Abstract
Previously, we have shown that p22, an EF-hand Ca2+-binding protein, interacts indirectly with microtubules in an N-myristoylation-dependent and Ca2+-independent manner. In the present study, we report that N-myristoylated p22 interacts with several microtubule-associated proteins within the 30–100 kDa range using overlay blots of microtubule pellets containing cytosolic proteins. One of those p22-binding partners, a 35–40 kDa microtubule-binding protein, has been identified by MS as GAPDH (glyceraldehyde-3-phosphate dehydrogenase). Several lines of evidence suggest a functional relationship between GAPDH and p22. First, endogenous p22 interacts with GAPDH by immunoprecipitation. Secondly, p22 and GAPDH align along microtubule tracks in analogous punctate structures in BHK cells. Thirdly, GAPDH facilitates the p22-dependent interactions between microtubules and microsomal membranes, by increasing the ability of p22 to bind microtubules but not membranes. We have also shown a direct interaction between N-myristoylated p22 and GAPDH in vitro with a KD of ∼0.5 μM. The removal of either the N-myristoyl group or the last six C-terminal amino acids abolishes the binding of p22 to GAPDH and reduces the ability of p22 to associate with microtubules. In summary, we report that GAPDH is involved in the ability of p22 to facilitate microtubule–membrane interactions by affecting the p22–microtubule, but not the p22–membrane, association.
Keywords: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), membrane, microtubule, microtubule–membrane interactions, N-myristoylation, p22
Abbreviations: ER, endoplasmic reticulum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; MAP, microtubule-associated protein
INTRODUCTION
It is well documented that microtubules facilitate the assembly of membrane-bound organelles as well as several membrane-trafficking events [1]. However, the molecular mechanisms involved in the interactions between microtubules and intracellular membranes are still incomplete. Much of the work in the field has been focused on motor-mediated organelle movement along microtubules [2,3], but non-motor MAPs (microtubule-associated proteins) have also been shown to be involved in organelle assembly and organization [4,5]. An increasing number of non-motor-associated proteins have been implicated in microtubule–organelle interactions as well as in organelle assembly and movement. Rab GTPases and CLIPs (cytoplasmic linker proteins) interact with motors to modulate the binding of membrane vesicles to microtubules [6–8]. Other proteins such as ch-TOG, the human homologue of XMAP-215 and CLIMP-63, an ER (endoplasmic reticulum) integral membrane protein, act to link microtubules to ER membranes [9–11], whereas Hook3, a microtubule- and Golgi-binding protein, is required for the organization of the Golgi complex [12]. Although a more detailed picture of the interactions between membrane-bound organelles and microtubules is emerging, numerous gaps still exist in our knowledge.
p22, one of the less well-characterized members of the EF-hand Ca2+-binding superfamily, was isolated in a screen for proteins involved in the targeting/docking/fusion of membrane vesicles with the apical plasma membrane, and was subsequently shown to be required for this process [13]. p22 belongs to an EF-hand subfamily that is related to the calcineurin B subfamily, contains four EF-hand Ca2+-binding motifs, undergoes Ca2+-mediated conformational changes and is N-myristoylated [13]. p22 is widely expressed, evolutionarily conserved, and it has been shown to have multiple functions, including the regulation of microtubule–membrane interactions [14], ER and microtubule organization [14], DRAK2 kinase activity (death-associated protein kinase-related apoptosis inducing kinase) [15], Na+/H+-exchanger transport activity [16–19] and calcineurin phosphatase activity [17]. Other p22-binding partners have been identified, such as the kinesin-related motor KIF1Bβ2 [20] and the neural-specific epidermal growth factor-like repeat-containing protein NELL2, which appear to be involved in the development of the central nervous system [21].
We have shown that p22 associates with microtubules and membranes of the early secretory pathway using distinct mechanisms [14,22]. Ability of p22 to bind membranes increases in an N-myristoylation- and Ca2+-dependent manner, which is suggestive of a non-classical Ca2+-myristoyl switch [14]. In contrast, p22 associates with microtubules independent of its Ca2+-binding ability [22]. The p22–microtubule association is modulated by a low-affinity interaction with cytosolic microtubule-binding factors, which are distinct from classical microtubule motors, CLIP-170, MAPs, p58 and actin [22]. Moreover, p22 plays a role in microtubule and ER organization and dynamics with distinct Ca2+-binding requirements; Ca2+ binding and Ca2+-mediated conformational changes are required for the functioning of p22 in ER network formation, but not for its ability to modulate microtubule organization and dynamics [14].
To explore further the nature of interaction of p22 with microtubules, we identified GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a p22-binding protein that affects the ability of p22 to associate with microtubules and to facilitate microtubule–membrane interactions. GAPDH is a classical glycolytic enzyme, which has been shown to display several different activities, such as microtubule bundling, membrane fusion, phosphotransfer activity, nuclear RNA export, DNA replication and DNA repair [23]. Previously, it has been shown that GAPDH associates with the microtubule cytoskeleton within the early secretory pathway [24,25]. GAPDH has also been shown to interact with the actin cytoskeleton [26]. Taken together, our findings suggest that GAPDH facilitates the p22-dependent microtubule–membrane interactions by affecting the ability of p22 to associate with microtubules but not microsomal membranes.
EXPERIMENTAL
Microtubule co-sedimentation assays
Rat liver and kidney cytosols were prepared as in [22]. Pure bovine brain tubulin (final concentration of 0.4 mg/ml) (Cytoskeleton Inc., Denver, CO, U.S.A.) was incubated for 30 min at 37 °C with 20 μM taxol, protease inhibitors, PEM buffer (0.1 M Pipes, pH 6.6, 1 mM EGTA and 1 mM MgSO4) and 500 μg of cytosol or 2 μg of purified GAPDH from rabbit muscle (Sigma–Aldrich, St. Louis, MO, U.S.A.), in the presence or absence of 12 μg of myr-p22 (bacterially expressed N-myristoylated p22 protein), myr-p22-Δ190-195 (bacterially expressed N-myristoylated C-terminal deletion p22 mutant) or p22-rec (bacterially expressed non-myristoylated p22 mutant); microtubule-binding assays were then performed as described previously [22]. Equal amounts of supernatants and microtubule pellets were loaded on to a SDS/12%-(w/v)-polyacrylamide gel for Coomassie Blue staining or immunoblotting using polyclonal anti-p22 or monoclonal anti-GAPDH.
Blot overlay assays
Purified rabbit muscle GAPDH, BSA (Fisher Scientific, Fair Lawn, NJ, U.S.A.), or ovalbumin (Sigma–Aldrich), 5 μg of each, or 15 μl of microtubule-binding pellets containing tubulin and either rat liver or kidney cytosolic microtubule-binding proteins were run on a SDS/10%-polyacrylamide gel. The proteins were transferred to nitrocellulose and the blots were incubated overnight in blocking solution [2.5% (v/v) non-fat milk, 0.05% Tween 20 and 0.5× PBS] at 4 °C. Each lane was probed with 5 μg of myr-p22 in blocking solution for 1.5 h at room temperature (22 °C) in a Slot Blot apparatus (Idea Scientific, Minneapolis, MN, U.S.A.). The blot was washed with PBS/Tween (1× PBS and 0.05% Tween 20) and subsequently processed for immunoblotting using anti-p22 at higher dilutions that are unable to detect endogenous p22.
