Abstract
Spermidine, spermine and putrescine are essential for mammalian cell growth, and there has been a pervasive effort to synthesize analogues of these polyamines that will disrupt their function and serve as tools to inhibit cell proliferation. Recently, we demonstrated that a number of such polyamine analogues are also capable of inducing the regulatory protein AZ (antizyme). In the present study the incorporation of a few sample analogues [mimics of bis(ethyl)spermine] was shown to be significantly limited by a decrease in the Vmax for the polyamine transport system in response to analogue-induced AZ. This creates an unusual circumstance in which compounds that are being designed for therapeutic use actually inhibit their own incorporation into targeted cells. To explore the impact of this feedback system, cultures of rat hepatoma HTC cells were pre-treated to exhibit either low or high polyamine uptake activity and then exposed to polyamine analogues. As predicted, regardless of initial uptake activity, all cultures eventually achieved the same steady-state levels of the cellular analogue and AZ. Importantly, analogue-induced AZ levels remained elevated with respect to controls even after the native polyamines were reduced by more than 70%. To model the insufficient AZ expression found in certain tumours, GS-CHO (GS Chinese-hamster ovary) cells were transfected to express high levels of exogenic AZI (AZ inhibitor). As anticipated, this clone incorporated significantly higher levels of the polyamine analogues examined. This study reveals a potential limitation in the use of polyamine-based compounds as therapeutics, and strategies are presented to either circumvent or exploit this elegant transport feedback system.
Keywords: antizyme, antizyme inhibitor, bis(ethyl)polyamine, oligoamine, polyamine analogue, polyamine transport
Abbreviations: AZ, antizyme; AZI, AZ inhibitor; BENSPM, bis(ethyl)norspermine; CHO, Chinese-hamster ovary; DFMO, α-difluoromethylornithine; 6H, His6; M34R, Met34→Arg substitution; MGBG, 1,1′-[(methylethanediylidine)-dinitrilo]diguanidine; ODC, ornithine decarboxylase
INTRODUCTION
The polyamines spermidine and spermine, and their diamine precursor, putrescine, are aliphatic cations essential for normal cell physiology and growth. Cancer cells, especially highly metastatic tumours, require elevated polyamine levels and characteristically express abnormally high activity of ODC (ornithine decarboxylase), the initial enzyme in the polyamine biosynthetic pathway [1–6]. This necessity of polyamines for cell growth makes polyamine biosynthetic enzymes attractive targets for potential anticancer drugs. Unfortunately, even very specific inhibitors, such as the ODC inhibitor DFMO (α-difluoromethylornithine; eflornithine), are only mildly effective in inhibiting tumour growth, because mammalian tissues can also obtain needed polyamines by a very effective polyamine transport system [7,8]. Similar to ODC, the activity of polyamine uptake is generally enhanced in rapidly proliferating cells.
Disruption of cellular polyamine function, and thus growth, has been achieved by exposure to a variety of non-functional polyamine analogues, or analogues with modified function [9–12]. Many such polyamine-based compounds were found to be actively incorporated into cells by the polyamine transporter. As this transport system is generally more active in rapidly proliferating cells, this appeared to be an ideal mechanism by which to preferentially incorporate toxic or diagnostic compounds into cancer cells [13,14]. The specificity of the polyamine transporter has proven to be surprisingly permissive, as numerous polyamine-based compounds have been synthesized and most appear to be incorporated into cells using the polyamine transporter. Many of these analogues initiate feedback inhibition of polyamine biosynthetic enzymes, depressing native polyamine levels [15,16]. Some also stimulate polyamine degradative enzymes, causing extreme polyamine starvation [17,18], or interact with critical biomolecules to disrupt normal cell function [19–21]. Several of these analogues have shown specific activity against tumour cells and have been used in clinical trials [22,23]. The differential uptake of polyamines and polyamine analogues into cancer cells may also be useful in tumour imaging [24], and the targeting of boron-containing compounds to tumour cells for boron-neutron-capture therapy [25,26]. In another approach, cytotoxic compounds have been conjugated with polyamines to facilitate their entry into cancer cells, utilizing elevated polyamine transport activity [27–31].
Although up-regulated polyamine transport into cancer cells is a tempting target, the development of useful polyamine-based compounds has been impeded by the absence of information about either the mechanism by which cells incorporate polyamines or its control. Some suggest that cytoplasmic polyamine uptake involves an endocytotic event whereby polyamines bound to cell surface glypicans are internalized into endosomes and subsequently released to the cytoplasm [32,33]. Whatever the precise mechanism, the process is markedly inhibited when normal intracellular polyamine levels are exceeded, preventing accumulation of harmful amounts of these compounds. We and others have demonstrated that this sensitive feedback system is mediated by the same regulatory protein that is responsible for the rapid degradation of ODC [34–36]. As cellular polyamine levels increase, they induce the synthesis of a small regulatory protein called AZ (antizyme) [37]. In addition to inactivating ODC and blocking polyamine synthesis, AZ decreases the activity of the polyamine transporter by an, as yet, unknown mechanism [38,39]. The induction of AZ synthesis by the polyamines involves an unusual, obligatory translational frameshift [40]. Unfortunately, the precise mechanism whereby the polyamines promote this frameshift is not known.
Recently, we reported that the specificity of the polyamine-dependent mechanism of AZ induction, like that of polyamine transport, is surprisingly permissive [41]. After surveying 24 polyamine-like compounds, including spermine and homospermine analogues, pentamines and various oligoamines, we found that almost all stimulated AZ synthesis. It is quite likely that many, and perhaps most, of the polyamine analogues and conjugates that have been created to target the polyamine transport system for pharmacological advantage will also stimulate this feedback response. Although this AZ induction helps explain the polyamine-depressing activity of many of these analogues, it points out a potential problem in the use of such compounds. Analogues that stimulate AZ synthesis will down-regulate the very transport system that they need to enter a cell. Thus cellular uptake of such drugs may not be controlled by exposure level, affinity for the transporter or even the pre-treatment activity of the transport system. Instead, it may be determined solely by the integrity of the polyamine feedback system. In the present study we show that the uptake of representative polyamine analogues is indeed limited by their induction of AZ. Furthermore, we have examined the role of this AZ feedback system in short- and long-term responsiveness to sample polyamine analogues. This analysis reveals new aspects of this potential therapeutic target that should be considered in the design and application of effective polyamine analogues and polyamine-conjugate drugs.
EXPERIMENTAL
Chemicals
[14C]Spermidine was purchased from Amersham Bioscience. Polyamines, aminoguanidine and cycloheximide were purchased from Sigma Chemicals. Mifepristone, hygromycin B and zeocin were purchased from Invitrogen. DFMO was generously provided by the Marion Merrell Dow Research Institute.
