Abstract
Clinical practice reveals that osteoporotic women treated with BPs (bisphosphonates) show an increased bone mass density and a reduced risk of fractures. However, the mechanisms leading to these beneficial effects of BPs are still poorly understood. We hypothesized that ZOL (zoledronic acid), a potent third-generation BP, may induce the expression of proteins associated with the bone-forming potential of osteoblastic cells such as BSP (bone sialo-protein). Expression of BSP gene is up-regulated by hormones that promote bone formation and has been associated with de novo bone mineralization. Using real-time reverse transcriptase–PCR and Western-blot analysis, we demonstrated that ZOL increased BSP expression in Saos-2 osteoblast-like cells. Nuclear run-on and mRNA decay assays showed no effect at the transcriptional level but a stabilization of BSP transcripts in ZOL-treated cells. ZOL effect on BSP expression occurred through an interference with the mevalonate pathway since it was reversed by either mevalonate pathway intermediates or a Rho GTPase activator. We showed that ZOL impaired membrane localization of RhoA in Saos-2 cells indicating reduced prenylation of this protein. By the use of small interfering RNAs directed to RhoA and Rac1, we identified both Rho GTPases as negative regulators of BSP expression in Saos-2 cells. Our study demonstrates that ZOL induces BSP expression in osteoblast-like cells through inactivation of Rho GTPases and provides a potential mechanism to explain the favourable effects of ZOL treatment on bone mass and integrity.
Keywords: bisphosphonate, bone sialoprotein, osteoblast-like cells, post-transcriptional regulation, Rho GTPase, zoledronic acid
Abbreviations: BP, bisphosphonate; BSP, bone sialoprotein; CNF-1, cytotoxic necrotizing factor-1; DRB, 5,6-dichloro-1-β-D-ribofuranosylbenz-imidazole; FOH, farnesol; FPP, farnesyldiphosphate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GGOH, geranylgeraniol; GGPP, geranylgeranyldiphosphate; RT, reverse transcriptase; siRNA, small interfering RNA; ZOL, zoledronic acid
INTRODUCTION
BPs (bisphosphonates) are synthetic compounds capable of suppressing bone resorption in vivo. These PPi analogues are preferentially taken up by the skeleton, where they are potent inhibitors of bone resorption mediated by osteoclasts. For this reason, BPs have become the most important class of drugs used to treat diseases involving excessive osteoclast activity, such as Paget's disease, osteolytic bone disease, hypercalcaemia of malignancy and postmenopausal osteoporosis (see [1] for a review). Several structurally related BPs have been synthesized to optimize their anti-resorptive effects. The most potent BPs, such as ZOL (zoledronic acid) and risedronate, contain a nitrogen atom within a heterocyclic ring. In the early years of BPs' use, the efficacy of these compounds was supposed to depend entirely on the inhibition of osteoclastic activity. Later, several studies showed that cells from the osteoblastic lineage represent an alternative target for BPs [2–6]. Moreover, the use of BPs in animal models of bone metabolism demonstrated that these components have marked effects on bone formation and other parameters of osteogenesis [3,7,8]. Recently, Reinholz et al. [9] reported that BPs have direct effects on bone-forming human fetal osteoblast cells. In contrast with less potent BPs, ZOL inhibited osteoblast proliferation and favoured their differentiation in culture. This switch from a proliferating stage of development to a non-proliferating state occurred with an increase in the rate of bone formation as measured by an in vitro mineralization assay [9].
Although all the above-mentioned studies support the notion that BPs enhance bone formation, a direct effect of these compounds on the expression of proteins playing a pivotal role in bone matrix maturation process, such as BSP (bone sialoprotein), has not been investigated yet. BSP constitutes 12% of the non-collagenous proteins in the mineral compartment of human bone and is synthesized by skeletal-associated cell types, including hypertrophic chondrocytes, osteoblasts, osteocytes and osteoclasts [10–12]. Studies on the developmental expression of BSP in rat bones have revealed that high expression of BSP mRNA correlates with de novo bone formation [13]. Furthermore, BSP expression is spatiotemporally linked to the formation of mineralized matrix by bone-forming cells in vitro [14–17]. These studies, together with the demonstration that BSP induces hydroxyapatite crystallization from physiological concentrations of calcium and phosphate in a cell-free system [18], imply that BSP may play a key role in new bone matrix formation and its subsequent mineralization.
To explore the potential role of ZOL in bone formation, we examined the effect of ZOL on BSP expression by osteogenic cells. Because of its bone-inducing activity, Saos-2 human osteosarcoma cell line is considered to be a model of an osteoblastic cell's ability to secrete bone-related molecules including BSP [19,20]. In the present study, we show that ZOL induces BSP expression at both mRNA and protein levels in Saos-2 cells. We also demonstrate that the biochemical mechanism of this effect occurs through the inhibition of prenylation of Rho GTPases.
EXPERIMENTAL
Cell culture
Human osteosarcoma Saos-2 cells (85-HTB; A.T.C.C.) were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA, U.S.A.) containing 10% (v/v) fetal bovine serum (ICN, Costa Mesa, CA, U.S.A.) at 37 °C in a humidified atmosphere of 5% CO2 and passaged weekly using 0.5 g/l trypsin in Hanks balanced salt solution without Ca2+ and Mg2+, containing 0.2 g/l EDTA (Invitrogen). This cell line has been well characterized as osteoblast-like cells by the criteria of increased alkaline phosphatase activity, cAMP response to parathyroid hormone, osteonectin production, specific receptors for 1,25-dihydroxyvitamin D3, matrix vesicle-like release and osteogenic potentials [20]. Saos-2 cells were dispensed at a density of approx. 0.8×106 in 75 cm2 culture flasks (Nunc, Roskilde, Denmark) and were allowed to reach 50% of confluence before addition of ZOL or its vehicle for the indicated times. For mRNA stability experiments, Saos-2 cells were exposed to 65 μM DRB (5,6-dichloro-1-β-D-ribofuranosylbenzimidazole; ICN) after stimulation with ZOL to arrest transcription. Control and ZOL-treated RNA samples were collected initially (0 h) and at 8, 16 and 24 h after DRB addition for quantitative real-time PCR analysis.
