Abstract
During cell activation, mitochondria play an important role in Ca2+ homoeostasis due to the presence of a fast and specific Ca2+ channel in its inner membrane, the mitochondrial Ca2+ uniporter. This channel allows mitochondria to buffer local cytosolic [Ca2+] changes and controls the intramitochondrial Ca2+ levels, thus modulating a variety of phenomena from respiratory rate to apoptosis. We have described recently that SB202190, an inhibitor of p38 MAPK (mitogen-activated protein kinase), strongly activated the uniporter. We show in the present study that a series of natural plant flavonoids, widely distributed in foods, produced also a strong stimulation of the mitochondrial Ca2+ uniporter. This effect was of the same magnitude as that induced by SB202190 (an approx. 20-fold increase in the mitochondrial Ca2+ uptake rate), developed without measurable delay and was rapidly reversible. In intact cells, the mitochondrial Ca2+ peak induced by histamine was also largely increased by the flavonoids. Stimulation of the uniporter by either flavonoids or SB202190 did not require ATP, suggesting a direct effect on the uniporter or an associated protein which is not mediated by protein phosphorylation. The most active compound, kaempferol, increased the rate of mitochondrial Ca2+ uptake by 85±15% (mean±S.E.M., n=4) and the histamine-induced mitochondrial Ca2+ peak by 139±19% (mean±S.E.M., n=5) at a concentration of 1 μM. Given that flavonoids can reach this concentration range in plasma after ingestion of flavonoid-rich food, these compounds could be modulating the uniporter under physiological conditions.
Keywords: calcium uniporter, flavonoid, genistein, kaempferol, mitochondria, SB202190
Abbreviations: [Ca2+]c, cytosolic [Ca2+]; [Ca2+]M, mitochondrial [Ca2+]; ER, endoplasmic reticulum; MAPK, mitogen-activated protein kinase
INTRODUCTION
Cell stimulation often triggers an increase in [Ca2+]c (cytosolic [Ca2+]), either through activation of plasma membrane Ca2+ channels or through release of Ca2+ from intracellular stores, mainly the ER (endoplasmic reticulum). These [Ca2+]c changes are non-homogeneous on a fast timescale, being much larger in regions close to the Ca2+ source (the plasma membrane or ER Ca2+ channels). In the last few years it has become evident that these fast local changes in the [Ca2+]c in defined subcellular environments (the high-Ca2+ microdomains) are specially relevant from a physiological point of view [1]. In particular, the extent of Ca2+ uptake by mitochondria is critically dependent on these high-Ca2+ microdomains [2–5]. This is because the mitochondrial Ca2+ uniporter has low-Ca2+ affinity, in the micromolar range, so that its activation requires a rise in [Ca2+]c much larger than the mean global [Ca2+]c transient. Ca2+ uptake by mitochondria fulfils a variety of functions in cell physiology. The increase in [Ca2+]M (mitochondrial [Ca2+]) activates several dehydrogenases, leading to an increase in the respiratory rate and ATP production [6,7]. Moreover, the fast mitochondrial Ca2+ uptake can modulate the local rise in [Ca2+]c, thus controlling associated phenomena such as secretion [3,8]. Finally, large and prolonged increases in [Ca2+]M can lead to opening of the permeability transition pore and apoptosis [9,10].
The mitochondrial Ca2+ uniporter is therefore the key controller of the amount of Ca2+ that enters into the mitochondria during cell stimulation, and thus of the different physiological consequences of mitochondrial Ca2+ uptake. However, there is little information on the possible mechanisms of physiological regulation of the activity of the uniporter. We have described recently that SB202190, an inhibitor of p38 MAPK (mitogen-activated protein kinase), strongly activated the uniporter by enhancing its Ca2+ affinity [11]. Our suggestion from this finding was that the activity of the uniporter could be modulated by phosphorylation. However, we show in the present paper that several natural plant flavonoids, widely available in foods [12–14], produce the same effects on the uniporter. In addition, activation of the uniporter by either flavonoids or SB202190 in permeabilized cells did not require ATP, thus excluding the involvement of any protein kinase. Rather, these compounds apparently produce a direct reversible activation of the uniporter, either acting on the channel molecule or on an associated protein. The observed effects of the flavonoids on mitochondrial Ca2+ uptake occur at concentrations that could be reached in human plasma under physiological conditions [15–17]. Thus this novel target may contribute to explain some of the biological effects of these phytochemicals.
EXPERIMENTAL
Materials
Wild-type coelenterazine, coelenterazine n and fura 2/AM (fura 2 acetoxymethyl ester) were from Molecular Probes (Eugene, OR, U.S.A.). SB202190 was from Tocris (Bristol, U.K.). Genistein and genistin were from LC laboratories (Woburn, MA, U.S.A.). The other flavonoids used were from Sigma (Madrid, Spain). Other reagents were from Sigma or Merck (Darmstadt, Germany).
