Abstract
The anti-apoptotic effect of Bcl-2 is well established, but the detailed mechanisms are unknown. In the present study, we show in vitro a direct interaction of Bcl-2 with the rat skeletal muscle SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase), leading to destabilization and inactivation of the protein. Recombinant human Bcl-2Δ21, a truncated form of Bcl-2 with a deletion of 21 residues at the C-terminal membrane-anchoring region, was expressed and affinity-purified as a glutathione S-transferase fusion protein. Bcl-2Δ21 co-immunoprecipitated and specifically interacted with SERCA in an in vitro-binding assay. The original level of Bcl-2 in sarcoplasmic reticulum vesicles was very low, i.e. hardly detectable by immunoblotting with specific antibodies. The addition of Bcl-2Δ21 to the sarcoplasmic reticulum resulted in the inhibition of the Ca2+-ATPase activity dependent on the Bcl-2Δ21/SERCA molar ratio and incubation time. A complete inactivation of SERCA was observed after 2.5 h of incubation at approx. 2:1 molar ratio of Bcl-2Δ21 to SERCA. In contrast, Bcl-2Δ21 did not significantly change the activity of the plasma-membrane Ca2+-ATPase. The redox state of the single Cys158 residue in Bcl-2Δ21 and the presence of GSH did not affect SERCA inhibition. The interaction of Bcl-2Δ21 with SERCA resulted in a conformational transition of SERCA, assessed through a Bcl-2-dependent increase in SERCA thiols available for the labelling with a fluorescent reagent. This partial unfolding of SERCA did not lead to a higher sensitivity of SERCA towards oxidative inactivation. Our results suggest that the direct interaction of Bcl-2 with SERCA may be involved in the regulation of apoptotic processes in vivo through modulation of cytoplasmic and/or endoplasmic reticulum calcium levels required for the execution of apoptosis.
Keywords: apoptosis, Bcl-2, Ca2+-ATPase, calcium, sarcoplasmic/endoplasmic reticulum
Abbreviations: CaM, calmodulin; DTNB, 5,5′-dithiobis-(2-nitrobenzoic acid); ER, endoplasmic reticulum; ESI-MS, electrospray ionization mass spectrometry; GST, glutathione S-transferase; MALDI–TOF, matrix-assisted laser-desorption ionization–time-of-flight; NESI-MS/MS, nanoelectrospray ionization tandem mass spectrometry; PMCA, plasma-membrane Ca2+-ATPase; SERCA, sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase; SPM, synaptic plasma membranes; SR, sarcoplasmic reticulum; STE, Tris-buffered saline; TG, thapsigargin
INTRODUCTION
Bcl-2 is a prominent anti-apoptotic protein, but its mechanism of action is currently not completely understood. In the present study, we will show that one of the anti-apoptotic functions of Bcl-2 may be the direct association with and destabilization of the SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase), potentially lowering ER (endoplasmic reticulum) Ca2+ levels below the threshold required for an apoptotic signal.
Calcium is an important second messenger regulating various cellular processes. Depending on the amplitude, Ca2+ signals can trigger apoptosis [1]. The apoptotic signal requires an increase in mitochondrial Ca2+ levels, which induces the release of mitochondrial cytochrome c and activation of executioner caspase proteases [2,3]. Resting cells display low concentrations of cytoplasmic Ca2+, whereas most of the cellular Ca2+ is stored in the ER. Ca2+ is transported into the ER by SERCA and is released through inositol-1,4,5-trisphosphate-activated channels and/or the ryanodine receptor [4]. Along with various Ca2+-binding proteins, SERCA is responsible for the maintenance of high luminal Ca2+ concentrations, which are essential for normal ER function [5,6]. Recent results indicate that mitochondrial Ca2+ concentrations triggering an apoptotic signal strictly depend on the ER Ca2+ levels [7–9]. Stimulation of inositol-1,4,5-trisphosphate-mediated Ca2+ release from the ER leads to the rapid uptake of Ca2+ by closely juxtaposed mitochondria [10]. TG (thapsigargin), an irreversible and selective inhibitor of SERCA, causes the passive release of Ca2+ from the ER and an increase in cytosolic Ca2+, which may induce apoptosis [11]. However, the overexpression of SERCA can also result in conditions inducing apoptosis [12].
The proteins of the Bcl-2 gene family play an essential role in the regulation of apoptosis [13–16]. It was proposed that Bcl-2 acts either as a channel-forming protein, permeabilizing the outer mitochondrial membrane, or as an adaptor docking protein [17–19]. The localization of Bcl-2 to the ER and the mitochondria [8,20,21] suggests that Bcl-2 may play a role in the regulation of intracellular Ca2+ homoeostasis. For example, the overexpression of Bcl-2 in some cell types caused a 30% decrease in the amount of Ca2+, which could be released from intracellular stores (ER and Golgi apparatus), thus attenuating the potential apoptogenic increase in Ca2+ levels in the mitochondria [22,23]. Bcl-2 overexpression abolished the increase in cytosolic Ca2+ concentration induced by the inhibition of SERCA with TG [24,25]. Using different pharmacological and molecular approaches to mimic the Bcl-2 effect on ER, Pinton et al. [26] could show that conditions lowering the ER Ca2+ levels (similar to those induced by Bcl-2 overexpression) protected cells from ceramide, a Bcl-2-sensitive apoptotic stimulus. Accordingly, stimuli that increased the Ca2+ levels in the ER had the opposite effect. The Bcl-2-induced depletion of ER Ca2+ might ultimately suppress the Ca2+ activation of mitochondrial processes, in particular the production of ATP and the release of cytochrome c. However, the mechanisms leading to decreased ER Ca2+ in Bcl-2-overexpressing cells are not established.
The aim of the present study was to examine the direct interaction of purified SERCA and Bcl-2 in vitro as one potential mechanism to alter ER Ca2+ levels. A truncated form, Bcl-2Δ21, lacking the C-terminal membrane-anchoring domain was used for bacterial expression and affinity purification that yields the appropriate amounts of the protein. It is known that this truncated protein retains approx. 50% of the anti-apoptotic activity when compared with the full-length protein when overexpressed in cells [27].
We will demonstrate that Bcl-2Δ21 destabilizes native SERCA in rat skeletal-muscle SR (sarcoplasmic reticulum) vesicles. These vesicles predominantly contain the SERCA1 isoform, which is, however, highly homologous with the other SERCA isoforms present in cardiac and smooth muscles. Our results will suggest that the direct interaction of Bcl-2 with SERCA may be involved in some aspects of the regulation of apoptotic processes in cells through modulation of cytoplasmic and/or ER calcium levels.
EXPERIMENTAL
Protein expression and purification
The clone of human Bcl-2Δ21 was kindly provided by Professor S. J. Korsmeyer (Harvard Medical School, Boston, MA, U.S.A.). Plasmids encoding GST–Bcl-2Δ21 fusion proteins (where GST stands for glutathione S-transferase) were constructed by subcloning the human Bcl-2Δ21 cDNA into the pGEX3T vector (Amersham Biosciences, Piscataway, NJ, U.S.A.). Bcl-2Δ21 was produced as GST fusion protein from the pGEX3T vector using Escherichia coli DH1 as the host strain. A 10 ml overnight culture was used to inoculate 1 litre of Luria–Bertani medium, which was further incubated at 37 °C until an absorbance A600 0.4 was achieved. The cells were induced with 0.1 mM isopropyl β-D-thiogalactoside and incubated at 30 °C for an additional 6 h. The cells were harvested by centrifugation and re-suspended in 15 ml of ice-cold Tris-buffered saline (STE) solution containing 7.5 mM Tris (pH 8.0), 150 mM NaCl and 3 mM EDTA. Cells were disrupted by sonication in the presence of 1% (w/v) Triton X-100, 5 mM dithiothreitol, 100 μM PMSF and protease inhibitors (Roche Diagnostics, Indianapolis, IN, U.S.A.) after the incubation for 20 min with lysozyme (0.1 mg/ml) on ice. The resulting lysate was centrifuged at 12000 g for 15 min at 4 °C to pellet the cellular debris. Glutathione–agarose beads were added to the supernatant and incubated at 4 °C with gentle rotation for 4 h. The beads were washed twice with 50 ml of ice-cold PBS without Triton X-100. After incubation with thrombin (5 units) for 1 h at room temperature (20 °C), the cleaved proteins were eluted with 0.5 ml of the STE buffer. To 0.5 ml of elution fractions 20 μl of prewashed thrombin-binding beads (Sigma, St. Louis, MO, U.S.A.) was added, followed by incubation for 30 min at 4 °C and removal of the beads by centrifugation. This procedure yields approx. 150 μg of Bcl-2Δ21 (0.5 ml of 0.3 mg/ml solution in the STE buffer if not stated otherwise) as measured by microassay with Coomassie Blue Plus protein reagent (Pierce, Rockford, IL, U.S.A.). Purified proteins were characterized by SDS/PAGE and Western-blot analysis.
