V. Severe morbidity and Short- and mid- to long-term mortality in older adults hospitalized with respiratory syncytial virus infection. J Infect Dis. 2020;222(8):1298–1310. doi: 10.1093/infdis/jiaa361. [DOI] [PubMed] [Google Scholar]
- 17.Shi T, Vennard S, Jasiewicz F, Brogden R, Nair H, Investigators R. Disease burden estimates of respiratory syncytial virus related acute respiratory infections in adults with comorbidity: a systematic review and meta-analysis. J Infect Dis. 2022;226(Suppl 1):17–21. doi: 10.1093/infdis/jiab040. [DOI] [PubMed] [Google Scholar]
- 18.Njue A, Nuabor W, Lyall M, Margulis A, Mauskopf J, Curcio D, Kurosky S, Gessner BD, Begier E. Systematic literature review of risk factors for poor outcomes among adults with respiratory syncytial virus infection in high-income countries. Open Forum Infect Dis. 2023;10(11):ofad513. doi: 10.1093/ofid/ofad513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Z, Warren JL, Shapiro ED, Pitzer VE, Weinberger DM. Estimated incidence of respiratory hospitalizations attributable to RSV infections across age and socioeconomic groups. Pneumonia (Nathan). 2022;14(1):6. doi: 10.1186/s41479-022-00098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Kulkarni D, Begier E, Wahi-Singh P, Wahi-Singh B, Gessner B, Nair H. Adjusting for case under-ascertainment in estimating RSV hospitalisation burden of older adults in high-income countries: a systematic review and modelling study. Infect Dis Ther. 2023;12(4):1137–49. doi: 10.1007/s40121-023-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branche AR, Falsey AR. Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs Aging. 2015;32(4):261–269. doi: 10.1007/s40266-015-0258-9. [DOI] [PubMed] [Google Scholar]
- 22.Nuwer R. Better awareness of RSV in older adults is needed to fight a growing burden. Nature. 2023;621(7980):58–59. doi: 10.1038/d41586-023-02958-y. [DOI] [PubMed] [Google Scholar]
- 23.Hurley LP, Allison MA, Kim L, O’Leary ST, Crane LA, Brtnikova M, Beaty BL, Allen KE, Poser S, Lindley MC, et al. Primary care physicians’ perspectives on respiratory syncytial virus (RSV) disease in adults and a potential RSV vaccine for adults. Vaccine. 2019;37(4):565–570. doi: 10.1016/j.vaccine.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Rozenbaum MH, Judy J, Tran D, Yacisin K, Kurosky SK, Begier E. Low levels of RSV testing among adults hospitalized for lower respiratory tract infection in the United States. Infect Dis Ther. 2023;12(2):677–685. doi: 10.1007/s40121-023-00758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardach A, Rey-Ares L, Cafferata ML, Cormick G, Romano M, Ruvinsky S, Savy V. Systematic review and meta-analysis of respiratory syncytial virus infection epidemiology in Latin America. Rev Med Virol. 2014;24(2):76–89. doi: 10.1002/rmv.1775. [DOI] [PubMed] [Google Scholar]
