Abstract
Introduction
Anti-vascular endothelial growth factor (VEGF) agents have been the standard treatment for retinal diseases for almost two decades. These treatments are administered via intravitreal injection using single-use vials or prefilled syringes (PFS). In this systematic review, we evaluate health care resource use and clinical outcomes and experiences with PFS for intravitreal injection of anti-VEGF treatments.
Methods
MEDLINE, EMBASE, and The Cochrane Library were searched from January 1, 2015 to February 8, 2024 to identify literature reporting outcomes regarding procedural efficiency, health care resource use, patient and clinician experiences, and safety for currently approved anti-VEGFs (ranibizumab, aflibercept, brolucizumab) administered using PFS. Comparators were vial-based injections of the same anti-VEGFs.
Results
A total of 36 publications met the criteria for inclusion in the systematic literature review; the majority were non-randomized studies, with a small number of reviews, case series, survey studies, and opinion articles. Publications reported that preparation times were significantly shorter for PFS (40.3–57.9 s) versus vials (ranibizumab, 62.8–98.0 s; aflibercept, 71.9–79.5 s), with no differences in product stability between PFS and vials. Clinicians expressed a preference for PFS and thought PFS were faster, easier to use, and had increased safety versus vials. Publications consistently reported significantly lower rates of endophthalmitis per injection with PFS versus vials (ranibizumab PFS, 0–0.02%; aflibercept PFS, 0.01–0.02%; ranibizumab vial, 0.02–0.05%; aflibercept vial, 0.02–0.06%). Four publications reported increased rates of transient vision loss after aflibercept PFS injection versus vial-based injection. No publications reported outcomes regarding health care resource use or patient experiences.
Conclusion
The available literature supports the increased procedural efficiency of PFS versus vial-based intravitreal injection of anti-VEGFs. PFS are positively perceived by clinicians and have a safety benefit in the form of a decreased risk of endophthalmitis versus vials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-024-01002-0.
Keywords: Aflibercept, Injection, Intravitreal, Outcomes, Prefilled, Ranibizumab, Safety, Syringe, VEGF, Vial
Plain Language Summary
Anti-vascular endothelial growth factor (VEGF) drugs, given by injection into the eye, are commonly used to treat diseases that affect the back of the eye (the retina). Anti-VEGF drugs are provided in small containers (vials) or in syringes that are already filled with the drug (prefilled syringes). When someone is treated with an anti-VEGF drug from a vial, the drug must first be taken from the vial using a needle and syringe, and then injected. When someone is treated with an anti-VEGF drug from a prefilled syringe, the drug is injected directly from the prefilled syringe, i.e., there are fewer steps involved when a prefilled syringe is used. We searched the medical literature to see if there were differences in clinical outcomes and experiences between prefilled syringes and vials when used to inject anti-VEGF drugs. Clinicians spent about 50% less time getting ready for injections when prefilled syringes were used than when vials were used. Clinicians also preferred to use prefilled syringes than vials for injecting anti-VEGF drugs. Clinicians reported that prefilled syringes were easier to use, faster, and safer than vials. Patients who were given injections from prefilled syringes had a lower rate of infection of the inside of the eye (endophthalmitis) than patients who were given injections from vials. These results indicate that using prefilled syringes for injecting drugs into the eye can improve efficiency at ophthalmology clinics and improve safety for patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-024-01002-0.
Key Summary Points
| Why carry out this study? |
| Anti-vascular endothelial growth factor (VEGF) agents for treating retinal diseases are given by intravitreal injection using single-use vials or prefilled syringes (PFS). |
| We performed a systematic review of the literature to assess procedural efficacy, health care resource use, patient and clinician experiences, and the safety outcomes associated with approved anti-VEGF injections administered using PFS versus single-use vials. |
| What was learned from the study? |
| Compared with vials, PFS are associated with increased procedural efficacy, improved safety, and greater clinician preference. |
| The time saving and safety benefits of PFS have positive implications for clinic capacity. |
Introduction
The introduction of anti-vascular endothelial growth factor (VEGF) agents from the mid-2000s onwards was a landmark development in the history of treating retinal diseases, including neovascular age-related macular degeneration, diabetic macular edema, and retinal vein occlusion [1, 2]. Pegaptanib, since discontinued, was the first anti-VEGF to be approved in 2004, closely followed by ranibizumab in 2006, aflibercept in 2011, and brolucizumab in 2019. Most recently, in 2022, faricimab, a dual angiopoietin-2/VEGF inhibitor, was also approved. These treatments have allowed patients to achieve improvements in vision and retinal anatomy, which are clear life-changing outcomes [3].
Anti-VEGF treatments for retinal diseases have traditionally been administered via vial-based intravitreal injection. Vial-based administration is a multistep process, which, in brief, comprises disinfecting the single-use vial, withdrawing the drug into a syringe using a sterile transfer needle, replacing the transfer needle with an injection needle, removing air bubbles and adjusting volume (as necessary), and then injecting [4]. In a subsequent significant development in the field of anti-VEGF intravitreal injection, a single-dose, prefilled ranibizumab 0.5-mg syringe was first approved for use by the US Food and Drug Administration in 2016. Approvals for ranibizumab 0.3 mg (2018), aflibercept 2.0 mg (2019), and brolucizumab 6.0 mg (2019) followed thereafter. The process of intravitreal injection using prefilled syringes (PFS) comprises attaching an injection needle, inspecting for air bubbles and adjusting the dose as necessary, and injecting [5–10]. Hence, a number of preparation steps are removed with PFS versus vial-based administration, with obvious time-saving implications for busy ophthalmology clinics. The reduced number of steps involved, and by association decreased likelihood of contamination, has also been purported to result in improved procedural safety, specifically the risk of endophthalmitis [11]. Increased accuracy of dosing has also been suggested to be a benefit of PFS anti-VEGF injection [11].
To date, there has been no comprehensive summary of the available evidence concerning the use of PFS for intravitreal injection of anti-VEGF treatments. Hence, we performed a systematic review of the literature to evaluate the procedural efficacy, health care resource use, patient and clinician experiences, and the safety outcomes associated with approved anti-VEGF injections administered using PFS versus single-use vials.
Methods
This systematic literature review was carried out in accordance with the PRISMA guidelines [12]. The search protocol was prospectively registered on February 2, 2024 on the INSPLASY International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY202420007).
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Sources
MEDLINE, EMBASE, and The Cochrane Library were searched on February 8, 2024. Search strategy details are provided in the Supplementary Material.
