Abstract
Introduction
Anti-vascular endothelial growth factor (VEGF) is generally given using pro re nata or “treat-and-extend” (T&E) regimens for neovascular age-related macular degeneration (nAMD). Randomized clinical trials have reported that T&E is superior to Pro re nata (PRN), but results from clinical trials may not always be replicated in clinical practice. Real-world data comparing T&E and PRN regimens for nAMD are limited. The objective of this work was to report 24-month outcomes of PRN versus T&E regimens for ranibizumab and aflibercept to treat nAMD in routine clinical practice.
Methods
We conducted a retrospective analysis of data from a prospectively designed observational outcomes registry, the Fight Retinal Blindness! Project (FRB). Treatment-naïve eyes starting nAMD treatment with at least three injections using a T&E or PRN regimen were tracked by using the FRB. The primary outcome was the mean change in visual acuity (VA) measured by the number of letters read on a logarithm of the minimum angle of resolution chart at 2 years versus baseline. The secondary outcome was the number of injections at 2 years.
Results
From January 1, 2015 to January 31, 2019, 3313 eyes from 2948 patients with nAMD were included: 1243 eyes from 1065 patients were classified as PRN and 2070 eyes from 1935 patients started a T&E regimen. At 24 months, patients on the T&E regimen experienced significantly greater mean (95% confidence interval) improvement in VA than those on PRN (+ 4.2 [3.1, 5.2] vs. + 1.3 [0.1, 2.6] letters; p < 0.001), with more injections (14.9 standard deviation(SD) 4.3) vs. 9.8(SD 4.3); p < 0.001).
Conclusions
Eyes treated with a T&E regimen had better VA outcomes from VEGF inhibitors than eyes treated PRN. This large real-world data assessment supports previous data from randomized clinical trials that the T&E regimen delivers better outcomes than PRN.
Keywords: Neovascular AMD, Treat-and-extend, Pro re nata, Intraocular injection
Plain Language Summary
This study focused on comparing two methods of treating neovascular age-related macular degeneration, a common eye condition. The treatments used were ranibizumab and aflibercept. We looked at the reactive “pro re nata” method, where treatment is given sporadically and only when the condition reactivates, and the proactive “treat-and-extend” method, which aims to keep the disease inactive with the fewest treatments at regular intervals. The main aim was to determine which method provides the best vision outcomes over a 24-month period and the frequency of treatment required. We found that the treat-and-extend method resulted in a greater improvement in vision than the pro re nata method, although it did require more injections. This study highlights the effectiveness of the treat-and-extend method for neovascular age-related macular degeneration, suggesting it gets better outcomes despite requiring more injections.
Key Summary Points
| Why carry out this study? |
| Our aim was to compare the effectiveness of pro re nata (PRN) and treat-and-extend (T&E) regimens in treating neovascular age-related macular degeneration (nAMD) using real-world data, addressing gaps in existing clinical practice evidence. |
| We sought to identify optimal anti-vascular endothelial growth (VEGF) treatment regimens, addressing the balance between treatment efficacy and the burden on patients and healthcare systems. |
| What was learned from this study? |
| The T&E regimen showed superior visual acuity improvement over 24 months (+ 4.2 vs. + 1.3 logMAR letters, p < 0.001) compared to PRN, with more injections (15 vs. 10 mean injections). |
| These results are significant for guiding clinical decisions and optimizing treatment approaches, considering both the clinical efficacy and the implications for health policy and economics. |
Introduction
Pivotal phase III clinical trials only demonstrated that ranibizumab [1, 2] and aflibercept [3] were effective for neovascular age-related macular degeneration (nAMD) when they were given strictly every 4 weeks. The efficacy of the variable treatment regimens, which evolved due to the impracticality of monthly treatment for entire populations, is less well-established.
The “reactive” pro re nata regimen (PRN; as needed) is based on fixed monthly visits after the induction phase, in which monthly treatments are given until the choroidal neovascular (CNV) lesion is first graded as inactive by the practitioner. Treatment is then withheld and given only if the CNV reactivates. The HARBOR randomized clinical trial reported that patients who received the PRN regimen after three initial monthly injections had a somewhat lower visual acuity (VA) gain at 12 months than those who continued on a fixed monthly regimen [4] (9.1 vs. 7.9 letters at 24 months) with fewer treatments. However, the Inhibition of VEGF in Age-related choroidal Neovascularisation (IVAN) trial [5] and Comparison of Age-related macular degeneration Treatment Trial (CATT) [6] reported more encouraging results: The visual outcomes of a strict PRN treatment regimen could approach those of a fixed monthly treatment schedule, even if there was a slight increase in central thickness, with fewer injections, but monthly monitoring was still required.
The “proactive” “treat-and-extend” (T&E) protocol increases the intervals between treatments after the CNV has been stabilized to keep the lesion inactive (the macula remains dry, without any leakage) with the fewest possible treatments [7]; in other words, the next injection is given just before the CNV lesion is expected to reactivate. Some studies have reported the effectiveness of the T&E regimen [8–13], but, as pointed out by the American Academy of Ophthalmology, larger, better-quality studies of the effectiveness of the T&E protocol in real-world practice are required [14]. Assessing which protocol, PRN or T&E, produces the best outcomes remains relevant, since clinical effectiveness must be balanced against societal healthcare costs.
The primary aim of this study was to compare VA change and number of intravitreal therapy (IVT) doses of ranibizumab or aflibercept with the PRN versus T&E regimen at 24 months after starting therapy for nAMD.
