Abstract
CDK11p110 (cyclin-dependent kinase 11p110, formerly known as PITSLRE) is a member of the CDK superfamily. It associates with cyclin L and is involved in the regulation of transcription and in premRNA splicing. During staurosporine-, Fas- and tumour necrosis factor α-induced apoptosis, CDK11p110, is cleaved by caspases to generate smaller 46–50 kDa proteins containing the catalytic kinase domain. Ectopic expression of the caspase-processed form CDK11p46 induces apoptosis. The mechanisms that regulate activation and stability of CDK11 isoforms are still unclear. In the present study, we demonstrate that in human melanoma cells CDK11p110 and CDK11p46 interact with Hsp90 (heat-shock protein 90) and its co-chaperone cdc37. Furthermore, we show that the treatment of cells with the Hsp90-specific inhibitor geldanamycin leads to ubiquitination and enhanced degradation of both CDK11p110 and CDK11p46 through a proteasome-dependent pathway. We also determined that geldanamycin-triggered degradation of CDK11p46 slows down the progression of apoptosis. These results indicate that Hsp90 and cdc37 stabilize CDK11 kinase, and suggest that this stabilization is crucial for its pro-apoptotic function.
Keywords: apoptosis, chaperone, cyclin-dependent kinase 11, heat-shock protein 90 (Hsp90), PITSLRE, proteasome
Abbreviations: CDK, cyclin-dependent kinase; CK2, casein kinase 2; ERK, extracellular-signal-regulated kinase; GA, geldanamycin; Hsp, heat-shock protein; MOK, MAPK (mitogen-activated protein kinase)/MAK (male germ cell-associated kinase)/MRK (MAK-related kinase) overlapping kinase
INTRODUCTION
The CDK11 (cyclin-dependent kinase 11) kinases belong to a large family of p34cdc2-related kinases [1]. There are at least 20 CDK11 isoforms that are differentially expressed in mammalian tissues and regulate diverse cellular functions. The larger CDK11p110 isoforms are associated with cyclin L and various splicing factors, and are involved in the regulation of transcription and RNA splicing in proliferating cells [2–4]. The smaller CDK11p58 isoforms result from translation initiation at an internal ribosome entry site in the CDK11p110 mRNA, and play an important role in mitosis [1,5]. Ectopic expression of CDK11p58 in Chinese-hamster ovary fibroblasts leads to telophase delay, abnormal cytokinesis and a reduced rate of cell growth. The decrease in cell growth rate is linked to apoptosis [1].
During Fas- and tumour necrosis factor α-induced apoptosis, CDK11 isoforms are activated by proteolytic cleavage by caspases. Caspase processing removes the N-terminal part of the protein and generates smaller 46–50 kDa proteins containing the catalytic kinase domain [6–9]. Similar to CDK11p58, ectopic expression of CDK11p46 induces apoptosis [10–12].
CDK11 kinases have also been implicated in tumorigenesis. Deletion of the chromosome region 1p36.3 that encodes CDK11 kinases and complete loss of expression of specific isoforms occur in neuroblastomas [13], childhood endormal sinus tumours [14], a subset of malignant melanoma [15] and in non-Hodgkin lymphoma [16]. Since deletion of the chromosome region encoding CDK11 occurs late in oncogenesis and is correlated with aggressive tumour growth, CDK11 kinases have been suggested to act as tumour supressors [17].
Although the CDK11p46 form is clearly involved in apoptotic signalling, little is known about its function in this process. Recently, it has been shown that during apoptosis CDK11p46 interacts with and phosphorylates eIF3f (eukaryotic initiation factor 3f) and inhibits translation [12]. Another study revealed that CDK11p46 associates with PAK1 (p21-activated kinase) and inhibits its activity [11]. To date, processing by caspases is the only known mechanism of regulation of CDK11p110 activity during apoptosis. At least two different caspase activities, caspases 3 and 8, are involved in the proteolysis of the CDK11p110 isoform during Fas-induced apoptosis. It was also shown that CDK11p110 is rapidly serine-phosphorylated after induction of apoptosis. It was suggested that phosphorylation of an N-terminal portion of CDK11p110 may enhance caspase cleavage in this region [8].
To elucidate the regulation of CDK11p110 as well as the caspase-processed form CDK11p46, we searched for cellular proteins that specifically associate with CDK11p46 by co-immunoprecipitation experiments. In the present study, we provide evidence that CDK11p46 interacts with a set of molecular chaperones including Hsp90 (heat-shock protein 90), cdc37 (a mammalian homologue of the yeast cell-cycle control protein) and Hsp70. We also determined that treatment of cells with GA (geldanamycin), an Hsp90-specific inhibitor, leads to ubiquitination and enhanced degradation of both CDK11p110 and CDK11p46 through proteasome-dependent pathways. These results demonstrate that molecular chaperones are involved in regulating the stability of both CDK11p110 and CDK11p46.
MATERIALS AND METHODS
Cell culture and transfection
The human melanoma cell line A375 was obtained from A.T.C.C. The cells were cultured at 37 °C with 5% CO2 in RPMI 1640 medium (Mediatech, Herndon, VA, U.S.A.), supplemented with 5% (v/v) fetal bovine serum (Omega Scientific, Tarzana, CA, U.S.A.), 1% L-glutamine and 1% penicillin/streptomycin (Invitrogen). All transfections were performed using LIPOFECTAMINE™ 2000 (Invitrogen) according to the manufacturer's instructions.
Antibodies
Anti-CDK11 antibody (GN1) is an affinity-purified rabbit polyclonal antibody obtained by injection of glutathione S-transferase-CDK11 containing amino acids 341–413 of CDK11 (Rockland, Gilbertsville, PA, U.S.A.). Rabbit polyclonal antibody recognizing Hsp90 (#sc-7947) and goat polyclonal antibody anti-cdc37 (#sc-6396) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Monoclonal anti-c-myc antibody (clone 9E10) was obtained from Sigma. Polyclonal anti-ERK1/ERK2 (where ERK stands for extracellular-signal-regulated kinase; #9102) and monoclonal anti-ubiquitin (#3936) antibodies were purchased from Cell Signaling.