Cell culture and transfection
The construction strategy of the mutated mitochondrially targeted aequorin chimera has been described previously [18]. HeLa cells were grown in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum. The HeLa cell clone MM5, which stably expresses mitochondrially targeted mutated aequorin, has been described previously [11]. Similar results were obtained using wild-type cells transiently transfected with a pcDNA3.1 plasmid containing the construct for mitochondrially targeted mutated aequorin. The construct for cytosolic aequorin has been also described previously [11]. It was cloned into the pcDNA3.1. plasmid and used to transfect wild-type HeLa cells. Transfections were carried out using Metafectene (Biontex, Munich, Germany).
[Ca2+]M and [Ca2+]c measurements with aequorin
The HeLa cell clone MM5 expressing mitochondrially targeted mutated aequorin was used for [Ca2+]M measurements. [Ca2+]c measurements were carried out using HeLa cells transiently transfected with the plasmid for cytosolic aequorin. Cells were plated on to coverslips (diameter, 13 mm). For aequorin reconstitution in experiments using intact cells, HeLa cells expressing either cytosolic or mitochondrially targeted aequorin were incubated for 1–2 h at room temperature (22 °C) in standard medium (145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM glucose and 10 mM Hepes, pH 7.4) with 1 μM wild-type coelenterazine. Cells were then placed in the perfusion chamber of a purposebuilt luminometer maintained at 37 °C. For experiments with permeabilized cells, MM5 cells expressing mitochondrially targeted mutated aequorin were reconstituted with 1 μM coelenterazine n for 1–2 h and then placed in the luminometer as above. Then standard medium containing 0.5 mM EGTA instead of Ca2+ was perfused for 1 min, followed by 1 min of intracellular medium (130 mM KCl, 10 mM NaCl, 1 mM MgCl2, 1 mM K3PO4, 0.5 mM EGTA, 1 mM ATP, 20 μM ADP, 2 mM succinate and 20 mM Hepes, pH 7) containing 100 μM digitonin. Then intracellular medium without digitonin was perfused for 5–10 min, followed by buffers of known [Ca2+] prepared in intracellular medium using HEDTA (N-hydroxyethylethylenediaminetriacetic acid)/Ca2+/Mg2+ mixtures. The basal [Ca2+]M was not modified by cell permeabilization. However, it should be noted that mitochondrially targeted mutated aequorin reconstituted with coelenterazine n is an aequorin chimera of very low Ca2+ affinity, which allows the measurement of [Ca2+] up to the near millimolar range, but is insensitive to [Ca2+] values below 10–20 μM. Because of this, residual background luminescence makes the basal [Ca2+] measured with this probe appear artificially high compared with the basal [Ca2+] measured using aequorin chimeras of higher Ca2+ sensitivity (as in the measurements in intact cells). When cells expressing mitochondrially targeted mutated aequorin reconstituted with native coelenterazine were permeabilized, the basal Ca2+ was the same as in intact cells expressing the same aequorin chimera (results not shown). Calibration of the luminescence data into [Ca2+] was carried out using an algorithm as described previously [18]. Statistical data are given as the means±S.E.M. EC50 values were calculated using the four-parameter (log EC50) fitting routine of the BioDataFit program (Chang Bioscience, Castro Valley, CA, U.S.A.).
RESULTS
In intact cells, the rate of Ca2+ uptake by mitochondria during cell stimulation depends not only on the activity of the uniporter, but also on additional variables that influence the magnitude of the local high-Ca2+ microdomain, e.g., the proximity of mitochondria and the Ca2+ source (the Ca2+ channel in the plasma membrane or the ER) or the rate of Ca2+ flux through that channel. Thus structural modifications of the mitochondrial network or changes in the activity of any of the Ca2+ channels may lead to secondary variations in mitochondrial Ca2+ uptake independent of the activity of the uniporter. In fact, because of the steep dependence of the activity of the uniporter with the level of extramitochondrial Ca2+, minor changes in [Ca2+]c may lead to much larger changes in mitochondrial Ca2+ uptake [19]. For this reason, we have investigated the kinetics of the uniporter using permeabilized cells expressing mitochondrially targeted aequorin and perfused with controlled Ca2+ buffers. This method allows the direct monitoring of the activity of the uniporter with no interference from other steps of the Ca2+ homoeostatic machinery. Mitochondrially targeted aequorin has been widely used before to monitor mitochondrial [Ca2+] both in transformed cell lines and in primary cultures [3,11,19–22].