Methods
Study Design and Setting
This was an international, multicenter (Australia, New Zealand, Ireland, Italy, France, Spain, and Switzerland), observational, longitudinal, retrospective study of treatment-naive eyes that had received IVT for nAMD in routine clinical practice and had been tracked in the Fight Retinal Blindness! (FRB!) outcomes database. The FRB! system is designed to collect data from each clinical visit. Physicians who participate in the FRB! project agreed to report 80% of their patients to avoid reporting biases. Treating physicians determined the treatment decision and visit schedules in consultation with the patient, which reflects real-world practice.
Approval to access to the FRB! outcomes database was obtained. Institutional ethics approval was obtained from the Human Research Ethics Committees of the University of Sydney; the Royal Victorian Eye and Ear Hospital, Mater Private Hospital Institutional Review Board, Ireland; Ethics Committee of Fondazione IRCCS Ca Granda Ospedale Maggiore Policlinico, Milan, Italy; the Royal Australian and New Zealand College of Ophthalmologists; the canton of Zurich; and the French Society of Ophthalmology. Ethics committees in Australia and New Zealand approved the use of “opt-out” patient consent. The “opt-out” consent procedure was applied at all centers. The research described follows the principles of the Declaration of Helsinki.
Study Population
Participants had to be treatment-naive and to have started and continued on aflibercept or ranibizumab for nAMD under a T&E or PRN regimen between January 1, 2015 and January 31, 2019. Patients received three loading doses before initiating the T&E or PRN regimen. They had to have received at least three injections in the first 12 months. Eyes that had fewer than three injections within the first 12 months were not included in this analysis.
Treatment decisions, including the choice of drug and treatment regimen, were by the clinician in consultation with the patient. Retreatments, applied consistently across groups, were based on the clinicians’ judgment of anatomical and functional ineffectiveness after several monthly injections.
The treatment regimen is not recorded in the database, so this was inferred based on the proportion of visits in which an injection was administered; T&E (injections in ≥ 83% of visits).
Completers were defined as eligible eyes that completed 24 months of follow-up; non-completers were eyes that did not. The last observation carried forward (LOCF) method was used for missing visual outcomes. For completers, VA at 24 months was the last VA reading within 24 months. Results, including VA, are presented for all eyes under treatment, but the number of visits and injections is presented for completers only.
Study Measurements
Age (years), smoking status, sex, VA in logMAR letters and lesion type were recorded at the index visit for the first injection. All treatments were recorded, along with VA, CNV lesion activity, and ocular adverse events at each visit. The lesion type was locally determined by the treating ophthalmologists.
Outcome Measures
The main outcome was mean VA change over 24 months after beginning anti-vascular endothelial growth factor (VEGF) therapy to compare T&E vs PRN. Secondary outcomes were the mean number of injections and visits over 24 months.
Statistical Analysis
Descriptive statistics included the mean (SD), median (interquartile range [IQR]), number (%), and 95% confidence interval (CI) as appropriate. Baseline characteristics and unadjusted outcomes were compared between treatment regimens with t tests, Wilcoxon rank-sum tests, and chi-squared tests as appropriate. Locally weighted scatterplot smoothing curves were used to analyze VA throughout the follow-up.
Linear mixed-effects models were used to compare the change in VA between treatment regimens. Generalized Poisson mixed-effects models were used to compare the number of visits and injections. Non-completion rates were analyzed using Cox proportional-hazards models. Changes in visual acuity were adjusted for age and baseline visual acuity using fixed effects, with random effects applied for both individual subjects (to account for bilateral involvement) and clinical center, ensuring accurate and generalizable treatment effect estimates.
p < 0.05 was considered statistically significant. All analyses were performed with R 4.0.5 with the glmmTMB package (v1.0.2.1) [15] for linear mixed-effects and generalized Poisson models and the coxme package (v2.2-16) [16] for Cox proportional hazards models.
Results
From January 1, 2015 to January 31, 2019, 3313 eyes from 2948 patients with nAMD were included: 1243 eyes from 1065 patients were classified as PRN (895 eyes were completers and 348 eyes were non-completers), and 2070 eyes from 1935 patients started a T&E regimen (1388 eyes were completers and 682 eyes were non-completers).
Patient characteristics are summarized in Table 1. Also, there were fewer type 1 and type 2 lesions and more type 3 and other lesion types treated with PRN versus T&E (p < 0.001).
Table 1.
