Abstract
Seed dispersal is an important ecological process and has important implications for plant population expansion and regeneration. Seed dispersal not only reduces the probability of death due to seed density but also facilitates seedling establishment. Many studies have focused on the effect of one or two factors on seed dispersal. However, little is known about studies on the effect of multiple factors and their interactions on seed dispersal. Here, we conducted a field experiment to explore how seed size, soil burial, and seed peeling affect the dispersal and hoarding of seeds of Quercus liaotungensis in dispersal animals. We found that large seeds were preferentially selected by animals, and the predation after dispersal, hoarding after dispersal, predation distance after dispersal, and hoarding distance after dispersal of large seeds were significantly greater than small seeds, which is more beneficial to the plant expansion and regeneration. Soil burial increased the time of seed intact in situ, significantly increased predation in situ, and reduced predation after dispersal, predation distance after dispersal, and hoarding distance after dispersal, which is not beneficial to the plant population expansion and regeneration. Seed peeling reduced the time of seed intact in situ, and the predation after dispersal was significantly greater than that of unpeeled seeds, which is not beneficial to the plant population. We did not find the interactions between seed size, soil burial, and seed peeling on dispersal. The effects of a single factor may be more than their interactions between seed size, soil burial and seed peeling on dispersal. These results implied that seed size, soil burial and seed peeling may affect plant population expansion and regeneration by affecting the dispersal and hoarding of animals.
Keywords: Plant expansion, Plant regeneration, Seed sizes, Soil burial, Seed peeling, Scatter-hoarding
1. Introduction
Seed dispersal is essential for the successful expansion and regeneration of plants. The Janzen-Connell hypothesis [1,2] indicates that seed mortality is affected by density and distance, and the farther away the seed is from the mother tree, the greater the chance of escape, and the higher the probability of seedling establishment. Small mammals’ cache behavior of scatter-hoarding (which means that small mammals cache seed in many points where only one or a few seeds per point) is beneficial for seed germination and seedling establishment [3,4]. Seeds dispersed by animals could keep the seeds away from the mother tree and reduce the seed density, thus reducing seed predation and seedling competition with the mother tree. In addition, seeds have a higher probability of being dispersed to suitable habitats, which is beneficial to seedling establishment and growth [5,6]. Previous studies mainly focused on the effect of one or two factors on rodent-mediated seed secondary dispersal. However, many factors can affect the process of seed dispersal, which can cause large differences in seed consumption, scatter-hoarding and dispersal distance, thus affecting plant expansion and regeneration. For example, in the field conditions, the half-retention time of Armeniaca sibirica and Prunus davidiana seeds at the release point is affected by the interaction between habitat and season, habitat and seed category, and seed category and season, respectively [5]. It is essential to fully understand how multiple factors and the interactions among them affect seed dispersal.
Acorn-rodent interactions are critical for oak population expansion and regeneration. Previous studies have indicated that some factors may influence the oak seed dispersal process. For example, intraspecific seed size is an important indicator of seed quality and nutritional value [7]. Larger seeds have more nutrients, which helps animals to obtain higher nutrient returns while retrieving the same number of seed points during food shortage [6,8,9]. Therefore, large seeds with high nutritional value are usually scatter-hoarded by animals and dispersed to further places, while small seeds with lower nutritional value are eaten in situ [[10], [11], [12], [13], [14]]. Soil burial is related to the survival rate of seeds [5]. Unburied seeds are more likely to be found by hoarding animals and/or predators [15,16]. Soil burial not only reduces the chance for animals to find seeds through olfaction, but also makes it more difficult for animals to search for seeds through vision [4,5,16,17]. Seed peeling affects seed germination and seedling establishment. Seed coat may limit seed germination, for example, Yan et al. [18] found that seed coat has a significant inhibitory effect on seed germination in Liaodong oak (Quercus liaotungensis). In addition, seed peeling is usually related to the identification of seed quality [19]. For example, Tamias sibiricus removes the acorn pericarp mainly to distinguish the health of seeds to select healthy acorns for scatter-hoarding [19]. Although there have been some studies demonstrating the effects of seed size, soil burial, and seed peeling on seed dispersal, there still needs more evidence on how the interaction between these factors affects seed dispersal and thus plant expansion and regeneration.