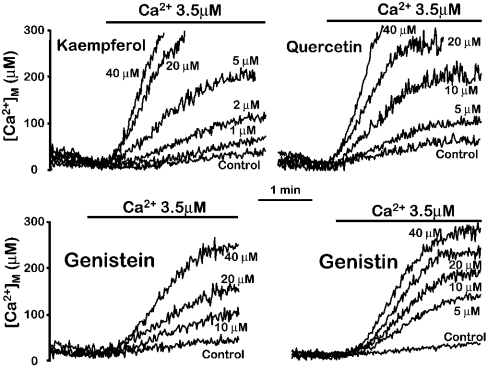
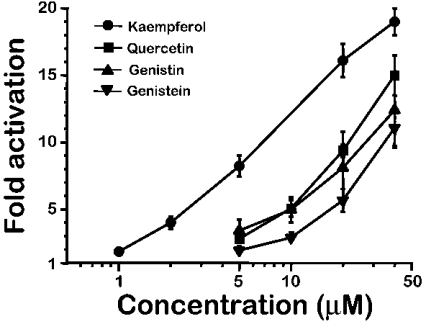
Figure 1 shows the effects of several of the most naturally abundant flavonoids on mitochondrial Ca2+ uptake in permeabilized HeLa cells, and Figure 2 shows the corresponding dose–response curves. These compounds produced a concentration-dependent activation of the rate of Ca2+ entry into mitochondria induced by perfusion of a Ca2+ buffer. The most active compound was kaempferol, which increased mitochondrial Ca2+ uptake by 85±15% (mean±S.E.M., n=4), already at 1 μM. However, the maximum effect was not completely reached, even at a concentration of 40 μM which stimulated mitochondrial Ca2+ uptake by 19±1-fold (mean±S.E.M., n=4) over the control values. The large magnitude of this activation is consistent with that previously shown for SB202190, suggesting that both kinds of compounds acted by the same mechanism. SB202190 activated mitochondrial Ca2+ uptake by 10.4±0.5-fold at 10 μM and by 13.8±0.3-fold at 20 μM (means±S.E.M., n=4; see Figure 7 in [11]), a value that was also below saturation. Each compound was added in each case together with the Ca2+ buffer, without pre-incubation, and activated Ca2+ uptake into mitochondria without measurable delay. The rate of mitochondrial Ca2+ uptake was not significantly modified by pre-incubation with the drug. In parallel experiments, 5 μM kaempferol activated mitochondrial Ca2+ uptake by 7.1±0.1-fold when added simultaneously to the Ca2+ buffer, and by 8.0±0.5-fold when it was pre-incubated for 5 min prior to perfusion of the Ca2+ buffer (mean±S.E.M., n=3, P>0.05).
Figure 1. Stimulation of mitochondrial Ca2+ uptake by several natural flavonoids in permeabilized cells.
MM5 cells expressing mitochondrially targeted mutated aequorin reconstituted with coelenterazine n were permeabilized as described in the Experimental section. Then a Ca2+ buffer containing 3.5 μM [Ca2+] was perfused either in the absence (Control) or in the presence of different concentrations of each compound, as indicated. The results are representative of 3–5 experiments for each compound.
Figure 2. Concentration dependence of the activation of mitochondrial Ca2+ uptake by several natural flavonoids.
Activation of mitochondrial Ca2+ uptake was calculated as the fold increase in the rate of Ca2+ uptake in the presence of the drug compared with the rate of Ca2+ uptake in the control.
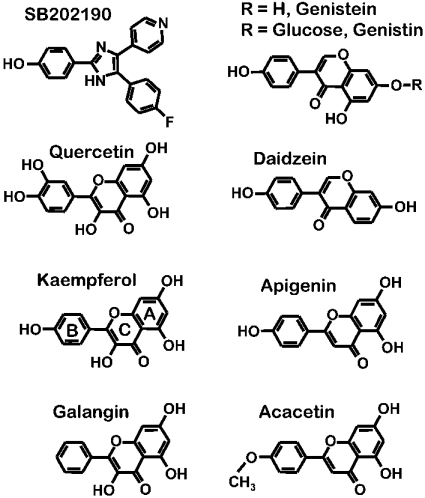
Figure 7. Chemical structures of SB202190 and the natural flavonoids used in this study.
The structure of kaempferol shows the standard letter code used to name flavone rings.
