Abstract
Recently, research has investigated the role of the ruminant native microbiome, and the role microbes play in methane (CH4) production and mitigation. However, the variation across microbiome studies makes implementing impactful strategies difficult. The first objective of this study is to identify, summarize, compile, and discuss the current literature on CH4 mitigation strategies and how they interact with the native ruminant microbiome. The second objective is to perform a meta-analysis on the identified16S rRNA sequencing data. A literature search using Web of Science, Scopus, AGRIS, and Google Scholar will be implemented. Eligible criteria will be defined using PICO (population, intervention, comparator, and outcomes) elements. Two independent reviewers will be utilized for both the literature search and data compilation. Risk of bias will be assessed using the Cochrane Risk Bias 2.0 tool. Publicly available 16S rRNA amplicon gene sequencing data will be downloaded from NCBI Sequence Read Archive, European Nucleotide Archive or similar database using appropriate extraction methods. Data processing will be performed using QIIME2 following a standardized protocol. Meta-analyses will be performed on both alpha and beta diversity as well as taxonomic analyses. Alpha diversity metrics will be tested using a Kruskal-Wallis test with a Benjamini-Hochberg multiple testing correction. Beta diversity will be statistically tested using PERMANOVA testing with multiple test corrections. Hedge’s g standardized mean difference statistic will be used to calculate fixed and random effects model estimates using a 95% confidence interval. Heterogeneity between studies will be assessed using the I2 statistic. Potential publication bias will be further assessed using Begg’s correlation test and Egger’s regression test. The GRADE approach will be used to assess the certainty of evidence. The following protocol will be used to guide future research and meta-analyses for investigating CH4 mitigation strategies and ruminant microbial ecology. The future work could be used to enhance livestock management techniques for GHG control. This protocol is registered in Open Science Framework (https://osf.io/vt56c) and available in the Systematic Reviews for Animals and Food (https://www.syreaf.org/contact).
Background
Agriculture, forestry, and land use sectors represent 22% of the global anthropogenic greenhouse gas (GHG) emissions with enteric fermentation of methane (CH4) contributing 5% of the total direct contribution to global anthropogenic GHG [1]. Among global agricultural food systems, CH4 emissions from livestock operations accounts for 30–40% of the total global anthropogenic GHG emissions [2]. Enteric fermentation, primarily from beef and dairy cattle, represents 46% of the total livestock generated CH4 emissions [2]. Due to a growing world population, an increase in demand for food products that come from ruminant livestock could be seen (i.e., meat, meat products, dairy products, etc.). An increase in ruminant livestock production could therefore lead to an increase in ruminant based CH4 emissions, resulting in an increase in anthropogenic GHG. Additionally, due to the relatively short atmospheric lifespan of CH4, its mitigation has been suggested as the most promising means to limit climate warming in the short-term [3–5]. To combat the anthropogenic-livestock effects on climate change, CH4 mitigation strategies have been explored via dietary manipulation, rumen manipulation, and animal breeding [6].
Promising enteric CH4 mitigation strategies and techniques include the use of inhibitory compounds such as 3-nitrooxypropanol (3-NOP; [7]) and halogenated bromoform from seaweed [8]; red algae [9]; plant secondary compounds [10, 11], early-life microbiome engineering [12]; supplemental feed to grazing cattle [13–16]; changes to the diet composition [17]; and breeding and genetics programs designed to select for low-emissions animals [18]. Interestingly, researchers have turned their attention to understanding how these mitigation strategies affect ruminant microbial ecology. To date, several studies have investigated how various CH4 mitigation techniques impact the rumen microbiome including inhibitory compounds such as 3-NOP [19] and halogenated bromoform from algae [20, 21], dietary manipulation [22, 23], breeding programs [24, 25], and early-life microbiome manipulation [12].
Currently, there remains a gap in the literature for an updated comprehensive and systemic review and meta-analysis on the effects of CH4 mitigation strategies on the ruminant microbiome. One of the reasons for this is the variation between microbiome datasets makes comparisons between studies difficult. This variation stems from differences in sampling procedures, a lack of consensus in computational methods, and differences in preprocessing methods [26, 27]. However, compiling multiple 16S rRNA amplicon sequencing datasets and analyzing them together could elucidate key large-scale patterns and results. Therefore, the results from the proposed study are crucial to increasing our understanding of how current CH4 mitigation strategies influence the rumen microbiome and how the native microbiome could be used for CH4 reduction. Our results could also enhance on-farm guidelines for future management decisions on best practices for reducing livestock GHG emissions.