- 26.Ali A, Lopardo G, Scarpellini B, Stein RT, Ribeiro D. Systematic review on respiratory syncytial virus epidemiology in adults and the elderly in Latin America. Int J Infect Dis. 2020;90:170–180. doi: 10.1016/j.ijid.2019.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correa RA, Arancibia F, De Avila Kfouri R, Chebabo A, Garcia G, Gutierrez Robledo LM, Lopardo G, Nemerovsky J, Perez CM, Rendon A, et al. Understanding the burden of respiratory syncytial virus in older adults in Latin America: an expert perspective on knowledge gaps. Pulm Ther. 2024. doi: 10.1007/s41030-024-00253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantarino L, Merchan-Hamann E. Influenza in Brazil: surveillance pathways. J Infect Dev Ctries. 2016;10(1):13–23. doi: 10.3855/jidc.7135. [DOI] [PubMed] [Google Scholar]
- 29.da Silva ADD, Veiga A, Cruz OG, Bastos LS, Gomes M. Severe acute respiratory infection surveillance in Brazil: the role of public, private and philanthropic healthcare units. Health Policy Plan. 2022;37(9):1075–1085. doi: 10.1093/heapol/czac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azambuja HCS, Carrijo MF, Martins TCR, Luchesi BM. The impact of influenza vaccination on morbidity and mortality in the elderly in the major geographic regions of Brazil, 2010 to 2019. Cad Saude Publica. 2020;36(Suppl 2):e00040120. doi: 10.1590/0102-311X00040120. [DOI] [PubMed] [Google Scholar]
- 31.Frohlich GC, Gregianini TS, Pinheiro FG, Nascimento R, Cezar TM, Pscheidt VM, Selayaran T, Martins LG, Gomes M, Salvato RS, et al. Resurgence of human respiratory syncytial virus during COVID-19 pandemic in Southern Brazil. J Med Virol. 2024;96(3):e29551. doi: 10.1002/jmv.29551. [DOI] [PubMed] [Google Scholar]
- 32.Comissão de Saúde . Audiência pública para debater acerca da conscientização do vírus sincicial respiratório. 2023. Oct 19. [accessed 2024 Apr 4]. https://www2.camara.leg.br/atividade-legislativa/comissoes/comissoes-permanentes/cssf/apresentacoes-em-eventos/eventos-2023/19-10-2023-audiencia-publica-para-debater-acerca-da-conscientizacao-do-virus-sincicial-respiratorio.
- 33.Melgar M, Britton A, Roper LE, Talbot HK, Long SS, Kotton CN, Havers FP. Use of respiratory syncytial virus vaccines in older adults: recommendations of the advisory committee on immunization practices - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793–801. doi: 10.15585/mmwr.mm7229a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papi A, Ison MG, Langley JM, Lee DG, Leroux-Roels I, Martinon-Torres F, Schwarz TF, van Zyl-Smit RN, Campora L, Dezutter N, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595–608. doi: 10.1056/NEJMoa2209604. [DOI] [PubMed] [Google Scholar]
- 35.Walsh EE, Perez Marc G, Zareba AM, Falsey AR, Jiang Q, Patton M, Polack FP, Llapur C, Doreski PA, Ilangovan K, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465–1477. doi: 10.1056/NEJMoa2213836. [DOI] [PubMed] [Google Scholar]
- 36.GSK . AREXVY [vacina vírus sincicial respiratório (recombinante, adjuvada)]. [accessed 2024 Feb 15]. https://br.gsk.com/media/8155/arexvy.pdf.
- 37.Pfizer . Abrysvo [vacina vírus sincicial respiratório A e B (recombinante)] [accessed 2024 Apr 29]. https://www.pfizer.com.br/bulas/abrysvo.