Websites from the following organizations were also searched using free-text terms to identify relevant gray literature: US Food and Drug Administration, European Medicines Association, National Institute for Health and Care Excellence, Scottish Medicines Consortium, Pharmaceutical Benefits Advisory Committee, Canadian Agency for Drugs and Technologies in Health, The Professional Society for Health Economics and Outcomes Research, EconLit Database, American Society of Retinal Specialists, European Society of Retina Specialists, The Association for Research and Vision in Ophthalmology, American Academy of Ophthalmology, The Retina International World Congress of Ophthalmology, The American Macular Degeneration Foundation, The Royal Australian and New Zealand College of Ophthalmologists, Asia-Australia Controversies in Ophthalmology, The Royal College of Ophthalmologists, and United Kingdom Ophthalmology Alliance.
Reference lists were hand searched to identify additional potentially relevant literature.
Study Screening and Selection
Eligibility criteria for inclusion in the systematic review were defined according to the Population, Intervention, Comparator, Outcome, and Study Design (PICOS) framework and are summarized in Table 1. Any literature reporting on the outcomes of interest in patients treated with intravitreal injections using PFS containing ranibizumab, aflibercept, or brolucizumab, or literature reporting on health care providers preparing and administering such injections were considered for inclusion. The databases were searched on February 8, 2024 and were restricted to literature published from 2015 onwards.
Table 1.
Systematic literature review eligibility criteria
| Eligibility criteria | |
|---|---|
| Populations |
•Patients treated with intravitreal injections using prefilled syringes containing single-pathway anti-VEGF treatments •Clinicians preparing and administering the above |
| Interventions |
Anti-VEGF prefilled syringes approved for treating retinal diseases •Ranibizumab •Aflibercept •Brolucizumab |
| Comparators |
Anti-VEGF vials approved for treating retinal diseases •Ranibizumab •Aflibercept •Brolucizumab |
| Outcomes |
Procedural efficiency •Preparation and/or injection time •Preparation and/or injection costs •Preparation and/or injection steps •Precision of intravitreal dosing •Convenience/procedural simplicity •Handling errors or contamination •Wastage (drug or other components) Health care resource use •Supplies used per injection (e.g., needles, syringes) •Effects on staff/clinic management/health system •Cost-effectiveness Patient and clinician experience •Patient preference or satisfaction •Clinician preference or satisfaction •Patient health-related quality of life Safety •Transient vision loss •Increased intraocular pressure •Intraocular inflammation/endophthalmitis •Vitreous floaters •Intraocular air bubbles/silicone oil droplets/subvisible particles •Treatment discontinuation/withdrawal because of adverse events •Preventable adverse events (associated with handling and administration) |
| Studies/publication types |
•Any literature reporting on the outcomes of interest in patients treated with intravitreal injections using prefilled syringes containing ranibizumab, aflibercept, or brolucizumab •Any literature reporting on health care providers preparing and administering such injections •Literature reporting results involving off-label compounding of prefilled syringe or splitting practices will be excluded |
| Publication dates | •January 1, 2015 to February 8, 2024 |
VEGF vascular endothelial growth factor
After removal of duplicates, abstracts were screened by two reviewers to determine eligibility.
Data Extraction and Quality Assessment
A single reviewer extracted relevant information/data from each eligible publication into a prespecified Excel spreadsheet. The quality of publications was assessed using the Joanna Briggs Institute Critical Appraisal Checklists for Analytical Cross-Sectional Studies, Case Series, and Expert Opinion [13–15].
Results
Overview
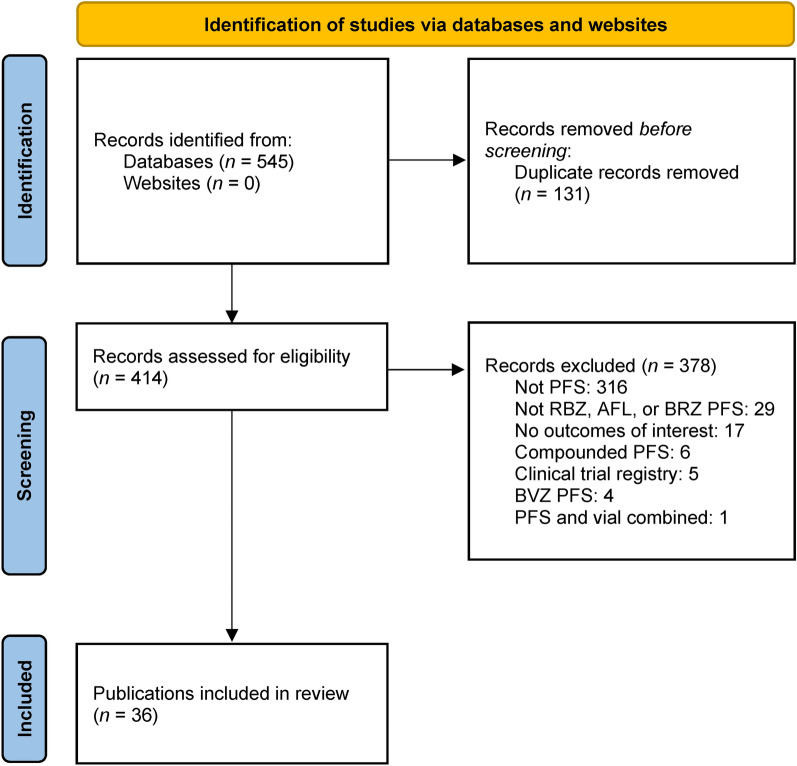
A total of 36 publications from the databases searches met the criteria for the systematic literature review (Table 1) and were included (Fig. 1). Of these, the majority were cross-sectional studies (n = 26) [16–41], with a small number of reviews (n = 4) [4, 11, 42, 43], case series (n = 3) [44–46], surveys (n = 2) [47, 48], and opinion (n = 1) [49] publications. Overall, 17 publications reported comparisons of ranibizumab PFS with ranibizumab or aflibercept vials [17–25, 27–31, 33, 34, 41], 10 publications reported comparisons of aflibercept PFS with aflibercept vials [26, 35, 37–40, 44–46, 48], and two publications reported comparisons of ranibizumab and aflibercept PFS and ranibizumab and aflibercept vials [32, 36].
Fig. 1.
Study selection flow diagram. AFL aflibercept, BRZ brolucizumab, BVZ bevacizumab, PFS prefilled syringe
Quality Assessment
Most of the publications in the review included the key information as stipulated in the Joanna Briggs Institute Critical Appraisal Checklists (Tables S1, S2, and S3). Exceptions (where the majority of checklist items were marked either “unclear” because of a lack of information or “no”) were published abstracts [16, 17, 20, 29–31, 37, 38]. The majority of publications (19 of 27) reporting results from cross-sectional studies did not disclose strategies to deal with potential confounding factors.
