Abstract
Cytokines, phorbol esters, radiation and chemotherapeutic drugs up-regulate the expression of MnSOD (manganese superoxide dismutase). Using the VA-13 cell line, we studied the regulation of SOD2 upon treatment with PMA. Pre-treatment with CHX (cycloheximide) followed by PMA led to significantly higher levels of MnSOD mRNA compared with those with either agent alone, suggesting de novo synthesis of an inhibitory protein. PMA treatment modulates redox-sensitive transcription factors, therefore we evaluated the effects of this combination treatment upon AP-1 (activator protein 1) and NF-κB (nuclear factor κB), two trans-acting factors suggested to play a role in SOD2 regulation. Co-administration of CHX and PMA led to a time-dependent increase in the binding activity of NF-κB. Therefore we evaluated IκBα (inhibitory κBα) and found that co-administration decreased its steady-state level compared with either agent alone, suggesting that enhanced NF-κB activation is due to inhibition of IκBα synthesis. PMA activates PKC (protein kinase C) enzymes which phosphorylate IκBα, leading to its degradation, therefore we used GF109203X to inhibit PKC activity. Stable transfection utilizing a PMA-responsive element in the human SOD2 gene, showed a concentration-dependent decrease in luciferase and NF-κB-binding activity with GF109203X. Western blot analysis indicated the presence of several PKC isoforms in the VA-13 cell line; however, PMA pre-treatment specifically down-regulated α and βI, suggesting a role for one or more of these proteins in SOD2 induction. Taken together, these results indicate that the PKC pathway leading to SOD2 induction proceeds at least in part through NF-κB and that inhibition of IκBα synthesis might serve as a potential pharmacological approach to up-regulate MnSOD.
Keywords: cycloheximide, gene regulation, manganese superoxide dismutase (MnSOD), nuclear-factor-κB-binding protein (NF-κB-binding protein), phorbol ester, protein kinase C (PKC)
Abbreviations: AP-1, activator protein 1; BME, Eagle's basal medium; CHX, cycloheximide; dn, dominant-negative; EMSA, electrophoretic mobility-shift assay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; IκBα, inhibitory κBα; I2E, intron 2 enhancer; IL, interleukin; MnSOD, manganese superoxide dismutase; NF-κB, nuclear factor κB; PKC, protein kinase C; RNS, reactive nitrogen species; ROS, reactive oxygen species; SOD, superoxide dismutase; TBST, Tris-buffered saline with Tween 20; TNF-α, tumour necrosis factor α
INTRODUCTION
MnSOD (manganese superoxide dismutase) is an essential antioxidant enzyme that catalyses the conversion of superoxide radical into hydrogen peroxide and molecular oxygen within the mitochondrial matrix [1]. ROS (reactive oxygen species), including the superoxide radical, are normal by-products of cellular metabolism and aerobic respiration. ROS and RNS (reactive nitrogen species) have been implicated in various pathological conditions, including aging, cancer, and vascular and neurodegenerative diseases, such as Parkinson's, amyotrophic lateral sclerosis and Alzheimer's disease (reviewed in [2]). An increase in ROS/RNS formation and/or an ineffective system for removal could contribute to the pathological hallmarks associated with the aforementioned diseases. Animals genetically modified to knockout expression of individual SOD (superoxide dismutase) enzymes have provided valuable information on the relative contributions of each isoform in cellular homoeostasis. Knockout models of the SOD2 gene clearly distinguished MnSOD from both the cytosolic (Cu,Zn SOD) and extracellular (ECSOD) copper- and zinc-containing SOD isoenzymes. In knockout models of the SOD2 gene, neonatal lethality was observed as a result of cardiomyopathy [3] and neurodegeneration [4]. Knockout of either the SOD1 or SOD3 gene did not result in neonatal lethality [5,6].
Transgenic cell lines have shown MnSOD to attenuate injury or death under conditions of enhanced oxidative stress by exposure to TNF-α (tumour necrosis factor α) [7], iron [8], NO-generating systems [8,9], alkalosis [10], chemical hypoxia [11] or 5-azacytidine treatment [12]. MnSOD has been shown to protect against oxygen-induced lung injury [13], acute doxorubicin-induced cardiac injury [14], as well as ischaemia and trauma-induced brain injuries in a transgenic animal model [8,15].
Numerous studies suggest that MnSOD activity may play a role in tumorigenesis. Tumour cells, which normally express low levels of MnSOD activity, undergo phenotypic changes as a result of transfection and subsequent overexpression of MnSOD [16–18]. Enhanced MnSOD activity has been shown to correlate with suppression of tumour growth in nude mice [16,17] and inhibition of metastatic potential of transplanted tumours [19]. Evaluation of redox-sensitive oncogenes in a murine fibrosarcoma cell line engineered to express high MnSOD activity showed selective down-regulation of DNA binding and transcriptional activation of Jun-associated transcription factors [AP-1 (activator protein-1) and CREB (cAMP-response element binding protein)] [20]. Taken together, these studies provide in vitro and in vivo evidence of tumour suppressor effects of MnSOD.
A variety of compounds such as TNF-α [21], IL (interleukin)-1β [22], dinitrophenol [23], paraquat [24] and PMA [25], which cause oxidative stress, lead to the up-regulation of SOD2 gene transcription. We have identified an enhancer element within intron 2 of the human SOD2 gene which is responsible for TNF-α- and IL-1β-mediated induction [26]. In addition, we have shown that the enhancer is responsive to PMA treatment [27]. In the present study, evaluation of the mechanism of PMA induction in the VA-13 cell line showed that de novo protein synthesis was not required; however, CHX (cycloheximide) in combination with PMA synergistically increased MnSOD mRNA levels, suggesting the presence of a ‘labile repressor’. We have identified the IκBα (inhibitory κBα) protein to be a repressor of MnSOD expression. Furthermore, we show that the responsiveness of the intronic enhancer to PMA is PKC (protein kinase C)-dependent and is mediated, at least in part, through PKCα and PKCβI. Taken together, these studies suggest potential pharmacological targets in the regulation of human MnSOD expression.