Plasmids
The construct pGBD(-T)AZ, which was derived from AZ-1 of rat hepatoma HTC cells, was obtained from G. Judd (Department of Biological Sciences, Northern Illinois University) [42,43]. The mRNA sequence in this construct differs from rat AZ-1 (GenBank® accession number D10706) in the deletion of one base, T-205, thereby eliminating the requirement for the +1 frameshift in its translation. This sequence was further modified by the introduction of an arginine residue in place of methionine at position 34, which eliminates any initiation of translation at this second start site (Sandra Moore, personal communication). AZ-M34R (AZ with a Met34→Arg substitution) was amplified by PCR using PCR Master Mix (Promega) with the forward primer TTC GGT ACC ATG CCG CTT CTT AG and the reverse primer ATT GGG CCC TCA GTC CTC CTC AC (restriction sites are underlined), thus inserting a stop codon before the V5–6H tag sequences (where 6H is the His6 epitope). The pGene expression vector and AZ-M34R insert were digested with KpnI and ApaI restriction enzymes, ligated using Ligafast (Promega) and the sequences confirmed by a capillary fluorescent sequencer, CEQ 8000 (Beckman Coulter).
Mouse AZI (AZ inhibitor) cDNA in pET19b (Novagen) was provided by J. Nilsson (Department of Cell and Molecular Biology, Umea University, Umea, Sweden) [44]. The complete open reading frame was amplified by PCR using the forward primer ACG CAG GTA CCA TGA AAG GAT TTA TTG ACG and the reverse primer TTT TAG GGC CCA GCT TCA GTG GAA. These were digested with KpnI and ApaI respectively and ligated into the expression vector pGene-V5-6H-A (Invitrogen), which had been digested with the same enzymes. This inserted AZI coding sequence is in frame with the C-terminal V5 epitope and 6H tag of this expression vector. The entire sequence was confirmed by sequence analysis.
Cell culture
Rat HTC cells were grown in monolayer and suspension cultures in Swim's 77 medium containing 10% calf serum, as described previously [34]. AZ- and AZI-derived proteins were expressed in CHO (Chinese-hamster ovary) cells using the GeneSwitch™ (Invitrogen) two-component, mifepristone-inducible mammalian expression system, which is noted for very low background expression. Regulated expression requires the target cells to be co-transfected with a regulatory plasmid (pSwitch) and an expression plasmid (pGene/V5-6H), containing the gene of interest. CHO cells that have been permanently transfected with the regulatory plasmid (GS-CHO) were obtained from Invitrogen and maintained in Swim's 77 medium supplemented with 5% foetal and 5% (v/v) calf serum containing 100 μg/ml hygromycin B. Monolayer cultures were maintained in air/3% CO2 at 37 °C. GS-CHO cells were transfected with pGene-AZ-M34R and pGene-AZI-V5-6H using SuperFect transfection reagent (Qiagen), and stably transfected clones, GS-CHO(AZ-M34R) and GS-CHO(AZI–V5–6H) respectively, were selected using 200 μg/ml zeocin. Expression of AZI–V5–6H was demonstrated in clones exposed to 10 nM mifepristone for 4 h by the appearance of a 53 kDa band that reacted with antibody to the V5 epitope (Invitrogen) on Western blots.
In experiments where polyamines were added to cell growth media, 2 mM aminoguanidine was also added to minimize degradation due to amine oxidases of calf serum. Previous studies have shown that the bis(ethyl)polyamine analogues used in this study are effective for at least 6 days, even in medium containing calf serum [16].
Synthetic polyamine analogues
The ability of spermine analogues symmetrically ethylated on their primary amino residues to be transported into cells and induce AZ has been reported previously [41]. As examples of this set of analogues, the present study utilizes BENSPM [bis(ethyl)norspermine] and two conformationally-restricted analogues, SL-11047 and SL-11102. SL-11047 is essentially bis(ethyl)spermine with a double bond between the central carbons. Similarly, SL-11102 is bis(ethyl)homospermine, but with a double bond between its central carbons. Preparation of BENSPM and SL-11047, as well as SL-11102, have been described previously in [16] and [45] respectively.
Spermidine uptake kinetic studies
Exponential phase GS-CHO(AZ-M34R) cells in suspension culture were harvested for study either after 24 h of growth (controls) or after exposure to 10 nM mifepristone for an additional 4 h (AZ-induced culture). In both cases the cells were pelleted at 1000 g for 5 min at 25 °C and resuspended to a concentration of 5×105 cells/ml in serum-free medium containing 1 mM aminoguanidine. Additional cell pellets were removed from each culture and frozen for later analysis of AZ protein by immunodetection. Control and AZ-induced cell cultures were divided into multiple labelling reaction mixtures (4 ml) to which 0.05 μCi of [14C]spermidine was added, in addition to sufficient unlabelled spermidine to establish total spermidine concentrations ranging from 0.25 to 10 μM. After incubation at 37 °C for 20 min, triplicate 1.0 ml samples were removed and diluted immediately with 5 ml of ice-cold PBS containing 1 mM unlabelled spermidine. The cells were pelleted, washed twice and radioactivity was subsequently counted in a LS1701 liquid-scintillation counter (Beckman).
Immunodetection of AZ and AZI
Affinity-purified polyclonal rabbit antibody specific for AZ-1 was prepared by using an AZ-fusion protein expressed in bacteria [34], and the immunodetection of AZ on Western blots was as described previously [34]. Expression of epigenic AZI was detected using alkaline-phosphatase-conjugated monoclonal antibody recognizing the V5 epitope (Invitrogen). As with AZ, bands were revealed using AP-conjugate substrate kit from Bio-Rad Laboratories. In order to evaluate the relative band intensities, the blots were scanned using Adobe Photoshop 6.0 and a Hewlett Packard ScanJet 3C scanner. The images were then analysed using Scion Image software. Although three AZ genes have been identified in mammalian cells [46,47], only two are likely to be expressed in this cell line. Of these we have only found AZ-1 to be expressed to a significant level in HTC and CHO cells, and this AZ isoform accounts for both the 29 and 24 kDa bands detected by this immunodetection procedure. Quantifications and comparisons of AZ band intensities in these studies were routinely performed on the more intense 24 kDa AZ bands.
Assays of polyamines and polyamine analogues
Levels of the polyamines and the polyamine analogues were determined by HPLC analysis of dansylated derivatives, as described previously [41,48].
RESULTS
Polyamine analogue incorporation is limited by AZ
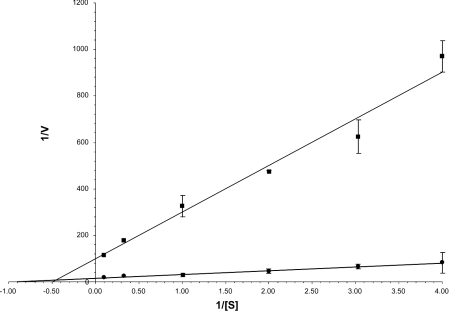
Exogenous sources of the polyamines are not over-accumulated in cells due to a feedback control mediated by AZ. There have been conflicting reports concerning the nature of this AZ-induced inhibition of the transporter. By comparing uptake kinetics before and after induction of a modified AZ fragment, antizyme-Z1, He et al. [35] concluded that AZ decreased the transport of spermine mainly by increasing the Km value for this substrate. However, depletion of cellular polyamine levels, and thereby AZ, has long been noted to increase transport velocity without affecting the Km for substrate polyamines [49]. To resolve this disparity we compared spermidine uptake kinetics in GS-CHO cells before and after induction of the native full-length isoform of AZ, the 29 kDa protein produced from the first translational start site [40]. The study presented in Figure 1 is typical of three repeats of this experiment where the average decrease in Vmax was 90%, whereas the Km approximately doubled from 0.84 to 1.7 μM. Thus it appears that induction of unmodified full-length AZ does affect both the Km and Vmax of transport, but, as expected from polyamine-deprivation studies, the predominant change is in the Vmax.