- 38.de Veras BMG, Pinto T, Holst AG, Lepetic A, Michelin L, da Costa Gomes MF. Casos graves de Virus Sincicial Respiratorio em anos de pandemia: Uma analise retrospectiva da base de dados do SIVEP-GRIPE no Brasil (2020-2022). Presented at the XXIII Congresso Brasileiro de Infectologia, September 19–22, 2023; Salvador, Bahia, Brazil. doi: 10.1016/j.bjid.2023.103129. [DOI] [Google Scholar]
- 39.Kaler J, Hussain A, Patel K, Hernandez T, Ray S. Respiratory syncytial virus: a comprehensive review of transmission, pathophysiology, and manifestation. Cureus. 2023;15(3):e36342. doi: 10.7759/cureus.36342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvajal JJ, Avellaneda AM, Salazar-Ardiles C, Maya JE, Kalergis AM, Lay MK. Host components contributing to respiratory syncytial virus pathogenesis. Front Immunol. 2019;10:2152. doi: 10.3389/fimmu.2019.02152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cantu-Flores K, Rivera-Alfaro G, Munoz-Escalante JC, Noyola DE. Global distribution of respiratory syncytial virus A and B infections: a systematic review. Pathog Glob Health. 2022;116(7):398–409. doi: 10.1080/20477724.2022.2038053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vianna LA, Siqueira MM, Volpini LPB, Louro ID, Resende PC. Seasonality, molecular epidemiology, and virulence of respiratory syncytial virus (RSV): a perspective into the Brazilian influenza surveillance program. PLOS ONE. 2021;16(5):e0251361. doi: 10.1371/journal.pone.0251361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbosa G, Perosa AH, Bellei N. Atypical interseasonal respiratory syncytial virus infection in hospitalized children up to 12 years old. Influenza Other Respir Viruses. 2023;17(8):e13183. doi: 10.1111/irv.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaves T, Perosa A, Bellei N. RSV pattern in adult population during COVID-19 pandemic in a tertiary hospital in São Paulo, Brazil. Presented at the 9th ESWI Influenza Conference, September 17–20, Valencia, Spain. 2023. [Google Scholar]
- 45.Anderson LJ, Peret TC, Piedra PA. RSV strains and disease severity. J Infect Dis. 2019;219(4):514–516. doi: 10.1093/infdis/jiy498. [DOI] [PubMed] [Google Scholar]
- 46.Scotta MC, Stein RT. Current strategies and perspectives for active and passive immunization against respiratory syncytial virus in childhood. J Pediatr (Rio J). 2023;99(Suppl 1):4–11. doi: 10.1016/j.jped.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staadegaard L, Caini S, Wangchuk S, Thapa B, de Almeida WAF, de Carvalho FC, Fasce RA, Bustos P, Kyncl J, Novakova L, et al. Defining the seasonality of respiratory syncytial virus around the world: national and subnational surveillance data from 12 countries. Influenza Other Respir Viruses. 2021;15(6):732–41. doi: 10.1111/irv.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freitas AR, Donalisio MR. Respiratory syncytial virus seasonality in Brazil: implications for the immunisation policy for at-risk populations. Mem Inst Oswaldo Cruz. 2016;111(5):294–301. doi: 10.1590/0074-02760150341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards KM. The impact of social distancing for severe acute respiratory syndrome coronavirus 2 on respiratory syncytial virus and influenza burden. Clin Infect Dis. 2021;72(12):2076–8. doi: 10.1093/cid/ciaa1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fry S, Chokephaibulkit K, Pallem S, Henry O, Pu Y, Akawung A, Kim JH, Yanni E, Tullio AN, Aurpibul L, et al. Incidence of respiratory syncytial virus-associated lower respiratory tract illness in infants in low- and middle-income regions during the coronavirus disease 2019 pandemic. Open Forum Infect Dis. 2023;10(12):ofad553. doi: 10.1093/ofid/ofad553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garg I, Shekhar R, Sheikh AB, Pal S. Impact of COVID-19 on the changing patterns of respiratory syncytial virus infections. Infect Dis Rep. 2022;14(4):558–68. doi: 10.3390/idr14040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abu-Raya B, Vineta Paramo M, Reicherz F, Lavoie PM. Why has the epidemiology of RSV changed during the COVID-19 pandemic? EClinicalMedicine. 