Co-immunoprecipitation assays
Rat liver cytosolic proteins (500 μg) were incubated overnight at 4 °C with 10 μg of monoclonal antibodies raised against GAPDH or GFP (green fluorescent protein) (Stressgen, Victoria, Canada) in the presence of PEM buffer. Then, samples were incubated for 4 h at room temperature with Protein G–Sepharose (Amersham Biosciences, Piscataway, NJ, U.S.A.) and 1% Triton X-100. After several washes, bead-containing fractions were resuspended in SDS/PAGE buffer, boiled and separated into supernatant and pellet fractions by centrifugation. Supernatants were analysed by SDS/PAGE (12% gel) and immunoblotting using anti-GAPDH and anti-p22.
Immunofluorescence microscopy
BHK cells were fixed with 4% (w/v) paraformaldehyde and processed for immunofluorescence using anti-p22 APpep2 antibodies [14,22]. Monoclonal anti-α-tubulin (clone DM1A; Sigma–Aldrich) and polyclonal anti-GAPDH (generously provided by Dr E. Tisdale, Department of Pharmacology, Wayne State University School of Medicine) were used in immunofluorescence to detect tubulin and GAPDH respectively. Cy5- and FITC-labelled secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA, U.S.A.) were used for double-label immunofluorescence. Then, cells were washed briefly in PBS and mounted on glass slides using ProLong anti-fade kit mounting reagent (Molecular Probes, Eugene, OR, U.S.A.). Cells were visualized with a Zeiss Axiovert S100 inverted microscope. Images were collected using Openlab software version 3.0.9 (Improvision Warwick, U.K.) with a Hamamatsu ORCA-ER digital camera (Hamamatsu Photonics, Hamamatsu City, Japan). Image analysis was performed using Adobe Photoshop 5.5.
Microtubule–membrane-binding assay
The effect of p22 and GAPDH on microtubule–membrane interactions was assayed as described in [14]. Microtubule-covered beads were incubated with 500 μg of rat liver cytosol and 12 μg of myr-p22 or 2 μg of purified rabbit muscle GAPDH in PEMT buffer for 30 min at 37 °C. Beads were washed and incubated for 30 min at 37 °C with 100 μg of microsomal membranes. Samples were assayed for calnexin, tubulin, GAPDH and p22 by immunoblotting and quantification using NIH-Image version 1.62.
Membrane-binding assay
Microsomal membranes were centrifuged at 174000 g for 30 min and resuspended in PBS to remove traces of cytosol as described in [14]. Then, 30 μg of the prewashed membranes were incubated with 2 μg of myr-p22 and/or 2 μg of purified rabbit muscle GAPDH in 100 μl of PEM (100 mM Pipes, pH 6.6, 1 mM EGTA and 1 mM MgSO4) plus protease inhibitor cocktail and 0.2 mg/ml PMSF for 10 min at 37 °C. Samples were centrifuged at 174000 g for 30 min. Membrane pellets were resuspended in equal amounts of SDS/PAGE loading buffer and analysed by SDS/PAGE and immunoblotting using anti-p22, anti-GAPDH and anti-calnexin. ECL®-treated immunoblots were quantified using NIH-Image version 1.62.
Affigel-10 bead-coupled proteins
After washing Affigel-10 beads (Bio-Rad Laboratories, Hercules, CA, U.S.A.) with distilled water, 2 ml of beads were incubated overnight at 4 °C with 1.5 mg of GAPDH, BSA, aldolase or ovalbumin (Sigma–Aldrich) in 100 mM Mops (pH 7.5). Then, 0.1 vol. of 1 M ethanolamide (pH 8.0) was added and the beads were incubated for 1 h at room temperature. Beads were first washed with distilled water and then with PEM/Triton (1× PEM plus 1% Triton X-100). For the binding assay, 50 μg of each specific protein coupled to the beads (GAPDH, BSA, aldolase or ovalbumin) was incubated with different amounts of myr-p22, myr-p22-Δ190-195 or p22-rec for 1 h at 37 °C in PEM/Triton. Beads were washed with PBS/Triton and resuspended in SDS/PAGE loading buffer. The amount of p22 proteins bound to the beads was detected by immunoblotting using anti-p22. For determining KD values, 50 μg of purified rabbit muscle GAPDH coupled with beads was incubated with different molar concentrations of myr-p22. The KD and Bmax values were calculated using the Prism 3.03 program by fitting a hyperbola directly to the saturation isotherm using non-linear regression.
SDS/PAGE, immunoblotting and antibodies
Immunoblots were processed by chemiluminescence using ECL® reagents (Amersham Biosciences) as described previously [13]. For quantification, non-saturated films were scanned and densitometric analysis was performed using NIH Image, version 1.62. Anti-p22 affinity-purified antibodies against full-length (APp22) and peptide p22 (APpep2) were characterized previously [14,22]. Monoclonal anti-GAPDH was purchased from Chemicon International (Temecula, CA, U.S.A.).
Preparation of bacterially expressed p22 proteins
To construct a p22 deletion mutant in which the last six C-terminal amino acid residues were deleted (p22-Δ190-195), we paired a downstream primer containing a stop codon followed by a BamHI site after residue 190 (5′-CGGGATCCTTACATCTTCTGTTCTACATCC-3′), with an upstream N-terminal primer using p22 as a template for PCR mutagenesis. The resulting PCR fragment was cloned into pET3a and then checked by DNA sequencing. Bacterial expression of myr-p22, myr-p22-Δ190-195 and p22-rec was performed as described previously [14,22]. All bacterially expressed p22 proteins showed 80–95% purity and the expected molecular mass by SDS/PAGE and immunoblotting analysis (J. Andrade, S. T. Pearce, H. Zhao and M. Barroso, unpublished work).
RESULTS
p22 binds microtubule-associated GAPDH
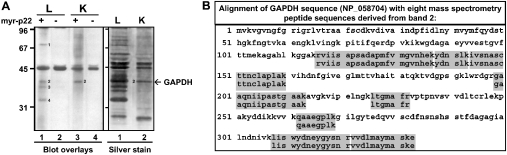
Previously, we have shown that the majority of p22 associates with microtubules in a cytosol-dependent manner [22]. To gain insight into the cytosolic proteins that may be involved in the p22–microtubule association, we have determined the number and molecular mass of the microtubule-associated cytosolic p22-binding partners. myr-p22 was used as a probe in blot overlays of microtubule pellets prepared from co-sedimentation assays that were performed in the presence of purified tubulin and rat liver cytosolic proteins (Figure 1A, left panel, lanes 1 and 2). Microtubule pellets were separated by SDS/PAGE, transferred to nitrocellulose, incubated in the presence or absence of myr-p22 and immunoblotted with anti-p22 (lane 1 versus lane 2). myr-p22 recognizes several protein bands in the 30–100 kDa range in microtubule pellets containing liver cytosolic proteins (lane 1). Bands detected in the absence of myr-p22 are due to the non-specific binding of anti-p22 to liver microtubule pellets (lane 2). The profile of p22-binding partners in the 30–100 kDa range was the same in the presence or absence of 1 mM CaCl2 (J. Andrade, S. T. Pearce, H. Zhao and M. Barroso, unpublished work), as expected, since the p22–microtubule association occurs in a Ca2+-independent manner [22]. It is important to note that the potential p22-binding partners in microtubule pellets are only a subset of the total proteins present in rat liver cytosol, since they consist of MAPs. p22 interacts with at least eight proteins in total rat liver cytosol (J. Andrade, S. T. Pearce, H. Zhao and M. Barroso, unpublished work), in contrast with four targets in microtubule pellets (Figure 1A, left panel, lane 1).
Figure 1. p22 interacts with microtubule-associated GAPDH.
(A) Left panel: blot overlays of microtubule pellets from co-sedimentation assays performed in the presence of either liver (L) or kidney (K) cytosolic proteins were probed with (+) or without (−) myr-p22. Bands 1–4 indicate p22-interacting partners. Values on the left are molecular masses in kDa. Right panel: microtubule pellets from co-sedimentation assays performed in the presence of either liver (L) or kidney (K) cytosol were analysed by SDS/PAGE and silver staining. (B) MS was used to identify band 2 as GAPDH. Alignment of GAPDH sequence (accession no. NP_058704) with MS peptide sequences derived from band 2 (shaded boxes).
