Abstract
Background
Out-of-hospital patients presenting with atypical chest pain and complete left bundle branch block (LBBB) have to be stratified for the presence of coronary artery disease and the risk of developing heart failure (HF). We investigated the prognostic role of coronary CT-angiography (CTA) and echocardiographic global longitudinal strain (GLS) in those patients in a mid-term follow-up.
Methods
Out-of-hospital patients with LBBB underwent echocardiography and a 64-slice CT angiography were evaluated retrospectively. Development of HF or a cardiovascular death were the events scheduled.
Results
Seventy-eight patients (32 female; mean age: 66.0 ± 10.4 years were enrolled. During a follow-up of 33 months (IQR: 17-77), one patient (1.5%) experienced a cardiovascular death, 14 patients (17.9%) required urgent outpatient visits due to acute decompensated HF (12 hospitalizations). Echocardiography showed a slightly reduced left ventricular ejection fraction (LVEF) (50.0% ± 9.8%) and GLS within the normal range (-16.2% ± 4.1%). CTA analysis showed coronary stenosis > 50% in 28 patients (35.9%). A high Agatston score (> 100) was observed in 29.5%. Notably, 25 patients (32.1%) were diagnosed with left main coronary artery disease and 15 patients (16.7%) underwent revascularization during the follow up. Significant associations were observed between events and LVEF (P = 0.001), diastolic dysfunction grade ≥ 2 (P = 0.02), GLS (P < 0.001), multiple coronary stenosis (P = 0.04) and Agatston score (P = 0.05). Multivariate analysis confirmed the relationships with LVEF (R2 = 0.89, P < 0.001), diastolic dysfunction (R2 = 3.30, P = 0.04), GLS (R2 = 1.43, P < 0.001), and Agatston score (R2 = 1.01, P = 0.05).
Conclusions
In patients with complete LBBB, CTA and GLS identified those at a high risk of development HF.
A middle-aged man was referred to the cardiology clinic for atypical thoracic discomfort and presented a complete left bundle branch block (LBBB) on a 12-lead ECG. The only cardiovascular risk factor collected was the father's history of myocardial infarction. The patient, an agonist tennis player, inquired about any contraindication to high-intensity exercise. He had no comorbidities and was not on any prescribed drugs. Echocardiography revealed a left ventricular ejection fraction (LVEF) of 50% with abnormal septal motion due to rebound stretching. The left ventricle's deformation was also examined with GLS, a sensitive marker of myocardial dysfunction, and a value of -19.6% was calculated. Additionally, coronary computed tomography angiography (CTA) excluded significant coronary artery disease. Over a 4-year follow-up, no cardiovascular events were observed. The article discusses the implications of LBBB on patient management and prognosis, emphasizing the need for enhanced risk stratification and an appropriate plan for ambulatory follow-up. It also highlights the association of LBBB with heart failure and the potential use of echocardiographic GLS for improved risk stratification in patients at risk for heart failure.