Isolation of SR
Native SR vesicles (light fraction) were prepared from 5–6-month-old Fisher 344 rat hindlimb skeletal muscles (fast-twitch fibres) as described earlier [28]. Briefly, muscles (usually 20–30 g) were homogenized at 4 °C in a Waring blender for 1 min at maximal speed in 3 vol. of buffer containing 0.1 M KCl, 0.1 mM EDTA and 20 mM Mops (pH 7.4). The homogenate was centrifuged at 5000 g for 20 min to remove cell debris, the pellet washed again under the same conditions and the pooled supernatants were centrifuged at 11800 g for 20 min to pellet the mitochondria. To dissolve myosin, the supernatant was filtered through six layers of cheesecloth and solid KCl was added to make a final concentration of 0.6 M. After 20 min incubation, the SR was pelleted at 23500 g for 1 h. The supernatant was decanted and the pellets were resuspended in a medium containing 0.3 M sucrose and 20 mM Mops (pH 7.0) and centrifuged at 100000 g for 30 min. SR vesicles were re-suspended in a small volume of medium consisting of 0.3 M sucrose and 20 mM Mops (pH 7.0) using a Dounce homogenizer, divided into aliquots, quickly frozen in liquid nitrogen and stored at −70 °C. Protein concentration was determined by the bicinchoninic acid assay using BSA as a standard according to the manufacturer's instructions (Pierce).
Incubations of SERCA with Bcl-2Δ21
For incubations, the concentrated SR vesicle suspension (≥12 mg of protein/ml) was diluted to the final concentration of 0.6 mg/ml in the appropriate buffer solution so that the contribution of the SR storage buffer to the incubation medium was <5%. Since the STE buffer is preferred for the affinity isolation and storage of Bcl-2Δ21 (at concentrations of ≤0.3 mg/ml), the incubations of SR with Bcl-2Δ21 were generally conducted in the STE buffer if not stated otherwise. To study the effect of EDTA contained in the buffer, in control experiments, Bcl-2Δ21 was isolated in ST buffer (150 mM NaCl and 7.5 mM Tris, pH 8.0) lacking EDTA, and the respective incubations were also performed in the ST buffer. Incubations were conducted in Eppendorf tubes without agitation at 37 °C in a dry thermostat.
SERCA activity assays
Total, Ca2+-dependent and basal ATPase activities of SERCA in SR were determined at 25 °C by colorimetric assay of Pi in the presence or absence of the calcium ionophore A23187 [29]. The reaction was started by the addition of 5 mM ATP to a medium containing 0.6 μg/ml SR, 100 mM KCl, 5 mM MgCl2, 4 μM A23187, 25 mM Mops (pH 7.0) and either 0.1 mM CaCl2 or 0.1 mM EGTA, and the initial rate of Pi release for 1 min was used to calculate the activity. Activity assayed in the presence of EGTA (basal activity) was subtracted from that assayed in the presence of CaCl2 (total activity) to obtain Ca2+-dependent ATPase (Ca2+-ATPase) activity.
To assess the Ca2+ dependence of SERCA activity, the assay was conducted at 25 °C in the assay medium composed of 0.6 μg/ml SR, 1 mM EGTA, 50 mM Mops (pH 7.0), 120 mM KCl, 10 mM MgCl2 and 4 μM A23187. CaCl2 (0.2–4 mM) was added to achieve the desired Ca2+ concentrations, as calculated using the CHELATOR program [30] (http://www.stanford.edu/~cpatton/other.htm). The calculated free Ca2+ concentrations under different experimental conditions used in the present study are listed in Table 1. Reactions were initiated by adding ATP to a final concentration of 5 mM, and the rate of ATP hydrolysis was calculated from the initial rate of Pi release for 1 min as described above. Basal ATPase activities, measured without added Ca2+, were subtracted from total activities to yield the Ca2+-ATPase activities reported. In all our experiments, the measured Ca2+-dependent ATP hydrolysis was attributed to SERCA because the Ca2+-ATPase activity was completely inhibited by the addition of 20 μM TG (Sigma).
Table 1. Calculated free Ca2+ concentrations under different conditions applied for the SERCA activity and Ca2+-affinity measurements.
| Conditions | Total added Ca2+ (mM) | Free Ca2+ (M) | Corrected* free Ca2+ (M) |
|---|---|---|---|
| SERCA incubation in the STE buffer† | 0.01 | 3.41×10−11 | − |
| 0.1 | 3.51×10−10 | − | |
| 2.5 | 5.12×10−8 | − | |
| SERCA activity measurements‡ | 0.1 | 1.65×10−5 | 6.95×10−6 |
| SERCA Ca2+-affinity measurements§ | 0.2 | 1.95×10−7 | 1.95×10−7 |
| 0.4 | 5.18×10−7 | 5.19×10−7 | |
| 0.6 | 1.16×10−6 | 1.16×10−6 | |
| 0.8 | 3.04×10−6 | 2.98×10−6 | |
| 1 | 2.22×10−5 | 1.66×10−5 | |
| 1.2 | 1.32×10−4 | 9.35×10−5 | |
| 1.4 | 2.61×10−4 | 2.06×10−4 | |
| 1.6 | 3.92×10−4 | 3.30×10−4 | |
| 1.8 | 5.26×10−4 | 4.59×10−4 | |
| 2 | 6.63×10−4 | 5.92×10−4 | |
| 2.4 | 9.43×10−4 | 8.66×10−4 | |
| 3 | 1.37×10−3 | 1.29×10−3 | |
| 4 | 2.12×10−3 | 2.04×10−3 |
* After the incubation of SR with Bcl-2Δ21 in STE buffer, contributing additional 0.04 mM Mops, 0.12 mM EDTA, 0.3 mM Tris and 7.5 mM NaCl to the measurement buffers.
† 0.6 μg/ml SR protein in 150 mM NaCl, 3 mM EDTA and 7.5 mM Tris (pH 8.0) at 37 °C.
‡ 0.6 μg/ml SR protein in 5 mM ATP, 4 μM A23187, 60 mM KCl, 5 mM MgCl2 and 25 mM Mops (pH 7.0) at 25 °C.
§ 0.6 μg/ml SR protein in 5 mM ATP, 4 μM A23187, 1 mM EGTA, 120 mM KCl, 10 mM MgCl2 and 50 mM Mops (pH 7.0) at 25 °C.
Preparation of brain SPM (synaptic plasma membranes)
The SPM were obtained from the brains of male adult Sprague–Dawley rats (3–5 months old). The animals were killed under CO2 anaesthesia; the brains were taken out quickly and used for the preparation of synaptosomes as described previously [31]. Synaptosomes were lysed in ice-cold 3 mM Tris/HCl and 3 mM EDTA (pH 8.5). The SPM were precipitated, washed in buffer to remove the EDTA and stored in 10 mM Tris/HCl, 50 μM MgCl2 and 0.32 M sucrose (pH 7.4) in small aliquots at −80 °C. Protein concentrations were determined using the bicinchoninic acid method.
