Abstract
Water deficit stress reduces crop yield in field crops, including sunflowers, at any growth stage. In response, most plants activate hormonal and gene expression patterns to mitigate damage. In this study, we evaluated changes in the physiological and gene transcription levels of two sunflower (Helianthus annuus L.) inbred lines -one sensitive (B59 line) and one water stress-tolerant (B71)–in response to water stress, by using mannitol to simulate water deficit conditions, which provides moderate stress in both sunflower lines. The analyses of the accumulation of various phytohormones under this stress revealed that Jasmonic acid (JA) significantly increased in the shoots of both lines. Similarly, Salicylic acid (SA) increased in the shoots of both lines, although it also accumulated in B71 roots. In addition, Abscisic acid (ABA) and Indole-3-acetic acid (IAA) showed a considerable increase in the B59 shoots. Regarding the JA and SA pathways, the WRKY70 transcription levels were higher in the shoots of both lines and the roots of B71. The B59 line showed overtranscription of a gene related to the ABA pathway (XERICO) and genes associated with IAA (ARF9 and ARF16 genes). The B71 line, on the other hand, simultaneously triggered the JA, SA and ABA hormonal pathways in response to this stress condition. The ABA and JA hormonal pathways activated different TFs, such as RD20, RD22, RD26, ANAC19 and ANAC29, through MYC2. Both the JA and SA hormonal pathways activated the WRKY70 transcription factor. Altogether, each line triggered the hormonal and transcriptional pathways in response to water stress, although at varying intensities. The results suggest that the hormonal pathways of JA, SA, IAA and ABA, along with their primary associated genes, are activated in response to water deficit at the early growth stage in sunflower seedlings, which mitigates damage.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-024-01497-8.
Keywords: Gene expression, Hormones, Inbred lines, Sunflower, Water stress
Introduction
Drought seems to be the most relevant factor limiting crop quality and productivity and causes losses in terms of economic output and human food supply (UNDRR 2021). Most crops are susceptible to drought stress, which leads to yield losses of 30–90% (Hussain et al. 2019). For this reason, researchers have focused on studying the impact of water deficit on crops at the physiological and molecular levels.
The sunflower is a major worldwide crop capable of growing in different climatic regions (Forleo et al. 2018). In Argentina, sunflower cultivation covers a vast area of the country. In the 2022/2023 agricultural cycle, the sunflower crop reached 22 million/hectare, with a 14% increase in the planting area compared to the previous period (Bolsa de Comercio de Rosario 2023). Argentina is the fourth-largest producer of sunflowers and the third-largest exporter of sunflower oil after Ukraine and Russia (USDA 2022). However, in some agroecological zones of Argentina, sunflowers can face some degree of water stress. Specifically, the Pampas area in the Central West Region of Argentina experiences water deficit periods, which hinder crop expansion and lead to irregular germination and unsynchronized seedling establishment. Therefore, it is crucial to determine actions to enhance sunflower performance in these marginal areas.
Plants have developed regulatory mechanisms to acquire tolerance towards abiotic stress factors and, ultimately, guarantee optimal growth. One of the main stress factors affecting crop production worldwide is water deficit, which is critically harmful. Other detrimental abiotic stress factors affecting varied physiological and molecular functions in plants include heavy metals and salt (Basit et al. 2021; El-Sawah et al. 2023).
On the other hand, external plant growth regulators (PGR) can reduce the effects produced by these stress factors on plants. For instance, the exogenous treatment with nitric oxide (NO) and 24-epibrassinolide (EBL) can considerably reduce the toxicity caused by Chromium (Cr) by activating enzymatic and non-enzymatic defense mechanisms in soybean plants grown in Cr-contaminated soils (Basit et al. 2023a). The use of ascorbic acid (AsA) and selenium (SeNPs), either alone or in combination, mitigated Cr-phytotoxicity by reducing the uptake and translocation of Cr and this substantially improved seedling growth in rice and potentially in other crops (Basit et al. 2023b).
In response to various concentrations of diluted seawater, the inoculation with arbuscular mycorrhizal fungi (AMF) reduced the injurious effect of salinity by reducing hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents, which increased antioxidant enzymes, amino acids and polyamines content and the transcription of genes involved in polyamine biosynthesis (El-Sawah et al. 2023).
Stress conditions produce alterations in the production and distribution of phytohormones. These phytohormones, in turn, regulate stress responses via hormone- and stress-responsive transcription factors. Thus, the interaction of hormones and transcription factors establishes an interconnected network (Xie et al. 2019; Wang et al. 2023).
When facing a water deficit, plants produce multiple phytohormones that transduce the stressful signal and, therefore, mediate the prevention or tolerance to the detrimental effects of this stress (Hamayun et al. 2018). This response includes induction of stress hormones such as jasmonic acid (JA), abscisic acid (ABA), salicylic acid (SA), brassinosteroids (BRs), and changes in the synthesis of growth-promoting hormones, such as gibberellins (GAs), cytokinins (CK) and auxins (AUX), among others, to facilitate positive responses to water deficit (Salvi et al. 2021; Iqbal et al. 2022).
Studies on Arabidopsis, barley, wheat, maize, and pearl millet have demonstrated positive effects of the phytohormones ABA and JA on drought tolerance. For instance, these phytohormones improved the growth of pearl millet (Pennisetum glaucum L.) seedlings by mitigating the adverse effects of polyethylene glycol (PEG-6000)-induced drought (Awan et al. 2020). The exogenous application of ABA and JA increased leaf relative water content (RWC), root and shoot lengths, as well as fresh and dry weight. Furthermore, these phytohormones help maintaining proline levels and minimizing lipid peroxidation through the enhancement of the activities of antioxidant enzymes (Awan et al. 2020).
Likewise, SA levels increased in plants under water deficit, as detected in evergreen shrubby plants Phillyrea augustifolia (up to five-time increases) (Munné-Bosch and Peñuelas 2003) and leaves of citrus species, such as Carrizo and Cleopatra (Hamayun et al. 2018). The stomatal closure observed in Arabidopsis under stressed conditions was also due to SA accumulation; which suggests that the plant achieves drought tolerance through SA-regulated induction of PR gene transcription and under the influence of SUMO E3 ligase SIZ1 (Miura and Nozawa 2014). In addition, BRs are essential in regulating plant physiological and molecular responses under various environmental stresses such as drought, salinity, and extreme temperatures (Manghwar et al. 2022). In response to heavy metal stress, BRs can reduce the uptake of these types of ions from the roots, thereby decreasing their content inside the plant cells (Basit et al. 2021).
Regarding plant growth hormones, the endogenous indole-3-acetic acid (IAA) plays an essential role in mediating plant tolerance to drought stress (Iqbal et al. 2022). For instance, researchers have reported that exogenous IAA and overexpression of the IAA biosynthesis gene improved drought stress tolerance in Arabidopsis, while mutants expressing lower endogenous IAA levels showed lower tolerance to drought stress not only than wild-type plants pretreated with IAA, but also than non-pretreated wild-type plants (Shi et al. 2014). In addition, at least in tomatoes and clovers, the transcription factors ARFs regulate transcriptionally auxin-responsive genes involved in drought stress tolerance, such as DREB4, MYB14, WRKY108715 and bZIP107 (Zhang et al. 2020; Iqbal et al. 2022). Gibberellins can also enhance plant drought tolerance (Chen et al. 2019; Liao et al. 2023). On the other hand, Salvi et al. (2021) have reported that osmotic or drought stress reduced the GA content and enhanced DELLA accumulation, which improved stress tolerance in plants with retarded growth.
Transcription factors (TFs) are effector proteins that can be activated by hormones and stress signals. These proteins have a crucial function in regulating gene transcription and signal transduction, thus acting as nodes in regulation networks of numerous biological processes (Hrmova and Hussain 2021; Yin et al. 2023). Some of these TFs are core members of networks regulating numerous downstream genes (Tripathi et al. 2014). The classification of these TFs into different gene families, such as AREB, DREB, MYB, WRKY, NAC, and bZIP, is based on the distinct structure of their DNA-binding domain (Jin et al. 2014). Some TF genes respond to water deficit through pathways dependent or independent of ABA (Cerda and Alvarez 2024).
This work addresses an integrated approach between the physiological and molecular response to water stress conditions, with emphasis on the role of phytohormones such as JA, SA and IAA and their crosstalk with ABA and GAs. We also assessed associations with metabolism and signaling genes in the regulation of the response of sunflower seedlings to water stress in early vegetative growth (V2).
According to a previous study by our group, B59 and B71 sunflower inbred lines (sensitive and tolerant to water stress, respectively) have differential gene transcription profiles. For instance, B59 showed more genes differentially expressed under water stress than B71. Most of the water stress-responding genes in both lines displayed an upregulated profile. In addition, genes related to hormone signaling pathways, components of the redox system, and secondary metabolites showed an enriched profile in the aerial part and roots of both lines (Escalante et al. 2020). However, to the best of our knowledge, no previous report addresses the differences in the water deficit response of these sensitive and tolerant sunflower inbred lines in terms of endogenous hormonal content, expression of hormone-related genes, and transcription factors in the shoot and roots.
We hypothesize that water stress alters the endogenous content of different hormones, and this modification is associated with changes in the transcription levels of genes and transcription factors involved in hormone metabolism and signaling pathways. To evaluate this, we analyzed phytohormone contents and the transcription patterns and functionality of various genes and transcription factors associated with hormonal metabolism and signaling. Another aim was to evaluate associations between hormonal and transcriptomic profiles and the regulation of the response of sunflower seedlings to water stress during their early vegetative growth (V2).
Material and methods
Plant material
Sunflower seeds (Helianthus annuus L., Asteraceae) of sensitive B59 and tolerant B71 inbred lines were sown at an experimental field of EEA-INTA Manfredi (31° 51′ 9.00″ South latitude and 63° 44′ 55.91″ West longitude), Argentina. MSc. Daniel Alvarez (EEA-INTA Manfredi) kindly supplied this material.
Germination and early growth assays
These assays were performed as described by Andrade et al. (2013) and Escalante et al. (2020). Briefly, water stress was simulated using mannitol (Biopack; Buenos Aires), which generated a moderate stress of −0.989 MPa. At the fourth day of sown in sand, and thereafter every three days, the seedlings were watered to field capacity (FC) with the following solutions (treatments): (1) Hoagland’s 50% full-strength (control); (2) mannitol 400 mM (Fig. 1). The seedlings were harvested after 20 days of sowing and carefully sectioned into the shoot and roots, immediately frozen in liquid nitrogen, and stored at −80 °C. The assays were performed in quadruplicate.
Fig.1.