EXPERIMENTAL
Cell culture
The SV40 (simian virus 40)-transformed human lung fibroblast cell line (VA-13) which was purchased from the A.T.C.C. (Manassas, VA, U.S.A.) was maintained in BME (Eagle's basal medium) supplemented with 10% (v/v) foetal bovine serum, 1% (w/v) glutamine and 1% (w/v) antibiotics. Cells were grown at 37 °C in a humidified atmosphere containing 5% CO2.
Plasmids
A BamHI fragment containing a 3.4 kb 5′-flanking region was used to generate the minimal promoter fragment (P7). To create the P7 fragment, PCR primers with recognition sequences for the KpnI and BglII restriction enzymes were added to allow subcloning upstream of the luciferase reporter gene. The following primer set was used (the 9 bp shown underlined indicates the KpnI-recognition site in the upper-strand primer and the BglII recognition site in the lower-strand primer starting at +24) [upper strand (−210), 5′-CGGGGTACCGCCTCCTTTCTCCCGTGCCCTGCCCTGG-3′; lower strand (+24), 5′-GGAAGGATCTGCCGAAGCCACCACCACAGCCACGGAGT-3′].
The P7 fragment was subcloned into a PGL3 luciferase vector (Promega). BamHI digestion of a 39 b λ phage clone containing the entire human SOD2 gene resulted in a 8074 bp fragment which was subsequently used as template for generation of a 342 bp fragment within the second intron (12E). The primers were designed to amplify the 12E region KpnI sites. PCR was carried out in a 100 μl master mixture containing 10 μl of 10×buffer [200 mM Tris/HCl, pH 8.8, 20 mM MgSO4, 100 mM KCl, 100 mM (NH4)2SO4, 1% Triton X-100, 1 mg/ml nuclease-free BSA], 4 μl of 10 mM dNTP mixture (2.5 mM each of dATP, dGTP and dTTP), 1 μl of 10 pM of each primer [upper strand, 5′-CGGGGTACCGGGGTTATGAAATTTGTTGAGTA-3′ and lower strand, 5′-CGGGGTACCCCACAAGTAAAGGACTGAAATTAA-3′ (KpnI recognition sites underlined)], 100 ng of plasmid DNA and 1 μl of 2.5 units/μl of a high-fidelity Pfu DNA polymerase (Stratagene). The thermal cycling settings were, after initial denaturation at 95 °C for 5 min, 30 amplification cycles of denaturation at 94 °C for 1 min, annealing at 60 °C for 1 min and extension at 72 °C for 1 min, and final extension at 72 °C for 10 min. After amplification, the I2E (intron 2 enhancer) region was subcloned into the modified PGL3 vector (Promega) containing the luciferase reporter gene driven by the minimal human MnSOD promoter (P7/PGL3). Nucleotide composition was verified using sequencing methods.
We reported previously that when the 342 bp I2E fragment was linked to the basal promoter (P7) of the SOD2 gene, transcriptional activities were increased significantly in response to cytokines in an orientation- and position-independent manner [26]. Based on these findings, we chose to transfect a construct containing the I2E element upstream of both the basal promoter (P7) and the luciferase reporter. Further information, including construct maps, as well as the location of transcription-factor-binding sites in the SOD2 gene (5′-I2E-3′), are provided by Xu et al. [26].
Stable transfection
We reported previously the generation of stable VA-13 clones by transfection of a linearized plasmid (I2E-P7/PGL3) [27]. In brief, the plasmid DNA (I2E-P7/PGL3) was linearized with BamHI and co-transfected with the pSV2-NEO plasmid using Lipofectin. After 48 h, the cells were exposed to 400 μg/ml G418 sulphate. Clones were obtained and propagated from single transfected cells plated on to 60-mm-diameter dishes. After isolation of three clones (VA13I2E4, VA13I2E7 and VA13I2E8), preliminary experiments were conducted comparing the effects of CHX±PMA (mRNA) and PMA-mediated differences in luciferase expression. No differences were observed among the individual clones, therefore clone VA13I2E7 was used for all subsequent experiments. Cell lines obtained from individual clones were maintained in BME with 10% (v/v) foetal bovine serum, 1% (w/v) glutamine, 1% (w/v) penicillin–streptomycin and 400 μg/ml G418. Integration of DNA was verified by restriction digestion followed by Southern blot analysis. Luciferase activity was measured using the Luciferase Assay System (Promega) according to the manufacturer's instructions. All luciferase assays were normalized to protein concentration.
Northern blot analysis
Cells (1×106/p150 plate) were plated, grown to approx. 90% confluence, pre-treated with CHX (10 μg/ml) for 1 h, followed by a 6 h treatment of PMA (100 nM). Total RNA was isolated, and the concentration was estimated spectrophotometrically at 260 nm. Total RNA (30 μg) was loaded on a 1% agarose formaldehyde gel for electrophoresis and then transferred on to a nylon membrane. The membrane was baked at 80 °C for 2 h, pre-hybridized at 42 °C in pre-hybridization solution [50% formamide, 5×saline/sodium phosphate/EDTA (150 mM NaCl, 10 mM NaH2PO4 and 1 mM EDTA)], and then hybridized for 72 h at 42 °C. Probes were 32P-labelled using the random priming method. The filters were washed twice at room temperature (22 °C) for 15 min with 2×SSC (1×SSC is 0.15 M NaCl/0.015 M sodium citrate) and 0.1% (w/v) sodium pyrophosphate, and twice for 20 min at 65 °C with 0.1×SSC, 0.1% (w/v) SDS and 0.1% (w/v) sodium pyrophosphate. The blots were then exposed to X-ray film at −70 °C. The probes used were a human MnSOD cDNA and a chicken β-actin cDNA.