Liaodong oak (Q. liaotungensis) is one of the important dominant tree species of warm temperate deciduous forest in China and plays an important role in regional climate regulation, soil and water conservation, and maintenance of ecosystem stability [14,20]. Its seeds mature and disperse in late August to late September, and become seedlings next spring [13]. As the seeds contain a lot of starch and other nutrients, they are subjected to a lot of predation by small rodents after they are shed, and it has been confirmed that the germination rate of Liaodong oak is less than 0.1 % in natural environments, even in the acorn mast-years [21,22]. Seed dispersal, germination, and seedling establishment are important for the expansion and regeneration of the Liaodong oak population. Previous studies indicated that seed dispersal of Liaodong oak is affected by intraspecific seed size and soil burial, respectively [13,14,23]. However, whether a single factor or multiple factors interactions have a greater impact on rodent-mediated seed dispersal has not been well studied. Here, we conducted a field experiment to explore how seed size, soil burial, seed peeling and their interactions affect plant population expansion and regeneration by affecting animal predation and dispersal. We expect that (1) large seeds are more likely to be scatter-hoarding to further places, soil burial will cause animals to consume more seeds and cache seeds nearby, peeled seeds are more likely to be recovered and eaten by animals, and (2) One factor of seed size, soil burial, and seed peeling may affect the seed dispersal process more than their interactions. The results contribute to a deeper understanding of expansion and regeneration mechanisms in Quercus species, and reveal the coevolutionary relationships between animals and plants.
2. Materials and methods
2.1. Study sites
The study was conducted in Liupanshan National Nature Reserve in Ningxia (106°09′ to 106°30′ E, 35°15′ to 35°41′ N). This area is located at the edge of the farming-pastoral ecotone in the north and belongs to the continental monsoon climate. The annual rainfall in the area is about 760 mm and the mean annual temperature is 5.8 °C. The soil type is mainly gray cinnamon, with a small amount of red soil. The most dominant species is Q. liaotungensis, and small rodents mainly include Apodemus peninsulae, Niviventer confucianus, Apodemus agrarius, and Sciurotamias davidianus [14,24].
2.2. Seed Collection and Marking
We harvested seeds from 30-year-old Q. liaotungensis shrubs (The tree's age based on information provided by the Nature Reserve). On September 20, 2018, in Q. liaotungensis shrubs, 40 canopy plants of relatively large size (approximately 2.5 m tall) and vigorous canopy growth were selected to harvest mature seeds from different directions, with about 500 seeds per tree. All the seeds harvested were thoroughly mixed, and we selected 1080 large seeds and 1080 small seeds separately by observation none of them were infested, and randomly selected 100 large and 100 small seeds each to measure fresh weight, long axis, and short axis diameter [13]. The large seeds were 3.05 ± 0.38 g weight (mean standard deviation (SD)) (n = 100), 21.78 ± 1.20 mm long axis diameter, and 15.95 ± 0.85 mm short axis diameter, while the small seeds were 1.46 ± 0.27 g weight, 16.65 ± 1.33 mm long axis diameter and 12.13 ± 0.70 mm short axis diameter. There were significant differences in fresh weight and long and short axes between the large and small seeds, and the method of distinguishing the seed sizes was referred by Zhang et al. [13].
We used the seed-tagging method to mark seeds [25]. A 7.2 cm long copper wire with a diameter of 0.8 mm was attached to a pink plastic label with a size of 2 cm × 1.2 cm [containing the mass of the copper wire (0.16 ± 0.001, n = 100) g]. Pertinent informations such as plot location, seed size, peeled, unpeeled, bare ground and buried were written on each label in pencil. This information was used to locate and record the fate of the seeds [25]. A small hole was drilled at the base of each seed with an electric drill of 1 mm in diameter; then, a copper wire was put through the hole, and connected with a red plastic label [25].
2.3. Seed experiment
We randomly selected three transects in the secondary forest of Q. liaotungensis, each transect is separated by more than 100 m. Next, we chose three large plots along the slope, maintaining a distance of approximately 25 m between neighboring large plots on each transect to prevent interactions [24]. Each large plot was 7 m × 7 m, and then four 1 m × 1 m plots were set at four corners of the large plot, with a 5 m interval between each small plot (Fig. 1). The seeds were categorized into unpeeled large seeds, peeled large seeds, unpeeled small seeds, and peeled small seeds, and four treatments were employed: unpeeled bare ground (after removing the litter), peeled bare ground, unpeeled soil burial (at a depth of 5 mm), and peeled soil burial. These four treatments were randomly assigned to the four small plots, the large and small seeds were in a 1:1 ratio (Fig. 1). The total number of seeds used was calculated as follows: 60 seeds (30 large and 30 small seeds) × 3 plots × 4 treatments × 3 replicates = 2160.
Fig. 1.
Distribution diagram of release plots in sample transect.