Total RNA isolation and real-time RT (reverse transcriptase)–PCR analysis
Total RNA was isolated from Saos-2 cells by using RNeasy columns (Qiagen Sciences, MD, U.S.A.) according to the manufacturer's instructions. First-strand cDNA was synthesized using 2 μg of total RNA in 20 μl of RT reaction mixture containing 0.2 μg of pd(N)6 random hexamer (Amersham Biosciences, Little Chalfont, Bucks., U.K.), 2 mM of each deoxynucleotide triphosphate (Eurogentec, Seraing, Belgium), 1× first-strand buffer [50 mM Tris/HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2] (Invitrogen), 10 mM dithiothreitol (Invitrogen), and 100 units of SuperScript™ II RNase H RT (Invitrogen). The RT reaction was performed at 42 °C for 50 min before a 15 min inactivation step at 70 °C. Quantitative real-time PCR was performed in triplicate using the ABI Prism 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA, U.S.A.) according to the manufacturer's instructions. BSP primers and TaqMan probes were designed using the primer design software Primer Express (PE Applied Biosystems) as follows: BSP forward 5′-TGCCTTGAGCCTGCTTCCT-3′, BSP reverse 5′-CTGAGCAAAATTAAAGCAGTCTTCA-3′, BSP probe FAM-5′-CCAGGACTGCCAGAGGAAGCAATCA-3′-TAMRA. The TaqMan GAPDH (glyceraldehyde-3-phosphate dehydrogenase) control reagents kit (PE Applied Biosystems) was used for GAPDH detection. cDNA samples (100 ng each) were mixed with 100 nM of each primer and TaqMan Universal PCR Master Mix containing 1×PCR buffer, 5.5 mM MgCl2, 0.8 mM dNTPs mix, 100 nM probe and 1 unit of AmpliTaq Gold® thermostable DNA polymerase (PE Applied Biosystems) in a total volume of 25 μl. The PCR was conducted using the following parameters: 94 °C for 10 min, and 45 cycles at 94 °C for 45 s, 57 °C for 45 s and 72 °C for 30 s, and 72 °C for 2 min after the last cycle. Quantitative real-time PCR was performed for BSP and normalized to the copies of GAPDH mRNA from the same sample, except for half-life experiments, which were normalized to 18 S rRNA (PE Applied Biosystems). Acquired data were analysed by Sequence Detector software version 1.9 (PE Applied Biosystems).
Nuclear run-on transcription assays
To determine the effects of ZOL on BSP gene transcription, nuclear run-on analysis was performed as described by Overall and Sodek [21] with some modifications. Briefly, Saos-2 cells were cultured to 50% confluence in 75 cm2 flasks for 48 h in the presence or absence of 20 μM ZOL. Cell layers were scraped from the flasks and nuclei isolated by centrifugation and washed. Nascent transcripts were radiolabelled by incubation of the nuclei in transcription run-on buffer containing ribonucleotides [1 mM ATP, 1 mM CTP, 1 mM GTP, 3.5 μM UTP and 125 μCi [32P]UTP (3000 Ci/mmol; ICN)] for 45 min at 32 °C. The nuclei were lysed and the [32P]RNA precipitated and collected by centrifugation. Equal amounts of [32P]RNA (1×106 c.p.m.) from each sample were hybridized to blotted BSP and GAPDH cDNAs and control plasmid DNA (pUC18) that had been immobilized on to a nylon membrane Hybond-N+ (Amersham Biosciences). Blots were hybridized at 42 °C for 96 h and washed with 0.1×SSC (7.5 mM NaCl/15 mM sodium citrate)/0.1% SDS at 55 °C. Hybridization of nascent transcripts to different cDNAs was visualized by autoradiography.
Production of antiserum against human BSP
A bacterial recombinant fragment of human BSP (amino acids 158–301) was made by PCR using the B6-5g plasmid [22] as template. The forward oligonucleotide included the NdeI restriction site as well as an in-frame cysteine residue. The reverse oligo included a BamHI restriction site. The PCR product was cloned into the NdeI and BamHI sites of a pET-15b expression vector (Novagen, Madison, WI, U.S.A.) and expressed in BL-21 (DE3) Escherichia coli cells. The recombinant protein was purified on a His-bind resin (Novagen) according to the manufacturer's instructions, conjugated through the cysteine residue to activated keyhole limpet haemocyanin (Pierce, Rockford, IL, U.S.A.) and injected into mice. Monoclonal antibodies were produced using standard mouse hybridoma technology under an established animal protocol at an AAALAC-approved facility. Recombinant full-length human BSP, made using human marrow fibroblasts and a BSP-expressing adenovirus construct [23], was used to select positive clones. The final monoclonal antibody, LFMb-24, was purified on a Protein G column, isotyped as an IgG1 and adjusted to 1 mg/ml concentration.
Western-immunoblot analysis
After treatment with ZOL, cell layers were rinsed three times with PBS and solubilized in 1% SDS. Protein concentrations of the samples were determined by utilizing BCA Protein Assay kit (Pierce). Equal amounts of cellular protein were electrophoresed on 10% SDS/polyacrylamide gel under reducing conditions and then transferred to a PVDF Western-blotting membrane (Roche, Mannheim, Germany). Membranes were blocked with blocking solution [50 mM Tris/HCl, 150 mM NaCl, 5% (w/v) non-fat dry milk and 0.1% Tween 20] for 2 h at room temperature (∼22 °C), and incubated with 10 μg/ml LFMb-24 mouse anti-human BSP monoclonal antibody for 2 h at room temperature. For some experiments, membranes were incubated with 0.8 μg/ml mouse anti-RhoA monoclonal antibody (26C4; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), 0.3 μg/ml mouse anti-Rac1 monoclonal antibody (clone 23A8; Upstate Biotechnology, VA, U.S.A.) or 0.7 μg/ml mouse anti-α-tubulin monoclonal antibody (clone B-5-1-2; Sigma). After washing, blots were incubated with 0.4 μg/ml peroxidase-conjugated rabbit anti-mouse immunoglobulins (Dako, Glostrup, Denmark) for 30 min. Blots were washed again and incubated in ECL® detection reagent (Amersham Biosciences).
Rho translocation assay
A Rho translocation assay was performed as described previously [24]. Briefly, Saos-2 cells were incubated in a lysis buffer containing 50 mM Hepes (pH 7.4), 50 mM NaCl, 1 mM MgCl2, 2 mM EDTA, 1 mM PMSF, 10 μg/ml leupeptin, 1 mM Na3VO4, 5 mM NaF, 1 mM dithiothreitol and 0.1% Triton X-100 for 5 min on ice. The cell lysates were centrifuged at 24000 g for 15 min. After collecting the supernatant as the cytosol fraction, the pellet was resuspended in 1% Triton X-100 in the lysis buffer and centrifuged at 24000 g for 15 min. The supernatant was then collected as the membrane fraction. Equal amounts (10 μg) of protein from each fraction were electro-phoresed on 12.5% SDS/polyacrylamide gel under reducing conditions followed by immunoblotting with anti-RhoA antibody as described above.
siRNA (small interfering RNA) transfection
RNAs (21 nt) were chemically synthesized and purified by reversed-phase HPLC (Eurogentec, Liège, Belgium). To inhibit RhoA and Rac1 synthesis, we used respectively the 5′-GAAGUCAAGCAUUUCUGUCTT-3′ and 5′-GACAGAAAUGCUUGACUUCTT-3′ and the 5′-GUUCUUAAUUUGCUUUUCCTT-3′ and 5′-GGAAAAGCAAAUUAAGAAC-3′ oligoribonucleotide sets [25]. The 5′-CUUACGCUGAGUACUUCGATT-3′ and 5′-UCGAAGUACUCAGCGUAAGTT-3′ oligoribonucleotides from GL3 Luciferase gene were used as siRNA unrelated control. Each pair of oligoribonucleotides was annealed at a concentration of 20 μM in 200 mM NaCl and 100 mM Tris/HCl (pH 7.5). Transfection was performed using the calcium phosphate method in 100 mm Petri dishes with a final concentration of 20 nM siRNA. Total RNA and protein were isolated from Saos-2 cells 48 h post-transfection.