Effect of water stress on the growth of sunflower seedlings. A Representative photograph of B59 seedlings growing under control conditions; B Representative photograph of B59 seedlings growing under water stress generated by mannitol 400 mM application; C Representative photograph of B71 seedlings growing under water stress generated by mannitol 400 mM application; D Representative photograph of B71 seedlings growing under control conditions
Extraction, purification, identification, and quantification of endogenous hormones
The phytohormones 12-oxo-phytodienoic acid (OPDA), JA, ABA, ABA glucose ester (ABA-GE), SA, IAA, Gibberellin 1 (GA1) and Gibberellin 3 (GA3) were extracted from shoot or root tissues (0.2 g dry weight plant-1), separated by reversed-phase HPLC and finally quantified by a quadrupole tandem mass spectrometer (MS/MS), as described by Andrade et al. (2021).
Briefly, the samples were extracted with 5 mL of deionized water and homogenized in an Ultraturrax T25 basic homogenizer (IKA, Staufen; Germany). Fifty ng of deuterated internal standard of each phytohormone were added to the samples. The samples with the added standard were centrifuged at 10750 × g for 15 min and the resulting supernatant was adjusted to pH 2.8–3.2 with 30% (v/v) acetic acid. Each supernatant was extracted twice with diethyl ether. The organic fraction was evaporated under vacuum and the dried extracts were dissolved in 1 mL methanol, and then filtered on a vacuum manifold. Finally, each eluate was evaporated in a SpeedVac SC110 (Savant Instruments, New York, NY, USA) at 35 °C. The extracts were separated by HPLC using a gradient of increasing methanol concentration, constant glacial acetic acid concentration (0.2% in water) in an Alliance 2695 separation module (Waters; Milford, MA, USA) equipped with a Restek Ultra C18 3 μm column. The identification and quantification of the phytohormones were performed with a quadrupole tandem mass spectrometer (MS/MS) (Quattro pt Ultima, Micromass, Manchester, UK) fitted with an electrospray ion (ESI-) source, in multiple reaction monitoring mode (MRM). The spectrometry software for data analysis was MassLynx version 4.1 (Waters). The assays were performed in quadruplicate.
Statistical analysis
A factorial experiment was set up in a completely randomized design. The hormone data were analyzed using the Infostat® statistical software (Di Rienzo et al. 2014). The obtained data were subjected to analysis of variance (ANOVA) and significant differences between means were evaluated by pair-wise comparisons using Tukey’s test at p < 0.05 level. Normality and analysis of variance assumptions were corroborated to select the statistical analysis. In case of absence of homogeneity, a non-parametric test (Kruskal Wallis) was performed, followed by non-parametric multiple comparisons.
Transcriptomic analysis
RNA isolation, quantification, and quality controls
RNA isolation was performed from the shoot and roots of 15 seedlings of each inbred line under each water status (irrigated and water stressed). High-quality total RNA was isolated from 100 mg of frozen tissue using the extraction kit of RNAqueous Plant RNA Isolation Aid (Ambion, Life Technologies Corporation, USA) according to the manufacturer’s instructions. The RNA concentration, integrity and quality were evaluated as described by Escalante et al. (2020). A Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) was used to check RNA concentration. The integrity of total RNA was assessed in agarose gel electrophoresis. Finally, a NanoBioanalyzer RNA-6000 (Agilent Technologies, Palo Alto, CA) was used to evaluate the quality of total RNA.
Microarray analysis
The processing of the transcriptomic profiles and bioinformatic data were performed according to Escalante et al. (2020). Briefly, data preprocessing was performed using the limma library (Smyth 2005) of the R language (R Core Team 2013). The backgroundCorrect function was employed for Background correction through the “rma” algorithm. Normalization was performed between arrays by using the normalized Between-Array function with “quantile” method (Bolstad et al. 2003). Finally, gene transcription profiles were transformed to log2 scale and summarization of technical replicates was done calculating the median. The Ward clustering method was applied to the Euclidean distance matrix calculated from the first three principal components of the (microarrays by genes) transcription matrix to group the treatment replicates (arrays) and finally check the reliability of the dataset. The raw data are available from the GEO repository, accession number GSE 128556.
Differential gene expression analysis
The transcriptomic analysis, identity of differentially expressed genes and statistical analysis were conducted according to Escalante et al. (2020). Briefly, the statistical analysis was performed using ad hoc routines, written in R, to fit gene by gene, a linear mixed effect model. In addition, two bioinformatic tools, MapMan and the repository of the sunflower unigene collection (SUR) (Moschen et al. 2016), and the probes associated to each unigene and functional annotation information were employed to carry out the transcriptomic analysis and the identification of differentially expressed genes (DEGs). For each experimental condition, the log2 ratio of fold change (water stress/irrigated) was calculated. The transcription cut-off was set to a log2 fold change higher than 1 or lower than − 1 and with a p value lower than 0.05 (Supplementary Tables S1–4).
qRT-PCR for differential gene expression analysis
This technical procedure was examined as described by Escalante et al. (2020). Briefly, two-elongation factor-1α (EF – 1α) and Tubulin-reference genes were used to assess the transcription of ten selected genes for each line in order to reduce the variance in real-time PCR results. Specific primer pairs were designed using Primer3 software (Rozen and Skaletsky 2000) with default parameters (Supplementary Table S5). Five hundred ng of RNA was treated with DNase and reverse transcribed (Superscript III first strand synthesis system, Invitrogen, Buenos Aires, Argentina) using random hexamer primers, according to the manufacturer’s instructions (Moschen et al. 2014).
Results
Hormonal profile
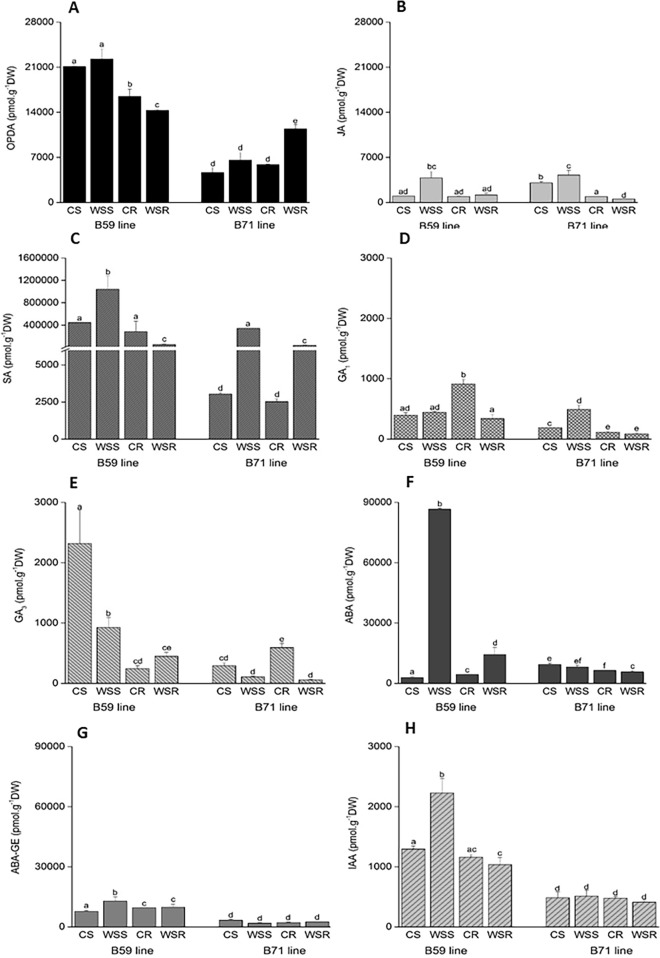
Regarding JAs & OPDA decreased significantly under water stress in B59 roots, although it remained unaltered in the shoot of this line under the stress condition. On the other hand, this phytohormone increased in B71 roots (11431.95 pmol.g-1; 2-fold increase with respect to the control seedlings) under water deficit (Fig. 2A). The JA content increased in the shoot of water-stressed B59 seedlings but remained unchanged in the root. In the water-stressed B71 line, JA slightly, although significantly, increased in the shoot but decreased in the roots (Fig. 2B).
Fig.2.
Endogenous hormone profiles in the shoot and roots of the control (C) and water-stressed seedlings (WS) of B59 and B71 lines. A 12-oxo-phytodienoic acid (OPDA); B Jasmonic acid (JA); C Salicylic acid (SA); D Gibberellin 1 (GA1); E Gibberellin 3 (GA3); F Abscisic acid (ABA); G Abscisic acid glucose ester (ABA-GE) and H Indole-3-acetic acid (IAA); CS: control shoot; WSS: water-stress shoot; CR: control roots; WSR: water-stressed roots. Mean values (±S.E.) with different letters above bars are significantly different at p < 0.05 (n = 4)
Under water stress, SA levels significantly increased in the shoot of B59 seedlings but decreased in the roots (Fig. 2C). The B71 line showed an important and significant increase of SA in response to water stress in both the shoot and roots, but the increase was sharper in the shoot.
With respect to GAs, the content of GA1 (Fig. 2D) decreased significantly in water-stressed roots of the B59 line. By contrast, GA1 displayed a remarkable increase (2.6 times) in the shoot of the B71 line in relation to the control condition. The GA3 content significantly decreased (2.5-fold decrease) in the B59 shoot upon water deficit, while it slightly increased in the roots of this line. Both the shoot and roots of B71 seedlings subjected to water deficit showed a decrease in GA3, although the decrease was only significant in roots (Fig. 2E).
The ABA content sharply increased (31-fold increase) in the shoot of water-stressed B59 seedlings (Fig. 2F) and increased in the roots, although to a lower extent. In B71 seedlings, both the shoot and roots displayed a decrease in ABA content under water deficit, although the differences were only significant in the roots (Fig. 2F).
On the other hand, the ABA-GE content showed a significant increase (1.7 times) in the water-stressed shoot of B59 seedlings, with respect to the control condition, but remained almost unchanged in B71 seedlings throughout the experiment (Fig. 2G).
The content of IAA under water deficit increased significantly in the shoot of the B59 line, whereas it remained unaltered both in the shoot and roots of B71 seedlings (Fig. 2H).
Transcription of genes related to hormonal metabolism and signaling pathways
By microarray analysis, we then analyzed the transcription of genes related to hormonal metabolism and signaling pathways in the shoot and roots of B59 and B71 seedlings subjected to water deficit. Validation of the microarray data, as mentioned in Escalante et al. (2020), was performed by qRT-PCR analysis (Supplementary Fig. S1), obtaining consistent expression patterns in 15 of the 20 transcripts evaluated, indicating similar expression levels between methodologies and a high level of reproducibility.
Genes involved in JA metabolism
Regarding the transcription profiles of genes related to JA biosynthesis, in the water-stressed B59 line, the transcription levels of LOX2, LOX3, HPL1, OPR1, and OPR3 increased in the shoot, with LOX2 showing the highest increase (3.3-fold change) (Fig. 3). In the roots, the transcription level of LOX3, LOX5, HPL1, OPR1 and OPR2 genes increased, with LOX3 showing the highest fold change (1.6). By contrast, the transcript level of LOX2 was downregulated (Fig. 3).
Fig.3.
