Abstract
The effects of ER (endoplasmic reticulum) Ca2+ on cytosolic Ca2+ oscillations in pancreatic acinar cells were investigated using mathematical models of the Ca2+ oscillations. We first examined the mathematical model of SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) to reproduce the highly co-operative inhibitory effect of Ca2+ in the ER lumen on ER Ca2+ uptake in the acinar cells. The model predicts that luminal Ca2+ would most probably inhibit the conversion of the conformation state with luminal Ca2+-binding sites (E2) into the conformation state with cytoplasmic Ca2+-binding sites (E1). The SERCA model derived from this prediction showed dose–response relationships to cytosolic and luminal Ca2+ concentrations that were consistent with the experimental data from the acinar cells. According to a mathematical model of cytosolic Ca2+ oscillations based on the modified SERCA model, a small decrease in the concentration of endoplasmic reticulum Ca2+ (approx. 20% of the total) was sufficient to abolish the oscillations. When a single type of IP3R (IP3 receptor) was included in the model, store depletion decreased the spike frequency. However, the frequency became less sensitive to store depletion when we added another type of IP3R with higher sensitivity to the concentration of free Ca2+ in the cytosol. Bifurcation analysis of the mathematical model showed that the loss of Ca2+ from the ER lumen decreased the sensitivity of cytosolic Ca2+ oscillations to IP3 [Ins(1,4,5)P3]. The addition of a high-affinity IP3R did not alter this property, but significantly decreased the sensitivity of the spike frequency to IP3. Our mathematical model demonstrates how luminal Ca2+, through its effect on Ca2+ uptake, can control cytosolic Ca2+ oscillations.
Keywords: Ca2+ signalling, computational model, endoplasmic reticulum, pancreatic acinar cell, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA)
Abbreviations: ACh, acetylcholine; cADPR, cADP ribose; [Ca2+]c, concentration of free Ca2+ in the cytosol; [Ca2+]ER, concentration of free Ca2+ in the endoplasmic reticulum; CCK, cholecystokinin; E1, conformation state of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase with cytoplasmic high-affinity Ca2+-binding sites; E2, conformation state of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase with luminal low-affinity Ca2+-binding sites; ER, endoplasmic reticulum; IP3, Ins(1,4,5)P3; IP3R, IP3 receptor; NAADP, nicotinic acid–adenine dinucleotide phosphate; PMCA, plasma membrane Ca2+-ATPase; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase
INTRODUCTION
Cytosolic Ca2+ oscillation is a widespread phenomenon, found in a variety of excitable and non-excitable cells. In excitable cells, periodic changes of the plasma membrane potential can drive cytosolic Ca2+ oscillations by stimulating voltage-dependent Ca2+ channels. On the other hand, oscillations in non-excitable cells are based on periodic release of Ca2+ from the intracellular Ca2+ store and Ca2+ uptake into the store. The release of Ca2+ from the internal stores, which are mainly in the ER (endoplasmic reticulum), is mediated by Ca2+ channels, such as IP3Rs {IP3 [Ins-(1,4,5)P3] receptors} and RyRs (ryanodine receptors), in the ER membrane. Opening of the channels is regulated by cytosolic second messengers [e.g. IP3, cADPR (cADP-ribose) and NAADP (nicotinic acid–adenine dinucleotide phosphate)]. The uptake of cytosolic Ca2+ into the ER, which restores [Ca2+]c (concentration of free Ca2+ in the cytosol) to the resting level, is mediated by Ca2+-ATPases located in the ER membrane [SERCAs (sarcoplasmic/endoplasmic reticulum Ca2+-ATPases)].
Ca2+-transport mechanisms in pancreatic acinar cells have been well characterized, and the kinetics of both cytosolic and ER Ca2+ have been investigated in detail by the combined use of ER-trapped fluorescent calcium indicators [1] and measurement of Ca2+-dependent Cl− current [2]. We have demonstrated previously that an incubation of acinar cells in a Ca2+-free medium during Ca2+ oscillations caused a continuous decrease in [Ca2+]ER (concentration of free Ca2+ in the ER), accompanied by a reduction of the amplitude of Ca2+ oscillations [3]. Ca2+ lost from the ER to the cytosol is excluded by PMCA (plasma membrane Ca2+-ATPase), since there is rapid activation of this pump during each cytosolic Ca2+ spike, as demonstrated for this cell type [4]. In the acinar cells, a decrease of spike amplitude was seen as soon as store depletion became detectable. A relatively small decrease in [Ca2+]ER [compared with the store depletion evoked by supramaximal doses of ACh (acetylcholine)] was sufficient to abolish cytosolic Ca2+ oscillations completely. Such phenomena imply a robust effect of Ca2+ in the ER lumen on cytosolic Ca2+ oscillations.
One of the possible mechanisms by which [Ca2+]ER affects cytosolic Ca2+ oscillations is inhibition of Ca2+ uptake by ER Ca2+. We have demonstrated previously that in intact acinar cells, an increase in [Ca2+]ER suppressed ER Ca2+ uptake [2]. The inhibition by high [Ca2+]ER was highly co-operative (Hill coefficient >4) and the uptake rate was halved when [Ca2+]ER was increased by approx. 50 μM. Such high co-operativity has also been shown in HL-60 cells [5] and dorsal root ganglia neurons [6]. High sensitivity of Ca2+ uptake to [Ca2+]ER would allow modulation of Ca2+ oscillations by a relatively small decrease of [Ca2+] in the store. The major subtype of SERCA in both pancreatic acinar cells and HL-60 cells is SERCA2b [7,8]. The activity of SERCA2b is known to be regulated by direct interaction with ER resident chaperones, including calreticulin [9,10], calnexin [11] and ERp57 [12]. The interaction between SERCA2b and the ER chaperones is sensitive to the condition of the Ca2+ store, suggesting that SERCA2b may respond to a change of the luminal [Ca2+] by using ER chaperones as ER Ca2+ sensors. These data indicate that the luminal sensitivity of Ca2+ uptake in the acinar cells and HL-60 cells reflects the property of SERCA2b.
A variety of mathematical models are available to analyse qualitative and quantitative properties of Ca2+ oscillations [13]. Although the stimulatory effect of cytosolic Ca2+ on Ca2+ uptake was included in most of these models, the inhibitory effect of luminal Ca2+ has rarely been taken into account. On the other hand, mathematical models have been developed to study the activity of SERCAs in isolated microsomes [14,15], and could reproduce the effect of luminal Ca2+ on the turnover rate of the pump, although the effects of luminal Ca2+ in these models were quantitatively different from what has been observed in pancreatic acinar cells. However, it should be possible to modify these models using experimental data from the acinar cells to obtain a mathematical model of ER Ca2+ uptake that can be used in Ca2+ oscillation models for pancreatic acinar cells.