Procedural Efficiency Outcomes
Overall, 11 publications [16–20, 23, 29, 30, 41, 48, 49] included outcomes related to the procedural efficiency of PFS (Table 2). Five publications [18–20, 29, 30] reported preparation times for ranibizumab PFS compared with vial-based ranibizumab and/or aflibercept preparation. For each comparison, preparation times for PFS (40.3–57.9 s) were significantly faster than vial-based preparation times (ranibizumab, 62.8–98.0 s; aflibercept, 71.9–79.5 s) [18, 20, 29, 30]. Subhi et al. [19] also reported significantly faster preparation times for PFS versus vial-based preparation times; however, the times for both (ranibizumab PFS, 16.9 s; ranibizumab vial, 40.3 s; aflibercept vial, 45.1 s) were considerably faster than the range of times reported in the other studies. Two publications [16, 17] reported that there were no differences in product stability between ranibizumab PFS and vials and two publications [16, 41] described the ease of use of ranibizumab PFS. Some publications [48, 49] reported problems with the aflibercept PFS in terms of lack of tactile responsiveness when injecting, potentially affecting accuracy of dosing.
Table 2.
Systematic literature review results: procedural efficiency outcomes
| First author, year |
Study design | Interventions | Number of patients/ eyes/injections/ clinicians |
Outcome(s) | Key results |
|---|---|---|---|---|---|
|
Michaud 2014 [16] |
Cross-sectional |
RBZ PFS RBZ vial |
Not specified |
Silicon oil migration Product stability |
Minimal silicone oil migration into solution in RBZ PFS No relevant difference in product stability between RBZ PFS and vial RBZ PFS is easy to hold and may increase the injection preparation efficiency by saving clinicians' time vs. vial |
|
Woodcock 2014 [17] |
Cross-sectional |
RBZ PFS RBZ vial |
Not specified | Product stability | No difference in product stability between RBZ PFS and vial |
|
Souied 2015 [18] |
Cross-sectional |
RBZ PFS RBZ vial |
Syringe preparations Center 1 RBZ PFS 39 RBZ vial 24 Center 2 RBZ PFS 18 RBZ vial 16 |
Syringe preparation time (mean ± SD) |
Center 1 RBZ PFS 46.0 ± 7.3 s RBZ vial 75.3 ± 14.7 s (p < 0.001) Center 2 RBZ PFS 45.8 ± 9.8 s RBZ vial 62.8 ± 15.6 s (p < 0.01) |
|
Subhi 2016 [19] |
Cross-sectional |
RBZ PFS RBZ vial AFL vial |
Syringe preparations RBZ PFS 56 RBZ vial 56 AFL vial 60 |
Syringe preparation time (mean ± SD) |
RBZ PFS 16.9 ± 3.6 s RBZ vial 40.3 ± 6.7 s (p < 0.0001) AFL vial 45.1 ± 6.9 s (p = 0.0014) |
|
Ayan 2017 [20] |
Cross-sectional |
RBZ PFS RBZ vial |
Syringe preparations RBZ PFS 24 RBZ vial 24 |
Syringe preparation time (mean ± SD) |
Syringe preparation time RBZ PFS 40.3 ± 7.62 s RBZ vial 98.0 ± 25.23 s Difference − 57.8 s; 95% CI − 67.67 to − 47.91 (p < 0.0001) |
|
Antoszyk 2018 [41] |
Simulated and actual use human factor | RBZ PFS |
Simulated use Retina specialists 15 Ophthalmic medical personnel 15 Actual use Retina specialists 3 Assistants 3 Patients 35 |
12 tasks specific to the unpacking, preparing, and proper administration | All participants successfully performed all essential and safety–critical tasks without use error in both the simulated use and actual use human factors usability studies |
|
Loewenstein 2019 [23] |
Cross-sectional |
RBZ PFS AFL vial |
Injections RBZ PFS 125 AFL vial 112 |
Injection volume | Volume: RBZ PFS more precise vs. AFL vial |
|
Ugurlu 2021 [29] |
Cross-sectional |
RBZ PFS RBZ vial AFL vial |
Injections RBZ PFS 45 RBZ vial 36 AFL vial 36 |
Syringe preparation time (mean ± SD) |
RBZ PFS 46.5 ± 4.8 s RBZ vial 64.2 ± 5.1 s AFL vial 74.7 ± 8.5 s Time significantly lower for RBZ PFS |
|
Ulaş 2021 [30] |
Cross-sectional |
RBZ PFS RBZ vial AFL vial |
Injections 90 Patients injected RBZ PFS 15 RBZ vial 15 AFL vial 15 |
Syringe preparation + injection time (mean ± SD) |
Clinician 1 RBZ PFS 50.29 ± 11.31 s RBZ vial 82.31 ± 21.52 s (p < 0.001) AFL vial 71.90 ± 16.57 s (p < 0.001) Clinician 2 RBZ PFS 57.86 ± 8.80 s RBZ vial 91.80 ± 17.69 s (p < 0.001) AFL vial 79.50 ± 14.22 s (p < 0.001) |
|
Lee 2022 [48] |
Survey |
AFL PFS AFL vial |
Ophthalmologists 78 |
Experiences using AFL PFS |
49/72 (68%) of respondents felt that more force was required to use AFL PFS plunger vs. traditional 1-ml syringes Common comments Lack of tactile feedback when pushing AFL PFS plunger Priming syringe difficult because of ambiguity in determining where to align the plunger tip |
|
Raevis 2022 [49] |
Letter to editor | AFL PFS | Not applicable | Author’s opinion | Reports of overdosing with PFS may be due to the syringe stopper deforming into the syringe dead space, allowing for more medication to be delivered |
AFL aflibercept, CI confidence interval, PFS prefilled syringe, RBZ ranibizumab
Health Care Resource Use Outcomes
No publications reported on health care resource use.
Patient and Clinician Experience Outcomes
Six publications reported outcomes related to clinician experiences with PFS (Table 3) [18–20, 41, 47, 48]. Clinicians, including physicians, retinal specialists, ophthalmologists, and nurses consistently reported that intravitreal injection using PFS was easier, faster, and had increased safety compared with vial-based intravitreal injection [18, 19, 41, 48]. Clinicians also expressed either increased satisfaction or a preference for using PFS over vials [18, 20, 47].
Table 3.