We also investigated the ability of myr-p22 to interact differentially with MAPs depending on their tissue of origin. Blot overlays with myr-p22 were performed on microtubule pellets containing either liver or kidney cytosolic microtubule-binding proteins (Figure 1, left panel, lanes 1 and 2 versus lanes 3 and 4). Four interacting partners in the 30–100 kDa range were detected in liver microtubule pellets (lane 1, bands 1–4). In contrast, only one p22-binding partner with a similar molecular mass to band 2 in liver was detected in kidney microtubule pellets (lane 3, band 2). Bands detected in the absence of myr-p22 are due to the non-specific binding of anti-p22 to liver and kidney microtubule pellets (lanes 2 and 4). Rat liver and kidney microtubule pellets were analysed by SDS/PAGE and silver staining (Figure 1A, right panel). Whereas 15–20 liver cytosolic proteins are found associated with microtubule pellets (lane 1), only four predominant cytosolic kidney proteins appear to be interacting with microtubules (lane 2). Significantly, a protein of similar molecular mass to band 2 in overlay blots is found associated with liver and kidney microtubule pellets using silver staining analysis (Figure 1A, right panel, lanes 1 and 2, band 2). We subjected band 2 (∼35–40 kDa band) to MS at the W. M. Keck Biomedical Mass Spectrometry Laboratory at the University of Virginia. Eight peptides were analysed by database searches and the protein was identified as GAPDH, with the sequenced peptides covering 30.4% of the total protein sequence and each peptide matching rat GAPDH with 100% homology (Figure 1B; grey boxes).
p22 and GAPDH associate with microtubule pellets in the presence of liver or kidney cytosolic proteins
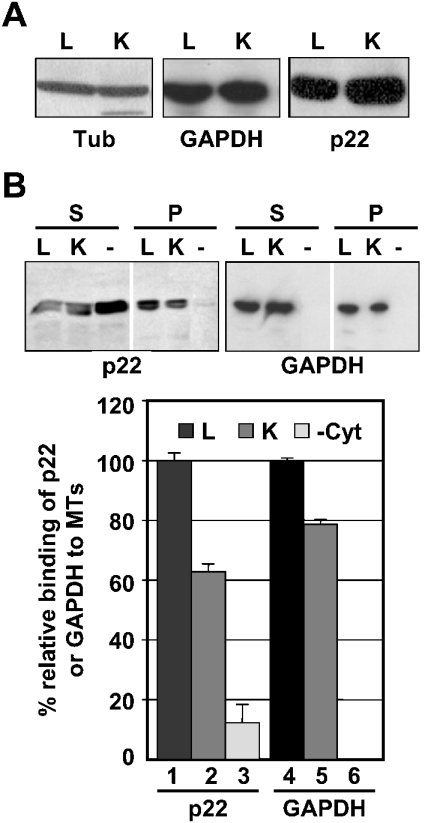
If p22 interacts with liver and kidney GAPDH, we would expect both p22 and GAPDH to be expressed in liver and kidney cytosols. Cytosolic fractions, isolated from liver and kidney organs, were subjected to SDS/PAGE and immunoblotting using p22, GAPDH and tubulin antibodies. Similar levels of p22, GAPDH and tubulin expression were detected in liver and kidney cytosolic proteins (Figure 2A). These results are in agreement with previous results showing that p22 and GAPDH protein and mRNA are expressed in every adult rat tissue tested [13,16,18,27].
Figure 2. p22 and GAPDH associate with microtubule pellets in the presence of liver or kidney cytosolic proteins.
(A) Rat liver (L) and kidney (K) cytosolic proteins were subjected to immunoblots using tubulin, GAPDH and p22 antibodies. (B) Upper panels: microtubule co-sedimentation assays were incubated with myr-p22 in the absence (lane-) or presence of rat liver (lane L) or kidney (lane K) cytosols and processed as described previously [22]. Supernatants (S) and microtubule pellets (P) were analysed by immunoblotting using anti-p22 and anti-GAPDH. (B) Lower panel: p22 and GAPDH immunoblots were quantified as described in the Experimental section, and the percentage binding of p22 or GAPDH to microtubule pellets in the presence of liver cytosol was normalized to 100% respectively. Results represent means±S.D. for three experiments. MTs, microtubules.
Since myr-p22 binds microtubule-associated GAPDH in overlay blots of microtubule pellets prepared with purified tubulin and liver or kidney cytosolic proteins (Figure 1A), we have tested the association of myr-p22 with microtubules in co-sedimentation assays performed in the presence of liver or kidney cytosolic proteins. In agreement with our previous results [22], 55–60% of the total amount of myr-p22 added to co-sedimentation assays is found associated with microtubule pellets in the presence of liver cytosol; these values were normalized to 100% (Figure 2B, lane 1) to allow for comparisons between different experiments. As expected, a significant reduction (∼8×) is detected when comparing the relative percentage of myr-p22 binding with microtubules in the presence or absence of liver cytosol (Figure 2B, lane 1 versus lane 3). Furthermore, myr-p22 associates with microtubule pellets in the presence of 1% Triton X-100 but not in the presence of cytosol pretreated with trypsin, indicating that p22 interacts with microtubules in a lipid-independent and protein-dependent manner (J. Andrade, S. T. Pearce, H. Zhao and M. Barroso, unpublished work).
As shown in Figure 2(B), both rat liver and kidney cytosols support the ability of myr-p22 to associate with microtubule pellets (lane 1 versus lane 2). Nevertheless, kidney cytosolic proteins have a slightly decreased ability (∼1.6×) to support the p22–microtubule association when compared with that of the liver cytosolic proteins. Since GAPDH has been shown to bind microtubules directly [23,28,29], we have tested the ability of liver or kidney cytosolic GAPDH to associate with the microtubule pellets. As shown in Figure 2(B) (lane 4 versus lane 5), kidney GAPDH also binds microtubule pellets at slightly lower levels than liver GAPDH (∼1.3×).
p22 and GAPDH co-distribute along tracks of microtubules
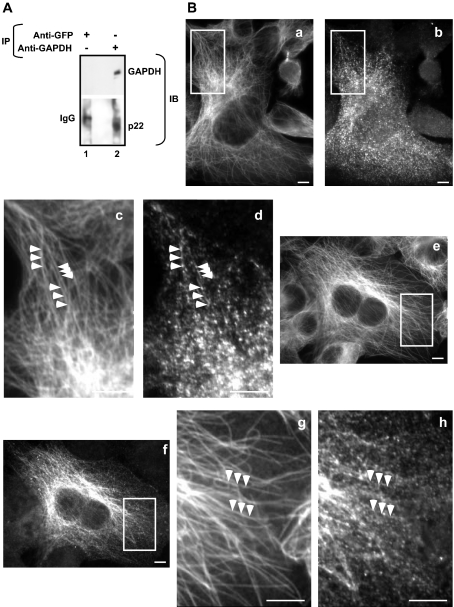
To test whether endogenous p22 and GAPDH interact in rat liver cytosol, immunoprecipitation assays were performed using anti-GAPDH or anti-GFP (Figure 3A, IP), followed by immunoblotting with anti-p22 and anti-GAPDH (Figure 3A, IB). Endogenous p22 and GAPDH were co-immunoprecipitated from rat liver cytosol using anti-GAPDH (lane 2), but not anti-GFP (lane 1). To test whether GAPDH and p22 co-localize in cells, we analysed the intracellular distribution of GAPDH and compared it with that of the endogenous p22. GAPDH plays multiple cellular roles and shows a complex immunofluorescence pattern with a wide distribution throughout the cell [23]. Nevertheless, if GAPDH affects the p22–microtubule association, we would expect at least a fraction of GAPDH to localize along the microtubule tracks, as shown previously for p22 [22]. Technical reasons prevent double-label immunofluorescence for p22 and GAPDH, since the monoclonal anti-GAPDH shows high non-specific background in BHK cells and there is no available monoclonal anti-p22. As an alternative, we performed double-label immunofluorescence of tubulin and p22 and of tubulin and GAPDH, using anti-p22 or anti-GAPDH respectively. As expected, p22 distributes clearly along microtubule tracks (Figure 3B, panels e–h). While the distribution of GAPDH appears mainly cytosolic, a fraction of GAPDH co-localizes with microtubules (panels a–d). At higher magnifications, both GAPDH (panels c and d) and p22 (panels g and h) are detected as small punctate structures that distribute along microtubule tracks.