In the present study, we first developed a mathematical model of SERCA2b to reproduce the effect of luminal Ca2+ on ER Ca2+ uptake that was seen in the pancreatic acinar cell. Then the SERCA2b model was incorporated into a computational model of cytosolic Ca2+ oscillations in the acinar cells. An attempt was made to reproduce the observed effect of luminal Ca2+ on the cytosolic Ca2+ oscillations by comparing the kinetics of both [Ca2+]c and [Ca2+]ER with the experimental data from the acinar cells. Thereafter bifurcation analysis was utilized to analyse the effects of luminal Ca2+ on the properties of cytosolic Ca2+ oscillations.
METHODS
Mathematical models were designed using Maple (Maplesoft, Ontario, Canada) and implemented in SIMULINK (The Mathworks, Natick, MA, U.S.A.). The flux control coefficients in the kinetic model of SERCA were calculated using GEPASI [16]. All the bifurcation analyses were carried out using XPPAUT [17].
RESULTS
Kinetic models of SERCAs
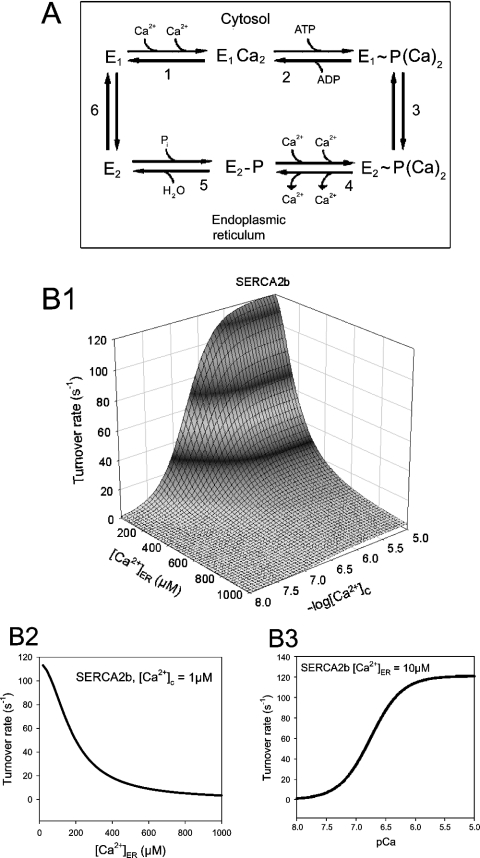
The kinetic models for the reaction cycle of SERCAs were based on the model developed by Dode et al. [14]. In this model, the reversible catalytic cycle was composed of the following reactions (see Figure 1A): (1) binding of two Ca2+ to the SERCA pump from the cytoplasmic side of the ER membrane, (2) phosphorylation of the pump by ATP, (3) conversion from the E1 (cytoplasmic high-affinity Ca2+-binding sites) into the E2 (luminal low-affinity Ca2+-binding sites) conformation state and translocation of Ca2+ from the cytoplasmic side to the luminal side of the ER membrane, (4) release of Ca2+ into the ER lumen, (5) dephosphorylation of the pump and (6) conversion from the E2 into the E1 conformation state. All the reactions are described as first-order reactions (except for the binding of Ca2+).
Figure 1. Reaction cycle and dose–response of turnover rate to cytosolic and luminal Ca2+ in SERCA2b model.
(A) Reaction cycle of SERCA. Clockwise direction of a reaction in the cycle is defined as a ‘forward’ reaction. The numbers shown with the reactions are used in the text in order to refer to individual reactions. (B1) Estimated dose–response of the turnover rate of SERCA2b against [Ca2+]c and [Ca2+]ER. Note that [Ca2+]c is expressed on a logarithmic scale and [Ca2+]ER is on a linear scale (this representation is chosen for visual convenience). (B2) Turnover rate at different [Ca2+]ER when [Ca2+]c=1 μM. (B3) Turnover rate at different [Ca2+]c when [Ca2+]ER=10 μM.
Since the model of Dode et al. [14] was based on experimental data relating to SERCA1a, we needed to adjust the parameters to reproduce the property of SERCA2b in pancreatic acinar cells. It has been shown that SERCA2b sensitivity to cytosolic Ca2+ is higher than for SERCA1a {Ka,0.5 (half maximum activation by cytosolic Ca2+)=0.27 μM and 0.44 μM respectively, when expressed in COS cells [18]}. Therefore we increased the affinity for cytosolic Ca2+ in reaction 1 from 1.79 μM to 1.1 μM. The data from HL-60 and pancreatic acinar cells suggest that luminal Ca2+ can inhibit ER Ca2+ uptake, both at high and low cytosolic Ca2+ concentrations [2,5]. On the other hand, the data from Dode et al. [14] indicate that the effect of luminal Ca2+ on SERCA1a is much more predominant at high [Ca2+]c. This difference in the relationship between cytoplasmic Ca2+ and luminal Ca2+ indicates that SERCA2b responds to luminal Ca2+ using a mechanism that is distinct from that employed by SERCA1a.
We hypothesized that there is another reaction in the cycle that is affected by luminal Ca2+. The reaction should affect the turnover rate to a similar degree at both low and high [Ca2+]c in order to be consistent with the above observation in the acinar cell. The strength of the effect resulting from a change of the rate constant of a local reaction vi (e.g. an individual reaction in the catalytic cycle of SERCA) on the global flux J (e.g. the turnover rate of the pump) can be quantified as
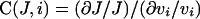 |
where (∂J/J) is the fractional change of the global flux, (∂vi/vi) is the fractional change of the rate constant of the local reaction and C(J,i) is the ratio of the fractional changes, called flux control coefficient [19]. By calculating the control coefficients of the individual steps in the catalytic cycle of SERCA, we can determine which step has the greatest potential to influence the overall turnover rate. It has been shown that, when a parameter (such as [Ca2+]ER) acts on the global flux through its direct influence on a local reaction, the strength of the response of the global flux to the parameter (or response coefficient) is determined by the control coefficient of the local reaction multiplied by the strength of the response of the local reaction to the parameter (or elasticity) [19]. Therefore a sufficient response of the global flux to the parameter can be achieved only at a reaction step that has a sufficiently large flux control coefficient.
We assumed that the luminal Ca2+ could only affect the E2 conformation and, therefore, only the control coefficients for the reactions that involve E2 were calculated (Table 1). In the majority of cases, forward reactions had larger control coefficients than backward reactions, which would be expected if the forward reactions were dominant at the steady state. A cytosolic Ca2+ rise from 100 nM to 10 μM increased the control coefficients (except for reaction 6) approx. 10-fold, which may explain why luminal Ca2+ has a larger effect at higher concentrations of cytosolic Ca2+. The forward direction of reaction 6 (E2→E1 conversion) had the highest control coefficient at a cytosolic Ca2+ concentration of 100 nM among the reactions in the catalytic cycle, which was relatively insensitive to cytosolic Ca2+ changes. This suggests that the E2→E1 conversion is most likely to be responsible for the sensitivity of ER Ca2+ uptake to luminal Ca2+ in HL-60 and pancreatic acinar cells.