Our overall objective is to address this knowledge gap by generating a systematic review and meta-analysis identifying CH4 mitigation strategies that impact the rumen microbiome. To accomplish this, we present this protocol that will accomplish two preliminary aims: 1) to outline how we will identify, compile, summarize, and discuss the current literature on CH4 mitigation strategies and their effects on the native rumen microbiome; and 2) to detail how we will perform a meta-analysis on the selected studies from the literature curation. The future study will address the research question of how CH4 mitigation strategies influence the rumen microbiome of post-weaned cattle.
Methods
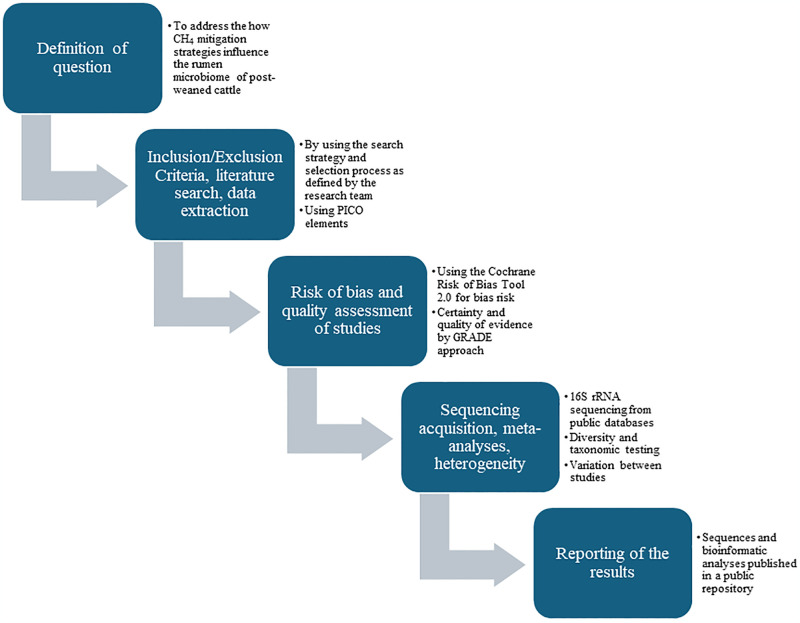
Reporting of this protocol is in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) statement [28]. The PRISMA-P checklist is used to guide researchers while writing the protocol by providing the essential and minimum component requirements. The PRISMA-P checklist is included in S1 Table. The protocol is registered in Open Science Framework (https://osf.io/vt56c) and available in the Systematic Reviews for Animals and Food (https://www.syreaf.org/contact). The proposed systematic review and the meta-analysis will follow the recommendations of the Cochrane Collaboration Handbook for Systematic Reviews [29]. The Cochrane method is a transparent and reproducible method for scientific investigation. The future meta-analysis will correspond to the steps outlined in Fig 1.
Fig 1. Above is a brief flowchart of the steps to be performed in the future systematic review and meta-analysis study.
Eligibility criteria
The eligibility criteria were defined using Population, Intervention, Comparison, Outcome Study Design (PICO) elements (Table 1).
Table 1. The eligibility criteria as defined by the PICO elements.
| Category | Inclusion | Exclusion |
|---|---|---|
| Population | Post-weaned cattle (> 6 mo.) | Pre-weaned calves |
| Intervention | Dietary and rumen manipulation CH4 mitigation strategies | Animal and feed management strategies |
| Comparator | Studies demonstrating significant changes in microbial ecology as well as significant CH4 reduction between control and treatment groups | Either no difference in microbial composition or no significant CH4 reduction |
| Outcomes | Taxonomic and diversity changes where CH4 reduction is seen | |
| Sequencing Protocol | Earth Microbiome Project Protocols will be prioritized | |
| Sequencing Region | 16S rRNA SSU V4 or V4-V5 |
Study design, characteristics, and population
The design of the systematic review is a thorough literature search for primary research studies wherein 16S rRNA gene amplicon datasets are publicly available in a national database such as the National Center for Biotechnology (NCBI), European Bioinformatics Institute (EBI), or similar [30, 31]. Eligible studies will also be determined based on the CH4 mitigation strategy used with the target strategies being dietary interventions and rumen manipulations as defined by previous research [6]. Our initial search for papers will include all age ranges within post-weaned cattle (six months of age or older) to determine our population and the availability of published studies that meet our inclusion criteria. Pre-weaned cattle will be initially excluded as to not confound the microbiome datasets found within the literature review. If the number of studies found is insufficient, the parameters will be widened to include pre-weaned cattle and the appropriate measures in analysis will be taken accordingly. No restrictions will be placed on breed or sex as we are not comparing microbiomes between different breeds or sex; instead, we aim to address how CH4 mitigation strategies alter the rumen microbiome regardless of these parameters. Only studies published in English will be included.