Purification of PMCA (plasma-membrane Ca2+-ATPase)
Erythrocyte ghosts isolated from fresh porcine blood were depleted of CaM (calmodulin) and used to purify PMCA by affinity chromatography using CaM–Sepharose as described in our previous study [32]. Briefly, the supernatant obtained after solubilization of the membranes with Triton X-100 (1 mg/mg of protein) was stabilized with phosphatidylcholine (Sigma) and Ca2+ (final concentrations of 0.5 mg/ml and 100 μM respectively) and applied to a CaM–Sepharose column (Amersham Biosciences), equilibrated with a buffer containing 10 mM Hepes (pH 7.2), 120 mM KCl, 1 mM MgCl2, 0.1 mM CaCl2, 0.5 mg/ml phosphatidylcholine and 0.32% C12E8 (Calbiochem, La Jolla, CA, U.S.A.). After application of the supernatant, the column was washed with several bed volumes of equilibration buffer lacking Ca2+ to remove the non-specifically bound proteins. The retained PMCA was eluted in a buffer containing 10 mM Hepes (pH 7.2), 120 mM NaCl, 1 mM MgCl2, 0.5 mg/ml phosphatidylcholine, 0.05% C12E8, 2 mM EDTA and 5% (v/v) glycerol, and the protein was monitored by measuring the absorbance at 280 nm. Each of the fractions was checked for PMCA activity using the colorimetric assay of Pi [29]. Active fractions were pooled, and MgCl2 and CaCl2 were added to neutralize the EDTA and yield a final free concentration of 1.5 mM MgCl2 and 4.8 μM CaCl2. The enzyme was stored at −80 °C without loss of activity. Protein concentrations were estimated by the Coomassie Brilliant Blue dye-binding method according to the manufacturer's instructions (Pierce).
Measurement of PMCA activity
The activity of PMCA was determined in 96-well microplates in which each well contained the following in a final volume of 100 μl: 25 mM Tris/HCl (pH 7.4), 50 mM KCl, 1 mM MgCl2, 0.1 mM ouabain, 4 μg/ml oligomycin, 200 μM EGTA, and 230 μM CaCl2 was added to yield a final free Ca2+ concentration of 10 μM [30]. For SPM, 4 μg of membrane protein was added, but for the purified protein only 0.14 μg of protein was added per well. After a 5 min preincubation of the PMCA with the other components of the assay, the reaction was started by the addition of 1 mM ATP and continued for 20 min at 37 °C. For SPM, the reaction was stopped by the addition of a solution containing 2% (w/v) ammonium molybdate in 1.8 M H2SO4 and 5% (v/v) W-1 (polyoxyethylene ether), and the yellow-coloured phosphomolybdate complex was read immediately at 405 nm in a microwell plate reader [32]. For purified PMCA, the reaction was stopped by the addition of Malachite Green dye solution; the contents were made acidic by the addition of 19.5% H2SO4, incubated for 45 min and the colour was read at 650 nm [29]. PMCA activity was defined as the Ca2+-activated ATP hydrolysis and expressed as nmol of Pi liberated·(mg of protein)−1·min−1, based on the values from a standard curve of the absorbance using various concentrations of free Pi. The PMCA activity measured in the presence of Ca2+, but in the absence of CaM, is referred to as ‘basal’ activity and that in the presence of Ca2+ and 120 nM CaM is referred to as ‘CaM-stimulated’ activity.
SDS/PAGE and Western-blot analyses
The samples were separated on precast Novex 4–20% gradient gels using Tris/glycine running and sample buffers (Invitrogen, Carlsbad, CA, U.S.A.). Protein separation was conducted for 90 min at 200 V and 12 °C using a mini-gel electrophoresis unit (Hoefer Scientific Instuments, San Francisco, CA, U.S.A.). The gels were then either stained for proteins by Coomassie Colloidal Blue (Pierce) or electroblotted (400 mA, 2 h at 4 °C) on to a 0.45 μm PVDF membrane (Millipore, Billerica, MA, U.S.A.) before Western-blot analysis. The membranes were incubated overnight at 4 °C in 5% (v/v) dry milk T-TBS solution (20 mM Tris, 150 mM NaCl and 0.05% Tween 20, pH 7.5). After blocking, the membrane was rinsed with several changes of T-TBS and exposed to the primary antibody solution (1:4000 dilution in 1% BSA/T-TBS) for 1 h at room temperature. After incubation, the membrane was washed with T-TBS and subjected to peroxidase-conjugated secondary antibody diluted 1:10000 in T-TBS for 1 h at room temperature. The spots were visualized by the ECL®-Plus detection kit (Amersham Biosciences) according to the manufacturer's instructions, and the images were captured on a Kodak X-ray film using a Kodak-developer/fixer kit (Fisher, Pittsburgh, PA, U.S.A.).
Antibodies
Mouse monoclonal anti-Bcl-2 (sc-7382) and rabbit polyclonal phospho-specific antibodies p-Bcl-2(Ser70), p-Bcl-2(Ser87), p-Bcl-2(Thr56) and p-Bcl-2(Thr74) (sc-16647, sc-16323, sc-16321 and sc-16322 respectively) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Anti-SERCA1 (MA3-912) rabbit polyclonal antibodies were from Affinity Bioreagents (Golden, CO, U.S.A.). Secondary horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgG antibodies were from Sigma and Amersham Biosciences respectively.
Immunoprecipitation and solution-binding assay
To monitor the association between SERCA and Bcl-2, 150 μg of SR in lysis buffer containing 10 mM Tris/HCl (pH 7.4), 10 mM EDTA, 1% Nonidet P40, 1 mM PMSF and protease inhibitors (Roche Diagnostics) was incubated with 3 μg of GST–Bcl-2 or GST alone for 2 h at 4 °C. After the incubation with 10 μl (packed volume) of glutathione–Sepharose beads for 2 h, the beads were separated by centrifugation at 7500 g for 10 min and washed six times with the lysis buffer. For the immunoprecipitation of Bcl-2, 300 or 700 μg of SR protein was solubilized in the above buffer. After preclearing by Protein A–agarose (30 μl), the supernatant was mixed with anti-Bcl-2 antibodies (1 μl) and incubated for 1 h at 4 °C. Prewashed Protein A–agarose (50 μl) was then added to the samples, and after 1 h incubation, Protein A–agarose with the immunocomplex was obtained by centrifugation and resuspended in a sample buffer before SDS/PAGE and immunoblotting with anti-Bcl-2 and anti-SERCA1 antibodies.
Bcl-2 reduction and alkylation
For protein cysteine reduction, Bcl-2Δ21 solutions were incubated with 2 mM dithiothreitol for 30 min at 37 °C. Carboxymethylation of cysteine residues was achieved by subsequent incubation with 5 mM iodoacetic acid for 30 min at room temperature followed by an exhaustive dialysis with several changes of a buffer for complete removal of the reagents.
Quantification of thiol groups
Protein-bound free thiol groups were assayed by quantitative titration with Ellman's reagent, DTNB [5,5′-dithiobis-(2-nitrobenzoic acid)] [33]. Samples containing 100 μg of protein/ml were incubated with 0.2 mM DTNB and 1% (w/v) SDS in 0.1 M Tris/HCl buffer (pH 8.0) for 20 min, and A412 was measured. GSH standard dilutions were used for the calibration.
Fluorescent labelling of protein thiols
The reactive cysteine residues in proteins were monitored using the maleimide-based fluorescent label ThioGlo1 (Covalent Associates, Woburn, MA, U.S.A.). The fluorescence of ThioGlo1–protein cysteine adducts was measured in 1 ml samples at excitation and emission wavelengths of 379 and 513 nm respectively with a Shimadzu RF5000U fluorescent spectrophotometer. Protein samples were incubated with 20 μM ThioGlo1 for 1 h at 37 °C and pH 7.4 in the absence and presence of 1% SDS to monitor cysteine residues in the native and denaturated protein. DTNB titration was used for calibration of the absolute amount of protein thiols as described above.
Reaction with peroxynitrite
Peroxynitrite was prepared by the reaction of ozone with cooled aqueous sodium azide as described previously [34], divided into aliquots and kept at −70 °C and pH 12 until use. The concentration of the stock peroxynitrite (approx. 50 mM) was calculated by determining the molar absorption coefficient (ε302=1670 M−1·cm−1) [34] and all dilutions were made in 0.1% NaOH (pH 12). SR (10 mg/ml of protein) was resuspended in a buffer containing 20 mM Na2HPO4, 20 mM NaHCO3 and 1 mM diethylenetriaminepenta-acetic acid (pH 7.4). A small volume of the stock peroxynitrite was quickly added while vortex-mixing to get desirable final concentrations of peroxynitrite up to 3 mM. For control experiments, peroxynitrite was added to the buffer 5 min before the mixing with SR membranes (reverse-order-of-addition experiment).
MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight) and HPLC–ESI-MS (electrospray ionization mass spectrometry) analyses of proteins
For MALDI–TOF MS, protein samples were desalted using C18 ZipTips (Millipore, Bedford, MA, U.S.A.) and either eluted directly on to the stainless-steel sample plate with 2 μl of 50% (v/v) acetonitrile/0.1% trifluoroacetic acid saturated with α-cyano-4-hydroxycinnamate. Mass spectra were acquired in the mass range between 10000 and 50000 a.m.u. (atomic mass units) on a Voyager-DE STR mass spectrometer (PerSeptive Biosystems, Framingham, MA, U.S.A.) [35]. ESI-MS analysis of the whole protein has been performed on a Q-TOF mass spectrometer (Micromass, Manchester, U.K.) essentially as described earlier [36]. Protein samples were desalted and concentrated on a C18 protein trap before HPLC–ESI-MS measurements. Deconvolution of multiply charged protein ions was performed with the MasLinx 3.5 software.
NESI-MS/MS (nanoelectrospray ionization-tandem mass spectrometry) of in-gel tryptic digests
Protein bands of interest were excised from the gel and processed as described elsewhere [35,36]. In-gel tryptic digests (2 μl) were submitted to NESI-MS/MS analysis on a ThermoElectron LCQ Duo (San Jose, CA, U.S.A.) equipped with a nanospray source (ThermoElectron). Separation of tryptic peptides was achieved on-line before MS/MS analysis on a BioBasic C18 nanoflow column [300 Å (1 Å=0.1 nm), 10 cm×75 μm, 15 μm tip size; New Objective, Woburn, MA, U.S.A.] as outlined in our previous study [35]. Protein sequence analysis was achieved with the ThermoElectron Bioworks 3.1 software package and NCBI protein database (ftp.ncbi.nlm.nih.gov/blast/db). Additionally, MS/MS spectra of interest were manually examined for the presence of phosphopeptides.
RESULTS
Expression, purification and characterization of Bcl-2Δ21
Human Bcl-2Δ21, lacking the membrane-anchoring region, was produced in E. coli as a GST fusion protein. SDS/PAGE analysis showed that the induced bacteria produced large amounts of a protein with an approx. 47 kDa apparent molecular mass that corresponds to the mass of GST–Bcl-2Δ21 (Figure 1A, lane 2). Incubation with thrombin yields a protein with apparent molecular mass of approx. 25 kDa (SDS/PAGE) and two minor bands smaller than 15 kDa (Figure 1A, lane 3). Western blotting confirms that the affinity-purified 25 kDa protein is immunoreactive to anti-Bcl-2 antibodies and does not react with anti-GST antibodies (results not shown). Mass-spectrometric analysis identifies the 25 kDa protein as Bcl-2Δ21, in agreement with an earlier report [37]. MALDI–TOF MS analysis (Figure 1B) shows a peak of m/z 24860, which corresponds well to the theoretical average mass of 24865.0 Da (Bcl-2Δ21), obtained from the SwissProt database (accession number P10415), taking into account the C-terminal loss of 21 amino acids and the additional N-terminal sequence GSPNSPR remaining from the thrombin-cleavable linker of the GST fusion protein. This result was confirmed by ESI-MS, which yielded a molecular mass of 24864.1±0.7 Da. There is no notable presence of any other proteins in the mass spectrum (Figure 1B).
Figure 1. Expression and purification of Bcl-2Δ21.
(A) SDS/PAGE of the protein purified from the E. coli lysate: lane 1, molecular-mass standard; lane 2, Bcl-2Δ21–GST bound to glutathione–agarose beads after 4 h incubation of beads with bacterial lysate; lane 3, purified Bcl-2Δ21 in STE buffer; and lane 4, glutathione–agarose beads with remaining GST after thrombin cleavage of the fusion proteins. (B) Analysis of purified Bcl-2Δ21 by MALDI–TOF MS.
Since the anti-apoptopic function of Bcl-2 is regulated through residue-specific phosphorylation [38], we performed Western-blot analysis of phosphorylation of Bcl-2Δ21 at Thr56, Ser70, Thr74 and Ser86 with respective sequence- and modification-specific antibodies and tryptic mapping of the protein sequence. Western-blot analysis did not show any phosphorylation at Ser70 or Ser86, but demonstrated that a fraction of the bacterially expressed Bcl-2Δ21 protein is phosphorylated at Thr56 and Thr74 (results not shown). However, NESI-MS/MS analysis of Bcl-2Δ21 after in-gel tryptic digestion only detected the native, non-phosphorylated peptides containing Thr56 and Thr74, indicating that the fraction of phosphorylated peptides is probably <5% when compared with the native peptides. In addition, both MALDI–TOF and ESI-MS analysis of the whole protein excluded significant fractions of phosphorylated isoforms (+80 a.m.u). Hence, the expressed Bcl-2Δ21 is not significantly phosphorylated.
Effect of Bcl-2Δ21 on SERCA activities
The incubation of rat muscle SR alone at 37 °C up to 2.5 h did not significantly affect the activity of SERCA. Co-incubation of the SR with Bcl-2Δ21 resulted in a decrease in SERCA activity, which was concentration- and time-dependent. Figure 2(A) shows the kinetics of inactivation obtained at different molar ratios of Bcl-2Δ21/SERCA. A complete inactivation of SERCA occurred at a molar ratio of 2:1 of Bcl-2Δ21/SERCA within 2 h of co-incubation (Figure 2B). The levels of SERCA in the SR (approx. 50% relative to the total protein) were determined based on the densitometric analysis of gels and reversed-phase HPLC of solubilized SR proteins [36].
Figure 2. Effect of Bcl-2Δ21 on SERCA activities in rat skeletal-muscle SR.
(A) Time-dependent inactivation of SERCA activity. SR vesicles (0.6 mg of protein/ml) were incubated in STE buffer alone (▪) or with 19 (⋄), 38 (▾), 75 (▵) and 150 (•) μg/ml Bcl-2Δ21. At the indicated times, aliquots were withdrawn and assayed for Ca2+-ATPase activity as described in the Experimental section. (B) The dependence of the Ca2+-ATPase activity on the molar ratio of Bcl-2Δ21/SERCA. SR vesicles (0.6 mg of protein/ml) were incubated for 2.5 h at 37 °C in STE buffer with different concentrations of Bcl-2Δ21 before the measurement of Ca2+-ATPase activity as shown in (A).
The SERCA activity is inhibited specifically by Bcl-2Δ21. Several important control experiments were performed, and are summarized in Figure 3. The incubation of the SR in the STE buffer instead of storage buffer did not inactivate SERCA. Comparable levels of reference proteins, BSA or GST alone, showed no effect on SERCA activity. To determine whether residual contaminating thrombin (used in the cleavage of GST–Bcl-2Δ21) was responsible for the inactivation of SERCA, SR vesicles were exposed to up to 4-fold higher concentrations of thrombin when compared with those used for GST–Bcl-2Δ21 fusion protein cleavage. These conditions resulted in no significant changes of the SERCA activity. To exclude any effect of low-molecular-mass compounds co-purified during the isolation of Bcl-2Δ21, we performed a control experiment with bacterial lysate of E. coli, which had been transformed with pGEX3T expressing only GST. The bacterial lysate was obtained under exactly the same conditions of protein isolation and purification that were used for the GST–Bcl-2Δ21 fusion proteins. Adsorption of GST to glutathione beads and exposure to thrombin did not release any detectable amount of protein, and the addition of the resulting protein-free medium to SR did not change the SERCA activity. In addition, the dialysis of the samples did not attenuate the inhibition effect (results not shown).
Figure 3. Bcl-2Δ21 specifically inhibits SERCA activity in rat skeletal-muscle SR.
SR vesicles (12 mg of protein/ml in isolation medium containing 0.3 M sucrose and 20 mM Mops, pH 7.0) were diluted to 0.6 mg of protein/ml and incubated either in buffer containing 120 mM KCl and 20 mM Mops, pH 7.0 (1), or in the STE buffer (pH 8.0) in the absence (2) and in the presence of 150 μg/ml Bcl-2Δ21 (3), GST (4) or BSA (5), or with 4 units/ml thrombin (6), or with the addition of a comparable volume of protein-free medium obtained from E. coli transformed with pGEX3T and expressing GST alone but following the method used for Bcl-2Δ21 purification (7). The aliquots after 2.5 h incubation were withdrawn and assayed for the Ca2+-ATPase activity as described in the Experimental section. The activity of control sample 1 [2.50±0.24 μmol of Pi·min−1·(mg of protein)−1] was set equal to unity.