Table 1. Flux control coefficients of turnover rate of SERCA1a model.
[Ca2+]ER was fixed at 100 μM.
| Flux control coefficients (forward/backward) | ||
|---|---|---|
| Reaction | [Ca2+]c=100 nM | [Ca2+]c=10 μM |
| 3 | 3.15×10−2/−1.67×10−3 | 2.11×10−1/−1.11×10−2 |
| 4 | 2.14×10−2/−2.23×10−3 | 1.43×10−1/−1.46×10−2 |
| 5 | 3.86×10−2/−9.73×10−4 | 2.53×10−1/−4.75×10−4 |
| 6 | 4.59×10−1/−4.30×10−1 | 2.24×10−1/−2.56×10−2 |
For estimation of the parameters, we assumed that SERCA1a and SERCA2b would show the same property (except for the sensitivity to cytosolic Ca2+) when the store was empty. On the other hand, an increase in [Ca2+]ER would inhibit the E2→E1 conversion with a Hill coefficient of 2. The dose-dependency of the turnover rate against [Ca2+]ER at [Ca2+]c=100 nM was fitted to the data from [2] to obtain the apparent affinity of the pump to luminal Ca2+. This gave us the SERCA2b kinetic model, whose estimated dose–response curves against [Ca2+]c and [Ca2+]ER are shown in Figure 1(B1). The rate constants of the reactions are shown in Table 2.
Table 2. Rate constants for the kinetic model of SERCA2b.
Reaction numbers refer to Figure 1. c, [Ca2+]c; e, [Ca2+]ER.
| Rate constants (s−1) | ||
|---|---|---|
| Reaction | Forward | Backward |
| 1 | 5000×c1.65/(c1.65+1.11.65) | 10 |
| 2 | 700 | 5 |
| 3 | 600 | 50 |
| 4 | 1000 | 13580×e2/(e2+15502) |
| 5 | 500 | 1 |
| 6 | 4.64×106/(e2+87.42) | 600 |
Figure 1(B1) shows that the turnover rate of SERCA2b was inhibited strongly by luminal Ca2+ both at low and high cytosolic [Ca2+], which is in agreement with the experiments performed in pancreatic acinar and HL-60 cells. With [Ca2+]c=1 μM, the turnover rate was 114.3 s−1 at [Ca2+]ER=10 μM and 3.28 s−1 at [Ca2+]ER=1 mM, and Ki,0.5 (half maximum inhibition by luminal Ca2+)=176 μM (Figure 1B2). The estimated Ki,0.5 was close to the resting [Ca2+]ER in the pancreatic acinar cell (approx. 150 μM [20]). The Ka,0.5 was 0.18 μM (Figure 1B3, [Ca2+]ER=10 μM).
Effect of [Ca2+]ER and SERCA activity on cytosolic Ca2+ oscillations in the whole-cell model
We constructed a ‘whole-cell model’ for Ca2+ oscillations in pancreatic acinar cells. This whole-cell model was composed of two compartments: the cytoplasm and the ER. The model included the following transport mechanisms: (i) Ca2+ release from the store via IP3Rs, (ii) Ca2+ leak from the store, (iii) Ca2+ extrusion by PMCA, (iv) Ca2+ uptake by SERCA2b. The details of the transport mechanisms are given in the Appendix.
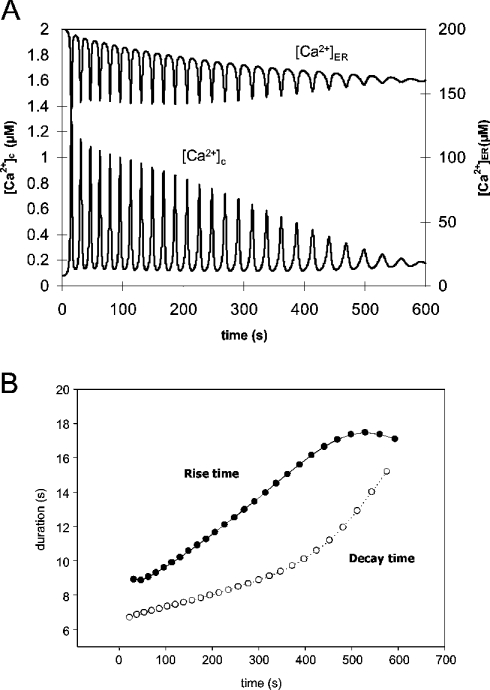
Figure 2(A) shows Ca2+ spike changes during continuous Ca2+ extrusion without compensation by Ca2+ influx through the plasma membrane. This would correspond to the experimental observation of cytosolic Ca2+ oscillations with no external Ca2+. Ca2+ oscillations were initiated by an increase of [IP3] from 0 to 0.254 μM at t=0. In the model, the amplitudes of the cytosolic Ca2+ responses decreased as [Ca2+]ER decreased, and oscillations eventually disappeared at [Ca2+]ER=160 μM. The periods between the spikes gradually increased as the store depletion progressed. We could see an increase in both rise time (time from the base to the peak of the next spike, 8.9 s in the first spike and 17.1 s in the last spike), and decay time (the spike period minus the rise time, 6.7 s in the first spike and 15.2 s in the last) (Figure 2B).
Figure 2. Simulation of cytosolic Ca2+ oscillations in a pancreatic acinar cell during gradual store depletion.
The gradual depletion of the Ca2+ store in the model was achieved by the extrusion of cytosolic Ca2+ by PMCA and the lack of compensating Ca2+ influx through the plasma membrane. (A) [Ca2+]c (lower trace) and [Ca2+]ER (upper trace) during the simulation. At the resting condition [Ca2+]c=0.08 μM and [Ca2+]ER=200 μM. The cell was stimulated by increasing [IP3] from 0 to 0.254 μM. (B) The rise time (•) and the decay time (○) of cytosolic Ca2+ spikes during the simulation.
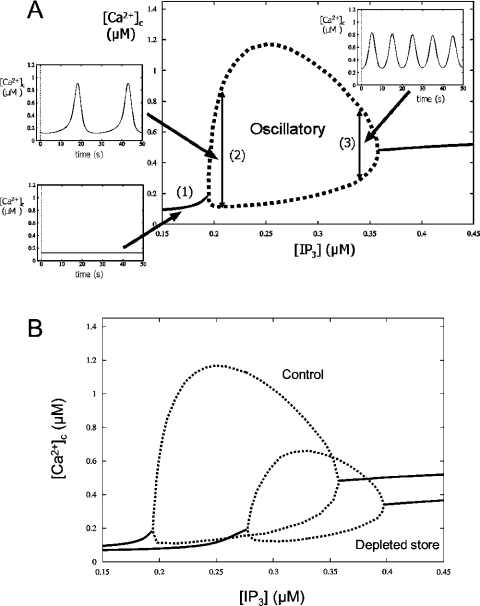
In order to learn more about the mechanism of spike modulation by luminal Ca2+, we carried out bifurcation analysis. Figure 3(A) shows a typical shape of a bifurcation curve for IP3-induced Ca2+ oscillations. The diagram displays [Ca2+]c at different [IP3]. At low and high [IP3], [Ca2+]c does not oscillate, and the diagram shows the steady state [Ca2+]c (solid lines). At an intermediate [IP3], [Ca2+]c oscillates and the diagram shows the maximum (peak) and the minimum (baseline) [Ca2+]c (broken lines). Therefore this diagram is showing the range of [IP3] which gives oscillations as well as the range of [Ca2+]c when it is oscillatory.