Figure 3. Characterization of p22–GAPDH interaction.
(A) p22 co-immunoprecipitates with GAPDH. Rat liver cytosolic proteins (500 μg) were incubated with anti-GAPDH (lane 2) or anti-GFP as a negative control (lane 1). Immunoprecipitates (IP) were analysed by immunoblotting (IB) using anti-GAPDH or anti-p22. (B) BHK cells were processed for double-label immunofluorescence microscopy using anti-tubulin (panels a, c, e and g) and anti-GAPDH (panels b and d) or anti-p22 APpep2 antibodies (panels f and h). Panels c, d, g and h are magnifications of the regions of interest (white squares) in panels a, b, e and f respectively. Arrowheads indicate co-localization between microtubule and GAPDH or p22 tracks. Scale bars, 10 μm.
p22-dependent interactions between microtubules and microsomal membranes are facilitated by GAPDH
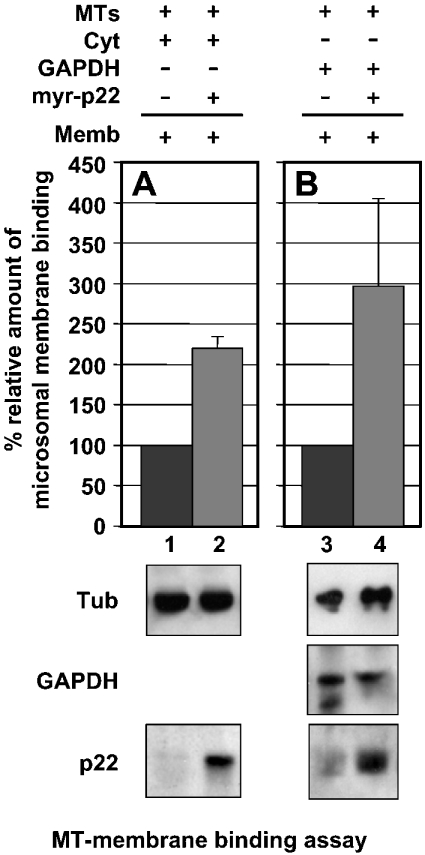
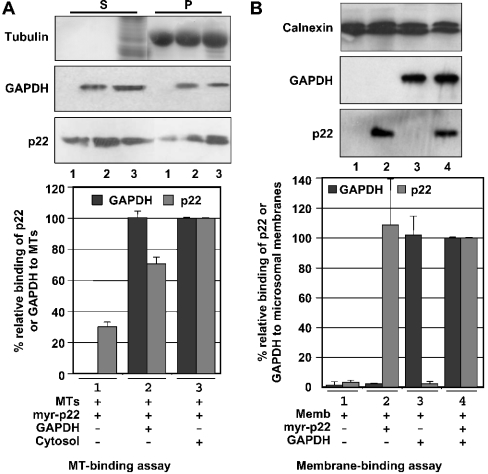
Recently, we have shown that p22 plays an important role in the interactions between microtubules and microsomal membranes [14]. To test whether GAPDH can facilitate the p22-dependent microtubule–membrane interactions, we have used the two-step microtubule–membrane-binding assay that has been described previously and characterized [14]. First, magnetic beads were covered with taxol-polymerized microtubules and incubated in the presence or absence of myr-p22, cytosol and/or GAPDH. Secondly, these microtubule-covered beads were incubated with microsomal membranes and immunoblotted with p22, GAPDH, calnexin and tubulin antibodies to assay the amount of p22, GAPDH, ER membranes and microtubules that are bound to the beads (Figure 4). First, immunoblotting using anti-GAPDH showed that the amount of GAPDH in 500 μg of rat liver cytosolic proteins corresponds to that of 2 μg of purified rabbit muscle GAPDH (J. Andrade, S. T. Pearce, H. Zhao and M. Barroso, unpublished work). Secondly, tubulin, p22 and GAPDH immunoblots confirm the presence of tubulin in magnetic beads incubated in the presence of taxol-polymerized microtubules of p22 in beads incubated with myr-p22 and of GAPDH in beads incubated with purified rabbit muscle GAPDH (Figure 4, lower panels). Thirdly, microsomal membrane binding is detected by the presence of calnexin, an ER membrane marker, bound to microtubule-covered beads (Figure 4, upper panels), as described previously [14]. In Figure 4(A) lane 1, a relative value of 100% has been assigned to the basal microsomal membrane binding supported by microtubule-covered beads that were preincubated with cytosolic proteins. In agreement with our previous results, the addition of myr-p22 increased to approx. 220% of the amount of microsomal membranes that are found associated with microtubule-covered beads (Figure 4A, lane 2). In Figure 4(B), lane 3, a relative value of 100% has been assigned to the basal microsomal membrane binding supported by microtubule-covered beads that were preincubated with purified rabbit muscle GAPDH. Addition of GAPDH in the absence of cytosol and myr-p22 was able to support the binding of microtubule-covered beads to microsomal membranes at a level similar to that of the cytosolic proteins (J. Andrade, S. T. Pearce, H. Zhao and M. Barroso, unpublished work). The well-known ability of GAPDH to bind microtubules and membranes could allow for its role in the modulation of microtubule–membrane interactions [23]. Microtubule-covered beads preincubated with myr-p22 and GAPDH are able to bind significantly higher amounts of microsomal membranes (∼300%) than that of the microtubule-covered beads preincubated with only GAPDH (Figure 4B, lane 4 versus lane 3). Our results suggest that GAPDH supports p22-dependent microtubule–membrane interactions.
Figure 4. Role of GAPDH in the p22-dependent interactions between microsomal membranes and microtubules.
(A) Lower panels: a two-step microtubule–membrane-binding assay [14] was used to assay the role of GAPDH in p22-dependent microtubule–membrane interactions. First, DYNABEADS® M-280 Tosylactivated were covered with taxol-polymerized microtubules and incubated with rat liver cytosol in the presence (lane 2) or absence of myr-p22 (lane 1). Secondly, beads were incubated with microsomal membranes. Equal amounts of reaction mixtures were analysed by SDS/PAGE and immunoblotting using antibodies against tubulin, calnexin and p22. (A) Upper panel: a value of 100% was assigned to the relative binding of microsomal membranes to microtubule-covered beads after incubation with cytosol in the absence of myr-p22 (lane 1). Results represent means±S.D. for three experiments. (B) Lower panels: DYNABEADS® M-280 Tosylactivated were covered with taxol-polymerized microtubules and incubated with purified rabbit muscle GAPDH in the presence (lane 2) or absence of myr-p22 (lane 1). Then, beads were incubated with microsomal membranes. Equal amounts of reaction mixtures were analysed by SDS/PAGE and immunoblotting using antibodies against tubulin, calnexin, p22 and GAPDH. (B) Upper panel: a value of 100% was assigned to the relative binding of microsomal membranes to microtubule-covered beads after incubation with GAPDH in the absence of myr-p22 (lane 1). Results represent means±S.D. for three experiments.