Effect of Bcl-2Δ21 on the apparent Ca2+ affinity
Ca2+-concentration dependences of the Ca2+-ATPase activity have been used to characterize the structural and functional integrity of SERCA and its interactions with specific ligands providing the allosteric regulation of Ca2+ transport [39]. Without addition of Bcl-2Δ21, an increase in the concentration of added Ca2+ from 0.2 to 1 mM (in the presence of 1 mM EGTA) stimulated the Ca2+-ATPase SERCA activity approx. 20-fold, showing that the protein in our SR preparation is functionally and structurally intact (Figure 4A). The descending segment of the curve obtained in the presence of the Ca2+ ionophore A23187, at concentrations higher than saturating concentrations for the cytosol-facing high-affinity Ca2+-binding sites (E1 state of the enzyme), apparently reflects the inhibition of Ca2+ transport by saturation of the lumen-facing low-affinity Ca2+-binding sites in the E2 conformational state of SERCA [39]. Incubation for 2.5 h with Bcl-2Δ21 at a molar ratio of approx. 1:1 (Bcl-2Δ21/SERCA) inhibited the Ca2+-ATPase activity at all Ca2+ concentrations by approx. 50% (Figure 4A). Importantly, the analysis of normalized curves in the presence of Bcl-2Δ21 (Figure 4B) does not show a significant deviation of the Ca2+ concentration for half-maximal activation from the control value (K0.5∼2×10−6 M under our conditions), which characterizes the apparent Ca2+ affinity of SERCA in the E1 conformational state [40]. Therefore the inhibition of SERCA activity by Bcl-2Δ21 is not related to either competition for the Ca2+-binding domain of SERCA or allosteric regulation of the high-affinity Ca2+-binding domains, as was demonstrated for phospholamban and sarcolipin [40,41].
Figure 4. Effect of Bcl-2Δ21 on the Ca2+-dependent activation of SERCA.
SR (0.6 mg of protein/ml) was incubated at 37 °C for 2.5 h alone (▪) or in the presence of 75 (•) or 150 (♦) μg/ml Bcl-2Δ21 in the STE buffer. (A) Samples were analysed for Ca2+-ATPase activity in the presence of 1 mM EGTA and different concentrations of added CaCl2 (0.2–4 mM) as described in the Experimental section. (B) The concentration dependences normalized to the maximal activities obtained after incubations in the absence or in the presence of 75 μg/ml Bcl-2Δ21 [2.33±0.23 or 1.42±0.12 μmol of Pi·min−1·(mg of protein)−1 respectively] and plotted against calculated free Ca2+ concentrations shown in Table 1. Broken lines illustrate the determination of the Ca2+ concentrations for half-maximal SERCA activation (K0.5).
Effect of protease inhibitors
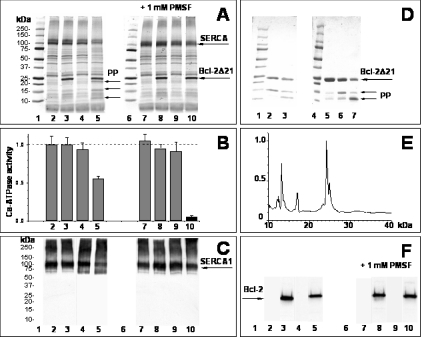
Some degradation of Bcl-2 on storage of the protein has been reported and attributed to proteolysis by contaminating bacterial enzymes [37]. We also observed a slow disappearance of the 25-kDa Bcl-2Δ21 band along with the formation of lower-molecular-mass bands that was more pronounced when keeping the protein at 4 °C than at −20 °C, which is not completely prevented by EDTA (results not shown). Degradation of Bcl-2Δ21 resulted in a decrease in the effect on SERCA activity, suggesting that structurally intact Bcl-2 is a prerequisite for SERCA inactivation. However, to test a potential role of contaminating proteases in the inhibition of SERCA, we studied the effect of protease inhibitors on Ca2+-ATPase activity and SERCA integrity by SDS/PAGE and Western blotting (Figure 5). Incubation of the SR for 2.5 h without Bcl-2Δ21 (sample 4 in Figures 5A–5C and 5F) did not change the 110 kDa SERCA band (Figure 5A), the immunoreactivity towards anti-SERCA1 antibodies (Figure 5C) or the Ca2+-ATPase activity (Figure 5B), which did not significantly differ from the control (sample 2 in the respective panels). On the other hand, after the incubation of the SR with Bcl-2Δ21 at a molar ratio of 1:1, we observed a decrease in both Coomassie staining and anti-SERCA1 immunostaining of the SERCA band, accompanied by the formation of a series of lower molecular mass products (cf. samples 3 and 5 in Figures 5A, 5C and 5D) and by a approx. 50% decrease in Ca2+-ATPase activity (cf. columns 3 and 5 in Figure 5B). However, SERCA is not necessarily the main target for proteolytic cleavage, as very similar products (PP in Figures 5A and 5D) originate from the degradation of Bcl-2Δ21 on its own. No anti-SERCA1 immunoreactivity could be detected for the low-molecular-mass region (Figure 5C); rather, increased anti-SERCA1 immunostaining is apparent for the higher-molecular-mass products.
Figure 5. Proteolytic degradation of SERCA and Bcl-2Δ21 and protection by protease inhibitors.
(A–C, F) Rat skeletal-muscle SR (0.6 mg/ml) was incubated in absence (samples 2–5) or presence (samples 7–10) of 1 mM PMSF. Samples without (2, 4, 7, 9) and with 75 μg/ml Bcl-2Δ21 (3, 5, 8, 10) were incubated at 37 °C for 0 (2, 3, 7, 8) or 2.5 h (4, 5, 9, 10) in the STE buffer and separated by SDS/PAGE (A), analysed for Ca2+-ATPase activity (B), and monitored by Western blotting with anti-SERCA1 (C) or anti-Bcl-2 (F) antibodies. In (A, C, F), lanes 1 and 6 show molecular-mass standards. Samples 2–5 and 7–10 are labelled under the respective lanes (in A, C, F) or columns (B). Bands named PP and labelled by arrows indicate proteolytic degradation products. (D) SDS/PAGE analysis of Bcl-2Δ21 (150 μg/ml in STE buffer) incubated without protease inhibitors for 1 (lane 2) and 3 h (lane 3) at 37 °C or stored at 4 °C for 1 (lane 5), 2 (lane 6) or 3 days (lane 7). Bands named PP and labelled by arrows indicate proteolytic degradation products. Lanes 1 and 4 show molecular-mass-standards. (E) MALDI–TOF spectrum of Bcl-2Δ21 after storage for 3 days at 4 °C (corresponds to lane 7 in D).
Importantly, the addition of 1 mM PMSF (either alone or in combination with the 1×Roche Molecular Biochemicals complete protease inhibitor cocktail; results not shown) completely prevented the proteolytic degradation of both SERCA and Bcl-2Δ21 (cf. samples 8 and 10 in Figures 5A, 5C and 5F). This protection against proteolysis did not abolish, but, instead, significantly enhanced the inhibition of SERCA activity by Bcl-2Δ21 (cf. columns 5 and 10 in Figure 5B). Therefore proteolysis of SERCA has a negligible effect on SERCA inactivation within our experimental time window. In fact, proteolytic degradation of Bcl-2Δ21 appears to decrease its inhibitory effect due to the loss of intact Bcl-2Δ21 during either the storage or co-incubation with the SR at 37 °C (Figures 5D and 5E).
Effect of Bcl-2Δ21 on the activity of the PMCA
To evaluate further the specificity of Bcl-2 inhibition of SERCA, we incubated Bcl-2Δ21 with the PMCA. This protein shares structural (32% identity at the primary sequence level) and mechanistic properties with SERCA, and regulates free intracellular calcium levels by transporting Ca2+ from the cytosol to the extracellular space. We exposed both SPM and purified erythrocyte membrane PMCA to Bcl-2Δ21 at a molar ratio of 1:1 of PMCA/Bcl-2. Figure 6 shows that the incubation of Bcl-2Δ21 neither with SPM nor with purified PMCA affects the PMCA activity both in the absence and in the presence of CaM. Hence, the effect of Bcl-2 appears to be rather specific for SERCA.