Systematic literature review results: clinician experiences outcomes
| First author, year |
Study design | Interventions | Number of patients/ eyes/injections/ clinicians |
Outcome(s) | Key results |
|---|---|---|---|---|---|
|
Souied 2015 [18] |
Cross-sectional |
RBZ PFS RBZ vial |
Syringe preparations Center 1 RBZ PFS 39 RBZ vial 24 Center 2 RBZ PFS 18 RBZ vial 16 |
Physician and nurse opinions |
Physicians and nurses thought PFS was an improvement vs. vial owing to fewer injection steps and fewer bubbles Physicians and nurses preferred PFS owing to improved safety, increased dose accuracy, and increased efficiency |
|
Subhi 2016 [19] |
Cross-sectional |
RBZ PFS RBZ vial AFL vial |
Syringe preparations RBZ PFS 56 RBZ vial 56 AFL vial 60 |
Clinicians self-perceived experience | Clinicians thought the PFS was faster and offered higher safety, including less risk of needle-related injuries |
|
Ayan 2017 [20] |
Cross-sectional |
RBZ PFS RBZ vial |
Syringe preparations RBZ PFS 24 RBZ vial 24 |
Clinician questionnaire | Clinicians were more satisfied with PFS over vial |
|
Antoszyk 2018 [41] |
Simulated and actual use human factor | RBZ PFS |
Simulated use Retina specialists 15 Ophthalmic medical personnel 15 Actual use Retina specialists 3 Assistants 3 Patients 35 |
12 tasks specific to the unpacking, preparing, and proper administration | The majority of participants rated the tasks required to use the RBZ PFS as “easy” or “very easy” |
|
Couturier 2021 [47] |
Delphi method questionnaire | nAMD treatments | Retina experts 93 | First-line treatment selection and the importance of long-term risk/benefit ratio | Retina experts expressed a preference for PFS over vials |
|
Lee 2022 [48] |
Survey |
AFL PFS AFL vial |
Ophthalmologists 78 |
Experiences using AFL PFS |
Majority of respondents reported Improved efficiency of use (73/78; 94%) Safer delivery (38/78; 49%) Ease of use (40/78; 51%) with AFL PFS vs. AFL vial |
AFL aflibercept, nAMD neovascular age-related macular degeneration, PFS prefilled syringe, RBZ ranibizumab
No publications reported on outcomes related to patient experiences.
Safety Outcomes
A total of 21 publications [21, 22, 24–28, 31–40, 44–46, 48] reported outcomes related to the safety of PFS (Table 4), including endophthalmitis rate [21, 22, 24, 25, 28, 31, 33, 34, 36, 38, 40], vision and transient vision loss [24, 35, 38, 45, 46, 48], intraocular pressure (IOP) after injection [26, 32, 37, 39, 48], the presence of intravitreal air bubbles [27], ocular hypertension [37], and intraocular inflammation rate [40]. Endophthalmitis rates per injection were consistently lower (and generally statistically significantly) with PFS injection (ranibizumab, 0–0.02% [0 to ~ 1 in 5000]; aflibercept, 0.01–0.02% [~ 1 in 10,000 to ~ 1 in 5000]) compared with vial-based injection (ranibizumab, 0.02–0.05% [~ 1 in 5000 to ~ 1 in 2500]; aflibercept, 0.02–0.06% [~ 1 in 5000 to ~ 1 in 1600]) [21, 22, 24, 25, 28, 31, 33, 34, 36, 38]. When evaluated, the difference between ranibizumab PFS injection and vial-based injection of ranibizumab or aflibercept was also evident for rates of culture-positive endophthalmitis [24, 34]. One publication [27] reported a lower rate of intravitreal air bubbles after ranibizumab PFS injection compared with vial-based aflibercept injection.
Table 4.
Systematic literature review results: safety outcomes
| First author, year |
Study design | Interventions | Number of patients/ eyes/injections/ clinicians |
Outcome(s) | Key results |
|---|---|---|---|---|---|
|
Baudin 2018 [21] |
Cross-sectional |
RBZ PFS RBZ vial AFL vial |
Injections RBZ PFS 315,037 RBZ vial 969,997 AFL vial 392,176 |
Endophthalmitis rate |
RBZ PFS 0.0133% RBZ vial 0.0213% AFL vial 0.0240% Incident risk ratio (95% CI) vs. RBZ PFS AFL vial 1.82 (1.25–2.66); p < 0.001 RBZ vial 1.63 (1.15–2.30); p < 0.001 Rate with RBZ PFS decreased by 46% vs. AFL vial and 40% vs. RBZ vial |
|
Tauqeer 2018 [22] |
Cross-sectional |
RBZ PFS RBZ vial |
Injections RBZ PFS 54,585 RBZ vial 131,808 |
Endophthalmitis rate |
RBZ PFS 0.02% RBZ vial 0.03% p = 0.254 OR (95% CI) for vial vs. PFS 1.45 (0.76–2.75); p = 0.257 |
|
Storey 2019 [24] |
Cross-sectional |
RBZ PFS RBZ vial |
Injections RBZ PFS 78,407 RBZ vial 165,347 |
Endophthalmitis rate Microbial spectrum Visual acuity |
Suspected endophthalmitis RBZ PFS 0.015% RBZ vial 0.026% OR (95% CI) for PFS vs. vial 0.59 (0.31–1.12); p = 0.10 Culture-positive endophthalmitis RBZ PFS 0.0026% RBZ vial 0.013% OR (95% CI) for PFS vs. vial 0.19 (0.045–0.82); p = 0.025 Oral-associated flora found in 27.3% of RBZ vial culture-positive cases vs. 0 RBZ PFS cases Mean logMAR vision loss at final follow-up significantly worse for eyes that developed endophthalmitis after RBZ vial vs. RBZ PFS 4.45 vs. 0.38 lines lost; p = 0.0062 |
|
Dhoot 2021 [25] |
Cross-sectional |
RBZ PFS RBZ vial AFL vial |
Injections RBZ PFS 427,763 RBZ vial 884,061 AFL vial 1,412,699 |
Suspected endophthalmitis rate |
RBZ PFS 0.022% RBZ vial 0.026% AFL vial 0.049% All p < 0.05 vs. AFL |
|
Gallagher 2021 [44] |
Case series | AFL PFS | 5 eyes, 4 patients | Case descriptions | 5 eyes (not treatment naïve) in 4 patients developed transient CRAO immediately after AFL injection via PFS |