Construction of c-myc-CDK11p46 and c-myc-CDK11p46K451R vectors
The pCMV/c-myc-CDK11p46 vector was constructed by inserting the CDK11p46 coding sequence (GenBank® accession no. U04824) into pCMV/myc (ClonTech Laboratories) using EcoRI and XhoI restriction enzyme sites. Substitution of Lys-451 with Arg (K451R) was achieved by PCR using the Quik Change™ site-directed mutagenesis kit (Stratagene) with the c-myc-CDK11p46 construct as a template and the mutagenesis primers 5′-GAAATTGTGGCTCTAAGCCGGCTGAAGATGG-3′ and 5′-CCATCTTCAGCCGCCTTAGAGCCACAATTTC-3′. Verification of the proper mutation and the integrity of the remainder of the construct were accomplished by DNA sequencing.
Immunoprecipitation and Western blotting
Cells were harvested, washed twice with cold PBS and lysed in lysis buffer (25 mM Tris/HCl, pH 7.5, 150 mM NaCl, 2 mM EGTA, 50 mM glycerophosphate and 1% Nonidet P40) or RIPA buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 0.1% SDS, 1% Nonidet P40, 1% sodium deoxycholate and 5 mM EDTA) containing 1 mM dithiothreitol, 1 mM sodium orthovanadate, 1 mM PMSF and 1% protease inhibitor cocktail (Sigma) for 30 min on ice. After lysis, cells were centrifuged at 13000 g for 10 min at 4 °C and the protein content was determined using the bicinchoninic acid assay (Pierce). Total cell extracts (0.5 mg of protein) were immunoprecipitated using rabbit normal IgG, anti-CDK11 or anti-c-myc antibody for 4 h at 4 °C. Subsequently, Protein A–agarose or Protein G–agarose beads (Oncogene, La Jolla, CA, U.S.A.) were added, and the incubation was continued for another 2 h. Immunocomplexes were then washed four times with lysis buffer and subjected to SDS/PAGE. The proteins were transferred on to a PVDF membrane (Bio-Rad Laboratories, Hercules, CA, U.S.A.) and the blots were probed with different antibodies. A secondary probe with horseradish peroxidase-conjugated antibodies (Sigma) was detected by enhanced chemiluminescence (ECL®; Amersham Biosciences).
Treatment of cells with GA and MG132 (benzyloxycarbonyl-Leu-Leu-Leu-aldehyde)
Cells transiently expressing c-myc-CDK11p46 were grown on six-well dishes. Cells were treated 36 h post-transfection with Hsp90 inhibitor, GA (Sigma) at a final concentration of 2 μM, or with proteasome inhibitor, MG132 (Sigma) at a final concentration of 25 μM for the indicated times (in control experiments an equivalent volume of DMSO was added).
Pulse–chase analysis
Cells transiently expressing c-myc-CDK11p46 were washed with PBS, and the culture medium was replaced with methionine/cysteine-free RPMI 1640 medium (Sigma) containing 5% fetal bovine serum, 1% L-glutamine and 1% penicillin/streptomycin. After 1 h, the cells were pulse-labelled with 85 μCi/ml [35S]methionine/[35S]cysteine (MP Biomedicals, Irvine, CA, U.S.A.) in fresh methionine/cysteine-free medium for 4 h. At this point, the cells were washed three times in RPMI 1640 medium and then either lysed immediately or incubated in complete medium in the presence or absence of 2 μM GA for the indicated times. CDK11p46 was immunoprecipitated with anti-c-myc antibody as described above and subjected to SDS/PAGE. Gels were dried and the radiolabelled c-myc-CDK11p46 was visualized by autoradiography.
Apoptosis assay
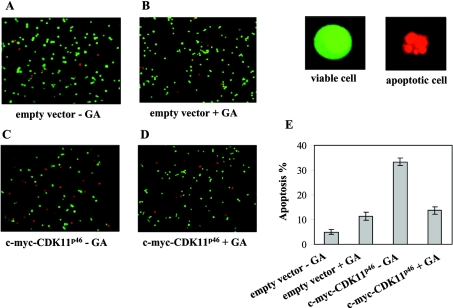
Progression of apoptosis was assessed by ethidium bromide and Acridine Orange staining followed by fluorescent microscopy. The assay was performed as described previously [18]. Briefly, A375 cells transiently expressing c-myc-CDK11p46 were grown on 24-well dishes. Cells were treated 24 h post-transfection with 0.5 μM GA for 16 h. Cells were harvested and 10 μl of cell suspension containing approx. 1×106 cells/ml in RPMI 1640 medium was mixed with a dye mix containing 100 μg/ml each of ethidium bromide and Acridine Orange. At least 300 cells were counted under the fluorescent microscope at 40× magnification. Viable cells fluoresce green and have an organized chromatin structure. Apoptotic cells have chromatin that is highly condensed or fragmented and stains orange. Necrotic cells have chromatin with an organized structure that also stains orange. Each set of experiments was repeated three times.
MS analysis
Total cell extracts (2 mg of protein) from cells transfected with empty vector or expressing c-myc-CDK11p46 were subjected to immunoprecipitation with anti-c-myc antibody and proteins were separated on 12% SDS/polyacrylamide gel. After Bio-Safe Coomassie (Bio-Rad Laboratories) staining, the gel was washed with water and protein bands were excised. Proteins were subjected to digestion with trypsin and peptides were analysed by matrix-assisted laser-desorption ionization–time-of-flight MS (Proteomics Core Facility, University of Arizona, AZ, U.S.A.).