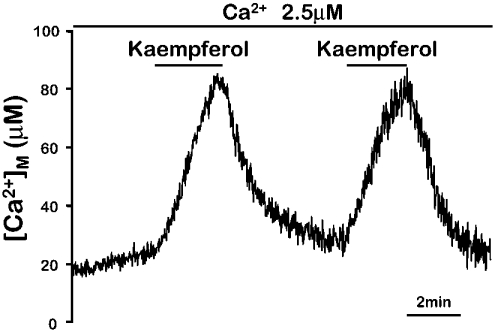
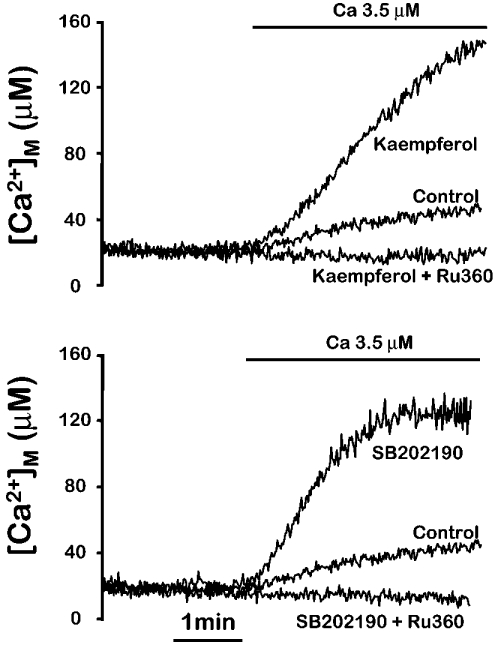
The effect of the flavonoids on mitochondrial Ca2+ uptake was rapidly reversible. Figure 3 shows that the activation of Ca2+ uptake induced by kaempferol disappeared rapidly after washing the compound, and could be induced again by re-introducing the activator. Both the fast activation and reversibility of the effect were present also in the case of SB202190 [11], providing further evidence for a common mechanism. Figure 4 shows that activation of mitochondrial Ca2+ uptake by both kaempferol and SB202190 was fully inhibited by 100 nM Ru360, the specific inhibitor of the mitochondrial Ca2+ uniporter. In conclusion, the flavonoids appear to stimulate the mitochondrial Ca2+ uniporter by the same mechanism as SB202190.
Figure 3. Reversible activation of mitochondrial Ca2+ uptake by kaempferol in permeabilized cells.
MM5 cells expressing mitochondrially targeted mutated aequorin reconstituted with coelenterazine n were permeabilized as described in the Experimental section. Then a Ca2+ buffer containing 2.5 μM [Ca2+] was perfused as indicated. Addition of 5 μM kaempferol to that buffer induced a large increase in the rate of Ca2+ entry into mitochondria, which was rapidly reversible when the compound was taken out. The experiment shown is representative of 5 similar ones.
Figure 4. Inhibition by Ru360 of the mitochondrial Ca2+ uptake induced by kaempferol and SB202190.
MM5 cells expressing mitochondrially targeted mutated aequorin reconstituted with coelenterazine n were permeabilized as described in the Experimental section. Then a Ca2+ buffer containing 3.5 μM [Ca2+] was perfused either in the absence (Control) or in the presence of 5 μM kaempferol, 10 μM SB202190, 5 μM kaempferol plus 100 nM Ru360 or 10 μM SB202190 plus 100 nM Ru360, as indicated. The experiments shown are representative of 3 similar ones of each type.
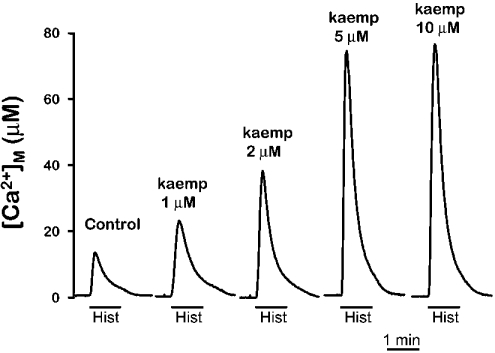
The results obtained above demonstrate that flavonoids activate mitochondrial Ca2+ uptake in permeabilized cells. To study their effect in intact cells, we measured the histamine-induced mitochondrial [Ca2+] peak in the presence or in the absence of the flavonoids. Figure 5 shows the effect of increasing concentrations of kaempferol on the mitochondrial [Ca2+] peak induced by histamine. It can be observed that kaempferol produced a large increase in the [Ca2+]M peak induced by histamine at the same concentrations shown above for the stimulation of mitochondrial Ca2+ uptake in permeabilized cells. A concentration of kaempferol of 1 μM produced also here an increase in the histamine-induced [Ca2+]M peak of near 100% (in 5 similar experiments the peak was increased by 139±19%; mean±S.E.M.). Quercetin and genistein, but not genistin, also increased the [Ca2+]M peak induced by histamine. The reason for the lack of effect of genistin in intact cells probably lies in the inability of this compound to enter the cells, because of the presence of the attached glucose molecule. In contrast with the effects on the [Ca2+]M peak, the [Ca2+]c peak showed little change in the presence of kaempferol. In a series of experiments carried out in parallel, the [Ca2+]c peak induced by histamine was 0.80±0.03 μM (n=13) in the control cells and 0.85±0.05 μM (n=15) in cells treated with 10 μM kaempferol with the same protocol used in Figure 5.