Statistical analysis
For all experiments, mRNA level fold induction is relative to control values, which were set to a value of 1. Both one-way ANOVA and Student's t test (unpaired) were used to compare differences between experimental conditions. To calculate the half-life of mRNA, BSP mRNA decay was analysed by linear regression of the percentage RNA remaining at each time-point of DRB treatment. For all tests, P≤0.05 was considered statistically significant. Stat View 4.0 software (Abacus Concepts, Berkeley, CA, U.S.A.) was used for statistical analysis.
RESULTS
ZOL enhances BSP expression at the protein and mRNA levels in Saos-2 cells
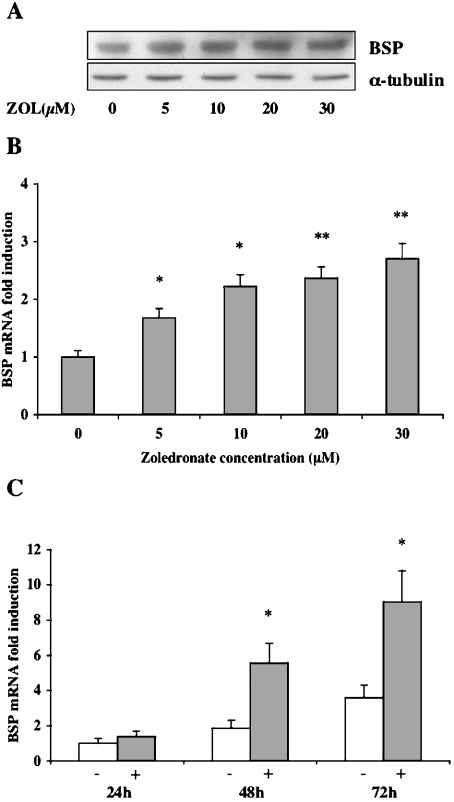
We used Saos-2 human osteoblast-like cells to examine the effects of ZOL on BSP expression. It has been previously suggested that the confluency status of Saos-2 cells may influence the expression of bone-related genes such as BSP [20] and bone morphogenetic proteins 1, 2 and 6 [26]. Therefore we avoided the interference of cell density by using preconfluent Saos-2 cells expressing a basal level of BSP in all the experiments. Saos-2 cells were treated with concentrations ranging from 5 to 30 μM ZOL for 48 h. The expression of BSP was examined by Western-blot analysis using a monoclonal antibody (LFMb24) directed against human BSP. Treated cells showed a dose-dependent increase in BSP protein levels relative to non-treated cells. Protein loading was normalized using both a total protein assay and a monoclonal anti-α-tubulin antibody (Figure 1A). Densitometric analysis reveals that the amount of BSP in cells stimulated with ZOL (20 μM) during 48 h was 2.6-fold higher than in control cells (results not shown). Then, BSP mRNA expression was quantified using the real-time RT–PCR technique. Consistent with the induction at the protein level, the maximum stimulatory effect (3-fold) of ZOL on BSP mRNA level was reached at a concentration of 20 μM and after 48 h of treatment (Figure 1C). We observed that ZOL increased the steady-state level of BSP mRNA in a dose- (Figure 1B) and time-dependent manner that was significantly different (P<0.005) from the up-regulation attributable to cell density (Figure 1C).
Figure 1. ZOL up-regulates BSP expression at both protein and mRNA levels in Saos-2 cells.
(A) Western-blot analysis of BSP expression in Saos-2 cells was performed using LFMb-24 mouse anti-human BSP monoclonal antibody. The cells were cultured in the presence of ZOL, concentrations ranging from 5 to 30 μM, during 48 h. Equal loading of total protein extracts (20 μg/lane) was assessed using an anti-α-tubulin antibody. (B) ZOL-induced changes in BSP mRNA levels in Saos-2 cells are given as fold-induction relative to untreated control cells. Total RNA from control and ZOL-treated cultures was analysed using real-time RT–PCR. The levels of BSP mRNA were normalized for variations in GAPDH mRNA levels. (C) Time-course study of BSP mRNA level in Saos-2 cells exposed to 20 μM ZOL for 24, 48 and 72 h. BSP mRNA levels of treated cells (+) at the different time points are represented as fold-induction relative to untreated cells (−) at 24 h and normalized for variations in GAPDH mRNA levels. Each experiment was performed three times with similar results. *P<0.005 and **P<0.0001 represent significant difference compared with control cell cultures.
ZOL up-regulates BSP expression by mRNA stabilization in Saos-2 cells
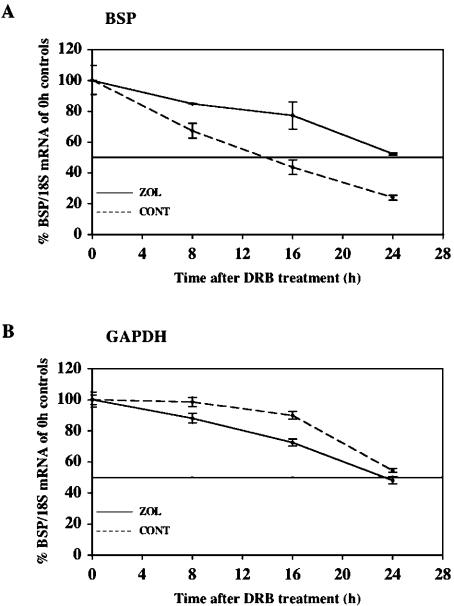
To determine whether the increase in BSP mRNA levels by ZOL was caused by changes in mRNA stability, we calculated the rate of decay of BSP transcripts after transcriptional arrest. Subconfluent Saos-2 cells were treated with DRB, a specific RNA polymerase II inhibitor [27], and the decrease in specific mRNA levels over a 24 h period was recorded. The levels of BSP mRNA from control and ZOL-treated cultures were quantified and normalized with respect to ribosomal 18 S using real-time RT–PCR analysis. As shown in Figure 2(A), the levels of BSP mRNA decreased less rapidly in ZOL-treated cells when compared with that in control cells after DRB exposure. The half-life of BSP mRNA was approx. 14 h in control cells and it was estimated, by linear extrapolation, to be approx. 27 h in ZOL-treated cells, indicating that the ZOL-induced increase in steady-state BSP mRNA level mainly reflects an increased BSP transcript stability. This prolongation of BSP mRNA half-life was specific because the half-life of GAPDH transcripts, used as control (evaluated to be approx. 24 h), was not significantly changed by ZOL treatment (Figure 2B).