Additionally, studies that meet the above criteria must also meet certain amplicon sequencing method criteria. Eligible studies must adhere to the best of their abilities to the criteria within the Earth Microbiome Project protocol [32]. These criteria include but are not limited to 16S rRNA amplicon sequencing that use primers that target the V4 or V4-V5 region of the 16S small subunit rRNA; using Illumina sequencing technology; and reports amplicons at least 390 base-pairs in length.
Intervention, comparison groups, and outcomes
All dietary and rumen manipulation CH4 mitigation strategies will be included in the initial search for the intervention group. Eligible studies must have investigated the effects of CH4 mitigation strategies on the native rumen microbiome with no restriction for the method of inhibition, dose, or concentration. No limits will be placed on the length of the study. Our comparison group will include studies that have a significant change in microbial ecology from the control group. Additionally, studies must include outcomes where significant CH4 reduction was seen between control and treatment groups as well as microbial analyses. The main outcomes will include alpha and beta diversity changes as well as taxonomic profile differences between studies where CH4 reduction was seen.
Search strategy
Our search strategy will include structured terms based on our PICO elements. The primary bibliographic databases to be used will include Web of Science, Scopus, International Information Systems for Agricultural Sciences and Technology (AGRIS), and Google Scholar. A preliminary literature search was conducted in October 2023 and the results of this search are described in S1 File. The preliminary search allowed us to refine our search terms which utilize the Boolean operators “AND” and “OR” to connect the search terms. The strategy for the preliminary search was “ruminant OR rumen OR ruminants OR cattle AND methane AND inhibition AND microbiome”. The search strategy to be used in the literature search stage of the study will use the above strategy with refinements used as necessary. These refinements might include but are not limited to the use of synonyms to the keywords, the use of various spellings of keywords (i.e., British versus American spelling in Standard English), and the addition of other keywords not yet listed.
Selection and screening process
Two independent reviewers (ANF and ADB) will be responsible for carrying out the literature screening process. Each reviewer will independently screen the above-mentioned databases using the defined search strategy in two stages. The first stage will include screening the titles and abstracts of identified articles for descriptions detailed in the PICO elements. The second stage will examine the text of the studies for the PICO elements and any study not meeting the inclusion criteria will be excluded and cataloged at this stage. If at any point there is a discrepancy between the two independent reviewers during the two-staged literature screening process, a third independent reviewer will be responsible for determination of study eligibility.
Data extraction and management
The selected eligible studies will be cataloged, and metadata will be managed using Microsoft Excel. Data extraction will be done by one reviewer and a second reviewer will be responsible for assuring data accuracy and completeness of the data collection to avoid measurement bias. The study-level data that will be extracted will include the article title, the authors’ names, the article DOI, year of publication, journal title, geographic location, the bioproject database, the bioproject number, and the study type (i.e., in-vitro v. in-vivo). Population-level data will include animal species, animal breed, animal sex, herd size, housing or pen information, feed type, methane collection system, and if applicable the in-vitro system used. Intervention and comparator level data will include the description of the CH4 intervention used, the concentration and/or dosage, and the length of the trial or study. Finally, outcome level data will include the hypervariable region(s) sequenced, primer sets, the sequencing platform, sequencing geographic location, sequencing analysis pipeline and version number, and the number of reads sequenced in the study.
Risk of bias and quality assessment
Quality and bias risk will be assessed by one reviewer using the Cochrane Risk of Bias Tool 2.0 (RoB 2) with adaptations to the signal questioning to fit animal science studies as previously described [33, 34]. For example, questions that consider if participants are aware of their inclusion of the study will be either dropped or amended as the population of interest are ruminant livestock animals. A precedent has been described for this amendment to RoB 2 signaling questioning in previous livestock studies [34, 35]. The five domains of bias to be evaluated include the randomization process, deviations from intended interventions, missing outcome data, measurement of outcome data, and the selection of reported results. A preliminary analysis will be conducted to ensure that the criteria can be applied appropriately and consistently. Once the preliminary analysis is completed, risk assessment will be conducted by one reviewer with a second reviewer responsible for analyzing the results of the assessment for criteria application consistency. If needed, a third reviewer will be consulted to resolve any discrepancies between the first two reviewers in the process. The evaluation will be scored according to the criteria established in the RoB 2 worksheet (S2 File) and will address each domain based on “low risk”, “high risk” or “some concern for risk” of bias in each study. The overall evaluation of the article will be scored according to the following points system: between 7–10 points means there is low risk of bias; between 3–6 points indicates there is some concern for risk of bias; and below 2 points is considered high risk for bias. All articles will then be ranked according to their risk of bias evaluation score, and the level of bias will influence the degree of importance for each study in evidence synthesis.