Figure 1. Effect of exogenic expression of 29 kDa AZ isoform on the kinetics of spermidine incorporation into CHO cells.
GS-CHO(AZ-M34R) cells were cultured for 4 h in the absence (■) or presence (●) of 10 nM mifepristone, and then the kinetics of [14C]spermidine uptake was analysed, as described in the Experimental section. The induction of the 29 kDa AZ form was confirmed by immunodetection. The results are expressed as the mean±S.D. for 3 replicate experiments. In three repeats of this experiment, AZ induction resulted in an average loss of 90% in the initial Vmax, whereas average Km values increased from 0.84 to 1.68 μM. (V, nmol/min per 106 cells; [S], μM)
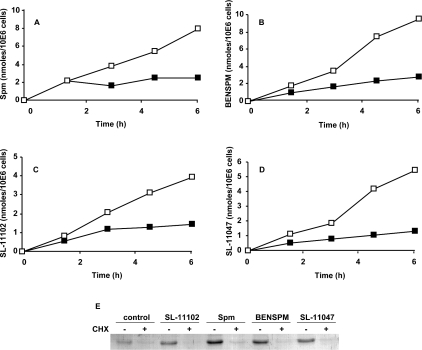
AZ is not only sufficient, but it also is absolutely necessary, for feedback regulation of the polyamine transporter. We demonstrated previously [34,50] that extensive depletion of cellular AZ levels by inhibitors of protein synthesis allowed polyamine uptake from exogenous sources to continue unabated, eventually causing cell death. As shown in Figure 2(A), HTC cells exposed to 10 μM spermine incorporate about 2 nmol/106 cells, an approx. 30% increase in their spermine content, before sufficient AZ is synthesized to prevent additional incorporation. When AZ synthesis is blocked by the addition of cycloheximide, the incorporation of spermine continues for at least 6 h, more than doubling the spermine levels. Polyamine analogues that we have previously shown to stimulate AZ production also appear to be limited in cellular uptake by this same AZ-mediated feedback system (Figures 2B–2E). In each case 1–3 nmol/106 cells of analogue was incorporated within the first 2 h and the subsequent rate of incorporation was greatly diminished. Coincident with the change in uptake velocity, induced AZ was obvious at 1.5 h and new AZ protein steady-state levels were established by 3 h (Figure 2E). In this Figure we show the uptake of a spermine analogue with only three carbons between its central nitrogens, BENSPM, an analogue of spermine that contains a double bond between its central carbons (SL-11047), and an analogue of homospermine that contains a double bond between its central carbons (SL-11102). In our previous study [41] we had reported that short-term AZ inductions by these compounds were about 75%, 105% and 80% respectively of that induced by spermine. Other analogues of the group we previously demonstrated to induce various levels of AZ [41] were similarly tested and found to also rely on AZ to limit their uptake (results not shown).
Figure 2. Feedback regulation of spermine and spermine analogue uptake blocked by cycloheximide.
Suspension cultures of HTC cells were exposed to 10 μM spermine (A), BENSPM (B), SL-11102 (C) or SL-11047 (D) in the absence (■) or presence (□) of 0.2 mM cycloheximide. Samples removed at the indicated times were analysed for increase in cell spermine level or incorporated analogue. Each data point is an average of duplicate analyses. (E) Western blot showing relative levels of AZ protein present in each culture after 3 h. CHX, cycloheximide; Spm, spermine; 10E6 cells, 106 cells.
Extended induction of AZ by polyamine analogues
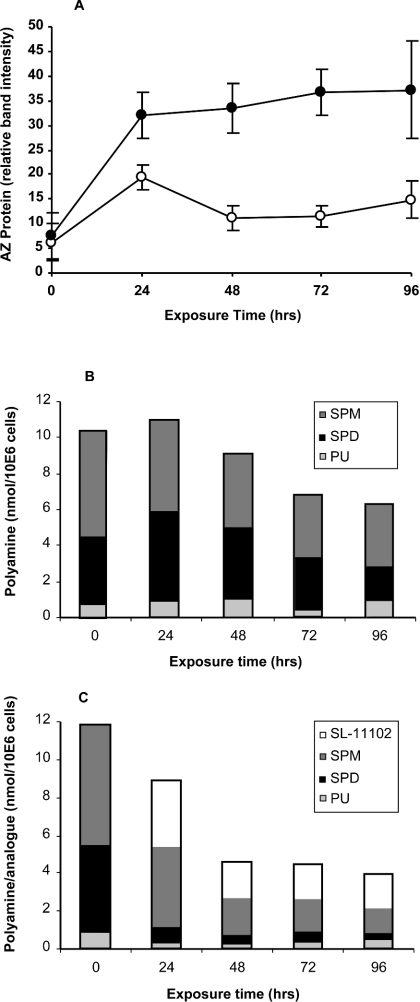
Since the uptake of polyamine analogues is limited by their AZ-mediated inhibition of the polyamine transport system, this may be expected to limit their effectiveness in altering polyamine homoeostasis during long-term exposures. Surprisingly, this does not appear to be the case. As shown in Figure 3(A), cells maintained in 10 μM SL-11102 exhibited elevated levels of AZ, with respect to control cultures, for at least the duration of a 4-day experiment. AZ is a labile protein with a half-life in these cells of about 75 min for the 24 kDa isoform, and even shorter for the 29 kDa form, and this lability does not appear to be affected by the polyamine analogues [41]. Thus the observed increase in the steady-state level of AZ protein implies a constant elevation in the rate of AZ synthesis. Coincident with this increased level of AZ protein, the treated cells exhibited markedly depressed polyamine levels (Figure 3B). This decrease in polyamines is consistent with the known ODC-inhibitory activity of AZ and the observation that AZ may facilitate polyamine export [38]. Polyamine pool reduction might also be associated, at least in part, to increased polyamine catabolism, as some analogues have been noted to stimulate the activity of spermidine/spermine acetyltransferase [17,18]. Regardless of the mechanism of cell polyamine reduction, it is noteworthy that AZ synthesis remains accelerated even after the second day of the experiment. At that time the native polyamines are reduced to about 25% of their initial value, a level at which no AZ synthesis would be anticipated in the absence of analogue. The level of the incorporated analogue is less than that of either spermidine or spermine in the control cells. Even the total of native polyamines and analogue within treated cells is strikingly less than the total of polyamines in untreated control cells. Importantly, this cellular analogue concentration is established dynamically, for when the exogenous analogue is removed, the intracellular levels decrease and AZ levels decline accordingly (results not shown). This fact might help direct the appropriate scheduling of analogue administration.