|
Karatsai 2021 [26] |
Cross-sectional |
AFL PFS AFL vial |
Injections AFL PFS 748 AFL vial 565 |
IOP (recorded before and at least 15 min after injection) |
No difference in baseline IOP IOP increase ≥ 20 mmHg after injection AFL PFS, n = 13 AFL vial, n = 3 p < 0.05 |
|
Krauthammer 2021 [27] |
Cross-sectional |
RBZ PFS AFL vial |
Injections RBZ PFS 36 AFL vial 61 |
Presence of intravitreal air bubbles |
RBZ PFS 0/36 (0%) AFL vial 9/61 (14.7%) p < 0.0001 |
|
Pancholy 2021 [28] |
Cross-sectional |
RBZ PFS RBZ vial |
Injections RBZ PFS 21,254 RBZ vial 50,413 |
Suspected endophthalmitis rate |
RBZ PFS 0.014% RBZ vial 0.038% p = 0.16 |
|
Scruggs 2021 [45] |
Survey | AFL PFS | Retina specialists 13 | Transient vision loss after AFL PFS |
10 specialists (76.9%) treated patients who reported a perceived increase in post-injection vision loss with AFL PFS There were 16 reported events of no light perception or light perception vision immediately after aflibercept PFS |
|
Aparicio Carreno 2022 [31] |
Cross-sectional |
RBZ PFS AFL vial (preloaded in clinic) |
Injections RBZ PFS 4292 AFL 7765 |
Endophthalmitis rate |
RBZ PFS 0 AFL vial 0.058% |
|
Dingerkus 2022 [32] |
Cross-sectional |
RBZ PFS AFL PFS AFL vial |
Injections RBZ PFS 36 AFL PFS 86 AFL vial 51 |
IOP |
No difference in baseline IOP IOP increase ≥ 30 mmHg after injection RBZ PFS 8/36 (22%) AFL PFS 35/86 (41%) AFL vial 16/51 (31%) p = 0.129 |
|
Finkelstein 2022 [33] |
Cross-sectional |
RBZ PFS RBZ vial AFL vial |
Injections RBZ PFS 14,177 RBZ vial 14,729 AFL 25,178 |
Endophthalmitis rate |
RBZ PFS 0.014% RBZ vial 0.054% (p = 0.066 vs. RBZ PFS) AFL vial 0.055% |
|
Lee 2022 [48] |
Survey |
AFL PFS AFL vial |
Ophthalmologists 78 |
IOP Vision |
At least 1 episode of IOP spike AFL PFS 51/76 (67%) AFL vial 27/76 (36%) p < 0.0001 11 ophthalmologists reported having at least 6 patients experience significant transient vision loss following AFL PFS (5 × more vs. AFL vial) |
|
Lee 2022 [46] |
Case series | AFL PFS |
Retina specialists 13 Patients 12 |
Vision loss |
10/13 (76.9%) retina specialist reported patients had a perceived increase in vision loss after AFL PFS injection 16 events of light perception or worse vision were reported immediately after AFL PFS |
|
Feng 2023 [34] |
Cross-sectional |
RBZ PFS RBZ vial AFL vial |
Injections RBZ PFS 77,925 RBZ vial 93,073 AFL vial 122,947 |
Endophthalmitis rate |
RBZ PFS 0.0154% RBZ vial 0.0226% AFL vial 0.0366% (p = 0.006 vs. RBZ PFS) Culture-positive RBZ PFS 0.0026% RBZ vial 0.0064% AFL vial 0.0187% (p < 0.05 vs. RBZ PFS and RBZ vial) |
|
Klaas 2023 [35] |
Cross-sectional |
AFL PFS AFL vial |
Injections AFL PFS 878 AFL vial 842 |
Transient vision loss rate |
AFL PFS 1.25% AFL vial 0.24% OR (95% CI) 5.33 (1.2–24.1); p = 0.0298 |
|
Louis 2023 [36] |
Cross-sectional |
RBZ PFS RBZ vial AFL PFS AFL vial |
Injections RBZ PFS 112,809 RBZ vial 34,790 AFL PFS 29,899 AFL vial 68,076 |
Endophthalmitis rate |
RBZ PFS 0.015% RBZ vial 0.037% AFL PFS 0.023% AFL vial 0.060% Relative risk (95% CI) vs. vial RBZ PFS 0.403 (0.196–0.830); p = 0.017 AFL PFS 0.389 (0.174–0.866); p = 0.018 |
|
Rana 2023 [37] |
Cross-sectional |
AFL PFS AFL vial |
Eyes 305 |
IOP Ocular hypertension |
Sustained IOP increase rate AFL PFS 1.6% AFL vial 2.3% p > 0.05 Ocular hypertension rate AFL PFS 3.9% AFL vial 9.5% p > 0.05 |
|
Rosanky 2023 [38] |
Cross-sectional |
AFL PFS AFL vial |
Injections AFL PFS 72,579 AFL vial 71,442 |
Endophthalmitis rate Vision |
AFL PFS 0.010% AFL vial 0.052% p < 0.0001 Mean visual acuity was similar at time of presentation, 3 months post-infection, and 6 months post-infection for AFL PFS vs AFL vial |
|
Russel 2023 [39] |
Cross-sectional |
AFL PFS AFL vial |
Eyes AFL PFS 109 AFL vial 148 |
IOP over 1 year after start of treatment |
Sustained IOP increase AFL PFS 1.8% AFL vial 2.7% p = 0.65 Ocular hypertension AFL PFS 2.8% AFL vial 8.8% p = 0.048 |
|
Schmidt-Ott 2023 [40] |
Cross-sectional |
AFL PFS AFL vial |
IOI Endophthalmitis |
IOI rate per units sold outside of USA AFL PFS 0.3 events per 10,000 units AFL vial 1.2 events per 10,000 units Endophthalmitis rate per units sold outside of USA AFL PFS 0.1 events per 10,000 units AFL vial 0.6 events per 10,000 units |
AFL aflibercept, CI confidence interval, CRAO central retinal artery occlusion, IOI intraocular inflammation, IOP intraocular pressure, OR odds ratio, PFS prefilled syringe, RBZ ranibizumab
Several publications [35, 45, 46, 48] reported increased rates of transient vision loss after aflibercept PFS injection compared with vial-based aflibercept injection. There was mixed evidence regarding the effect of aflibercept PFS injection on IOP, with several publications reporting no differences in the proportion of patients experiencing an IOP increase of at least 30 mmHg or sustained IOP increases after injection [32, 37, 39]. In contrast, one publication [26] reported a significantly higher rate of patients with an IOP increase greater than 20 mmHg after aflibercept PFS injection versus vial-based aflibercept injection, and another reported a significantly higher proportion of patients with at least one IOP spike after aflibercept PFS injection compared with vial-based aflibercept injection [48].