2023;61(102089). doi: 10.1016/j.eclinm.2023.102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varela FH, Scotta MC, Polese-Bonatto M, Sartor ITS, Ferreira CF, Fernandes IR, Zavaglia GO, de Almeida WAF, Arakaki-Sanchez D, Pinto LA, et al. Absence of detection of RSV and influenza during the COVID-19 pandemic in a Brazilian cohort: likely role of lower transmission in the community. J Glob Health. 2021;11(5007. doi: 10.7189/jogh.11.05007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albuquerque DAR, Melo MDT, Sousa TLF, Normando PG, Fagundes JGM, Araujo-Filho JAB. Hospital admission and mortality rates for non-COVID-19 respiratory diseases in Brazil’s public health system during the COVID-19 pandemic: a nationwide observational study. J Bras Pneumol. 2023;49(1):e20220093. doi: 10.36416/1806-3756/e20220093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anglemyer A, Rutherford G, Walls T, Maldonado Y. Unusual interseasonal RSV activity in the southern and northern hemispheres. J Infect Dis. 2022;225(9):1680–2. doi: 10.1093/infdis/jiab620. [DOI] [PubMed] [Google Scholar]
- 56.Teirlinck AC, Broberg EK, Stuwitz Berg A, Campbell H, Reeves RM, Carnahan A, Lina B, Pakarna G, Boas H, Nohynek H, et al. Recommendations for respiratory syncytial virus surveillance at the national level. Eur Respir J. 2021;58(3): doi: 10.1183/13993003.03766-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.French CE, McKenzie BC, Coope C, Rajanaidu S, Paranthaman K, Pebody R, Nguyen-Van-Tam JS, Noso RSVSG, Higgins JP, Beck CR. Risk of nosocomial respiratory syncytial virus infection and effectiveness of control measures to prevent transmission events: a systematic review. Influenza Other Respir Viruses. 2016;10(4):268–90. doi: 10.1111/irv.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hababou Y, Taleb A, Recoing A, Moreau F, Simon I, Muller de Schongor F, Gault E, Rameix-Welti MA. Molecular investigation of a RSV outbreak in a geriatric hospital. BMC Geriatr. 2021;21(1):120. doi: 10.1186/s12877-021-02064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coultas JA, Smyth R, Openshaw PJ. Respiratory syncytial virus (RSV): a scourge from infancy to old age. Thorax. 2019;74(10):986–93. doi: 10.1136/thoraxjnl-2018-212212. [DOI] [PubMed] [Google Scholar]
- 60.Estofolete CF, Banho CA, Verro AT, Gandolfi FA, Dos Santos BF, Sacchetto L, Marques BC, Vasilakis N, Nogueira ML. Clinical characterization of respiratory syncytial virus infection in adults: a neglected disease? Viruses. 2023;15(9): doi: 10.3390/v15091848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Openshaw PJM, Chiu C, Culley FJ, Johansson C. Protective and harmful immunity to RSV infection. Annu Rev Immunol. 2017;35(501–32. doi: 10.1146/annurev-immunol-051116-052206. [DOI] [PubMed] [Google Scholar]
- 62.Bellei N, Moreira LP, Godoy H, Almeida DR, De Paola AAV. Respiratory syncytial virus infection is associated with acute decompensation in adult patients with congestive heart failure. Presented at the European Society of Cardiology Annual Congress, 29 August to 2 September; London (UK), 2015. [Google Scholar]
- 63.SIVEP-Gripe – Sistema de Informação da Vigilância Epidemioĺogica da Gripe . 2020. Brasília, Brasil: Secretaria de Vigilância em Saúde. [accessed 2023 Sep 22]. https://sivepgripe.saude.gov.br/sivepgripe. [Google Scholar]
- 64.Ministério da Saúde . Gripe (Influenza) 2023. [accessed 2024 Apr 4]. https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/g/gripe-influenza/.
- 65.Ministério da Saúde . Secretaria de Vigilância em Saúde. Guia de vigilância epidemiológica: emergência de saúde pública de importâncianacional pela doença pelo coronavírus 2019; 3 Apr 2020. [accessed 2024 Feb 15]. https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_epidemiologica_emergencia_saude_publica_importancia_nacional_doenca_coranvirus.pdf.