Figure 2. ZOL increases BSP mRNA stability in Saos-2 cells.
DRB (65 μM), a transcription inhibitor, was added to ZOL-treated and -untreated cell cultures, and RNA was extracted at 0, 8, 16 and 24 h. The steady-state levels of BSP mRNA were analysed using real-time RT–PCR, normalized to 18 S rRNA, expressed as the percentage of the initial 0 h control and plotted as a function of time. The decay of (A) BSP mRNA and (B) GAPDH mRNA in Saos-2 cells. The half-lives of BSP and GAPDH mRNA were calculated by linear extrapolation. Each experiment was performed three times with similar results.
ZOL has no effect on the transcription rate of BSP gene
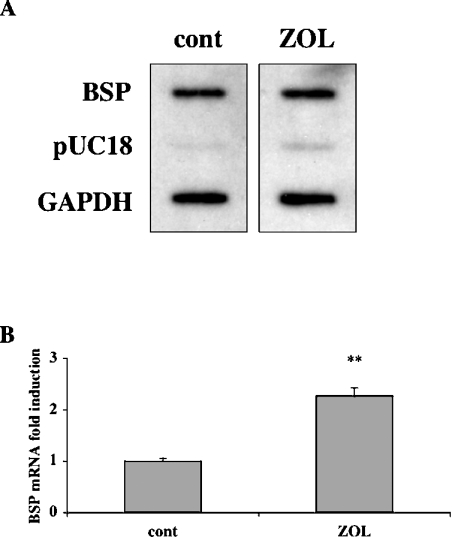
Our demonstration of a ZOL regulation of BSP gene expression through a post-transcriptional mechanism does not exclude the possibility of an effect at the transcriptional level. Therefore we next examined the effects of ZOL on the rate of BSP gene transcription by performing a nuclear run-on assay. Transcriptionally active nuclei were isolated from Saos-2 cells maintained for 48 h in the absence (control cells) or presence of 20 μM ZOL. The nascent transcripts were hybridized to filter-bound BSP and GAPDH cDNAs, and pUC18 plasmid DNA was used as negative control. Nuclear run-on hybridization signals revealed comparable transcription rates in treated Saos-2 cultures in comparison with control cultures, for both BSP and GAPDH genes (Figure 3A). Thus ZOL did not increase the rate of BSP gene transcription, whereas ZOL-induced up-regulation of BSP mRNA steady-state level was effective (Figure 3B).
Figure 3. ZOL has no effect on BSP gene transcription rate in Saos-2 cells.
(A) After 48 h of treatment with ZOL (20 μM), nuclear run-on assay was performed by labelling nascent transcripts in vitro with [α-32P]UTP, and by hybridizing the radiolabelled RNA to immobilized cDNAs for BSP, GAPDH and vector DNA pUC18. (B) Total RNA isolated from the treated and non-treated cells was analysed by real-time RT–PCR to control ZOL-induced up-regulation of BSP mRNA steady-state level. ZOL-induced changes in BSP mRNA levels in Saos-2 cells are given as fold-induction relative to untreated control cells and normalized for variations in GAPDH mRNA levels. These results are representative of three separate experiments. **P<0.0001 represents significant difference compared with control cell cultures.
ZOL-stimulated BSP expression occurs through the inhibition of geranylgeranylation and not farnesylation
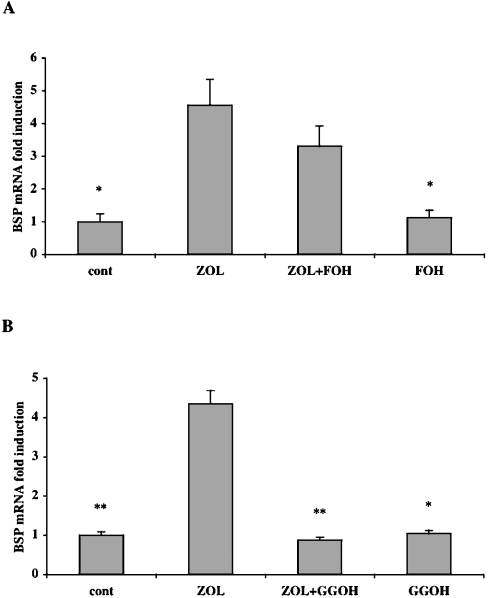
Previous studies have demonstrated that ZOL is capable of interfering with the mevalonate pathway of many cell types, including osteoblastic cells. ZOL inhibits the synthesis of FPP (farnesyl-diphosphate) and GGPP (geranylgeranyldiphosphate), two mevalonate pathway intermediates, and as a consequence decreases prenylation of small GTPases like Ras and Rho [28]. Therefore we next explored the possibility that ZOL-induced BSP expression is related to an alteration of prenylation. To determine which isoprenoid intermediate in the mevalonate pathway could participate in the regulation of BSP mRNA expression, Saos-2 cells were treated with 20 μM ZOL in the presence of 20 μM GGOH (geranylgeraniol) or 20 μM FOH (farnesol). Co-treatment with FOH did not significantly reverse the effects of ZOL on BSP mRNA expression (Figure 4A). In contrast, co-treatment with GGOH completely reversed the ZOL-induced up-regulation of BSP mRNA expression (P<0.0001) (Figure 4B). Treatment with either FOH (20 μM) or GGOH (20 μM) alone did not alter the basal BSP mRNA level when compared with control. These results suggest that BSP mRNA expression is negatively regulated by a geranylgeranylated protein rather than a farnesylated protein.
Figure 4. GGOH, and not FOH, reverses ZOL-mediated BSP up-regulation in Saos-2 cells.
(A) Saos-2 cells were cultured with ZOL (20 μM) in the presence or absence of FOH (20 μM) for 48 h. (B) Saos-2 cells were cultured with ZOL (20 μM) in the presence or absence of GGOH (20 μM) for 48 h. Steady-state levels of BSP mRNA were analysed using real-time RT–PCR and normalized for variations in GAPDH mRNA levels. The expression of BSP mRNA is given as fold-induction relative to untreated control cells (cont). Experiments were performed three times with comparable results. *P<0.005 and **P<0.0001 represent significant difference compared with ZOL-treated cells.
Rho GTPases intervene in ZOL-induced BSP gene up-regulation
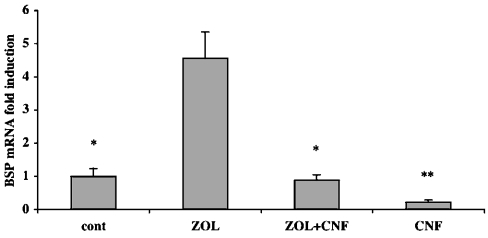
Since Rho GTPases need to be geranylgeranylated to be active at the cell membrane, the potential contribution of Rho in BSP mRNA up-regulation was investigated. We tested the effect of the E. coli CNF-1 (cytotoxic necrotizing factor-1), which is known to activate directly and specifically Rho GTPases [29]. Co-treatment with ZOL (20 μM) and CNF-1 (200 ng/ml) completely reversed ZOL-mediated up-regulation of BSP mRNA (Figure 5), suggesting that the ZOL effect on BSP expression occurs through a Rho-inhibitory mechanism. Furthermore, treatment with CNF-1 (200 ng/ml) alone decreased BSP mRNA expression by approx. 80% when compared with non-treated cells, indicating that direct activation of Rho GTPases leads to the down-regulation of BSP mRNA expression.