Figure 6. Effect of Bcl-2Δ21 on Ca2+-ATPase activities of brain SPM (A) and purified PMCA (B).
Samples were incubated at room temperature at a molar ratio of 1:2 of Bcl-2Δ21/PMCA with or without 120 nM CaM. ○ and •, CaM-stimulated activities; □ and ▪, basal activities measured without (open symbols) or with (solid symbols) Bcl-2Δ21 respectively as described in the Experimental section.
Association of Bcl-2Δ21 and SERCA
The specific inhibition of SERCA activity by Bcl-2Δ21 suggests that both proteins may form a stable complex after co-incubation. To demonstrate this interaction, we used a binding assay based on the co-purification of both proteins when recombinant GST–Bcl-2Δ21 protein was incubated with SR vesicles. Western-blot analysis reveals that SERCA specifically associates with the Bcl-2Δ21–GST fusion protein, whereas GST alone does not interact with SERCA (Figure 7A). To ensure the interaction of native Bcl-2Δ21 with SERCA in our SR preparations, co-immunoprecipitation of the proteins was employed (Figure 7B). Without addition of Bcl-2Δ21, only traces of native Bcl-2 were shown in our SR preparation, hardly detectable after immunoprecipitation with anti-Bcl-2 antibodies from large quantities of SR (Figure 7C). However, the resulting immunocomplex also contained SERCA1 that was co-purified with the native Bcl-2 protein (Figure 7B).
Figure 7. Association of Bcl-2 and SERCA in rat skeletal-muscle SR.
(A) Lysates of SR were incubated with GST–Bcl-2Δ21 (lane 2) or GST alone (lane 1), followed by affinity purification using glutathione–agarose beads as described in the Experimental section. GST–Bcl-2Δ21 and associated proteins were subsequently resolved by SDS/PAGE and immunoblotted with anti-SERCA1 and anti-GST antibody. WB, Western blot. (B) Western-blot analysis of 5 μg of SR (lane 1) with anti-SERCA1 antibodies and of proteins immunoprecipitated from 700 μg of SR with anti-Bcl-2 antibodies (lane 2). (C) Western-blot analysis of 17 (lane 1), 35 (lane 2), 70 (lane 3) μg of SR with anti-Bcl-2 antibodies, and of protein immunoprecipitated from 700 μg of SR (lane 4) with anti-Bcl-2 antibodies.
Role of the Bcl-2Δ21 redox state in the inhibition of SERCA activity
SERCA activity is controlled, in part, through the redox modification of some of its cysteine residues [42]. The formation of mixed disulphides of SERCA thiols with the only Cys158 residue of Bcl-2Δ21 may represent a potential mechanism of protein association and inhibition of SERCA activity. To evaluate the role of Cys158, we studied native, oxidized and S-alkylated Bcl-2Δ21 (Table 2). By means of DTNB titration, we verified that native Bcl-2Δ21 contained one free thiol group, and that oxidation by peroxynitrite or S-alkylation resulted in an almost complete loss of reduced Cys158. Peroxynitrite (ONOO−) was selected as an antioxidant as SERCA is a subject to peroxynitrite modification in vivo [42]. However, the modification of the cysteine residue in Bcl-2Δ21 did not significantly affect the inhibition of SERCA activity. Moreover, the additional presence of GSH at up to 5 mM concentration did not affect the inhibition of SERCA activity. Therefore we can rule out mixed-disulphide formation of SERCA thiols with either GSH or Bcl-2Δ21 as a putative mechanism of SERCA inhibition. Moreover, the redox status of Cys158 in Bcl-2Δ21 does not control SERCA inactivation.
Table 2. Effect of Bcl-2Δ21 thiol modification on the inhibition of SERCA activity.
| Sample | SERCA activity (ratio to control) | Thiol group content in Bcl-2Δ21 (mol/mol of protein)* |
|---|---|---|
| Control (no Bcl-2Δ21)† | 1.0±0.03 | − |
| Bcl-2Δ21‡ | 0.03±0.03 | 0.98±0.07 |
| Bcl-2Δ21 carboxymethylated§ | 0.05±0.02 | 0.05±0.02 |
| 5 mM GSH | 0.98±0.04 | − |
| Bcl-2Δ21+5 mM GSH | 0.04±0.03 | 0.98±0.07 |
| Bcl-2Δ21 oxidized¶ | 0.05±0.04 | 0.08±0.04 |
* Measured as DTNB-reactive thiols.
† 0.6 mg/ml SR in the STE buffer.
‡ At a molar ratio of 2:1 to SERCA.
§ Obtained by the reaction with 5 mM iodoacetic acid followed by exhaustive dialysis as described in the Experimental section.
¶ With 3 mM ONOO−.
Conformational transition of SERCA
To evaluate whether a conformational transition of SERCA is induced by Bcl-2Δ21 binding, we quantified the amount of free, reactive SERCA thiols, which can be derivatized with a maleimide-based fluorescent label, ThioGlo1. Figure 8 reveals a complete labelling of Bcl-2Δ21 with ThioGlo1, insensitive to the presence of SDS, suggesting that Cys158 is rather surface-exposed. On the other hand, ThioGlo1 only labels, on average, approx. three cysteine residues of native SERCA in the absence of SDS. Importantly, the co-incubation of Bcl-2Δ21 and SERCA in the absence of SDS results in the ThioGlo1 labelling of approx. two additional cysteine residues, which must originate from SERCA. Hence, the incubation of SERCA with Bcl-2Δ21 appears to trigger a partial unfolding of the membrane protein.
Figure 8. Conformational transition of SERCA on incubation with Bcl-2Δ21 determined by ThioGlo1 labelling of protein free cysteine residues.
Protein samples were incubated with 20 μM ThioGlo1 for 1 h at 37 °C and pH 7.4 with and without 1% SDS to assay the surface-exposed and total protein free cysteine residues respectively. Fluorescent ThioGlo1–cysteine adducts were measured in 1 ml samples at excitation and emission wavelengths of 379 and 513 nm respectively (left ordinate). DTNB titration was used for quantification of protein thiols (right ordinate). a.u., arbitrary units.
SERCA inactivation is not due to increased sensitivity of partially unfolded protein to oxidation
To assess whether the additional surface-exposed cysteine residues of SERCA could be vulnerable to oxidation during the incubation of SERCA with Bcl-2Δ21, we tested the direct effect of different oxidants, H2O2, ONOO− and NO/O2, which are known to modify SERCA thiols [42]. In these experiments, we compared the loss of SERCA activity during the incubation with oxidants in the presence and in the absence of Bcl-2Δ21 (Table 3). The co-incubation of SR with Bcl-2Δ21 resulted in an approx. 50% inhibition of SERCA activity within 1 h. However, the addition of the oxidants did not cause any accelerated loss of SERCA activity. Therefore the conformational transition in SERCA induced by Bcl-2 is not accompanied by a higher sensitivity of functionally important cysteine residues towards oxidation.
Table 3. Effect of Bcl-2Δ21 on the oxidative inactivation of SERCA.
| Sample | SERCA activity (ratio to control) |
|---|---|
| SR in STE* (before incubation) | 1.0±0.05 |
| SR in STE (1 h incubation at 37 °C) | 1.03±0.06 |
| SR+400 μM DEA/NO† | 0.91±0.04 |
| SR+500 μM H2O2 | 1.08±0.11 |
| SR+1 mM ONOO− | 0.57±0.05 |
| SR+Bcl-2Δ21‡ (1 h incubation at 37 °C) | 0.54±0.05 |
| SR+Bcl-2Δ21‡ +400 μM DEA/NO | 0.50±0.03 |
| SR+Bcl-2Δ21‡ +500 μM H2O2 | 0.59±0.04 |
| SR+Bcl-2Δ21‡ +1 mM ONOO− | 0.49±0.04 |
* 0.6 mg/ml SR protein in STE buffer.