Figure 3. Bifurcation analysis of the modifications of Ca2+ oscillations by store depletion.
(A) A typical bifurcation diagram of [Ca2+]c against [IP3] in IP3-induced Ca2+ oscillations is presented. Steady-state solutions (solid line) and the maxima and the minima of [Ca2+]c during Ca2+ oscillations (broken line) are shown. [Ca2+]c will be stationary when [IP3] is outside the ‘oscillatory’ area (1). [Ca2+]c oscillates when [IP3] is in the ‘oscillatory’ area, which is encompassed by the broken line (2 and 3). Oscillation frequency is faster at higher [IP3] (3) than lower [IP3] (2). (B) Bifurcation diagrams of [Ca2+]c against [IP3] in the whole-cell model. Steady-state solutions (solid line), the maxima and the minima of [Ca2+]c during Ca2+ oscillations (broken line) are shown. S=200 μM in control and S=160 μM in depleted store.
Figure 3(B) shows the bifurcation diagram of cytosolic Ca2+ in the whole-cell model with [IP3] as a bifurcation parameter. A decrease of S (resting [Ca2+]ER at [Ca2+]c=0.08 μM) from 200 μM to 160 μM decreased the maximum oscillation amplitude of the oscillations by 0.515 μM (from 1.05 μM to 0.535 μM), but did not abolish the oscillations. It also raised both the minimum and maximum [IP3] for Ca2+ oscillations from [IP3]min=0.196 μM, [IP3]max=0.358 μM (S=200 μM) to [IP3]min=0.278 μM, [IP3]max=0.397 μM (S=160 μM). This shift of the bifurcation curve towards higher [IP3] can be interpreted as ‘desensitization’ of Ca2+ response to IP3, because a higher [IP3] will be required to obtain Ca2+ oscillations when the store is depleted.
Multiple subtypes of IP3R may decrease the sensitivity of spike frequency to store depletion
Although store depletion has normally been observed to decrease both spike frequency and amplitudes, there are some cases with little frequency change, even when the spike amplitude was reduced strongly due to store depletion [3,4,21]. Since minor parameter modifications in our whole-cell model failed to reproduce such stability of spike frequency, we decided to incorporate an extra mechanism in the model: an additional subtype of the IP3R. It has been shown that there are at least three distinct subtypes (types 1, 2 and 3) of IP3R in mammalian cells, including pancreatic acinar cells [22,23]. The proportion of the IP3R subtypes varies between cell types [23], and it has been suggested that changes in this proportion can affect cytosolic Ca2+ signals [24,25]. The properties of the IP3R subtypes differ in many ways, including the sensitivity to activation by cytosolic Ca2+ [26,27] and IP3 [25,28], inhibition by cytosolic Ca2+ [29,30], and susceptibility to phosphorylation by protein kinase A [31].
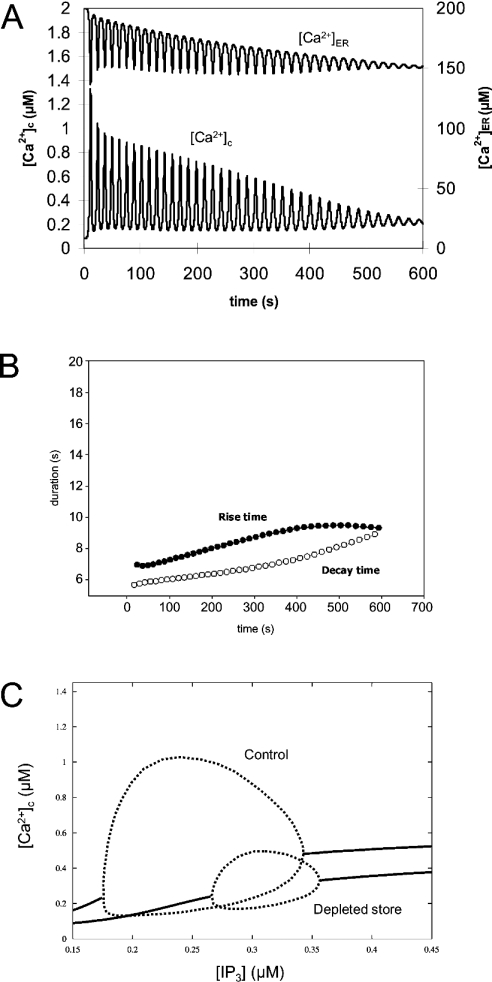
We included in our whole-cell model the sensitivity difference between the IP3R subtypes to activation by cytosolic Ca2+ and that was sufficient to reproduce the behaviour of spike frequency when the store was depleted. It has been suggested that the type-3 IP3R plays the major role in the generation of agonist-induced cytosolic Ca2+ oscillations in pancreatic acinar cells [31,32]. However, the type-2 IP3R, which has a higher affinity for cytosolic Ca2+ than the type-3 IP3R [25,27,29], could also contribute to Ca2+ oscillations. Significant levels of expression of the type-2 IP3R subtype were observed in the pancreas [23,33], whereas the level of the type-1 IP3R is reported to be low [23] and unlikely to contribute to the Ca2+ signalling in this cell type. In order to model the heterogeneous expressions of IP3R subtypes qualitatively, we incorporated an IP3R with higher affinity for cytosolic Ca2+ in the whole-cell model, in addition to the IP3R used in the previous model. Figure 4(A) shows Ca2+ spike changes during continuous Ca2+ extrusion without Ca2+ influx through the plasma membrane. As before, store depletion decreased the spike amplitude and abolished spiking at [Ca2+]ER of approx. 150 μM, which was slightly lower than in the whole-cell model with a single type of IP3R. Rise time (from 6.5 s to 10.7 s) and decay time (from 5.7 s to 9.0 s) (Figure 4B) also increased, but the magnitude of the changes was smaller than in the simulation based on a single IP3R. This effect of a high-affinity IP3R on the stability of spike frequency was seen only when its contribution to Ca2+ release was relatively small (20% of total release in the example shown in Figure 4A) and having a larger proportion of high-affinity IP3R inhibited Ca2+ oscillations (results not shown).
Figure 4. Simulation of cytosolic Ca2+ oscillations in a pancreatic acinar cell during gradual store depletion when two subtypes of IP3R are present.