Figure 3. Effect of extended exposure of HTC cells to 10 μM SL-11102 on AZ protein and cellular polyamine levels.
Suspension cultures of HTC cells were exposed to 10 μM SL-11102 (A, ●; C) or not (A, ○; B) for 4 days and sampled daily to determine AZ protein (A) and cell content of polyamines and analogue (B, C). The results are expressed as the means±S.D. for 3 replicate experiments. SPM, spermine; SPD, spermidine; PU, putrescine; 10E6 cells, 106 cells.
Preferential targeting of tumour cells
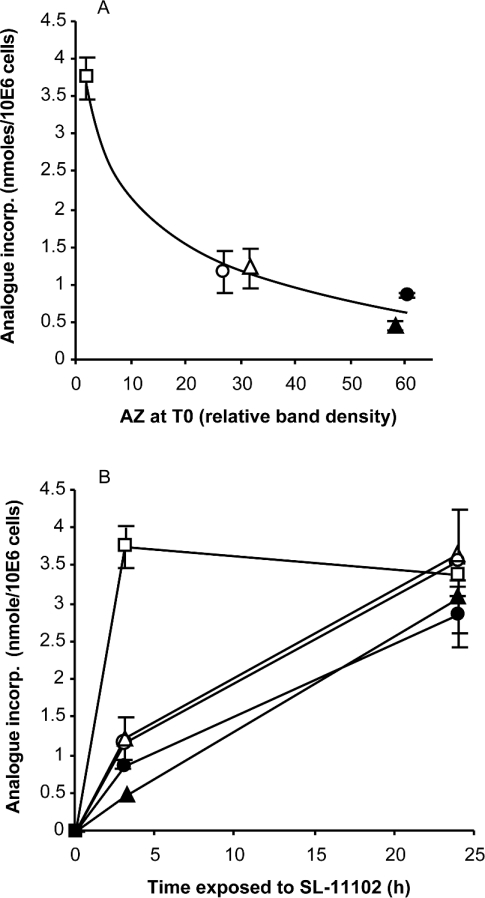
Tumours and other actively proliferating cell populations require polyamines for cell growth and generally exhibit markedly increased polyamine uptake activity, in addition to the up-regulation of biosynthesis. This, of course, has made a very attractive target for the design of anticancer drugs. Conversely, tumour cells that are not growing rapidly and have a low mitotic index would be expected to be much more resistant to drugs designed as polyamine analogues [51]. This reasoning is substantiated by the type of experiment shown in Figure 4(A). In this Figure, cultures of HTC cells were pre-treated to induce either high or low levels of AZ, and thus low and high activity respectively of the polyamine transporter. When mammalian cells are suspended in fresh media there is a transient increase in polyamine levels that induces a temporary elevation in the concentration of cellular AZ, peaking between 3 and 6 h, and returning to a low steady-state level by 24 h. Conversely, previous studies have demonstrated that cells exposed to DFMO, an inhibitor of ODC activity, have decreased putrescine and spermidine concentrations and greatly depressed levels of AZ [34,49]. Cultures were thus exposed to fresh medium for selected times or treated with DFMO to induce variable levels of native AZ prior to being exposed to the analogue SL-11102. These were then evaluated for the incorporated level of this compound after a 3 h and a 24 h exposure. As anticipated, cultures expressing less AZ at the time of SL-11102 addition allowed more analogue to be incorporated at the end of the 3 h period. This differential incorporation, however, did not persist upon longer exposures to the analogue, and by 24 h all cultures contained approximately the same level of analogue (Figure 4B). In agreement with this, by 24 h all cultures also expressed approximately the same level of AZ protein (results not shown). Thus it appears that the level of AZ-inducing analogue that is eventually achieved by cells in culture is independent of how rapidly this level was attained. Once sufficient polyamine analogue is incorporated to induce AZ synthesis, then additional uptake is limited and a steady-state analogue concentration is established. Therefore, elevations in polyamine transport velocity generally associated with rapidly dividing cells may not necessarily produce an additional accumulation of AZ-inducing polyamine analogues upon extended exposures.
Figure 4. Initial versus steady-state levels of incorporated analogue.
Variable levels of native AZ were established in cells by exposing HTC cultures to fresh media for 0.5 h (△), 3 h (●), 6 h (▲) or 24 h (○) before addition of 10 μM SL-11102. One culture was exposed to fresh medium containing 4 mM DFMO for 24 h (□) prior to treatment with analogue. (A) The level of analogue incorporated into cells by 3 h was inversely related to the relative amount of AZ protein observed in the cells at the time the SL-11102 was added. (B) Change in cellular contents of analogue in all cultures measured at 3 h and 24 h of exposure. 10E6 cells, 106 cells.
The concept that tumour cells can be preferentially targeted by AZ-inducing polyamine analogues may still be correct if the tumour cells are defective in the transport feedback system itself. For example, there is evidence that the invasiveness of prostate cancer cells is associated with insufficient expression of AZ [52]. Similarly, relative to adjacent normal colon cells, human colon cancer cells appear to over-express a protein, AZI, which sequesters AZ [53]. In both instances, tissue polyamine levels, and therefore growth potential, appear to increase in response to a decrease in AZ activity. To examine the effect of such an alteration in the polyamine uptake feedback system, a stable clone of GS-CHO cells was developed to express mouse V5–6H-tagged AZI in response to the hormone mifepristone. As shown in Figure 5, these cells were pre-treated for 5 h with and without mifepristone, and then exposed to either 10 μM spermidine or SL-11047. The induction of AZI allowed for over a 2-fold increase in the level of spermidine incorporated, and about the same increase in the analogue SL-11047 was noted. AZ was not completely inactivated by the induction of AZI, as net uptake of each compound was eventually blocked. This is in contrast to the continuing uptake of polyamines in cells where AZ was eliminated by cycloheximide, as in Figure 2.
