Abstract
BLM (bleomycin) is effective in combination therapy against various cancers including testicular cancer. However, several other cancers such as colon cancer are refractory to BLM treatment. The exact mechanism for this differential response of cancer cells to the drug is not known. In the present study, we created fluorescently labelled BLM-A5, which retained nearly full genotoxic potential, and used this molecule to conduct the first study to understand the transport pathway of the drug in Saccharomyces cerevisiae. Uptake studies revealed that fluoro-BLM-A5 is transported into the cell in a concentration-dependent manner. Transport of a non-saturating concentration of fluoro-BLM-A5 was modest for the first 90 min, but thereafter it was sharply induced until 300 min. The inducible transport was completely abolished by the addition of cycloheximide, suggesting that BLM-A5 uptake into the cell is dependent on new protein synthesis. Interestingly, transport of fluoro-BLM-A5 was blocked if the cells were preincubated with increasing concentrations of spermine. Moreover, a mutant lacking the Ptk2 kinase, necessary for positively regulating polyamine transport, was defective in fluoro-BLM-A5 uptake and exhibited extreme resistance to the drug. A simple interpretation of these results is that BLM-A5 may enter the cell through the polyamine transport system. We showed further that after the uptake, fluoro-BLM-A5 accumulated into the vacuole of the parent, but localized to the cytoplasm of mutants disrupted for the END3 gene required for an early step of the endocytotic pathway. In general, mutants with a defect in the endocytic pathway to the vacuole were hypersensitive to BLM-A5. We suggest that BLM-A5 is transported across the yeast plasma membrane and sequestered into the vacuole for detoxification.
Keywords: anticancer, bleomycin, drug sensitivity, polyamine, transporter, yeast
Abbreviations: BLM, bleomycin; F-BLM, FITC coupled with BLM-A5; CHX, cycloheximide; FITC, [5-(and-6)-carboxyl fluorescein succinimidyl ester (5(6)-FAM,SE)]; FM4-64, N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino)phenyl)-hexatrienyl)pyridiniumdibromide; SPM, spermine; F-SPM, FITC coupled with SPM; TFA, trifluoroacetic acid
INTRODUCTION
The antibiotic BLM (bleomycin) was initially isolated from Streptomyces verticillus and subsequently shown to exhibit powerful genotoxic and antitumour properties [1,2]. BLM is administered systemically and used only in combination therapy with other antineoplastic agents [3–6]. While BLM is effective against lymphomas, testicular carcinomas, and squamous cell carcinomas of the cervix, head and neck, it has no effect on other types of cancers including colon carcinomas [7,8]. A number of mechanisms have been proposed to account for tumour resistance to BLM, and these include increased drug efflux, decreased uptake, increased degradation by BLM-hydrolase and increased DNA repair [2,9,10]. However, the exact mechanism contributing to BLM-resistant tumours is unknown.
The structure of BLM is characterized by metal- and DNA-binding domains, a carbohydrate moiety and a polyamine-like region (see Figure 1A) [1,11]. Unlike the other domains, very little is known about the carbohydrate moiety, except that BLM lacking this region exerts much less powerful genotoxic effect [12]. BLM binds to the reduced form of iron (Fe II), and in the presence of oxygen forms a free radical reactive complex that can attack several macromolecules in the cells including RNA and DNA [13]. For DNA, activated BLM induces the formation of a characteristic set of lesions, which closely resembles those produced by ionizing radiation [11,14]. These lesions include oxidized apurinic/apyrimidinic (AP) sites and single-strand breaks, where the 3′-end is terminated with a portion of the deoxyribose ring to form 3′-phosphoglycolate that effectively blocks DNA synthesis [11,15]. In the absence of oxygen, BLM can also produce bi-stranded DNA lesions at specific sequences, e.g. CGCC [16,17].
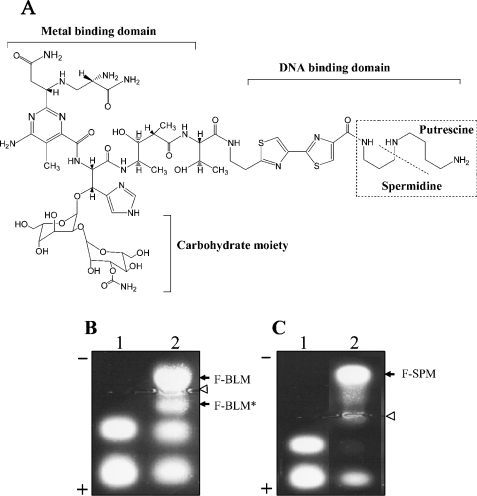
Figure 1. Structure of BLM-A5 and agarose gel electrophoresis analysis of fluorescently labelled forms.
(A) Depiction of BLM-A5 domains. The metal-binding domain binds to reduce iron and in the presence of oxygen forms a free radical that attacks the DNA. While the polyamine-like region is involved in DNA binding, the function of the carbohydrate moiety is unknown. This structure has been adapted from Leitheiser et al. [58]. (B, C) Resolution of fluorescently labelled BLM (F-BLM) and SPM (F-SPM) by agarose gel electrophoresis. The products formed by reacting either BLM (B, lane 2) or SPM (C, lane 2) with activated succinimidyl FITC (lane 1) were loaded on 1% agarose gel in 40 mM Mes buffer and allowed to migrate for 2 h at 50 V (see the Materials and methods section). Bands were detected by long-wavelength UV light. Open arrows indicate the loading lanes and closed arrows indicate positions of the reaction products F-BLM, F-BLM* and/or F-SPM. The * indicates the inactive form of F-BLM.
It is well established that BLM-induced DNA lesions are mutagenic [18–21]. Thus normal cells of cancer patients exposed to BLM must rely on enzymes to repair efficiently the drug-induced DNA lesions, as well as defence mechanisms to detoxify rapidly the drug, to prevent lethal mutations leading to toxic side effects and secondary tumours. A strategy that cells might use to detoxify BLM involves multidrug resistance pumps. In the yeast Saccharomyces cerevisiae, the known drug efflux pumps belonging to the ATP-binding cassette and the major facilitator superfamilies, e.g. Snq2, Yor1, Atr1 and Flr1, are not involved in the extrusion of BLM [22–25]. Indeed, mutants lacking these drug efflux pumps are not sensitive to the BLM [25]. Moreover, a mutant AD1-8 bearing deletion in seven genes (yor1Δ, snq2Δ, pdr5Δ, pdr10Δ, pdr11Δ, ycf1Δ, pdr15Δ), encoding ATP-dependent drug transporters, was not sensitive to BLM (D. Ramotar, unpublished work). Therefore these observations prompted us to investigate if a mechanism exists to transport BLM into yeast cells. We therefore initially synthesized a fluorescently labelled form of BLM (using BLM-A5) and rigorously showed that this molecule retained nearly the same genotoxic potential as the natural drug in yeast cells. We showed that fluoro-BLM-A5 (F-BLM) is transported into the cells in a concentration- and time-dependent manner, and that this process requires new protein synthesis. Additionally, transport of F-BLM was greatly reduced in a mutant deleted for the PTK2 gene, encoding a kinase that positively regulates polyamine transport. These findings raise the interesting possibility that both BLM and SPM (spermine) may utilize a common transport mechanism. Finally, we show that F-BLM accumulates in the vacuole, and that its distribution is altered in mutants defective in the endocytotic pathway, causing these mutants to display hypersensitivity to the drug. Collectively, our results clearly indicate that BLM is transported into yeast cells and directed to the vacuole for detoxification.
MATERIALS AND METHODS
Strains, media and transformation
The S. cerevisiae strains used in the present study are listed in Table 1. Yeast cells were grown at 30 °C in either YPD [1% (w/v) yeast extract, 2% (w/v) peptone, 2% (w/v) dextrose] or minimal synthetic (SD: 0.65% yeast nitrogen base without amino acids, 2% dextrose, 0.17% dropout mix) medium used for transformation [26,27]. Yeast cells were transformed by the lithium-acetate method [28].