The first finding of LBBB in a medium-to-high-risk patient can be concerning, prompting us to question how we might identify those at risk of future cardiovascular death or heart failure development. LBBB is a cardiac conduction abnormality characterized by a delay or blockage of electrical impulses through the left bundle branch. While the identification of LBBB via surface electrocardiograms is relatively straightforward, understanding its implications for patient management and prognosis can be more complex, often requiring additional investigations.[1] LBBB is more likely to occur in patients with underlying heart disease (approximately 33%), but it can also present in a healthy population (with a prevalence of 0.06%).[2] The management of patients with suspected cardiac symptoms and incidental findings of LBBB outside of a hospital setting remains a contentious issue. There is a need to improve risk stratification and establish an appropriate plan for ambulatory follow-up. Given that previous studies have associated LBBB with the presence of coronary artery disease,[3,4] it is advisable to screen most patients with LBBB (for instance, with anatomical testing to rule out CAD).[5,6] However, official recommendations do not provide a precise description of the diagnostic workup for incidental LBBB with suspected cardiac symptoms.[7] Furthermore, the use of echocardiographic GLS has been explored for its potential prognostic role in this context. Evidence suggests that the analysis of longitudinal deformation with speckle-tracking echocardiography could detect subclinical dysfunction and identify the initial alteration of left ventricular myocardial contraction. This method is gaining recognition for its prognostic value.[8] Therefore, GLS might serve as a valuable clinical tool to enhance risk stratification in patients at risk for HF, according to the severity of the condition, and can assist in identifying different cardiomyopathy phenotypes.[9]
Left ventricular ejection fraction (LVEF) evaluated on echocardiography could be the first relevant data but could be difficult to assess due to septal-lateral wall dissynchrony. Patients with complete LBBB and moderately reduced LVEF proved to have a poor long-term outcome, suggesting that patients in this population would potentially benefit from a closer follow-up.[10] Unfortunately, clinical experience evaluating long term follow-up in LBBB patients are lacking.
After echocardiography, patients with LBBB should be referred for anatomical testing to rule out CAD (especially left main and proximal LAD disease). Stress testing in the presence of LBBB could be difficult because of the higher rate of false-positive results.[11] In contrast, CTA appears to be unaffected by image quality from the LBBB and could be considered as an alternative to invasive coronary angiography.[12,13]
In addition, complete LBBB patients claiming atypical chest pain have been demonstrated to be an intriguing clinical challenge considering that the presence of a complete LBBB masked the signs of ischemia at rest or during effort, and septal dissynchrony does not allow a correct diagnosis of CAD during stress-echo.
The primary objective of this analysis was then to assess the prognostic/diagnostic role of CTA and GLS in patients with complete LBBB experiencing atypical chest pain during a long-term follow-up.[14]
METHODS
We conducted a retrospective observational single-center study involving consecutive adult patients without known CAD who were diagnosed with complete LBBB. These patients attended outpatient visits for atypical chest pain (as per European guidelines)[15] from 2011 to 2022, and subsequently underwent transthoracic echocardiography (TTE) and CTA.
The endpoint was defined as an episode of acute decompensated Heart Failure (HF) or cardiovascular death. The exclusion criteria included age < 18 years, known history of CAD, known non-ischemic cardiomyopathy, severe valvular disease, heart transplantation, and pregnancy. Patients exhibiting signs of heart failure during their initial visit were also excluded. LBBB was characterized by wide QRS complexes with a duration > 120 ms, a dominant S wave in V1, a broad monophasic R wave in lateral leads (I, aVL, and V5-6), absence of Q waves in lateral leads, and a prolonged R wave peak time > 60 ms in leads V5-6.[15]
Patients were evaluated through subsequent outpatient visits and through electronic medical records. Transthoracic echocardiography (TTE) was conducted using General Electric VIVID E9 with an S5-1 1.5/3.6 MHz transducer. Three consecutive cardiac cycles of all apical views were digitally recorded for subsequent post-processing analysis to assess LVEF and GLS, in accordance with the recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging[15].