RESULTS
Identification of proteins that are co-immunoprecipitated with CDK11p46
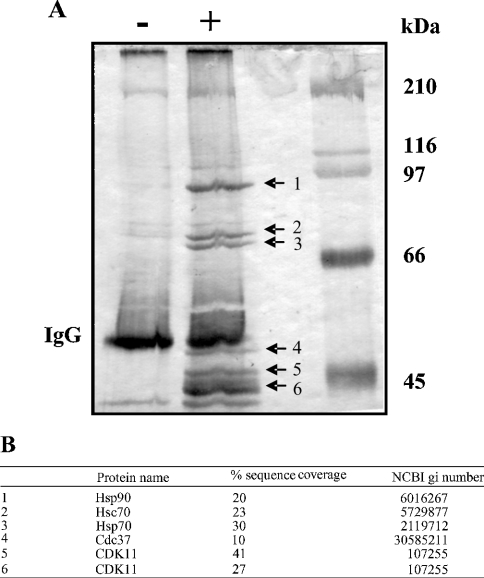
To identify proteins associated with CDK11, A375 cells were transiently transfected with the pCMV/c-myc-CDK11p46 vector. The CDK11p46 protein is the final product of caspase processing of CDK11p110. CDK11p46 contains amino acids 388–779 and possesses the entire kinase domain, which covers amino acids 422–707. CDK11p46 was immunoprecipitated with anti-c-myc antibody from total cell extracts and the immunocomplexes were analysed by SDS/PAGE. As shown in Figure 1(A) several proteins were co-immunoprecipitated with the c-myc-CDK11p46 fusion protein. Of these, a few proteins were also immunoprecipitated from cells transfected with the empty vector and thus reflect non-specific binding. Six proteins with approximate masses of 90, 72, 70, 50, 46 and 44 kDa were immunoprecipitated in a CDK11p46-dependent manner. The identity of proteins was determined by tryptic peptide MS fingerprinting (Figure 1B). This analysis revealed that the approx. 90 kDa protein is Hsp90; the approx. 72–70 kDa proteins are Hsc70 (constitutive form) and Hsp70 (inducible form) respectively; the approx. 50 kDa protein is cdc37 and the approx. 46–44 kDa proteins are CDK11. The following studies focused on the functional importance of the association of Hsp90 and CDK11p110/p46.
Figure 1. Identification of proteins interacting with CDK11p46.
(A) Cell extracts from A375 cells transfected with empty vector (−) or c-myc-CDK11p46 (+) were subjected to immunoprecipitation with anti-c-myc antibody, followed by SDS/PAGE. Protein bands were visualized with silver stain. (B) Bands were excised from the gel and, after in-gel digestion with trypsin, their identities were determined by tryptic peptide MS analysis. Percentage of sequence coverage and NCBI gi numbers for each protein identified are indicated.
Interaction of CDK11p110 and CDK11p46 with Hsp90 and its co-chaperone, cdc37, in vivo
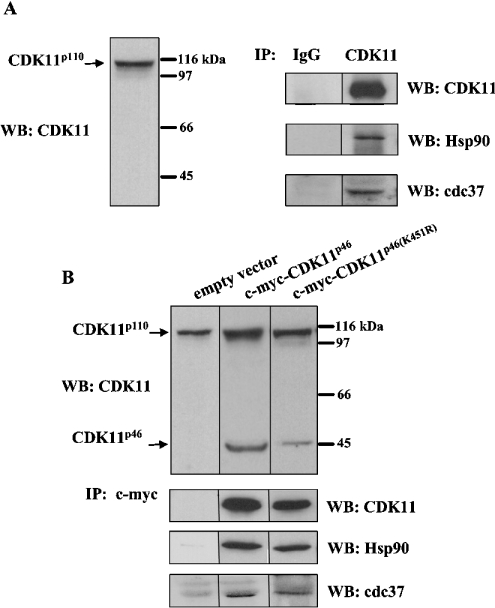
To determine whether endogenous CDK11p110 forms a stable complex with Hsp90, we immunoprecipitated CDK11p110 with anti-CDK11 antibody and subsequently performed Western blotting with anti-Hsp90 antibody. As shown in Figure 2(A), CDK11p110 associates with Hsp90 in A375 cells and does not associate in the control. To confirm that CDK11p46 interacts with Hsp90, we repeated the co-immunoprecipitation assay of total cell extracts from A375 cells transfected with c-myc-CDK11p46 using anti-c-myc antibody, followed by immunodetection with anti-Hsp90 antibody. As shown in Figure 2(B), Hsp90 was co-immunoprecipitated with CDK11p46. As a control, we used total cell extracts from cells transfected with empty vector.
Figure 2. CDK11p110 and CDK11p46 are associated with Hsp90 and cdc37 in A375 cells.
(A) Cell extracts from A375 cells were subjected to Western blotting (WB) with anti-CDK11 antibody or immunoprecipitation (IP) with normal rabbit IgG or anti-CDK11, followed by Western blotting with anti-CDK11, anti-Hsp90 or anti-cdc37 antibody. (B) Cell extracts from A375 cells transfected with empty vector, c-myc-CDK11p46 or c-myc-CDK11p46K451R were subjected to Western blotting with anti-CDK11 antibody or immunoprecipitation with anti-c-myc antibody, followed by Western blotting with anti-CDK11, anti-Hsp90 or anti-cdc37 antibody.
Hsp90 proteins are required for the stability and functional maturation of numerous proteins involved in cell-cycle regulation, steroid hormone responsiveness and signal transduction (reviewed in [19]). Hsp90 functions in association with its co-chaperone partners, which are responsible for the specificity of interaction between Hsp90 and its targets. One of these co-chaperones, cdc37, interacts through its N-terminal domain with a number of protein kinases, whereas the C-terminal domain interacts with Hsp90 [20–22]. To examine whether CDK11p110 associates with cdc37 in A375 cells, we immunoprecipitated CDK11p110 with anti-CDK11 antibody and subsequently performed Western blotting with anti-cdc37 antibody. As shown in Figure 2(A), cdc37 interacts with CDK11p110 and does not interact in the control. We also confirmed that CDK11p46 specifically associates with cdc37 (Figure 2B).