Figure 5. Effect of kaempferol on the mitochondrial Ca2+ peak induced by histamine.
MM5 cells were reconstituted with native coelenterazine as described in the Experimental section. Then cells were placed in the luminometer and stimulated with 100 μM histamine (Hist) either in control cells or in cells treated for 2 min prior and during stimulation with different concentrations of kaempferol (kaemp), as indicated. The experiments shown are representative of 3–6 similar ones of each type.
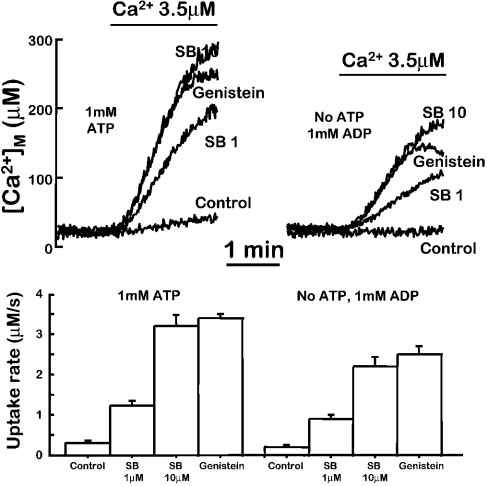
We now addressed the questions regarding the mechanism of the activation of mitochondrial Ca2+ uptake by flavonoids or SB202190. Given that this compound is considered a specific inhibitor of p38 MAPK [23], we proposed that this kinase could be involved in the regulation. On the other hand, some flavonoids, such as genistein, are known to inhibit tyrosine kinases. However, its glucose-derivative genistin, generally considered to be an inactive analogue as a tyrosine kinase inhibitor, was highly active here to activate mitochondrial Ca2+ uptake. To obtain more definitive evidence on the possible participation of a protein kinase in the effects of flavonoids or SB202190, we decided to test if their effect requires the presence of ATP. In permeabilized cells, we can control the composition of the intracellular medium. ATP at a concentration of 1 mM is normally included in the medium, together with 2 mM succinate as mitochondrial substrate. We then investigated if the activation of mitochondrial Ca2+ uptake by flavonoids or SB202190 could be somehow modified in the absence of ATP. Several experimental conditions were used: cells were permeabilized and then washed for at least 15 min (and in some experiments for 30 min) prior to the addition of the Ca2+ buffer with (i) medium with no ATP added; (ii) no ATP added and in the presence of the non-hydrolysable ATP analogues ATP[S] (50 μM) or adenosine 5′-[β,γ-imido]triphosphate (‘AMP-PNP’, 100 μM); and (iii) no ATP added and in the presence of 1 mM ADP to wash out intramitochondrial ATP via ATP/ADP exchanger. In this case, although addition of ADP may allow some ATP synthesis through the myokinase reaction, the large excess and continuous perfusion of ADP assures that the phosphate transfer potential remains low enough to avoid any phosphorylation taking place. In all the cases, 5 μM oligomycin was also included to inhibit local mitochondrial ATP production. Figure 6 shows the results obtained using medium containing 1 mM ADP and no ATP, but similar results were obtained using the other options. SB202190 and genistein similarly activated mitochondrial Ca2+ uptake both in the presence and in the absence of ATP. SB202190 (10 μM), for example, increased mitochondrial Ca2+ uptake by 10.7±0.9-fold (mean±S.E.M., n=3) in the presence of ATP and by 10.9±0.9-fold (mean±S.E.M., n=3) in the absence of ATP. This suggests that phosphorylation is not involved. Moreover, the same effects were also obtained when a concentration of SB202190 producing a half maximal effect was tested both in the presence or in the absence of ATP (Figure 6). Given that this compound inhibits p38 MAPK by competition with ATP, the large decrease in ATP concentration (in case any residual ATP could be still present under our experimental conditions) should have strongly potentiated its effect if it were mediated by kinase inhibition. This was found not to be the case.
Figure 6. Effect of the absence of ATP in the activation of mitochondrial Ca2+ uptake by genistein or SB202190.
MM5 cells expressing mitochondrially targeted mutated aequorin reconstituted with coelenterazine n were permeabilized as described in the Experimental section. After permeabilization, cells were perfused for 15 min either with the usual intracellular medium containing 1 mM ATP (upper left panel) or with intracellular medium containing 1 mM ADP instead of ATP and 5 μM oligomycin (upper right panel). Then, when indicated, 3.5 μM Ca2+ buffers made in the corresponding intracellular medium (with or without ATP) were perfused either in the absence (Control) or in the presence of 10 μM SB202190 (SB10), 40 μM genistein or 1 μM SB202190 (SB1). The lower panel shows the mean uptake rates obtained in 3 similar experiments of each type. SB202190 activated mitochondrial Ca2+ uptake similarly when ATP was substituted with nothing or by the non-hydrolysable ATP analogues ATP[S] (50 μM) or (adenosine 5′-[β,γ-imido]triphosphate (AMP-PNP) (100 μM) (results not shown).