Cell fractionation
Cells were placed on ice, washed twice with PBS, and scraped into homogenization buffer (20 mM Hepes, 5 mM EGTA, 10 mM 2-mercaptoethanol, 100 μg/ml leupeptin and 1 mM PMSF). Cells were homogenized in a pre-cooled Dounce homogenizer. The lysate was centrifuged (2 min, 50 g) to remove intact cells. Cell homogenates were fractionated into cytosolic or membrane components by centrifugation (1 h, 100000 g). The resulting supernatant was stored at −70 °C. The membrane pellet was rinsed in homogenization buffer and then resuspended in the same buffer containing 0.5% (v/v) Triton X-100. The sample was incubated on ice for 30 min with occasional vortex-mixing. Following resuspension, the fraction was centrifuged (30 min, 14000 g) and the supernatant was stored at −70 °C.
EMSA (electrophoretic mobility-shift assay)
Cells were plated at a density of 6×105 cells/100-mm-diameter dish. Cells were treated as described above, and nuclear extracts, as well as the cytoplasmic fraction, were isolated as described previously with the inclusion of 35% (v/v) glycerol and protease inhibitors (pepstatin, aprotinin and leupeptin) at 1 μg/ml in the extraction buffer. The phosphatase inhibitors sodium orthovanadate and sodium fluoride were included at concentrations of 5 mM and 1 mM respectively. Protein concentration was determined by a colorimetric assay (Bio-Rad). Oligonucleotides corresponding to AP-1 or the NF-κB (nuclear factor κB) element within the I2E fragment were either purchased from Santa Cruz (AP-1) or synthesized by Life Technologies as follows: AP-1, 5′-CGCTTGATGACTCAGCCGGAA-3′; NF-κB, 5′-GAGACTGGGGAATACCCCAGT-3′.
After annealing, each oligonucleotide was radioactively end-labelled with [γ-32P]ATP (3000 Ci/mmol at 10 mCi/ml) and T4 polynucleotide kinase (New England Biolabs, Beverly, MA, U.S.A.). The probes were purified on a 20% native PAGE gel. The gel was exposed to Kodak film and the band corresponding to the double strand was excised. The DNA was eluted overnight at 37 °C in 300 μl of 10 mM Tris/HCl, 1 mM EDTA buffer (pH 7.4). The activity of labelled probe was counted and stored at −70 °C. Nuclear extract (10 μg) protein was used, and the final volume of each reaction was 20 μl. In each reaction, 4 μl of 5-fold binding buffer [20% glycerol, 5 mM MgCl2, 2.5 mM EDTA, 5 mM DTT (dithiothreitol), 50 mM Tris/HCl, pH 7.5, 0.25 mg/ml poly(deoxyinosinic-deoxycytidylic acid)] and 40000 c.p.m. of labelled probe were used. Samples were incubated at room temperature for 20 min. The reaction was stopped by addition of 2 μl of 10×DNA-loading buffer [25 mM Tris/HCl (pH 7.5), 0.02% (w/v) Bromophenol Blue and 4% (v/v) glycerol]. DNA–protein complexes were separated from unbound probe on a native 6% PAGE gel in 0.5×Tris/borate/EDTA buffer. The specificity of binding has been reported previously either using corresponding mutated oligonucleotides or supershift experiments [6,13]. Gels were vacuum-dried and exposed to Kodak film at −70 °C.
Western blot analysis: IκBα
Cytoplasmic extracts (25 μg) of control and treated cells were analysed for levels of IκBα. Samples were separated on an SDS/12.5% PAGE gel and transferred on to nitrocellulose. Transfer efficiency was assessed by staining with 0.1% (w/v) Ponceau S. The membrane was washed with distilled water to remove the excess stain and blocked in Blotto [5% (w/v) dried milk, 10 mM Tris/HCl, 150 mM NaCl (pH 8.0) and 0.5% (v/v) Tween 20] for 1 h at room temperature. An affinity-purified rabbit anti-IκBα antibody (1:1000) purchased from Santa Cruz Biotechnology was used. After two washes in TBST [Tris-buffered saline (10 mM Tris/HCl and 150 nM NaCl) with Tween 20], the blot was incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology) at a 1:5000 dilution in Blotto for 1.5 h at room temperature. The blot was washed three times with TBST and once with TBS (Tris-buffered saline). Protein bands were visualized using the ECL® (enhanced chemiluminescence) detection system (Amersham Biosciences).
PKC isoforms
Cytoplasmic, membrane or total cell lysates were analysed for various PKC isoforms. All antibodies were used at a 1:1000 dilution and were purchased from Santa Cruz Biotechnology. Secondary antibodies (Santa Cruz Biotechnology) were used at a 1:5000 dilution.
Inhibitor study
Cells were plated (1×105/well) in a 24-well plate for reporter assays or (6×105) in a p100 plate for EMSA analysis. After 72 h, cells were pre-treated for 2 h with GF109203X (0–1 μM; Calbiochem) followed by either a 1 h (EMSA) or 12 h (reporter assay) administration of PMA. Cells were collected as described previously, and luciferase activity or DNA-binding studies were performed.
Adenoviral-mediated gene transfer
Cells were plated (1.5×105/well) in a six-well plate. After 24 h, cells were washed twice, and a 1 h adenoviral infection of 108 pfu (plaque-forming units)/ml at 37 °C in Optimem was performed. Dominant-negative expression vectors for the human PKCα, β1 and β2 isoforms were obtained from Dr George King (Joslin Diabetes Center, Boston, MA, U.S.A.). After 1 h, BME containing 10% (v/v) foetal bovine serum was added. At 48 h following transfection, cells were treated with PMA. Samples were collected and assayed for luciferase activity or the presence of PKC.
Statistical analysis
All experiments were replicated in triplicate and representative findings are shown. ANOVA was performed for multiple samples, and statistical significance was determined using Bonferroni's test.