We recorded the number of seeds that were intact in situ, predation, dispersal, and burial on the 1st, 2nd, 3rd, 5th, 15th and 30th days after the set up of the plots and within a radius of 30 m around each plot [5,13,26]. Here, we recorded the categories of seed fates [13,27]: predation in situ (PIS), predation after dispersal (PAD), and hoarding after dispersal (HAD). No further analysis was conducted for missed seeds (may be in a burrow or not seen or beyond 30 m) as no significant differences were observed between different seed sizes, soil burial and seed peeling. We also recorded predation distance after dispersal (PDAD), and hoarding distance after dispersal (HDAD) [5].
2.4. Data analysis
We computed generalized linear mixed-effects models (GLMMs) for seed fate, predation distance, and hoarding distance. We used seed sizes, burial state and seed peeling as explanatory variables, the plot as a random effect, and seed fates, and dispersal distance as dependent variables. We fitted generalized linear mixed-effects models for Gaussian variables (family = Gaussian, link = identity) and count variables (family = Poisson, link = log) in the “lmer4 package” [28]. Furthermore, we used generalized linear mixed-effects models to test the pairwise interaction between seed sizes, burial state and seed peeling, and the three factors’ interactions on seed fates and dispersal distance. These analyses were performed with R 4.2.2 [29]. T-test for paired samples with SPSS (Version 21.0) was used to evaluate the difference between seed fates (PIS, PAD and HAD) and dispersal distances (PDAD and HDAD) in a single variable (seed sizes, burial state and seed peeling), and the least significant difference method (LSD) was used to detect differences [24]. The normality test and standardization of data are conducted in SPSS 21.0.
We conducted an inter-sample similarity analysis (decorana) on the data to understand the similarities and differences between the samples to make the subsequent redundancy analysis (RDA) more reliable. We conducted redundancy analysis (RDA) using the vegan package in the R 4.2.2, and performed two different permutation tests to assess the ability of the independent variable on each dependent variable, and performed 999 permutation tests on the RDA model using the permutes function to assess the overall independent variable interpreted each dependent variable. Then 9999 permutation tests were performed for each factor using the envfit function to assess the amount of interpretation of the individual independent variable for the dependent variable.
We used SigmaPlot version 12.5 and R 4.2.2 to create all the figures.
3. Results
From field experiments, we found that soil burial was associated with predation in situ; seed size and seed peeling were associated with predation after dispersal; seed size and soil burial were associated with hoarding after dispersal, predation and hoarding distance after dispersal, respectively. However, we did not find significant effects of the interaction between seed size, seed peeling, and soil burial on intact in situ, predation and hoarding after dispersal, predation and hoarding distance after dispersal.
3.1. Intact seeds
The peeled large seeds on the bare ground had the shortest intact in situ time and the lowest intact in situ rate. In contrast, the buried unpeeled small seeds had the intact in situ time and the highest intact in situ rate (Fig. 2). The intact in situ rate of large seeds was lower than small seeds, and especially on the 2nd day, the intact in situ rate of large seeds was significantly higher than small seeds (P < 0.05). The intact in situ rate of seeds on bare ground was significantly lower than soil buried (P < 0.01). The bare-peeled seeds were the fastest removed by rodents, after the 1st day of seed release, the intact in situ rates of large and small seeds were 27.22 % and 33.33 % (Fig. 2). The intact in situ rate of peeled seeds was significantly higher than unpeeled seeds on the 1st and 2nd days (P < 0.05 and P < 0.01). After 6th day of seed release, except for a few unpeeled and buried seeds, other seeds were completely consumed by rodents (Fig. 2).
Fig. 2.
Intact in situ rate of different seeds with different characteristics in bare land and soil.
3.2. Effects of seed sizes, soil burial, and seed peeling on seed fates
Seed size, soil burial and seed peeling had different effects on the three seed fates. Specifically, large seeds generally had higher rates of predation and hoarding after dispersal than small seeds (F = 12.80, P < 0.01 and F = 4.06, P < 0.05 respectively) (Table 1, Fig. 3). Soil burial significantly increased predation in situ and reduced predation after dispersal of seeds (F = 21.37, P < 0.001 and F = 24.76, P < 0.001 respectively) (Table 1, Fig. 3). Peeled seeds had higher rates of predation after dispersal than unpeeled seeds (F = 20.81, P < 0.001) (Table 1, Fig. 3). Whether the seed peeled or not, the predation in situ rate of small seeds was significantly greater than large seeds on bare ground (P < 0.05) (Fig. 3a). The predation after dispersal rate of large and peeled seeds was significantly greater than small and peeled seeds on bare ground (P < 0.05) (Fig. 3c), and large and unpeeled seeds also was significantly greater than small and unpeeled seeds under the soil buried (P < 0.001) (Fig. 3d). The predation after dispersal rate of peeled seeds was significantly greater than unpeeled seeds under the soil buried (P < 0.05) (Fig. 3d). The hoarding after dispersal rate of large and peeled seeds was significantly greater than small seeds under the soil buried (P < 0.01) (Fig. 3f).