Table 1. Strains used in the present study.
| Strain | Genotype | Source |
|---|---|---|
| BY4741 (parent) | MATa his3Δ leu2Δ met15Δ ura3Δ | Research Genetics |
| BY4741 (end3Δ) | Isogenic to BY4741, except end3Δ::KAN | Research Genetics |
| BY 4741 (vma2Δ) | Isogenic to BY4741, except vma2Δ::KAN | Research Genetics |
| BY 4741 (vps27Δ) | Isogenic to BY4741, except vps27Δ::KAN | Research Genetics |
| BY 4741 (ptk2Δ) | Isogenic to BY4741, except ptk2Δ::HIS3 | The present study |
| BY 4741 (sky1Δ) | Isogenic to BY4741, except sky1Δ::HIS3 | The present study |
| BY 4741 (agp2Δ) | Isogenic to BY4741, except agp2Δ::URA3 | The present study |
| BY 4741 (agp2Δ end3Δ) | Isogenic to BY4741, except agp2Δ::URA3 end3Δ::KAN | The present study |
| BY 4741 (ptk2Δ end3Δ) | Isogenic to BY4741, except ptk2Δ::HIS3 end3Δ::KAN | The present study |
| YW465 (parent) | MATα ade2Δ0 his3Δ-200 leu2Δ-1 met15Δ0 trp1Δ-63 ura3Δ0 | [35] |
| YW778 | Isogenic to YW465, except tpp1Δ::MET15 apn1Δ::HIS3 apn2Δ::KanMX4 | [35] |
Gene disruption
Deletion of PTK2, SKY1 and AGP2 genes in the indicated strain was performed by one-step gene targeting using universal upstream and downstream primers [29]. The primer sequences used for deleting either the PTK2, SKY1 or AGP2 gene and confirming the deletion alleles were the following: PTK2-F1, GGTAGATAAAAGTCCCTCTGTTAGTACTTTGAAACTATTGGGAAAACGCCTGTTCAGATTGTACTGAGAGTGCAC; PTK2-R1,CTGTTGGTAATGTCCAAGTGGTGGTGAATTACCTTCTTCTTTTTCGAGGATGACCTGTGCGGTATTTCACACCGC; PTK2-V1, CTATTGGAAAGACCCTTCCAC; SKY1-F1, CCTGGGTTTGTGACTAAAAGCGCTCATTTGGCTGACACTAGTACGCAGATTGTACTGAGAGTGCAC; SKY-R1, CTTTTATGATCGCGGACTTCTTCAAACCATCCGGGGATATCGGAACCTGTGCGGTATTTCACACCGC; SKY-V1, GAGGGATCTATTTGTAGCTGGCAAG; AGP2-F1, GTTCCAATACTTTGCATTACTGTGTCTACAGCGGAAATGGTCTGCTCCATGGAAAAGAGAAG; and AGP2-R1, GTCAACGCACTTCGTTGCTAATCTCAAAAAGGGGGAACTTACAGGTACGACTCACTATAGGG.
Coupling of FITC with BLM and SPM
A 100 μl aliquot of 2.1 mM of the fluorescent molecule succinimidyl-FITC [5-(and-6)-carboxyl fluorescein succinimidyl ester (5(6)-FAM,SE; Molecular Probes] in 0.2 M NaHCO3 (pH 9.0) was added to 300 μl of 0.6 mM BLM-A5 (Calbiochem; prepared in 0.2 M NaHCO3, pH 8.3) and the mixture was incubated for 2 h at room temperature (25 °C). The reaction was stopped by the addition of 10 μl of 1.5 M hydroxylamine (pH 8.5). The reaction products were resolved on 1% agarose gel for 2 h at 100 V using 40 mM Mes buffer (Amersham Biosciences) at pH 6.0. The product (slower migrating band towards the cathode) containing (FITC coupled with BLM) was visualized by a hand-held long-wavelength (365 nm) UV lamp, excised, placed at −80 °C until frozen, centrifuged at 10080 g for 5 min in an Eppendorf centrifuge, freeze-dried and resuspended in 300 μl of sterile distilled water. F-BLM was aliquoted and stored at −20 °C. Using the same method, FITC was coupled with SPM (Sigma) to generate the molecule F-SPM.
HPLC analysis of F-BLM
Reversed-phase HPLC was performed using a Vydac C18 column [250 mm×0.4 mm, 5 μm, 300 Å (1 Å=0.1 nm); Mandel Scientific, St-Laurent, QC, Canada] connected to a Beckman Gold system (Beckman, Missisauga, ON, Canada). This system includes a 126 solvent module, a 168 detector equipped with deuterium lamp, an external injector (Rheodyne, Rohnert Park, CA, U.S.A.) and a 32 Karat analysis software. F-BLM derived from the agarose gel and resuspended in sterile deionized water (see above) was diluted in 1 ml of 0.1% TFA (trifluoroacetic acid) and filtered through a 0.45 μm filter (4 mm Nylon syringe filter; Fisher, Montreal, Quebec, Canada) and loaded on to the column. The product was separated using a 14–20% (v/v) acetonitrile/0.1% TFA linear gradient at a flow rate of 1 ml/min and analysed at a wavelength of 254 nm.
The concentration of F-BLM was assessed against natural BLM (BLM-A5) by reading the absorbance at 292 nm and by the extent of fragmentation of purified pBluescript plasmid DNA when compared with the unmodified drug. The cytotoxic effect of F-BLM was determined by clonogenic assays of a parent strain YW465 and the DNA-repair-deficient mutant YW778 (see text).
Uptake analyses
For F-BLM uptake studies, exponentially growing cultures were washed twice in sterile distilled water and resuspended in uptake buffer [50 mM citrate acetate buffer, pH 5.5, 2% (w/v) glucose and 0.05% Tween 20] at a density of 2×108 cells/ml. F-BLM (0.0–4.2 μg/ml) was added to 100 μl of the cells, incubated at 30 °C in the dark with mild shaking (New Brunswick Floor model shaker at 50 rev./min) for the indicated times, and uptake was stopped by adding 1 ml of stop buffer (50 mM citrate acetate buffer, pH 5.5, and 2 mM sodium azide). Cells were washed three times with sodium phosphate buffer (0.1 M Na2HPO4/NaH2PO4, pH 7.0), resuspended in 500 μl of the buffer, sonicated at 30% for 15 s (model W185D; Heat System, Ultrasonic, New York, NY, U.S.A.), and the extent of F-BLM uptake was measured using a fluorescent spectrophotometer at 495/525 nm (excitation/emission) (PerkinElmer LS-S fluoresence spectrophotometer). An identical method was used for monitoring F-SPM uptake. L-[4,5-3H]leucine (specific activity 63.0 Ci/mmol; Amersham Biosciences) transport was also performed under the same conditions, and the level of labelled leucine uptake was monitored by a scintillation counter.
Fluorescence microscopy
Cells were grown to a density of 2×108 cells/ml, washed twice in sterile distilled water and resuspended in uptake buffer. An aliquot of 100 μl of cells was incubated with either F-BLM or F-SPM (0.36 μg/ml), and the cells were incubated at 30 °C in the dark with mild shaking for 1 h. FM4-64 dye [N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino)phenyl)-hexatrienyl)pyridiniumdibromide; Molecular Probes] was used as a positive control to localize the vacuoles [30]. Cells were washed three times with 1 ml of sodium phosphate buffer, resuspended in 100 μl of the buffer, and 3 μl was mounted on microscope slides for fluorescent microscopy. Cells were photographed at 100×magnification by imaging camera (Retiga GX 32-002TB-303) attached to a Leica DMRE fluorescent microscope and images were processed by the MacIntosh OpenLab program. FM4-64 (Molecular Probes) was used to identify the vacuoles.
Survival curves, spot tests and mutation assays were performed as described previously [31]. For nystatin (Sigma) treatment, cells were preincubated for 1 h with 10 units/ml.
Primer extension assay
Primer extension assay was performed as described previously [25]. Briefly, exponentially growing cultures were tested with and without BLM (20 μg/ml for 1 h). Chromosomal DNA was isolated and assessed for the ability to permit incorporation of [methyl-3H]dTMP by purified Escherichia coli DNA polymerase I (Promega). Apn1 used for pretreating the chromosomal DNA was purified in our laboratory [32]. The labelled [methyl-3H]dTPP (NET221X from NEN Life Sciences Products; 37.0 MBq) was added to the reaction mixture to a specific activity of 1230 c.p.m./pmol [25].
Statistical analysis
Results are expressed as means±S.D. for three separate experiments with duplicate or triplicate determinations for each experiment.
RESULTS
Preparation and analysis of FITC-labelled BLM
Radioactively labelled BLM is not commercially available for drug uptake studies, and we therefore conjugated activated succinimidyl-FITC to bleomycin-A5 (BLM) by using a similar approach reported for attaching FITC to tallysomycins, which is structurally related to BLM but contains an additional talose sugar (see the Materials and methods section) [33,34]. Since BLM contains a few nitrogen groups that are accessible to react with the amine-reactive FITC (Figure 1A), only a limited number of products could be expected. The products were readily resolved from the free BLM and FITC by electrophoresis on agarose gels, as the latter two starting reagents migrated in opposite directions. Figure 1(B, lane 2) shows the result of a typical agarose gel depicting four distinct fluorescent bands visualized by long-wavelength UV light (365 nm). The two upper bands, a major and minor compound, designated as F-BLM and F-BLM* respectively, were the result of covalent linkage of BLM to succinimidyl-FITC, whereas the two lower bands were due to the uncoupled activated succinimidyl-FITC (Figure 1B, lane 2 versus lane 1). Since the highly positively charged free BLM migrated towards the cathode and completely out of the gel, and the free FITC migrated towards the anode, neither F-BLM nor F-BLM* is expected to contain any contaminating starting material. In a similar synthesis, we have also coupled SPM with activated FITC (Figure 1C, see below).