- 66.Niquini RP, Lana RM, Pacheco AG, Cruz OG, Coelho FC, Carvalho LM, Villela DAM, Gomes M, Bastos LS. Description and comparison of demographic characteristics and comorbidities in SARI from COVID-19, SARI from influenza, and the Brazilian general population. Cad Saude Publica. 2020;36(7):e00149420. doi: 10.1590/0102-311x00149420. [DOI] [PubMed] [Google Scholar]
- 67.Menezes RC, Ferreira IBB, Sobral L, Garcia SL, Pustilnik HN, Araujo-Pereira M, Andrade BB. Severe viral lower respiratory tract infections in Brazilian children: Clinical features of a national cohort. J Infect Public Health. 2024;17(1):1–9. doi: 10.1016/j.jiph.2023.09.015. [DOI] [PubMed] [Google Scholar]
- 68.Martins-Filho PR, Junior JMO, Santos CAD. Case-fatality rates and risk of death from COVID-19 and influenza A/H3N2 in Brazil: A nationwide ecological study. Enferm Infecc Microbiol Clin (Engl Ed). 2023;41(3):199–201. doi: 10.1016/j.eimce.2022.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martins Goncalves T, Mitsue Saruhashi Shimabukuro P, Nakamura Hiraki KR, Braz-Silva PH, Giannecchini S, Kai-Wang to K, Taminato M, Borges de Morais R. Severe acute respiratory syndrome by influenza and factors associated with death in older adults: a population study. J Infect Dev Ctries. 2023;17(2):241–50. doi: 10.3855/jidc.16801. [DOI] [PubMed] [Google Scholar]
- 70.Instituto Brasileiro de Geografia e Estatística . Population projections for Brazil and Federation Units by simple sex and age: 2010-2060. Rio (de) Janeiro: Brazilian Institute of Geography and Statistics; 2020. [accessed 2023 Sep 29]. https://www.ibge.gov.br/en/statistics/social/population/18176-population-projection. [Google Scholar]
- 71.Instituto Todos pela Saúde (ITpS) . Monitoramento de patógenos respiratórios (2022-23). [accessed 2024 Feb 22]. https://www.itps.org.br/pesquisa/monitoramento-de-patogenos-respiratorios.
- 72.Kujawski SA, Whitaker M, Ritchey MD, Reingold AL, Chai SJ, Anderson EJ, Openo KP, Monroe M, Ryan P, Bye E, et al. Rates of respiratory syncytial virus (RSV)-associated hospitalization among adults with congestive heart failure-United States, 2015-2017. PLOS ONE. 2022;17(3):e0264890. doi: 10.1371/journal.pone.0264890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Branche AR, Saiman L, Walsh EE, Falsey AR, Sieling WD, Greendyke W, Peterson DR, Vargas CY, Phillips M, Finelli L. Incidence of Respiratory Syncytial Virus Infection Among Hospitalized Adults, 2017-2020. Clin Infect Dis. 2022;74(6):1004–11. doi: 10.1093/cid/ciab595. [DOI] [PubMed] [Google Scholar]
- 74.Wyffels V, Kariburyo F, Gavart S, Fleischhackl R, Yuce H. A Real-World Analysis of Patient Characteristics and Predictors of Hospitalization Among US Medicare Beneficiaries with Respiratory Syncytial Virus Infection. Adv Ther. 2020;37(3):1203–1217. doi: 10.1007/s12325-020-01230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boattini M, Almeida A, Christaki E, Marques TM, Tosatto V, Bianco G, Iannaccone M, Tsiolakkis G, Karagiannis C, Maikanti P, et al. Severity of RSV infection in Southern European elderly patients during two consecutive winter seasons (2017-2018). J Med Virol. 2021;93(8):5152–5157. doi: 10.1002/jmv.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osei-Yeboah R, Johannesen CK, Egeskov-Cavling AM, Chen J, Lehtonen T, Fornes AU, Paget J, Fischer TK, Wang X, Nair H, et al. Respiratory syncytial virus-associated hospitalisation in adults with comorbidities in two European countries: a modelling study. J Infect Dis. 2023;229(Supplement_1):70–77. doi: 10.1093/infdis/jiad510. [DOI] [PubMed] [Google Scholar]