The ability of p22 to associate with microtubules, but not with microsomal membranes, is supported by GAPDH
Previously, we have shown that the Ca2+-mediated increase in p22-dependent microtubule–membrane interactions is due to the ability of Ca2+ to increase the p22–membrane, but not the p22–microtubule, association [14]. In the present study, we propose that the GAPDH-mediated increase in p22-dependent microtubule–membrane interactions is due to the ability of GAPDH to facilitate the p22–microtubule but not the p22–membrane association. To test this hypothesis, we have assayed the ability of GAPDH to facilitate the p22–microtubule association in the absence of cytosol. As shown in Figure 5(A) (upper panels), addition of 2 μg of GAPDH and/or 500 μg of liver cytosol to microtubule co-sedimentation assays stimulates the ability of myr-p22 to bind microtubules (lanes 2 and 3). When the percentage of myr-p22 bound to microtubules in the presence of rat liver cytosol was normalized to 100%, the relative percentage binding of myr-p22 to microtubules in the presence of GAPDH is approx. 70% (Figure 5A, lower p22 panel, lanes 2 and 3). As expected, purified commercially available GAPDH associates with microtubule pellets at a level similar to that of the cytosolic GAPDH (Figure 5A, lower GAPDH panel, lanes 2 and 3).
Figure 5. The association of p22 with microtubules, but not with microsomal membranes, is stimulated by GAPDH.
(A) Upper panels: microtubule co-sedimentation assays were performed in the presence of myr-p22 (lane 1), p22-myr plus rabbit muscle GAPDH (lane 2) or myr-p22 plus rat liver cytosol (lane 3). Equal amounts of supernatants (S) and microtubule pellets (P) were analysed by immunoblotting using anti-p22, anti-tubulin and anti-GAPDH. Lower panel: the percentage of p22 or GAPDH binding to microtubule pellets in the presence of rat liver cytosol was normalized to 100%. Results represent means±S. D. for three experiments. (B) Upper panels: microsomal membranes were incubated in the presence or absence (lane 1) of rabbit muscle GAPDH (lanes 3 and 4) and/or myr-p22 (lanes 2 and 4) and re-pelleted. Equal amounts of membrane pellets were analysed by SDS/PAGE and immunoblotting using p22, GAPDH and calnexin antibodies. Lower panel: immunoblots were quantified as described in the Experimental section. A value of 100% was assigned to the binding of p22 or GAPDH to microsomal membrane pellets on incubation of microsomal membranes in the presence of myr-p22 and GAPDH (lane 4). Results represent means±S. D. for three experiments.
We have shown that GAPDH supports the p22-dependent microtubule–membrane interactions (Figure 4B) and p22–microtubule interactions (Figure 5A). In Figure 5(B), we have tested whether GAPDH can modulate the ability of p22 to associate with microsomal membranes, by incubating microsomal membranes with myr-p22 in the presence or absence of GAPDH. After ultracentrifugation, membrane pellets were assayed by SDS/PAGE and immunoblotting using anti-p22, anti-GAPDH and anti-calnexin (Figure 5B, upper panel). Under these conditions, we were unable to detect significant amounts of endogenous membrane-associated p22 (J. Andrade, S. T. Pearce, H. Zhao and M. Barroso, unpublished work), making this assay suitable to determine the requirements of association of p22 with microsomal membrane pellets as described previously [14]. Calnexin is detected in all membrane pellets independent of GAPDH (Figure 5B, calnexin panels). In the presence or absence of GAPDH, p22 associates with membrane pellets at similar levels (Figure 5B, lower panel), suggesting that p22 binds to microsomal membranes in a GAPDH-independent manner. Similar results were obtained when binding myr-p22 to salt-washed microsomal membranes in the presence or absence of GAPDH (J. Andrade, S. T. Pearce, H. Zhao and M. Barroso, unpublished work).
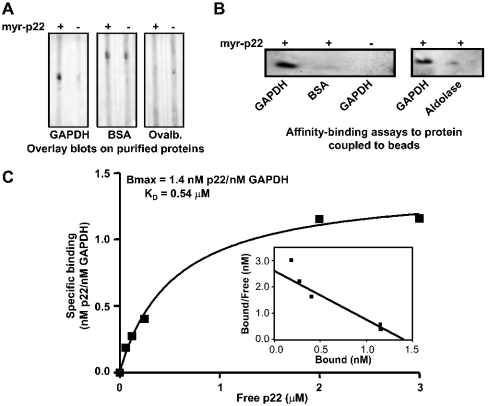
p22 binds directly to GAPDH
To characterize the specificity of the p22–GAPDH interaction in vitro, myr-p22 overlay blots were performed on equal amounts of purified GAPDH, BSA and ovalbumin. As shown in Figure 6(A), only GAPDH binds myr-p22 specifically at detectable levels. To demonstrate a direct interaction between myr-p22 and GAPDH, we have shown that myr-p22 binds to GAPDH, but not BSA or aldolase coupled with Affigel-10 beads (Figure 6B). To determine the affinity-binding constant of the p22–GAPDH interactions, a similar in vitro-binding assay was performed. Binding of myr-p22 to GAPDH-containing beads was dose-dependent and saturable, and the calculated KD and Bmax values were approx. 0.5 μM and 1.4 nM p22/nM GAPDH respectively (Figure 6C).
Figure 6. p22 interacts directly with GAPDH in vitro.
(A) Blot overlays were performed on 5 μg of purified rabbit muscle GAPDH, BSA or ovalbumin (Ovalb.) in the presence (+) or absence (–) of myr-p22. (B) Affigel-10 beads coupled with GAPDH, BSA or aldolase were incubated in the presence (+) or absence (–) of myr-p22. The amount of myr-p22 bound to the beads was detected by immunoblotting using anti-p22. (C) KD analysis of the binding of myr-p22 to GAPDH was performed by incubating the indicated concentrations of myr-p22 (molar amounts) with 50 μg of GAPDH-coupled with beads. The amount of bound myr-p22 was detected by immunoblotting with anti-p22. The values for KD and Bmax were obtained from three repeat experiments as described in the Experimental section.
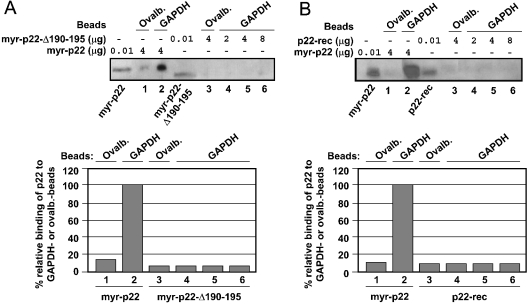
The N-myristoyl group and the last six C-terminal amino acid residues of p22 are required for their interaction with GAPDH
Since the extreme N- and C-termini of p22 are conserved in all p22 homologues, they probably have a functional role. We have analysed the effect of deleting the N-myristoyl group or the last six C-terminal amino acid residues of p22 on its ability to bind GAPDH. myr-p22-Δ190-195 (Figure 7A) or p22-rec (Figure 7B) did not bind GAPDH coupled with Affigel-10 beads, even when double the amount of these mutants are used, compared with that of the myr-p22 (upper panels, lane 6 versus lane 2). When the amount of GAPDH-bound myr-p22 is normalized to 100% (lower panels, lane 2), the amount of GAPDH-bound myr-p22-Δ190-195 or p22-rec is lower than 10%, (lower panels, lanes 4–6). Non-specific binding of myr-p22, myr-p22-Δ190-195 or p22-rec to ovalbumin-coupled Affigel-10 beads is approx. 5–15% (lower panel, lanes 1 and 3).