RESULTS
Induction of MnSOD mRNA in VA-13 cells by phorbol ester is independent of protein synthesis and is superinduced by inhibition of protein synthesis
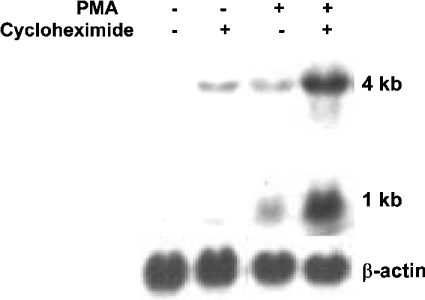
We have shown previously that PMA can induce MnSOD mRNA levels in VA-13 cells, and that this induction may be mediated through co-ordinated efforts of C/EBP (CCAAT/enhancer-binding protein) and NF-κB transcription factors, which bind to an enhancer element within the second intron of the human SOD2 gene (I2E) [27]. In the present study, we evaluated whether protein synthesis was required for the PMA-mediated induction using the VA-13 cell line. After all treatments, an increase in both the 4 kb and 1 kb transcripts was seen. Blots were stripped and reprobed with a chicken β-actin cDNA probe as a control for RNA integrity and loading. Using densitometry, we quantified fold-change among treatments after normalization to β-actin. Experiments were conducted in triplicate and averaged, with Figure 1 being a representation of our findings. We showed a 3.8±0.2-fold increase in MnSOD mRNA levels with CHX alone (Figure 1). PMA treatment resulted in a 4.9±0.3-fold increase in mRNA levels; however, pre-treatment with CHX followed by the phorbol ester resulted in a 6.8±1.4-fold increase compared with control.
Figure 1. MnSOD mRNA levels increase synergistically with PMA+CHX treatment.
Northern blot analysis of MnSOD mRNA in VA-13 cells as a result of CHX (10 μg/ml) exposure for 1 h followed by a 6 h treatment with PMA (100 nM). The 1 kb and 4 kb human MnSOD transcripts are indicated. The blots were stripped and reprobed with a β-actin probe.
CHX enhances PMA-mediated increases in NF-κB DNA-binding activity
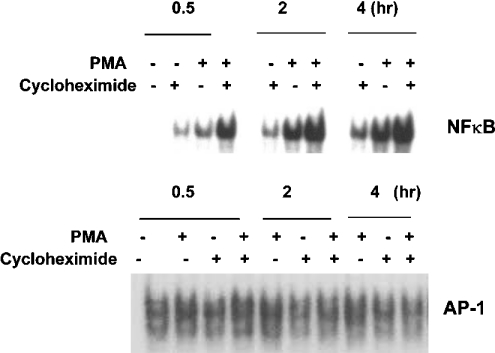
Previously, we have shown that PMA treatment can lead to an increase in NF-κB DNA-binding activity within the intronic enhancer of the SOD2 gene [27]. Since changes in the activity of NF-κB are thought to be a primary mechanism of increased transcription of the SOD2 gene, we evaluated the binding pattern with respect to CHX±PMA over 4 h. Figure 2 shows a time-dependent increase in NF-κB DNA-binding activity with CHX alone. In addition CHX+PMA treatment led to substantial increases in the DNA-binding activity of NF-κB compared with that with PMA or CHX alone. Furthermore, phorbol esters are known to modulate AP-1-dependent transcriptional events in vitro [30]. AP-1 has been suggested to play a role in regulation of MnSOD, as binding sites for this transcription factor have been described in the promoter region [31]. Therefore we evaluated the binding pattern with respect to CHX, PMA and CHX+PMA treatment. No changes were observed in the DNA-binding pattern of AP-1 (Figure 2) upon administration of CHX.
Figure 2. CHX+PMA selectively enhances NF-κB DNA-binding activity in VA-13 cells.
EMSA of nuclear extracts (10 μg) with radiolabelled oligonucleotides. Cells were pre-treated with CHX (10 μg/ml) for 1 h before PMA (100 nM) administration. Extracts were prepared at 30 min, 2 h or 4 h after PMA treatment.
CHX enhances PMA-mediated IκBα degradation in VA-13 cells
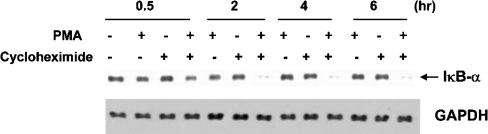
Western blot analysis of cytoplasmic extracts prepared 30 min, 2 h, 4 h or 6 h after co-administration with the phorbol ester revealed an 85% decrease in IκBα protein at 2 h compared with control. With only CHX, there was a 19% decrease, and with PMA treatment alone, a 30% decrease in IκBα protein levels compared with control was seen (Figure 3). Lower levels of IκBα with the combined treatment paralleled the increase in NF-κB-binding activity described previously. Protein loading was assessed using GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a control.
Figure 3. CHX+PMA enhances IκBα degradation in VA-13 cells.
Western blot analysis of IκBα levels in the cytoplasmic extracts of cells pre-treated for 1 h with CHX (10 μg/ml) followed by PMA (100 nM). Extracts were prepared 30 min, 2 h, 4 h or 6 h after PMA administration. GAPDH was used as a loading control.
GF109203X inhibits PMA-mediated inducibility of I2E-dependent luciferase expression and MnSOD protein
Administration of GF109203X inhibited, in a dose-dependent manner, the effect of PMA on I2E-dependent luciferase expression, suggesting a PKC-dependent mechanism of transcription activation (Figure 4A). Furthermore, analysis of AP-1 and NF-κB DNA-binding activity after GF109203X/PMA treatment revealed a dose-dependent decrease in NF-κB DNA-binding activity within the intronic fragment, but no change in AP-1 DNA-binding activity, suggesting that the PKC is involved in increased NF-κB binding (Figure 4B). In addition, MnSOD protein level was decreased upon GF109203X/PMA treatment compared with that with PMA alone (Figure 4C), consistent with reporter and EMSA analysis. GAPDH was used as a protein-loading control.
Figure 4. Inhibition of PKC decreases PMA-mediated effects on NF-κB DNA-binding activity within the intronic enhancer as well as MnSOD protein.