CTA was performed using IQon Spectral 64-slice CT Version with Philips Medical Systems. Patients were pretreated with up to 25 mg of intravenous metoprolol to achieve a target heart rate of < 65 beats/min and were given a 2.5 mg sublingual dose of isosorbide dinitrate to enhance coronary artery visualization. CTA images were obtained with an initial unenhanced scan for calcium scoring (using the Agatston method with prospective ECG triggering, 2.5-mm slice thickness, 120 kV tube voltage, and 200 mA tube current) to determine the amount of calcified plaques.[15]
Images were captured with retrospective ECG triggering at 35%-75% of the R-R cycle and intravenous administration of a nonionic contrast agent (Ultravist 370 mg/mL; Schering AG, Berlin, Germany) followed by 30 mL of saline injection using a two-phase injection protocol at a rate of 5 mL/s. Tube voltage and current were adjusted according to the body mass index (BMI). The coronary arteries were segmented, and each coronary segment was visually analyzed for the presence of stenosis (visually quantified as > 50% of the diameter of the coronary vessel).[15]
Statistical analysis was conducted using SPSS Statistics 26 (IBM, Armonk, NY, USA). Categorical variables are presented as frequency with percentages and were compared using Fisher’s exact test. Scale variables are presented as mean ± SD or median [interquartile range (IQR)] and were compared using the Mann–Whitney U test or Kruskal-Wallis test. Kaplan-Meier analysis and univariate and multivariate Cox models were performed. We considered results with a P ≤ 0.05 to be statistically significant. Sex, age, and the number of risk factors were treated as confounders. Follow-up events included the development of acute HF (requiring hospitalization and/or urgent cardiological consultation for HF symptoms) and cardiovascular mortality.
The local ethics committee approved our study protocol that complied with the Declaration of Helsinki. All patients signed an informed consent prior to performing the CTA examination.
RESULTS
We enrolled a total of 78 consecutive patients, with a mean age of 66.0 ± 10.4 years, 32 (41.0%) were female. Table 1 presents the risk factors, therapy, and anthropometric characteristics of patients. Hypertension was the most common risk factor (69% of cases) and the antihypertensive treatment has been described. During a mean follow-up of 33 months (IQR: 17-77), four patients (6.1%) died from any cause. Only one patient (1.5%) experienced cardiovascular death. Urgent outpatient visits due to acute decompensated HF were required in 14 (17.9%) patients, and 12 of these 14 patients (15.4%) were hospitalized (Table 2).
Table 1. The risk factors, therapy, and anthropometric characteristics of the patients.
| Total (n = 78) | Male (n = 46) | Female (n = 32) | |
| Data were expressed as n (%) or mean ± SD. *P < 0.05. ACE: angiotensin-converting enzyme; ARB: angiotension II receptor blocker; eGFR: estimated glomerular filtration rate; LDL: low density lipoprotein. | |||
| Age | 66.0 ± 10.0 | 64.6 ± 10.9 | 68.1 ± 9.4 |
| Hypertension | 54 (69.2%) | 33 (71.7%) | 21 (65.6%) |
| Atrial fibrillation | 9 (11.5%) | 6 (13.0%) | 3 (9.4%) |
| Carotid atherosclerosis | 32 (41.0%) | 22 (47.8%) | 10 (31.3%) |
| Diabetes | 9 (11.5%) | 6 (13.0%) | 3 (9.4%) |
| Smoke | 22 (28.2%) | 17 (37.0%)* | 5 (15.6%)* |
| Number of risk factors | 2.3 ±1.4 | 2.6 ±1.5* | 1.8 ±1.3* |
| ASA | 23 (29.5%) | 14 (30.4%) | 9 (28.1%) |
| Statin | 10 (12.8%) | 8 (17.4%) | 2 (6.2%) |
| ACE/ARB | 30 (38.4%) | 21 (45.7%) | 9 (28.1%) |
| Betablockers | 34 (43.6%) | 20 (43.5%) | 14 (43.8%) |
| Diuretics | |||
| LDL | 124.8 ± 31.6 | 130.5 ± 32.1* | 116.8 ± 29.6* |
| eGFR | 74.3 ± 14.1 | 72.2 ± 15.2 | 77.3 ± 11.9 |
Table 2. Differences between patients with hospitalization for HF and patients without.