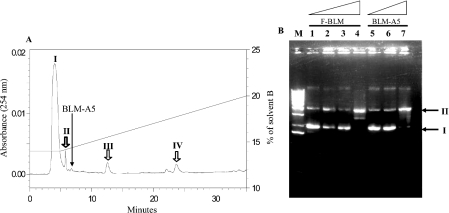
The major compound, F-BLM, was isolated from the agarose gel and further analysed for additional products by HPLC (see the Materials and methods section). This analysis resolved the F-BLM into four separate peaks (I, II, III and IV) by reading the BLM absorbance at 254 nm (Figure 2A), which also exhibited the same excitation/emission (495/525 nm) wavelength as FITC (results not shown). Peak I contained the major portion of F-BLM and it eluted 3 min earlier than purified BLM-A5 (Figure 2A). Each of the four peaks of F-BLM was separately assessed for the ability to damage purified plasmid DNA by monitoring the conversion of covalently closed circular form (I) into the nicked form (II). All four peaks contained F-BLM, which was active at damaging the plasmid DNA (Figure 2B, lane 4, showing data only for peak I), when compared with purified BLM-A5 (lane 7). None of the F-BLM peaks caused damage to the plasmid in the presence of EDTA, suggesting that F-BLM action on DNA is metal-dependent (results not shown). In short, F-BLM isolated from the agarose gel consists of at least four forms, perhaps representing different covalent linkage of FITC to BLM, which can damage DNA. Since peaks II, III and IV did not contain sufficient quantity of F-BLM for subsequent studies, all ensuing experiments were performed with F-BLM derived from peak I.
Figure 2. Purification of F-BLM by HPLC and F-BLM-induced DNA damage analysis.
(A) HPLC analysis of F-BLM. F-BLM isolated from agarose gel was diluted in 0.1% TFA and subjected to HPLC using a Vydac C18 column at a flow rate of 1 ml/min. F-BLM elution was monitored by reading the absorbance at 254 nm. Peaks I, II, III and IV are fractions containing F-BLM. The long arrow indicates the elution position of purified BLM-A5. (B) F-BLM-induced DNA damage. The F-BLM in peak I was tested for the ability to convert covalently closed circular DNA (form I) into the nicked DNA (form II). Lanes 1–7, 50 ng each of pBluescript plasmid incubated with 0, 0.1, 1.0 and 10 μg/ml of F-BLM (lanes 1–4 respectively) and 0.1, 1.0 and 10 μg/ml of BLM-A5 (lanes 5–7 respectively) at 25 °C for 30 min. Lane M, DNA size standard. Samples were loaded on 1% agarose gel, migrated for 1 h at 100 V, and photographed after staining with ethidium bromide.
F-BLM induces cell killing and damages DNA in vivo
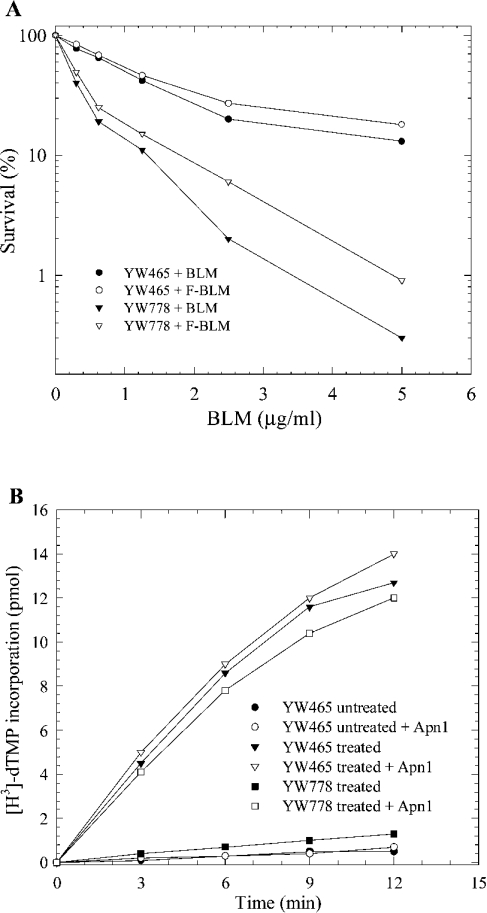
To examine if F-BLM is functionally active, we compared the survival of exponentially growing cells challenged with increasing concentrations of either F-BLM or the unmodified drug (see the Materials and methods section). The strains examined were the parent YW465 and a BLM-hypersensitive mutant strain YW778 (apn1Δ apn2Δ tpp1Δ) known to be defective in the repair of BLM-induced DNA lesions [35]. Results revealed that F-BLM decreased the survival of both the parent and the mutant strains, but it was slightly less effective than the natural drug BLM-A5 (Figure 3A). On the basis of this finding, it would appear that F-BLM possesses nearly full capacity to act as a cytotoxic agent.
Figure 3. F-BLM decreases cell survival and induces DNA damage.
(A) Exponentially growing cells were treated with increasing concentrations of either F-BLM or the natural drug BLM-A5. Cultures were serially diluted and scored for survivors on solid YPD medium after 2 days of growth at 30 °C. (B) In vitro incorporation of [methyl-3H]dTMP by DNA polymerase I into chromosomal DNA isolated from untreated and F-BLM-treated yeast cells. Exponentially growing cells were either treated with or without 20 μg/ml of F-BLM for 2 h. Where indicated (open symbols), the chromosomal DNA was preincubated with 20 ng of purified Apn1 for 20 min before monitoring [methyl-3H]dTMP incorporation. YW465 is the parent strain (Wt) and YW778 is the mutant defective in DNA repair. Results are representative of two independent experiments.
We next examined if F-BLM-mediated cell killing is a direct result of damage to DNA. In this experiment, the parent and the BLM-hypersensitive strain YW778 were treated with or without F-BLM (20 μg/ml for 2 h), and chromosomal DNA was isolated and examined for the ability to permit in vitro incorporation of [methyl-3H]dTMP [25]. In this analysis, we expect that if F-BLM damages the DNA, the lesions will be processed in the parent, but not in the mutant, to produce free hydroxy groups, which can serve as substrate in a second step to allow the in vitro incorporation of [methyl-3H]dTMP by purified E. coli DNA polymerase I [25]. The DNA isolated from the F-BLM-treated parent strain showed a substantial level of [methyl-3H]dTMP incorporation, but not the DNA derived from the treated mutant strain YW778 (Figure 3B). Analogous data were obtained when cells were treated with unmodified BLM [25]. As shown previously, DNA isolated from untreated cells showed no [methyl-3H]dTMP incorporation (Figure 3B) [25]. These results suggest that F-BLM is acting in a manner similar to the unmodified drug in generating DNA lesions, which must be processed by the enzymes that are deficient in strain YW778. To examine further, the F-BLM-damaged chromosomal DNA derived from strain YW778 was pretreated with purified Apn1 (a DNA repair enzyme that can process BLM-induced DNA lesions to create 3′-hydroxy groups) and then monitored for [methyl-3H]dTMP incorporation by DNA polymerase I. Preincubation of F-BLM-damaged DNA derived from strain YW778 with purified Apn1 greatly stimulated [methyl-3H]dTMP incorporation (Figure 3B). Apn1 did not permit additional [methyl-3H]dTMP incorporation into the damaged DNA isolated from the parent strain (Figure 3B). Consistent with these findings, introduction of a multicopy plasmid YEpAPN1 expressing the Apn1 protein into strain YW778 restored full parental resistance to F-BLM (results not shown). Taken together, these results indicate that the cytotoxic effects of F-BLM are related to its ability to produce DNA lesions that can be processed by Apn1.
We also examined if F-BLM acted similarly to BLM-A5 in the induction of mutations. In this experiment, the parent strain was challenged with increasing concentrations of either F-BLM or BLM-A5 and scored for canavanine-resistant colonies (CanR+). These colonies arose as a result of mutation in the CAN1 gene encoding the arginine permease, which permits entry of the arginine analogue canavanine into the cell. This analysis revealed that F-BLM caused a concentration-dependent increase in the frequency of canR mutants and to the same magnitude as cells exposed to BLM-A5 (Table 2). Thus, taken together, these results clearly indicate that attachment of FITC to BLM-A5 did not interfere with the ability of the drug to enter yeast cells and to act as a genotoxic agent.