Figure 7. The last six C-terminal amino acids and the N-myristoyl moiety are required for the binding of p22 to GAPDH.
(A) Upper panel: Affigel-10 beads coupled with GAPDH (lanes 2, 4–6) were incubated in the presence of 4 μg of myr-p22 (lanes 2) or 2, 4 or 8 μg of myr-p22-Δ190-195 (lanes 4–6). Affigel-10 beads coupled with ovalbumin (lanes 1 and 3) were incubated in the presence of 4 μg of myr-p22 (lanes 1) or myr-p22-Δ190-195 (lanes 3). Equal amounts of beads were assayed by immunoblotting with anti-p22. myr-p22 or myr-p22-Δ190-195 (0.01 μg each) was used as the control. (B) Upper panel: Affigel-10 beads coupled with ovalbumin (lanes 1 and 3) were incubated in the presence of 4 μg of myr-p22 (lanes 1) or p22-rec (lanes 3). Affigel-10 beads coupled with GAPDH (lanes 2, 4–6) were incubated in the presence of 4 μg of myr-p22 (lanes 2) or 2, 4 or 8 μg of p22-rec (lanes 4–6). Equal amounts of beads were assayed by immunoblotting with anti-p22. myr-p22 (0.05 μg) or p22-rec (0.01 μg) was used as the control. (A, B) Lower panels: quantification of relative percentage of myr-p22, myr-p22-Δ190-195 or p22-rec binding to GAPDH- or ovalbumin-beads. In (A, B) lanes 1–6 in upper panels correspond to lanes 1–6 in lower panels. In (A, B) lower panels, the amount of myr-p22 associated with GAPDH-coupled beads when 4 μg of myr-p22 was incubated with 50 μg of GAPDH is considered as 100%.
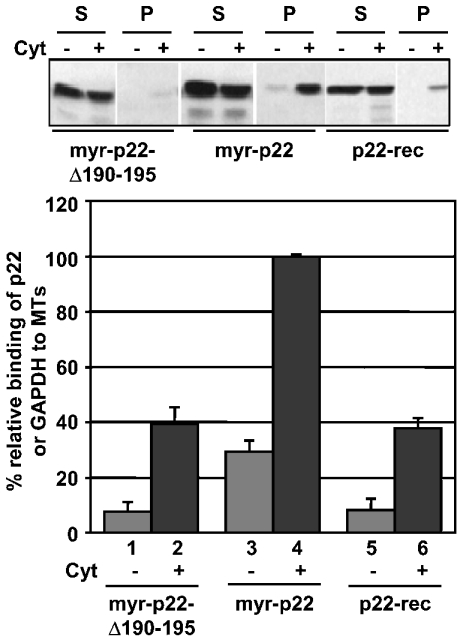
The N-myristoyl group and the last six C-terminal amino acid residues are involved in the p22–microtubule association
If GAPDH positively affects the p22–microtubule association, p22 mutants unable to bind GAPDH should show a reduced ability to associate with microtubule pellets. Thus we have compared the ability of myr-p22-Δ190-195 or p22-rec to bind microtubule pellets in the presence or absence of liver cytosol with that of the myr-p22 (Figure 8, upper panel). When normalizing the percentage binding of myr-p22 to microtubules in the presence of rat liver cytosol to 100%, p22-rec and myr-p22-Δ190-195 associate with microtubule pellets at a relative level of approx. 37.8±3.5 and 39.4±5.8% respectively (Figure 8, lower panel). Thus a significant decrease (∼2.6×) is detected in the ability of either p22-rec or myr-p22-Δ190-195 to associate with microtubule pellets in the presence of cytosol when compared with that of the myr-p22. A similar decrease is detected in the absence of cytosol; whereas myr-p22 associates with microtubules at a relative level of 29.3±3.9%, p22-rec and myr-p22-Δ190-195 bind microtubules at 8.2±4.0 and 7.6±3.0% respectively.
Figure 8. p22 associates with microtubule pellets in a N-myristoylation- and C-terminal-dependent manner.
Upper panel: microtubule co-sedimentation assays were performed in the presence (lanes +Cyt) or absence (lanes −Cyt) of rat liver cytosol and myr-p22-Δ190-195, myr-p22 and/or p22-rec. Equal amounts of supernatants (S) and pellets (P) were analysed by immunoblotting using anti-p22. Lower panel: quantification of percentage relative binding of myr-p22, myr-p22-Δ190-195 or p22-rec to microtubule pellets in the presence or absence of liver cytosol. The percentage of p22 binding to microtubule pellets in the presence of cytosol was normalized to 100%. Results represent means±S.D. for four experiments. MTs, microtubules.
DISCUSSION
Previously, p22 has been shown to modulate microtubule–membrane interactions due to its ability to associate with microtubules and microsomal membranes [14,22]. In agreement with the ability of GAPDH to interact directly with p22 and to facilitate the p22–microtubule, but not the p22–membrane association, we have shown that GAPDH supports the p22–microtubule-binding step of the p22-dependent microtubule–membrane interactions. These results indicate that GAPDH facilitates the p22-dependent microtubule–membrane interactions through its ability to stimulate the binding of p22 to microtubules.
Previously, GAPDH has been shown to display several different activities unrelated to its glycolytic function [23]. In agreement with its potential ability to affect the p22–microtubule association, GAPDH has been shown to interact with the C-terminal region of α-tubulin (residues 409–451) and stimulate the bundling activity of microtubules [30–33]. Furthermore, GAPDH isoforms have been implicated in membrane fusion and trafficking [25,34–37]. Several lines of evidence indicate a connection between the functions of GAPDH in membrane fusion and microtubule organization. First, a heterozygous mutation of GAPDH in Chinese-hamster ovary cells affects membrane trafficking by increasing the interaction of GAPDH with microtubules [34]. Secondly, GAPDH plays a role in microtubule organization and dynamics within the early secretory pathway [24]. Thirdly, tubulin was identified as an endogenous inhibitor of the GAPDH isoform that catalyses membrane fusion [38,39]. Taken together, these and our results suggest that p22 and GAPDH co-operate to facilitate microtubule–membrane interactions within the early secretory pathway.
Previously, we have shown that cytosolic microtubule-binding proteins are involved in the p22–microtubule association [22]. To search for these cytosolic proteins, we took advantage of the capacity of kidney and liver cytosolic proteins to stimulate the p22–microtubule association in vitro. Our blot overlay results indicate that only one p22-binding partner (∼35–40 kDa) is present in the microtubule pellets containing either liver or kidney cytosolic proteins. This ∼35–40 kDa protein was identified as GAPDH using MS. In agreement with a p22-GAPDH co-localization in vivo, antibodies against GAPDH co-immunoprecipitate GAPDH and p22 from rat liver cytosol, and a fraction of endogenous GAPDH and p22 localize to analogous small punctate structures distributed along microtubule tracks. These results suggest that cytosolic GAPDH interacts with p22 along microtubule tracks.