Figure 5. CNF-1, an activator of Rho GTPases, is capable of reversing ZOL-induced BSP mRNA up-regulation.
Saos-2 cells were cultured with ZOL (20 μM) alone or in combination with CNF-1 (200 ng/ml) for 48 h. Steady-state levels of BSP mRNA were analysed using real-time RT–PCR and normalized for variations in GAPDH mRNA levels. The expression of BSP mRNA is given as fold-induction relative to non-treated control cells (cont). The figure is representative of three separate experiments with similar results. *P<0.005 and **P<0.0001 represent significant difference compared with ZOL-treated cells.
ZOL impairs RhoA membrane localization in Saos-2 cells
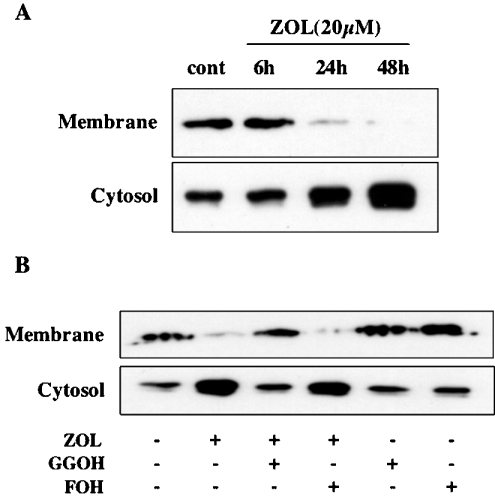
The geranylgeranylation of the small GTPases is essential for their membrane translocation from the cytosol [30]. We examined the effect of ZOL on the translocation of RhoA protein from the cytosol to the membrane in Saos-2 cells after separation of cytosolic and membrane fractions. In untreated cells, equivalent amounts of RhoA are present in both fractions. Treatment of Saos-2 cells with ZOL (20 μM) decreased membrane localization of RhoA with a reciprocal concomitant increase in RhoA in the cytosol (Figure 6A). ZOL clearly attenuated the translocation from the cytosol (inactive form) to the plasma membrane (active form) in a time-dependent manner. This inhibition of translocation from the cytosol to the membrane became evident after 24 h of ZOL treatment and reached a maximum at 48 h (Figure 6A). Co-treatment with GGOH (20 μM), but not FOH (20 μM), reversed the effects of ZOL and completely restored the amount of cytosolic and membrane-associated RhoA to basal levels (Figure 6B).
Figure 6. ZOL prevents RhoA protein translocation from the cytosol to the cell membrane in Saos-2 cells.
(A) Saos-2 cells were treated with ZOL (20 μM) for the indicated times and (B) in the presence or absence of GGOH (20 μM) or FOH (20 μM) for 48 h. Cells were extracted and separated into membrane and cytosolic fractions to detect RhoA by immunoblotting as described in the Experimental section. These results are representative of three independent experiments.
Direct inhibition of RhoA up-regulates BSP mRNA expression
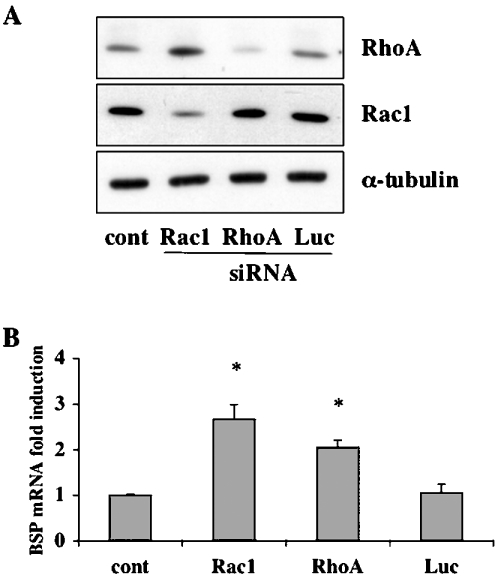
Our results demonstrate that ZOL is capable of inhibiting RhoA geranylgeranylation in Saos-2 cells. Therefore it is reasonable to hypothesize that blocking RhoA expression should lead to an increase in BSP expression. We used siRNA targeting specifically either RhoA or control GL3 luciferase gene (Luc). Western-blot analysis of cells transfected with these siRNAs revealed that the RhoA-specific siRNA (20 nM) inhibited RhoA protein expression to undetectable levels, whereas Luc siRNA (20 nM) was ineffective (Figure 7A). In agreement with the hypothesis, the total repression of RhoA synthesis in Saos-2 cells significantly increased (2.5-fold) the level of BSP mRNA in comparison with non-transfected cells (P<0.005). Luc siRNA transfection did not alter BSP mRNA basal level of expression (Figure 7B). To investigate further the role of Rho GTPases in the regulation of BSP expression, we also used an siRNA directed against Rac1 (Figure 7A). The blocking of Rac1 expression induced a significant increase in BSP mRNA level (Figure 7B). These results indicate that Rho GTPases are negative regulators of BSP gene expression in osteoblast-like cells. Our findings demonstrate that ZOL acts as an enhancer of BSP expression through the suppression of Rho GTPase geranylgeranylation.
Figure 7. RhoA and Rac1 silencing induce BSP mRNA expression.
(A) Saos-2 cells were transfected with calcium phosphate alone (cont), a siRNA targeting RhoA (RhoA), a siRNA targeting Rac1 (Rac1) or a siRNA targeting GL3 Luciferase (Luc). Cells were lysed 48 h post-transfection and analysed by immunoblotting with a specific antibody to RhoA or Rac1 to control RhoA and Rac1 protein synthesis blockade. Anti-α-tubulin antibody was used for normalization. (B) Saos-2 cells were transfected as described above and total RNA was extracted 48 h post-transfection. Steady-state levels of BSP mRNA were analysed using real-time RT–PCR and normalized for variations in GAPDH mRNA levels. BSP mRNA expression is given as fold-induction relative to control. Each experiment was performed three times with similar results. *P<0.005 represents significant difference compared with control cell cultures.