Data synthesis and meta-analysis
The certainty and quality of evidence will be assessed by two independent reviewers (ANF and ADB) using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach [36, 37]. GRADE is used to evaluate the certainty of evidence across the PICO elements. The parameters used in the GRADE method include risk of bias, imprecision, inconsistency, indirectness, and publication bias. Given any discrepancies between the two independent reviewers regarding the results of the GRADE assessment, a third reviewer will be consulted for resolution to evidence evaluation.
If there are sufficient studies (i.e., more than three) having similar definitions of the PICO elements, meta-analyses will be conducted. If in the case that pre-weaned cattle were included due to insufficient studies of post-weaned cattle, meta-analyses will be performed adjusting for age within the microbial analysis. Publicly available 16S rRNA amplicon sequencing data will be extracted using NCBI’s Sequence Read Archive using SRA Toolkit [38], QIIME2’s fondue plugin [39], or other extraction methods as necessary. Data processing will be performed using a modified protocol adapted from previously published methods [26, 40]. Briefly, the catalogued 16S rRNA gene datasets will be initially processed using a standardized protocol in QIIME2 version 2023.9 [41]. Demultiplexed sequences will undergo quality control using the DADA2 plugin [42] or DEMUX plugin [41] depending on original sequence quality. The resulting feature table will be filtered for mitochondria and chloroplasts. Taxonomic analyses will be assessed at the genus level by first assigning taxonomy to the amplicon sequence variants (ASVs) using the pre-trained SILVA 138 99% database via the q2-feature-classifier plugin [43, 44]. Phylogenetic diversity analyses will be conducted by creating a phylogenetic insertion tree using the q2-fragment-insertion plugin, rarefying the sequences to an acceptable level, and finally using the q2-core-diversity plugin for alpha and beta diversity analyses [41, 45, 46].
For diversity testing, Shannon’s Diversity Index [47], Faith’s Phylogenetic Diversity [48], and richness [49] will be assessed for alpha diversity and tested statistically using the Kruskal-Wallis test with a Benjamini-Hochberg multiple testing correction [50]. Meta-analyses of the above alpha diversity metrics will be performed in RStudio using the packages meta and metafor following previously published protocols [40, 51]. Hedge’s g standardized mean difference statistic will be used to calculate fixed (i.e., CH4 reduction) and random effects (i.e., difference in mitigation strategy) model estimates using a 95% confidence interval [52]. Heterogeneity between studies will be assessed using the I2 statistic used for calculating the percentage of variation reflecting true heterogeneity [53]. Heterogeneity varies between 0–100% which will be interpreted as follows: 0%– 39% could not be important, 40%– 59% could represent moderate heterogeneity, 60%– 90% could indicate substantial heterogeneity, and anything greater could suggest considerable heterogeneity [29, 52]. Potential publication bias will be further assessed using Begg’s correlation test [54] and Egger’s regression test [55]. Potential bias will be indicated if the number of studies is greater than 10 with a P-value < 0.1. For beta diversity, both weighted and unweighted UniFrac distance will be analyzed and statistically tested using PERMANOVA testing with multiple test corrections [56, 57]. All raw sequencing data used within the future study will be published to EBI, a publicly available database, using the QIITA platform [58]. The data and R scripts used for statistical analyses will be presented and described within the future manuscript as well as deposited into a GitHub repository.