To examine further whether the association of CDK11p110/p46 with Hsp90–cdc37 complex depends on its kinase activity, we overexpressed kinase-dead mutant, c-myc-CDK11p46K451R. Immunoprecipitation of CDK11p46K451R with anti-c-myc antibody followed by Western blotting with anti-Hsp90 and anti-cdc37 antibody revealed that K451R mutation did not have an effect on the association with Hsp90–cdc37 complex (Figure 2B).
Regulation of CDK11p110 and CDK11p46 stability by Hsp90
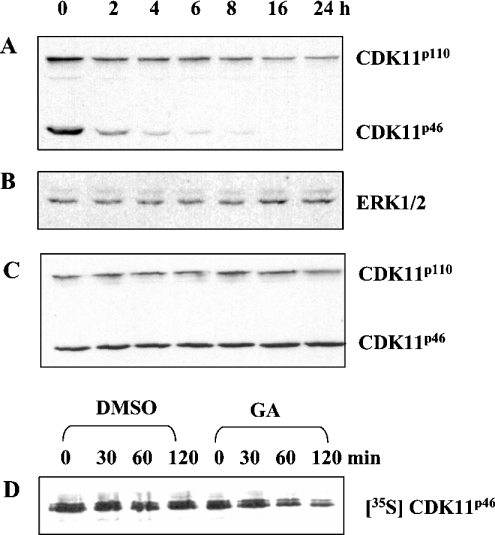
Hsp90 possesses a nucleotide-binding pocket near the N-terminus [23,24]. Binding and hydrolysis of ATP regulates the conformational states of Hsp90, which in turn regulate its function. GA, an ansamycin antibiotic, binds to the nucleotide-binding domain within Hsp90 and prevents the formation of the client protein–cdc37–Hsp90 heterocomplex [23,25]. To examine whether the inhibition of Hsp90 function has any effect on CDK11p46 stability, A375 cells transfected with c-myc-CDK11p46 were treated with GA for 0–24 h (Figure 3). Western blotting of cell extracts with anti-CDK11 antibody revealed the amounts of both CDK11p46 and endogenous CDK11p110. As shown in Figure 3(A), the level of CDK11p46 was significantly decreased after 2 h of treatment with GA, whereas 16 h post-treatment, CDK11p46 was not detectable with anti-CDK11 antibody. The amounts of endogenous CDK11p110 were also significantly reduced, although after 16 h of treatment with GA approx. 25% of protein still remained. The results suggest that the molecular chaperone Hsp90 stabilizes both c-myc-CDK11p46 and CDK11p110. However, longer treatment with GA is required to lower the levels of endogenous CDK11p110 compared with c-myc-CDK11p46. As a control, we examined the levels of ERK1/ERK2, which are known not to interact with Hsp90. As shown in Figure 3(B), the amounts of ERK1/ERK2 did not change during GA treatment. As an additional control, we treated A375 cells with DMSO instead of GA. As shown in Figure 3(C), DMSO treatment had no negative effect on CDK11p46 and CDK11p110 levels.
Figure 3. Hsp90 regulates CDK11p110 and CDK11p46 stability in A375 cells.
A375 cells transfected with c-myc-CDK11p46 were treated with (A) 2 μM GA or (C) DMSO for the indicated periods of time. Cell extracts were prepared and the amounts of CDK11p46 and CDK11p110 were monitored by Western blotting with anti-CDK11 antibody. (B) Equal loading of proteins was shown by stripping the blot (A) and performing anti-ERK1/ERK2 blotting. (D) A375 cells expressing c-myc-CDK11p46 were pulse-labelled with [35S]methionine/[35S]cysteine for 4 h and than chased in non-radioactive medium with DMSO or 2 μM GA for up to 2 h. At the indicated times, cell extracts were prepared and c-myc-CDK11p46 was immunoprecipitated with anti-c-myc antibody, resolved by SDS/PAGE and visualized by autoradiography.
Next, we determined the half-life of CDK11p46 on GA treatment. A375 cells transfected with c-myc-CDK11p46 were pulse-labelled with [35S]methionine/[35S]cysteine for 4 h and then chased in non-radioactive, complete medium with or without GA for up to 2 h. Cell extracts were prepared and CDK11p46 was immunoprecipitated with anti-c-myc antibody. As shown in Figure 3(D), newly synthesized CDK11p46 was stable in vehicle control treatment with DMSO, whereas its half-life was shortened to approx. 2 h on GA treatment. These results show that CDK11p110/p46 is rapidly degraded when the Hsp90–CDK11 complex dissociates, indicating that Hsp90 is necessary for CDK11p110/p46 stability.
Proteasome-dependent degradation of CDK11p110 and CDK11p46
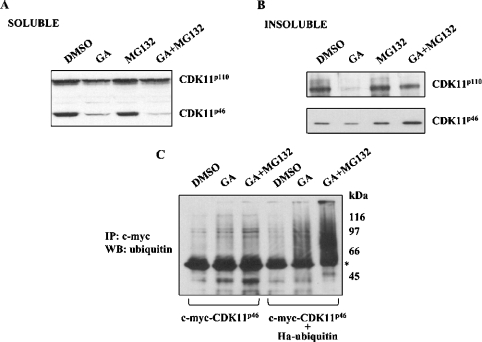
Although several protease systems are involved in protein degradation, most proteins whose stability is regulated by Hsp90 are degraded by the proteasome complex (reviewed in [26]). To examine whether dissociation of the Hsp90–CDK11p110/p46 complex indeed leads to degradation of CDK11p110/p46 by proteasome, we treated A375 cells expressing c-myc-CDK11p46 with the Hsp90 inhibitor GA and the proteasome inhibitor MG132. Cell extracts were prepared, and Western blotting with anti-CDK11 antibody revealed the amounts of both endogenous CDK11p110 and c-myc-CDK11p46. As shown in Figure 4(A), treatment with both GA and MG132 did not lead to accumulation of CDK11p110 and CDK11p46 in the soluble fraction. Since it was previously reported that some protein kinases, which interact with Hsp90, such as PDK1 (phosphoinositide-dependent kinase 1) [27], MOK [MAPK (mitogen-activated protein kinase)/MAK (male germ cell-associated kinase)/MRK (MAK-related kinase) overlapping kinase] [28] and LKB1 (a serine/threonine protein kinase 11 incoded by LKB1/STK11 gene) [29], accumulate in the insoluble fraction on treatment with both GA and MG132, we also examined the amounts of CDK11p110 and CDK11p46 in the insoluble fraction derived from A375 cells. As shown in Figure 4(B), amounts of CDK11p110 and CDK11p46 in the insoluble fraction decreased with GA treatment, whereas treatment with both GA and MG132 blocked degradation of both CDK11p110 and CDK11p46. These results indicate that a proteasome-mediated pathway degrades CDK11p110/p46 in the absence of Hsp90.