Table 2. Frequency of spontaneous and induced CanR mutations.
| 10−8× Mutation frequency of canR | |||
|---|---|---|---|
| Strain | [F-BLM] or [BLM-A5] (μg/ml) | F-BLM | BLM-A5 |
| YW465 (parent) | 0.0 | 16±2 | 14±3 |
| YW465 | 0.1 | 423±30 | 467±20 |
| YW465 | 1.0 | 2800±110 | 3200±160 |
| YW778 (apn1Δ, apn2Δ, tpp1Δ) | 0.0 | 44±5 | 58±6 |
| YW778 | 0.1 | 3600±200 | 3500±300 |
F-BLM is transported into parent yeast cells and inhibited by CHX (cycloheximide)
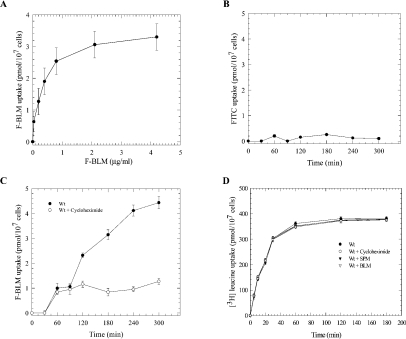
We next investigated the kinetic parameters for the uptake of F-BLM into the parental strain. Exponential-phase cells showed a concentration-dependent uptake of F-BLM, which reached a Vmax of 3.4±0.5 pmol·h−1·(107 cells)−1 (Figure 4A). In contrast, no detectable uptake was observed for the activated succinimidyl-FITC (Figure 4B). At the non-saturating concentration of 0.72 μg/ml, F-BLM entered the cells in a time-dependent manner (Figure 4C). Drug entry occurred after 30 min and increased linearly to 60 min, but began to plateau between 60 and 90 min (Figure 4C). After 90 min, F-BLM uptake resumed and linearly accelerated over the next 150 min (Figure 4C). These findings indicate that F-BLM is transported into the cells in a biphasic manner and that the latter phase might be inducible (see the Discussion section).
Figure 4. Kinetics of F-BLM uptake and inhibition by CHX in the parent strain.
(A) Concentration-dependent transport of F-BLM. Exponential-phase cells were incubated with increasing concentrations of F-BLM for 1 h at 30 °C, washed and the amount of F-BLM uptake was quantified using a fluorescent spectrophotometer. (B) Analysis of FITC uptake in the parent strain. (C) Time-dependent transport of F-BLM and inhibition by CHX. (B, C) Exponential-phase cells were incubated with a fixed concentration of FITC (10.0 μg/ml) and F-BLM (0.72 μg/ml) respectively, and samples were processed for drug uptake at the indicated time. CHX (10 μg/ml) was added to the cells for 30 min before the analysis of F-BLM uptake. (D) Uptake of [3H]leucine in the parent strain. Conditions for [3H]leucine uptake were the same as for F-BLM. SPM (1.0 mM for 16 h) and BLM (0.72 μg/ml for 1 h) were used to examine for interference of [3H]leucine uptake. Results are representative of three independent experiments. Wt, parent strain BY4741.
To test if F-BLM transport is dependent on new protein synthesis, cells were preincubated with the protein synthesis inhibitor CHX (10 μg/ml) and then assessed for the drug uptake. The results revealed that CHX did not interfere with the initial uptake of F-BLM, which occurred during the first 60–90 min, but after this time interval uptake was completely inhibited (Figure 4C). In control experiments, CHX treatment did not block [3H]leucine uptake into the cell, nor did BLM (0.72 μg/ml) exposure cause any effects (Figure 4D). Thus it would appear that the late phase of F-BLM uptake into yeast cells is performed by a transport process that is dependent on new protein synthesis.
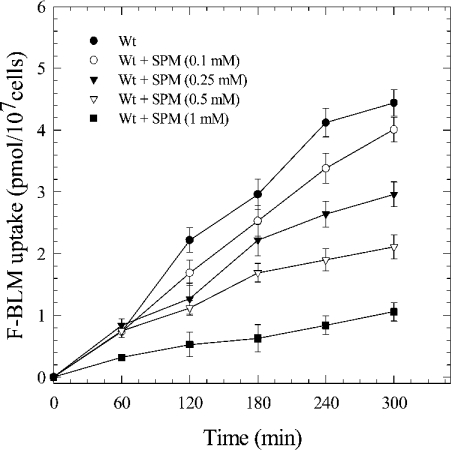
F-BLM transport is diminished by SPM
Since BLM-A5 contains a carbohydrate moiety, as well as a region that bears the same chemical composition as polyamines (Figure 1A), we tested whether cells preincubated with either various carbon sources or SPM could interfere with F-BLM transport. No alteration in F-BLM transport was observed if the cells were preincubated with either 2% galactose, maltose or raffinose (results not shown). Similarly, cells preincubated with increasing and non-toxic levels of SPM (0.1–1.0 mM) for a brief period (<1 h) showed no defect in F-BLM uptake (results not shown). However, if the cells were preincubated with SPM (0.1–1.0 mM) for a longer time (16 h), F-BLM uptake was reduced in a concentration-dependent fashion (Figure 5). F-BLM uptake was similarly decreased with non-toxic concentrations of spermidine, suggesting that this effect was not specific for SPM (results not shown). In control experiments, SPM (1.0 mM for 16 h) did not interfere with [3H]leucine uptake (see Figure 4D). A simple interpretation of these findings is that polyamines and BLM-A5 may utilize a common transport process (see the Discussion section).
Figure 5. Inhibition of F-BLM uptake in the parent strain by SPM.
Exponential-phase cells (Wt) were first incubated with increasing concentrations of SPM (0.1–1.0 mM for 16 h) followed by analysis for F-BLM uptake. Results represent the average of three independent experiments.
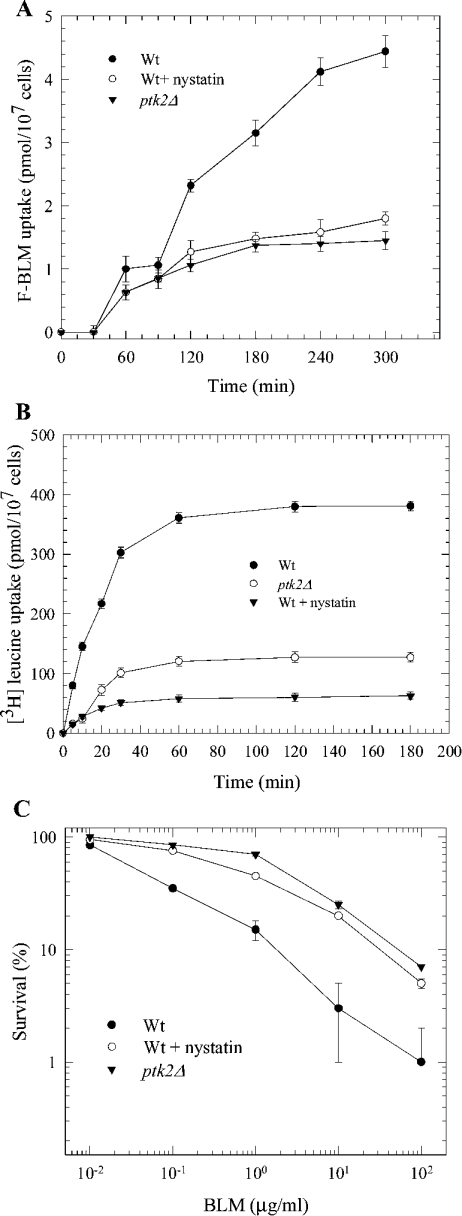
ptk2Δ mutants are defective in F-BLM uptake
To explore if there is indeed a relationship between F-BLM and SPM transport, we examined if F-BLM uptake is altered in a ptk2Δ mutant. This mutant is deleted for the PTK2 gene encoding a kinase that is involved in positively regulating the transport of polyamine [36]. ptk2Δ mutants were previously shown to be deficient in polyamine transport and displayed resistance to toxic levels of SPM [36]. Interestingly, Figure 6(A) shows that the ptk2Δ mutant exhibited a sharp decrease in F-BLM uptake, at least 4-fold lower when compared with the parental strain. Moreover, this mutant did not display the typical biphasic transport for F-BLM, as seen for the parent (Figure 6A). Thus it would appear that the transport of BLM and SPM into yeast cells is governed by the same mechanism (see the Discussion section).
Figure 6. Analysis of F-BLM and [3H]leucine transport and cell survival.