Our previous results suggest that p22 interacts with microtubules through a cytosolic protein with a molecular mass over the range of 30–70 kDa and a low affinity for microtubules [22]. GAPDH has a molecular mass of 38 kDa, and previous studies have shown that KD is approx. 1 μM for the GAPDH–microtubule binding [23,29,40]. GAPDH associates directly and specifically with p22, and KD is approx. 0.5 μM for the p22–GAPDH interaction. The relatively low affinity of p22 towards GAPDH could be explained in several different ways. First, the intracellular amount of GAPDH is extremely high (∼2% of total liver cytosolic proteins), when compared with that of the p22. Secondly, affinity of GAPDH towards p22 and microtubules is similar. Thirdly, KD for the p22–GAPDH interaction was determined for bacterially expressed p22 and commercially available rabbit muscle GAPDH; it is possible that KD for the interaction between endogenous p22 and GAPDH is actually lower, due to post-translational modifications. Although our results suggest that GAPDH acts to mediate the interaction of p22 with microtubules, they do not exclude the involvement of other proteins in the p22–microtubule association (see below) and the existence of a low-affinity direct interaction between p22 and microtubules, which would allow for the consistent and reproducible ability of a minor fraction of p22 to bind microtubules independent of cytosol (15–30%).
We have shown that, whereas N-myristoylation and other yet unknown p22 regions are involved in the p22–microtubule association, ability of p22 to bind Ca2+ and to undergo Ca2+-mediated conformational changes is not involved in the p22–microtubule association [14,22]. In the present study, we have confirmed and expanded these observations by demonstrating that the N-myristoyl group and the last six C-terminal amino acid residues participate in the binding of p22 to GAPDH and microtubules. These regions appear to be required for the indirect GAPDH-mediated p22–microtubule association and for the minor direct binding of p22 to microtubules. p22 may bind GAPDH directly through a non-linear binding site, which should include at least the N-myristoyl group and the extreme C-terminal region. Furthermore, our results also suggest that p22 interacts with microtubules through cytosolic proteins other than GAPDH in an N-myristoylation- and C-terminal-independent manner.
Several lines of evidence suggest that liver GAPDH should be responsible for approx. 70% of the p22–microtubule-binding activity with other yet unknown proteins accounting for the remaining approx. 30%. First, GAPDH or kidney cytosol can only recover approx. 60–70% of the p22–microtubule-binding activity detected in liver cytosol. Secondly, GAPDH acts as the only microtubule-associated p22-binding partner in kidney cytosol, whereas liver cytosol contains at least four p22-binding partners, including GAPDH. Thirdly, the absence of either the N-myristoyl group or the last six C-terminal residues reduces the p22–microtubule association to approx. 36–38%. These results suggest that other yet unknown p22–microtubule-binding modulators are capable of stimulating the p22–microtubule association in a tissue-specific manner; they act independent of N-myristoylation and C-terminal region of p22, and thus their mechanism of action should be distinct from that of GAPDH. Alternatively, GAPDH may be subjected to different post-translational modifications in liver and kidney. The reason for the tissue specificity of p22–microtubule-binding activity is not known, but may be related to a distinct organization of the microtubule network in epithelial cells with different morphology, shape and secretory functions [41]. Together our results suggest that the p22–microtubule association occurs in a complex manner that depends on the protein conformation of p22 as well as on its ability to interact with GAPDH and other yet unknown p22–microtubule-binding protein(s).
Acknowledgments
We thank Dr E. Tisdale for the GAPDH antibodies and Dr E. Sztul for her review of our paper.
References
- 1.Bloom G. S., Goldstein L. S. Cruising along microtubule highways: how membranes move through the secretory pathway. J. Cell Biol. 1998;140:1277–1280. doi: 10.1083/jcb.140.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan V. J., Schroer T. A. Membrane motors. Curr. Opin. Cell Biol. 1999;11:476–482. doi: 10.1016/s0955-0674(99)80068-4. [DOI] [PubMed] [Google Scholar]
- 3.Karcher R. L., Deacon S. W., Gelfand V. I. Motor-cargo interactions: the key to transport specificity. Trends Cell Biol. 2002;12:21–27. doi: 10.1016/s0962-8924(01)02184-5. [DOI] [PubMed] [Google Scholar]
- 4.Cassimeris L., Spittle C. Regulation of microtubule-associated proteins. Int. Rev. Cytol. 2001;210:163–226. doi: 10.1016/s0074-7696(01)10006-9. [DOI] [PubMed] [Google Scholar]
- 5.Schuyler S. C., Pellman D. Microtubule ‘plus-end-tracking proteins’: the end is just the beginning. Cell (Cambridge, Mass.) 2001;105:421–424. doi: 10.1016/s0092-8674(01)00364-6. [DOI] [PubMed] [Google Scholar]
- 6.Hammer J. A., III, Wu X. S. Rabs grab motors: defining the connections between Rab GTPases and motor proteins. Curr. Opin. Cell Biol. 2002;14:69–75. doi: 10.1016/s0955-0674(01)00296-4. [DOI] [PubMed] [Google Scholar]
- 7.Perez F., Pernet-Gallay K., Nizak C., Goodson H. V., Kreis T. E., Goud B. CLIPR-59, a new trans-Golgi/TGN cytoplasmic linker protein belonging to the CLIP-170 family. J. Cell Biol. 2002;156:631–642. doi: 10.1083/jcb.200111003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard J., Hyman A. A. Dynamics and mechanics of the microtubule plus end. Nature (London) 2003;422:753–758. doi: 10.1038/nature01600. [DOI] [PubMed] [Google Scholar]
- 9.Charrasse S., Schroeder M., Gauthier-Rouviere C., Ango F., Cassimeris L., Gard D. L., Larroque C. The TOGp protein is a new human microtubule-associated protein homologous to the Xenopus XMAP215. J. Cell Sci. 1998;111:1371–1383. doi: 10.1242/jcs.111.10.1371. [DOI] [PubMed] [Google Scholar]
- 10.Klopfenstein D. R., Klumperman J., Lustig A., Kammerer R. A., Oorschot V., Hauri H. P. Subdomain-specific localization of CLIMP-63 (p63) in the endoplasmic reticulum is mediated by its luminal alpha-helical segment. J. Cell Biol. 2001;153:1287–1300. doi: 10.1083/jcb.153.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klopfenstein D. R., Kappeler F., Hauri H. P. A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 1998;17:6168–6177. doi: 10.1093/emboj/17.21.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walenta J. H., Didier A. J., Liu X., Kramer H. The Golgi-associated hook3 protein is a member of a novel family of microtubule-binding proteins. J. Cell Biol. 2001;152:923–934. doi: 10.1083/jcb.152.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barroso M. R., Bernd K. K., DeWitt N. D., Chang A., Mills K., Sztul E. S. A novel Ca2+-binding protein, p22, is required for constitutive membrane traffic. J. Biol. Chem. 1996;271:10183–10187. doi: 10.1074/jbc.271.17.10183. [DOI] [PubMed] [Google Scholar]