Figure 4. Degradation of CDK11p110 and CDK11p46 is mediated by the proteasome pathway.
A375 cells transfected with c-myc-CDK11p46 were treated for 3 h with the vehicle DMSO, 2 μM GA, 25 μM MG132, or 2 μM GA and 25 μM MG132. Cell extracts were prepared and the amounts of CDK11p110 and CDK11p46 in (A) soluble supernatants and (B) insoluble fractions were determined by Western blotting with anti-CDK11 antibody. (C) Cells transfected with c-myc-CDK11p46 or c-myc-CDK11p46 and haemagglutinin–ubiquitin were treated with DMSO, 2 μM GA, or 2 μM GA and 25 μM MG132 for 6 h. Proteins were extracted with RIPA buffer and c-myc-CDK11p46 was immunoprecipitated (IP) using anti-c-myc antibody and immunoblotted (WB) with anti-ubiquitin antibody. *Heavy chain of mouse IgG.
The majority of proteins degraded by the proteasome pathway are first modified by covalently attached polyubiquitin chains, which serve as a recognition signal for the proteasome (reviewed in [30]). To determine whether ubiquitination is involved in CDK11p46 degradation on disruption of its association with Hsp90, A375 cells were either transfected with c-myc-CDK11p46 or co-transfected with c-myc-CDK11p46 and haemagglutinin-tagged ubiquitin. Cells were then treated with GA or GA and MG132, and c-myc-CDK11p46 was immunoprecipitated with anti-c-myc antibody, followed by Western blotting with anti-ubiquitin antibody. As shown in Figure 4(C), treatment of GA and MG132 resulted in the formation of polyubiquitinated forms of c-myc-CDK11p46. Stronger signals were visible when cells were co-transfected with c-myc-CDK11p46 and haemagglutinin-tagged ubiquitin and treated with GA and MG132. Taken together, these results suggest that CDK11p46 is ubiquitinated and degraded by a proteasome pathway when the CDK11–Hsp90 complex dissociates.
GA-induced degradation of CDK11p46 decreases the progression of apoptosis
It was previously established that ectopic expression of CDK11p46 induces apoptosis [10–12]. To examine whether GA-induced degradation of CDK11p46 has any effect on the rate of apoptosis, we treated A375 cells expressing c-myc-CDK11p46 or an empty vector as control, with 0.5 μM GA for 16 h (treatment with 0.5 μM GA for 4 h is sufficient to induce degradation of CDK11p46, results not shown). Next, we identified and counted apoptotic cells by a quantitative fluorescent assay. As shown in Figures 5(C) and 5(E), overexpression of c-myc-CDK11p46 significantly promoted apoptosis (33±1.04%). CDK11p46-triggered apoptosis was significantly blocked by the Hsp90 inhibitor GA (14±1.5%; Figures 5D and 5E). In the control, overexpression of the empty vector resulted in 5±1.00% of apoptotic cells, whereas treatment with GA caused 11±1.52% of apoptosis (Figures 5A, 5B and 5E). Taken together, these results indicate that disruption of association of Hsp90 and CDK11p46 leads to degradation of the latter, which consequently decreases the amount of apoptotic cells.
Figure 5. GA-induced degradation of CDK11p46 causes a decrease in apoptosis level.
A375 cells transfected with empty vector were treated with (A) DMSO or (B) 0.5 μM GA for 24 h. Cells transfected with c-myc-CDK11p46 were treated with (C) DMSO or (D) 0.5 μM GA for 16 h. Apoptotic cells were identified by staining with ethidium bromide/Acridine Orange as described in the Materials and methods section. (E) Percentage of apoptotic cells from three independent experiments (A–D).
DISCUSSION
The main goal of the present study was to identify cellular proteins that interact with and regulate CDK11p46. CDK11p46 possesses the entire catalytic kinase domain and is the final caspase-processed product derived from a larger protein CDK11p110. CDK11p46 is generated in human cells undergoing apoptosis. Since ectopic expression of CDK11p46 stimulates apoptosis, a pro-apoptotic role of CDK11p46 was proposed. In the present study, we demonstrated that the caspase-processed form CDK11p46 is associated with the molecular chaperones Hsp90, cdc37 and two members of the Hsp70 family. We also determined that endogenous CDK11p110 interacts with the Hsp90–cdc37 complex. Furthermore, we showed that Hsp90 is responsible for the stabilization of CDK11p110 as well as CDK11p46 in human cells, and that these proteins are degraded by a proteasome-dependent pathway. We also showed that CDK11p46-induced apoptosis is decreased on GA-triggered disruption of the CDK11p46–Hsp90 complex.