(A) The effect of GF109203X on PMA (100 nM) responsiveness of the I2E/P7-dependent luciferase reporter gene. Results are means±S.E.M. for three independent experiments. +P≤0.0001, significant difference between PMA and non-treatment with each concentration of GF109203X; *P≤0.0001, significant difference compared with PMA alone. (B) EMSA of nuclear extracts (10 μg) with radiolabelled oligonucleotides. Cells were pre-treated with GF109203X (0–1 μM) for 1 h before PMA (100 nM) administration. Extracts were prepared 1 h after PMA treatment. (C) Western blot analysis of cytosolic extracts (100 μg) of cells pre-treated with GF109203X (0–1 μM) for 1 h before PMA (100 nM) administration. Extracts were prepared 24 h after PMA treatment. GAPDH was used as a loading control.
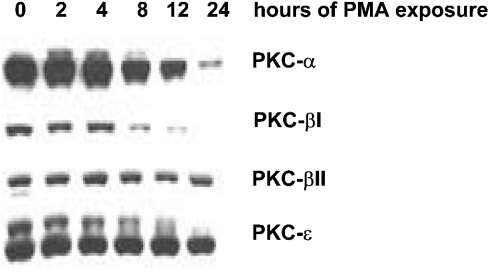
Time-dependent down-regulation of PKC isoforms with sustained PMA treatment
Continuous exposure of some cell lines to PMA will down-regulate PKC isoforms of the conventional (α, βI, βII and γ) and novel types (δ, ε, η and θ), but not the atypical isoforms (ζ, λ, τ and μ) [28,29]. Therefore we treated the VA-13 cell line for 24 h with PMA, and collected cells at 2, 4, 8, 12 and 24 h. Total cell lysates were analysed for the predominant conventional and novel PKC isoforms in the VA-13 cell line (α, βI, βII and ε). Within 8 h, a decline in α (38.9%), βI (84.8%) and βII (42.9%) was observed compared with the non-treated cells (0 h). The level of PKCε did not decline until after 12 h (Figure 5).
Figure 5. Time-dependent down-regulation of PKC isoforms with PMA treatment.
Total cell lysates were collected at various time points (0–24 h) after PMA (100 nM) treatment and PKC isoforms determined by Western blot analysis.
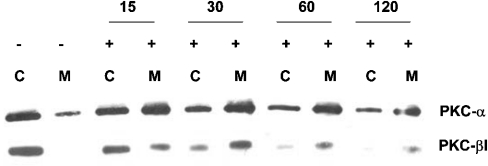
Time-dependent translocation
Conventional PKC isoforms are known to translocate to the plasma membrane following phorbol ester stimulation. Therefore, to verify activation of the α and βI isoforms in the VA-13 cell line upon PMA treatment, both cytosolic and membrane fractions were analysed by Western blotting over a 2 h time period (Figure 6). In the VA-13 cell line, some membrane-associated PKCα was detected in the untreated VA-13 cells. Within 15 min of PMA exposure, an increase in the membrane-associated PKCα isoform was noted, with sustained levels through 2 h of treatment. No membrane-associated PKCβI was observed in unstimulated VA-13 cells; however, within 30 min of PMA exposure, the cytosolic content of PKCβI had decreased, with a concomitant increase in the membrane-associated level.
Figure 6. Time-dependent translocation of PKC isoforms after PMA treatment.
Western blot analysis of PKC isoforms after PMA (100 nM) administration. Cytoplasmic (C) and membrane (M) content of each PKC isoform is represented over a 2 h time course.
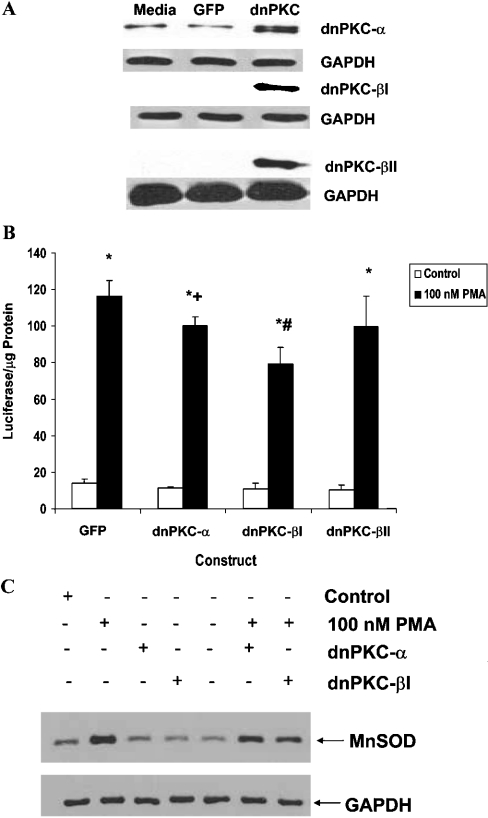
Overexpression of dn (dominant-negative) PKCα and βI decreases I2E and MnSOD protein level responsiveness to PMA
To determine if PKCα, βI or βII contributed to the responsiveness of the I2E fragment to PMA, dominant-negative expression vectors were transfected into the I2E stable clone described previously [27]. Expression of the dominant-negative isoforms was verified by Western blot analysis (Figure 7A). I2E stable clones expressing dnPKCα, dnPKCβI or dnPKCβII were treated with 100 nM PMA for 12 h, and luciferase values were compared with the control [GFP (green fluorescent protein)] vector. Figure 7(B) shows a significant decrease in reporter gene expression with PMA treatment in the cells which overexpressed the dnPKCα and βI isoforms. Overexpression of the dnPKCβII isoform did not alter luciferase expression compared with the GFP control. Figure 7(C) shows a decrease in MnSOD protein level in cells transfected with either the dnPKCα or the dnPKCβI expression construct, followed by phorbol ester treatment, compared with PMA alone, which is consistent with luciferase analysis. GAPDH was used as a loading control.
Figure 7. PKCα and βI are involved in PMA-mediated increases in MnSOD protein.