† All the oxidants were added after preincubation of the SR for 30 min at 37 °C with or without Bcl-2Δ21. Oxidation reactions were allowed to proceed for 30 min before samples were assayed for Ca2+-ATPase activity. DEA, diethylamine.
† At a molar ratio of 2:1 to SERCA1.
DISCUSSION
The present study shows that recombinant Bcl-2Δ21, a truncated form of the anti-apoptotic protein Bcl-2, inhibits SERCA activity in rat skeletal-muscle SR. This inhibition correlates with a partial unfolding of SERCA, but not with proteolytic degradation or increased sensitivity of SERCA to oxidation. Experiments with this model protein may have physiological significance, as a naturally occurring splice variant, Bcl-2β, has been identified, which lacks the C-terminal membrane-anchoring domain [25]. Moreover, overexpression of Bcl-2Δ21 in certain cell types has confirmed an anti-apoptotic effect of this truncated protein despite the lack of the C-terminal sequence [27], indicating that the anti-apoptotic mechanism may not be dependent on this part of the molecule. Rather, the C-terminal domain targets Bcl-2 to cell compartments [43] and promotes Bcl-2 homodimerization [37,44], i.e. appears to be involved in the modulation of concentration and location of the Bcl-2 monomers available for anti-apoptotic action.
There is growing experimental evidence that both Bcl-2 and SERCA are involved in the signalling pathways leading to apoptosis. Currently, the anti-apoptotic mechanism(s) of Bcl-2 are not clearly known. Numerous mechanisms have been proposed such as antioxidant activity [45], membrane pore-forming properties [37,46] and specific protein–protein interactions with pro-apoptotic regulators or effectors of the caspase-signalling cascade [17–19]. The overexpression and targeting of Bcl-2 to the ER, which protects cells from apoptosis, is associated with the partial depletion of the ER Ca2+ store [22–26]. This depletion effect can result from a reduction of SERCA activity, and our results suggest that Bcl-2 may achieve a reduction of SERCA activity through direct interaction with the protein.
At least three SERCA isoforms are expressed in tissue-specific and developmentally regulated patterns [47,48]. The fast-twitch muscle isoform (SERCA1) is expressed at high levels in striated skeletal muscles. The cardiac/slow-twitch muscle isoform (SERCA2a) is expressed in slow-twitch skeletal muscles, heart and smooth muscles, whereas SERCA2b is widely expressed. SERCA3, the non-muscle isoform, is expressed in specialized cells. Two tissue-specific protein regulators of SERCA activity in muscle tissue have been identified as sarcolipin and phospholamban [41]. Through a phosphorylation-dependent association with SERCA2a, phospholamban regulates SERCA activity in slow-twitch skeletal muscles and heart. Nevertheless, a similar regulation can be observed with SERCA1 when phospholamban is artificially reconstituted with the SERCA1 isoform [49]. This is not surprising given the high sequence homology between the SERCA isoforms. The similarity of SERCA1 and SERCA2 in their responses to phospholamban suggests that SERCA1 may also be a good model for the interaction of SERCA and Bcl-2, in general. Importantly, a related Ca2+ transporter, PMCA, does not exhibit a similar response to Bcl-2Δ21. Hence, we believe that the effect of Bcl-2Δ21 is specific to SERCA. At present, we have evidence that the interaction of Bcl-2Δ21 with SERCA leads to a conformational transition of the Ca2+ pump, suggested by an increased fraction of SERCA thiols available for derivatization with ThioGlo1. This conformational change does not resemble the inhibitory interaction of sarcolipin and phospholamban with SERCA, resulting in the lowering of the apparent Ca2+ affinity and stabilization of SERCA in E2 conformational state [41,50]. The important feature of the phospholamban and sarcolipin inhibition of SERCA is the reversibility of the effect by the increase in Ca2+ concentration and by phosphorylation of the modulator proteins. Besides, the transmembrane sequence of phospholamban is essential for the inhibition of SERCA suggesting that the regulatory proteins modulate the apparent Ca2+ affinity through intramembrane interactions [50]. Our results show that Bcl-2 is not a functional homologue of phospholamban and sarcolipin. Rather, Bcl-2 inhibits SERCA through different types of interactions (probably, cytoplasmic) because the transmembrane C-terminal domain is not required for the interaction with SERCA, and this interaction cannot be reversed by increasing Ca2+ concentration. Although at present we have no data on the potential role of Bcl-2 phosphorylation, there is no other in vitro evidence of the reversibility of SERCA inhibition, i.e. regulatory function of Bcl-2 in this process.
The extent to which Bcl-2 would interact with SERCA in vivo is certainly controlled by various additional factors such as the availability of other Bcl-2-binding proteins (including Bcl-2 itself for homodimer formation), the potential competition of other SERCA-binding proteins, the phosphorylation status of Bcl-2 and the expression levels of Bcl-2. Importantly, Kuo et al. [51] were able to co-immunoprecipitate Bcl-2 and SERCA2 from lysates of breast epithelial and lymphoma cells. Obviously, a total inactivation of SERCA is not desirable as such a condition can trigger apoptosis, concluded from experiments with the specific SERCA inhibitor TG [11,12]. Hence, the anti-apoptotic role of Bcl-2 in vivo may rely on a small fraction of SERCA inhibited through binding to Bcl-2, sufficient to lower the ER Ca2+ content to levels which are below the threshold to execute/induce pro-apoptotic Ca2+ levels in the mitochondria. Future studies will identify the contact areas between SERCA and Bcl-2 and possibly the regulatory effect of Bcl-2 phosphorylation.
Acknowledgments
This research was supported by the American Heart Association (0355555Z) and the NIH (PO1AG12993). We thank Dr M. Alterman and Dr T. Duzhak (Biochemical Research Service Laboratory, University of Kansas, KS, U.S.A.) for the MALDI–TOF measurements, Dr T. Williams and Dr B. Drake (Mass Spectrometry Laboratory, University of Kansas) and Dr J. Kanski (Pharmaceutical Chemistry Department, University of Kansas) for the assistance with ESI-MS and NESI-MS/MS respectively.