- 77.Moyes J, Cohen C, Pretorius M, Groome M, von Gottberg A, Wolter N, Walaza S, Haffejee S, Chhagan M, Naby F, et al. Epidemiology of respiratory syncytial virus-associated acute lower respiratory tract infection hospitalizations among HIV-infected and HIV-uninfected South African children, 2010-2011. J Infect Dis. 2013;208 (Suppl 3):S217–S226. doi: 10.1093/infdis/jit479. [DOI] [PubMed] [Google Scholar]
- 78.Chuaychoo B, Ngamwongwan S, Kaewnaphan B, Athipanyasilp N, Horthongkham N, Kantakamalakul W, Muangman N. Clinical manifestations and outcomes of respiratory syncytial virus infection in adult hospitalized patients. J Clin Virol. 2019;117:103–108. doi: 10.1016/j.jcv.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoon JG, Noh JY, Choi WS, Park JJ, Suh YB, Song JY, Cheong HJ, Kim WJ. Clinical characteristics and disease burden of respiratory syncytial virus infection among hospitalized adults. Sci Rep. 2020;10(1):12106. doi: 10.1038/s41598-020-69017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Havers FP, Whitaker M, Melgar M, Chatwani B, Chai SJ, Alden NB, Meek J, Openo KP, Ryan PA, Kim S, et al. Characteristics and Outcomes Among Adults Aged >/=60 Years Hospitalized with Laboratory-Confirmed Respiratory Syncytial Virus - RSV-NET, 12 States, July 2022-June 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1075–1082. doi: 10.15585/mmwr.mm7240a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.ElSherif M, Andrew MK, Ye L, Ambrose A, Boivin G, Bowie W, David MP, Gruselle O, Halperin SA, Hatchette TF, et al. Leveraging Influenza Virus Surveillance From 2012 to 2015 to Characterize the Burden of Respiratory Syncytial Virus Disease in Canadian Adults >/=50 Years of Age Hospitalized With Acute Respiratory Illness. Open Forum Infect Dis. 2023;10(7):ofad315. doi: 10.1093/ofid/ofad315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wiseman DJ, Thwaites RS, Drysdale SB, Janet S, Donaldson GC, Wedzicha JA, Openshaw PJ, Investigators R. Immunological and Inflammatory Biomarkers of Susceptibility and Severity in Adult Respiratory Syncytial Virus Infections. J Infect Dis. 2020;222(Suppl 7):584–591. doi: 10.1093/infdis/jiaa063. [DOI] [PubMed] [Google Scholar]
- 83.Tin Tin Htar M, Yerramalla MS, Moisi JC, Swerdlow DL. The burden of respiratory syncytial virus in adults: a systematic review and meta-analysis. Epidemiol Infect. 2020;148(e48. doi: 10.1017/S0950268820000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Branche AR, Saiman L, Walsh EE, Falsey AR, Jia H, Barrett A, Alba L, Phillips M, Finelli L. Change in functional status associated with respiratory syncytial virus infection in hospitalized older adults. Influenza Other Respir Viruses. 2022;16(6):1151–1160. doi: 10.1111/irv.