Discussion
Methane is a potent GHG with a global warming potential that is estimated to be 28-times higher than CO2 per unit mass on a 100-year time horizon and 82 times higher on a 20-year time horizon [59, 60]. Ruminants in agriculture, primarily dairy and beef cattle, contribute to anthropogenic GHG sources and therefore, measures have been taken to address enteric CH4 emissions. Recently, research has reported that manipulation of the ruminant microbiome could prove a promising avenue for CH4 reductions [12, 19–20, 24, 61]. Unfortunately, utilizing the information from the various microbiome studies has many technical challenges due to the variation in methodologies employed in microbial ecological studies [26, 27]. One potential work around is compiling the data from the various studies and re-analyzing the data in a more cohesive manner. Therefore, the future systematic review and meta-analysis will summarize and compile data investigating the effects of CH4 mitigation strategies on the rumen microbiome as well as re-analyze the compiled data from 16S rRNA amplicon sequencing studies to assess the abilities of CH4 mitigation strategies to manipulate the rumen microbiome. The future results from the proposed study could help both researchers and producers formulate more streamlined microbiome engineering protocols for CH4 reduction.
The proposed review and meta-analysis will have several strengths in that the study will follow guidelines previously reported for systematic reviews and meta-analyses studies in animal science and veterinary medicine [33, 62, 63]. Additionally, the members of the study will comprise scientists from various research backgrounds in animal science including microbiology, ruminant nutrition, food and meat safety, and agricultural engineering. The diverse nature of the research team will allow for a more robust systemic review and methodological approach. Bias will be minimized by using two independent reviewers for literature screening and data compilation. However, the study will be limited due to the various technical aspects of microbiome studies including the use of targeted primers (i.e., 16S primers), the differences in sampling methods, and the availability of sequencing data to the public. Another limitation could be only selecting studies that investigate cattle microbiomes. The data elucidated in the future study might not translate between different ruminant species. However, results could translate between ruminant species given recent evidence that there is a shared and heritable core ruminant microbiome irrespective of species [64]. Still, our preliminary search has yielded papers that meet our requirements and therefore indicate we are likely to have success with this study.
Supporting information
(DOC)
(XLSX)
(DOCX)
Data Availability
Deidentified research data will be made publicly available when the study is completed and published.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Dhakal S., Minx J.C., Toth F.L., Abdel-Aziz A., Figueroa Meza M.J., Hubacek K., et al., 2022: Emissions Trends and Drivers. In IPCC, 2022: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Shukla P.R., Skea J., Slade R., Al Khourdajie A., van Diemen R., McCollum D., Pathak M., Some S., Vyas P., Fradera R., Belkacemi M., Hasija A., Lisboa G., Luz S., Malley J., (eds.)]. Cambridge University Press, Cambridge, UK and New York, NY, USA. [Google Scholar]
- 2.FAO. 2023. Pathways towards lower emissions—A global assessment of the greenhouse gas emissions and mitigation options from livestock agrifood systems. Rome. 10.4060/cc9029en [DOI]
- 3.Ocko I.B., Sun T., Shindell D., Oppenheimer M., Hristov A.N., Pacala S.W., et al. Acting rapidly to deploy readily available methane mitigation measures by sector can immediately slow global warming. Environ. Res. Lett. 2021. 16(054042). doi: 10.1088/1748-9326/abf9c8 [DOI] [Google Scholar]
- 4.Beck M.R., Thompson L.R., Campbell T.N., Stackhouse-Lawson K.A. and Archibeque S.L. Implied climate warming contributions of enteric methane emissions are dependent on the estimate source and accounting methodology. Appl. Anim. Sci. 2022. 38. 639–647. doi: 10.15232/aas.2022-02344 [DOI] [Google Scholar]
- 5.Beck M.R., Thompson L.R., Rowntree J.E., Thompson T.N., Koziel J.A., Place S.E. et al. U.S. manure methane emissions represent a greater contributor to implied climate warming than enteric methane emissions using the global warming potential methodology. Front. Sust. Food Sys. 2023. 7(1209541). doi: 10.3389/fsufs.2023.1209541 [DOI] [Google Scholar]