Differential transcription levels of genes in sunflower seedlings under water stress. Heat maps showing the response of different jasmonic acid (JA) metabolism and signal transduction-related genes in the root and shoots of B59 and B71 seedlings subjected to water stress. Color intensity corresponds to the transcription ratio at logarithmic scale (red upregulated, blue downregulated)
In the water-stressed B71 line, the transcription level of LOX3 and HPL1 increased in the shoot (1.2- and 1.8-fold change, respectively), while the transcript level of LOX2 was lower than that of the control. The roots showed increased LOX, OPR1 and OPR2 gene transcription levels. In contrast to the shoot, LOX2 showed an increased level in the roots (2.1 of fold change; Fig. 3).
With respect to the genes related to JA inactivation, the ATST2a gene transcript was the only upregulated transcript in the shoot of water-stressed B59. The transcription of this gene stayed unaltered in water-stressed B71 (Fig. 3).
The transcription levels of TOPLESS, MYC5, and JAZ6, genes associated with the regulation of JA signal transduction, increased upon water deficit in the shoot of B59, with MYC5 reaching the highest level (15-fold change). In the water-stressed B71 line, MYC2 was the only differentially transcribed gene, with an increased transcription level in the shoot compared with the roots (Fig. 3). The shoots and roots of water-stressed B71 seedlings as well as the shoot of water-stressed B59 seedlings presented an upregulation of WRKY70 transcription levels (Fig. 3).
Genes involved in SA metabolism
Two genes associated with SA biosynthesis, CM1 and PAL1, showed significantly higher transcription levels (2.9 and 2.1-fold change, respectively) in the shoot of water-stressed B59 seedlings. Water-stressed B59 roots showed no changes in these transcripts (Fig. 4). The B71 line under water deficit displayed a similar transcription pattern to that of water-stressed B59 seedlings both in the shoot and roots.
Fig.4.
Differential transcription of genes in sunflower seedlings under water stress. Heat maps showing the response of different salicylic acid (SA) metabolism and signal transduction-related genes in the shoot and roots of B59 and B71 seedlings under water stress. Color intensity corresponds to the transcription ratio at logarithmic scale (red upregulated, blue downregulated)
Regarding the genes associated with the inactivation of this pathway, the shoot of both lines showed increased transcription levels of AT4G36470, SAMT1 and BSMT1 genes, with SAMT1 (3.1-fold change) and BSMT1 (22-fold change) showing the highest levels of transcription in B59 and B71 upon water deficit, respectively (Fig. 4). In the roots of B59, the SAMT1 and BSMT1 transcription levels increased under water deficit, with BSMT1 transcripts reaching the highest levels (3.7-fold change). Like in B59, the transcription level of SAMT1 and BSMT genes increased in the roots of water-stressed B71 seedlings, with values of 1.2 and 1.6-fold change, respectively. The shoots of both lines and the B71 roots showed decreased transcription levels of MES when exposed to the evaluated stress (Fig. 4).
With respect to the transduction/regulation of SA signal upon water stress, the transcription level of the RRTF1 gene decreased in the B59 shoot and B71 roots but increased in the B71 shoot (Fig. 4). The transcription levels of the WRKY4 gene were lower in the shoot and roots of B59 and the B71 shoot. The WRKY70 gene transcription was higher in the shoots of B59 and B71 and B71 roots (Fig. 4).
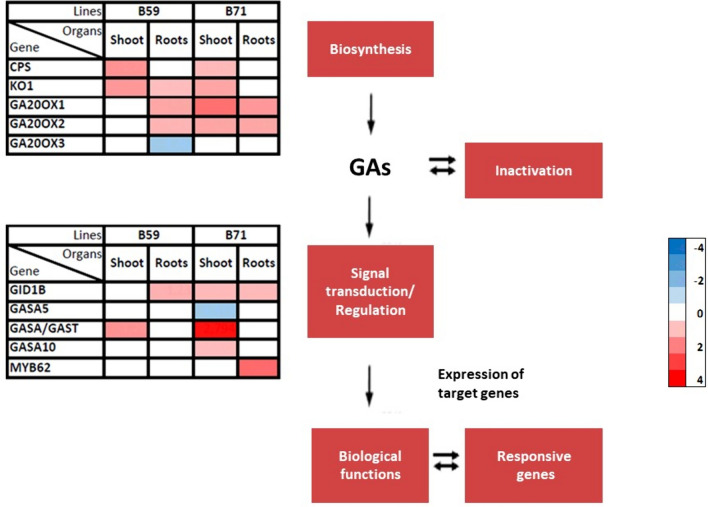
Genes involved in GAs metabolism
The genes involved in the biosynthesis of GAs (Fig. 5) CPS and KO1 showed increased levels in the shoot and roots of water-stressed B59. On the other hand, the GA20ox1 and GA20ox2 transcript levels were higher only in the roots of this line. Conversely, the GA20ox3 transcript levels were lower in the roots under water stress. The water-stressed B71 line displayed elevated levels of the genes CPS, KO1, GA20ox1 and GA20ox2 in the shoot, and GA20ox1 and GA20ox2 in the roots.
Fig.5.
Differential transcription level of genes in sunflower seedlings under water stress. Heat maps showing the response of different gibberellins (GAs) metabolism and signal transduction-related genes in the shoot and roots of B59 and B71 seedlings subjected to water stress. Color intensity corresponds to the transcription ratio at logarithmic scale (red upregulated, blue downregulated)
In terms of the genes involved in the regulation of the signal transduction/regulation of GAs (Fig. 5), the transcription level of the GID1B gene was higher in B59 roots and in both evaluated organs of B71 seedlings exposed to water deficit, compared to the controls. In the shoot of water-stressed B59, the transcription level of the GASA family genes increased. In addition, the shoot of water-stressed B71 showed an upregulation of GASA (2.7-fold change) and GASA10, while the transcription level of GASA5 diminished. In roots, only the transcription of the MYB62 gene showed an increased level.
Genes involved in ABA metabolism
The genes involved in ABA biosynthesis showed variations in transcription levels when the evaluated plant lines were exposed to water deficit compared to the controls (Fig. 6). For instance, the XEP gene and the gene of the isoform SDR4 evidenced increased transcription levels in the shoot of water-stressed B59 seedlings, with SDR4 transcripts showing the highest values. However, although the transcription of the ZEP gene increased in the roots (as in the shoot), the SDR4 transcription decreased in this aerial organ. Similarly, the water-stressed B71 line showed increased transcription levels of the ZEP and SDR4 genes in the shoot. In the roots, the transcription levels of ZEP, NCED1 and NCED4 also increased, with NCED1 showing the highest fold change.
Fig.6.
Differential transcription of genes in sunflower seedlings under water stress. Heat maps showing the response of different abscisic acid (ABA) metabolism and signal transduction-related genes in the shoot and roots of B59 and B71 seedlings subjected to water stress. Color intensity corresponds to the transcription ratio at logarithmic scale (red upregulated, blue downregulated)
According to the analysis of genes associated with ABA inactivation, the B59 shoot showed increased levels of UGT1 transcripts and decreased levels of ABA8OH under water deficit conditions, while the roots displayed higher levels of UGT1 transcripts. On the other hand, the B71 line showed a downregulation of the ABA8OH gene transcription levels in the shoot and an upregulation of UGT1 gene transcripts in the roots (Fig. 6).
Among the genes regulating the ABA signal transduction pathway with differential transcription levels under water deficit conditions, ABI2, XERICO, ABF3, ABF4 and ANAC083 showed increased transcription levels in the B59 shoot. Specifically, ABI2 and XERICO showed fold-change values greater than 3.5. Several genes showed downregulated transcription patterns, such as RAV1, MYB44, and ANAC019, among others (Fig. 6). The genes with downregulated transcription patterns were RAV1, MYB44, ANAC019, ANAC055, RRTF1, WRKY33 and CBF4. The roots of B59, on the other hand, showed higher transcription levels of AKS3, ABI2, ANAC029, ATAF1 and XERICO than the control. Moreover, XERICO showed a fold change above 3. Conversely, the transcription of RAV1 decreased. In the shoot of B71, we registered upregulation of ABF3, ABF4, ABI2, SnRK2.8, XERICO, ANAC019, ANAC029, RRTF1 and MYC2 genes, and downregulation of AKS3 (Fig. 6). In the roots of this line, AKS3, ABI2, PP2C, XERICO, ANAC019, ANAC029 and ANAC055 showed increased transcription levels; conversely, RAV1, RRTF1, MYC2 and CBF4 transcript levels were lower than in the control (Fig. 6).
Regarding the ABA-responsive genes, the HVA22, HVA22E and RD22 genes increased their transcription level in the shoot of water-stressed B59 seedlings, while in the roots, RD26 increased and RD20 decreased. In the water-stressed B71 line, the transcription levels of the HVA22E, RD20 and RD22 genes increased in the shoot and RD26 gene in the roots (Fig. 6).
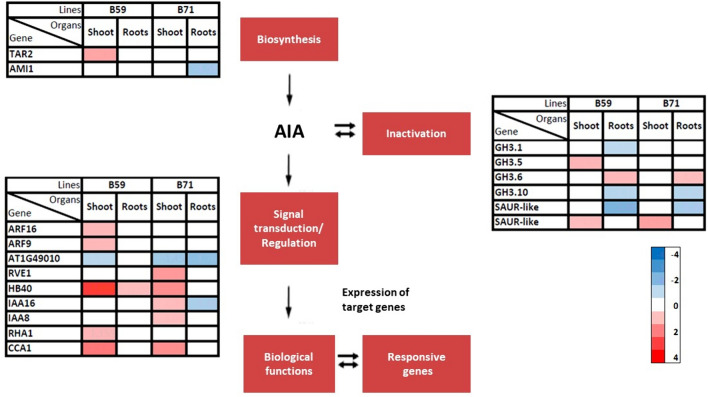
Genes involved in IAA metabolism
Concerning IAA biosynthesis genes, the TAR2 transcription level increased in the B59 shoot but remained unchanged in its roots when exposed to water deficit. In B71, only the AMI1 gene showed decreased transcription levels in roots (Fig. 7).
Fig.7.
Differential transcription of genes in sunflower seedlings under water stress. Heat maps showing the response of different indole-3-acetic acid (IAA) metabolism and signal transduction-related genes in the shoot and roots of B59 and B71 seedlings subjected to water stress. Color intensity corresponds to the transcription ratio at logarithmic scale (red upregulated, blue downregulated)
Among the genes related to IAA inactivation, the B59 shoot showed increased levels of GH 3.5 and a member of the SAUR-Like gene family, while the B59 roots had increased levels of the GH3.6 gene. Conversely, the transcription levels of GH3.1, GH3.10 and a member of the SAUR-like family decreased in the roots of this line when exposed to water deficit (Fig. 7). Regarding B71, the SAUR-like gene and GH3.6 showed increased levels in the shoot and roots of stressed seedlings, respectively; however, the GH3.10 and SAUR-like genes were downregulated in these roots (Fig. 7).