Table 1.
The effects of seed size (SS), burial state (BS), and seed peeling (SP) on predation in situ (PIS), predation after dispersal (PAD), hoarding after dispersal (HAD), predation distance after dispersal (PDAD), and hoarding distance after dispersal (HDAD) of rodents.
| Variables | SS | BS | SP | SS × BS | SS × SP | BS × SP | SS × BS × SP |
|---|---|---|---|---|---|---|---|
| PIS | 1.96 | 21.37c | 1.33 | 0.14 | 0.17 | 4.19 | 0 |
| PAD | 12.80b | 0.05 | 20.81c | 0.37 | 0.09 | 3.012 | 3.32 |
| HAD | 4.06a | 24.76c | 0.54 | 0.09 | 0.05 | 1.51 | 0.11 |
| PDAD | 13.31b | 12.84b | 2.59 | 0 | 0 | 1.32 | 0.1 |
| HDAD | 129.34c | 8.17a | 1.03 | 1.27 | 0.37 | 1.11 | 0.05 |
Note.
P < 0.05.
P < 0.01.
P < 0.001.
Fig. 3.
Effect of seed sizes, soil burial, and seed peeling on seed fate. Note: Different capital letters indicate significant differences between peeled and unpeeled seeds in the same state of placement, * indicates significant differences between seed sizes in the same treatment, *P < 0.05, **P < 0.01 and ***P < 0.001.
3.3. Effects of seed sizes, soil burial, and seed peeling on seed dispersal distance
Seed size and soil burial had effects on predation and hoarding distance after dispersal, but seed peeling did not. The average predation and hoarding distance after dispersal of seeds were 5.4 ± 0.7 m and 5.0 ± 0.6 m. Large, unpeeled, and bare seeds had maximum predation and hoarding distance (21 and 22 m, respectively). Large seeds have farther predation and hoarding distance after dispersal than small seeds (F = 129.34, P < 0.001 and F = 13.31, P < 0.01 respectively) (Table 1, Fig. 4). Soil burial significantly reduced predation and hoarding distance after the dispersal of seeds (F = 8.17, P < 0.05 and F = 12.84, P < 0.01 respectively) (Table 1, Fig. 4). Seed peeling had no significant effect on predation and hoarding distance after dispersal of seeds (Table 1). The predation distance after the dispersal of large seeds was significantly greater than small seeds (P < 0.001) (Fig. 4a and b). The hoarding distance after the dispersal of large seeds was significantly greater than small seeds under the soil buried (P < 0.05) (Fig. 4d).
Fig. 4.
Effect of seed sizes, soil burial, and seed peeling on seed dispersal distance. Note: Different capital letters indicate significant differences between peeled and unpeeled seeds in the same state of placement, * indicates significant differences between seed sizes in the same treatment, *P < 0.05 and ***P < 0.001.
3.4. Interpretation of seed sizes, soil burial, and seed peeling on the dependent variable
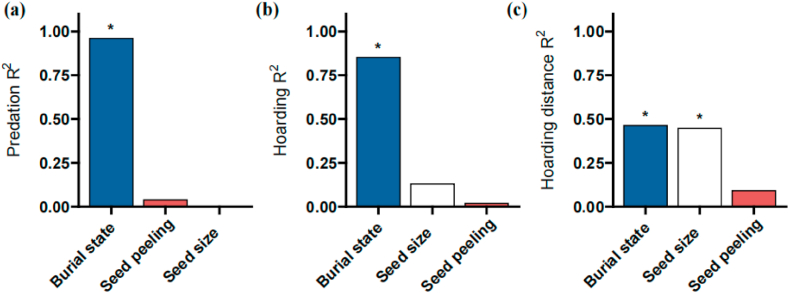
The overall interpretation of seed size, soil burial, and seed peeling for predation (includes predation in situ and predation after dispersal), hoarding and hoarding distance was 42.62 %, 58.64 %, and 62.26 %, respectively (Fig. 5). Soil burial was significantly explained by both predation and hoarding (P < 0.05), and seed size and peeling were not significantly explained by both predation and hoarding (Fig. 5a and b). Soil burial and seed size were both significant levels (P < 0.05), and peeling was not significant (Fig. 5c).