Baseline and demographic characteristics of eligible eyes stratified by treatment regimen
| Pro re nata | Treat and extend | p value | |
|---|---|---|---|
| Eyes | 1243 | 2070 | |
| Patients | 1065 | 1935 | |
| Age, years | |||
| Mean (SD) | 79.8 (8.1) | 80.2 (8.4) | 0.187 |
| Median (range) | 81 (36–99) | 81 (44–104) | 0.139 |
| Sex, n (%) females | 669 (62.8%) | 1163 (60.1%) | 0.081 |
| Left eyes, n (%) | 608 (48.9%) | 1008 (48.7%) | 0.932 |
| Angiographic lesion type, n (%) | |||
| Type I | 444 (35.7%) | 827 (40%) | < 0.001 |
| Type II | 200 (16.1%) | 420 (20.3%) | |
| Type III | 83 (6.7%) | 92 (4.4%) | |
| Othera | 82 (6.6%) | 72 (3.5%) | |
| Unknown | 434 (34.9%) | 659 (31.8%) | |
| Year of treatment initiation, n (%) | |||
| 2015 | 192 (15.4%) | 360 (17.4%) | 0.020 |
| 2016 | 286 (23%) | 510 (24.6%) | |
| 2017 | 339 (27.3%) | 501 (24.2%) | |
| 2018 | 407 (32.7%) | 641 (31%) | |
| 2019 | 19 (1.5%) | 58 (2.8%) |
PRN pro re nata or “as needed” regimen, SD standard deviation, T&E “treat and extend”
aOther: Polypoidal choroidal vasculopathy or mixed types 1–2 neovascularization or atypical neovascularization
Results in bold indicate those for wich the p-value was < 0.05
VA Outcomes at 24 Months
Visual outcomes at 24 months are summarized in Table 2, and the longitudinal VA over 24 months is shown in Fig. 1. Mean (SD) VA at baseline was lower in the PRN than T&E group (56.9 [23.2] vs. 58.9 [18.9] letters; p = 0.011). Also, the mean (SD) VA at 24 months was lower with PRN than T&E (58.1 [25.9] vs. 62.2 [21.1] letters; p < 0.001). The mean (95% CI) change in VA adjusted for differences in baseline characteristics, including baseline vision, was significantly worse with PRN than T&E (+ 1.3 [0.1, 2.6] vs. + 4.2 [3.1, 5.2] letters; p < 0.001).
Table 2.
Visual outcomes at 24 months by treatment regimen
| Pro re nata | Treat and extend | p values | |
|---|---|---|---|
| Eyes | 1243 | 2070 | |
| Baseline VA letters | |||
| Mean (SD) | 56.9 (23.2) | 58.9 (18.9) | 0.011 |
| Median (range) | 63 (0, 100) | 63 (0, 90) | 0.980 |
| ≤ 35 letters (20/200), n (%) | 244 (19.6%) | 266 (12.9%) | < 0.001 |
| ≥ 70 letters (20/40), n (%) | 491 (39.5%) | 771 (37.2%) | 0.209 |
| Final VA, letters | |||
| Mean (SD) | 58.1 (25.9) | 62.2 (21.1) | < 0.001 |
| Median (range) | 69 (1, 100) | 70 (0, 90) | 0.030 |
| ≤ 35 letters (20/200), n (%) | 268 (21.6%) | 277 (13.4%) | < 0.001 |
| ≥ 70 letters (20/40), n (%) | 608 (48.9%) | 1091 (52.7%) | 0.038 |
| VA change, letters | |||
| Mean (95% CI) | 1.3 (0.3, 2.3) | 3.4 (2.6, 4.1) | 0.001 |
| Adjusted mean (95% CI) | 1.3 (0.1, 2.6) | 4.2 (3.1, 5.2) | < 0.001b |
| Gain ≥ 10 letters, n (%) | 323 (26%) | 678 (32.8%) | < 0.001 |
| Gain ≥ 15 letters, n (%) | 232 (18.7%) | 469 (22.7%) | 0.007 |
| Loss ≥ 10 letters, n (%) | 229 (18.4%) | 333 (16.1%) | 0.092 |
| Loss ≥ 15 letters, n (%) | 168 (13.5%) | 239 (11.5%) | 0.106 |
| Duration of treatment, days | |||
| Mean (SD) | 972.3 (519) | 898.7 (498.7) | - |
| Median (range) | 931 (56, 2256) | 882 (44, 2266) | - |
| Number of visitsa | |||
| Mean (SD) | 16.3 (5.5) | 15.8 (4.7) | 0.025b |
| Median (range) | 16 (3, 40) | 15 (3, 32) | |
| Number of injectionsa | |||
| Mean (SD) | 9.8 (4.3) | 14.9 (4.3) | < 0.001b |
| Median (range) | 10 (3, 25) | 14 (3, 30) | |
| Non-completers, n (%) | 348 (28%) | 682 (32.9%) | 0.389b |
Outcomes included 24-month completers and non-completers by using the last observation carried forward
CI confidence interval, VA visual acuity, SD standard deviation
aCompleters only
bp value adjusted for baseline vision, age, and nesting of outcomes in bilateral patients and within patients attending the same practice
Results in bold indicate those for wich the p-value was < 0.05
Fig. 1.
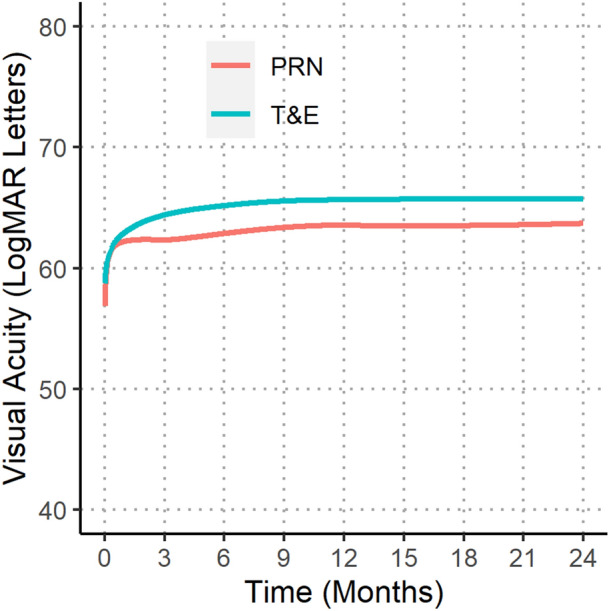
Locally weighted scatterplot smoothing (LOESS) of 24-month visual outcomes comparing pro re nata (PRN) and treat-and-extend (T&E) regimens
24-Month Visit and Injection Frequency Outcomes
The mean (SD) number of injections over 24 months was significantly lower with PRN than T&E (9.8 [4.3] vs. 14.9 [4.3]) (Table 2; p < 0.001). The mean (SD) number of visits was greater with PRN than T&E (16.3 [5.5] vs. 15.8 [4.7]) (p = 0.025). We found little or no linear correlation between the number of injections and the VA gain (Pearson r = 0.10; Fig. 2).