The main alternative mechanism is a direct effect of flavonoids or SB202190 on the uniporter. However, because flavonoids are strong antioxidants, it could be proposed that reactive oxygen species play a role in the process. To investigate this possibility, we have tested the effects on mitochondrial Ca2+ uptake of several classical antioxidants (both lipid and water soluble): α-tocopherol (100 μM), lipoic acid (100 μM), ascorbic acid (1 mM) and N-methyl-cysteine (5 mM). None of them produced any effect on mitochondrial Ca2+ uptake (results not shown), suggesting that the effect of flavonoids is not mediated by their antioxidant properties.
If, as the above results suggest, the effects of flavonoids are due to a direct interaction with the uniporter or an associated protein, there should be a structural motif in the molecules responsible for the interaction. To obtain information on the structure–activity relationship of the effect of these compounds on the mitochondrial uniporter, we have measured the activity of several closely related flavonoids. Figure 7 shows the structural formulae of the compounds used, together with that of SB202190, and Table 1 shows the apparent EC50 for each one assuming that full activation corresponds to 20-fold stimulation over the control value. As shown above, full saturation of the effect could not be reached even at 40 μM kaempferol, which activated Ca2+ uptake by 19-fold. Thus 20-fold is probably an underestimation for the maximum possible activation. The value shown in Table 1 is therefore not a true EC50, but indicates the concentration of the drug that activates mitochondrial Ca2+ uptake by 10-fold. In the case of the less active compounds, the activation observed at 50 μM is shown instead. Two structural motifs can be seen. (i) A 4′-monohydroxy phenyl group in the B-ring of the flavone. Both addition of a second hydroxy group (as in quercetin) or the absence of hydroxy groups (as in galangin) lead to a 3–5-fold decrease in activity. The same happened in the case of apigenin which lost all the activity when its 4′-hydroxy group was methylated (as in acacetin). (ii) The hydroxyl group in the 3 position of the C-ring of the flavone. Its absence decreased by approx. 7-fold the activity (compare kaempferol with apigenin). The loss of activity from genistein to daidzein could perhaps be explained by the same reason, although the fact that genistin is even more active than genistein suggests that the A-ring is less involved in the binding.
Table 1. Activation of mitochondrial [Ca2+] uptake by several flavonoids.
The Table shows the concentration of each compound producing 10-fold activation of mitochondrial Ca2+ uptake. EC50 and S.D. values were calculated using a four-parameter fitting of log EC50 after fixing the maximum effect at 20-fold stimulation. When EC50 values were above 50 μM, the activation obtained at 50 μM (means±S.E.M., n=4) is shown instead.
| Compound | Apparent EC50 (μM) |
|---|---|
| Kaempferol | 7±1 |
| Quercetin | 21±1 |
| Genistin | 29±2 |
| Genistein | 37±2 |
| Galangin | 33±2 |
| Apigenin | 50±2 |
| Acacetin | 1.3±0.2* |
| Daidzein | 4.1±0.3* |
| SB202190 | 10±1 |
* Fold activation at 50 μM.
DISCUSSION
In the present paper we show that natural plant flavonoids produce a direct activation of the mitochondrial Ca2+ uniporter. Flavonoids constitute a large series of compounds found ubiquitously in foods of plant origin and therefore they constitute an integral part of the human diet. The dietary intake of flavonoids has been significantly associated in epidemiological studies with a reduced risk of heart disease and cancer [13,14,16,24]. Substantial evidence supports also the potential beneficial and neuromodulatory effects of flavonoid-rich gingko biloba extracts in the central nervous system [25,26]. Kaempferol, the stronger activator of the mitochondrial Ca2+ uniporter in this study and one of the main flavonoids in gingko biloba extracts, has been shown to protect neuronal cell cultures from β-amyloid-induced toxicity [27]. However, little information exists on the actual molecular mechanisms of action of these compounds.