- 14.Andrade J., Zhao H., Titus B., Timm P. S., Barroso M. The EF-hand Ca2+-binding protein p22 plays a role in microtubule and endoplasmic reticulum organization and dynamics with distinct Ca2+-binding requirements. Mol. Biol. Cell. 2004;15:481–496. doi: 10.1091/mbc.E03-07-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuwahara H., Kamei J., Nakamura N., Matsumoto M., Inoue H., Kanazawa H. The apoptosis-inducing protein kinase DRAK2 is inhibited in a calcium-dependent manner by the calcium-binding protein CHP. J. Biochem. (Tokyo) 2003;134:245–250. doi: 10.1093/jb/mvg137. [DOI] [PubMed] [Google Scholar]
- 16.Lin X., Barber D. L. A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12631–12636. doi: 10.1073/pnas.93.22.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin X., Sikkink R. A., Rusnak F., Barber D. L. Inhibition of calcineurin phosphatase activity by a calcineurin B homologous protein. J. Biol. Chem. 1999;274:36125–36131. doi: 10.1074/jbc.274.51.36125. [DOI] [PubMed] [Google Scholar]
- 18.Pang T., Su X., Wakabayashi S., Shigekawa M. Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J. Biol. Chem. 2001;276:17367–17372. doi: 10.1074/jbc.M100296200. [DOI] [PubMed] [Google Scholar]
- 19.Di Sole F., Cerull R., Babich V., Quinones H., Gisler S. M., Biber J., Murer H., Burckhardt G., Helmle-Kolb C., Moe O. W. Acute regulation of Na/H exchanger NHE3 by adenosine A1 receptors is mediated by calcineurin homologous protein (CHP) J. Biol. Chem. 2003;279:2962–2974. doi: 10.1074/jbc.M306838200. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura N., Miyake Y., Matsushita M., Tanaka S., Inoue H., Kanazawa H. KIF1Bbeta2, capable of interacting with CHP, is localized to synaptic vesicles. J. Biochem. (Tokyo) 2002;132:483–491. doi: 10.1093/oxfordjournals.jbchem.a003246. [DOI] [PubMed] [Google Scholar]
- 21.Kim H., Ha C. M., Choi J., Choi E. J., Jeon J., Kim C., Park S. K., Kang S. S., Kim K., Lee B. J. Ontogeny and the possible function of a novel epidermal growth factor-like repeat domain-containing protein, NELL2, in the rat brain. J. Neurochem. 2002;83:1389–1400. doi: 10.1046/j.1471-4159.2002.01245.x. [DOI] [PubMed] [Google Scholar]
- 22.Timm S., Titus B., Bernd K., Barroso M. The EF-hand Ca2+-binding protein p22 associates with microtubules in an N-myristoylation-dependent manner. Mol. Biol. Cell. 1999;10:3473–3488. doi: 10.1091/mbc.10.10.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirover M. A. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta. 1999;1432:159–184. doi: 10.1016/s0167-4838(99)00119-3. [DOI] [PubMed] [Google Scholar]
- 24.Tisdale E. J. Glyceraldehyde-3-phosphate dehydrogenase is phosphorylated by protein kinase Ciota/lambda and plays a role in microtubule dynamics in the early secretory pathway. J. Biol. Chem. 2002;277:3334–3341. doi: 10.1074/jbc.M109744200. [DOI] [PubMed] [Google Scholar]
- 25.Tisdale E. J. Glyceraldehyde-3-phosphate dehydrogenase is required for vesicular transport in the early secretory pathway. J. Biol. Chem. 2001;276:2480–2486. doi: 10.1074/jbc.M007567200. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz H. D., Bereiter-Hahn J. Glyceraldehyde-3-phosphate dehydrogenase associates with actin filaments in serum deprived NIH 3T3 cells only. Cell Biol. Int. 2002;26:155–164. doi: 10.1006/cbir.2001.0819. [DOI] [PubMed] [Google Scholar]
- 27.Pang T., Wakabayashi S., Shigekawa M. Expression of calcineurin B homologous protein 2 protects serum deprivation-induced cell death by serum-independent activation of Na+/H+ exchanger. J. Biol. Chem. 2002;277:43771–43777. doi: 10.1074/jbc.M208313200. [DOI] [PubMed] [Google Scholar]
- 28.Somers M., Engelborghs Y., Baert J. Analysis of the binding of glyceraldehyde-3-phosphate dehydrogenase to microtubules, the mechanism of bundle formation and the linkage effect. Eur. J. Biochem. 1990;193:437–444. doi: 10.1111/j.1432-1033.1990.tb19357.x. [DOI] [PubMed] [Google Scholar]
- 29.Walsh J. L., Keith T. J., Knull H. R. Glycolytic enzyme interactions with tubulin and microtubules. Biochim. Biophys. Acta. 1989;999:64–70. doi: 10.1016/0167-4838(89)90031-9. [DOI] [PubMed] [Google Scholar]
- 30.Kumagai H., Sakai H. A porcine brain protein (35 K protein) which bundles microtubules and its identification as glyceraldehyde 3-phosphate dehydrogenase. J. Biochem. (Tokyo) 1983;93:1259–1269. doi: 10.1093/oxfordjournals.jbchem.a134260. [DOI] [PubMed] [Google Scholar]
- 31.Huitorel P., Pantaloni D. Bundling of microtubules by glyceraldehyde-3-phosphate dehydrogenase and its modulation by ATP. Eur. J. Biochem. 1985;150:265–269. doi: 10.1111/j.1432-1033.1985.tb09016.x. [DOI] [PubMed] [Google Scholar]
- 32.Durrieu C., Bernier-Valentin F., Rousset B. Binding of glyceraldehyde 3-phosphate dehydrogenase to microtubules. Mol. Cell. Biochem. 1987;74:55–65. doi: 10.1007/BF00221912. [DOI] [PubMed] [Google Scholar]
- 33.Volker K. W., Knull H. A glycolytic enzyme binding domain on tubulin. Arch. Biochem. Biophys. 1997;338:237–243. doi: 10.1006/abbi.1996.9819. [DOI] [PubMed] [Google Scholar]
- 34.Robbins A. R., Ward R. D., Oliver C. A mutation in glyceraldehyde 3-phosphate dehydrogenase alters endocytosis in CHO cells. J. Cell Biol. 1995;130:1093–1104. doi: 10.1083/jcb.130.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa T., Hirano Y., Inomata A., Yokota S., Miyachi K., Kaneda M., Umeda M., Furukawa K., Omata S., Horigome T. Participation of a fusogenic protein, glyceraldehyde-3-phosphate dehydrogenase, in nuclear membrane assembly. J. Biol. Chem. 2003;278:20395–20404. doi: 10.1074/jbc.M210824200. [DOI] [PubMed] [Google Scholar]
- 36.Han X., Ramanadham S., Turk J., Gross R. W. Reconstitution of membrane fusion between pancreatic islet secretory granules and plasma membranes: catalysis by a protein constituent recognized by monoclonal antibodies directed against glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta. 1998;1414:95–107. doi: 10.1016/s0005-2736(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 37.Hessler R. J., Blackwood R. A., Brock T. G., Francis J. W., Harsh D. M., Smolen J. E. Identification of glyceraldehyde-3-phosphate dehydrogenase as a Ca2+-dependent fusogen in human neutrophil cytosol. J. Leukoc. Biol. 1998;63:331–336. doi: 10.1002/jlb.63.3.331. [DOI] [PubMed] [Google Scholar]
- 38.Glaser P. E., Han X., Gross R. W. Tubulin is the endogenous inhibitor of the glyceraldehyde 3-phosphate dehydrogenase isoform that catalyzes membrane fusion: implications for the coordinated regulation of glycolysis and membrane fusion. Proc. Natl. Acad. Sci. U.S.A. 2002;99:14104–14109. doi: 10.1073/pnas.222542999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glaser P. E., Gross R. W. Rapid plasmenylethanolamine-selective fusion of membrane bilayers catalyzed by an isoform of glyceraldehyde-3-phosphate dehydrogenase: discrimination between glycolytic and fusogenic roles of individual isoforms. Biochemistry. 1995;34:12193–12203. doi: 10.1021/bi00038a013. [DOI] [PubMed] [Google Scholar]
- 40.Muronetz V. I., Wang Z. X., Keith T. J., Knull H. R., Srivastava D. K. Binding constants and stoichiometries of glyceraldehyde 3-phosphate dehydrogenase-tubulin complexes. Arch. Biochem. Biophys. 1994;313:253–260. doi: 10.1006/abbi.1994.1385. [DOI] [PubMed] [Google Scholar]
- 41.Hamm-Alvarez S. F., Sheetz M. P. Microtubule-dependent vesicle transport: modulation of channel and transporter activity in liver and kidney. Physiol. Rev. 1998;78:1109–1129. doi: 10.1152/physrev.1998.78.4.1109. [DOI] [PubMed] [Google Scholar]