Fig. 2.
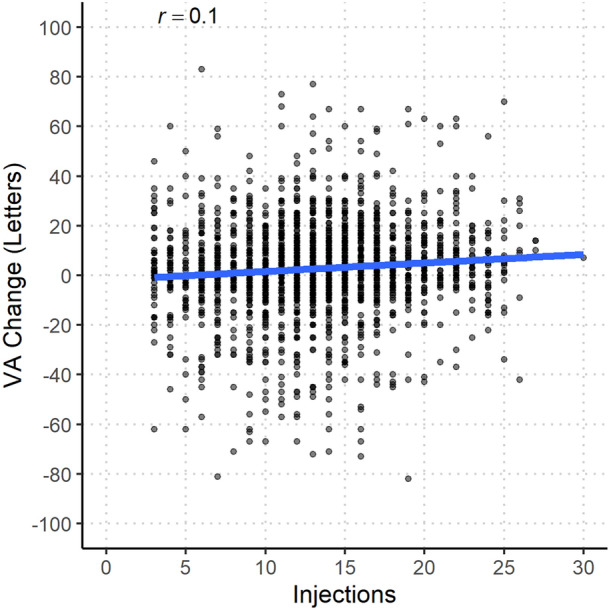
Scatterplot of injections received and change in visual acuity at 24 months for eyes that completed 24 months of follow-up. VA visual acuity. Pearson’s correlation coefficient, r, is presented at the top of the figure
The mean injection intervals under a T&E regimen increased from 33 days at 0–3 months to 61 days at 12–15 months and only increased slightly further to 70 days by 21–24 months (Fig. 3).
Fig. 3.
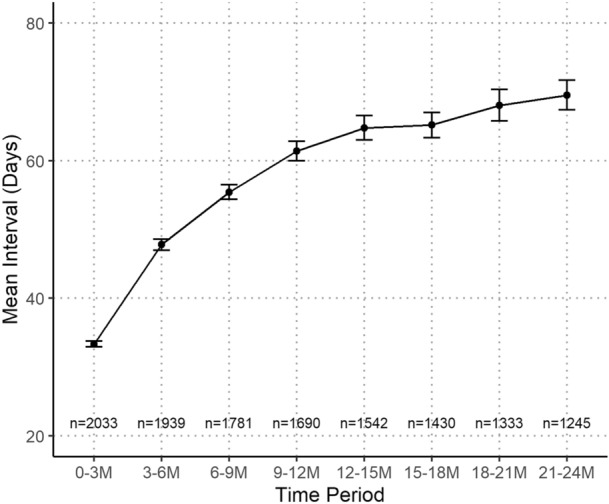
Treatment intervals over 24 months under a treat-and-extend (T&E) regimen. Data are mean (95% confidence interval). Sample sizes are shown above the x-axis
Discussion
We report 24-month outcomes of a real-life cohort of patients with nAMD who received ranibizumab or aflibercept IVT under a T&E or PRN regimen. Two years after starting IVT with ranibizumab or aflibercept, the T&E regimen delivered a better improvement in mean VA (+ 4.2 logMAR letters) than PRN (+ 1.3) with more injections (mean 9.8 vs. 14.9 injections) over 24 months.
Our results are consistent with a meta-analysis of real-world observational studies of ranibizumab IVT for nAMD that suggested that T&E is superior to PRN for nAMD. For studies based purely on PRN regimens (n = 21,612), at 2 years, the mean VA change was + 1.3 letters (n = 14,408) as in our study. The mean VA change was higher for studies based on T&E regimens (n = 2566) at 2 years: + 6.7 letters (n = 2521) [17] versus + 4.2 letters in our study. This finding is also consistent with a recent prospective randomized trial, the Canadian Treat-and-Extend Analysis Trial With Ranibizumab (CANTREAT), designed to evaluate and compare the monthly administration of ranibizumab with T&E over 24 months, which reported a mean gain of 6.8 letters at 2 years in the T&E arm [18].
Our results agree with the RAINBOW study that investigated the effectiveness and safety of aflibercept injection for treatment-naïve patients with nAMD in real-life clinical practice in France. At 2 years, the mean VA change was + 3.0 letters in the overall population. The change from baseline in VA in our analysis and the RAINBOW study was not as large as in the VIEW clinical trial at week 96 (+ 7.6 letters) [19]. However, patients had more severe disease in the VIEW study than in our study (mean [SD] VA at baseline 53.6 [13.5] vs. 56.9 [23.2] letters with PRN and 58.9 [18.9] letters with T&E in our study).
The difference in mean number of injections between T&E (n = 14.9) and PRN (n = 9.8) was likely due to the more regular nature of treatments with the injection scheme in the T&E regimen, which is also the likely explanation for greater improvement in VA with a T&E regimen [20]. Also, the “proactive” T&E model, which aims at minimizing anatomic damage from recurrent fluid and minimizing exudative recurrences, may be more efficient for restoring and then preserving VA than the more reactive PRN regimen, which only allows treatment if the lesion reactivates [21]. In a recent review, people with fewer anti-VEGF injections may have had slightly worse vision at 1 year than those with monthly injections [22]. This was a difference of 1 or 2 more letters read on a vision test chart and an approximately 10% increased chance of gaining 15 or more letters of vision with monthly injections. The study found no evidence of difference between monthly injections and T&E regimens (nine injections, on average).