(A) Comparison of F-BLM uptake in the parent and ptk2Δ mutant. F-BLM uptake was analysed as in Figure 4. (B) Comparison of [3H]leucine uptake in the parent and ptk2Δ mutant. (C) Sensitivity of parent and ptk2Δ mutant to BLM. Exponentially growing cells were treated as in Figure 4. For nystatin treatment, cells were preincubated with the drug (10 units/ml for 1 h) following uptake measurements.
The exact role of Ptk2 in regulating polyamine transport is not known, but a previous study demonstrated that Ptk2 may function by regulating the activity of Pma1, an essential plasma membrane proton pump [37]. Pma1 creates a voltage gradient that is used as an energy source by transporters to drive uptake of nutrients, ions and other compounds into the cells [37,38]. As such, we tested if abrogation of the electrochemical gradient by the drug nystatin could alter F-BLM uptake. When parental cells were exposed to a non-lethal concentration of nystatin followed by uptake analysis of F-BLM, only a modest level of the drug was transported into the cell (Figure 6A). This observation strongly supports the notion that transport of F-BLM into the cell appears to be an energy-dependent process that could be regulated by Ptk2. It is noteworthy that the Ptk2 effect is not specific for BLM-A5 uptake, as ptk2Δ mutants were also defective in the transport of [3H]leucine into the cells (Figure 6B, and also see the Discussion section).
ptk2Δ mutants are resistant to the genotoxic effects of BLM
Since the ptk2Δ mutant manifests decreased levels of F-BLM transport, we tested if it also displays altered sensitivity to the drug. Thus we performed clonogenic survival assays using exponentially growing cultures that were treated with increasing concentrations of BLM. As shown in Figure 6(C), the ptk2Δ mutant was extremely resistant to BLM, when compared with the parent. While 80% of the ptk2Δ mutant survived a BLM concentration of 0.5 μg/ml for 1 h, only 20% of parental cells survived this treatment (Figure 6C). This finding was not restricted to cells treated under acute conditions, as exponentially growing cultures that were serially diluted and spotted on to YPD solid medium containing BLM also revealed that the ptk2Δ mutant was extremely resistant to the drug (see below). Treatments that reduced F-BLM transport such as exposure to SPM or nystatin, protected the parent strain from BLM-induced killing (Figure 6C, shown only for nystatin). Taken together, these findings strongly support the notion that the lethality of BLM is dependent on efficient transport of the drug into the cell.
F-BLM accumulates into the vacuole of the parent strain, but only weakly in the ptk2Δ mutant
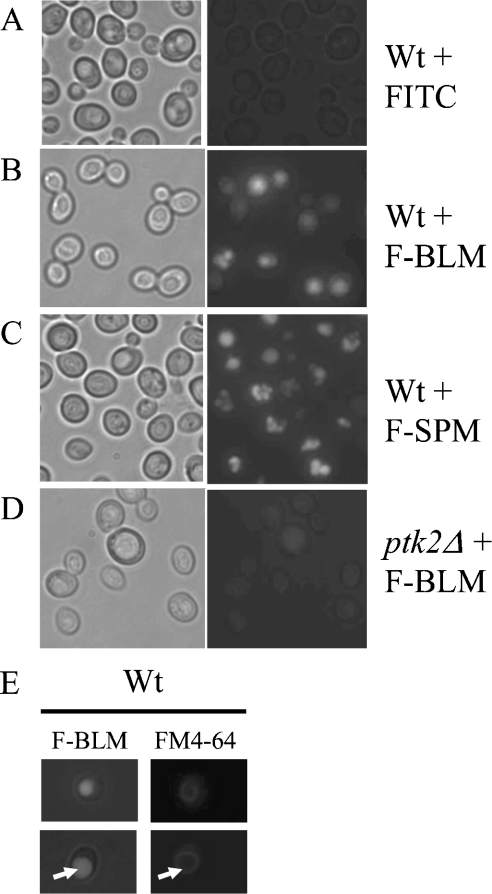
We next determined the intracellular fate of F-BLM by monitoring the distribution of the drug using fluorescent microscopy. Parental cells incubated with F-BLM showed an initial weak cytoplasmic staining within 20 min. After 60 min, the intensity of F-BLM was greatly increased and the drug was found to be compartmentalized into the vacuole (Figure 7B), as the staining pattern coincided with the location of FITC-labelled SPM (F-SPM) (Figure 7C), which is known to accumulate in the vacuoles, and FM4-64, that stains the endocytic pathway, as well as the vacuolar membranes (Figure 7E) [30,39]. In contrast with the parental cells, F-BLM staining was extremely weak in the vacuole of the ptk2Δ mutant, suggesting that indeed the mutant is defective in the drug transport (Figure 7D). In control experiments, neither the activated succinimidyl-FITC nor FITC blocked with hydroxylamine showed any significant staining in the cell under the same conditions (Figure 7A). On the basis of our findings, it appears that after F-BLM is transported across the plasma membrane, the drug is predominantly sequestered into the vacuoles.
Figure 7. Fluorescent analysis showing F-BLM distribution in the parent and ptk2Δ mutant.
(A–D) Cells were incubated with FITC (0.72 μg/ml), F-BLM (0.36 μg/ml) or F-SPM (0.36 μg/ml) for 1 h and then photographed at a magnification of 100× with a Leica fluorescent microscope equipped with a digital camera (Retiga GX 32-002TB-303). FITC and F-SPM were used as negative and positive controls respectively. (E) Identification of the vacuoles with FM4-64 dye. Vacuoles are shown with arrows.
end3Δ mutants show decreased accumulation of F-BLM, but display hypersensitivity to the drug
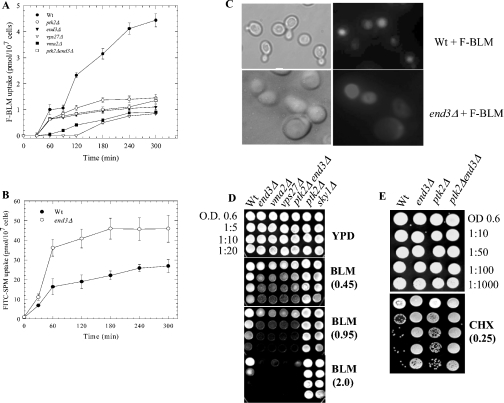
Since several plasma membrane proteins, such as transporters of amino acids, sugars and drugs are known to undergo internalization by endocytosis and subsequent degradation in the vacuoles, we tested if F-BLM transport is affected by mutations in the endocytic pathway [40–42]. End3, a cytoskeletal protein, plays a key role in the early steps of endocytic internalization, and end3Δ mutants are defective in both fluid-phase and plasma membrane receptor/transporter internalization [43]. As such, turnover of the putative BLM transporter might be blocked in the end3Δ mutant, causing a higher level of F-BLM uptake than the parent, as reported for SPM transport [44]. The results revealed that F-BLM uptake was not increased, and instead decreased in the end3Δ mutant, when compared with the parent (Figure 8A). In control experiments, F-SPM uptake was substantially higher in the end3Δ mutant, as reported previously (Figure 8B).
Figure 8. F-BLM uptake and distribution, and cytotoxic effects on mutants defective in endocytosis.
(A) Comparison of F-BLM uptake in the parent and various mutant strains. Cells were monitored for F-BLM transport as described in Figure 4. (B) F-SPM was used as a control to monitor SPM uptake into the parent and end3Δ mutant. F-SPM uptake was monitored as for F-BLM as described in the Materials and methods section. (C) Fluorescent analysis of F-BLM distribution in the parent and end3Δ mutant. Cells were processed for immunofluorescent microscopy as in Figure 7. (D, E) Spot-test analysis of the parent and various mutant strains for BLM and CHX sensitivity respectively. Exponentially growing parent and the isogenic mutants were serially diluted and spotted on YPD solid agar plates containing the indicated concentrations of BLM (0.45–2.0 μg/ml) or CHX (0.25 μg/ml). The plates were photographed after 2 days of growth at 30 °C. Results are representative of three independent experiments.