DISCUSSION
BPs are a very important family of pharmacological agents with major clinical and socioeconomic impacts. Their initial and still main clinical field of prescription is the prevention of excessive bone destruction. Investigations aiming to elucidate the mechanisms of action of BPs have been mainly related to bone resorption inhibition through a direct or indirect inhibition of osteoclast formation and activity. Few studies have addressed the potential impact of BPs on osteogenesis [31,32]. Recently, it has been shown that BPs directly regulate proliferation and differentiation of osteoblast cells. In fact, BPs increase the expression of type I collagen and stimulate alkaline phosphatase activity, two markers of osteoblastic cell differentiation [33], in normal human osteoblasts [9,34,35] and MC3T3 osteoblast-like cells [36]. Recent clinical data indicate that ZOL, one of the latest BPs, has a positive effect on bone mineral density comparable with other nitrogen-containing BPs in postmenopausal osteoporosis [37]. Histomorphometric studies performed for assessing the quality of bone after ZOL [38] or alendronate [39,40] treatments showed a positive bone balance at the tissue level that could justify the observed increase in bone mineral density. In the present study, we demonstrated that ZOL stimulates the expression of BSP, a key bone matrix glycoprotein involved in the maturation of bone matrix.
BSP is a highly glycosylated and sulphated phosphoprotein almost exclusively found in mineralized connective tissues. In these tissues, high levels of BSP are coincident with de novo bone formation suggesting that BSP functions in early bone matrix processes. BSP is up-regulated by glucorticoids and bone morphogenetic proteins that support bone formation and down-regulated by factors which promote bone resorption such as 1,25-dihydroxy-vitamin D3 (see [41] for a review). We found that ZOL increases BSP expression at the mRNA and protein levels in human Saos-2 osteoblast-like cells. This observation is in accordance with the recent in vitro studies demonstrating that ZOL enhances the differentiation and thus the osteogenic potential of osteoblasts in culture [9,34]. Indeed, it is well established that BSP expression in osteoblasts is linked to the differentiation process leading to mineralization [17,42].
In the present study, we show that ZOL increased the half-life of BSP mRNA and did not affect BSP gene transcriptional rate. Varghese and Canalis [43] have recently shown that alendronate increased the steady-state level of collagenase 3 mRNA in osteoblast cells because it prolonged its half-life, although they did not provide a mechanism of action. This study and our results suggest that the increase in mRNA stability may be an important mechanism for BP-regulated gene expression in osteoblasts. The concentrations used in this study are relevant in vivo since it has been estimated that concentrations of BPs in the space under resorbing osteoclasts might reach up to 10−3 mol/l [44].
Biochemical studies describing the mechanism of action of BPs demonstrate that nitrogen-containing BPs such as ZOL act by inhibiting FPP synthase in the mevalonate pathway, the biosynthetic pathway for cholesterol and isoprenoid lipids such as FPP and GGPP [45–47]. FPP and GGPP are required for post-translational prenylation of several classes of proteins including the family of small GTPases Ras, Rho and Rac proteins, which are important signalling proteins that regulate a variety of cell processes including control of cytoskeletal organization, cell morphology, integrin signalling and apoptosis (see [48] for a review). Statins are inhibitors of the HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase, which is the rate-limiting step of the mevalonate pathway. Both statins and ZOL interfere with the mevalonate pathway and one common consequence of their action at the cellular level is a deficiency in key prenylated proteins such as Rho GTPases. Interestingly, pitavastatin, a newly developed HMG-CoA reductase inhibitor, increased BMP (bone morphogenetic protein)-2 and osteocalcin mRNA through the inhibition of Rho–Rho-kinase pathway [49].
In the present study, we have shown that the effect of ZOL on BSP expression was overcome by the addition of GGOH (used by the cells for protein geranylgeranylation) but not by FOH (which restores protein farnesylation). Hence it appears that although ZOL can prevent both geranylgeranylation and farnesylation of proteins, loss of geranylgeranylated proteins is of greater consequence than farnesylated proteins for BSP expression in osteoblastic cells. van Beek et al. [47] have previously demonstrated that protein geranylgeranylation is more important than protein farnesylation to explain ZOL anti-resorptive action on osteoclastic cells. This is consistent with the known role of geranylgeranylated proteins such as Rho, Rac and Rab in the processes that are fundamental to osteoclast cell activity and survival [46,50]. The role of small GTPases in the functioning of osteoblasts is less well documented. In the present study, we did demonstrate the importance of Rho GTPases in ZOL-mediated regulation of BSP expression in osteoblast cells. Indeed, Saos-2 cells co-treated with ZOL and CNF-1, a specific activator of Rho GTPases, completely reversed the up-regulation of BSP mRNA by ZOL. Furthermore, the use of CNF-1 alone was sufficient to diminish significantly BSP mRNA. These findings indicate that up-regulation of BSP expression by ZOL occurs by inhibiting Rho GTPases. Moreover, BSP gene expression appears to be under the direct control of Rho GTPases since their activation induces the down-regulation of BSP mRNA levels in Saos-2 cells.
Prenylation of small GTPases is required for their normal membrane localization and function [30,51]. We show in this study that ZOL impairs RhoA translocation from the cytosol to cell membrane in Saos-2 cells. The time course of ZOL effects on Rho prenylation demonstrates a total disappearance of RhoA from the membrane after 48 h, which coincides with the maximal increase in BSP mRNA level. Furthermore, we demonstrated that the lack of either RhoA or Rac1 expression significantly stimulated the expression of BSP mRNA. This observation confirms the importance of Rho GTPases in the direct regulation of BSP gene expression in Saos-2 cells. The mechanism(s) by which Rho GTPases decrease the stability of BSP mRNA, however, remains to be elucidated. In a study demonstrating the stabilization of the endothelial nitric oxide synthase mRNA by mevastatin, the researchers propose that Rho-mediated cytoskeletal changes may affect the stability of mRNAs [52]. Indeed, previous reports indicate that mRNA localization in the cytoplasm is an important component of gene expression regulation and requires cytoskeletal systems of both microtubules and microfilaments [53,54]. Considering the major role played by Rho GTPases in the organization of the cytoskeleton it is probable from these results and our findings that Rho-mediated cytoskeletal changes affect BSP mRNA stability in osteogenic cells.
In conclusion, we demonstrate for the first time that ZOL induces an up-regulation of BSP expression in osteoblast-like cells through the inactivation of Rho small GTPases. Our findings propose a potential mechanism to explain the increased bone mineralization and density observed in BP-treated patients. Furthermore, this study identifies ZOL as a regulator of gene expression and it is probable that it may also affect the expression of several other genes. The identification of such regulated genes will help in the understanding of the effects of ZOL in both osseous and non-osseous cells.
Acknowledgments
We thank Dr J. Green (Novartis, Basel, Switzerland) and Professor K. Aktories (Albert-Ludwigs-Universität, Freiburg, Germany) for the gift of ZOL and CNF-1 respectively. We also acknowledge Professor A. Albert (University of Liège, Belgium) for his help with statistical analysis and S. Thonard for expert technical assistance. A. B. is a Research Associate and C. D. is a ‘Télévie’ Research Fellow at the National Fund for Scientific Research (NFSR, Belgium). This work was partially supported by grants from the NFSR, the Inter University Attraction Pole (IAP-P5/31) and the University of Liège (Belgium). We also acknowledge the support of the European Commission through contracts CEE LSHC-CT-2004-503049 and CEE LSHC-CT-2003-505233.