Discussion
The aim of this systematic review was to summarize the available evidence regarding the use of PFS for intravitreal injection of approved anti-VEGF treatments for retinal diseases. Overall, we identified 36 publications reporting relevant evidence, including that related procedural efficiency, clinician experiences, and safety outcomes. Five publications reported that the preparation time for intravitreal injection was significantly faster (approximately 50%) for PFS compared with vial-based injections, and two publications reported no differences in product stability between PFS and vials. Another noteworthy finding in the literature is that clinicians have expressed positive opinions regarding PFS, including ease of use, speed, and safety. Furthermore, on the topic of safety, a large number of publications have reported data demonstrating a significantly lower risk of endophthalmitis following intravitreal injection with PFS compared with vials. This is likely because of the inadvertent transfer of contaminants from the rubber bung to the injection fluid when the drug is withdrawn from the vial and the lack of clear guidance on adequate disinfection procedures [50, 51]. Several publications noted an increased frequency of post-injection transient vision loss with aflibercept PFS versus vials. These instances of transient vision loss are likely a consequence of acute retinal arterial occlusion due to a sharp rise in IOP, which may necessitate emergency paracentesis. As this has not been reported with other PFS, it is thought to be related to the aflibercept PFS design, including the wide barrel diameter and the lack of tactile responsiveness associated with the high dead-space plunger design, affecting dosing accuracy [35, 44, 46, 52].
We did not identify any publications specifically reporting on health care resource use outcomes; hence, this represents a gap in the literature. The faster preparation time and decreased rate of endophthalmitis with PFS use have implications for clinic capacity and economics. The need to spend less time preparing injections and treating post-injection endophthalmitis would free up time for clinicians to spend with other patients and/or on other clinic-related activities. As an example, nearly 45,000 intravitreal injections were administered at Moorfields Eye Hospital (UK) in 2019 [53]. If this number of injections were administered via PFS rather than vial, the time saved (assuming 30 s saved per PFS vs. vial injection) would be approximately 375 h. Less experienced clinicians may particularly appreciate the less complicated PFS injection process, which may increase their treatment capacity. Furthermore, the use of PFS means that, in some settings, nurses/ancillary staff may no longer be required to disinfect the vial and transfer the injection fluid to the syringe. In addition to reducing the risk of endophthalmitis, this decreases the number of staff required during the treatment process and enables nurses/ancillary staff to prepare for the next patient, thereby optimizing patient flow. Because of the nature of the process (i.e., number of steps potentially involving multiple clinic staff and the need for communication between such staff), there would appear to be an increased likelihood of medication wastage during drug preparation with vials compared with PFS. There is also a possibility of insufficient medication being withdrawn from the vial on the first attempt because of the viscosity of the drug; use of PFS means that this complication is avoided. The decreased number of materials required for PFS injection may be of benefit in situations where the supply of medical consumables is limited. For example, the COVID-19 pandemic severely disrupted the National Health Service supply chain in the UK, leading to a shortage of needles and syringes, which are required for vial-based injection. The economic effects of the purported benefits of PFS use warrant investigation.
Another gap in the available literature is the absence of evidence on patient experiences with PFS. We hypothesize that differences in preparation time and safety would have an effect on patient satisfaction (e.g., decreased treatment contact time with PFS injection may decrease anxiety) and hence adherence. Given the increasing importance of patient-reported outcomes, studies are required to examine these possibilities and obtain a comprehensive understanding of the patient perspective regarding PFS injection.
The results of our systematic review are limited by the fact that the available evidence comes predominantly from non-randomized studies and case series. To our knowledge, no randomized controlled trials have compared outcomes between PFS and vial-based intravitreal injection approaches. Nevertheless, the main findings of increased procedural efficiency and decreased rates of endophthalmitis with PFS versus vial-based administration were consistently reported across publications. As for all systematic reviews, another limitation is the possibility that relevant literature were not identified in the search or were published after the search was completed. Given the relatively small body of literature on this topic, we are confident that all relevant publications were captured.
Conclusions
The results of our systematic review of the literature demonstrate that the use of PFS for intravitreal injection has benefits over vial-based intravitreal injection for the clinician, patients, and health care systems. Compared with vials, PFS are associated with increased procedural efficacy, improved safety (specifically decreased rates of endophthalmitis), and greater clinician preference, all of which support the use of PFS for administering intravitreal treatments. The time saving and safety benefits of PFS have positive implications for capacity, an important consideration for increasingly busy ophthalmology clinics.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
Writing assistance in the preparation of this manuscript was provided by Luke Carey, PhD, CMPP, of Envision Pharma Group and funded by F. Hoffmann-La Roche Ltd.
Author Contribution
Joel Uzzan and Adam Mapani contributed to the interpretation of results, writing of search protocol, writing/reviewing of the manuscript, and the reading and approval of the final version of the manuscript. Oliver Cox contributed to the design of the review, interpretation of results, writing of search protocol, writing/reviewing of the manuscript, and the reading and approval of the final version of the manuscript. Marloes Bagijn contributed to the conception of the review, design of the review, interpretation of results, writing of search protocol, writing/reviewing of the manuscript, and the reading and approval of the final version of the manuscript. Insaf Saffar contributed to the conception of the review, design of the review, interpretation of results, writing of search protocol, writing/reviewing of the manuscript, and the reading and approval of the final version of the manuscript.
Funding
Funding was provided by F. Hoffmann-La Roche Ltd. for the systematic literature review, the journal’s Rapid Service fee, and third-party writing assistance, which was provided by Luke Carey, PhD, CMPP, of Envision Pharma Group.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Joel Uzzan: Consultant and Investigator: Apellis, Bayer, Bausch + Lomb, F. Hoffmann-La Roche Ltd., Horus, and Novartis. Adam Mapani: Educational, travel, and research grants, advisory board, and speaker’s fees: AbbVie, Alimera, Apellis, Bayer, Biogen, BVI, F. Hoffmann-La Roche Ltd., Gyroscope, Novartis, Scope International, and Veni-Vidi. Marloes Bagijn, Oliver Cox, and Insaf Saffar: Employee: F. Hoffmann-La Roche Ltd.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Kim LA, D’Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol. 2012;181(2):376–9. 10.1016/j.ajpath.2012.06.006. 10.1016/j.ajpath.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller JW. VEGF: from discovery to therapy: the Champalimaud award lecture. Transl Vis Sci Technol. 2016;5(2):9. 10.1167/tvst.5.2.9. 10.1167/tvst.5.2.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hang A, Feldman S, Amin AP, Ochoa JAR, Park SS. Intravitreal anti-vascular endothelial growth factor therapies for retinal disorders. Pharmaceuticals (Basel). 2023;16(8):1140. 10.3390/ph16081140. 10.3390/ph16081140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parenky AC, Wadhwa S, Chen HH, Bhalla AS, Graham KS, Shameem M. Container closure and delivery considerations for intravitreal drug administration. AAPS PharmSciTech. 2021;22(3):100. 10.1208/s12249-021-01949-4. 10.1208/s12249-021-01949-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucentis [package insert]. Genentech, Inc: South San Francisco, CA; 2024.