Fig. 5.
Explanation of seed size, soil burial, and seed peeling on seed predation (includes predation in situ and predation after dispersal), hoarding and hoarding distance. * indicates the interpretation of independent variable has a significant effect on dependent variable (P < 0.05).
4. Discussion
Seed dispersal is an important process of plant expansion and regeneration. Specifically, fewer seeds are eaten and more seeds scatter-hoarding farther away, the more beneficial to seed germination and seedling establishment, and facilitates plant expansion and regeneration [1,2,5]. Our field experiments demonstrated that seed size, soil burial, and seed peeling influence seed dispersal, thus potentially affecting plant expansion and regeneration. Our results are consistent with our hypothesis that a single factor influences seed dispersal more than their interactions, and the effect of each factor on seed fates and dispersal distance was inconsistent. In which, large peeled and bare ground seeds were removed first. Although large seeds were eaten more than small seeds after dispersal, more large seeds were scatter-hoarding farther away. Soil burial leads to more seeds being eaten in situ and reduced dispersal distance. Seed peeling increased the probability of predation after dispersal. Our results demonstrated that some factors favor seed dispersal and some do not, emphasizing that it is very important to consider multiple factors in seed dispersal.
Differences in seed size during dispersal can influence plant expansion and regeneration. In general, larger seeds tend to offer more nutrients, leading to increased dispersal and hoarding activities [5,6,8,13,14]. In this study, large seeds were preferentially selected by animals, in line with the optimal foraging theory [30]. Large seeds have a higher nutrient content and are more attractive to animals. This result is in line with other studies [5,6,8,14,31]. For example, Zhang et al. [31] found that rodents dispersed large seeds faster and dispersed small seeds slower. This study found that large seeds have higher predation after dispersal rates than small seeds, in line with the findings of Brewer [32] but not with the findings of Xiao et al. [11] and Zhang et al. [13]. Brewer [32] found that Heteromys desmarestianus ate more large seeds of Astrocaryum mexicanum. It may be animals eating large seeds can replenish more energy, while small seeds may not be enough to compensate for the energy input of rodents during dispersal and hoarding [8,10]. Our study found that hoarding after dispersal of large seeds was significantly greater than small seeds, which supports the findings of Wang et al. [7,33]. Zhang et al. [13] also found that large acorns had a higher proportion of seed caching than small acorns. After scatter-hoarding large seeds, animals can search for fewer storage points to retrieve food returns with the same nutritional value [34,35].
Furthermore, our study also found that the predation distance after dispersal and hoarding distance after dispersal of large seeds were significantly greater than small seeds, which further provided support for the findings of Jansen et al. [10] and Xiao et al. [12]. For example, seed dispersal distances after dispersal significantly increased with seed size in five rodent-dispersed fagaceous species [12]. Zhang et al. [13] found that large acorns were dispersed longer after removal. To mitigate the risk of food loss at their newly established food hoarding sites, animals opt to transport large seeds with high nutritional value to more distant locations for establishing additional food storage sites. Large seeds are preferentially selected by animals, although being more consumed, they also be more scatter-hoarding to farther distances. More retained small seeds have a high probability of death restricted by density and distance [2,36], while fewer large seeds have a high probability of germination and seedlings establishment [5,37].
Soil burial can affect seed survival rates. We found that the buried seeds were generally retained more in situ than in bare ground, this result is in line with Zhang and Wang [16] and Cheng et al. [17]. Soil burial not only reduces the chance for animals to find seeds through olfaction but also makes it more difficult for animals to search for seeds through vision [4,5,16,17]. Cheng et al. [17] also found that soil-buried seeds have a higher retention time than seeds at the surface. In addition, soil burial provides a better living environment for seeds, facilitating seed germination and seedling establishment [4,15]. However, soil burial increases the predation risk as well as the time and handling costs of finding food for animals [16,17], so the buried seeds are found by the animals, which can be devastating. This study found that soil burial increased predation in situ and reduced hoarding after dispersal, which is consistent with the findings of Yan et al. [23]. Due to rodents consuming more energy in early search and digging, they choose to consume more seeds in situ and reduce the scatter-hoarding of seeds, thereby maintaining their energy balance of income and expenses.
In comparison to bare ground, soil burial significantly reduces the predation distance after dispersal and the hoarding distance after dispersal. This observation suggests that rodents conserve energy by decreasing the distance they travel to disperse seeds. Additionally, rodents invest more time in searching for seeds buried in the soil, increasing their exposure to predation. Previous studies support the idea that rodents reduce seed hoarding in response to heightened predation risk [38,39]. In high predation risk scenarios, hoarding animals have limited available space, leading to shorter food hoarding distances. These findings are in line with the results of our study [38,39]. While soil burial benefits seed germination and reduces the likelihood of animals discovering seeds, once buried seeds are located by animals, they are more likely to be consumed and hoarded in proximity.