Among the genes associated with the regulation of the IAA signal transduction/regulation system, in the B59 shoot, the ARF16, ARF9, RHA1, CCA1 and ATHB40 genes were upregulated under water deficit, with ATHB40 having values higher than a 3-fold change. The only gene with a detected reduction in its transcription level was AT1G49010. The shoot of the B71 line, on the other hand, showed an increase in RVE1, ATHB40, IAA16, IAA8 and CCA1, and a decrease in AT1G49010, while the roots had decreased transcription levels of the AT1G49010 and IAA16 genes (Fig. 7).
Discussion
The physiological and molecular analyses of B59 and B71 seedlings growing under moderate water deficit conditions allowed us to investigate the relationships between the plant hormone content and the transcription profiles of genes involved in hormone metabolism and signaling.
The decrease in OPDA in the water-stressed roots of the B59 line may be related to an increase in primary root length, as reported previously by Andrade et al. (2017). In line with our results, Canales et al. (2021) have reported that drought stress led to lower levels of OPDA in two oat genotypes promoting radical growth primarily roots with smaller diameters. This radical growth promotion is critical to regulate proper water status. By contrast, OPDA increased in the water-stressed roots of B71. This increase could correlate with shorter primary roots in this line with a diminution in the primary root length of this line (Andrade et al. 2017).
The analysis of JA accumulation dynamics suggests that the increase of JA in the shoots of both lines after water-stress treatment is linked to an inhibition of shoot growth, as previously informed by Andrade et al. (2017).
A cDNA microarray analysis has shown that treatments with compounds of the JA family, such as jasmonic acid methyl ester (JAMe) and 12-hydroxyjasmonic acid (12-OH-JA), stimulate the transcription of genes involved in the synthesis and metabolism of JA, such as LOXs and OPR3 (Miersch et al. 2008). In addition, various environmental factors, such as drought stress, lead to the induction of LOX gene transcription, thus contributing to JA biosynthesis.
Our study suggests that the upregulation of the LOX2 gene in the B59 shoot is related to increased endogenous JA contents and that the transcription of this gene in B71 roots may be associated with increased OPDA levels. Thus, both lines triggered the expression of different members of the JA family to cope with water stress through the 13-LOX pathway. Similarly, the upregulation of the LOX3 gene in the shoot of both lines was accompanied by a JA increment. Furthermore, LOX3 participates in the production of JA under osmotic stress (Grebner et al. 2013) and lateral root development (Vellosillo et al. 2007). In Arabidopsis thaliana, LOX5 participates in lateral root development (Caldelari et al. 2011). In our study, LOX5 transcription was restricted to the B59 roots. According to Vellosillo et al. (2007), LOX5 may be a key factor for root development as a regulator for the formation of lateral roots via 9-HOT production.
The HPL1 gene followed the same transcription trend as LOX3 in the shoot and roots of B59 and B71. This finding is consistent with the early over-expression of the HPL1 gene in the drought-tolerant variety chickpea after 2 h of drought stress (De Domenico et al. 2012). Altogether, HPL and AOS branches may regulate coordinately the full activation of the response to drought stress in the shoot and roots of the sensitive B59 line and the B71 shoot.
Among the six OPRs encoded in A. Thaliana, OPR3 is the only protein involved in JA biosynthesis (Wasternack and Hause 2013). In our study, the transcription level of OPR3 in B59 shoots subjected to water deficit correlated with an increase in JA in this organ. This finding is consistent with a study on wheat leaves showing a high transcription level of OPR3 after 0.5 h and 1 h of drought priming with 10% PEG 6000 (Wang et al. 2021).
In the context of the jasmonates (JAs) signaling pathway, Myelocytomatosis (MYC) transcription factors are the regulators involved in plant development and responses to various stresses, such as cold and drought (Song et al. 2022). Our results showed a correlation between the JA content and MYC2 transcription levels: both increase or decrease simultaneously. Specifically, an increase in JA in the B71 shoot was linked with higher MYC2 transcription levels, while a JA decrease in the roots of this line was associated with decreased MYC2 transcription levels. In addition, MYC2 participates in JA-mediated drought tolerance. In the B59 line, the increase in JA in water-stressed shoots was associated with higher MYC5 transcription levels. Previous reports have shown a redundancy function between MYC5 and MYC2/3/4 regarding the regulation of several JA responses (Song et al. 2022). Therefore, we suggest that MYC5 and MYC2 jointly mediate water-stress responses in sunflower seedlings.
Extensive research on WRKY family genes has demonstrated the involvement of these transcriptional factors in responding to drought stress (Wang et al. 2022). In our study, the upregulation of WRKY70 by water stress in the shoot and roots of B71 suggests its involvement in mediating water stress responses in sunflowers. In Myrothamnus flabellifolia, WRKY70 improved root growth and water retention, enhanced the antioxidant enzyme system, and maintained reactivated oxygen species (ROS) homeostasis and membrane-lipid stability; all of which increased drought tolerance (Xiang et al. 2021). In our study, the JAZ6 and TOPLESS (TPR1) upregulation in the B59 shoot could explain the lack of MYC2 transcription.
In our research, SA accumulation in the shoot seemed to stimulate stomatal closure in both lines, enhancing water stress tolerance as in Arabidopsis (Miura et al. 2013). The accumulation of SA in water-stressed roots of B71 suggests that this phytohormone participates in drought tolerance. As with other phytohormones. SA can also accumulate in roots upon abiotic stresses like drought (Khan et al. 2015). Studies involving the applications of different phytohormones have revealed that SA regulates root morphology, such as the development of lateral and adventitious roots, in a concentration-dependent manner (Bagautdinova et al. 2022).
Various abiotic stressors regulate primary enzymes involved in SA biosynthesis. In this study, SA showed increased levels in the shoot of both lines, which correlated with an over-transcription of genes related to this phytohormone, such as CM1 and PAL1. Moreover, Shao et al. (2023) have shown significant upregulation of many genes under drought, including CM. Thus, the increased SA content owing to the high activity of SA biosynthetic pathway enzymes helps plants cope with environmental stresses. Our study also revealed over-expression of both SA methyltransferase genes, SAMT1 and BSMT1, in the shoot and roots of B59, respectively. This upregulation suggests that SAMT1 plays a significant role in maintaining SA and methyl salicylate (MeSA) ratio in sunflowers under water stress. In addition, heat, alamethicin treatment, physical wounding and JAMe upregulated the SA catabolic gene BSMT1 (Kim et al. 2022). Conversely, in Camellia sinensis, the gene encoding BSMT1 displayed downregulated levels under cold or drought stress (Zheng et al. 2016).
Regarding the regulation of signal transduction, both lines showed downregulation of WRKY4, which is consistent with the findings of Wang et al. (2022). These researchers reported that treatment with 40% PEG6000 led to the downregulation of LoWRKY4 in the roots, stems and leaves of Larix olgensis. This finding indicates the involvement of LoWRKY4 in regulating the response to drought stress. In addition, the transcription levels of the WRKY70 gene increased in both the shoot and roots of B71. A dehydration-induced gene encoding a WRKY TF, MfWRKY70, from the resurrection plant M. flabellifolia, was involved in improved tolerance to various stress factors, including drought and salinity (Xiang et al. 2021). MfWRKY70 responded by inducing root growth, which favors the retention of water. This protein also enhanced the antioxidant enzyme system and kept the homeostasis of reactive oxygen species (ROS) and the stability of the membrane upon different stresses.
Gibberellin plays a fundamental role in regulating critical processes during plant development and participates in responses to abiotic stress, including drought (Iqbal et al. 2022). According to our results, water deficit led to lower levels of bioactive GA3 in the shoots of both lines, and this response could be related to the reduction in the stomatal conductance, as previously described in the B59 and B71 lines (Andrade et al. 2021). Research has reported contrasting effects (promotion or suppression) of GAs on root growth, depending on the type of root and species (Fonouni-Farde et al. 2019). In this study, the low GA3 content in water-stressed roots of B71 seems to be associated with promoting tolerance to this stress.
The GA20ox subfamilies of the GA oxidases (GAoxs) are essential in the biosynthesis of GAs (Zhang et al. 2022). Our results showed an upregulation of GA20ox 1 and 2 in the roots of B59 and in both organs of B71, but this upregulation did not lead to a rise in the bioactive GA content. However, upon water deficit, Solanum lycopersicum (the tomato) showed an inhibited expression of GA20ox1 and GA20ox2 in guard cells and leaf tissue (Shohat et al. 2021). In maize, Liu et al. (2024) reported the repression of ZmGA20ox3 expression under drought stress. Therefore, this gene can regulate the expression of other drought response genes by controlling endogenous GA content, which may negatively regulate maize drought responses. This finding is consistent with our results, as we observed a decrease in endogenous GA1 content alongside a downregulation of GA20ox3 in B59 roots.
Regarding the regulation of signal transduction, GA loss is a common phenomenon during drought stress response to allow growth regulation. The binding of DELLA to the GA receptor GID1 leads to its degradation and the activation of GA (Tyler et al. 2004). Tyler et al. described a downregulation of GID1B-like in two chickpeas (Cicer arietinum L.) cultivars viz., a drought-sensitive (ICC283; Desi type) cultivar and a drought-tolerant (ICC8261; Kabuli type) cultivar, under this stress. The findings of that study suggest that GID1 repression leads to lower GA responses, which would attenuate root growth under drought stress. In opposition to these findings, according to our results, GID1B was upregulated in the B71 shoot and the roots of both lines when exposed to water deficit conditions.
The Gibberellic acid-stimulated Arabidopsis (GASA) gene family takes part in regulating plant growth upon abiotic and biotic stresses (Li et al. 2022). Research has shown that different abiotic stresses, such as cold, heat, drought, osmotic, salt, and wounding can either induce or inhibit the expression of GASAs, as revealed by transcriptomic or qRT-PCR analyses (Qiao et al. 2021). In this study, we detected overexpression of the GASA gene in the shoot of B71 and an upregulation of GASA10 in the same organ. In addition, GASA genes participate in drought stress responses in Populus euphratica leaves. For instance, upon this stress, P. euphratica leaves showed induced (PeuGASA1, PeuGASA2, PeuGASA4, PeuGASA15, and PeuGASA19) or inhibited (e.g. PeuGASA10) expression of different GASA genes (Han et al. 2021).
On the other hand, in a study on two different genotypes of safflowers subjected to water stress, the transcription factor MYB62 was downregulated in the drought-tolerant accession PI401477 but not in the drought-intolerant accession PI560169 (Wei et al. 2020). In the present study, however, only the roots of the B71 tolerant line showed an upregulation of this gene.