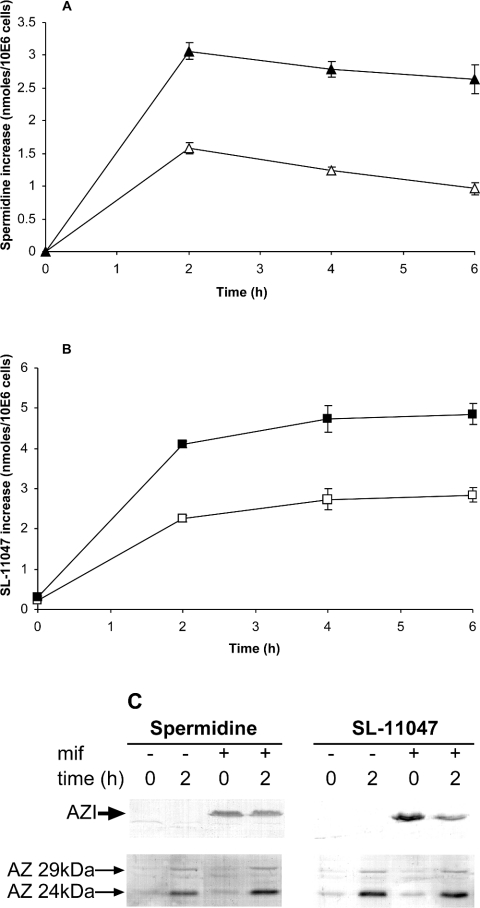
Figure 5. Effect of induction of exogenic AZI on cellular uptake of spermidine and SL-11047.
GS-CHO(AZI–V5–6H) cells were either untreated (△, □) or exposed to mifepristone (▲, ■) for 5 h to induce AZI. (A) Cultures of each were treated with 10 μM spermidine and the increase in cellular spermidine, with respect to controls, was measured at 2, 4 and 6 h. (B) Similar cultures were exposed to 10 μM SL-11047 and monitored for cellular levels of this analogue at the indicated times. The results are expressed as the means±S.D. for 3 replicate experiments. The presence of V5-tagged AZI in mifepristone-induced cultures is shown in (C), as well as the Western blot detection of AZ isoforms induced by spermidine and SL-11047. mif, mifepristone; 10E6 cells, 106 cells.
The induction of mouse AZI by mifepristone is shown in Figure 5(C) by immunodetection of the V5 tag on this construct. This expressed construct was verified by detection using monoclonal antibody prepared against AZI, and was found to be active at releasing ODC from AZ (results not shown). Theoretically, this artificial elevation of AZI levels in the mifepristone-induced cultures should bind AZ and prevent it from down-regulating the transport system. As the levels of cellular polyamines increased, AZ synthesis would also be expected to increase, until sufficient AZ became available to inhibit the transporter. Although the transport system was eventually inhibited, it does not appear that this was caused by elevated AZ levels. As seen in Figure 5(C), the level of AZ protein apparent in mifepristone-induced cells exposed to exogenous spermidine for 2 h was no greater than that in control cells exposed to spermidine, even though the former contain much higher spermidine concentrations. AZ levels at 4 and 6 h were essentially unchanged from those at 2 h (results not shown) and in 8 repeats of this experiment the average increase in AZ due to AZI during spermidine exposure was only 3%. Evidently, our comprehension of the interaction of AZ and AZI in the regulation of polyamine transport is still incomplete. Despite this uncertainty, the experiment shown in Figure 5 strongly suggests that abnormalities in the AZ-mediated feedback system reported for several tumour tissues will result in preferential uptake of polyamine analogues into such tissues.
DISCUSSION
The system for incorporation of polyamines into mammalian cells is ideal for drug uptake into cancer tissues, because of its efficiency (low Km), high capacity, minimal structural requirements and its specific enhancement in tumour cells. The potential downside of this transport system is the elegant feedback mechanism that has evolved to ensure polyamine homoeostasis. The present study has explored the extent to which this feedback mechanism limits the uptake and effectiveness of polyamine analogues designed to alter cell growth.
Before the connection between transport velocity and AZ levels had been established, Kramer et al. [54] demonstrated that at least some polyamine analogues could mimic native polyamines in regulating their transporter. Recently, we reported that many polyamine analogues are capable of stimulating AZ synthesis [41], and we speculated that this might limit their cellular incorporation, at least as represented by experiments in tissue culture. The experiments shown in the present study confirm this speculation by using representative analogues to demonstrate that AZ induction is necessary and sufficient to limit analogue transport into cells. Accordingly, when the AZ protein is diminished by cycloheximide, analogue incorporation continues unabated (Figure 2). Moreover, partial inhibition of AZ activity by induction of exogenic AZ-binding protein, AZI, was observed to increase the level of analogue incorporated into cells (Figure 5). Thus analogues that induce AZ synthesis actively limit their own uptake, similar to that of native polyamines. This may present an unusual problem for polyamine-based drugs in that the maximum cellular levels achieved are controlled by the feedback system and not by exposure dosage.
Although cellular levels of polyamine analogues are potentially limited, because of the AZ synthesis they facilitate, these levels appear sufficient to maintain elevated levels of AZ protein for an extended period, and appear to be sufficient to effect cell growth and evoke cell killing of many tumour cells. This sufficiency to maintain elevated levels of AZ protein was demonstrated for the conformationally restricted SL-11102 in Figure 3, and similar results obtained using several other AZ-inducing analogues (results not shown). Since polyamine analogues do not appear to alter the half-life of AZ [41], such elevated steady-state levels of this unstable protein must be due to continuous induction of AZ synthesis by the intracellular analogue concentrations maintained. However, it is not clear why AZ synthesis should continue at an elevated rate. If AZ synthesis is stimulated by polyamines in excess of the normal binding capacity of negatively-charged cell components, then an increase in AZ would be anticipated, as additional polyamines or polyamine analogues entered a cell. However, once the native polyamines are reduced such that the level of total polyamines (analogue included) is less than control cells (Figure 3C), then there should be no further stimulation of the translational frameshift required for AZ synthesis. Therefore, it is not clear why AZ synthesis appeared to continue even after the total cellular polyamine levels were so greatly reduced. Since native polyamines are less than 25% of control values by 48 h, they are not likely to be responsible for stimulating this elevated AZ synthesis. Perhaps the analogues that we have used in these studies are more potent than the native polyamines at stimulating AZ synthesis. Alternatively, the analogues may not interact with natural cellular binding partners as well as the native polyamines, such that the analogues remain more or less unbound in cells even at relatively low concentrations. Whatever the mechanism, this ability to continue AZ production may prove valuable in tumour prevention or management, as exogenic AZ expression studies have suggested [36,55,56].
A major reason the polyamine transporter has been chosen for targeting drugs to tumour cells is the widely reported enhancement of its activity in proliferating cells. The studies presented here, however, suggest that differences in initial activity of the transport system may only influence short-term analogue uptake. Regardless of initial transporter activity, by 24 h in tissue culture intracellular analogue concentration reached an equilibrium where further increases in analogue level were prevented by the AZ that it induced. This observation indicates the need to further study potential preferential targeting of rapidly proliferating cells with regard to transport. Obviously, it will be important to study these control mechanisms in in vivo models, in addition to tissue culture, to assure that these observations are relevant to clinical applications. For instance, although SL-11102 induces AZ in tissue culture, and subsequently effects transport, we have shown SL-11102 to be highly effective in controlling tumour growth in PANC-1 human pancreatic tumour cells implanted in nude mice (B. Frydman, A. Valasinas and L. Marton, unpublished work).