Seed peeling affected seed selection in animals. Rodents may choose healthy seeds to scatter-hoarding rather than moldy and worm-eaten seeds [19,40,41]. We found that peeled seeds had low intact in situ rates and would be dispersed by rodents faster. Seed peeling behavior increases the energy consumption and the risk of predation of dispersal animals in the wild, peeled seeds reduce the time to distinguish seeds of rodents, therefore, animals may preferentially choose peeled seeds [5,42,43]. Although peeling seeds increases energy consumption and handling time in animals, the return of hoarding healthy seeds is higher [30]. When animals retrieve peeled seeds, there is no need to distinguish whether the seeds are healthy, and they may preferentially choose peeled seeds. Our study also found that peeled seeds had higher predation after dispersal rates. This is due to the accelerated germination percentage and germination rate after seed coat removal [18], and many studies showed that animals tend to consume germinated seeds and store non-germinated seeds [5]. To avoid nutrient loss after seed germination, the peeled seeds are preferentially eaten when animals retrieve the peeled seeds.
In this study, we did not find the interactions of seed size, soil burial and seed peeling could affect the seed dispersal process. The overall interpretation of seed predation, hoarding, and hoarding distance was 42.62 % and 58.64 % and 62.26 %, indicating that other factors in the environment influence the seed dispersal process. Furthermore, soil burial was significantly explained both seed predation and hoarding; soil burial and seed size were also significantly explained hoarding distance, suggesting that this may be due to the strong influence of a single factor on seed dispersal, resulting in the interaction of multiple factors is not significant. In the future, we should consider more factors affecting seed dispersal and increase repeated experiments between years, to more comprehensively and reveal the complex ecological process of rodent-mediated seed dispersal.
5. Conclusions
Our study demonstrates that a single factor of seed size, soil burial and seed peeling have an important influence on the seed dispersal process more than their interactions. This study suggests that large seeds are dispersed longer distances and have a higher proportion of seed scatter-hoarding than small acorns. Large seeds have a greater chance of escape, implying that large seeds are more likely to germinate and establish seedlings, contributing to plant population expansion and regeneration. Soil burial decreases the likelihood of seeds being discovered by animals; however, once animals locate the seed release point, they tend to opt for seed consumption over seed dispersal. And even if the seeds are hoarded, they bury the seeds close to the release point. Soil burial is not beneficial to seed dispersal and plant population expansion and regeneration. Seed peeling facilitates seed germination and also can identify the quality of seeds. But when animals find peeled seeds, they consume more seeds, which may not be beneficial to plant population expansion and regeneration. More future studies are needed to reveal the interaction between seed size, soil burial, and peeling on plant population expansion and regeneration.
Funding
Funding for this study was supported by the Key Project of Key Research and Development Program of Ningxia Hui Autonomous Region, China (2018BEG02001). The National Natural Science Foundations of China (31760705).
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Jiming Cheng: Writing – review & editing, Writing – original draft, Methodology, Investigation, Conceptualization. Xingfu Yan: Writing – review & editing, Supervision, Conceptualization. Jinfeng Zhang: Investigation, Data curation. Chao Zhang: Data curation. Min Zhang: Writing – original draft. Shuhua Wei: Data curation. Jiazhi Wang: Data curation. Yonghong Luo: Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Contributor Information
Jiming Cheng, Email: chengjiming@mails.ccnu.edu.cn.
Xingfu Yan, Email: 2006048@nmu.edu.cn.
Jinfeng Zhang, Email: 20231034@qhnu.edu.cn.
Chao Zhang, Email: zhangchao2023@mails.ccnu.edu.cn.
Min Zhang, Email: minzhang@mails.ccnu.edu.cn.
Shuhua Wei, Email: weishuhua666@163.com.
Jiazhi Wang, Email: george0602@126.com.
Yonghong Luo, Email: 21915013@mail.imu.edu.cn.