The store was depleted gradually in the same way as in Figure 2. (A) [Ca2+]c (lower trace) and [Ca2+]ER (upper trace) in the whole-cell model with two subtypes of IP3R. In this simulation, it was assumed that the ‘high-affinity’ IP3R subtype had three times higher affinity to cytosolic Ca2+ than the subtype used for the simulation in Figure 2. (B) The rise time (•) and the decay time (○) of Ca2+ spikes during the simulation. (C) Bifurcation diagrams of [Ca2+]c against [IP3] in the whole-cell model. Steady-state solutions (solid line), the maxima and the minima of periodic orbits (broken line) are shown. S=200 μM in control and S=160 μM in depleted store.
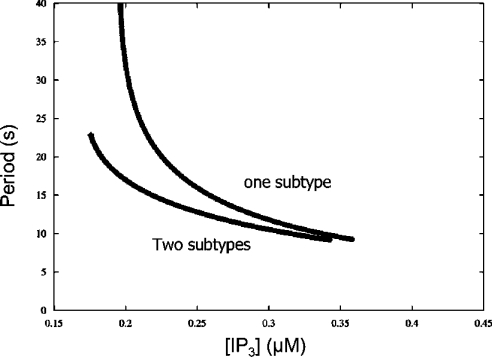
Figure 4(C) shows the bifurcation diagram of cytosolic Ca2+. The decrease of S (resting [Ca2+]ER at [Ca2+]c=0.08 μM) from 200 μM to 160 μM decreased the maximum amplitudes of the oscillations by 0.571 μM (from 0.897 to 0.326 μM) and shifted the curve to higher [IP3] ([IP3]min=0.176 μM, [IP3]max=0.342 μM with S=200 μM; [IP3]min=0.266 μM, [IP3]max=0.356 μM with S=160 μM). The extent of the amplitude changes of the oscillations and the curve shift were comparable with that of the whole-cell model with a single IP3R subtype. This suggests that the co-expression of two IP3R subtypes did not significantly alter the basic property of the inhibition of Ca2+ oscillations by store depletion. However, co-expression seems to affect the sensitivity of the Ca2+ spike frequency to [IP3]. Figure 5 shows the spike period at different [IP3]. When only a single IP3R was expressed, a decrease in [IP3] increased the spike period rapidly and went beyond 40 s at the lowest possible [IP3] for sustainable Ca2+ oscillations. The spike period in the model with two IP3R subtypes was similar compared with the single IP3R system when [IP3] was high, but was much smaller when [IP3] was low.
Figure 5. Relationship between [IP3] and oscillation periods when one or two IP3R subtypes were present.
The traces compare the relationship between the period of cytosolic Ca2+ oscillations and [IP3] in the mathematical models with one and two subtypes of IP3R.
DISCUSSION
In the present study, we have developed a mathematical model for SERCA2b, and analysed the effect of the ER luminal Ca2+ on cytosolic Ca2+ oscillations. Using data for the relationship between [Ca2+]ER and ER Ca2+ uptake rate in intact pancreatic acinar cells [2], as well as kinetic data from isolated ER vesicles [14,18], we have produced a portrait of the dual dependence of SERCA2b activity on cytosolic and ER Ca2+ as a three-dimensional plot. Our SERCA2b model is based on prediction from the flux control coefficients of the mathematical model for SERCA1a, indicating that there should be an additional effect of luminal Ca2+ on SERCA2b that inhibits ER Ca2+ uptake at low [Ca2+]c. Our mathematical model of SERCA is more complete than previously published models [14,15], because it reproduces accurately not only the sensitivity of ER Ca2+ uptake to luminal Ca2+ in the acinar cells, but also modulation of this sensitivity by cytosolic Ca2+, which was not addressed by previous models. Khan et al. [34] showed that a [Ca2+]ER rise increased the phosphorylation level of SERCAs by Pi. As a high concentration of Pi alone was not sufficient for maximum phosphorylation, they suggested that there must be binding sites for luminal Ca2+ in an unphosphorylated form. Therefore the effect of luminal Ca2+ would be shifting the equilibrium from E1 to E2 that was subsequently phosphorylated by Pi in their experiments. This is consistent with our hypothesis that the conversion from E2 into E1 is sensitive to luminal Ca2+.
In the computational models, store depletion decreased both the amplitude and frequency of cytosolic Ca2+ oscillations. In bifurcation diagrams, store depletion raised both the lower and higher thresholds of [IP3] and ‘desensitized’ Ca2+ oscillations to [IP3]. It has been shown experimentally that the spike frequency and amplitude are positively correlated to [IP3] in pancreatic acinar cells [35] and, therefore, this shift of the bifurcation curve would decrease both amplitude and frequency of Ca2+ oscillations. It may appear to be counter-intuitive that an increase of ER Ca2+ uptake stimulated by store depletion prolonged the decay time, as these two events are often considered to be negatively correlated [36,37]. This puzzling relationship between ER Ca2+ uptake rate and decay time is associated with the decrease of spike amplitude caused by store depletion. When the amplitude is smaller, the feedback inhibition of Ca2+ release by cytosolic Ca2+ and the stimulation of Ca2+ uptake by store depletion will be weaker. In this situation, both rise and decay times will become longer. The pattern of change in spike shapes during gradual store depletion in our model is consistent with the change during ACh-induced slow Ca2+ spikes (period >100 s) in the pancreatic acinar cell with decreased external Ca2+ [4].
Spike amplitudes decrease with constant spike frequency during gradual store Ca2+ depletion. This was observed both with regard to ACh-induced fast oscillations [3] and CCK (cholecystokinin)-induced slow oscillations in pancreatic acinar cells [4,38]. In the mathematical model, it is possible to enhance the stability of spike frequency by co-expressing two subtypes of IP3R. Therefore ACh and CCK may also be utilizing more than one release mechanism to generate Ca2+ oscillations. Pancreatic acinar cells have all three subtypes of IP3R [23], as well as cADPR- [39] and NAADP- [40] mediated release mechanisms, which are strongly linked to the RyR [41].The interaction between these Ca2+-release mechanisms is crucial for the generation of the spatial and temporal patterns of Ca2+ oscillations [42]. Changes in CCK concentration have much smaller effects on the frequency of Ca2+ oscillations than on the amplitude [43], which supports our model in which the spike frequency is stable when Ca2+ oscillations are governed by multiple Ca2+-release mechanisms. However, if the frequency is controlled by oscillations in the concentration of the Ca2+-releasing second messenger, store depletion would also decrease amplitude without affecting its frequency [44]. Such a mechanism has not been demonstrated in pancreatic acinar cells, where application of second messengers through a patch pipette is sufficient to cause Ca2+ oscillations [42]. However, oscillations of the messenger concentration cannot be ruled out in some types of agonist-induced Ca2+ oscillations.