Importantly, if the enhanced polyamine transporter activity of tumour tissue is associated with abnormalities in the AZ-mediated feedback system, then such cells are likely to preferentially incorporate polyamine-based compounds. Indeed, Tsuji et al. [57] observed that reduction or lack of expression of the AZ gene is an important event for the early deregulation of cellular proliferation in oral tumour development. Similarly, Jung et al. [53] have shown depression of AZ activity as a common event in human gastric carcinomas, and Koike et al. [52] present tantalizing evidence that “tumour progression in prostate carcinoma involves faulty AZ regulation”. It is quite likely that the commonly observed increase in polyamine levels of many invasive cancer cells is due to under-expression of the AZ gene or otherwise over-expression of AZ-binding proteins, such as AZI. Cells in which the AZ gene is defective would obviously exhibit enhanced uptake of polyamine-based drugs. Furthermore, the studies presented in Figure 5 confirm that repression of AZ activity by over-induction of AZI will also result in increased analogue incorporation and higher steady-state analogue levels.
Finally, the fact that AZ shuts off the transport of the natural polyamines may provide an advantage in the activity of analogue that is already transported into the cell. Thus there is a complex, but elegant, interplay between the various components of the control of transport that may provide challenges for therapeutic intervention, but may also provide for creative opportunities.
In view of this elegant feedback control of the polyamine transporter, and assuming that these mechanisms play an important role in intact tumours, what strategies can be employed to optimize preferential transport of polyamine-based drugs into tumour cells? First, this feedback might be avoided by selecting or designing compounds that efficiently use the polyamine transporter, but do not stimulate AZ synthesis. These two reactions must have distinct specificities, as MGBG {1,1′-[(methylethanediylidine)-dinitrilo]diguanidine}, an early anti-tumour agent, exhibited these divergent characteristics [49,58]. As with MGBG, such compounds are expected to accumulate preferentially in cells possessing elevated polyamine uptake activity. Regrettably, to date there has been no concerted effort to identify such compounds or the molecular characteristics that permit utilization of the polyamine transporter without inducing AZ.
A second strategy is to use an efficient AZ response to distinguish normal from cancer cells. If invasive tumour cells commonly exhibit depressed AZ expression or excessive AZI induction, as shown for oral, colon and prostate cancers [52,53,57], then growth-inhibitory or diagnostic polyamine analogues would clearly accumulate preferentially in such tumour cells. Unfortunately, investigators have just begun to look for such abnormalities in AZ and AZI expression in human tumours. Even where abnormalities in these regulatory proteins have been demonstrated, the impact of this on polyamine transporter activity has not been well studied. Cancer cells exhibiting major deficiencies in this feedback response should be very susceptible to polyamine-based growth inhibitors.
A third approach is to utilize polyamine-based compounds that affect cell viability at cell concentrations well below the levels that would induce uptake-limiting AZ synthesis. Such compounds would incorporate preferentially into actively proliferating cells, as these generally express elevated polyamine uptake activities. In this strategy, specificity for actively proliferating cells would be lost if it were necessary to increase dosage levels to where uptake became feedback inhibited.
A fourth approach involves understanding the time course for AZ induction and decay. Timing the administration of analogues to maximize entry, while not providing excess analogue that may only contribute to toxicity, is an obvious goal.
There is considerable interest in polyamine-based drugs, and several have already demonstrated efficacy in clinical trials. The present study draws attention to the fact that these compounds could potentially promote inactivation of the very transport system they require for entry into cells. Clearly the design and effective utilization of such compounds can benefit from additional consideration of the intricacies of this unusual transport system and the regulatory proteins that control its activity.
Acknowledgments
This work was supported by grant from National Institutes of Health (CA-92703) to J.L.A.M.
References
- 1.Cohen S. S. Oxford: Oxford University Press; 1998. A guide to the polyamines. [Google Scholar]
- 2.Manni A., Grove R., Kunselman S., Aldaz M. Involvement of the polyamine pathway in breast cancer progression. Cancer Lett. 1995;92:49–57. doi: 10.1016/0304-3835(95)03763-m. [DOI] [PubMed] [Google Scholar]
- 3.Pegg A. E., Shantz L. M., Coleman C. S. Ornithine decarboxylase as a target for chemoprevention. J. Cell. Biochem. 1995;22(suppl.):132–138. doi: 10.1002/jcb.240590817. [DOI] [PubMed] [Google Scholar]
- 4.Marton L. J., Pegg A. E. Polyamines as targets for therapeutic intervention. Annu. Rev. Pharm. Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 5.Kubota S. Ornithine decarboxylase and cancer. Cancer J. 1998;11:294–297. [Google Scholar]
- 6.Wallace H. M., Fraser A. V., Hughes A. A perspective of polyamine metabolism. Biochem. J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan N. A., Quemener V., Moulinoux J.-P. Characterization of polyamine transport pathways. In: Carter C., editor. Neuropharmacology of Polyamines. London: Academic Press Limited; 1994. pp. 37–60. [Google Scholar]
- 8.Seiler N., Delcros J. G., Moulinoux J. P. Polyamine transport in mammalian cells – an update. Int. J. Biochem. Cell Biol. 1996;28:843–861. doi: 10.1016/1357-2725(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 9.Seiler N., Atanassov C. L., Raul F. Polyamine metabolism as target for cancer chemoprevention. Int. J. Oncology. 1998;13:993–1006. doi: 10.3892/ijo.13.5.993. [DOI] [PubMed] [Google Scholar]
- 10.Kramer D. L. Polyamine inhibitors and analogs. In: Nishioka K., editor. Polyamines in Cancer: Basic Mechanisms and Clinical Approaches. Austin: R. G. Landes Company; 1996. pp. 151–189. [Google Scholar]