In our study, the ABA content significantly increased in the B59 shoot, which would be linked to a reduction in stomatal conductance under water deficit, as previously registered for this line by Andrade et al. (2021). Similarly, the ABA content significantly increased in various species, such as Arabidopsis, wheat, rice, and tomatoes, among others, when exposed to drought (Muhammad Aslam et al. 2022). In addition, in the water-stressed shoot of B59, we did not detect a reciprocal relation between the most increased free ABA content and the decreased ABA-GE content. This finding suggests that, at least in this line, the hydrolysis of ABA-GE is not involved in contributing to the free ABA pool when subjected to water deficit. In accordance with the research by Fang and Xiong (2015), in our study, ABA accumulation in the water-stressed roots of B59 may be involved in root growth modification. In this sense, under drought stress, ABA induces primary root elongation, enabling the roots to grow deeper into the soil in search of water (Hauser et al. 2017).
Plants can respond to different abiotic stresses, such as drought, by overexpressing genes related to ABA biosynthesis. Specifically, the upregulation expression of NCED genes is associated with an accumulation of ABA (He et al. 2018). The NCED1 gene can be up- or downregulated by water stress, depending on the species (Ye et al. 2011). For instance, different reports have demonstrated an induced expression of NCED1 under water deficit in tobacco (Pedrosa et al. 2017), tomato (Muñoz-Espinoza et al. 2015), or Arabidopsis plants (Tong et al. 2017). In rice, conversely, the transcription of NCED1 progressively decreased with increasing water deficit (Changan et al. 2018). In our study, only the B71 roots showed overtranscription of the NCED1 gene. However, this transcription profile did not correlate with increased endogenous ABA content. In line with our results, the accumulation of ABA was negatively correlated with NCED1 transcription in both roots and leaves of a drought-tolerant rice cultivar (N22) (Changan et al. 2018). A short-chain alcohol dehydrogenase/reductase (SDR) catalyzes the reaction that leads to the synthesis of the aldehyde ABA, the last step involved in the synthesis of ABA (Bajguz and Piotrowska-Niczyporuk 2023). In our study, an overexpression of the SDR4 isoform was detectable only in the shoot of B59. This effect could be due to the remarkable increase in ABA endogenous content in response to water stress.
During drought stress, ABA biosynthesis can be accompanied by an increase in the transcript level of genes encoding enzymes involved in its catabolism. Subsequently, the conversion of ABA to inactive forms can occur through the conjugation with ABA-glucose esters (ABA-GEs), a process catalyzed by ABA UDP-glucosyltransferase (UGT) (Ma et al. 2018). In our study, the increased expression of UGT1 in the shoot and roots of B59 only correlated with the increase of ABA-GE in the shoot of this line. A recent study in barley has reported that drought led to high levels of the UGT1 transcript in leaves; this finding suggests accumulated levels of a conjugated form of ABA (Nykiel et al. 2022).
Regarding the regulation of signal transduction, overexpressed levels of XERICO (XER), a stress-responsive RING E3 ligase, increase ABA content and enhance drought tolerance in maize (Brugiére et al. 2017). In other species, such as Arabidopsis, Oryza sativa, Zea mays or P. trichocarpa, which show hypersensitivity to ABA, XER overexpression also enhanced tolerance to drought stress through stomatal closure and lower transpiration (Vonapartis et al. 2022).
In agreement with our results in which both B59 and B71 seedlings expressed XER, Vonapartis et al. (2022) have recently reported the expression of XER in young Arabidopsis seedlings. Moreover, the shoot and roots of the B59 line displayed overtranscription of this gene, which coincided with ABA accumulation in both organs.
On the other hand, the NAC family transcription factors ANAC019, ANAC029, and ANAC055 regulate ABA response in Arabidopsis. Specifically, ANAC019 and ANAC055 regulate drought tolerance through increased transcription of a group of stress-inducible genes (Wu et al. 2009). ANAC029 is involved in senescence and responds to stress factors. For instance, transgenic Arabidopsis plants overexpressing TaNAC29 tolerated better salt stress and drought. Furthermore, these transgenic plants were hypersensitive to ABA, which could be due to decreased transpiration and faster stomatal closure (Huang et al. 2015). Our results showed that the expression of ANAC genes depended on the line and tissue. For example, whereas ANAC029 was upregulated in the roots of both lines, ANAC019 and ANAC055 were upregulated in the shoot and roots of B71 and B71 roots, respectively.
Among the ABA-responsive genes, the NAC transcription factor RD26 regulates the developmental plasticity of the root, e.g., root hair and lateral root growth under drought stress, by controlling the expression of specific genes (Kamranfar et al. 2021). In our study, water deficit resulted in overtranscription of the RD26 gene in the roots of both lines. On the other hand, the RD22 (responsive to desiccation 22) gene codes for a protein that participates in crosstalk with ABA to respond to abiotic stress responses, such as drought stress (Thakro et al. 2023). In this regard, the transcription of two RD22-like genes (RD22A and RD22B) increased upon drought in maize leaves (Phillips and Ludidi 2017). Similarly, our results showed an upregulation of the RD22 gene in the shoot of the B59 and B71 lines, which suggests the involvement of this gene to alleviate water stress in sunflower seedlings.
Auxin is a well-known phytohormone implicated in various plant growth processes and responses to stress factors (Brackmann et al. 2018). Recently, researchers have suggested a connection between auxin and tolerance to water deficit through regulation of ABA-responsive gene expression, root architecture and ROS metabolism (Schopfer et al. 2002). In this study, IAA accumulation may be associated with the sharp increase in ABA in the water-stressed shoot of the B59 line. This finding suggests that changes in IAA levels could affect ABA synthesis. Thus, both phytohormones would play a leading role in water-stress responses, as previously suggested (Du et al. 2013).
The TAA family genes are crucial for auxin biosynthesis. In A. thaliana, this family consists of three closely related genes (TAA1, TAR1 and TAR2) (Shao et al. 2017). In our study, the upregulation of TAR2 may be associated with the increase in IAA in the shoot of the B59 line. By contrast, Blakeslee et al. (2019) have reported a strong downregulation of TAR2 transcription in leaves following drought stress in Arabidopsis.
An important regulatory mechanism for maintaining IAA homeostasis involves the conjugation of IAA to amino acids, a process controlled by members of group II within the GRETCHEN HAGEN 3 (GH3) gene family, such as GH3.5 and GH3.6 (Staswick et al. 2005). Also, group II GH3 genes are involved in salinity and water deficit tolerance (Casanova-Sáez et al. 2022). In the present study, both lines showed an upregulation of GH3.5 and GH3.6 genes. Specifically, GH3.5 seems to enhance tolerance to salt and drought stresses in cotton plants (Kirungu et al. 2019), while GH3.6 negatively regulates water deficit in apples (He et al. 2022). In this sense, transgenic apple plants knocking down MdGH3.6 showed higher adventitious root numbers, longer roots, increased water use efficiency, and increased cuticular wax under water deficit (Jiang et al. 2022).
Regarding signal transduction regulation, the HB40 gene, which encodes a member of the d sub-class of HD-Zip I genes associated with drought stress, has been isolated from 12-day-old Arabidopsis roots (Henriksson et al. 2005). In this study, we detected an overexpression of the AtHB40 gene in the B59 shoot, which is probably linked to the sensitivity of this line to water stress.
Aux/IAAs and ARFs play critical roles in the IAA signaling transduction pathway. Prevailing research on the function, gene expression, and regulation of ARF genes has mainly been on rice, Arabidopsis, tomatoes, among others (Luo et al. 2018). In our study, the expression of IAA16 increased in the shoot of B71, while IAA8 and ARF9 increased in the shoot of the B71 tolerant line and the B59 line, respectively. Similarly, in white clover, PEG-induced drought stress led to the upregulation of the expression of IAA8 (among others), which promoted the development of more lateral roots to access more water (Zhang et al. 2020). Moreover, IAA8 participates in lateral root formation in Arabidopsis (De Ollas et al. 2015). Similarly, in Mesona chinensis Benth, ARF9 expression increased under drought stress conditions induced by different PEG concentrations (Tang et al. 2022). However, although the kernel had higher levels of IAA8 in response to drought stress, the levels of two IAA16 were downregulated (Wang et al. 2019).
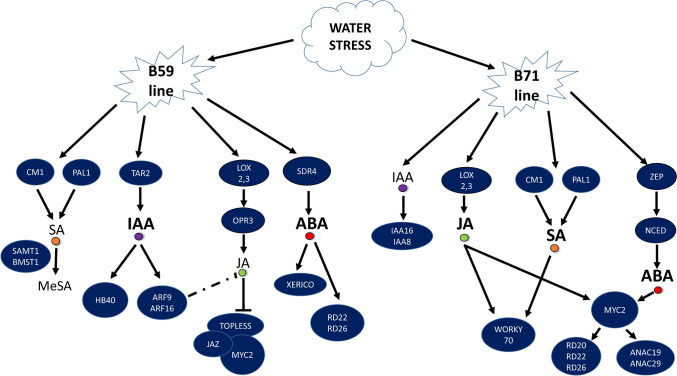
As proposed in the model shown in Fig. 8, each line responded to water stress by activating hormonal and transcriptional pathways, although at different magnitudes. In response to water stress, the B59 line triggered the activation of the hormonal pathways SA, JA, ABA and IAA, although ABA and IAA were the predominant activated pathways. The SA pathway starts with the transcription of the CM1 and PAL1 genes that trigger the biosynthesis of this phytohormone. Subsequently, through the conversion of SA to ME-SA, SAMT1 and BSMT1 would methylate SA. On the other hand, the TAR2 gene plays a crucial role in IAA biosynthesis, while the transcription factors ARF9, ARF10 and HB40 are critical in the signal transduction pathway of this phytohormone. The expression of the genes LOXs and OPR3 would regulate JA biosynthesis. We proposed that within this pathway ARF9 and ARF16 repress the biosynthesis of JA. Downstream, the JAZ negative regulator and its co-repressor TOPLESS would block MYC2 expression. Finally, ABA increased its biosynthesis mainly after the expression of the SDR4 gene. In addition, XERICO was one of the most highly expressed genes in the signal transduction pathway of ABA. The RD22 and RD26 genes were expressed as genes of response to abiotic stress and were related to the ABA hormonal pathway.
Fig. 8.
Model summarizing the relationship between the phytohormone contents and the transcription patterns of various genes and transcription factors related to hormonal metabolism and signaling in B59 and B71 sunflower inbred lines in response to water stress. The arrows represent positive regulation (accumulation of transcripts or hormones) and the blocked arrows represent negative regulation. The dashed lines indicate potential interactions. In each line, the hormones involved in the response to water stress are highlighted in bold and larger font. For abbreviations refer to the text
The B71 line induced the IAA, JA, SA and ABA hormonal pathways when exposed to water stress, although these last three were the main activated pathways. The signal transduction pathway of IAA was mainly activated through the genes IAA16 and IAA8. JA biosynthesis became activated in response to the expression of the LOXs biosynthetic genes. This phytohormone induced the expression of the MYC2 master regulator, which was also activated by ABA. Like in B59, in B71, the SA biosynthesis started with the expression of CM1 and PAL1. The SA signal transduction pathway involved the expression of WORKY70, which was also activated by JA. The biosynthesis of ABA was triggered by the expression of the genes ZEP and NCED. Then, the expression of the MYC2 transcription factor, a regulator of the ABA and JA pathways, was induced for downstream ABA-responsive gene expression. In turn, MYC2 activated the expression of RD20, 22 and 26 ABA-responsive genes and ANAC19 and 29 transcription factors belonging to the ABA signal transduction pathway.