References
- 1.Russell R. G., Rogers M. J., Frith J. C., Luckman S. P., Coxon F. P., Benford H. L., Croucher P. I., Shipman C., Fleisch H. A. The pharmacology of bisphosphonates and new insights into their mechanisms of action. J. Bone Miner. Res. 1999;14:53–65. doi: 10.1002/jbmr.5650140212. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Moreno C., Serrano S., Nacher M., Farre M., Diez A., Marinoso M. L., Carbonell J., Mellibovsky L., Nogues X., Ballester J., et al. Effect of alendronate on cultured normal human osteoblasts. Bone. 1998;22:233–239. doi: 10.1016/s8756-3282(97)00270-6. [DOI] [PubMed] [Google Scholar]
- 3.Giuliani N., Pedrazzoni M., Negri G., Passeri G., Impicciatore M., Girasole G. Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone. 1998;22:455–461. doi: 10.1016/s8756-3282(98)00033-7. [DOI] [PubMed] [Google Scholar]
- 4.Klein B. Y., Ben-Bassat H., Breuer E., Solomon V., Golomb G. Structurally different bisphosphonates exert opposing effects on alkaline phosphatase and mineralization in marrow osteoprogenitors. J. Cell. Biochem. 1998;68:186–194. [PubMed] [Google Scholar]
- 5.Plotkin L. I., Weinstein R. S., Parfitt A. M., Roberson P. K., Manolagas S. C., Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khokher M. A., Dandona P. Diphosphonates inhibit human osteoblast secretion and proliferation. Metabolism. 1989;38:184–187. doi: 10.1016/0026-0495(89)90260-6. [DOI] [PubMed] [Google Scholar]
- 7.Goziotis A., Sukhu B., Torontali M., Dowhaniuk M., Tenenbaum H. C. Effects of bisphosphonates APD and HEBP on bone metabolism in vitro. Bone. 1995;16:317S–327S. doi: 10.1016/8756-3282(95)00044-e. [DOI] [PubMed] [Google Scholar]
- 8.Little D. G., Smith N. C., Williams P. R., Briody J. N., Bilston L. E., Smith E. J., Gardiner E. M., Cowell C. T. Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J. Bone Miner. Res. 2003;18:1300–1307. doi: 10.1359/jbmr.2003.18.7.1300. [DOI] [PubMed] [Google Scholar]
- 9.Reinholz G. G., Getz B., Pederson L., Sanders E. S., Subramaniam M., Ingle J. N., Spelsberg T. C. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000;60:6001–6007. [PubMed] [Google Scholar]
- 10.Fisher L. W., Whitson S. W., Avioli L. V., Termine J. D. Matrix sialoprotein of developing bone. J. Biol. Chem. 1983;258:12723–12727. [PubMed] [Google Scholar]
- 11.Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J. Biol. Chem. 1987;262:9702–9708. [PubMed] [Google Scholar]
- 12.Bianco P., Fisher L. W., Young M. F., Termine J. D., Robey P. G. Expression of bone sialoprotein (BSP) in developing human tissues. Calcif. Tissue Int. 1991;49:421–426. doi: 10.1007/BF02555854. [DOI] [PubMed] [Google Scholar]
- 13.Chen J., Shapiro H., Sodek J. Developmental expression of bone sialoprotein mRNA in rat mineralized connective tissues. J. Bone Miner. Res. 1992;7:987–997. doi: 10.1002/jbmr.5650070816. [DOI] [PubMed] [Google Scholar]
- 14.Kasugai S., Nagata T., Sodek J. Temporal studies on the tissue compartmentalization of bone sialoprotein (BSP), osteopontin (OPN), and SPARC protein during bone formation in vitro. J. Cell. Physiol. 1992;152:467–477. doi: 10.1002/jcp.1041520305. [DOI] [PubMed] [Google Scholar]
- 15.Kasugai S., Todescan R., Jr, Nagata T., Yao K. L., Butler W. T., Sodek J. Expression of bone matrix proteins associated with mineralized tissue formation by adult rat bone marrow cells in vitro: inductive effects of dexamethasone on the osteoblastic phenotype. J. Cell. Physiol. 1991;147:111–120. doi: 10.1002/jcp.1041470115. [DOI] [PubMed] [Google Scholar]
- 16.Nagata T., Bellows C. G., Kasugal S., Butler W. T., Sodek J. Biosynthesis of bone proteins [SPP-1(secreted phosphoprotein-1, osteopontin), BSP (bone sialoprotein) and SPARC (osteonectin)] in association with mineralized-tissue formation by fetal-rat calvarial cells in culture. Biochem. J. 1991;274:513–520. doi: 10.1042/bj2740513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao K., Todescan R. J., Sodek J. Temporal changes in matrix protein synthesis and mRNA expression during mineralized tissue formation by adult rat bone marrow cells in culture. J. Bone Miner. Res. 1994;9:231–240. doi: 10.1002/jbmr.5650090212. [DOI] [PubMed] [Google Scholar]
- 18.Hunter G., Goldberg H. Nucleation of hydroxyapatite by bone sialoprotein. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8562–8565. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodan S. B., Imai Y., Thiede M. A., Wesolowski G., Thompson D., Bar-Shavit Z., Shull S., Mann K., Rodan G. A. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987;47:4961–4966. [PubMed] [Google Scholar]
- 20.McQuillan D. J., Richardson M. D., Bateman J. F. Matrix deposition by a calcifying human osteogenic sarcoma cell line (SAOS-2) Bone. 1995;16:415–426. doi: 10.1016/8756-3282(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 21.Overall C. M., Sodek J. Concanavalin A produces a matrix-degradative phenotype in human fibroblasts. Induction and endogenous activation of collagenase, 72-kDa gelatinase, and Pump-1 is accompanied by the suppression of the tissue inhibitor of matrix metalloproteinases. J. Biol. Chem. 1990;265:21141–21151. [PubMed] [Google Scholar]
- 22.Fisher L. W., McBride O. W., Termine J. D., Young M. F. Human bone sialoprotein. Deduced protein sequence and chromosomal localization. J. Biol. Chem. 1990;265:2347–2351. [PubMed] [Google Scholar]
- 23.Fedarko N. S., Fohr B., Robey P. G., Young M. F., Fisher L. W. Factor H binding to bone sialoprotein and osteopontin enables tumor cell evasion of complement-mediated attack. J. Biol. Chem. 2000;275:16666–16672. doi: 10.1074/jbc.M001123200. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida M., Sawada T., Ishii H., Gerszten R. E., Rosenzweig A., Gimbrone M. A., Jr, Yasukochi Y., Numano F. Hmg-CoA reductase inhibitor modulates monocyte-endothelial cell interaction under physiological flow conditions in vitro: involvement of Rho GTPase-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 2001;21:1165–1171. doi: 10.1161/hq0701.092143. [DOI] [PubMed] [Google Scholar]
- 25.Deroanne C., Vouret-Craviari V., Wang B., Pouyssegur J. EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J. Cell Sci. 2003;116:1367–1376. doi: 10.1242/jcs.00308. [DOI] [PubMed] [Google Scholar]
- 26.Raval P., Hsu H. H., Anderson H. C. Osteoinductive ability of confluent Saos-2 cell correlates with enhanced expression of bone morphogenetic proteins. J. Orthop. Res. 1996;14:605–610. doi: 10.1002/jor.1100140415. [DOI] [PubMed] [Google Scholar]