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Souza Luna LK, Cruz JS, Chaves T, Bellei N. Comparative analysis of Respiratory Syncytial Virus frequency rates and viral load in different patient cohorts in a University Hospital in Sao Paulo, Brazil, over an eight-year period (2005-2013). Braz J Infect Dis. 2023;27(6):103702. doi: 10.1016/j.bjid.2023.103702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rozenbaum MH, Begier E, Kurosky SK, Whelan J, Bem D, Pouwels KB, Postma M, Bont L. Incidence of Respiratory Syncytial Virus Infection in Older Adults: Limitations of Current Data. Infect Dis Ther. 2023;12(6):1487–1504. doi: 10.1007/s40121-023-00802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lucas PCC, Lorenz C, Florez-Montero GL, Palasio RGS, Portella TP, Monteiro PCM, Yu ALF, Carvalhanas T. Institutional outbreaks of influenza-like illnesses in the state of Sao Paulo: an analysis of the epidemiological profile during the COVID-19 pandemic. Public Health. 2023;221:142–149. doi: 10.1016/j.puhe.2023.06.018. [DOI] [PubMed] [Google Scholar]
- 88.Hogan CA, Caya C, Papenburg J. Rapid and simple molecular tests for the detection of respiratory syncytial virus: a review. Expert Rev Mol Diagn. 2018;18(7):617–629. doi: 10.1080/14737159.2018.1487293. [DOI] [PubMed] [Google Scholar]
- 89.Alvarez-Moreno CA, Araújo ESAD, Baumeister E, Crespo KAN, Kalergis AM, Medina JEM, Tsukayama P, Ugarte-Gil C. Differential Diagnosis in the Management of Acute Respiratory Infections through Point-of-Care Rapid Testing in a Post-Pandemic Scenario in Latin America: Special Focus on COVID-19, Influenza, and Respiratory Syncytial Virus. COVID. 2024;4(2):221–260. doi: 10.3390/covid4020017. [DOI] [Google Scholar]
- 90.Bernstein DI, Mejias A, Rath B, Woods CW, Deeter JP. Summarizing Study Characteristics and Diagnostic Performance of Commercially Available Tests for Respiratory Syncytial Virus: A Scoping Literature Review in the COVID-19 Era. J Appl Lab Med. 2023;8(2):353–371. doi: 10.1093/jalm/jfac058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramirez J, Carrico R, Wilde A, Junkins A, Furmanek S, Chandler T, Schulz P, Hubler R, Peyrani P, Liu Q, et al. Diagnosis of Respiratory Syncytial Virus in Adults Substantially Increases When Adding Sputum, Saliva, and Serology Testing to Nasopharyngeal Swab RT-PCR. Infect Dis Ther. 2023;12(6):1593–1603. doi: 10.1007/s40121-023-00805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Onwuchekwa C, Atwell J, Moreo LM, Menon S, Machado B, Siapka M, Agarwal N, Rubbrecht M, Aponte-Torres Z, Rozenbaum M, et al. Pediatric Respiratory Syncytial Virus Diagnostic Testing Performance: A Systematic Review and Meta-analysis. J Infect Dis. 2023;228(11):1516–1527. doi: 10.1093/infdis/jiad185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chartrand C, Tremblay N, Renaud C, Papenburg J. Diagnostic Accuracy of Rapid Antigen Detection Tests for Respiratory Syncytial Virus Infection: Systematic Review and Meta-analysis. J Clin Microbiol. 2015;53(12):3738–3749. doi: 10.1128/JCM.01816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mesquita FDS, Oliveira DBL, Crema D, Pinez CMN, Colmanetti TC, Thomazelli LM, Gilio AE, Vieira SE, Martinez MB, Botosso VF, et al. Rapid antigen detection test for respiratory syncytial virus diagnosis as a diagnostic tool. J Pediatr (Rio J). 2017;93(3):246–252. doi: 10.1016/j.jped.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 95.La EM, Bunniran S, Garbinsky D, Reynolds M, Schwab P, Poston S, Harrington L. Respiratory syncytial virus knowledge, attitudes, and perceptions among adults in the United States. Hum Vaccin Immunother. 2024;20(1):2303796. doi: 10.1080/21645515.2024.2303796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Q, Xiu S, Yang L, Li L, Yang M, Wang X, Shen Y, Wang W, Lin L. Perceptions about respiratory syncytial virus (RSV) and attitudes toward the RSV vaccine among the general public in China: A cross-sectional survey. Hum Vaccin Immunother. 