Hsp90 interacts with and regulates a number of proteins involved in cell signalling, among which protein kinases represent a large group. It is clear now that the interaction between Hsp90 and protein kinases is not direct. Hsp90 functions in association with its co-chaperone, cdc37, which acts as an adaptor protein that facilitates the interaction between protein kinase and Hsp90 and is responsible for the specificity of the interaction. It is worth mentioning that there are differences in the association of closely related protein kinases with cdc37–Hsp90 complexes. Conventional mitogen-activated protein kinases (ERK, p38 and c-Jun N-terminal kinase/stress-activated protein kinase) do not associate with the cdc37–Hsp90 complex, whereas their homologues, MOK, MAK and MRK do associate [28]. Similarly, CDK4, CDK6 and CDK9 associate with the cdc37–Hsp90 complex, whereas CDK2, CDK3 and CDK5 do not [20,31–34]. In this study, we showed that another member of the CDK family, CDK11, forms a stable complex with cdc37–Hsp90. Furthermore, we could detect the CDK11p46–cdc37–Hsp90 heterocomplex using only the C-terminal portion of CDK11p110. Studies on several different protein kinases including pp60v−src [35], Raf [36], CK2α (casein kinase 2α) [37], Plk [38], Akt [39], MOK [28], PDK1 [27], IKK [40] and LKB1 [29,41] revealed that the association of protein kinases with the cdc37–Hsp90 complex usually occurs through the catalytic kinase domain. Our results suggest that in CDK11, it is also the catalytic kinase domain that is responsible and sufficient for association with the cdc37/Hsp90 heterodimer.
In many cases, association of the cdc37–Hsp90 complex with a protein kinase is necessary for the stability of the latter. The ansamycin antibiotic GA blocks the ATP/ADP-binding site within the N-terminal part of Hsp90 [23,24,42] and prevents the conformational changes necessary for the chaperoning functions of Hsp90 [43,44], which leads to the disruption of client protein activation [45–47]. Treatment with GA caused rapid destabilization of both CDK11p110 and CDK11p46. Metabolic labelling and pulse–chase experiments confirmed that after GA treatment, the half-life of CDK11p46 was significantly shortened. Binding of GA to the Hsp90 chaperone complex prevents refolding of the client protein, which results in its degradation. The degradation of proteins that fail to fold correctly is frequently regulated by proteasome. To determine whether the proteasome pathway is indeed involved in CDK11p110/p46 degradation, we inhibited proteasome-mediated degradation with the proteasome inhibitor MG123. Treatment of cells with both GA and MG132 blocked degradation of CDK11p110/p46 and increased their amounts in the insoluble fraction. Many proteins destined for degradation are labelled with polyubiquitin chains and then targeted to the proteasome. In agreement with this observation, we found that treatment of cells with GA and MG132 resulted in the formation of polyubiquitinated forms of CDK11p46. These results suggest that the proteasome is responsible for the degradation of CDK11p110/p46 after dissociation from Hsp90 and that ubiquitination is involved in this process.
It should be pointed out that the treatment with GA results not only in rapid degradation of CDK11p46 but also in decrease in the amount of apoptotic A375 cells. Since CDK11p46 is generated during Fas-induced apoptosis and ectopic expression of CDK11p46 induces apoptosis, CDK11p46 is considered as a pro-apoptotic protein. Disruption of the CDK11p46–Hsp90 complex may therefore cause deregulation of cell death and prevent apoptosis. These findings lead to the conclusion that molecular antagonists of CDK11p46–Hsp90 interaction may prevent apoptosis of cells, which are programmed to undergo apoptosis.
In addition to acting as protein chaperones, Hsc70/Hsp70 and Hsp90 may also regulate the activity of interacting protein kinases. It was shown that Hsc70 associates with newly synthesized cyclin D1 and is a component of a mature, active cyclin D1–CDK4 holoenzyme complex [48]. Another protein kinase, Raf-1, forms a complex with Hsp90 which is necessary for its kinase activity and mitogen-activated protein kinase pathway signalling [21]. Furthermore, Hsp90 interacts with and activates CK2 [49]. The effect of association of CDK11p110 and CDK11p46 with a Hsp complex on its kinase activity is under investigation. It is possible that the activity of endogenous CDK11p110 and its association with cyclin L or CK2 [50,51] is modulated when the association with Hsp90 and/or Hsc70/Hsp70 is disrupted.
Acknowledgments
We thank Dr D. Bohmann (Center for Cancer Biology, University of Rochester Medical Center, Rochester, NY, U.S.A.) for the gift of the plasmid-expressing haemagglutinin-tagged ubiquitin. We also thank Dr G. Tsaprailis of the Southwest Environmental Health Center/Arizona Cancer Center Proteomics Core Facility. This work was supported by National Institutes of Health grants CA70145, ESO 66694 and CA 23074 to the Arizona Cancer Center.
References
- 1.Bunnell B. A., Heath L. S., Adams D. E., Lahti J. M., Kidd V. J. Increased expression of a 58-kDa protein kinase leads to changes in the CHO cell cycle. Proc. Natl. Acad. Sci. U.S.A. 1990;87:7467–7471. doi: 10.1073/pnas.87.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loyer P., Trembley J. H., Lahti J. M., Kidd V. J. The RNP protein, RNPS1, associates with specific isoforms of the p34cdc2-related PITSLRE protein kinase in vivo. J. Cell Sci. 1998;111:1495–1506. doi: 10.1242/jcs.111.11.1495. [DOI] [PubMed] [Google Scholar]