We next examined the cellular location of F-BLM in the end3Δ mutant. Fluorescent microscopy revealed that the drug was not contained within the vacuole, but was found mostly distributed in the cytoplasm of the mutant (Figure 8C). We then tested if the altered drug distribution has any consequences in cell survival. Exponentially growing end3Δ mutants spotted on to YPD solid media containing BLM displayed remarkable sensitivity to the drug (Figure 8D). In contrast, the end3Δ mutant showed resistance to CHX (Figure 8E), which is expelled from cells by multidrug ATP-binding-cassette transporters, e.g. Pdr5 that probably accumulated in this mutant [24]. Thus it would appear that the non-compartmentalization of BLM in the cell is the most probable cause of the decreased survival and, consequently, decreased drug uptake. It is noteworthy that defects along the endocytosis pathway to the vacuoles also led to the BLM-hypersensitive phenotype (Figure 8D). Mutants lacking the Vma2 protein required for the activity of the vacuolar H+ ATPase to acidify this compartment, or Vps27 protein required for endosomal transport from the cell surface to the vacuole, were also hypersensitive to BLM (Figure 8D) [43]. As such, we suggest that the endocytic pathway to the vacuole is required to internalize a plasma membrane BLM transporter, and that this pathway plays a crucial role in the detoxification of BLM.
ptk2Δ end3Δ double mutant displays hypersensitivity to BLM
Since Ptk2-deficient mutants were resistant to BLM (Figures 6C and 8D), we tested if deletion of the PTK2 gene could rescue the end3Δ mutant from BLM lethality. Interestingly, the ptk2Δ end3Δ double mutant was as sensitive to BLM as the end3Δ single mutant on spot-test analysis (Figure 8D). In addition, analysis of F-BLM uptake in the ptk2Δ end3Δ double mutant showed that it was not significantly different from the end3Δ single mutant (Figure 8A). One interpretation of these results is that the BLM-transporter activity might be leaky in the absence of Ptk2, allowing accumulation of toxic levels of BLM in ptk2Δ end3Δ double mutant during the chronic drug exposure. Alternatively, the BLM lethality of the ptk2Δ end3Δ double mutant could also be the result of a defective End3-dependent fluid endocytosis pathway.
DISCUSSION
In the present study, we generated a fluorescently labelled form of the anticancer drug BLM, designated F-BLM, which retained nearly full capacity to act as a genotoxic agent. We rigorously showed that F-BLM induces cell killing by creating endogenous DNA lesions analogous to the unmodified drug. We then used the fluorescent molecule as a tool to probe the mechanism of uptake and cellular distribution of BLM. Our results revealed that F-BLM is transported into the cell in a concentration-dependent manner. Moreover, the transport of F-BLM occurred in two phases, an early phase that may be time-independent and possibly due to passive diffusion, and a late phase that is time-dependent and blocked by CHX. This latter phase of F-BLM uptake may depend on a transporter, or a regulatory component of the transporter, that requires new protein synthesis. If this is the case, then the induction of a putative BLM transporter would certainly pose a severe disadvantage for normal cells challenged with BLM, unless this transporter has diverse functions such as playing a role in the transport of nutrients. We have also observed that disruption of the electrochemical gradient by nystatin blocked the late phase of F-BLM uptake. Whether this signifies that F-BLM transport is an energy-dependent process awaits further investigations.
While this study was in progress, we performed a genome-wide screen on a haploid mutant collection to search for both BLM-hypersensitive and -resistant strains. This study revealed five BLM-resistant mutants, and three of these were deleted for genes involved in plasma membrane transport including the L-carnitine transporter Agp2, as well as the kinases Ptk2 and Sky1 [36,45,46]. We showed that Agp2 has the ability to transport BLM into the cell, as agp2Δ mutants exhibited a decreased level of F-BLM uptake [47]. Similarly, Ptk2 and Sky1 are also required to permit F-BLM uptake into the cells [47]. While Ptk2 has a general role in providing energy for transporters through maintaining the voltage gradient by positively influencing the activity of the proton pump Pma1 [37], the Sky1 kinase seems to perform a direct role in BLM transport as it is not defective in [3H]leucine uptake (results not shown). These additional observations are consistent with the possibility that at least an energy-dependent plasma membrane transporter exists to transport BLM into the cell. It is unlikely that Agp2 is the sole BLM transporter, as deletion of the AGP2 gene in the end3Δ mutant did not protect the resulting agp2Δend3Δ double mutant from BLM-induced lethality (results not shown), although we cannot exclude that the toxicity arose from a defect in the End3-dependent fluid endocytosis process. Nonetheless, it remains to be determined whether other BLM transporter(s), besides Agp2, exists in yeast. Indeed, plasma membrane derived from either yeast or human cells contains a protein that binds BLM-A2, and which could also act as the carrier of the drug [48–51]. This putative BLM-A2-binding protein (∼250 kDa in mass) was initially identified by its specific interaction with labelled [57Co]cobalt–BLM-A2 complex followed by analysis on native protein gels [48–51]. Whether this BLM-binding protein is also a transporter of the drug awaits isolation and characterization from either yeast or human cells. It is expected that cells lacking this protein should be defective in F-BLM uptake and exhibit extreme resistant to the drug. It is noteworthy that the previously identified BLM3 gene encoding a large integral membrane protein that may belong to the major facilitator family of permeases, and which is believed to play a role in preventing the lethal effects of BLM, is unlikely to be a BLM transporter [52]. In fact, deletion of the entire BLM3 gene, or an allele blm3-1 of the gene, causes some sensitivity to BLM, rather than resistance [52]. Moreover, several mutants lacking permeases including Gap1 (the general amino acid permease), Put4 (proline-specific permease) and Can1 (arginine permease) do not show any altered resistance to BLM when compared with the parental strain (results not shown), arguing that BLM entry into the cells is performed by specific transporter(s).
The observation that SPM diminishes F-BLM uptake, as well as protecting cells from the genotoxic effects of the drug, raises the possibility that SPM and BLM-A5 may utilize a common plasma membrane transporter to gain entry into the cell. Alternatively, SPM may act by triggering the down-regulation of the BLM-A5 transporter. As we cannot entirely exclude this latter possibility, three indications seem to support the former one. First, BLM-A5 used in the present study contains a region that is similar to the chemical composition of polyamines (Figure 1A), secondly, it is striking that the kinase-deficient mutants ptk2Δ and sky1Δ, which are resistant to SPM due to defects in regulating the activity of the plasma membrane polyamine transporter, were also resistant to BLM (Figure 8D) [36,46] and, finally, a genome-wide screen re-isolated ptk2Δ and sky1Δ as BLM-resistant mutants [47]. These facts suggest that the plasma membrane polyamine transporter might be involved in transporting BLM into the cells. However, there is one caveat, i.e. during short exposure, SPM does not block F-BLM uptake into the cell. While the reason for this is not obvious, it is noteworthy that SPM added to solid YPD media can effectively protect cells from toxicity caused by BLM-A5. Whether BLM-A5 and SPM indeed use a common transporter cannot be tested at the moment, as the plasma membrane polyamine transporter(s) has not yet been found, although Agp2 is being examined for possible involvement [39]. We note, however, that several vacuolar polyamine transporters have been discovered in yeast, but none of these played a role in regulating BLM resistance, as single mutants deficient in these transporters show parental resistance to BLM [39,53].
After F-BLM uptake, the drug begins to concentrate into the vacuoles of the parent strain within 60 min. Deletion of the END3 gene encoding a protein required for the initial step of endocytosis causes dispersal of the drug into the cytoplasm leading to rapid cell death [43]. On the basis of our findings, we propose a model (Figure 9) whereby the plasma membrane transporter Agp2, and possibly others, delivers BLM-A5 into the cell. The drug, which may be internalized with the membrane transporter, is then channelled through the endocytic pathway to the vacuoles where it is sequestered for detoxification [43,54]. Consistent with this model, we identified at least 40 genes, involved in vacuolar and vesicular transport, which when deleted cause BLM hypersensitivity [47]. Moreover, it has been shown that overexpression of the genes VPS3, VPS8 and PEP7, which encode proteins that function in vesicular trafficking between the endosomes and the vacuoles, were capable of protecting yeast cells against BLM lethality [55]. Thus leakage of BLM either during transport to the vacuole, or from the vacuole, may be responsible for the genotoxic effects of the drug. In this model, we propose that a fraction of the drug also reaches the vacuoles by the passive process of fluid-phase endocytosis, where internalization of molecules occurred without formation of a ligand [43]. If so, this might explain the presence of some drug in the vacuoles of the ptk2Δ mutant, although this could also be due to a leaky transport process caused by the absence of Ptk2.
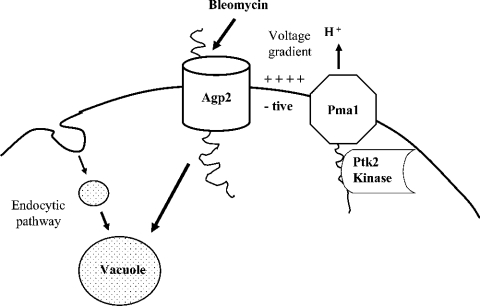
Figure 9. Model illustrating the transport and detoxification pathway of BLM.
The drug enters the cell through a BLM transporter. The activity of the transporter might be influenced by the kinases Ptk2 and Sky1, which are known to regulate the plasma membrane polyamine transporter (see text). After uptake, BLM is channelled to the vacuole for detoxification. Interruption of the endocytic pathway to the vacuoles resulted in mutants that are hypersensitive to BLM.