Strengths and Limitations
This study has several strengths and limitations. One strength is the high number of examined eyes and injections, along with the comparative results for different treatment protocols (T&E vs. PRN) and drugs. To exclude the effects of prior treatment, we included only treatment-naïve patients receiving initial anti-VEGF injections. The measurement of VA using logMAR VA strengthens our results because logMAR charts provide more reliable and discriminative results than do Snellen charts [23].
The observational, retrospective design of this study naturally implicates certain limitations of the data quality. Smoking status was omitted because data were available in only one-third of participants and lack of any evidence that suggested it had a direct influence on treatment choice. The omission of drug switch data introduces a non-differential bias across groups. Similarly, the lack of data on proportion use of ranibizumab or aflibercept limits the ability to adjust for drug selection differences between treatment strategies. The main limitation of our study is using an 83% injection-to-visit ratio to separate PRN from T&E treatments, which could lead to classification bias. This criterion was selected as the optimal approach within the limitations inherent to our retrospective dataset. Consequently, a patient discontinuing follow-up did not alter their visit/injection proportion, thereby not changing their assigned group. This approach ensures patients remain categorized according to their initial treatment pathway, effectively minimizing the risk of negative selection bias due to treatment discontinuation for reasons such as systemic comorbidities.
Despite the consecutive patient recruitment, implicit selection bias cannot be ruled out entirely. Similarly, better VA in both the affected and fellow eye at baseline has been associated with better final VA [11]. Moreover, while gathering data from multiple centers could potentially lead to classification bias, the involvement of experienced ophthalmologists in the analysis helps to reduce this risk.
Loss to follow-up may bias in favor of a treated group if the patients who drop out have poorer vision. Approximately, 33% of eyes in our T&E groups and 28% of eyes in our PRN group did not complete 24 months. High dropout rates are a feature and a limitation of observational studies. Dropouts had worse visual acuity at baseline and at their final follow-up visit compared to completers (mean 24-month/final VA 61.4 in completers vs. 49.7 in non-completers for PRN; 64.2 in completers and 58.3 in dropouts for T&E). Regardless, the T&E dropouts still performed better than PRN dropouts, with a mean (95% CI) crude change in VA of + 3.2 letters vs. + 1.1 letters, respectively. Reasons for discontinuation, which were given in 20% percent eyes, included unrelated or good outcomes (death [32 eyes], moves to another doctor [32 eyes], treatment successful [31 eyes], medically contraindicated [five eyes]) and poor outcomes (further treatment futile [89 eyes], patient declines [19 eyes]). There are weaknesses in all methods to account for missing data, LOCF is the most straightforward and it at least gives a much clearer picture of what is happening in routine clinical practice than a completers only analysis. Importantly, LOCF does not introduce differential bias between PRN and TAE, thus preserving the study’s internal validity.
Reasons for non-completion in the remaining 80% of non-completers were unknown so we can only speculate why these patients did not continue treatment. In clinical routine care, the monthly monitoring required in the PRN regimen is difficult to maintain. Delayed monitoring might lead to significant undertreatment of eyes. In our PRN group, clinical protocols such as dosing may have changed over time, and monthly follow-up was not possible. Patients were examined only 16 times instead of the 24 times initially expected over 24 months, probably because of the difficulty in traveling to clinics, especially for older patients living far away.
We found similar visit counts in PRN (16.3) and T&E (15.8). The reduction of visit numbers usually seen with a T&E regimen is only evident when the recommended frequent visits can be provided or adhered to. Evidently this was not the case in the population we studied. The challenge of delivering intravitreal therapies in large populations is universally acknowledged, which enhances the significance of our data.
The distribution of injection: visit ratios is bimodal. We previously used 83% as the most appropriate point to differentiate between PRN and T&E [12]. Only very rarely do patients have the expected 100% injection: visit ratio because they often attend the practice for reasons other than management of their nAMD such as a red eye or to have cataract surgery. Also, the 2-year observation period in our study limits the assessment of treatment efficacy; a 5-year duration would provide a more conclusive comparison.
Another limitation is the baseline disparity between PRN and T&E groups, notably in mean BCVA and the proportion with baseline VA below 35 letters. This difference may be due to different local conditions amongst the populations studied that use the different regions. While it may reflect more advanced disease, nevertheless, we and others have repeatedly shown that eyes with worse vision tend to gain more vision, which would tend to bias results in favor of the PRN regimen [24]. All our statistical models were adjusted for baseline VA (fixed effect).
Conclusions
In conclusion, this large observational study confirms prior data of randomized clinical trials on the efficacy of anti-VEGF treatment and provides additional data on the potential superiority of T&E over PRN protocols. Despite ongoing efforts to work out optimal treatment regimens for anti-VEGF treatment, a gold standard has not been determined, so the trade-off between treatment efficacy versus patient and health system burden and injection complications remains controversial. Optimization of the treatment regimen of nAMD has great importance to individual patients, their careers, and practitioners, and has significant implications for health policy and health economics. Any savings obtained by reduced injection rates must be weighed against worse health outcomes.