Since the rate of Ca2+ release from the ER should depend on the concentration gradient of Ca2+ across the ER membrane, [Ca2+]ER can also affect Ca2+ release. Caroppo et al. [45] reported recently that the extent of IP3-induced Ca2+ release, assessed by changes in the fluorescence of ER-trapped Mag-fura-2, was dependent on the luminal Ca2+ concentration. Falcke et al. [46] also showed that the overexpression of SERCA2b in Xenopus oocytes elevated [Ca2+]ER and increased the amplitude of Ca2+ oscillations. By comparing the experimental results with mathematical models, Falcke et al. [46] suggested that the enhancement of Ca2+ release caused by the elevation of [Ca2+]ER could offset the inhibitory effect of the increased Ca2+ uptake. Since only the IP3R was sensitive to the luminal Ca2+ in their mathematical model, their results indicate that [Ca2+]ER can modulate cytosolic Ca2+ oscillations in Xenopus oocytes through its effect on the IP3R when the Ca2+ uptake is insensitive to luminal Ca2+. If the rate of Ca2+ release is proportional to the Ca2+ concentration gradient between the ER and the cytosol, [Ca2+]ER would affect Ca2+ release much less than Ca2+ uptake, since [Ca2+]ER can inhibit Ca2+ uptake with high co-operativity. Instead, luminal Ca2+ may alter the open probability of Ca2+-release channels in a non-linear fashion by direct or indirect interactions with the channels.
Recently, Sneyd et al. [47] examined the relationship between cytosolic Ca2+ oscillations and plasma membrane Ca2+ influx both theoretically and experimentally. They argued, using mathematical models, that the total cellular Ca2+ during steady state Ca2+ oscillations can be lower than in the resting condition and just above the threshold level to maintain cytosolic Ca2+ oscillations. In this condition, the loss of cellular Ca2+ during a Ca2+ spike due to Ca2+ extrusion by PMCA would need to be recovered by Ca2+ entry from the plasma membrane in the inter-spike period before the next Ca2+ rise. Therefore blockage of Ca2+ influx during steady-state Ca2+ oscillations will keep the total Ca2+ below the threshold and thereby acutely abolish the oscillations. They supported this concept by showing experimentally that blockade of Ca2+ influx and efflux by La3+ during carbachol-induced Ca2+ oscillations could acutely block cytosolic Ca2+ oscillations in HEK-293 cells. The difference between their model and ours, in which the removal of external Ca2+ reduced Ca2+ spike amplitudes only gradually, can be explained by the difference in the kinetics of the Ca2+ oscillations observed. While we modelled relatively fast Ca2+ oscillations (period of approx. 10 s), Sneyd et al. [47] examined slow Ca2+ oscillations (period of approx. 100 s) in which each Ca2+ spike will be accompanied by significant Ca2+ extrusion. Park et al. [3] observed small, but detectable, decreases of [Ca2+]ER (approx. 20 μM) during each slow Ca2+ oscillation spike. In this case, the recovery of [Ca2+]ER would depend on Ca2+ influx. No such changes in [Ca2+]ER were observed during fast Ca2+ oscillation spikes. This suggests that, during the fast oscillations, the decrease in Ca2+ influx from the plasma membrane will not have an immediate effect on [Ca2+]ER. Therefore the effect of blocking Ca2+ influx observed by Sneyd et al. [47] is not directly applicable to our model. Nevertheless, it is remarkable that a small decrease in [Ca2+]ER, during an inter-spike period, can be sufficient to abolish Ca2+ oscillations, and this suggests that SERCA2b, known to be present in HEK-293 cells [48], may have a strong luminal sensitivity in this cell type, exactly as in the acinar cell.
Using computational models, we analysed the effect of changes in [Ca2+]ER on Ca2+ oscillations in pancreatic acinar cells. Our SERCA2b model successfully reproduced the highly non-linear inhibition of ER Ca2+ uptake by luminal Ca2+ in this cell type and demonstrated the dual dependence of Ca2+ uptake on [Ca2+]c and [Ca2+]ER. In the whole-cell model, we observed a high sensitivity of the amplitude of cytosolic Ca2+ oscillations to changes in [Ca2+]ER that was consistent with the experimental finding [3]. A whole-cell model with a single IP3R type did not reproduce the ability of Ca2+ oscillations to retain the spike frequency when the amplitude was being reduced by store depletion. However, we have found that the inclusion of an additional subtype of IP3R with higher affinity to cytosolic Ca2+ enhanced the stability of spike frequency during store depletion, suggesting a co-operative effect between the IP3R subtypes. We expect that our mathematical models will advance understanding of the regulation of Ca2+ oscillations by cytosolic and luminal Ca2+.
Acknowledgments
We thank Nina Burdakova and Mark Houghton for technical assistance. K. Y. is a Wellcome Trust Prize Ph.D. student.
APPENDIX
The structure of the whole-cell model
The differential equations for [Ca2+] in the cytosol (c) and the endoplasmic reticulum (e) were:
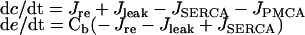 |
where Cb is the ratio of the Ca2+-buffering capacities of the cytosol and the ER, Jre is the Ca2+-release rate from the IP3R, Jleak is the Ca2+-leak rate from the ER, JSERCA is the Ca2+-uptake rate by SERCA and JPMCA is the Ca2+-extrusion rate by PMCA.
Ca2+ uptake by SERCA
Turnover rates were calculated from the kinetic models of SERCA2b and converted into uptake rate by multiplying them by conversion factors (Vp).
Ca2+ release from IP3R
We used the mathematical model of Ca2+ waves in pancreatic acinar cells of Sneyd et al. [49] for the IP3R model. This model assumed three states of the receptor (shut, open or inactive) where [IP3] and [Ca2+]c regulate the transit of the states. The differential equations for the probability of three states (x for shut, y for open and z for inactive state) were:
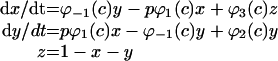 |
where φ1(c)=(k1R1+r2c)/(R1+c), φ−1(c)=(k−1+r−2)R3/(c+R3), φ2(c)=(k2R3+r4c)/(R3+c), φ3(c)=(k3R5+r6c)/(R5+c) and P(c)=y4.
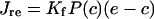 |
where p is [IP3], P(c) is open probability and Kf is the maximum Ca2+ release rate from the IP3R.
Ca2+ extrusion by PMCA
The rate of Ca2+ extrusion from the cytosol by the plasma membrane in a pancreatic acinar cell was measured as described by Camello et al. [50] using the droplet technique. We derived an empirical relationship between cytosolic Ca2+ and the extrusion rate from the measurements:
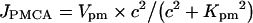 |
where Vpm is the maximum extrusion rate and Kpm is the apparent affinity of PMCA to cytosolic Ca2+.
Ca2+ leak from the ER
It has been shown that the leak rate does not change significantly when [Ca2+]ER is above 100 μM [2]. Therefore Jleak was set to be constant in the whole-cell model.