- 11.Porter C. W., McManis J., Casero R. A., Bergeron R. J. Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res. 1987;47:2821–2825. [PubMed] [Google Scholar]
- 12.Wallace H. M., Fraser A. V. Polyamine analogues as anticancer drugs. Biochem. Soc. Trans. 2003;31:393–396. doi: 10.1042/bst0310393. [DOI] [PubMed] [Google Scholar]
- 13.Porter C. W., Sufrin J. R. Interference with polyamine biosynthesis and/or function by analogs of polyamines or methionine as a potential anticancer chemotherapeutic strategy. Anticancer Res. 1986;6:525–542. [PubMed] [Google Scholar]
- 14.Casero R. A., Woster P. M. Terminally alkylated polyamine analogues as chemotherapeutic agents. J. Med. Chem. 2001;44:1–26. doi: 10.1021/jm000084m. [DOI] [PubMed] [Google Scholar]
- 15.Kramer D. L., Fogelpetrovic M., Diegelman P., Cooley J. M., Bernacki R. J., McManis J. S., Bergeron R. J., Porter C. W. Effects of novel spermine analogues on cell cycle progression and apoptosis in malme-3m human melanoma cells. Cancer Res. 1997;57:5521–5527. [PubMed] [Google Scholar]
- 16.Reddy V. K., Valasinas A., Sarkar A., Basu H. S., Marton L. J., Frydman B. Conformationally restricted analogues of n-1,n-12-bisethylspermine – synthesis and growth inhibitory effects on human tumor cell lines. J. Med. Chem. 1998;41:4723–4732. doi: 10.1021/jm980172v. [DOI] [PubMed] [Google Scholar]
- 17.Saab N. H., West E. E., Bieszk N. C., Preuss C. V., Mank A. R., Casero R. A., Woster P. M. Synthesis and evaluation of unsymmetrically substituted polyamine analogues as modulators of human spermidine spermine-N1-acetyltransferase (SSAT) and as potential antitumor agents. J. Med. Chem. 1993;36:2998–3004. doi: 10.1021/jm00072a020. [DOI] [PubMed] [Google Scholar]
- 18.Gabrielson E. W., Pegg A. E., Casero R. A. The induction of spermidine/spermine N-1-acetyltransferase (SSAT) is a common event in the response of human primary non-small cell lung carcinomas to exposure to the new antitumor polyamine analogue N-1,N-11-bis(ethyl) norspermine. Clin. Cancer Res. 1999;5:1638–1641. [PubMed] [Google Scholar]
- 19.Ghoda L., Basu H. S., Porter C. W., Marton L. J., Coffino P. Role of ornithine decarboxylase suppression and polyamine depletion in the antiproliferative activity of polyamine analogs. Mol. Pharmacol. 1992;42:302–306. [PubMed] [Google Scholar]
- 20.He Y., Suzuki T., Kashiwagi K., Kusamaeguchi K., Shirahata A., Igarashi K. Correlation between the inhibition of cell growth by bis(ethyl) polyamine analogues and the decrease in the function of mitochondria. Eur. J. Biochem. 1994;221:391–398. doi: 10.1111/j.1432-1033.1994.tb18751.x. [DOI] [PubMed] [Google Scholar]
- 21.Basu H. S., Pellarin M., Feuerstein B. G., Deen D. F., Marton L. J. Effect of N1,N14-bis-(ethyl)-homospermine (BE-4-4-4) on the growth of U-251 MG and SF-188 human brain tumor cells. Int. J. Cancer. 1991;48:873–878. doi: 10.1002/ijc.2910480614. [DOI] [PubMed] [Google Scholar]
- 22.Hahm H. A., Ettinger D. S., Bowling K., Hoker B., Chen T. L., Zabelina Y., Casero R. A. Phase I study of N-1,N-11-diethylnorspermine in patients with non-small cell lung cancer. Clin. Cancer Res. 2002;8:684–690. [PubMed] [Google Scholar]
- 23.Wolff J. C., Armstrong D. K., Fetting J. H., Carducci M. K., Riley C. D., Bender J. F., Casero R. A., Davidson N. E. A phase II study of the polyamine analog N-1,N-11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin. Cancer Res. 2003;9:5922–5928. [PubMed] [Google Scholar]
- 24.Martin B., Posseme F., Le Barbier C., Carreaux F., Carboni B., Seiler N., Moulinoux J. P., Delcros J. G. Z-1,4-diamino-2-butene as a vector of boron, fluorine, or iodine for cancer therapy and imaging: Synthesis and biological evaluation. Bioorganic Med. Chem. 2002;10:2863–2871. doi: 10.1016/s0968-0896(02)00147-5. [DOI] [PubMed] [Google Scholar]
- 25.Shuto J. C., Cai J., Soloway A. H., Barth R. F., Adams D. M., Ji W., Tjarks W. Synthesis and biological evaluation of boron-containing polyamines as potential agents for neutron cature therapy of brain tumors. J. Med. Chem. 1999;42:1282–1292. doi: 10.1021/jm980703f. [DOI] [PubMed] [Google Scholar]
- 26.Cai J., Soloway A. H., Barth R. F., Adams D. M., Hariharan J. R., Wyzlic I. M., Radcliffe K. Boron-containing polyamines as DNA targeting agents for neutron capture therapy of brain tumors: Synthesis and biological evaluation. J. Med. Chem. 1997;40:3887–3896. doi: 10.1021/jm960787x. [DOI] [PubMed] [Google Scholar]
- 27.Holley J., Mather A., Cullis P., Symons M. R., Wardman P., Watt R. A., Cohen G. M. Uptake and cytotoxicity of novel nitroimidazole-polyamine conjugates in Ehrlich ascites tumour cells. Biochem. Pharmacol. 1992;43:763–769. doi: 10.1016/0006-2952(92)90241-a. [DOI] [PubMed] [Google Scholar]
- 28.Lin P. T., Dance A. M., Bestwick C., Milen L. The biological activities of new polyamine derivatives as potential therapeutic agents. Biochem. Soc. Trans. 2003;31:407–410. doi: 10.1042/bst0310407. [DOI] [PubMed] [Google Scholar]
- 29.Bergeron R. J., McManis J. S., Franklin A. M., Yao H., Weimar W. R. Polyamine–iron chelator conjugate. J. Med. Chem. 2003;46:5478–5483. doi: 10.1021/jm0302694. [DOI] [PubMed] [Google Scholar]
- 30.Nagarajan M., Xiao X. S., Antony S., Kohlhagen G., Pommier Y., Cushman M. Design, synthesis, and biological evaluation of indenoisoquinoline topoisomerase I inhibitors featuring polyamine side chains on the lactam nitrogen. J. Med. Chem. 2003;46:5712–5724. doi: 10.1021/jm030313f. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy A. R., Kritchevsky D., Shen W. C. Effects of spermine-conjugated Bowman–Birk inhibitor (spermine-BBI) on carcinogenesis and cholesterol biosynthesis in mice. Pharm. Res. 2003;20:1908–1910. doi: 10.1023/b:pham.0000008035.02046.cb. [DOI] [PubMed] [Google Scholar]
- 32.Soulet D., Covassin L., Kaouass M., Charest-Gaudreault R., Audette M., Poulin R. Role of endocytosis in the internalization of spermidine-C-2-BODIPY, a highly fluorescent probe of polyamine transport. Biochem. J. 2002;367:347–357. doi: 10.1042/BJ20020764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belting M., Mani K., Jonsson M., Cheng F., Sandgren S., Jonsson S., Ding K., Delcros J. G., Fransson L. A. Glypican-1 is a vehicle for polyamine uptake in mammalian cells – A pivotal role for nitrosothiol-derived nitric oxide. J. Biol. Chem. 2003;278:47181–47189. doi: 10.1074/jbc.M308325200. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell J. L. A., Judd G. G., Bareyal-Leyser A., Ling S. Y. Feedback repression of polyamine transport is mediated by antizyme in mammalian tissue culture cells. Biochem. J. 1994;299:19–22. doi: 10.1042/bj2990019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Y., Suzuki T., Kashiwagi K., Igarashi K. Antizyme delays the restoration by spermine of growth of polyamine-deficient cells through its negative regulation of polyamine transport. Biochem. Biophys. Res. Commun. 1994;203:608–614. doi: 10.1006/bbrc.1994.2226. [DOI] [PubMed] [Google Scholar]