In short, in constructing a fluorescently labelled form of BLM-A5, our study provides a useful tool to investigate further the transport mechanism of BLM in yeast, as well as in mammalian cells. We believe that a similar BLM transport machinery may also exist in human cells, as preliminary results reveal that F-BLM can be transported into HeLa cells and that the drug accumulates in the lysosomes (results not shown). Moreover, a dominant-negative form of the human SRPK1, a counterpart of yeast Sky1 kinase, was recently shown to confer on HeLa and Chinese-hamster ovary cells resistance specifically to BLM [56]. If indeed a BLM transporter exists in mammalian cells, F-BLM could also be useful to identify among BLM-resistant tumours those that are defective in the drug uptake. So far, there is no evidence yet that BLM resistance might be linked to either multidrug efflux pumps or multidrug resistance-associated proteins [2,57], underscoring the importance of isolating and characterizing the human BLM transporter.
Acknowledgments
We thank members of the laboratory and Dr E. Drobetsky, Dr R. Poulin and Dr S. Labbe for useful discussions. We also thank M.-H. Crête for technical assistance with the HPLC. This study was supported by a grant to D.R. from the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society. D.R. was supported by a career scientist award from the NCIC, and currently by a senior fellowship from the Fonds de la Recherche en Sante du Quebec. M.A. received a fellowship from Maisonneuve-Rosemont hospital.
References
- 1.Hecht S. M. Bleomycin: new perspectives on the mechanism of action. J. Nat. Prod. 2000;63:158–168. doi: 10.1021/np990549f. [DOI] [PubMed] [Google Scholar]
- 2.Ramotar D., Wang H. Protective mechanisms against the antitumor agent bleomycin: lessons from Saccharomyces cerevisiae. Curr. Genet. 2003;43:213–224. doi: 10.1007/s00294-003-0396-1. [DOI] [PubMed] [Google Scholar]
- 3.Wharam M. D., Phillips T. L., Kane L., Utley J. F. Response of a murine solid tumor to in vivo combined chemotherapy and irradiation. Radiology. 1973;109:451–455. doi: 10.1148/109.2.451. [DOI] [PubMed] [Google Scholar]
- 4.Umezawa H. Natural and artificial bleomycins: chemistry and antitumor activities. Pure Appl. Chem. 1971;28:665–680. doi: 10.1351/pac197128040665. [DOI] [PubMed] [Google Scholar]
- 5.Jani J. P., Mistry J. S., Morris G., Davies P., Lazo J. S., Sebti S. M. In vivo circumvention of human colon carcinoma resistance to bleomycin. Cancer Res. 1992;52:2931–2937. [PubMed] [Google Scholar]
- 6.Einhorn L. H. Curing metastatic testicular cancer. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4592–4595. doi: 10.1073/pnas.072067999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Povirk L. F., Austin M. J. Genotoxicity of bleomycin. Mutat. Res. 1991;257:127–143. doi: 10.1016/0165-1110(91)90022-n. [DOI] [PubMed] [Google Scholar]
- 8.Lazo J. S., Sebti S. M., Schellens J. H. Bleomycin. Cancer Chemother. Biol. Response Modif. 1996;16:39–47. [PubMed] [Google Scholar]
- 9.Pei Z., Calmels T. P., Creutz C. E., Sebti S. M. Yeast cysteine proteinase gene ycp1 induces resistance to bleomycin in mammalian cells. Mol. Pharmacol. 1995;48:676–681. [PubMed] [Google Scholar]
- 10.Robertson K. A., Bullock H. A., Xu Y., Tritt R., Zimmerman E., Ulbright T. M., Foster R. S., Einhorn L. H., Kelley M. R. Altered expression of Ape1/ref-1 in germ cell tumors and overexpression in NT2 cells confers resistance to bleomycin and radiation. Cancer Res. 2001;61:2220–2225. [PubMed] [Google Scholar]
- 11.Burger R. M. Cleavage of nucleic acids by bleomycin. Chem. Rev. 1998;98:1153–1169. doi: 10.1021/cr960438a. [DOI] [PubMed] [Google Scholar]
- 12.Tounekti O., Kenani A., Foray N., Orlowski S., Mir L. M. The ratio of single- to double-strand DNA breaks and their absolute values determine cell death pathway. Br. J. Cancer. 2001;84:1272–1279. doi: 10.1054/bjoc.2001.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger R. M., Peisach J., Blumberg W. E., Horwitz S. B. Iron-bleomycin interactions with oxygen and oxygen analogues. Effects on spectra and drug activity. J. Biol. Chem. 1979;254:10906–10912. [PubMed] [Google Scholar]
- 14.Worth L., Jr, Frank B. L., Christner D. F., Absalon M. J., Stubbe J., Kozarich J. W. Isotope effects on the cleavage of DNA by bleomycin: mechanism and modulation. Biochemistry. 1993;32:2601–2609. doi: 10.1021/bi00061a018. [DOI] [PubMed] [Google Scholar]
- 15.Povirk L. F., Wubter W., Kohnlein W., Hutchinson F. DNA double-strand breaks and alkali-labile bonds produced by bleomycin. Nucleic Acids Res. 1977;4:3573–3580. doi: 10.1093/nar/4.10.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Absalon M. J., Wu W., Kozarich J. W., Stubbe J. Sequence-specific double-strand cleavage of DNA by Fe-bleomycin. 2. Mechanism and dynamics. Biochemistry. 1995;34:2076–2086. doi: 10.1021/bi00006a030. [DOI] [PubMed] [Google Scholar]
- 17.Steighner R. J., Povirk L. F. Bleomycin-induced DNA lesions at mutational hot spots: implications for the mechanism of double-strand cleavage. Proc. Natl. Acad. Sci. U.S.A. 1990;87:8350–8354. doi: 10.1073/pnas.87.21.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett R. A., Swerdlow P. S., Povirk L. F. Spontaneous cleavage of bleomycin-induced abasic sites in chromatin and their mutagenicity in mammalian shuttle vectors. Biochemistry. 1993;32:3188–3195. doi: 10.1021/bi00063a034. [DOI] [PubMed] [Google Scholar]
- 19.Dar M. E., Jorgensen T. J. Deletions at short direct repeats and base substitutions are characteristic mutations for bleomycin-induced double- and single-strand breaks, respectively, in a human shuttle vector system. Nucleic Acids Res. 1995;23:3224–3230. doi: 10.1093/nar/23.16.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steighner R. J., Povirk L. F. Effect of in vitro cleavage of apurinic/apyrimidinic sites on bleomycin-induced mutagenesis of repackaged lambda phage. Mutat. Res. 1990;240:93–100. doi: 10.1016/0165-1218(90)90012-q. [DOI] [PubMed] [Google Scholar]
- 21.Tates A. D., van Dam F. J., Natarajan A. T., Zwinderman A. H., Osanto S. Frequencies of HPRT mutants and micronuclei in lymphocytes of cancer patients under chemotherapy: a prospective study. Mutat. Res. 1994;307:293–306. doi: 10.1016/0027-5107(94)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Coleman S. T., Tseng E., Moye-Rowley W. S. Saccharomyces cerevisiae basic region-leucine zipper protein regulatory networks converge at the ATR1 structural gene. J. Biol. Chem. 1997;272:23224–23230. doi: 10.1074/jbc.272.37.23224. [DOI] [PubMed] [Google Scholar]
- 23.Decottignies A., Lambert L., Catty P., Degand H., Epping E. A., Moye-Rowley W. S., Balzi E., Goffeau A. Identification and characterization of SNQ2, a new multidrug ATP binding cassette transporter of the yeast plasma membrane. J. Biol. Chem. 1995;270:18150–18157. doi: 10.1074/jbc.270.30.18150. [DOI] [PubMed] [Google Scholar]
- 24.Katzmann D. J., Epping E. A., Moye-Rowley W. S. Mutational disruption of plasma membrane trafficking of Saccharomyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein. Mol. Cell. Biol. 1999;19:2998–3009. doi: 10.1128/mcb.19.4.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masson J. Y., Ramotar D. The Saccharomyces cerevisiae IMP2 gene encodes a transcriptional activator that mediates protection against DNA damage caused by bleomycin and other oxidants. Mol. Cell. Biol. 1996;16:2091–2100. doi: 10.1128/mcb.16.5.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guthrie C., Fink G. R. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:3–37. [PubMed] [Google Scholar]
- 27.Sherman F., Fink G., Hicks J. Plainview, NY: Cold Spring Harbor Laboratory; 1983. Laboratory Course Manual for Methods in Yeast Genetics. [Google Scholar]