Acknowledgements
We thank the study participants. We sincerely thank Hannah Crowdy, Clinical Research Associate, for her crucial role in data compilation for our study. Her expertise and technical assistance were essential to our research’s success. We thank all study investigators of the Fight Retinal Blindness! Study Group: Armadale Eye Clinic, Victoria (Dr A Cohn); Australian Eye Specialists (Bacchus Marsh), Victoria (Dr N Jaross); Blink, Australian Capital Territory (Dr R Barry); Bundaberg Eye Clinic, Queensland (Dr I McLean); CH Saint Brieuc, France (Dr T GUILLAUMIE, Dr A MIRI); CHU de Dijon, France (Dr P GABRIELLE); Centre Ophtalmologique Vincennes Vision, France (Dr S Tick); Cairns Eye Surgery, Queensland (Dr A Field); Camberwell Retina Specialists, Victoria (Dr S Wickremasinghe); Canberra Hospital, Australian Capital Territory (Dr C Dayajeewa, Dr J Wells); Care Foresight, New South Wales (Dr A Dunlop); Central Coast Eye Specialist, New South Wales (Dr S Young); Centre Ophtalmologique de l’Ecole Militaire, France (Dr G MIMOUN); Centre for Eye Research Australia, Victoria (Professor R Guymer); Centro de Ojos de La Coruña, Spain (Dr P Carnota); Clinica Oftalvist Valencia, Spain (Dr R Gallego-Pinazo); Clinica Universidad de Navarra, Spain (Dr A GARCÍA LAYANA, Dr M Saenz-de-Viteri); Coastwide Eye Surgery, New South Wales (Dr R Ferrier); Doncaster Eye Center, Victoria (Dr L Chow); Dorset Consultant Center, Victoria (Dr H Steiner); Dr Alex Amini’s Practice, Victoria (Dr A Amini); Dr Jern Yee Chen Practice, South Australia (Dr J Chen); Dr Niladri Saha GHR, South Australia (Dr N Saha); Dr Niladri Saha MV, South Australia (Dr N Saha); Dr. Phillip Windle, Queensland (Dr P Windle); Eye Associates, New South Wales (Dr M Gillies, Dr A Hunt); Eye Doctors Mona Vale, New South Wales (Dr P Beaumont); Eye Specialists Greensborough, Victoria (Dr L Chow); Eye Surgeons Miranda, New South Wales (Dr A Hunt); Eye Wide Bay, Queensland (Dr Z Louw); Eyeclinic Albury Wodonga, New South Wales (Dr A Luckie); Eyemedics (Wayville), South Australia ( S Lake, Dr D Qatarneh); FPHAG, Spain (Dr L Sararols, Dr J Suarez); Focus Eye Centre, New South Wales (Dr P Berdoukas); Fondazione IRCCS CA’GRANDA—Ospedale Maggiore Policlinico, Italy (Dr F Viola); Gladesville Eye Specialists, New South Wales (Dr S Young); Hospital Universitario Principe de Asturias, Spain (Dr R Montejano Milner, Dr C Arruabarrena); Hawthorn Eye Clinic, Victoria (Dr L Chow); Hospital Clinico Universitario Lozano Blesa, Spain (Dr F ASCASO, Dr A BonedBoned Murillo, Dr M DÍAZ, Mr G PEREZ RIVASES); Hospital Clínic de Barcelona, Spain (Ms S Alforja Castiella, Dr R Casaroli-Marano, Dr M Figueras-Roca, Mr J Zarranz-Ventura); Hospital Costa del Sol, Spain (Dr S GISMERO MORENO, Dr A González Escobar, Mr J Moreno Gutiérrez); Hospital Dos de Maig, Spain (Dr j escobar ); Hospital Punta de Europa, Spain (Dr F Lavid); Hospital San Juan de Dios del Aljarafe, Spain (Dr P Catalán Muñoz, Dr M Tena Sempere); Hospital Tor Vergata Roma, Italy (Professor F RICCI); Hospital Universitari Germans Trias i Pujol, Spain (Dr L Broc Iturralde, Dr S Gómez Sánchez ); Hospital Universitario Basurto, Spain (Dr G GARAY-ARAMBURU); Hospital Universitario Fundacion Jimenez Diaz, Spain (Mrs N Munoz Sanz); Hospital Universitario Miguel Servet, Spain (Dr P CALVO, Dr J Sanchez); Hospital Universitario Puerta de Hierro, Spain (Dr E Almazan Alonso, Mrs M Garcia Zamora); Hospital Universitario Ramon y Cajal, Support Spain (Dr E Ciancas, Dr J GONZALEZ-LOPEZ); Hospital Universitario de Bellvitge, Spain (Dr D Lorenzo); Hospital Universitario de La Princesa, Spain (Dr m Acebes, Dr S Aparicio-Sanchis); Hospital Universitario del Henares, Spain (Dr A Fernández Hortelano); Hospital Universitario del Vinalopo, Spain (Dr A Piñero Sánchez); Hospital de Torrevieja, Spain (Dr L García García, Dr E Salinas Martínez); Hospital do Meixoeiro, Spain (Dr A CAMPO GESTO, Dr M Rodriguez Núñez); Les Manning, Queensland (Dr L Manning); Luigi Sacco Hospital—University of Milan, Italy (Dr A Invernizzi); Maison rouge Ophthalmologic center, France (Dr L Castelnovo, Dr G Michel, Dr B Wolff); Mark Perks clinic, South Australia (Dr M Perks); Marsden Eye Specialists, New South Wales (Dr J Arnold, Dr H Cass); Mater Private Hospital, Ireland (Dr L OToole); Midwest Ophthalmology, New South Wales (Dr K Tang); Mona Vale Eye Centre, New South Wales (Dr C Chung); Montpellier CHU, France (Professor V DAIEN); Mosman Eye Centre, New South Wales (Dr C Chung); Nepean Valley Eye Surgeons, New South Wales (Dr G Banerjee); New England Eye Centre, New South Wales (Dr M Morgan); Port Macquarie Eye Centre, New South Wales (Dr J Game, Dr C Thompson); Retina & Macula Specialists (Hurstville), New South Wales (Dr R Chalasani, Dr M Chilov, Dr A Fung, Dr S Nothling ); Retina & Macula Specialists (Miranda), New South Wales (Dr M Chilov, Dr S Nothling ); Retina Associates, New South Wales (Dr R Chong, Dr S Fraser-Bell, Dr A Fung, Dr C Younan); Southern Eye Centre, Victoria (Dr D Louis); Specialist Eye Group, Victoria (Dr L Chow, Dr A Cohn); St John of God Hospital Geelong, Victoria (Dr P Lockie); Strathfield Retina Clinic, New South Wales (Dr C Chung, Dr J Wong); Sydney Eye Hospital, New South Wales (Dr R Chong, Dr S Fraser-Bell, Dr M Gillies); Tamworth Eye Centre, New South Wales (Dr P Hinchcliffe); University Hospital Zurich, Switzerland (Dr D Barthelmes); Unidad de Gestion Clinica de Oftalmologia, Hospital de Txagorritxu, Spain (Dr E DIAZ DE DURANA SANTA COLOMA, Dr G GARAY-ARAMBURU); University Hospital Maggiore della Carita, Italy (Dr S Vujosevic); Vall de Hebron University Hospital, Spain (Dr H Brosa Morros); Victoria Parade Eye Consultants, Victoria (Professor R Guymer, Dr A Harper, Dr J ODay); Victorian Eye Surgeons, Victoria (Dr A Cohn); Visionary Eye Specialists, New South Wales (Dr C Hooper); Dr Maria Jose Rodríguez Cid, Hospital de Conxo, Spain.
Author Contributions
Hélène Beylerian, Vincent Daien, Vuong Nguyen, Mark Gillies, Daniel Barthelmes, Catherine Creuzot-Garcher and Benjamin Wolff contributed to the study conception and design. Material preparation, data collection and analysis were performed by Vuong Nguyen. The first draft of the manuscript was written by Hélène Beylerian. Eloi Debourdeau, Mark Gillies, Vincent Daien, Pierre Henry Gabrielle, Stela Vujosevic, Louise O’Toole and Martin Puzo commented on previous versions of the manuscript and approved the final manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Data Availability
Data are accessible to all active FRB registry contributors.
Declarations
Conflict of Interest
Eloi Debourdeau, Hélène Beylerian, Vincent Daien, Vuong Nguyen, Mark Gillies, Daniel Barthelmes, Catherine Creuzot-Garcher, Pierre Henry Gabrielle, Stela Vujosevic, Louise O’Toole, Martin Puzo and Benjamin Wolff have nothing to disclose.
Ethical Approval
Approval to access to the FRB! outcomes database was obtained for this research. Institutional ethics approval was obtained from the Human Research Ethics Committees of the University of Sydney; the Royal Victorian Eye and Ear Hospital, Mater Private Hospital Institutional Review Board, Ireland; Ethics Committee of Fondazione IRCCS Ca Granda Ospedale Maggiore Policlinico, Milan, Italy; the Royal Australian and New Zealand College of Ophthalmologists; the canton of Zurich; and the French Society of Ophthalmology. Ethics committees in Australia and New Zealand approved the use of “opt-out” patient consent. The “opt-out” consent procedure was applied at all centers. The research described follows the principles of the Declaration of Helsinki.
Footnotes
The Fight Retinal Blindness! Study Group is listed in the Acknowledgements section.
Contributor Information
Eloi Debourdeau, Email: e-debourdeau@chu-montpellier.fr.
The Fight Retinal Blindness! Study Group:
A. Cohn, Bacchus Marsh, N. Jaross, R. Barry, I. McLean, T. Guillaumie, A. Miri, P. Gabrielle, S. Tick, A. Field, S. Wickremasinghe, C. Dayajeewa, J. Wells, A. Dunlop, S. Young, G. Mimoun, R. Guymer, P. Carnota, R. Gallego-Pinazo, A. García Layana, M. Saenz-de-Viteri, R. Ferrier, L. Chow, H. Steiner, A. Amini, J. Chen, N. Saha, P. Windle, M. Gillies, A. Hunt, P. Beaumont, Z. Louw, A. Luckie, S. Lake, D. Qatarneh, L. Sararols, J. Suarez, P. Berdoukas, F. Viola, R. Montejano Milner, C. Arruabarrena, F. Ascaso, A. Boned Murillo, M. Díaz, G. Perez Rivases, S. Alforja Castiella, R. Casaroli-Marano, M. Figueras-Roca, J. Zarranz-Ventura, S. Gismero Moreno, A. González Escobar, JMoreno Gutiérrez, J. Escobar, F. Lavid, P. Catalán Muñoz, M. Tena Sempere, F. Ricci, L. Broc Iturralde, S. Gómez Sánchez, G. Garay-Aramburu, N. Munoz Sanz, P. Calvo, J. Sanchez, E. Almazan Alonso, M. Garcia Zamora, E. Ciancas, J. Gonzalez-Lopez, D. Lorenzo, M. Acebes, S. Aparicio-Sanchis, A. Fernández Hortelano, A. Piñero Sánchez, L. García García, E. Salinas Martínez, A. Campo Gesto, M. Rodriguez Núñez, L. Manning, A. Invernizzi, L. Castelnovo, G. Michel, B. Wolff, M. Perks, J. Arnold, H. Cass, L. OToole, K. Tang, C. Chung, V. Daien, G. Banerjee, M. Morgan, J. Game, C. Thompson, R. Chalasani, M. Chilov, A. Fung, S. Nothling, R. Chong, S. Fraser-Bell, C. Younan, D. Louis, P. Lockie, J. Wong, P. Hinchcliffe, D. Barthelmes, E. Diaz De Durana Santa Coloma, G. Garay-Aramburu, S. Vujosevic, H. Brosa Morros, A. Harper, J. ODay, C. Hooper, and Maria Jose Rodríguez Cid
References