Conclusions
When subjected to water stress, B59 showed activation of ABA and IAA hormonal pathways, which, in turn, induced TFs such as XERICO and ARF9 and ARF16, respectively. On the other hand, the B71 line simultaneously triggered the JA, SA and ABA hormonal pathways in response to water stress. The ABA and JA hormonal pathways activated, through MYC2, different TFs such as RD20, RD22, RD26, ANAC19 and ANAC29. Additionally, both the JA and SA hormonal pathways activated theWRKY70 transcription factor.
These findings highlight the importance of studying the interaction of the hormones quantified in this study with other phytohormones, such as BRs, to deepen the understanding of drought tolerance in sunflowers. Further research is still necessary to understand how BRs regulate responses to water stress in sunflower seedlings at an early growth stage as V2, specifically, research on the active synthesis routes of BRs and the main responsive genes that regulate these responses.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure S1. Validation of microarrays of parental lines B71 and B59 under conditions of water stress. Log2 (FC) of the ten selected genes. Elongation factor (Ha.EF1α) and α-TUB were used as reference genes (JPG 787 KB)
Supplementary Table S1. Description of unique differentially expressed genes (DEGs) in Shoots of B59 line in water stress vs. control contrast (XLSX 95 KB)
Supplementary Table S2 Description of unique differentially expressed genes (DEGs) in Roots (R) of B59 line in water stress vs. control contrast(XLSX 34 KB)
Supplementary Table S3 Description of unique differentially expressed genes (DEGs) in Shoots of B71 line in water stress vs. control contrast (XLSX 51 KB)
Supplementary Table S4 Description of unique differentially expressed genes (DEGs) in Roots (R) of B71 line in water stress vs. control contrast (XLSX 57 KB)
Supplementary Table S5 List of primer sequences for validation of the gene expression by qRT-PCR (DOCX 14 KB)
Acknowledgements
We thank Dr. Julia Sabio y Garcia for critical reading of this manuscript.
Author contributions
S.A. contributed to the study conception and design. A.A and M.E prepared the material and analyzed the data. F.R conducted the statistical analyses. A.A and A.V. wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto (SECYT-UNRC) (Grant C538). S.A. has received research support from UNRC.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andrade A, Vigliocco A, Alemano S, Llanes A, Abdala G (2013) Comparative morpho-biochemical responses of sunflower lines sensitive and tolerant to water stress. Am J Plant Sci 4:156–167. 10.4236/ajps.2013.4.12A3018 10.4236/ajps.2013.4.12A3018 [DOI] [Google Scholar]
- Andrade A, Escalante M, Vigliocco A, Tordable MC, Alemano S (2017) Involvement of jasmonates in responses of sunflower (Helianthus annuus) seedlings to moderate water stress. Plant Growth Regul 83(3):501–511. 10.1007/s10725-017-0317-9 10.1007/s10725-017-0317-9 [DOI] [Google Scholar]
- Andrade A, Boero A, Escalante M, Llanes A, Arbona V, Gómez-Cádenas A, Alemano S (2021) Comparative hormonal and metabolic profile analysis based on mass spectrometry provides information on the regulation of water-deficit stress response of sunflower (Helianthus annuus L.) inbred lines with different water-deficit stress sensitivity. Plant Phys Biochem 168:432–446. 10.1016/j.plaphy.2021.10.015 10.1016/j.plaphy.2021.10.015 [DOI] [PubMed] [Google Scholar]
- Awan SA, Khan I, Rizwan M, Zhang X, Brestic M, Khan A, El-Sheikh MA, Alyemeni MN, Ali S, Huang L (2020) Exogenous abscisic acid and jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol Plant 172(2):809–819. 10.1111/ppl.13247 10.1111/ppl.13247 [DOI] [PubMed] [Google Scholar]
- Bagautdinova ZZ, Omelyanchuk N, Tyapkin AV, Kovrizhnykh VV, Lavrekha VV, Zemlyanskaya EV (2022) Salicylic acid in root growth and development. Int J Mol Sci 23(4):2228. 10.3390/ijms23042228 10.3390/ijms23042228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajguz A, Piotrowska-Niczyporuk A (2023) Biosynthetic pathways of hormones in plants. Metabolites 13(8):884. 10.3390/metabo13080884 10.3390/metabo13080884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basit F, Liu J, An J, Chen M, He C, Zhu X, Li Z, Hu J, Guan Y (2021) Brassinosteroids as a multidimensional regulator of plant physiological and molecular responses under various environmental stresses. Environ Sci Pollut Res 28:44768–44779. 10.1007/s11356-021-15087-8 10.1007/s11356-021-15087-8 [DOI] [PubMed] [Google Scholar]
- Basit F, Tao J, An J, Song X, Sheteiwy MS, Holford P, Hu J, Jośko I, Guan Y (2023a) Nitric oxide and brassinosteroids enhance chromium stress tolerance in Glycine max L (Merr) by modulating antioxidative defense and glyoxalase systems. Environ Sci Pollut Res Int 30(18):51638–51653. 10.1007/s11356-023-25901-0 10.1007/s11356-023-25901-0 [DOI] [PubMed] [Google Scholar]
- Basit F, Abbas S, Zhu M, Tanwir K, El-Keblawy A, Sheteiwy MS, Raza A, Hu J, Hu W, Guan Y (2023b) Ascorbic acid and selenium nanoparticles synergistically interplay in chromium stress mitigation in rice seedlings by regulating oxidative stress indicators and antioxidant defense mechanism. Environ Sci Pollut Res Int 30(57):120044–120062. 10.1007/s11356-023-30625-2 10.1007/s11356-023-30625-2 [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, Spatola Rossi T, Kriechbaumer V (2019) Auxin biosynthesis: spatial regulation and adaptation to stress. J Exp Bot 70(19):5041–5049. 10.1093/jxb/erz283 10.1093/jxb/erz283 [DOI] [PubMed] [Google Scholar]
- Bolsa de Comercio de Rosario (2023) Informativo semanal de la Bolsa de Comercio de Rosario. Balance regional de Girasol en Argentina. ISSN. 2796-7824
- Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193 10.1093/bioinformatics/19.2.185 [DOI] [PubMed] [Google Scholar]
- Brackmann K, Qi J, Gebert M, Jouannet V, Schlamp T, Grünwald K, Wallner ES, Novikova DD, Levitsky VG, Agustí J, Sanchez P, Lohmann JU, Greb T (2018) Spatial specificity of auxin responses coordinates wood formation. Nat Commun 9(1):875. 10.1038/s41467-018-03256-2 10.1038/s41467-018-03256-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugière N, Zhang W, Xu Q, Scolaro EJ, Lu C, Kahsay RY, Kise R, Trecker L, Williams RW, Hakimi S, Niu X, Lafitte R, Habben JE (2017) Overexpression of RING domain E3 ligase ZmXerico1 confers drought tolerance through regulation of ABA homeostasis. Plant Physiol 175(3):1350–1369. 10.1104/pp.17.01072 10.1104/pp.17.01072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldelari D, Wang G, Farmer EE, Dong X (2011) Arabidopsis lox3 lox4 double mutants are male sterile and defective in global proliferative arrest. Plant Mol Biol 75:25–33. 10.1007/s11103-010-9701-9 10.1007/s11103-010-9701-9 [DOI] [PubMed] [Google Scholar]
- Canales FJ, Montilla-Bascón G, Rispail N, Arbona V, Prats E (2021) OPDA (12-oxo phytodienoic acid) modulate root growth in oats as a drought tolerance response.
- Casanova-Sáez R, Mateo-Bonmatí E, Šimura J, Pěnčík A, Novák O, Staswick P, Ljung K (2022) Inactivation of the entire Arabidopsis group II GH3s confers tolerance to salinity and water deficit. New Phytol 235(1):263–275. 10.1111/nph.18114 10.1111/nph.18114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda A, Alvarez JM (2024) Insights into molecular links and transcription networks integrating drought stress and nitrogen signaling. New Phytol 241(2):560–566. 10.1111/nph.19403 10.1111/nph.19403 [DOI] [PubMed] [Google Scholar]
- Changan SS, Ali K, Kumar V, Garg NK, Tyagi A (2018) Abscisic acid biosynthesis under water stress: anomalous behavior of the 9-cis-epoxycarotenoid dioxygenase1 (NCED1) gene in rice. Biol Plant 62:663–670. 10.1007/s10535-018-0807-2 10.1007/s10535-018-0807-2 [DOI] [Google Scholar]
- Chen Z, Liu Y, Yin Y, Liu Q, Li N, Li X, He W, Hao D, Liu X, Guo C (2019) Expression of AtGA2ox1 enhances drought tolerance in maize. Plant Growth Regul 89:203–215. 10.1007/s10725-019-00526-x 10.1007/s10725-019-00526-x [DOI] [Google Scholar]
- De Domenico S, Bonsegna S, Horres R, Pastor V, Taurino M, Poltronieri P, Imtiaz M, Kahl G, Flors V, Winter P, Santino A (2012) Transcriptomic analysis of oxylipin biosynthesis genes and chemical profiling reveal an early induction of jasmonates in chickpea roots under drought stress. Plant Physiol Biochem 61:115–122. 10.1016/j.plaphy.2012.09.009 10.1016/j.plaphy.2012.09.009 [DOI] [PubMed] [Google Scholar]
- De Ollas C, Arbona V, Gómez-Cadenas A (2015) Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant Cell Environ 38:2157–2170. 10.1111/pce.12536 10.1111/pce.12536 [DOI] [PubMed] [Google Scholar]
- Du H, Wu N, Chang Y, Li X, Xiao J, Xiong L (2013) Carotenoid deficiency impairs ABA and IAA biosynthesis and differentially affects drought and cold tolerance in rice. Plant Mol Biol 83:475–488. 10.1007/s11103-013-0103-7 10.1007/s11103-013-0103-7 [DOI] [PubMed] [Google Scholar]
- Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M (2014) InfoStat versión 2014. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina.