- 6.Arndt C., Hristov A.N., Price W.J., McClelland S.C., Pelaez A.M., Cueva S.F., et al. Full adoption of the most effective strategies to mitigate methane emissions by ruminants can help meet the 1.5°C target by 2030 but not 2050. Proc. Natl. Acad. Sci. USA. 2022. 119(e2111294119). doi: 10.1073/pnas.2111294119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitta D.W., Indugu N., Melgar A., Hristov A., Challa K., Vecchiarelli B., et al. The effect of 3-nitrooxypropanol, a potent methane inhibitor, on ruminal microbial gene expression profiles in dairy cows. Microbiome. 2022. 10(146). doi: 10.1186/s40168-022-01341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roque B.M., Salwen J.K., Kinley R. and Kebreab E. Inclusion of Asparagopsis armata in lactating dairy cows diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 2019a. 234. 132–138. doi: 10.1016/j.jclepro.2019.06.193 [DOI] [Google Scholar]
- 9.Narvaez-Izquiedo J., De Ka Hoz J.F., Kannan G. and Bohorquez-Herrera J. Use of macroalgae as a nutritional supplement for sustainable production of ruminants: A systematic review and an insight on the Colombian Caribbean region. Algal Res. 2024. 77:103359. doi: 10.1016/j.algal.2023.103359 [DOI] [Google Scholar]
- 10.Kozlowska M., Cieślak A., Jóźwik A., El-Sherbiny M., Stochmal A., Oleszek W., et al. The effect of total and individual alfalfa saponins on rumen methane production. J. Sci. Food Agric. 2020. 100. 1922–1930. doi: 10.1002/jsfa.10204 [DOI] [PubMed] [Google Scholar]
- 11.Beck M.R. and Gregorini P. Animal design through functional dietary diversity for future productive landscapes. Front. Sustain. Food Sys. 2021. 5(546581). doi: 10.3389/fsufs.2021.546581 [DOI] [Google Scholar]
- 12.Meale S.J., Popova M., Saro C., Martin C., Bernard A., Lagree M., et al. Early life dietary intervention in dairy calves results in a long-term reduction in methane emissions. Sci. Rep. Nat. 2021. 11(3003). doi: 10.1038/s41598-021-82084-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck M.R., Thompson L.R., White J.E., Williams G.D., Place S.E., Moffet C.A., et al. Whole cottonseed supplementation improves performance and reduces methane emission intensity of grazing beef steers. Prof. Anim. Sci. 2018. 34. 339–345. doi: 10.15232/pas.2018-01722 [DOI] [Google Scholar]
- 14.Beck M.R., Thompson L.R., Williams G.D., Place S.E., Gunter S.A. and Reuter R.R. Fat supplements differing in physical form improve performance but divergently influence methane emissions of grazing beef cattle. Anim. Feed Sci. Technol. 2019. 254(114210). doi: 10.1016/j.anifeedsci.2019.114210 [DOI] [Google Scholar]
- 15.Thompson L.R., Beck M.R., Gunter S.A., Williams G.D., Place S.E. and Reuter R.R. An energy supplement with monensin reduces methane emission intensity of stocker cattle grazing winter wheat. Appl. Anim. Sci. 2019. 35. 433–440. doi: 10.15232/aas.2018-01841 [DOI] [Google Scholar]
- 16.Beck M.R., Garrett K., Fleming A.E., Maxwell T.M.R., Greer A.W., Bunt C., et al. Effects of Lactobacillus fermented plant products on dairy cow health, production, and environmental impact. Anim. Feed Sci. Technol. 294(115514). doi: 10.1016/j.anifeedsci.2022.115514 [DOI] [Google Scholar]
- 17.Han X., Li B., Wang S., Chen Y. and Yang Y. Effect of dietary concentrate to forage ratios on ruminal bacterial and anaerobic fungal populations of cashmere goats. Anaerobe. 2019. 59. 118–125. doi: 10.1016/j.anaerobe.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 18.de Haas Y., Pszczola M., Soyeurt H., Wall E. and Lassen J. Invited review: Phenotypes to genetically reduce greenhouse gas emissions in dairying. J. Dairy Sci. 2017. 100. 855–870. doi: 10.3168/jds.2016-11246 [DOI] [PubMed] [Google Scholar]
- 19.Zhang X.M., Gruninger R.J., Alemu A.W., Wang M., Tan Z.L., Kindermann M. et al. 3-Nitrooxyproponal supplementation had little effect on fiber degradation and microbial colonization of forage particles when evaluated using the in situ ruminal incubation technique. J. Dairy Sci. 2020. 103(10). 8986–8997. doi: 10.3168/jds.2019-18077 [DOI] [PubMed] [Google Scholar]
- 20.Roque B.R., Brooke C.G., Ladau J., Polley T., Marsh L.J., Najafi N., et al. Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Ani. Microbiome. 2019b. 1(3). doi: 10.1186/s42523-019-0004-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizrahi I., Wallace R.J. and Moraïs S. The rumen microbiome: balancing food security and environmental impacts. Nat. Rev. 2021. 19. doi: 10.1038/s41579-021-00543-6 [DOI] [PubMed] [Google Scholar]