All the parameter values for the whole-cell model are summarized in Table A1. These values were used in all the simulations unless otherwise stated.
Table A1. Parameters for the whole-cell model of Ca2+ oscillations in pancreatic acinar cells.
| Parameter | Value (unit) | Parameter | Value (unit) |
|---|---|---|---|
| Kf | 54 (μM·s−1) | r6 | 0 |
| k1 | 0 | R1 | 6 (μM) |
| k−1 | 0.88 (s−1) | R3 | 50 (μM) |
| k2 | 0.53 (s−1) | R5 | 1.6 (μM) |
| k3 | 1 (s−1) | Jleak | 0.2 (μM·s−1) |
| r2 | 100 (s−1) | Vp | 0.0257 (μM) |
| r−2 | 0 | Vpm | 0.0028 (μM·s−1) |
| r4 | 20 (s−1) | Kpm | 0.23 (μM) |
References
- 1.Hofer A. M., Landolfi B., Debellis L., Pozzan T., Curci S. Free [Ca2+] dynamics measured in agonist-sensitive stores of single living intact cells: a new look at the refilling process. EMBO J. 1998;17:1986–1995. doi: 10.1093/emboj/17.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mogami H., Tepikin A. V., Petersen O. H. Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J. 1998;17:435–442. doi: 10.1093/emboj/17.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park M. K., Petersen O. H., Tepikin A. V. The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movements and equilibration. EMBO J. 2000;19:5729–5739. doi: 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tepikin A. V., Voronina S. G., Gallacher D. V., Petersen O. H. Pulsatile Ca2+ extrusion from single pancreatic acinar cells during receptor-activated cytosolic Ca2+ spiking. J. Biol. Chem. 1992;267:14073–14076. [PubMed] [Google Scholar]
- 5.Favre C. J., Schrenzel J., Jacquet J., Lew D. P., Krause K. H. Highly supralinear feedback inhibition of Ca2+ uptake by the Ca2+ load of intracellular stores. J. Biol. Chem. 1996;271:14925–14930. doi: 10.1074/jbc.271.25.14925. [DOI] [PubMed] [Google Scholar]
- 6.Solovyova N., Veselovsky N., Toescu E. C., Verkhratsky A. Ca2+ dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca2+-induced Ca2+ release triggered by physiological Ca2+ entry. EMBO J. 2002;21:622–630. doi: 10.1093/emboj/21.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favre C. J., Jerstrom P., Foti M., Stendhal O., Huggler E., Lew D. P., Krause K. H. Organization of Ca2+ stores in myeloid cells: association of SERCA2b and the type-1 inositol-1,4,5-trisphosphate receptor. Biochem. J. 1996;316:137–142. doi: 10.1042/bj3160137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee M. G., Xu X., Zeng W., Diaz J., Kuo T. H., Wuytack F., Raeymaekers L., Muallem S. Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells: role in initiation and propagation of [Ca2+]i waves. J. Biol. Chem. 1997;272:15771–15776. doi: 10.1074/jbc.272.25.15771. [DOI] [PubMed] [Google Scholar]
- 9.Camacho P., Lechleiter J. D. Calreticulin inhibits repetitive intracellular Ca2+ waves. Cell. 1995;82:765–771. doi: 10.1016/0092-8674(95)90473-5. [DOI] [PubMed] [Google Scholar]
- 10.John L. M., Lechleiter J. D., Camacho P. Differential modulation of SERCA2 isoforms by calreticulin. J. Cell Biol. 1998;142:963–973. doi: 10.1083/jcb.142.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roderick H. L., Lechleiter J. D., Camacho P. Cytosolic phosphorylation of calnexin controls intracellular Ca2+ oscillations via an interaction with SERCA2b. J. Cell Biol. 2000;149:1235–1248. doi: 10.1083/jcb.149.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Camacho P. Ca2+-dependent redox modulation of SERCA 2b by ERp57. J. Cell Biol. 2004;164:35–46. doi: 10.1083/jcb.200307010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherman A. S., Li Y., Keizer J. E. Whole-cell models. In: Fall C. P., Marland E. S., Wagner J. M., Tyson J. J., editors. Computational Cell Biology. New York: Springer-Verlag; 2002. pp. 101–139. [Google Scholar]
- 14.Dode L., Vilsen B., Van Baelen K., Wuytack F., Clausen J. D., Andersen J. P. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 3 isoforms by steady-state and transient kinetic analyses. J. Biol. Chem. 2002;277:45579–45591. doi: 10.1074/jbc.M207778200. [DOI] [PubMed] [Google Scholar]
- 15.Inesi G., de Meis L. Regulation of steady state filling in sarcoplasmic reticulum: roles of back-inhibition, leakage, and slippage of the calcium pump. J. Biol. Chem. 1989;264:5929–5936. [PubMed] [Google Scholar]
- 16.Mendes P. GEPASI: a software package for modelling the dynamics, steady states and control of biochemical and other systems. Comput. Appl. Biosci. 1993;9:563–571. doi: 10.1093/bioinformatics/9.5.563. [DOI] [PubMed] [Google Scholar]
- 17.Ermentrout B. Philadelphia: SIAM; 2002. Simulating, Analyzing and Animating Dynamical Systems: A Guide to XPPAUT for Researchers and Students. [Google Scholar]
- 18.Lytton J., Westlin M., Burk S. E., Shull G. E., MacLennan D. H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 1992;267:14483–14489. [PubMed] [Google Scholar]
- 19.Fell D. A. Understanding the Control of Metabolism. London: Portland Press; 1997. Metabolic control analysis; pp. 101–134. [Google Scholar]
- 20.Park M. K., Tepikin A. V., Petersen O. H. The relationship between acetylcholine-evoked Ca2+-dependent current and the Ca2+ concentrations in the cytosol and the lumen of the endoplasmic reticulum in pancreatic acinar cells. Pflügers Arch. 1999;438:760–765. doi: 10.1007/s004249900128. [DOI] [PubMed] [Google Scholar]
- 21.Shuttleworth T. J., Thompson J. L. Ca2+ entry modulates oscillation frequency by triggering Ca2+ release. Biochem. J. 1996;313:815–819. doi: 10.1042/bj3130815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee M. G., Xu X., Zeng W., Diaz J., Wojcikiewicz R. J., Kuo T. H., Wuytack F., Raeymaekers L., Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells: correlation with initiation and propagation of [Ca2+]i waves. J. Biol. Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- 23.Wojcikiewicz R. J. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J. Biol. Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 24.Hattori M., Suzuki A. Z., Higo T., Miyauchi H., Michikawa T., Nakamura T., Inoue T., Mikoshiba K. Distinct roles of inositol 1,4,5-trisphosphate receptor types 1 and 3 in Ca2+ signaling. J. Biol. Chem. 2004;279:11967–11975. doi: 10.1074/jbc.M311456200. [DOI] [PubMed] [Google Scholar]