With regard to concentrations of these compounds in plasma, accurate measurements have been hindered by the large diversity of this family of compounds (more than 4000 identified in plant sources) and the uncertainty of the metabolism and biodisponibility of each one. However, the global daily intake with normal food is around 1–2 g, and concentrations in the low micromolar range of the main flavonoids may be reached in plasma after consumption of flavonoid-rich food or dietary supplements [15–17]. According to our results, these concentrations may be enough to produce a significant activation of mitochondrial Ca2+ uptake. The apparent EC50 for kaempferol was 7 μM, but, because of the large magnitude of the stimulation (maximum effect of at least 20-fold increase in the uptake rate), much smaller concentrations were required to increase the uptake rate by 100% (which is only 5% of the maximum effect). In particular, a concentration of kaempferol as low as 1 μM was able to nearly double the rate of mitochondrial Ca2+ uptake. The long-term effect of such activation is presently unclear. Mitochondrial Ca2+ uptake has been shown to perform multiple roles in the regulation of the respiratory rate and ATP production [6,7], in the opening of the permeability transition pore and subsequently in apoptosis [9,10], or in the regulation of processes dependent on local cytosolic high-Ca2+ microdomains, such as secretion [3,8,21]. However, there is no information available yet on the possible effect of in vivo changes in the rate of mitochondrial Ca2+ uptake.
On the other hand, it is important to highlight that these compounds are essentially not toxic to mammalian cells [13,14,16,17]. For example, concentrations of kaempferol that produce maximal activation of mitochondrial Ca2+ uptake have been shown to produce little effects in HeLa cell cultures over several days [17]. Therefore, these compounds may now provide an opportunity to investigate the role of specific changes in mitochondrial Ca2+ uptake in the regulation of [Ca2+]c-dependent processes, such as secretion or in the triggering of apoptosis. In addition, the low toxicity in vivo will be essential in case a possible pharmacological use of their effects in mitochondrial Ca2+ homoeostasis is developed.
Regarding the mechanism of the activation, our results indicate that phosphorylation is not involved. If the effect of flavonoids or SB202190 was mediated by the inhibition of any protein kinase, we would have expected to obtain a similar stimulation of mitochondrial Ca2+ uptake simply by removing ATP. In addition, these compounds should not produce any further stimulation under those conditions. This was found not to be the case. Perfusion of the Ca2+ buffer in the absence of ATP or in the presence of ADP or non-hydrolysable ATP analogues did not modify the rate of mitochondrial Ca2+ uptake, and both flavonoids and SB202190 stimulated mitochondrial Ca2+ uptake similarly in the presence or in the absence of ATP. An alternative mechanism of action for the flavonoids could be an interaction with reactive oxygen species, thanks to their widely known antioxidant properties [28,29]. However, the lack of effect of a series of classical antioxidants (α-tocopherol, lipoic acid, ascorbic acid and N-methyl-cysteine) argues against this hypothesis. Finally, the simplest alternative is a direct stimulation of the uniporter. Structure–activity relationship suggests that both the 4′-hydroxyphenyl group in the B-ring and the 3-hydroxy group in the C-ring are involved in binding. Interestingly, SB202190 shares the first motif, but the rest of the molecule differs considerably.
In conclusion, we have found a new molecular target for natural flavonoids at low concentrations, which may be important to interpret some of the physiological effects of this important class of plant compounds. In addition, our finding suggests that modulation of mitochondrial Ca2+ uptake may occur in vivo depending on the dietary intake of these compounds. Of course, a highly attractive hypothesis would be the possible presence of endogenous substances able to act through the same mechanism as local modulators of mitochondrial Ca2+ uptake, but we have no evidence for the presence of these substances at present.
Acknowledgments
We thank Elena González and Beatriz Tena for excellent technical assistance. This work was supported by grants from Dirección General de Enseñanza Superior (BFI2002-01397) and from Junta de Castilla y León (VA 005/02). J.S. holds a FPI fellowship from the Spanish Ministerio de Ciencia y Tecnología. E.H.-S. holds a FPU fellowship from the Spanish Ministerio de Educación.