- 22.Snelling T.J., Auffret M.D., Duthie C.-A., Stewart R.D., Watson M., Dewhurst R.J., et al. Temporal stability of the rumen microbiota in beef cattle, and response to diet and supplements. Anim. Microbiome. 2019. 1(16). doi: 10.1186/s42523-019-0018-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H., Wang C., Huasai S. and Chen A. Effects of dietary forage to concentrate ratio on nutrient digestibility, ruminal fermentation and rumen bacterial composition in Angus cows. Sci. Rep. Nat. 2021. 11(17023). doi: 10.1038/s41598-021-96580-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herd R.M., Arthur P.F., Donoghue K.A., Bird S.H., Bird-Gardiner T. and Hegarty R.S. Measures of methane production and their phenotypic relationships with dry matter intake, growth, and body composition traits in beef cattle. J. Anim. Sci. 2014. 92. 5267–5274. doi: 10.2527/jas.2014-8273 [DOI] [PubMed] [Google Scholar]
- 25.Wallace R.J., Rooke J.A., McKain N., Duthie C.-A., Hyslop J.J., Ross D.W., et al. The rumen microbial metagenome associated with high methane production in cattle. BMC Genomics. 2015. 16(839). doi: 10.1186/s12864-015-2032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holman D.B. and Gzyl K.E. A meta-analysis of the bovine gastrointestinal tract microbiota. FEMS Microbiol. Ecol. 2019. 95(fiz072). doi: 10.1093/femsec/fiz072 [DOI] [PubMed] [Google Scholar]
- 27.Lam T.J. and Ye Y. Meta-analysis of microbiome association networks reveal patterns of dysbiosis in diseased microbiomes. Sci. Rep. Nat. 2022. 12(17482). doi: 10.1038/s41598-022-22541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015. 4(1). doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J. et al. Cochrane handbook for systemic reviews of interventions version 6.4 (updated August 2023). Cochrane, 2023. www.training.cochrane.org/handbook. Accessed January 10, 2024. [Google Scholar]
- 30.Baker W., van den Broek A., Camon E., Hingamp P., Sterk P., Stoesser G. et al. The EMBL nucleotide sequence database. Nucl. Acids Res. 2000. 28(1). doi: 10.1093/nar/28.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayers E.W., Bolton E.E., Brister J.R., Canese K., Chan J., Comeau D.C., et al. Database resources of the national center for biotechnology information. Nucl. Acids Res. 2022. 50. doi: 10.1093/nar/gkab1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson L.R., Sanders J.G., McDonald D., Amir A., Ladau J., Locey K.J., et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature. 2017. 551:457–463 doi: 10.1038/nature24621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sterne J.A.C., Savović, Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. 2019. 366(l4898). 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 34.Branco Lopes R., Bernal-Córdoba C., Fausak E.D. and Silva-del-Río N. Effect of prebiotics on growth and health of dairy calves: a protocol for a systematic review and meta-analysis. PLoS ONE. 2021. 16(6). e0253379. doi: 10.1371/journal.pone.0253379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sargeant J.M., Bergevin M.D., Churchill K., Dawkins K., Deb B., Dunn J., et al. The efficacy of antibiotics to control colibacillosis in broiler poultry: a systematic review. Ani. Health Res. Rev. 2019. 20(2). 263–273. doi: 10.1017/S1466252319000264 [DOI] [PubMed] [Google Scholar]
- 36.Guyatt G.H., Oxman A.D., Kunz R., Woodcock J., Brozek J., Helfand M., et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J. Clin. Epidemiol. 2011. 64(12). 1303–1310. doi: 10.1016/j.jclinepi.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 37.Schünemann H. Brożek J., Guyatt G. and Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. BMJ. 2013. 332. 1089–1092. [Google Scholar]
- 38.Leinonen R., Sugawara H. and Shumway M on behalf of the International Nucleotide Sequence Database Collection. The sequence read archive. Nucl. Acids Res. 39. doi: 10.1093/nar/gkq1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziemski M., Adamov A., Kim L., Flörl L. and Bokulich N.A. Reproducible acquisition, management and meta-analysis of nucleotide sequence (meta)data using q2-fondue. Bioinformatics. 2022. 38(22). 5081–5091. doi: 10.1093/bioinformatics/btac639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng X., Zhang Y., Li Y., Wu Q., Wu J., Park S.-K., et al. Meta-analysis of 16S rRNA microbial data identified alterations of the gut microbiota in COVID-19 patients during the acute and recovery phases. BMC Microbiol. 2022. 22(274). doi: 10.1186/s12866-022-02686-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boylen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019. 87. 852–857. doi: 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A. and Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016. 13(5). 81–583. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012. 