- 6.Eylea [package insert]. Regeneron Pharmaceuticals, Inc: Tarrytown, NY; 2023.
- 7.Beovu [package insert]. Novartis Pharmaceuticals Corporation: East Hanover, NJ; 2023.
- 8.Lucentis [summary of product characteristics]. Novartis Europharm Limited: Dublin, Ireland; 2023.
- 9.Eylea [summary of product characteristics]. Bayer AG: Leverkusen, Germany; 2024.
- 10.Beovu [summary of product characteristics]. Novartis Europharm Limited: Dublin, Ireland; 2024.
- 11.Sassalos TM, Paulus YM. Prefilled syringes for intravitreal drug delivery. Clin Ophthalmol. 2019;13:701–6. 10.2147/opth.S169044. 10.2147/opth.S169044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350: g7647. 10.1136/bmj.g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 13.Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. JBI; 2020.
- 14.Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–33. 10.11124/JBISRIR-D-19-00099. 10.11124/JBISRIR-D-19-00099 [DOI] [PubMed] [Google Scholar]
- 15.McArthur A, Klugárová J, Yan H, Florescu S. Innovations in the systematic review of text and opinion. Int J Evid Based Healthc. 2015;13(3):188–95. 10.1097/XEB.0000000000000060. 10.1097/XEB.0000000000000060 [DOI] [PubMed] [Google Scholar]
- 16.Michaud JE, Sigg J, Boado L, et al. Ranibizumab pre-filled syringe approved in the European Union: innovation to improve dose accuracy, reduce potential infection risk, and offer more efficient treatment administration. Invest Ophthalmol Vis Sci. 2014;55(13):1949. [Google Scholar]
- 17.Woodcock C, Walsh M, Kurstjens N. Characteristics and potential benefits of the ranibizumab pre-filled syringe. Clin Exp Ophthalmol. 2014;42(S1):125. 10.1111/ceo.12451. 10.1111/ceo.12451 [DOI] [Google Scholar]
- 18.Souied E, Nghiem-Buffet S, Leteneux C, et al. Ranibizumab prefilled syringes: benefits of reduced syringe preparation times and less complex preparation procedures. Eur J Ophthalmol. 2015;25(6):529–34. 10.5301/ejo.5000629. 10.5301/ejo.5000629 [DOI] [PubMed] [Google Scholar]
- 19.Subhi Y, Kjer B, Munch IC. Prefilled syringes for intravitreal injection reduce preparation time. Dan Med J. 2016;63(4):A5214. [PubMed] [Google Scholar]
- 20.Ayan F, Jones C, Mohamed Q. Time differentiation for injection: ranibizumab vial vs pre-filled syringe (PFS) in real life clinical practice. Ophthalmic Res. 2017;58(1):2. 10.1159/000478717. 10.1159/000478717 [DOI] [Google Scholar]
- 21.Baudin F, Benzenine E, Mariet AS, et al. Association of acute endophthalmitis with intravitreal injections of corticosteroids or anti-vascular growth factor agents in a nationwide study in France. JAMA Ophthalmol. 2018;136(12):1352–8. 10.1001/jamaophthalmol.2018.3939. 10.1001/jamaophthalmol.2018.3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tauqeer Z, Storey P, Wolfe JD, et al. Endophthalmitis rates of ranibizumab in pre-filled syringes compared to vials. Invest Ophthalmol Vis Sci. 2018;59(9):5697. [Google Scholar]
- 23.Loewenstein I, Goldstein M, Moisseiev J, Moisseiev E. Accuracy and precision of intravitreal injections of anti-vascular endothelial growth factor agents in real life: what is actually in the syringe? Retina. 2019;39(7):1385–91. 10.1097/iae.0000000000002170. 10.1097/iae.0000000000002170 [DOI] [PubMed] [Google Scholar]
- 24.Storey PP, Tauqeer Z, Yonekawa Y, et al. The impact of prefilled syringes on endophthalmitis following intravitreal injection of ranibizumab. Am J Ophthalmol. 2019;199:200–8. 10.1016/j.ajo.2018.11.023. 10.1016/j.ajo.2018.11.023 [DOI] [PubMed] [Google Scholar]
- 25.Dhoot DS, Boucher N, Pitcher JD 3rd, Saroj N. Rates of suspected endophthalmitis following intravitreal injections in clinical practices in the United States. Ophthalmic Surg Lasers Imaging Retina. 2021;52(6):312–8. 10.3928/23258160-20210528-03. 10.3928/23258160-20210528-03 [DOI] [PubMed] [Google Scholar]
- 26.Karatsai E, Chandra S, Fasolo S, Sivaprasad S, Hamilton R. Prefilled Eylea syringe: our recent experience. Eye (Lond). 2021;35(8):2083–5. 10.1038/s41433-021-01523-z. 10.1038/s41433-021-01523-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krauthammer M, Trabelsi E, Moisseiev E. Intravitreal air bubbles following intravitreal injections: a comprehensive analysis. Graefes Arch Clin Exp Ophthalmol. 2021;259(12):3697–702. 10.1007/s00417-021-05302-0. 10.1007/s00417-021-05302-0 [DOI] [PubMed] [Google Scholar]
- 28.Pancholy M, Storey PP, Levin HJ, et al. Endophthalmitis following intravitreal anti-vascular endothelial growth factor therapy: changes in incidence and outcomes over a 9-year period. Curr Eye Res. 2021;46(9):1370–7. 10.1080/02713683.2021.1874023. 10.1080/02713683.2021.1874023 [DOI] [PubMed] [Google Scholar]
- 29.Ugurlu A. Comparison of the syringe preparation time for ranibizumab prefilled syringe versus bevacizumab, ranibizumab and aflibercept vial. Ophthalmologica. 2021;244(1):2. [Google Scholar]
- 30.Ulaş F, Özçil T, Bayrak A. Comparison of preparation and application duration of ranibizumab prefilled syringe, ranibizumab vial and aflibercept vial in real clinical setting. Ophthalmologica. 2021;244(1):2. [Google Scholar]
- 31.Aparicio Carreño C, Gándara Ande A, Fórneas Sangil A, et al. Endophthalmitis after intravitreal injection with anti-angiogenic drugs: a rare but serious complication. Eur J Hosp Pharm. 2022;29(Suppl. 1):A201. [Google Scholar]
- 32.Dingerkus VLS, Somfai GM, Kinzl S, Orgül SI, Becker MD, Heussen FM. Incidence of severe rise in intraocular pressure after intravitreous injection of aflibercept with prefilled syringes. Sci Rep. 2022;12(1):18136. 10.1038/s41598-022-23039-6. 10.1038/s41598-022-23039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkelstein M, Katz G, Zur D, Rubowitz A, Moisseiev E. The effect of syringe-filling technique on the risk for endophthalmitis after intravitreal injection of anti-VEGF agents. Ophthalmologica. 2022;245(1):34–40. 10.1159/000518236. 10.1159/000518236 [DOI] [PubMed] [Google Scholar]