References
- 1.Berridge M. J., Bootman M. D., Roderick H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Collins T. J., Lipp P., Berridge M. J., Bootman M. D. Mitochondrial Ca2+ uptake depends on the spatial and temporal profile of cytosolic Ca2+ signals. J. Biol. Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 3.Montero M., Alonso M. T., Carnicero E., Cuchillo-Ibañez I., Albillos A., Garcia A. G., Garcia-Sancho J., Alvarez J. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat. Cell. Biol. 2000;2:57–61. doi: 10.1038/35000001. [DOI] [PubMed] [Google Scholar]
- 4.Pacher P., Thomas A. P., Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2380–2385. doi: 10.1073/pnas.032423699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filippin L., Magalhaes P. J., Di Benedetto G., Colella M., Pozzan T. Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. J. Biol. Chem. 2003;278:39224–39234. doi: 10.1074/jbc.M302301200. [DOI] [PubMed] [Google Scholar]
- 6.Jouaville L. S., Pinton P., Bastianutto C., Rutter G. A., Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutter G. A., Rizzuto R. Regulation of mitochondrial metabolism by ER Ca2+ release: an intimate connection. Trends Biochem. Sci. 2000;25:215–221. doi: 10.1016/s0968-0004(00)01585-1. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci D. R., Hlubek M. D., Stuenkel E. L. Mitochondria regulate the Ca2+-exocytosis relationship of bovine adrenal chromaffin cells. J. Neurosci. 1999;19:9261–9270. doi: 10.1523/JNEUROSCI.19-21-09261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardi P., Petronilli V., Di Lisa F., Forte M. A mitochondrial perspective on cell death. Trends Biochem. Sci. 2001;26:112–117. doi: 10.1016/s0968-0004(00)01745-x. [DOI] [PubMed] [Google Scholar]
- 10.Hajnoczky G., Davies E., Madesh M. Calcium signaling and apoptosis. Biochem. Biophys. Res. Commun. 2003;304:445–454. doi: 10.1016/s0006-291x(03)00616-8. [DOI] [PubMed] [Google Scholar]
- 11.Montero M., Lobaton C. D., Moreno A., Alvarez J. A novel regulatory mechanism of the mitochondrial Ca2+ uniporter revealed by the p38 mitogen-activated protein kinase inhibitor SB202190. FASEB J. 2002;16:1955–1957. doi: 10.1096/fj.02-0553fje. [DOI] [PubMed] [Google Scholar]
- 12.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 13.Middleton E., Jr, Kandaswami C., Theoharides T. C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 14.Havsteen B. H. The biochemistry and medical significance of the flavonoids. Pharmacol. Therap. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 15.Janssen K., Mensink R. P., Cox F. J., Harryvan J. L., Hovenier R., Hollman P. C., Katan M. B. Effects of the flavonoids quercetin and apigenin on hemostasis in healthy volunteers: results from an in vitro and a dietary supplement study. Am. J. Clin. Nutr. 1998;67:255–262. doi: 10.1093/ajcn/67.2.255. [DOI] [PubMed] [Google Scholar]
- 16.Birt D. F., Hendrich S., Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol. Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 17.O'Prey J., Brown J., Fleming J., Harrison P. R. Effects of dietary flavonoids on major signal transduction pathways in human epithelial cells. Biochem. Pharmacol. 2003;66:2075–2088. doi: 10.1016/j.bcp.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez J., Montero M. Measuring [Ca2+] in the endoplasmic reticulum with aequorin. Cell Calcium. 2002;32:251–260. doi: 10.1016/s0143416002001860. [DOI] [PubMed] [Google Scholar]
- 19.Montero M., Lobaton C. D., Moreno A., Alvarez J. Modulation of histamine-induced Ca2+ release by protein kinase C. Effects on cytosolic and mitochondrial [Ca2+] peaks. J. Biol. Chem. 2003;278:49972–49979. doi: 10.1074/jbc.M308378200. [DOI] [PubMed] [Google Scholar]
- 20.Rizzuto R., Simpson A. W., Brini M., Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 21.Ainscow E. K., Rutter G. A. Mitochondrial priming modifies Ca2+ oscillations and insulin secretion in pancreatic islets. Biochem. J. 2001;353:175–180. doi: 10.1042/0264-6021:3530175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert V., Gurlini P., Tosello V., Nagai T., Miyawaki A., Di Lisa F., Pozzan T. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. EMBO J. 2001;20:4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies S. P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nestel P. Isoflavones: their effects on cardiovascular risk and functions. Curr. Opin. Lipidol. 2003;14:3–8. doi: 10.1097/00041433-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe C. M., Wolffram S., Ader P., Rimbach G., Packer L., Maguire J. J., Schultz P. G., Gohil K. The in vivo neuromodulatory effects of the herbal medicine ginkgo biloba. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6577–6580. doi: 10.1073/pnas.111126298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Y., Smith J. V., Paramasivam V., Burdick A., Curry K. J., Buford J. P., Khan I., Netzer W. J., Xu H., Butko P. Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. U.S.A. 2002;99:12197–12202. doi: 10.1073/pnas.182425199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth A., Schaffner W., Hertel C. Phytoestrogen kaempferol (3,4′,5,7-tetrahydroxyflavone) protects PC12 and T47D cells from β-amyloid-induced toxicity. J. Neurosci. Res. 1999;57:399–404. [PubMed] [Google Scholar]
- 28.Duthie G., Crozier A. Plant-derived phenolic antioxidants. Curr. Opin. Clin. Nutr. Metab. Care. 2000;3:447–451. doi: 10.1097/00075197-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Heim K. E., Tagliaferro A. R., Bobilya D. J. Flavonoid antioxidants: chemistry, metabolism and structure–activity relationships. J. Nutr. Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]