(A) Western blot analysis of PKC isoforms from total cell lysates of cells transfected with either the GFP adenoviral control construct or individual dnPKC isoform constructs. GAPDH was used as a loading control. (B) The effect of dnPKC expression vectors on the PMA-mediated increase in I2E-dependent luciferase expression. Results are expressed as means±S.E.M. for three independent experiments. *P≤0.0001, significant difference between treatment and non-treatment with same vector; +P≤0.05 and #P≤0.0001, significant difference between PMA treatment of dominant-negative construct and GFP construct. (C) At 48 h after adenoviral infection, cells were treated with PMA (100 nM) for 24 h. Cytosolic extracts were collected and analysed by Western blot using 100 μg of protein/lane. GAPDH was used as a loading control.
DISCUSSION
Previously, we reported the activation of the human SOD2 gene by TNF-α, IL-1β and PMA to be mediated, at least in part, through an NF-κB element within the second intron [26,27]. In the present study, we show that the protein synthesis inhibitor, CHX, can significantly increase MnSOD mRNA in the presence of PMA. In addition to NF-κB, phorbol esters can activate AP-1, a transcription factor with binding sites located in the SOD2 promoter [30,31]. Analysis of DNA-binding activity after administration of CHX±PMA revealed an increase in NF-κB compared with that of PMA alone. No additive effect of AP-1 DNA-binding activity was seen with CHX±PMA compared with phorbol ester alone. This finding suggests that the effect of CHX may involve modulation of proteins that bind the intronic NF-κB site.
Activation of NF-κB is a multistep process that can involve phosphorylation of the IκB family of inhibitors, followed by their ubiquitination and subsequent degradation by the 26 S proteasome. Superinduction of the c-fos and IL-2 genes in the presence of protein synthesis inhibitors and stimuli such as PMA can occur either through mRNA stabilization or loss of a labile repressor [32,33]. Previously, we reported that PMA treatment resulted in activation of NF-κB, mediated via degradation of the IκBα and β inhibitors [27]. Since IκBα is an NF-κB target gene [34], we chose this cytoplasmic inhibitor to investigate, being the most likely labile repressor candidate of SOD2 expression. We find lower levels of the IκBα inhibitor in the presence of CHX±PMA compared with either CHX or PMA alone. This finding is consistent with those of Novak et al. [35], who reported that a synergistic increase in the human urokinase gene, in the presence of CHX±PMA, was mediated via a labile repressor exerting its effect through a NF-κB site.
Previously, we reported that degradation of IκBα occurs rapidly in the presence of either cytokines or phorbol esters, with levels rebounding to greater than control within 90 min [27]. Re-synthesis of the non-phosphorylated IκBα inhibitor can bind p50–p65 heterodimers in the nucleus and inhibit NF-κB-mediated transcriptional activity, thereby providing an auto-inhibitory feedback loop for modulation of gene expression [36]. In addition, IκBα localized in the nucleus can enhance dissociation of p50–p65 from specific DNA consensus sequences [37]. Recently, Daosukho et al. [38] reported that modulation of the DNA-binding activity in favour of the p50–p65 complex enhances NF-κB-mediated induction of MnSOD, therefore inhibition of IκBα by CHX would enhance p50–p65 interaction at the intronic NF-κB site, thereby increasing transcription of MnSOD mRNA. This is the first study to our knowledge which suggests that the labile repressor, IκBα, might serve as a potential pharmacological target for modulation of MnSOD expression.
As mentioned previously, activation of NF-κB may be preceded by phosphorylation of IκBα. Intracellular targets of phorbol esters belong to the PKC family as do chimaerins, protein kinase D, RasGRPs (Ras guanine nucleotide-releasing proteins), Munc13s and diacylglycerol kinase γ [39]. Previous studies have identified IκBα as a downstream target of PKC phosphorylation [40–42], therefore we wanted to determine if the NF-κB-mediated transcriptional activation localized to the second intron was PKC-dependent in the presence of PMA.
PKC molecules contain both a regulatory and catalytic domain. The regulatory domain interacts with calcium, phosphatidylserine and diacylglycerol, whereas the catalytic domain contains ATP and protein substrate-binding sites. Both domains have served as targets for the design of pharmacological agents to modulate enzyme activity. The bisindolylmaleimide, GF109203X, first described by Toullec et al. [43], exhibits a high degree of selectivity for PKC isoenzymes by acting as a competitive ATP inhibitor in the catalytic domain of the enzyme. The use of GF109203X in the VA-13 cell line suggests a role for one or more of the PKC isoforms in the transcriptional regulation of the SOD2 gene upon PMA administration, as both NF-κB DNA-binding activity and I2E-dependent luciferase activation decreased in a concentration-dependent manner. Our results extend the findings of Kim et al. [44] who reported that inhibition of PKC decreased PMA-mediated MnSOD mRNA induction in a human lung adenocarcinoma cell line (A549). Bianchi et al. [45] reported a decrease in arachidonic-acid-mediated induction of MnSOD mRNA and protein levels in a human HepG2 hepatoma cell line with administration of calphostin C, a PKC inhibitor. Das et al. [46] showed calphostin C and GF109203X to block MnSOD induction by vinblastine, vincristine and paclitaxel in A549 cells. PKC translocation studies by Das et al. [46] revealed PMA induced PKCα, β, δ and μ movement to the A549 membrane; however, the anticancer drugs specifically stimulated the translocation of PKCδ, suggesting a role for this isoform in MnSOD induction in the A549 cell line. The present study cannot rule out the contribution of the PKCδ isoform to PMA-mediated induction; however, in the VA-13 model, the PKCδ isoform was expressed at low levels in comparison with the PKCα, βI, βII and ζ isoforms, as determined by Western blot analysis. Furthermore, translocation of the α and β isoforms of PKC to the cellular membrane upon phorbol ester stimulation, as well as a decrease in PMA-mediated I2E-dependent luciferase expression after transient transfection of dnPKCα or βI, suggests a probable role for these enzymes in the regulation of the SOD2 gene in the VA-13 cell line. Studies have shown that prolonged treatment with PMA will selectively down-regulate some PKC isoforms, while others are unaffected [47]. In the present study, we show that prolonged PMA administration has a greater effect on the PKCα and βI isoforms than others. When the stable clones expressing the I2E-dependent luciferase construct were pre-treated with PMA for 8 h and then rechallenged with another 100 nM phorbol ester, there was no further increase in activity, suggesting that the PKC isoforms responsible for the NF-κB activation were unavailable (results not shown). Taken together, these results suggest a role for multiple PKC isoforms in up-regulation of MnSOD and suggest that the isoenzymes contribution may be stimulus- and/or cell-type-dependent.