- 1.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31. 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57-65.e5. 10.1016/j.ophtha.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 3.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48. 10.1016/j.ophtha.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 4.Busbee BG, Ho AC, Brown DM, Heier JS, Suñer IJ, Li Z, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046–56. 10.1016/j.ophtha.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 5.IVAN Study Investigators, Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–411. 10.1016/j.ophtha.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 6.CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–908. 10.1056/NEJMoa1102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freund KB, Korobelnik JF, Devenyi R, Framme C, Galic J, Herbert E, et al. Treat-and-extend regimens with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina (Philadelphia, PA). 2015;35(8):1489–506. 10.1097/IAE.0000000000000627 [DOI] [PubMed] [Google Scholar]
- 8.Oubraham H, Cohen SY, Samimi S, Marotte D, Bouzaher I, Bonicel P, et al. Inject and extend dosing versus dosing as needed: a comparative retrospective study of ranibizumab in exudative age-related macular degeneration. Retina. 2011;31(1):26–30. 10.1097/IAE.0b013e3181de5609 [DOI] [PubMed] [Google Scholar]
- 9.Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122(1):146–52. 10.1016/j.ophtha.2014.07.041 [DOI] [PubMed] [Google Scholar]
- 10.Augsburger M, Sarra GM, Imesch P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1889–95. 10.1007/s00417-019-04404-0 [DOI] [PubMed] [Google Scholar]
- 11.Aurell S, Sjövall K, Paul A, Morén Å, Granstam E. Better visual outcome at 1 year with antivascular endothelial growth factor treatment according to treat-and-extend compared with pro re nata in eyes with neovascular age-related macular degeneration. Acta Ophthalmol. 2019;97(5):519–24. 10.1111/aos.13989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barthelmes D, Nguyen V, Daien V, Campain A, Walton R, Guymer R, et al. Two year outcomes of “treat and extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina. 2018;38(1):20–8. 10.1097/IAE.0000000000001496 [DOI] [PubMed] [Google Scholar]
- 13.Arnold JJ, Campain A, Barthelmes D, Simpson JM, Guymer RH, Hunyor AP, et al. Two-year outcomes of “treat and extend” intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology. 2015;122(6):1212–9. 10.1016/j.ophtha.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 14.Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, et al. Age-related macular degeneration preferred practice pattern®. Ophthalmology. 2020;127(1):P1–65. 10.1016/j.ophtha.2019.09.024 [DOI] [PubMed] [Google Scholar]
- 15.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017;9(2):378–400. 10.32614/RJ-2017-066 [DOI] [Google Scholar]
- 16.No ID: Therneau, T. (2012). coxme: mixed effects Cox models. R package version 2.2-3. Vienna, Austria: R Foundation for Statistical Computing. | BCO-DMO [Internet]. [cité 24 janv 2023]. Disponible sur: https://www.bco-dmo.org/related-resource/770714
- 17.Kim L, Mehta H, Barthelmes D, Nguyen V, Gillies M. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36:1. 10.1097/IAE.0000000000001142 [DOI] [PubMed] [Google Scholar]
- 18.Kertes PJ, Galic IJ, Greve M, Williams G, Baker J, Lahaie M, et al. Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA Ophthalmol. 2020;138(3):244–50. 10.1001/jamaophthalmol.2019.5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201. 10.1016/j.ophtha.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 20.Lee AY, Lee CS, Egan CA, Bailey C, Johnston RL, Natha S, et al. UK AMD/DR EMR REPORT IX: comparative effectiveness of predominantly as needed (PRN) ranibizumab versus continuous aflibercept in UK clinical practice. Br J Ophthalmol. 2017;101(12):1683–8. 10.1136/bjophthalmol-2016-309818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DM, Regillo CD. Anti-VEGF agents in the treatment of neovascular age-related macular degeneration: applying clinical trial results to the treatment of everyday patients. Am J Ophthalmol. 2007;144(4):627–37. 10.1016/j.ajo.2007.06.039 [DOI] [PubMed] [Google Scholar]
- 22.Li E, Donati S, Lindsley KB, Krzystolik MG, Virgili G. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2020;5:CD012208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott DB. The good (logMAR), the bad (Snellen) and the ugly (BCVA, number of letters read) of visual acuity measurement. Ophthal Physiol Opt. 2016;36(4):355–8. 10.1111/opo.12310 [DOI] [PubMed] [Google Scholar]
- 24.Zarranz-Ventura J, Liew G, Johnston RL, Xing W, Akerele T, McKibbin M, et al. The neovascular age-related macular degeneration database: report 2: incidence, management, and visual outcomes of second treated eyes. Ophthalmology. 2014;121(10):1966–75. 10.1016/j.ophtha.2014.04.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are accessible to all active FRB registry contributors.