- 28.Gietz D., St. Jean A., Woods R. A., Schiestl R. H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., Hieter P., Boeke J. D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Dulic V., Egerton M., Elguindi I., Raths S., Singer B., Riezman H. Yeast endocytosis assays. Methods Enzymol. 1991;194:697–710. doi: 10.1016/0076-6879(91)94051-d. [DOI] [PubMed] [Google Scholar]
- 31.Leduc A., He C. H., Ramotar D. Disruption of the Saccharomyces cerevisiae cell-wall pathway gene SLG1 causes hypersensitivity to the antitumor drug bleomycin. Mol. Gen. Genomics. 2003;269:78–89. doi: 10.1007/s00438-003-0812-8. [DOI] [PubMed] [Google Scholar]
- 32.Jilani A., Vongsamphanh R., Leduc A., Gros L., Saparbaev M., Ramotar D. Characterization of two independent amino acid substitutions that disrupt the DNA repair functions of the yeast Apn1. Biochemistry. 2003;42:6436–6445. doi: 10.1021/bi034163m. [DOI] [PubMed] [Google Scholar]
- 33.Mistry J. S., Jani J. P., Morris G., Mujumdar R. B., Reynolds I. J., Sebti S. M., Lazo J. S. Synthesis and evaluation of fluoromycin: a novel fluorescence-labeled derivative of talisomycin S10b. Cancer Res. 1992;52:709–718. [PubMed] [Google Scholar]
- 34.Sznaidman M. L., Hecht S. M. Studies on the total synthesis of tallysomycin. Synthesis of the threonylbithiazole moiety containing a structurally unique glycosylcarbinolamide. Org. Lett. 2001;3:2811–2814. doi: 10.1021/ol0101178. [DOI] [PubMed] [Google Scholar]
- 35.Vance J. R., Wilson T. E. Repair of DNA strand breaks by the overlapping functions of lesion-specific and non-lesion-specific DNA 3′ phosphatases. Mol. Cell. Biol. 2001;21:7191–7198. doi: 10.1128/MCB.21.21.7191-7198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaouass M., Audette M., Ramotar D., Verma S., De Montigny D., Gamache I., Torossian K., Poulin R. The STK2 gene, which encodes a putative Ser/Thr protein kinase, is required for high-affinity spermidine transport in Saccharomyces cerevisiae. Mol. Cell. Biol. 1997;17:2994–3004. doi: 10.1128/mcb.17.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goossens A., de La Fuente N., Forment J., Serrano R., Portillo F. Regulation of yeast H(+)-ATPase by protein kinases belonging to a family dedicated to activation of plasma membrane transporters. Mol. Cell. Biol. 2000;20:7654–7661. doi: 10.1128/mcb.20.20.7654-7661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt A., Beck T., Koller A., Kunz J., Hall M. N. The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 1998;17:6924–6931. doi: 10.1093/emboj/17.23.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soulet D., Covassin L., Kaouass M., Charest-Gaudreault R., Audette M., Poulin R., Haimeur A., Guimond C., Pilote S., Mukhopadhyay R., et al. Role of endocytosis in the internalization of spermidine-C(2)-BODIPY, a highly fluorescent probe of polyamine transport. Biochem. J. 2002;367:347–357. doi: 10.1042/BJ20020764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egner R., Kuchler K. The yeast multidrug transporter Pdr5 of the plasma membrane is ubiquitinated prior to endocytosis and degradation in the vacuole. FEBS Lett. 1996;378:177–181. doi: 10.1016/0014-5793(95)01450-0. [DOI] [PubMed] [Google Scholar]
- 41.Gitan R. S., Luo H., Rodgers J., Broderius M., Eide D. Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. J. Biol. Chem. 1998;273:28617–28624. doi: 10.1074/jbc.273.44.28617. [DOI] [PubMed] [Google Scholar]
- 42.Beck T., Schmidt A., Hall M. N. Starvation induces vacuolar targeting and degradation of the tryptophan permease in yeast. J. Cell Biol. 1999;146:1227–1238. doi: 10.1083/jcb.146.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munn A. L. Molecular requirements for the internalisation step of endocytosis: insights from yeast. Biochim. Biophys. Acta. 2001;1535:236–257. doi: 10.1016/s0925-4439(01)00028-x. [DOI] [PubMed] [Google Scholar]
- 44.Kaouass M., Gamache I., Ramotar D., Audette M., Poulin R. The spermidine transport system is regulated by ligand inactivation, endocytosis, and by the Npr1p Ser/Thr protein kinase in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:2109–2117. doi: 10.1074/jbc.273.4.2109. [DOI] [PubMed] [Google Scholar]
- 45.van Roermund C. W., Hettema E. H., van den Berg M., Tabak H. F., Wanders R. J. Molecular characterization of carnitine-dependent transport of acetyl-CoA from peroxisomes to mitochondria in Saccharomyces cerevisiae and identification of a plasma membrane carnitine transporter, Agp2p. EMBO J. 1999;18:5843–5852. doi: 10.1093/emboj/18.21.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erez O., Kahana C. Screening for modulators of spermine tolerance identifies Sky1, the SR protein kinase of Saccharomyces cerevisiae, as a regulator of polyamine transport and ion homeostasis. Mol. Cell. Biol. 2001;21:175–184. doi: 10.1128/MCB.21.1.175-184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aouida M., Page N., Leduc A., Peter M., Ramotar D. A genome-wide screen in Saccharomyces cerevisiae reveals altered transport as a mechanism of resistance to the anticancer drug bleomycin. Cancer Res. 2004;64:1102–1109. doi: 10.1158/0008-5472.can-03-2729. [DOI] [PubMed] [Google Scholar]
- 48.Pron G., Belehradek J., Jr, Mir L. M. Identification of a plasma membrane protein that specifically binds bleomycin. Biochem. Biophys. Res. Commun. 1993;194:333–337. doi: 10.1006/bbrc.1993.1824. [DOI] [PubMed] [Google Scholar]
- 49.Pron G., Mahrour N., Orlowski S., Tounekti O., Poddevin B., Belehradek J., Jr, Mir L. M. Internalisation of the bleomycin molecules responsible for bleomycin toxicity: a receptor-mediated endocytosis mechanism. Biochem. Pharmacol. 1999;57:45–56. doi: 10.1016/s0006-2952(98)00282-2. [DOI] [PubMed] [Google Scholar]
- 50.Aouida M., Tounekti O., Belhadj O., Mir L. M. Comparative roles of the cell wall and cell membrane in limiting uptake of xenobiotic molecules by Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2003;47:2012–2014. doi: 10.1128/AAC.47.6.2012-2014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aouida M., Tounekti O., Leduc A., Belhadj O., Mir L., Ramotar D. Isolation and characterization of Saccharomyces cerevisiae mutants with enhanced resistance to the anticancer drug bleomycin. Curr. Genet. 2004;45:265–272. doi: 10.1007/s00294-004-0492-x. [DOI] [PubMed] [Google Scholar]
- 52.Evans Febres D., Pramanik A., Caton M., Doherty K., McKoy J., Garcia E., Alejo W., Wood Moore C. The novel BLM3 gene encodes a protein that protects against lethal effects of oxidative damage. Cell. Mol. Biol. (Noisy-le-grand) 2001;47:1149–1162. [PubMed] [Google Scholar]
- 53.Tomitori H., Kashiwagi K., Asakawa T., Kakinuma Y., Michael A. J., Igarashi K. Multiple polyamine transport systems on the vacuolar membrane in yeast. Biochem. J. 2001;353:681–688. doi: 10.1042/0264-6021:3530681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghosh M., Shen J., Rosen B. P. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5001–5006. doi: 10.1073/pnas.96.9.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez M., Pramanik A., Moto-Ndje S., Moore C. W. Overexpression of genes involved in vesicular trafficking to the vacuole defends against lethal effects of oxidative damage. Cell. Mol. Biol. (Noisy-le-grand) 2003;49:1025–1035. [PubMed] [Google Scholar]
- 56.Sanz G., Mir L., Jacquemin-Sablon A. Bleomycin resistance in mammalian cells expressing a genetic suppressor element derived from the SRPK1 gene. Cancer Res. 2002;62:4453–4458. [PubMed] [Google Scholar]
- 57.Berger W., Elbling L., Hauptmann E., Micksche M. Expression of the multidrug resistance-associated protein (MRP) and chemoresistance of human non-small-cell lung cancer cells. Int. J. Cancer. 1997;73:84–93. doi: 10.1002/(sici)1097-0215(19970926)73:1<84::aid-ijc14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 58.Leitheiser C. J., Rishel M. J., Wu X., Hecht S. M. Solid-phase synthesis of bleomycin group antibiotics. Elaboration of deglycobleomycin A(5) Org. Lett. 2000;2:3397–3399. doi: 10.1021/ol0002469. [DOI] [PubMed] [Google Scholar]