41(D) 590–596. doi: 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bokulich N., Dillon M., Bolyen E., Kaehler B.D., Huttley G.A. and Caporaso J.G.. Q2-sample-classifier: machine-learning tools for microbiome classification and regression. J. Open Source Soft. 2018. 3(934). doi: 10.21105/joss.00934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsen F.A., Kodner R.B. and Armbrust E.V. pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinform. 2010. 11(538). doi: 10.1186/1471-2105-11-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansen S., McDonald D., Gonzalez A., Navas-Molina J.A., Jiang L., Xu Z.Z., et al. Phylogenetic placement of exact amplicon sequences improves associations with clinical information. mSystems. 2018. 3. e00021–00018. doi: 10.1128/mSystems.00021-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shannon C.L. and Weaver W.. The mathematical theory of communication. Urbana: University of Illinois Press, 1949. Pp. vii, 117 [Google Scholar]
- 48.Faith D.P. Conservation evaluation and phylogenetic diversity. Biol. Cons. 1992. 61. 1–10. doi: 10.1016/0006-3207(92)91201-3 [DOI] [Google Scholar]
- 49.McIntosh R.P. An Index of Diversity and the Relation of Certain Concepts to Diversity. Ecology. 1967. 48(3). 392–404. doi: 10.2307/1932674 [DOI] [Google Scholar]
- 50.Kruskal W.H. and Wallis W.A.. Use of ranks in one-criterion variance analysis. J. American Stat. Associa. 1952. 260. 583–621. doi: 10.1080/01621459.1952.10483441 [DOI] [Google Scholar]
- 51.Balduzzi S., Rücker G. and Schwarzer G. How to perform meta-analysis with R: a practical tutorial. Evid. Based Ment. Heal. 2019. 22(4). 153–160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borenstein M., Hedges L.V., Higgins J.P.T. and Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 2010. 1. 97–111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 53.Higgins J.P.T., Thompson S.G., Deeks J.J. and Altman D.G. Measuring inconsistency in meta-analyses testing for heterogeneity. BMJ. 2003. 327. 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begg C.B. and Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994. 50(4). 1088–1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 55.Egger M., Smith G.D., Schneider M. and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997. 315(7109):629. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lozupone C. and Knight R.. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005. 71. 8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lozupone C.A., Hamady M., Kelley S.T. and Knight R.. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007. 73. 1576–1585. doi: 10.1128/AEM.01996-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez A., Navas-Molina J.A., Kosciolek T., McDonald D., Vázquez-Baeza Y., Ackermann G., et al. Qiita: rapid, web-enabled microbiome meta-analysis. Nat. Met. 2018. 15:796–798. doi: 10.1038/s41592-018-0141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edenhofer O. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press. Cambridge, MA, USA. 2015. Vol. 3. [Google Scholar]
- 60.Carnachan S.M., Bell T.J., Hinkley S.F. and Sims I.M. Polysaccharides from New Zealand native plants: a review of their structure, properties, and potential applications. Plants. 2019. 8(163). doi: 10.3390/plants8060163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinez-Álvaro M., Auffret M.D., Stewart R.D., Dewhurst R.J., Duthie C.-A., Rooke J.A., et al. Identification of complex rumen microbiome interaction within diverse functional niches as mechanisms affecting the variation of methane emissions in bovine. Front. Microbiol. 2020. 11(659). doi: 10.3389/fmicb.2020.00659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sargeant J.M. and O’Connor A.M. Conducting systematic reviews of intervention questions II: Relevance screening, data extraction, assessing risk of bias, presenting the results and interpreting the findings. Zoonoses Pub. Health. 2014. 61. 39–51. doi: 10.1111/zph.12124 [DOI] [PubMed] [Google Scholar]
- 63.O’Connor A.M., Sargeant J.M. and Wang C. Conducting systematic reviews of intervention questions III: Synthesizing data from intervention studies using meta-analysis. Zoonoses Pub. Health. 2014. 61. 52–63. doi: 10.1111/zph.12123 [DOI] [PubMed] [Google Scholar]
- 64.Henderson G., Cox F., Ganesh S., Jonker A., Young W., Global Rumen Census Collaborators et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 5:14567. doi: 10.1038/srep14567 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(XLSX)
(DOCX)
Data Availability Statement
Deidentified research data will be made publicly available when the study is completed and published.