2024;20(1):2310916. doi: 10.1080/21645515.2024.2310916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sousa MLA, Shimizu IS, Patino CM, Torres-Duque CA, Zabert I, Zabert GE, Perez-Padilla R, Varon-Vega F, Cohen M, Ferreira JC. COVID-19 knowledge, attitudes, and practices among health care workers in Latin America. J Bras Pneumol. 2022;48(5):e20220018. doi: 10.36416/1806-3756/e20220018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feldman RG, Antonelli-Incalzi R, Steenackers K, Lee DG, Papi A, Ison MG, Fissette L, David MP, Marechal C, Van der Wielen M, et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine Is Efficacious in Older Adults With Underlying Medical Conditions. Clin Infect Dis. 2023;78(1):202–209. doi: 10.1093/cid/ciad471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ison MG, Papi A, Athan E, Feldman RG, Langley JM, Lee DG, Leroux-Roels I, Martinon-Torres F, Schwarz, TF, van Zyl-Smit RN, et al. Efficacy and safety of respiratory syncytial virus prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin Infect Dis. 2024. Jan 22, online ahead of print. ciae010. doi: 10.1093/cid/ciae010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wilson E, Goswami J, Baqui AH, Doreski PA, Perez-Marc G, Zaman K, Monroy J, Duncan CJA, Ujiie M, Ramet M, et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N Engl J Med. 2023;389(24):2233–2244. doi: 10.1056/NEJMoa2307079. [DOI] [PubMed] [Google Scholar]
- 101.Molnar D, La EM, Verelst F, Poston S, Graham J, Van Bellinghen LA, Curran D. Public Health Impact of the Adjuvanted RSVPreF3 Vaccine for Respiratory Syncytial Virus Prevention Among Older Adults in the United States. Infect Dis Ther. 2024; doi: 10.1007/s40121-024-00939-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Global intiative for chronic obstructive lung disease . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2024 GOLD Report). [accessed 2024 Feb 22]. https://goldcopd.org/2024-gold-report/.
- 103.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention, 2024. Apr 2024. [accessed 2024 May 29]. https://ginasthma.org/.
- 104.Sociedade Brasileira de Imunizações (SBIm) . Vaccine recommendations – 2024/2025. [accessed 2024 Apr 29]. https://sbim.org.br/images/calendarios/calend-sbim-idoso.pdf.
- 105.Sociedade Brasileira de Pneumologia e Tisiologia (SBPT) . Sociedade Brasileira de Imunizações (SBIm). Guia de Imunização SBIm/SBPT – Pneumologia 2024/25. [accessed 2024 Apr 4]. https://sbim.org.br/publicacoes/guias.
- 106.Sociedade Brasileira de Oncologia Clínica (SBOC)/SBIm: . Guia de Vacinação no Paciente Onológico 2ª edição. [accessed 2024 Apr 29]. https://sboc.org.br/images/Guia_Vacinas_11.pdf.
- 107.Associação Brasileira de Transplante de Órgãos (ABTO) . VACINAÇÃO PRÉ e PÓS-TRANSPLANTES DE ÓRGÃOS ADULTO – 2024. [accessed 2024 Apr 29]. https://site.abto.org.br/wp-content/uploads/2024/02/ABTO2024_recomendacoes-vacinacao_18dez23.pdf.
- 108.Associação Brasileira para o Estudo da Obesidade e Síndrome Metabólica (ABESO)/SBIm . Nota Técnica, 10/ 01 /2024. Vacinação para pacientes com obesidade. [accessed 2024 Apr 29]. https://sbim.org.br/images/files/notas-tecnicas/nt-conjunta-sbim-abeso-vacinacao-obesidade-final-v2.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed are included in this published article or in the original cited data sources.