- 3.Berke J. D., Sgambato V., Zhu P. P., Lavoie B., Vincent M., Krause M., Hyman S. E. Dopamine and glutamate induce distinct striatal splice forms of Ania-6, an RNA polymerase II-associated cyclin. Neuron. 2001;32:277–287. doi: 10.1016/s0896-6273(01)00465-2. [DOI] [PubMed] [Google Scholar]
- 4.Dickinson L. A., Edgar A. J., Ehley J., Gottesfeld J. M. Cyclin L is an RS domain protein involved in pre-mRNA splicing. J. Biol. Chem. 2002;277:25465–25473. doi: 10.1074/jbc.M202266200. [DOI] [PubMed] [Google Scholar]
- 5.Cornelis S., Bruynooghe Y., Denecker G., Van Huffel S., Tinton S., Beyaert R. Identification and characterization of a novel cell cycle-regulated internal ribosome entry site. Mol. Cell. 2000;5:597–605. doi: 10.1016/s1097-2765(00)80239-7. [DOI] [PubMed] [Google Scholar]
- 6.Lahti J. M., Xiang J., Heath L. S., Campana D., Kidd V. J. PITSLRE protein kinase activity is associated with apoptosis. Mol. Cell. Biol. 1995;15:1–11. doi: 10.1128/mcb.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyaert R., Kidd V. J., Cornelis S., Van de Craen M., Denecker G., Lahti J. M., Gururajan R., Vandenabeele P., Fiers W. Cleavage of PITSLRE kinases by ICE/CASP-1 and CPP32/CASP-3 during apoptosis induced by tumor necrosis factor. J. Biol. Chem. 1997;272:11694–11697. doi: 10.1074/jbc.272.18.11694. [DOI] [PubMed] [Google Scholar]
- 8.Tang D., Gururajan R., Kidd V. J. Phosphorylation of PITSLRE p110 isoforms accompanies their processing by caspases during Fas-mediated cell death. J. Biol. Chem. 1998;273:16601–16607. doi: 10.1074/jbc.273.26.16601. [DOI] [PubMed] [Google Scholar]
- 9.Ariza M. E., Broome-Powell M., Lahti J. M., Kidd V. J., Nelson M. A. Fas-induced apoptosis in human malignant melanoma cell lines is associated with the activation of the p34(cdc2)-related PITSLRE protein kinases. J. Biol. Chem. 1999;274:28505–28513. doi: 10.1074/jbc.274.40.28505. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S., Cai M., Xu S., Chen S., Chen X., Chen C., Gu J. Interaction of p58 (PITSLRE), a G2/M-specific protein kinase, with cyclin D3. J. Biol. Chem. 2002;277:35314–35322. doi: 10.1074/jbc.M202179200. [DOI] [PubMed] [Google Scholar]
- 11.Chen S., Yin X., Zhu X., Yan J., Ji S., Chen C., Cai M., Zhang S., Zong H., Hu Y., et al. The C-terminal kinase domain of the p34cdc2-related PITSLRE protein kinase (p110C) associates with p21-activated kinase 1 and inhibits its activity during anoikis. J. Biol. Chem. 2003;278:20029–20036. doi: 10.1074/jbc.M300818200. [DOI] [PubMed] [Google Scholar]
- 12.Shi J., Feng Y., Goulet A. C., Vaillancourt R. R., Sachs N. A., Hershey J. W., Nelson M. A. The p34cdc2-related cyclin-dependent kinase 11 interacts with the p47 subunit of eukaryotic initiation factor 3 during apoptosis. J. Biol. Chem. 2003;278:5062–5071. doi: 10.1074/jbc.M206427200. [DOI] [PubMed] [Google Scholar]
- 13.Lahti J. M., Valentine M., Xiang J., Jones B., Amann J., Grenet J., Richmond G., Look A. T., Kidd V. J. Alterations in the PITSLRE protein kinase gene complex on chromosome 1p36 in childhood neuroblastoma. Nat. Genet. 1994;7:370–375. doi: 10.1038/ng0794-370. [DOI] [PubMed] [Google Scholar]
- 14.Perlman E. J., Valentine M. B., Griffin C. A., Look A. T. Deletion of 1p36 in childhood endodermal sinus tumors by two-color fluorescence in situ hybridization: a pediatric oncology group study. Genes Chromosomes Cancer. 1996;16:15–20. doi: 10.1002/(SICI)1098-2264(199605)16:1<15::AID-GCC2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Nelson M. A., Ariza M. E., Yang J. M., Thompson F. H., Taetle R., Trent J. M., Wymer J., Massey-Brown K., Broome-Powell M., Easton J., et al. Abnormalities in the p34cdc2-related PITSLRE protein kinase gene complex (CDC2L) on chromosome band 1p36 in melanoma. Cancer Genet. Cytogenet. 1999;108:91–99. doi: 10.1016/s0165-4608(98)00122-8. [DOI] [PubMed] [Google Scholar]
- 16.Dave B. J., Pickering D. L., Hess M. M., Weisenburger D. D., Armitage J. O., Sanger W. G. Deletion of cell division cycle 2-like 1 gene locus on 1p36 in non-Hodgkin lymphoma. Cancer Genet. Cytogenet. 1999;108:120–126. doi: 10.1016/s0165-4608(98)00138-1. [DOI] [PubMed] [Google Scholar]
- 17.Eipers P. G., Barnoski B. L., Han J., Carroll A. J., Kidd V. J. Localization of the expressed human p58 protein kinase chromosomal gene to chromosome 1p36 and a highly related sequence to chromosome 15. Genomics. 1991;11:621–629. doi: 10.1016/0888-7543(91)90069-q. [DOI] [PubMed] [Google Scholar]
- 18.Powell A. A., LaRue J. M., Batta A. K., Martinez J. D. Bile acid hydrophobicity is correlated with induction of apoptosis and/or growth arrest in HCT116 cells. Biochem. J. 2001;356:481–486. doi: 10.1042/0264-6021:3560481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pratt W. B., Toft D. O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 20.Stepanova L., Leng X., Parker S. B., Harper J. W. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- 21.Grammatikakis N., Lin J. H., Grammatikakis A., Tsichlis P. N., Cochran B. H. p50 (cdc37) acting in concert with Hsp90 is required for Raf-1 function. Mol. Cell. Biol. 1999;19:1661–1672. doi: 10.1128/mcb.19.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao J., Grammatikakis N., Scroggins B. T., Uma S., Huang W., Chen J. J., Hartson S. D., Matts R. L. Hsp90 regulates p50 (cdc37) function during the biogenesis of the active conformation of the heme-regulated eIF2 alpha kinase. J. Biol. Chem. 2001;276:206–214. doi: 10.1074/jbc.M007583200. [DOI] [PubMed] [Google Scholar]