- 36.Murakami Y., Matsufuji S., Miyazaki Y., Hayashi S. Forced expression of antizyme abolishes ornithine decarboxylase activity, suppresses cellular levels of polyamines and inhibits cell growth. Biochem. J. 1994;304:183–187. doi: 10.1042/bj3040183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi S., Murakami Y., Matsufuji S. Ornithine decarboxylase antizyme – a novel type of regulatory protein. Trends Biochem. Sci. 1996;21:27–30. [PubMed] [Google Scholar]
- 38.Sakata K., Kashiwagi K., Igarashi K. Properties of a polyamine transporter regulated by antizyme. Biochem. J. 2000;347:297–303. [PMC free article] [PubMed] [Google Scholar]
- 39.Sakata K., Fukuchishimogori T., Kashiwagi K., Igarashi K. Identification of regulatory region of antizyme necessary for the negative regulation of polyamine transport. Biochem. Biophys. Res. Commun. 1997;238:415–419. doi: 10.1006/bbrc.1997.7266. [DOI] [PubMed] [Google Scholar]
- 40.Matsufuji S., Matsufuji T., Miyazaki Y., Murakami Y., Atkins J. F., Gesteland R. F., Hayashi S. Autoregulatory frameshifting in decoding mammalian ornithine decarboxylase antizyme. Cell. 1995;80:51–60. doi: 10.1016/0092-8674(95)90450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell J. L. A., Leyser A., Holtorff M. S., Bates J. S., Frydman B., Valasinas A. L., Reddy V. K., Marton L. J. Antizyme induction by polyamine analogues as a factor in cell growth inhibition. Biochem. J. 2002;366:663–672. doi: 10.1042/BJ20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell J. L. A., Judd G. G., Leyser A., Choe C.-Y. Osmotic stress induces variation in cellular levels of ODC-antizyme. Biochem. J. 1998;329:453–459. doi: 10.1042/bj3290453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell J. L. A., Judd G. G. Antizyme modifications affecting polyamine homeostasis. Biochem. Soc. Trans. 1998;26:591–595. doi: 10.1042/bst0260591. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson J., Grahn B., Heby O. Antizyme inhibitor is rapidly induced in growth-stimulated mouse fibroblasts and releases ornithine decarboxylase from antizyme suppression. Biochem. J. 2000;346:699–704. [PMC free article] [PubMed] [Google Scholar]
- 45.Valasinas A., Sarkar A., Reddy V. K., Marton L. J., Basu H. S., Frydman B. Conformationally restricted analogues of N-1,N-14-bisethylhomospermine (BE-4-4-4): Synthesis and growth inhibitory effects on human prostate cancer cells. J. Med. Chem. 2001;44:390–403. doi: 10.1021/jm000309t. [DOI] [PubMed] [Google Scholar]
- 46.Ivanov I. P., Gesteland R. F., Atkins J. F. A second mammalian antizyme: conservation of programmed ribosomal frameshifting. Genomics. 1998;52:119–129. doi: 10.1006/geno.1998.5434. [DOI] [PubMed] [Google Scholar]
- 47.Ivanov I. P., Rohrwasser A., Terreros D. A., Gesteland R. F., Atkins J. F. Discovery of a spermatogenesis stage-specific ornithine decarboxylase antizyme: antizyme 3. Proc. Nat. Acad. Sci. U.S.A. 2000;97:4808–4813. doi: 10.1073/pnas.070055897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minocha S. C., Minocha R., Robie C. A. High-performance liquid chromatographic method for the determination of dansyl-polyamines. J. Chromatogr. 1990;511:177–183. [Google Scholar]
- 49.Byers T. L., Pegg A. E. Properties and physiological function of the polyamine transport system. Am. J. Physiol. 1989;257:C545–C553. doi: 10.1152/ajpcell.1989.257.3.C545. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell J. L. A., Diveley R. R., Jr, Bareyal-Leyser A. Feedback repression of polyamine uptake into mammalian cells requires active protein synthesis. Biochem. Biophys. Res. Commun. 1992;186:81–88. doi: 10.1016/s0006-291x(05)80778-8. [DOI] [PubMed] [Google Scholar]
- 51.Carlisle D. L., Devereux W. L., Hacker A., Woster P. M., Casero R. A. Growth status significantly affects the response of human lung cancer cells to antitumor polyamine-analogue exposure. Clin. Cancer Res. 2002;8:2684–2689. [PubMed] [Google Scholar]
- 52.Koike C., Chao D. T., Zetter B. R. Sensitivity to polyamine-induced growth arrest correlates with antizyme induction in prostate carcinoma cells. Cancer Res. 1999;59:6109–6112. [PubMed] [Google Scholar]
- 53.Jung M. H., Kim S. C., Jeon G. A., Kim S. H., Kim Y., Choi K. S., Park S. I., Joe M. K., Kimm K. Identification of differentially expressed genes in normal and tumor human gastric tissue. Genomics. 2000;69:281–286. doi: 10.1006/geno.2000.6338. [DOI] [PubMed] [Google Scholar]
- 54.Kramer D. L., Miller J. T., Bergeron R. J., Khomutov R., Khomutov A., Porter C. W. Regulation of polyamine transport by polyamines and polyamine analogs. J. Cell. Physiol. 1993;155:399–407. doi: 10.1002/jcp.1041550222. [DOI] [PubMed] [Google Scholar]
- 55.Iwata S., Sato Y., Asada M., Takagi M., Tsujimoto A., Inaba T., Yamada T., Sakamoto S., Yata J., Shimogori T., et al. Anti-tumor activity of antizyme which targets the ornithine decarboxylase (ODC) required for cell growth and transformation. Oncogene. 1999;18:165–172. doi: 10.1038/sj.onc.1202275. [DOI] [PubMed] [Google Scholar]
- 56.Feith D. J., Shantz L. M., Pegg A. E. Targeted antizyme expression in the skin of transgenic mice reduces tumor promoter induction of ornithine decarboxylase and decreases sensitivity to chemical carcinogenesis. Cancer Res. 2001;61:6073–6081. [PubMed] [Google Scholar]
- 57.Tsuji T., Todd R., Meyer C., Mcbride J., Liao P. H., Huang M. F., Chou M. Y., Donoff R. B., Wong D. W. Reduction of ornithine decarboxylase antizyme (Odc-az) level in the 7,12-dimethylbenz(A)anthracene-induced hamster buccal pouch carcinogenesis model. Oncogene. 1998;16:3379–3385. doi: 10.1038/sj.onc.1201887. [DOI] [PubMed] [Google Scholar]
- 58.Hölttä E., Hannonen P., Pispa J., Jänne J. Effect of methylglyoxal bis(guanylhydrazone) on polyamine metabolism in normal and regenerating rat liver and rat thymus. Biochem. J. 1973;136:669–676. doi: 10.1042/bj1360669. [DOI] [PMC free article] [PubMed] [Google Scholar]