- El-Sawah AM, Abdel-Fattah GG, Holford P, Korany SM, Alsherif EA, AbdElgawad H, Ulhassan Z, Jośko I, Ali B, Sheteiwy MS (2023) Funneliformis constrictum modulates polyamine metabolism to enhance tolerance of Zea mays L to salinity. Microbiol Res 266:127254. 10.1016/j.micres.2022.127254 10.1016/j.micres.2022.127254 [DOI] [PubMed] [Google Scholar]
- Escalante M, Vigliocco A, Moschen S, Fernández P, Heinz R, Garcia-Garcia F, Di Rienzo JA, Andrade A, Alemano S (2020) Transcriptomic analysis reveals a differential gene expression profile between two sunflower inbred lines with different ability to tolerate water stress. Plant Mol Biol Rep 38:222–237. 10.1007/s11105-020-01192-4 10.1007/s11105-020-01192-4 [DOI] [Google Scholar]
- Fang Y, Xiong L (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72:673–689. 10.1007/s00018-014-1767-0 10.1007/s00018-014-1767-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonouni-Farde C, Miassod A, Laffont C, Morin H, Bendahmane A, Diet A, Frugier F (2019) Gibberellins negatively regulate the development of Medicago truncatula root system. Sci Rep 9(1):2335. 10.1038/s41598-019-38876-1 10.1038/s41598-019-38876-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forleo MB, Suardi PN, A, Coaloa D, Pari L, (2018) The eco-efficiency of rapeseed and sunflower cultivation in Italy. Joining environmental and economic assessment. J Clean Prod 172:3138–3153. 10.1016/j.jclepro.2017.11.094 10.1016/j.jclepro.2017.11.094 [DOI] [Google Scholar]
- Grebner W, Stingl NE, Oenel A, Mueller MJ, Berger S (2013) Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiol 161(4):2159–2170. 10.1104/pp.113.214544 10.1104/pp.113.214544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamayun M, Hussain A, Iqbal A, Khan SA, Lee IJ (2018) Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. BioMed Res Internat. 10.1155/2018/7696831 10.1155/2018/7696831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Jiao Z, Niu MX, Yu X, Huang M, Liu C, Wang HL, Zhou Y, Mao W, Wang X, Yin W, Xia X (2021) Genome-Wide comprehensive analysis of the GASA gene family in populus. Int J Mol Sci 22:12336. 10.3390/ijms222212336 10.3390/ijms222212336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser F, Li Z, Waadt R, Schroeder JI (2017) SnapShot: abscisic acid signaling. Cell 171(7):1708–1708. 10.1016/j.cell.2017.11.045 10.1016/j.cell.2017.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Zhuang Y, Cai Y, Agüero CB, Liu S, Wu J, Deng S, Walker MA, Lu J, Zhang Y (2018) Overexpression of 9-cis-epoxycarotenoid dioxygenase cis gene in grapevine increases drought tolerance and results in pleiotropic effects. Front Plant Sci 3:9–970. 10.3389/fpls.2018.00970 10.3389/fpls.2018.00970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wang C, Jiao R, Ni Q, Wang Y, Gao Q, Zhang Y, Xu G (2022) Biochemical characterization of a novel glucose-tolerant GH3 β-glucosidase (Bgl1973) from Leifsonia sp ZF2019. Appl Microbiol Biot 106(13–16):5063–5079. 10.1007/s00253-022-12064-0 10.1007/s00253-022-12064-0 [DOI] [PubMed] [Google Scholar]
- Henriksson E, Olsson AS, Johannesson H, Johansson H, Hanson J, Engström P, Söderman E (2005) Homeodomain leucine zipper class I genes in Arabidopsis Expression patterns and phylogenetic relationships. Plant Physiol 139(1):509–518. 10.1104/pp.105.063461 10.1104/pp.105.063461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrmova M, Hussain SS (2021) Plant Transcription Factors Involved in Drought and Associated Stresses. Int J Mol Sci 22:5662. 10.3390/ijms22115662 10.3390/ijms22115662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Chen MH, Yang LT, Li YR, Wu JM (2015) Effects of exogenous abscisic acid on cell membrane and endogenous hormone contents in leaves of sugarcane seedlings under cold stress. Sugar Tech 17:59–64. 10.1007/s12355-014-0343-0 10.1007/s12355-014-0343-0 [DOI] [Google Scholar]
- Hussain HA, Men S, Hussain S, Chen Y, Ali S, Zhang S, Zhang K, Li Y, Xu Q, Liao C, Wang L (2019) Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci Rep 9(1):3890. 10.1038/s41598-019-40362-7 10.1038/s41598-019-40362-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S, Wang X, Mubeen I, Kamran M, Kanwal I, Díaz GA, Abbas A, Parveen A, Atiq MN, Alshaya H, Zin El-Abedin TK, Fahad S (2022) Phytohormones Trigger Drought Tolerance in Crop Plants: Outlook and Future Perspectives. Front Plant Sci 12:799318. 10.3389/fpls.2021.799318 10.3389/fpls.2021.799318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Shen W, Liu C, Tahir MM, Li X, Zhou S, Ma F, Guan Q (2022) Engineering Drought-Tolerant Apple by Knocking down Six GH3 Genes and Potential Application of Transgenic Apple as a Rootstock. Hortic Res. 9:122. 10.1093/hr/uhac122 10.1093/hr/uhac122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Zhang H, Kong L, Gao G, Luo J (2014) PlantTFDB 3 0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res 42(D1):D1182–D1187. 10.1093/nar/gkt1016 10.1093/nar/gkt1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamranfar I, Balazadeh S, Mueller-Roeber B (2021) NAC transcription factor RD26 is a regulator of root hair morphogenic plasticity. BioRxiv. 10.1101/2021.04.21.440803 10.1101/2021.04.21.440803 [DOI] [Google Scholar]
- Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:462. 10.3389/fpls.2015.00462 10.3389/fpls.2015.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Jeon SJ, Yanders S, Park SC, Kim HS, Kim S (2022) MYB3 plays an important role in lignin and anthocyanin biosynthesis under salt stress condition in Arabidopsis. Plant Cell Rep 41(7):1549–1560. 10.1007/s00299-022-02878-7 10.1007/s00299-022-02878-7 [DOI] [PubMed] [Google Scholar]
- Kirungu JN, Magwanga RO, Lu P, Cai X, Zhou Z, Wang X, Peng R, Wang K, Liu F (2019) Functional characterization of GhA08G1120 (GH3.5) gene reveal their significant role in enhancing drought and salt stress tolerance in cotton. BMC Genet 20(1):62. 10.1186/s12863-019-0756-6 10.1186/s12863-019-0756-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Gao J, Wang G, Wang S, Chen K, Pu W, Wang Y, Xia Q, Fan X (2022) Genome-wide identification and characterization of GASA gene family in Nicotiana tabacum. Front Genet 12:768942. 10.3389/fgene.2021.768942 10.3389/fgene.2021.768942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Zhang Y, Yu Q, Fang W, Chen M, Li T, Liu Y, Liu Z, Chen L, Yu S, Xia H, Xue HW, Yu H, Luo L (2023) Coordination of growth and drought responses by GA-ABA signaling in rice. New Phytol 240(3):1149–1161. 10.1111/nph.19209 10.1111/nph.19209 [DOI] [PubMed] [Google Scholar]
- Liu X, Liu Y, Chen Z, Zhang C, Guo J, Liu Q, Yin Y, Hu Y, Xia H, Li B, Sun X, Li Y (2024) Gene Editing of ZmGA20ox3 improves plant architecture and drought tolerance in maize. Plant Cell Rep 43:18. 10.1007/s00299-023-03090-x 10.1007/s00299-023-03090-x [DOI] [PubMed] [Google Scholar]
- Luo J, Zhou JJ, Zhang JZ (2018) Aux/IAA gene family in plants: molecular structure, regulation, and function. Int J Mol Sci 19(1):259. 10.3390/ijms19010259 10.3390/ijms19010259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Cao J, Jiahan H, Qiaoqiao C, Xufeng L, Yi Y (2018) Molecular mechanism for the regulation of ABA homeostasis during plant development and stress responses. Int J Mol Sci 19:3643. 10.3390/ijms19113643 10.3390/ijms19113643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manghwar H, Hussain A, Ali Q, Liu F (2022) Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int J Mol Sci 23(3):1012. 10.3390/ijms23031012 10.3390/ijms23031012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miersch O, Neumerkel J, Dippe M, Stenzel I, Wasternack C (2008) Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol 177(1):114–127. 10.1111/j.1469-8137.2007.02252.x 10.1111/j.1469-8137.2007.02252.x [DOI] [PubMed] [Google Scholar]
- Miura K, Nozawa R (2014) Overexpression of SIZ1 enhances tolerance to cold and salt stresses and attenuates response to abscisic acid in arabidopsis thaliana. Plant Biotechnol 31(2):167–172. 10.5511/plantbiotechnology.14.0109a 10.5511/plantbiotechnology.14.0109a [DOI] [Google Scholar]
- Miura K, Okamoto H, Okuma E, Shiba H, Kamada H, Hasegawa PM, Murata Y (2013) SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J 73(1):91–104. 10.1111/tpj.12014 10.1111/tpj.12014 [DOI] [PubMed] [Google Scholar]
- Moschen S, Bengoa Luoni S, Paniego NB, Hopp HE, Dosio GAA, Fernández P, Heinz RA (2014) Identification of candidate genes associated with leaf senescence in cultivated sunflower (Helianthus annuus L.). PLoS ONE. 10.1371/journal.pone.0104379 10.1371/journal.pone.0104379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschen S, Higgins J, Di Rienzo JA, Heinz RA, Paniego N, Fernández P (2016) Network and biosignature analysis for the integration of transcriptomic and metabolomic data to characterize leaf senescence process in sunflower. BMC Bioinformatics 17:174. 10.1186/s12859-016-1045-2 10.1186/s12859-016-1045-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad Aslam M, Waseem M, Jakada BH, Okal EJ, Lei Z, Saqib HSA, Yuan W, Xu W, Zhang Q (2022) Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int J Mol Sci 23:1084. 10.3390/10.3390/ijms23031084 10.3390/10.3390/ijms23031084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Peñuelas J (2003) Photo-and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 217(5):758–766. 10.1007/s00425-003-1037-0 10.1007/s00425-003-1037-0 [DOI] [PubMed] [Google Scholar]
- Muñoz-Espinoza VA, López-Climent MF, Casaretto JA, Gómez-Cadenas A (2015) Water Stress Responses of Tomato Mutants Impaired in Hormone Biosynthesis Reveal Abscisic Acid, Jasmonic Acid and Salicylic Acid Interactions. Front Plant Sci 6:997. 10.3389/fpls.2015.00997 10.3389/fpls.2015.00997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykiel M, Gietler M, Fidler J, Graska J, Rybarczyk-Płońska A, Prabucka B, Muszyńska E, Bocianowski J, Labudda M (2022) Differential water deficit in leaves is a principal factor modifying barley response to drought stress. Int J Mol Sci 23(23):15240. 10.3390/ijms232315240 10.3390/ijms232315240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa AM, Cidade LC, Martins CPS, Macedo AF, Neves DM, Gomes FP, Floh EI, Costa MG (2017) Effect of overexpression of citrus 9-cis-epoxycarotenoid dioxygenase 3 (CsNCED3) on the physiological response to drought stress in transgenic tobacco. Genet Mol Res. 10.4238/gmr16019292 10.4238/gmr16019292 [DOI] [PubMed] [Google Scholar]