- 34.Feng HL, Abdelwahab S, Imam N, Astafurov K, Roth DB. Reduced incidence of intravitreal injection-related endophthalmitis with prefilled syringes. J Vitreoretin Dis. 2023;7(4):305–9. 10.1177/24741264231159011. 10.1177/24741264231159011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klaas JE, Bui V, Maierhofer N, et al. Risk of transient vision loss after intravitreal aflibercept using vial-prepared vs the novel prefilled syringe formulation. Front Med. 2023;10:1295633. 10.3389/fmed.2023.1295633. [DOI] [PMC free article] [PubMed]
- 36.Louis AM, Ali AM, Patel SB, et al. Impact of prefilled syringes and masking on postintravitreal injection endophthalmitis. J Vitreoretin Dis. 2023;7(5):382–8. 10.1177/24741264231191339. 10.1177/24741264231191339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rana NA, Russell MW, Chalasani M, Sharma S, Singh RP, Muste J. Characterization of sustained increases in intraocular pressure after aflibercept in prefilled and vial draw syringes. Invest Ophthalmol Vis Sci. 2023;64(8):2706. [Google Scholar]
- 38.Rosanky C, Moon J, Khan S, Storey PP. Prefilled syringes and endophthalmitis following intravitreal injection of aflibercept. Invest Ophthalmol Vis Sci. 2023;64(8):3935. [Google Scholar]
- 39.Russell MW, Chalasani M, Rana N, et al. Effect of prefilled vs vial-drawn syringes on sustained increases in intraocular pressure in patients treated with aflibercept. J Vitreoretin Dis. 2023;7(6):498–503. 10.1177/24741264231200735. 10.1177/24741264231200735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt-Ott U, Fitzpatrick S, Hasanbasic Z, et al. Reported rates of intraocular inflammation with intravitreal aflibercept administered via pre-filled syringe or from vials in clinical practice between 2012 and 2022. Clin Ophthalmol. 2023;17:385–90. 10.2147/opth.S393519. 10.2147/opth.S393519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antoszyk AN, Baker C, Calzada J, et al. Usability of the ranibizumab 0.5 mg prefilled syringe: human factors studies to evaluate critical task completion by healthcare professionals. PDA J Pharm Sci Technol. 2018;72(4):411–9. 10.5731/pdajpst.2017.008342. 10.5731/pdajpst.2017.008342 [DOI] [PubMed] [Google Scholar]
- 42.Schargus M, Frings A. Issues with intravitreal administration of anti-VEGF drugs. Clin Ophthalmol. 2020;14:897–904. 10.2147/OPTH.S207978. 10.2147/OPTH.S207978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storey PP, Patel D, Garg S. Endophthalmitis following intravitreal injection of anti-vascular endothelial growth factor agents. Can J Ophthalmol. 2020;55(4):286–92. 10.1016/j.jcjo.2020.01.015. 10.1016/j.jcjo.2020.01.015 [DOI] [PubMed] [Google Scholar]
- 44.Gallagher K, Raghuram AR, Williams GS, Davies N. Pre-filled aflibercept syringes-variability in expressed fluid volumes and a case series of transient central retinal artery occlusions. Eye (Lond). 2021;35(10):2899–900. 10.1038/s41433-020-01211-4. 10.1038/s41433-020-01211-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scruggs B, Lee DJ, Thomas M, Faridi A. Transient vision loss associated with aflibercept pre-filled syringes. Invest Ophthalmol Vis Sci. 2021;62(8):457. [Google Scholar]
- 46.Lee DJ, Scruggs BA, Sánchez E, Thomas M, Faridi A. Transient vision loss associated with prefilled aflibercept syringes: a case series and analysis of injection force. Ophthalmol Sci. 2022;2(2): 100115. 10.1016/j.xops.2022.100115. 10.1016/j.xops.2022.100115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couturier A, Kodjikian L, Baillif S, et al. Treatment of exudative age-related macular degeneration: consensus of French experts for first-line treatment selection and the importance of long-term risk/benefit ratio. J Fr Ophtalmol. 2021;44(7):937–46. 10.1016/j.jfo.2021.01.001. 10.1016/j.jfo.2021.01.001 [DOI] [PubMed] [Google Scholar]
- 48.Lee DJ, Scruggs BA, Faridi A, Sánchez E, Thomas M. Survey of intravitreal injection outcomes amongst ophthalmologists using pre-filled aflibercept syringes. Clin Exp Ophthalmol. 2022;50(7):803–5. 10.1111/ceo.14125. 10.1111/ceo.14125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raevis J. Re: Lee et al.: Transient vision loss associated with prefilled aflibercept syringes (Ophthalmol Sci. 2022;2:100115). Ophthalmol Sci. 2022;2(4):100199. 10.1016/j.xops.2022.100199. [DOI] [PMC free article] [PubMed]
- 50.Bjornstad M, Kosinski T, Burlage R. Evaluation of the efficacy and superiority of different vial rubber closure disinfection techniques. Int J Pharm Compd. 2020;24(5):434–8. [PubMed] [Google Scholar]
- 51.Buckley T, Dudley SM, Donowitz LG. Defining unnecessary disinfection procedures for single-dose and multiple-dose vials. Am J Crit Care. 1994;3(6):448–51. 10.4037/ajcc1994.3.6.448 [DOI] [PubMed] [Google Scholar]
- 52.Guest JM, Malbin B, Abrams G, et al. Accuracy of intravitreal injection volume for aflibercept pre-filled syringe and BD Luer-Lok one-milliliter syringe. Int J Retina Vitreous. 2022;8(1):27. 10.1186/s40942-022-00375-3. 10.1186/s40942-022-00375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chopra R, Preston GC, Keenan TDL, et al. Intravitreal injections: past trends and future projections within a UK tertiary hospital. Eye (Lond). 2022;36(7):1373–8. 10.1038/s41433-021-01646-3. 10.1038/s41433-021-01646-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.