Acknowledgments
This work was supported by NIH (National Institutes of Health) grants R01CA49997, R01CA73599, and a research career award, P01AG05119, from the National Heart and Lung Institute.
References
- 1.Weisiger R. A., Fridovich I. J. Mitochondrial superoxide dismutase: site of synthesis and intramitochondrial localization. J. Biol. Chem. 1973;248:4793–4796. [PubMed] [Google Scholar]
- 2.Halliwell B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 3.Li Y., Huang T. T., Carlson E. J., Melov S., Ursell P. C., Olson J. L., Noble L. J., Yoshimura M. P., Berger C., Chan P. H., et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 4.Lebovitz R. M., Zhang H., Vogel H., Cartwright J., Dionne L., Lu N., Huang S., Matzuk M. M. Neurodegeneration, myocardial injury and perinatal death in mitochondria superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsson L. M., Jonsson J., Edlund T., Marklund S. L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reaume A. G., Elliott J. L., Hoffman E. K., Kowall N. W., Ferrante R. J., Siwek D. F., Wilcox H. M., Flood D. G., Beal M. F., Brown R. H., Jr, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 7.Wong G. H. W., Elwell J., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58:923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- 8.Keller J. N., Kindy M. S., Holtsberg F. W., St. Clair D. K., Yen H.-C., Germeyer A., Steiner S. M., Bruce-Keller A. J., Hutchins J. B., Mattson M. P. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J. Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Zulueta M., Ensz L. M., Mukhina G., Lebovitz R. M., Azacka R. M., Engelhardt J. F., Oberley L. W., Dawson V. L., Dawson T. M. Manganese superoxide dismutase protects nNOS neurons from NMDA and nitric oxide-mediated neurotoxicity. J. Neurosci. 1998;18:2040–2055. doi: 10.1523/JNEUROSCI.18-06-02040.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majima H. J., Oberley T. D., Furukawa K., Mattson M. P., Yen H.-C., Szweda L. I., St. Clair D. K. Prevention of mitochondrial injury by manganese superoxide dismutase reveals a primary mechanism for alkaline-induced cell death. J. Biol. Chem. 1998;273:8217–8224. doi: 10.1074/jbc.273.14.8217. [DOI] [PubMed] [Google Scholar]
- 11.Kiningham K. K., Oberley T. D., Lin S. M., Mattingly C. A., St. Clair D. K. Overexpression of manganese superoxide dismutase protects against mitochondrial-initiated poly(ADP-ribose) polymerase-mediated cell death. FASEB J. 1999;13:1601–1610. doi: 10.1096/fasebj.13.12.1601. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y., Kiningham K. K., Lin S. M., St. Clair D. K. Overexpression of MnSOD protects murine fibrosarcoma cells (FSa-II) from apoptosis and promotes a differentiation program upon treatment with 5-azacytidine: involvement of MAPK and NFκB pathways. Antioxid. Redox Signaling. 2001;3:375–386. doi: 10.1089/15230860152409022. [DOI] [PubMed] [Google Scholar]
- 13.Wispe J. R., Warner B. B., Clark J. C., Dey C. R., Neuman J., Glasser S. W., Crapo J. D., Chang L. Y., Whitsett J. A. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J. Biol. Chem. 1992;267:23937–23941. [PubMed] [Google Scholar]
- 14.Yen H. C., Oberley T. D., Vichitbandha S., Ho Y.-S., St. Clair D. K. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J. Clin. Invest. 1996;98:1253–1260. doi: 10.1172/JCI118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan P. G., Bruce-Keller A. J., Rabchevsky A. G., Christakos S., St. Clair D. K., Mattson M. P., Scheff S. W. Exacerbation of damage and altered NF-κB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J. Neurosci. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Church S. L., Grant J. W., Ridnour L. A., Oberley L. W., Swanson P. E., Meltzer P. S., Trent J. M. Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3113–3117. doi: 10.1073/pnas.90.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J. J., Oberley L. W., St. Clair D. K., Ridnour L. A., Oberley T. D. Phenotypic changes induced in human breast cancer cells by overexpression of manganese-containing superoxide dismutase. Oncogene. 1995;10:1989–2000. [PubMed] [Google Scholar]
- 18.Yan T., Oberley L. W., Zhong W., St. Clair D. K. Manganese containing superoxide dismutase overexpression causes phenotypic reversion in SV40-transformed human lung fibroblasts. Cancer Res. 1996;56:2864–2871. [PubMed] [Google Scholar]
- 19.Safford S. E., Oberley T. D., Urano M., St. Clair D. K. Suppression of fibrosarcoma metastasis by elevated expression of manganese superoxide dismutase. Cancer Res. 1994;54:4261–4265. [PubMed] [Google Scholar]
- 20.Kiningham K. K., St. Clair D. K. Overexpression of manganese superoxide dismutase selectively modulates the activity of Jun-associated transcription factors in fibrosarcoma cells. Cancer Res. 1997;57:5265–5271. [PubMed] [Google Scholar]
- 21.Wong G. H. W., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988;242:941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 22.Masuda A., Longo D. L., Kobayashi Y., Appella E., Oppenheim J. J., Matsushima K. Induction of mitochondrial manganese superoxide dismutase by interleukin 1. FASEB J. 1988;15:3087–3091. doi: 10.1096/fasebj.2.15.3263930. [DOI] [PubMed] [Google Scholar]
- 23.Dryer S. E., Dryer R. L., Autor A. P. Enhancement of mitochondrial, cyanide-resistant superoxide dismutase in the livers of rats treated with 2,4-dinitrophenol. J. Biol. Chem. 1980;255:1054–1057. [PubMed] [Google Scholar]