References
- 1.Hajnoczky G., Csordas G., Yi M. Old players in a new role: mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium. 2002;32:363–377. doi: 10.1016/s0143416002001872. [DOI] [PubMed] [Google Scholar]
- 2.Green D. R., Reed J. C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 3.Martinou J. C., Green D. R. Breaking the mitochondrial barrier. Nat. Rev. Mol. Cell. Biol. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- 4.Berridge M. J., Lipp P., Bootman M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell. Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 5.Berridge M. J. Cell signalling. A tale of two messengers. Nature (London) 1993;361:315–325. doi: 10.1038/365388a0. [DOI] [PubMed] [Google Scholar]
- 6.Clapham D. E. Calcium signaling. Cell (Cambridge, Mass.) 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 7.Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 8.Rizzuto R., Pinton P., Carrington W., Fay F. S., Fogarty K. E., Lifshitz L. M., Tuft R. A., Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 9.Csordas G., Thomas A. P., Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzuto R., Simpson A. W., Brini M., Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature (London) 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y. P., Teng D., Dralyuk F., Ostrega D., Roe M. W., Philipson L., Polonsky K. S. Apoptosis in insulin-secreting cells. Evidence for the role of intracellular Ca2+ stores and arachidonic acid metabolism. J. Clin. Invest. 1998;101:1623–1632. doi: 10.1172/JCI1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma T. S., Mann D. L., Lee J. H., Gallinghouse G. J. SR compartment calcium and cell apoptosis in SERCA overexpression. Cell Calcium. 1999;26:25–36. doi: 10.1054/ceca.1999.0049. [DOI] [PubMed] [Google Scholar]
- 13.Reed J. C., Cuddy M., Slabiak T., Croce C. M., Nowell P. C. Oncogenic potential of bcl-2 demonstrated by gene transfer. Nature (London) 1988;336:259–261. doi: 10.1038/336259a0. [DOI] [PubMed] [Google Scholar]
- 14.Nunez G., Hockenbery D., McDonnell T. J., Sorensen C. M., Korsmeyer S. J. Bcl-2 maintains B cell memory. Nature (London) 1991;353:71–73. doi: 10.1038/353071a0. [DOI] [PubMed] [Google Scholar]
- 15.Hockenbery D., Nunez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature (London) 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 16.Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell (Cambridge, Mass.) 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 17.Wei M. C., Lindsten T., Mootha V. K., Weiler S., Gross A., Ashiya M., Thompson C. B., Korsmeyer S. J. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng E. H., Wei M. C., Weiler S., Flavell R. A., Mak T. W., Lindsten T., Korsmeyer S. J. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 19.Farrow S. N., Brown R. New members of the Bcl-2 family and their protein partners. Curr. Opin. Genet. Dev. 1996;6:45–49. doi: 10.1016/s0959-437x(96)90009-x. [DOI] [PubMed] [Google Scholar]
- 20.Lithgow T., van Driel R., Bertram J. F., Strasser A. The protein product of the oncogene bcl-2 is a component of the nuclear envelope, the endoplasmic reticulum, and the outer mitochondrial membrane. Cell Growth Differ. 1994;5:411–417. [PubMed] [Google Scholar]
- 21.Landolfi B., Curci S., Debellis L., Pozzan T., Hofer A. M. Ca2+ homeostasis in the agonist-sensitive internal store: functional interactions between mitochondria and the ER measured in situ in intact cells. J. Cell Biol. 1998;142:1235–1243. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinton P., Ferrari D., Magalhaes P., Schulze-Osthoff K., Di Virgilio F., Pozzan T., Rizzuto R. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J. Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foyouzi-Youssefi R., Arnaudeau S., Borner C., Kelley W. L., Tschopp J., Lew D. P., Demaurex N., Krause K. H. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam M., Dubyak G., Chen L., Nunez G., Miesfeld R. L., Distelhorst C. W. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinton P., Ferrari D., Rapizzi E., Di Virgilio F., Pozzan T., Rizzuto R. A role for calcium in Bcl-2 action? Biochimie. 2002;84:195–201. doi: 10.1016/s0300-9084(02)01373-1. [DOI] [PubMed] [Google Scholar]
- 26.Pinton P., Ferrari D., Rapizzi E., Di Virgilio F., Pozzan T., Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell (Cambridge, Mass.) 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez J. L., Rosemblatt M., Hidalgo C. Highly purified sarcoplasmic reticulum vesicles are devoid of Ca2+-independent (‘basal’) ATPase activity. Biochim. Biophys. Acta. 1980;599:522–568. doi: 10.1016/0005-2736(80)90199-6. [DOI] [PubMed] [Google Scholar]
- 29.Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal. Biochem. 1979;100:95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- 30.Schoenmakers T. J., Visser G. J., Flik G., Theuvenet A. P. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- 31.Michaelis E. K., Michaelis M. L., Chang H. H., Kitos T. E. High affinity Ca2+-stimulated Mg2+-dependent ATPase in rat brain synaptosomes, synaptic membranes, and microsomes. J. Biol. Chem. 1983;258:6101–6108. [PubMed] [Google Scholar]
- 32.Zaidi A., Barron L., Sharov V. S., Schöneich C., Michaelis E. K., Michaelis M. L. Oxidative inactivation of purified plasma membrane Ca2+-ATPase by hydrogen peroxide and protection by calmodulin. Biochemistry. 2003;42:12001–12010. doi: 10.1021/bi034565u. [DOI] [PubMed] [Google Scholar]
- 33.Ellman G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 34.Pryor W. A., Cueto R., Jin X., Koppenol W. H., Ngu-Schwemlein M., Squadrito G. L., Uppu P. L., Uppu R. M. A practical method for preparing peroxynitrite solutions of low ionic strength and free of hydrogen peroxide. Free Radic. Biol. Med. 1995;18:75–83. doi: 10.1016/0891-5849(94)00105-s. [DOI] [PubMed] [Google Scholar]
- 35.Kanski J., Alterman M. A., Schöneich C. Proteomic identification of age-dependent protein nitration in rat skeletal muscle. Free Radic. Biol. Med. 2003;35:1229–1239. doi: 10.1016/s0891-5849(03)00500-8. [DOI] [PubMed] [Google Scholar]
- 36.Sharov V. S., Galeva N. A., Knyushko T. V., Bigelow D. J., Williams T. D., Schöneich C. Two-dimensional separation of the membrane protein sarcoplasmic reticulum Ca-ATPase for high-performance liquid chromatography-tandem mass spectrometry analysis of posttranslational protein modifications. Anal. Biochem. 2002;308:328–335. doi: 10.1016/s0003-2697(02)00261-0. [DOI] [PubMed] [Google Scholar]
- 37.Kim K. M., Giedt C. D., Basanez G., O'Neill J. W., Hill J. J., Han Y. H., Tzung S. P., Zimmerberg J., Hockenbery D. M., Zhang K. Y. Biophysical characterization of recombinant human Bcl-2 and its interactions with an inhibitory ligand, antimycin A. Biochemistry. 2001;40:4911–4922. doi: 10.1021/bi002368e. [DOI] [PubMed] [Google Scholar]
- 38.Ito T., Deng X., Carr B., May W. S. Bcl-2 phosphorylation required for anti-apoptosis function. J. Biol. Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 39.MacLennan D. H., Rice W. J., Green N. M. The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases. J. Biol. Chem. 1997;272:28815–28818. doi: 10.1074/jbc.272.46.28815. [DOI] [PubMed] [Google Scholar]
- 40.Toyofuku T., Kurzydlowski K., Lytton J., MacLennan D. H. The nucleotide binding/hinge domain plays a crucial role in determining isoform-specific Ca2+ dependence of organellar Ca2+-ATPases. J. Biol. Chem. 1992;267:14490–14496. [PubMed] [Google Scholar]
- 41.MacLennan D. H., Asahi M., Tupling A. R. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann. N.Y. Acad. Sci. 2003;986:472–480. doi: 10.1111/j.1749-6632.2003.tb07231.x. [DOI] [PubMed] [Google Scholar]
- 42.Viner R. I., Williams T. D., Schöneich C. Peroxynitrite modification of protein thiols: oxidation, nitrosylation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry. 1999;38:12408–12415. doi: 10.1021/bi9909445. [DOI] [PubMed] [Google Scholar]
- 43.Wang N. S., Unkila M. T., Reineks E. Z., Distelhorst C. W. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J. Biol. Chem. 2001;276:44117–44128. doi: 10.1074/jbc.M101958200. [DOI] [PubMed] [Google Scholar]
- 44.Conus S., Kaufmann T., Fellay I., Otter I., Rosse T., Borner C. Bcl-2 is a monomeric protein: prevention of homodimerization by structural constraints. EMBO J. 2000;19:1534–1544. doi: 10.1093/emboj/19.7.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voehringer D. W., Meyn R. E. Redox aspects of Bcl-2 function. Antioxid. Redox Signal. 2000;2:537–550. doi: 10.1089/15230860050192314. [DOI] [PubMed] [Google Scholar]
- 46.Reed J. C. Double identity for proteins of the Bcl-2 family. Nature (London) 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 47.Lytton J., Westlin M., Burk S. E., Shull G. E., MacLennan D. H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 1992;267:14483–14489. [PubMed] [Google Scholar]
- 48.East J. M. Sarco(endo)plasmic reticulum calcium pumps: recent advances in our understanding of structure/function and biology (review) Mol. Membr. Biol. 2000;17:189–200. doi: 10.1080/09687680010009646. [DOI] [PubMed] [Google Scholar]
- 49.Negash S., Yao Q., Sun H., Li J., Bigelow D. J., Squier T. C. Phospholamban remains associated with the Ca2+- and Mg2+-dependent ATPase following phosphorylation by cAMP-dependent protein kinase. Biochem. J. 2000;351:195–205. doi: 10.1042/0264-6021:3510195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura Y., Kurzydlowski K., Tada M., MacLennan D. H. Phospholamban regulates the Ca2+-ATPase through intramembrane interactions. J. Biol. Chem. 1996;271:21726–21731. doi: 10.1074/jbc.271.36.21726. [DOI] [PubMed] [Google Scholar]
- 51.Kuo T. H., Kim H.-R. C., Zhu L., Yu Y., Lin H.-M., Tsang W. Modulation of endoplasmic reticulum calcium pump by Bcl-2. Oncogene. 1998;17:1903–1910. doi: 10.1038/sj.onc.1202110. [DOI] [PubMed] [Google Scholar]