- Phillips K, Ludidi N (2017) Drought and exogenous abscisic acid alter hydrogen peroxide accumulation and differentially regulate the expression of two maize RD22-like genes. Sci Rep 7(1):8821. 10.1038/s41598-017-08976-x 10.1038/s41598-017-08976-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Yan W, He J, Liu X, Zhang Q, Wang X (2021) Identification, evolution and expression analyses of mapk gene family in Japanese flounder (Paralichthys olivaceus) provide insight into its divergent functions on biotic and abiotic stresses response. Aquat Toxicol 241:106005. 10.1016/j.aquatox.2021.106005 10.1016/j.aquatox.2021.106005 [DOI] [PubMed] [Google Scholar]
- R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http://www.R-project.org/
- Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386. 10.1385/1-59259-192-2:365 10.1385/1-59259-192-2:365 [DOI] [PubMed] [Google Scholar]
- Salvi P, Manna M, Kaur H, Thakur T, Gandass N, Bhatt D, Muthamilarasan M (2021) Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep 40:1305–1329. 10.1007/s00299-021-02683-8 10.1007/s00299-021-02683-8 [DOI] [PubMed] [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A (2002) Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214:821–828. 10.1007/s00425-001-0699-8 10.1007/s00425-001-0699-8 [DOI] [PubMed] [Google Scholar]
- Shao A, Ma W, Zhao X, Hu M, He X, Teng W, Li H, Tong Y (2017) The Auxin biosynthetic TRYPTOPHAN AMINOTRANSFERASE RELATED TaTAR2.1–3A increases grain yield of wheat. Plant Physiol 174(4):2274–2288. 10.1104/pp.17.00094 10.1104/pp.17.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao WB, Liao YM, Luo RS, Ji J, Xiao WL, Zhou X, Liu LW, Yang S (2023) Discovery of novel phenothiazine derivatives as new agrochemical alternatives for treating plant viral diseases. Pest Manag 79(11):4231–4243. 10.1002/ps.7623 10.1002/ps.7623 [DOI] [PubMed] [Google Scholar]
- Shi H, Chen L, Ye T, Liu X, Ding K, Chan Z (2014) Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol Biochem 82:209–217. 10.1016/j.plaphy.2014.06.008 10.1016/j.plaphy.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Shohat H, Cheriker H, Kilambi HV, Eliaz IN, Blum S, Amsellem Z, Tarkowská D, Aharoni A, Eshed Y, Weiss D (2021) Inhibition of gibberellin accumulation by water deficiency promotes fast and long-term ‘drought avoidance’ responses in tomato. New Phytol 232(5):1985–1998. 10.1111/nph.17709 10.1111/nph.17709 [DOI] [PubMed] [Google Scholar]
- Smyth GK (2005) Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W (eds) Bioinformatics and computational biology solutions using R and bioconductor. Springer, New York, pp 397–420 [Google Scholar]
- Song C, Cao Y, Dai J, Li G, Manzoor MA, Chen C, Deng H (2022) The multifaceted roles of MYC2 in plants: toward transcriptional reprogramming and stress tolerance by jasmonate signaling. Front Plant Sci 13:868874. 10.3389/fpls.2022.868874 10.3389/fpls.2022.868874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Lin Y, Wei F, Quan C, Wei K, Wei Y, Cai Z, Kashif MH, Miao J (2022) Characteristics and comparative analysis of Mesona chinensis Benth chloroplast genome reveals DNA barcode regions for species identification. Funct Integr Genom 22(4):467–479. 10.1007/s10142-022-00846-8 10.1007/s10142-022-00846-8 [DOI] [PubMed] [Google Scholar]
- Thakro V, Malik N, Basu U, Srivastava R, Narnoliya L, Daware A, Varshney N, Mohanty JK, Bajaj D, Dwivedi V, Tripathi S, Jha UC, Dixit GP, Singh AK, Tyagi AK, Upadhyaya HD, Parida SK (2023) A superior gene allele involved in abscisic acid signaling enhances drought tolerance and yield in chickpea. Plant Physiol 191(3):1884–1912. 10.1093/plphys/kiac550 10.1093/plphys/kiac550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong SM, Xi HX, Ai KJ, Hou HS (2017) Overexpression of wheat TaNCED gene in Arabidopsis enhances tolerance to drought stress and delays seed germination. Biol Plant 61:64–72. 10.1007/s10535-016-0692-5 10.1007/s10535-016-0692-5 [DOI] [Google Scholar]
- Tripathi P, Rabara RC, Rushton PJ (2014) A systems biology perspective on the role of WRKY transcription factors in drought responses in plants. Planta 239:255–266. 10.1007/s00425-013-1985-y 10.1007/s00425-013-1985-y [DOI] [PubMed] [Google Scholar]
- Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun TP (2004) DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol 135(2):1008–1019. 10.1104/pp.104.039578 10.1104/pp.104.039578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDRR (UNITED NATIONS OFFICE FOR DISASTER RISK REDUCTION) (2021) GAR Special Report on Drought 2021. https://www.undrr.org/publication/gar-special-report-drought-2021.
- USDA (2022) National Agricultural Statistics Service. USDA-NASS 1400 Independence Ave., SW Washington, DC 20250.
- Vellosillo T, Martínez M, López MA, Vicente J, Cascón T, Dolan L, Hamberg M, Castresana C (2007) Oxylipins produced by the 9-lipoxygenase pathway in arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell 19(3):831–846. 10.1105/tpc.106.046052 10.1105/tpc.106.046052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonapartis E, Mohamed D, Li J, Pan W, Wu J, Gazzarrini S (2022) CBF4/DREB1D represses XERICO to attenuate ABA, osmotic and drought stress responses in Arabidopsis. Plant J 110(4):961–977. 10.1111/tpj.15713 10.1111/tpj.15713 [DOI] [PubMed] [Google Scholar]
- Wang B, Liu C, Zhang D, He C, Zhang J, Li Z (2019) Effects of maize organ-specific drought stress response on yields from transcriptome analysis. BMC Plant Biol 19(1):1–19. 10.1186/s12870-019-1941-5 10.1186/s12870-019-1941-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li Q, Xie J, Huang M, Cai J, Zhou Q, Dai T, Jiang D (2021) Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop J 9(1):120–132. 10.1016/j.cj.2020.06.002 10.1016/j.cj.2020.06.002 [DOI] [PubMed] [Google Scholar]
- Wang C, Zhao Q, Zhang L, Zhang H (2022) Expression Pattern Analysis of Larch WRKY in Response to Abiotic Stress. Forests 13:2123. 10.3390/f13122123 10.3390/f13122123 [DOI] [Google Scholar]
- Wang B, Wang J, Yang T, Wang J, Dai Q, Zhang F, Xi R, Yu Q, Li N (2023) The transcriptional regulatory network of hormones and genes under salt stress in tomato plants (Solanum lycopersicum L). Front Plant Sci 14:1115593. 10.3389/fpls.2023.1115593 10.3389/fpls.2023.1115593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth, and development. An update to the, review in Annals of Botany. Ann Bot 111(6):1021–1058. 10.1093/aob/mct067 10.1093/aob/mct067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Hou K, Zhang H, Wang X, Wu W (2020) Integrating transcriptomics and metabolomics to studies key metabolism, pathways and candidate genes associated with drought-tolerance in Carthamus tinctorius L under drought stress. Ind Crops Prod 151:112465. 10.1016/j.indcrop.2020.112465 10.1016/j.indcrop.2020.112465 [DOI] [Google Scholar]
- Wu Y, Deng Z, Lai J, Zhang Y, Yang C, Yin B, Zhao Q, Zhang L, Li Y, Yang C, Xie Q (2009) Dual function of Arabidopsis ATAF1 in abiotic and biotic stress responses. Cell Res 19(11):1279–1290. 10.1038/cr.2009.108 10.1038/cr.2009.108 [DOI] [PubMed] [Google Scholar]
- Xiang XY, Chen J, Xu WX, Qiu JR, Song L, Wang JT, Tang R, Chen D, Jiang CZ, Huang Z (2021) Dehydration-induced WRKY transcriptional factor MfWRKY70 of Myrothamnus flabellifolia enhanced drought and salinity tolerance in Arabidopsis. Biomolecules 11(2):327. 10.3390/biom11020327 10.3390/biom11020327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Nolan TM, Jiang H, Yin Y (2019) AP2/ERF transcription factor regulatory networks in hormone and abiotic stress responses in arabidopsis. Front Plant Sci 10:228. 10.3389/fpls.2019.00228 10.3389/fpls.2019.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye N, Zhu G, Liu Y, Li Y, Zhang J (2011) ABA controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol 52(4):689–698. 10.1093/pcp/pcr028 10.1093/pcp/pcr028 [DOI] [PubMed] [Google Scholar]
- Yin L, Zander M, Huang SC, Xie M, Song L, Saldierna Guzmán JP, Hann E, Shanbhag BK, Ng S, Jain S, Janssen BJ, Clark NM, Walley JW, Beddoe T, Bar-Joseph Z, Lewsey MG, Ecker JR (2023) Transcription factor dynamics in cross-regulation of plant hormone signaling pathways. Bio Rxiv. 10.1101/2023.03.07.531630 10.1101/2023.03.07.531630 [DOI] [Google Scholar]
- Zhang Y, Lv Y, Hassan MJ, Li Z, Peng Y (2020) Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol 20(1):1–12. 10.1186/s12870-020-02354-y 10.1186/s12870-020-02354-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Nie X, Kong W, Deng X, Sun T, Liu X, Li Y (2022) Genome-Wide identification and evolution analysis of the gibberellin oxidase gene family in six gramineae crops. Genes (basel). 13(5):863. 10.3390/genes13050863 10.3390/genes13050863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Wang Y, Ding Z, Zhao L (2016) Global Transcriptional analysis reveals the complex relationship between Tea quality, leaf senescence and the responses to cold drought combined stress in Camellia sinensis. Front Plant Sci 7:1858. 10.3389/fpls.2016.01858 10.3389/fpls.2016.01858 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Validation of microarrays of parental lines B71 and B59 under conditions of water stress. Log2 (FC) of the ten selected genes. Elongation factor (Ha.EF1α) and α-TUB were used as reference genes (JPG 787 KB)
Supplementary Table S1. Description of unique differentially expressed genes (DEGs) in Shoots of B59 line in water stress vs. control contrast (XLSX 95 KB)
Supplementary Table S2 Description of unique differentially expressed genes (DEGs) in Roots (R) of B59 line in water stress vs. control contrast(XLSX 34 KB)
Supplementary Table S3 Description of unique differentially expressed genes (DEGs) in Shoots of B71 line in water stress vs. control contrast (XLSX 51 KB)
Supplementary Table S4 Description of unique differentially expressed genes (DEGs) in Roots (R) of B71 line in water stress vs. control contrast (XLSX 57 KB)
Supplementary Table S5 List of primer sequences for validation of the gene expression by qRT-PCR (DOCX 14 KB)