- 27.Zandomeni R., Bunick D., Ackerman S., Mittleman B., Weinmann R. Mechanism of action of DRB. III. Effect on specific in vitro initiation of transcription. J. Mol. Biol. 1983;167:561–574. doi: 10.1016/s0022-2836(83)80098-9. [DOI] [PubMed] [Google Scholar]
- 28.Russell R. G., Rogers M. J. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt G., Sehr P., Wilm M., Selzer J., Mann M., Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature (London) 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 30.Van Aelst L., D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 31.Sahni M., Guenther H. L., Fleisch H., Collin P., Martin T. J. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J. Clin. Invest. 1993;91:2004–2011. doi: 10.1172/JCI116422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishikawa M., Akatsu T., Katayama Y., Yasutomo Y., Kado S., Kugal N., Yamamoto M., Nagata N. Bisphosphonates act on osteoblastic cells and inhibit osteoclast formation in mouse marrow cultures. Bone. 1996;18:9–14. doi: 10.1016/8756-3282(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 33.Stein G. S., Lian J. B., Owen T. A. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4:3111–3123. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- 34.Fromigué O., Body J. J. Bisphosphonates influence the proliferation and the maturation of normal human osteoblasts. J. Endocrinol. Invest. 2002;25:539–546. doi: 10.1007/BF03345497. [DOI] [PubMed] [Google Scholar]
- 35.Viereck V., Emons G., Lauck V., Frosch K. H., Blaschke S., Grundker C., Hofbauer L. C. Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochem. Biophys. Res. Commun. 2002;291:680–686. doi: 10.1006/bbrc.2002.6510. [DOI] [PubMed] [Google Scholar]
- 36.Igarashi K., Hirafuji M., Adachi H., Shinoda H., Mitani H. Effects of bisphosphonates on alkaline phosphatase activity, mineralization, and prostaglandin E2 synthesis in the clonal osteoblast-like cell line MC3T3-E1. Prostaglandins Leukot. Essent. Fatty Acids. 1997;56:121–125. doi: 10.1016/s0952-3278(97)90508-1. [DOI] [PubMed] [Google Scholar]
- 37.Theriault R. L. Zoledronic acid (Zometa) use in bone disease. Expert Rev. Anticancer Ther. 2003;3:157–166. doi: 10.1586/14737140.3.2.157. [DOI] [PubMed] [Google Scholar]
- 38.Pataki A., Muller K., Green J. R., Ma Y. F., Li Q. N., Jee W. S. Effects of short-term treatment with the bisphosphonates zoledronate and pamidronate on rat bone: a comparative histomorphometric study on the cancellous bone formed before, during, and after treatment. Anat. Rec. 1997;249:458–468. doi: 10.1002/(SICI)1097-0185(199712)249:4<458::AID-AR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 39.Balena R., Toolan B. C., Shea M., Markatos A., Myers E. R., Lee S. C., Opas E. E., Seedor J. G., Klein H., Frankenfield D., et al. The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J. Clin. Invest. 1993;92:2577–2586. doi: 10.1172/JCI116872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavassieux P. M., Arlot M. E., Reda C., Wei L., Yates A. J., Meunier P. J. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J. Clin. Invest. 1997;100:1475–1480. doi: 10.1172/JCI119668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganss B., Kim R. H., Sodek J. Bone sialoprotein. Crit. Rev. Oral Biol. Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- 42.Bianco P., Riminucci M., Bonucci E., Termine J., Gehron Robey P. Bone sialoprotein (BSP) secretion and osteoblast differentiation: relationship to bromodeoxyuridine incorporation, alkaline phosphatase, and matrix deposition. J. Histochem. Cytochem. 1993;41:183–191. doi: 10.1177/41.2.8419458. [DOI] [PubMed] [Google Scholar]
- 43.Varghese S., Canalis E. Alendronate stimulates collagenase 3 expression in osteoblasts by posttranscriptional mechanisms. J. Bone Miner. Res. 2000;15:2345–2351. doi: 10.1359/jbmr.2000.15.12.2345. [DOI] [PubMed] [Google Scholar]
- 44.Sato M., Grasser W., Endo N., Akins R., Simmons H., Thompson D. D., Golub E., Rodan G. A. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J. Clin. Invest. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luckman S. P., Hughes D. E., Coxon F. P., Graham R., Russell G., Rogers M. J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J. Bone Miner. Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 46.Fisher J. E., Rogers M. J., Halasy J. M., Luckman S. P., Hughes D. E., Masarachia P. J., Wesolowski G., Russell R. G., Rodan G. A., Reszka A. A. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc. Natl. Acad. Sci. U.S.A. 1999;96:133–138. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Beek E., Lowik C., van der Pluijm G., Papapoulos S. The role of geranylgeranylation in bone resorption and its suppression by bisphosphonates in fetal bone explants in vitro: a clue to the mechanism of action of nitrogen-containing bisphosphonates. J. Bone Miner. Res. 1999;14:722–729. doi: 10.1359/jbmr.1999.14.5.722. [DOI] [PubMed] [Google Scholar]
- 48.Machesky L., Hall A. Rho: a connection between membrane receptor signalling and the cytoskeleton. Trends Cell Biol. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- 49.Ohnaka K., Shimoda S., Nawata H., Shimokawa H., Kaibuchi K., Iwamoto Y., Takayanagi R. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of Rho-associated kinase in human osteoblasts. Biochem. Biophys. Res. Commun. 2001;287:337–342. doi: 10.1006/bbrc.2001.5597. [DOI] [PubMed] [Google Scholar]
- 50.Coxon F. P., Helfrich M. H., Van't Hof R., Sebti S., Ralston S. H., Hamilton A., Rogers M. J. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J. Bone Miner. Res. 2000;15:1467–1476. doi: 10.1359/jbmr.2000.15.8.1467. [DOI] [PubMed] [Google Scholar]
- 51.Casey P. J. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 52.Laufs U., Liao J. K. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J. Biol. Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 53.Bassell G., Singer R. H. mRNA and cytoskeletal filaments. Curr. Opin. Cell Biol. 1997;9:109–115. doi: 10.1016/s0955-0674(97)80159-7. [DOI] [PubMed] [Google Scholar]
- 54.Nasmyth K., Jansen R. P. The cytoskeleton in mRNA localization and cell differentiation. Curr. Opin. Cell Biol. 1997;9:396–400. doi: 10.1016/s0955-0674(97)80013-0. [DOI] [PubMed] [Google Scholar]