- 24.Krall J., Bagley A. C., Mullenbach G. T., Hallewell R. A., Lynch R. E. Superoxide mediates the toxicity of paraquat for cultured mammalian cells. J. Biol. Chem. 1988;263:1910–1914. [PubMed] [Google Scholar]
- 25.Fujii J., Taniguchi N. Phorbol ester induces manganese superoxide dismutase in tumor necrosis factor-resistant cells. J. Biol. Chem. 1991;266:23142–23146. [PubMed] [Google Scholar]
- 26.Xu Y., Kiningham K. K., Devalaraja M. N., Yeh C.-C., Majima H., Kasarskis E. J., St. Clair D. K. An intronic NF-κB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-α and interleukin-1β. DNA Cell Biol. 1999;18:709–722. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]
- 27.Kiningham K. K., Xu Y., Daosukho C., Popova B., St. Clair D. K. Nuclear factor κB-dependent mechanisms coordinate the synergistic effect of PMA and cytokines on the induction of superoxide dismutase 2. Biochem. J. 2001;353:147–156. [PMC free article] [PubMed] [Google Scholar]
- 28.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 29.Bandyopadhyay G., Standaert M. L., Zhao L., Yu B., Avignon A., Galloway L., Karnam P., Moscat J., Farese R. V. Activation of protein kinase C (α, β, and ζ) by insulin in 3T3/L1 cells: transfection studies suggest a role for PKC-ζ in glucose transport. J. Biol. Chem. 1997;272:2551–2558. doi: 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- 30.Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- 31.Ho Y. S., Howard A. J., Crapo J. D. Molecular structure of a functional rat gene for manganese-containing superoxide dismutase. Am. J. Respir. Cell Mol. Biol. 1991;4:278–286. doi: 10.1165/ajrcmb/4.3.278. [DOI] [PubMed] [Google Scholar]
- 32.Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 33.Zubiaga A., Munoz E., Huber B. T. Superinduction of IL-2 gene transcription in the presence of cycloheximide. J. Immunol. 1991;11:3857–3863. [PubMed] [Google Scholar]
- 34.Tzen C.-Y., Cox R. L., Scott R. E. Coordinate induction of IκBα and NFκB genes. Exp. Cell Res. 1994;211:12–16. doi: 10.1006/excr.1994.1052. [DOI] [PubMed] [Google Scholar]
- 35.Novak U., Cocks B. G., Hamilton J. A. A labile repressor acts through the NFκB-like binding sites of the human urokinase gene. Nucleic Acids Res. 1991;19:3389–3393. doi: 10.1093/nar/19.12.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran K., Merika M., Thanos D. Distinct functional properties of IκBα and IκBβ. Mol. Cell. Biol. 1997;17:5386–5389. doi: 10.1128/mcb.17.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zabel U., Baeuerle P. A. Purified human IκB can rapidly dissociate the complex of the NF-κB transcription factor with its cognate DNA. Cell. 1990;61:255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]
- 38.Daosukho C., Kiningham K., Kasarskis E. J., Ittarat W., St. Clair D. K. Tamoxifen enhancement of TNF-α induced MnSOD expression: modulation of NF-κB dimerization. Oncogene. 2002;21:3603–3610. doi: 10.1038/sj.onc.1205448. [DOI] [PubMed] [Google Scholar]
- 39.Brose N., Rosenmund C. Move over protein kinase C, you've got company: alternative cellular effectors of diacylglycerol and phorbol esters. J. Cell Science. 2002;115:4399–4411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- 40.Henkel T., Machleidt T., Alkalay I., Kronke M., Ben-Neriah Y., Baeuerle P. A. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature (London) 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 41.Steffan N. M., Bren G. D., Frantz B., Tocci M. J., O'Neill E. A., Paya C. V. Regulation of IκBα phosphorylation by PKC- and Ca2+-dependent signal transduction pathways. J. Immunol. 1995;155:4685–4691. [PubMed] [Google Scholar]
- 42.Vertegaal A. C., Kuiperij H. B., Yamaoka S., Courtois G., van der Eb A. J., Zantema A. Protein kinase C-α is an upstream activator of the IκB kinase complex in the TPA signal transduction pathway to NFκB in U2OS cells. Cell. Signalling. 2000;12:759–768. doi: 10.1016/s0898-6568(00)00133-9. [DOI] [PubMed] [Google Scholar]
- 43.Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Bardet V., Boissin P., Boursier E., Loriolle F., et al. The bisindolylmaleimide GF109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 1991;266:15571–15781. [PubMed] [Google Scholar]
- 44.Kim H. P., Roe J. H., Chock P. B., Yim M. B. Transcriptional activation of the human manganese superoxide dismutase gene mediated by tetradecanoylphorbol acetate. J. Biol. Chem. 1999;274:37455–37460. doi: 10.1074/jbc.274.52.37455. [DOI] [PubMed] [Google Scholar]
- 45.Bianchi A., Becuwe P., Franck P., Dauca M. Induction of MnSOD gene by arachidonic acid is mediated by reactive oxygen species and p38 MAPK signaling pathway in human HepG2 hepatoma cells. Free Radical Biol. Med. 2002;32:1132–1142. doi: 10.1016/s0891-5849(02)00834-1. [DOI] [PubMed] [Google Scholar]
- 46.Das K. C., Guo X., White C. W. Protein kinase C-δ-dependent induction of manganese superoxide dismutase gene expression by microtubule-active anticancer drugs. J. Biol. Chem. 1998;273:34639–34645. doi: 10.1074/jbc.273.51.34639. [DOI] [PubMed] [Google Scholar]
- 47.Hsu S. L., Chou Y. H., Yin S. C., Liu J. Y. Differential effects of phorbol ester on growth and protein kinase C isoenzyme regulation in human hepatoma Hep3B cells. Biochem. J. 1998;333:57–64. doi: 10.1042/bj3330057. [DOI] [PMC free article] [PubMed] [Google Scholar]