- 23.Stebbins C. E., Russo A. A., Schneider C., Rosen N., Hartl F. U., Pavletich N. P. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell (Cambridge, Mass.) 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 24.Prodromou C., Roe S. M., O'Brien R., Ladbury J. E., Piper P. W., Pearl L. H. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell (Cambridge, Mass.) 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 25.Grenert J. P., Sullivan W. P., Fadden P., Haystead T. A., Clark J., Mimnaugh E., Krutzsch H., Ochel H. J., Schulte T. W., Sausville E., et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J. Biol. Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 26.Hohfeld J., Cyr D. M., Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001;2:885–890. doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita N., Sato S., Ishida A., Tsuruo T. Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-dependent kinase-1. J. Biol. Chem. 2002;277:10346–10353. doi: 10.1074/jbc.M106736200. [DOI] [PubMed] [Google Scholar]
- 28.Miyata Y., Ikawa Y., Shibuya M., Nishida E. Specific association of a set of molecular chaperones including HSP90 and Cdc37 with MOK, a member of the mitogen-activated protein kinase superfamily. J. Biol. Chem. 2001;276:21841–21848. doi: 10.1074/jbc.M010944200. [DOI] [PubMed] [Google Scholar]
- 29.Nony P., Gaude H., Rossel M., Fournier L., Rouault J. P., Billaud M. Stability of the Peutz-Jeghers syndrome kinase LKB1 requires its binding to the molecular chaperones Hsp90/Cdc37. Oncogene. 2003;22:9165–9175. doi: 10.1038/sj.onc.1207179. [DOI] [PubMed] [Google Scholar]
- 30.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai K., Kobayashi R., Beach D. Physical interaction of mammalian CDC37 with CDK4. J. Biol. Chem. 1996;271:22030–22034. doi: 10.1074/jbc.271.36.22030. [DOI] [PubMed] [Google Scholar]
- 32.Lamphere L., Fiore F., Xu X., Brizuela L., Keezer S., Sardet C., Draetta G. F., Gyuris J. Interaction between Cdc37 and Cdk4 in human cells. Oncogene. 1997;14:1999–2004. doi: 10.1038/sj.onc.1201036. [DOI] [PubMed] [Google Scholar]
- 33.Mahony D., Parry D. A., Lees E. Active cdk6 complexes are predominantly nuclear and represent only a minority of the cdk6 in T cells. Oncogene. 1998;16:603–611. doi: 10.1038/sj.onc.1201570. [DOI] [PubMed] [Google Scholar]
- 34.O'Keeffe B., Fong Y., Chen D., Zhou S., Zhou Q. Requirement for a kinase-specific chaperone pathway in the production of a Cdk9/cyclin T1 heterodimer responsible for P-TEFb-mediated tat stimulation of HIV-1 transcription. J. Biol. Chem. 2000;275:279–287. doi: 10.1074/jbc.275.1.279. [DOI] [PubMed] [Google Scholar]
- 35.Jove R., Garber E. A., Iba H., Hanafusa H. Biochemical properties of p60v-src mutants that induce different cell transformation parameters. J. Virol. 1986;60:849–857. doi: 10.1128/jvi.60.3.849-857.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stancato L. F., Chow Y. H., Hutchison K. A., Perdew G. H., Jove R., Pratt W. B. Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J. Biol. Chem. 1993;268:21711–21716. [PubMed] [Google Scholar]
- 37.Miyata Y., Yahara I. Interaction between casein kinase II and the 90-kDa stress protein, HSP90. Biochemistry. 1995;34:8123–8129. doi: 10.1021/bi00025a019. [DOI] [PubMed] [Google Scholar]
- 38.Simizu S., Osada H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nat. Cell Biol. 2000;2:852–854. doi: 10.1038/35041102. [DOI] [PubMed] [Google Scholar]
- 39.Sato S., Fujita N., Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen G., Cao P., Goeddel D. V. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol. Cell. 2002;9:401–410. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- 41.Boudeau J., Deak M., Lawlor M. A., Morrice N. A., Alessi D. R. Heat-shock protein 90 and Cdc37 interact with LKB1 and regulate its stability. Biochem. J. 2003;370:849–857. doi: 10.1042/BJ20021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roe S. M., Prodromou C., O'Brien R., Ladbury J. E., Piper P. W., Pearl L. H. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 43.Prodromou C., Panaretou B., Chohan S., Siligardi G., O'Brien R., Ladbury J. E., Roe S. M., Piper P. W., Pearl L. H. The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. EMBO J. 2000;19:4383–4392. doi: 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chadli A., Bouhouche I., Sullivan W., Stensgard B., McMahon N., Catelli M. G., Toft D. O. Dimerization and N-terminal domain proximity underlie the function of the molecular chaperone heat shock protein 90. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12524–12529. doi: 10.1073/pnas.220430297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitesell L., Mimnaugh E. G., De Costa B., Myers C. E., Neckers L. M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulte T. W., Blagosklonny M. V., Ingui C., Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J. Biol. Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- 47.Chavany C., Mimnaugh E., Miller P., Bitton R., Nguyen P., Trepel J., Whitesell L., Schnur R., Moyer J., Neckers L. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J. Biol. Chem. 1996;271:4974–4977. doi: 10.1074/jbc.271.9.4974. [DOI] [PubMed] [Google Scholar]
- 48.Diehl J. A., Yang W., Rimerman R. A., Xiao H., Emili A. Hsc70 regulates accumulation of cyclin D1 and cyclin D1-dependent protein kinase. Mol. Cell. Biol. 2003;23:1764–1774. doi: 10.1128/MCB.23.5.1764-1774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyata Y., Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J. Biol. Chem. 1992;267:7042–7047. [PubMed] [Google Scholar]
- 50.Trembley J. H., Hu D., Slaughter C. A., Lahti J. M., Kidd V. J. Casein kinase 2 interacts with cyclin-dependent kinase 11 (CDK11) in vivo and phosphorylates both the RNA polymerase II carboxyl-terminal domain and CDK11 in vitro. J. Biol. Chem. 2003;278:2265–2270. doi: 10.1074/jbc.M207518200. [DOI] [PubMed] [Google Scholar]
- 51.Sachs N. A., Vaillancourt R. R. Cyclin-dependent kinase 11p110 and casein kinase 2 (CK2) inhibit the interaction between tyrosine hydroxylase and 14-3-3. Biochim. Biophys. Acta. 2003;1624:98–108. [Google Scholar]