References
- 1.Janzen D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970;104:501–528. [Google Scholar]
- 2.Connell J.H. In: Dynamics of Populations. Boer P.J., Gradwell G., editors. Center for Agricultural Publication and Documentation; Wageningen, The Netherlands: 1971. On the role of natural enemies in preventing competitive exclusion in some marine mammals and in rain forest trees. [Google Scholar]
- 3.Howe H.F., Smallwood J. Ecology of seed dispersal. Annu. Rev. Ecol. Systemat. 1982;13:201–228. [Google Scholar]
- 4.Vander Wall S.B. University of Chicago Press; Chicago, America: 1990. Food Hoarding in Animals. [Google Scholar]
- 5.Zhang Z. Science Press; Beijing, China: 2019. Studies on the Rodent–Seed Interactions of Forest Ecosystems: Exploring the Secrets of Cooperation between Antagonists. [Google Scholar]
- 6.Cheng J.M., He H.M., Niu H.Y., Zhang H.M. Research progress on the effect of intraspecific personality differences on seed dispersal in rodents. Biodiv. Sci. 2023;31:171–185. [Google Scholar]
- 7.Wang B., Chen J., Corlett R.T. Factors influencing repeated seed movements by scatter-hoarding rodents in an alpine forest. Sci. Rep. 2014;4:4786. doi: 10.1038/srep04786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao Z.S., Zhang Z.B. Nut predation and dispersal of Harland Tanoak Lithocarpus harlandii by scatter-hoarding rodents. Acta Oecol. 2006;29:205–213. [Google Scholar]
- 9.Liang Z.L., Ma J.Z., Rong K. Animal scatter-hoarding behavior and its impact on the regeneration of plant populations. Acta Ecol. Sin. 2016;36:1162–1169. [Google Scholar]
- 10.Jansen P.A., Bongers F., Hemerik L. Seed mass and mast seeding enhance dispersal by a neotropical scatter-hoarding rodent. Ecol. Monogr. 2004;74:569–589. [Google Scholar]
- 11.Xiao Z.S., Zhang Z.B., Wang Y.S. Dispersal and germination of big and small nuts of Quercus serrata in a subtropical broad-leaved evergreen forest. For. Ecol. Manag. 2004;195:141–150. [Google Scholar]
- 12.Xiao Z.S., Zhang Z.B., Wang Y.S. Effects of seed size on dispersal distance in five rodent-dispersed fagaceous species. Acta Oecol. 2005;28:221–229. [Google Scholar]
- 13.Zhang H.M., Chen Y., Zhang Z.B. Differences of dispersal fitness of large and small acorns of Liaodong oak (Quercus liaotungensis) before and after seed caching by small rodents in a warm temperate forest, China. For. Ecol. Manag. 2008;255:1243–1250. [Google Scholar]
- 14.Cheng J.M., Zhang M., Yan X.F. Effects of seed size and cache density on the seed fate of Quercus wutaishanica mediated by rodents. Life. 2024;14:286. doi: 10.3390/life14030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z.B. Effects of burial and environmental factors on seedling recruitment of Quercus liaotungensis Koidz. Acta Ecol. Sin. 2001;21:374–384. [Google Scholar]
- 16.Zhang Z.B., Wang F.S. Effect of burial on acorn survival and seedling recruitment of Liaodong oak (Quercu liaotungensis) under rodent predation. Acta Theriol. Sin. 2001;21:35–43. [Google Scholar]
- 17.Cheng J.R., Xiao Z.S., Zhang Z.B. Effects of burial and coating on acorn survival of Quercus variabilis and Quercus serrata under rodent predation. Chinese J. Ecol. 2007;26:668–672. [Google Scholar]
- 18.Yan X.F., Qiu Z.H., Zhang Q., Zhang K.W., Zhou Y.F. Effects of coat and sowing depth on seed germination and early seedling growth of Quercus wutaishanica. Chin. J. Appl. Ecol. 2014;25:53–60. [PubMed] [Google Scholar]
- 19.Yi X.F., Steele M.A., Zhang Z.B. Acorn pericarp removal as a cache management strategy of the Siberian chipmunk, Tamias sibiricus. Ethology. 2012;118:87–94. [Google Scholar]
- 20.Yan M.J., Zhang J.G., He Q.Y., Shi W.Y., Otsuki K., Yamanaka N., Du S. Sapflow-Based stand transpiration in a semiarid natural oak forest on China's Loess Plateau. Forests. 2016;7:227. [Google Scholar]
- 21.Li H.J., Zhang Z.B. Effect of rodents on acorn dispersal and survival of the Liaodong oak (Quercus liaotungensis Koidz.) For. Ecol. Manag. 2003;176:387–396. [Google Scholar]
- 22.Li H.J., Zhang Z.B. Effects of mast seeding and rodent abundance on seed predation and dispersal by rodents in Prunus armeniaca (Rosaceae) For. Ecol. Manag. 2007;242:511–517. [Google Scholar]