- 25.Miyakawa T., Maeda A., Yamazawa T., Hirose K., Kurosaki T., Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehning D., Joseph S. K. Functional properties of recombinant type I and type III inositol 1,4,5-trisphosphate receptor isoforms expressed in COS-7 cells. J. Biol. Chem. 2000;275:21492–21499. doi: 10.1074/jbc.M001724200. [DOI] [PubMed] [Google Scholar]
- 27.Ramos-Franco J., Fill M., Mignery G. A. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys. J. 1998;75:834–839. doi: 10.1016/S0006-3495(98)77572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wojcikiewicz R. J., Luo S. G. Differences among type I, II, and III inositol-1,4,5-trisphosphate receptors in ligand-binding affinity influence the sensitivity of calcium stores to inositol-1,4,5-trisphosphate. Mol. Pharmacol. 1998;53:656–662. doi: 10.1124/mol.53.4.656. [DOI] [PubMed] [Google Scholar]
- 29.Mak D. O., McBride S., Foskett J. K. Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels. Ca2+ activation uniquely distinguishes types 1 and 3 InsP3 receptors. J. Gen. Physiol. 2001;117:435–446. doi: 10.1085/jgp.117.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swatton J. E., Taylor C. W. Fast biphasic regulation of type 3 inositol trisphosphate receptors by cytosolic calcium. J. Biol. Chem. 2002;277:17571–17579. doi: 10.1074/jbc.M200524200. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci D. R., Groblewski G. E., Sneyd J., Yule D. I. Targeted phosphorylation of inositol 1,4,5-trisphosphate receptors selectively inhibits localized Ca2+ release and shapes oscillatory Ca2+ signals. J. Biol. Chem. 2000;275:33704–33711. doi: 10.1074/jbc.M004278200. [DOI] [PubMed] [Google Scholar]
- 32.Straub S. V., Giovannucci D. R., Bruce J. I., Yule D. I. A role for phosphorylation of inositol 1,4,5-trisphosphate receptors in defining calcium signals induced by peptide agonists in pancreatic acinar cells. J. Biol. Chem. 2002;277:31949–31956. doi: 10.1074/jbc.M204318200. [DOI] [PubMed] [Google Scholar]
- 33.Fujino I., Yamada N., Miyawaki A., Hasegawa M., Furuichi T., Mikoshiba K. Differential expression of type 2 and type 3 inositol 1,4,5-trisphosphate receptor mRNAs in various mouse tissues: in situ hybridization study. Cell Tissue Res. 1995;280:201–210. doi: 10.1007/BF00307790. [DOI] [PubMed] [Google Scholar]
- 34.Khan Y. M., East J. M., Lee A. G. Effects of pH on phosphorylation of the Ca2+-ATPase of sarcoplasmic reticulum by inorganic phosphate. Biochem. J. 1997;321:671–676. doi: 10.1042/bj3210671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakui M., Potter B. V., Petersen O. H. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature (London) 1989;339:317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- 36.Camacho P., Lechleiter J. D. Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science. 1993;260:226–229. doi: 10.1126/science.8385800. [DOI] [PubMed] [Google Scholar]
- 37.Majewska A., Brown E., Ross J., Yuste R. Mechanisms of calcium decay kinetics in hippocampal spines: role of spine calcium pumps and calcium diffusion through the spine neck in biochemical compartmentalization. J. Neurosci. 2000;20:1722–1734. doi: 10.1523/JNEUROSCI.20-05-01722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yule D. I., Williams J. A. U73122 inhibits Ca2+ oscillations in response to cholecystokinin and carbachol but not to JMV-180 in rat pancreatic acinar cells. J. Biol. Chem. 1992;267:13830–13835. [PubMed] [Google Scholar]
- 39.Thorn P., Gerasimenko O., Petersen O. H. Cyclic ADP-ribose regulation of ryanodine receptors involved in agonist evoked cytosolic Ca2+ oscillations in pancreatic acinar cells. EMBO J. 1994;13:2038–2043. doi: 10.1002/j.1460-2075.1994.tb06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cancela J. M., Churchill G. C., Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature (London) 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- 41.Gerasimenko J. V., Maruyama Y., Yano K., Dolman N. J., Tepikin A. V., Petersen O. H., Gerasimenko O. V. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J. Cell Biol. 2003;163:271–282. doi: 10.1083/jcb.200306134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cancela J. M., Van Coppenolle F., Galione A., Tepikin A. V., Petersen O. H. Transformation of local Ca2+ spikes to global Ca2+ transients: the combinatorial roles of multiple Ca2+ releasing messengers. EMBO J. 2002;21:909–919. doi: 10.1093/emboj/21.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsunoda Y., Stuenkel E. L., Williams J. A. Oscillatory mode of calcium signaling in rat pancreatic acinar cells. Am. J. Physiol. 1990;258:C147–C155. doi: 10.1152/ajpcell.1990.258.1.C147. [DOI] [PubMed] [Google Scholar]
- 44.Hirose K., Iino M. Heterogeneity of channel density in inositol-1,4,5-trisphosphate-sensitive Ca2+ stores. Nature (London) 1994;372:791–794. doi: 10.1038/372791a0. [DOI] [PubMed] [Google Scholar]
- 45.Caroppo R., Colella M., Colasuonno A., DeLuisi A., Debellis L., Curci S., Hofer A. M. A reassessment of the effects of luminal [Ca2+] on inositol 1,4,5-trisphosphate-induced Ca2+ release from internal stores. J. Biol. Chem. 2003;278:39503–39508. doi: 10.1074/jbc.M305823200. [DOI] [PubMed] [Google Scholar]
- 46.Falcke M., Li Y., Lechleiter J. D., Camacho P. Modeling the dependence of the period of intracellular Ca2+ waves on SERCA expression. Biophys. J. 2003;85:1474–1481. doi: 10.1016/S0006-3495(03)74580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sneyd J., Tsaneva-Atanasova K., Yule D. I., Thompson J. L., Shuttleworth T. J. Control of calcium oscillations by membrane fluxes. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1392–1396. doi: 10.1073/pnas.0303472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poch E., Leach S., Snape S., Cacic T., MacLennan D. H., Lytton J. Functional characterization of alternatively spliced human SERCA3 transcripts. Am. J. Physiol. 1998;275:C1449–C1458. doi: 10.1152/ajpcell.1998.275.6.C1449. [DOI] [PubMed] [Google Scholar]
- 49.Sneyd J., LeBeau A., Yule D. I. Traveling waves of calcium in pancreatic acinar cells: model construction and bifurcation analysis. Phys. D. 2000;145:158–179. [Google Scholar]
- 50.Camello P., Gardner J., Petersen O. H., Tepikin A. V. Calcium dependence of calcium extrusion and calcium uptake in mouse pancreatic acinar cells. J. Physiol. 1996;490:585–593. doi: 10.1113/jphysiol.1996.sp021169. [DOI] [PMC free article] [PubMed] [Google Scholar]