- 23.Yan X.F., Zhou L.B., Liu J.L. Effects of different habitats and coverage treatments on the fates of Quercus wutaishanica seeds under the predation pressure of rodents. Acta Ecol. Sin. 2012;32:2778–2787. [Google Scholar]
- 24.Luo Y.H., Cheng J.M., Yan X.F., Yang H., Shen Y., Ge J.R., Zhang M., Zhang J.F., Xu Z.W. Density-dependent seed predation of Quercus wutaishanica by rodents in response to different seed states. Animals. 2023;13:1732. doi: 10.3390/ani13111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Z.S., Jansen P.A., Zhang Z.B. Using seed-tagging methods for assessing post-dispersal seed fate in rodent-dispersed trees. For. Ecol. Manag. 2006;223:18–23. [Google Scholar]
- 26.Lu J.Q., Zhang Z.B. Effects of high and low shrubs on acorn hoarding and dispersal of Liaodong oak Quercus liaotungensis by small rodents. Acta Zool. Sin. 2005;51:195–204. [Google Scholar]
- 27.Zhang H.M., Steele M.A., Zhang Z.B., Wang W., Wang Y. Rapid sequestration and recaching by a scatter-hoarding rodent (Sciurotamias davidianus) J. Mammal. 2014;95:480–490. [Google Scholar]
- 28.Bolker B.M., Brooks M.E., Clark C.J., Geange S.W., Poulsen J.R., Stevens H.H.M., White J.S. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 2019;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 29.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2023. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 30.Lewis A.R. Selection of nuts by gray squirrels and optimal foraging theory. Am. Midl. Nat. 1982;107:250–257. [Google Scholar]
- 31.Zhang M.M., Steele M.A., Yi X.F. Reconsidering the effects of tannin on seed dispersal by rodents: evidence from enclosure and field experiments with artificial seeds. Behav. Process. 2013;10:200–207. doi: 10.1016/j.beproc.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Brewer S.W. Predation and dispersal of large and small seeds of a tropical palm. Oikos. 2001;92:245–255. [Google Scholar]
- 33.Wang B., Wang G., Chen J. Scatter-hoarding rodents use different foraging strategies for seeds from different plant species. Plant Ecol. 2012;213:1329–1336. [Google Scholar]
- 34.Huang Z.Y., Wang Y., Zhang H.M., Wu F.Q., Zhang Z.B. Behavioural responses of sympatric rodents to complete pilferage. Anim. Behav. 2011;81:831–836. [Google Scholar]
- 35.Zhang H.M., Wang Y., Zhang Z.B. Response of seed-hoarding behaviour to conspecific audiences in scatter- and/or larder-hoarding rodents. Behaviour. 2011;148:825–842. [Google Scholar]
- 36.Janzen D.H. Seed predation by animals. Annu. Rev. Ecol. Evol. S. 1971;2:465–492. [Google Scholar]
- 37.Vander Wall S.B. On the relative contributions of wind vs. animals to seed dispersal of four Sierra Nevada pines. Ecology. 2008;89:1837–1849. doi: 10.1890/07-0409.1. [DOI] [PubMed] [Google Scholar]
- 38.Leaver L.A. Effects of food value, predation risk, and pilferage on the caching decisions of Dipodomys merriami. Behav. Ecol. 2004;15:729–734. [Google Scholar]
- 39.Wang W., Zhang H.M., Zhang Z.B. Effects of predation risk on cultivated walnut (Juglans regia) seeds hoarding behavior by David's rock squirrel (Sciurotamias davidianus) in enclosure. Acta Theriol. Sin. 2007;27:358–364. [Google Scholar]
- 40.Yi X.F., Yang Y.Q., Curtis R., Bartlow A.W., Agosta S.J., Steele M.A. Alternative strategies of seed predator escape by early-germinating oaks in Asia and North America. Ecol. Evol. 2012;2:487–492. doi: 10.1002/ece3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi X.F., Zhang M.M., Bartlow A.W., Dong Z. Incorporating cache management behavior into seed dispersal: the effect of pericarp removal on acorn germination. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi X.F., Wang Z.Y., Liu C.Q., Liu G.Q. Seed trait and rodent species determine seed dispersal and predation: Evidences from semi-natural enclosures. Iforest. 2015;8:207–213. [Google Scholar]
- 43.Chang G., Xiao Z.S., Zhang Z.B. Effects of burrow condition and seed handling time on hoarding strategies of Edward's long-tailed rat (Leopoldamys edwardsi) Behav. Process. 2010;85:163–166. doi: 10.1016/j.beproc.2010.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.