| Total (n = 78) | With CV death or HF (n = 15) | Without CV death or HF (n = 63) | |
| Data were expressed as number (%) or mean ± SD or median [interquartile range]. *P < 0.05, **P < 0.01. EF: ejection fraction; GLS: global longitudinal score; HF: heart failure; LAD: left anterior descending; LCX: left circumflex artery; LMCA: left main coronary artery; RCA: right coronary artery. | |||
| Age, yrs | 66.0 ± 10.0 | 68.9 ± 9.7 | 65.4 ± 10.5 |
| Male | 46 (59.0%) | 10 (67.7%) | 36 (57.1%) |
| Hypertension | 54 (69.2%) | 11 (73.3%) | 43 (68.3%) |
| Atrial fibrillation | 9 (11.5%) | 3 (20.0%) | 6 (9.5%) |
| Carotid atherosclerosis | 32 (41.0%) | 5 (33.3%) | 27 (42.9%) |
| Diabetes | 9 (11.5%) | 2 (13.3%) | 7 (11.1%) |
| Smoke | 22 (28.2%) | 5 (33.3%) | 17 (27.0%) |
| Number of risk factors | 2.3 ± 1.4 | 2.41 ± 1.4 | 2.3 ± 1.5 |
| ASA | 23 (29.5%) | 6 (40.0%) | 17 (27.0%) |
| Statin | 10 (12.8%) | 1 (6.7%) | 9 (14.3%) |
| ACE/ARB | 30 (38.4%) | 8 (53.3%) | 22 (34.9%) |
| Betablockers | 34 (43.6%) | 6 (40.0%) | 28 (44.4%) |
| LDL | 124.8 ± 31.6 | 135.5 ± 31.0 | 122.4 ± 31.5 |
| eGFR | 74.3 ± 14.1 | 74.5 ± 15.2 | 74.3 ± 13.9 |
| Heart Rate | 64.4 ± 10.9 | 64.7 ± 9.9 | 64.4. ± 10.2 |
| EF | 49.9 ± 9.9 | 41.7 ± 10** | 51.8 ± 8.9** |
| Diastolic disfunction grade ≥ 2 | 31 (39.7%) | 10 (66.7%)* | 21 (33.3%)* |
| GLS | -16.1 ± 4.1 | -11.9 ± 3.1** | -17.1 ± 3.4** |
| Agatston score | 3.5 [0.0-148.7] | 56.0 [0.0-720.0]* | 0.0 [0.0-90.0]* |
| Agaston score > 100 | 23 (29.5%) | 7 (46.7%) | 16 (25.4%) |
| Coronary stenosis | 28 (35.9%) | 7 (46.7%) | 21 (33.3%) |
| Multiple coronary stenosis | 12 (15.4%) | 5 (33.3%)* | 7 (11.1%)* |
| LMCA stenosis | 2 (2.6%) | 1 (6.7%) | 1 (1.6%) |
| LAD stenosis | 21 (26.9%) | 5 (33.3%) | 16 (25.4%) |
| LCX stenosis | 11 (14.1%) | 5 (33.3%)* | 6 (9.5%)* |
| RCA stenosis | 9 (11.5%) | 2 (13.3%) | 7 (11.1% |
| High risk plaque | 21 (26.9%) | 5 (33.3% | 16 (25.4%) |
| Urgent outpatient visits for HF | 14 (17.9%) | 14 (93.3%) | 0 (0.0%) |
| Hospitalization for HF | 12 (15.4%) | 12 (80.0%) | 0 (0.0%) |
| All cause death | 4 (5.1%) | 1 (6.7%) | 3 (4.8%) |
| Cardiovascular death | 1 (1.3%) | 1 (6.7%) | 0 (0.0%) |
| Follow up (months) | 33 [17-77] | 25 [8-31] * | 42 [17-78] * |
Echocardiography analysis revealed a slightly reduced LVEF (50.0% ± 9.8%) and a GLS within the normal range (-16.2% ± 4.1%). CTA showed obstructive (> 50%) coronary artery stenosis in 28 patients (35.9%). Of these, 16 patients (20.5% of the total) had single-vessel disease, while 12 patients (15.4% of the total) had multivessel disease. During the follow-up period, 15 patients (16.7% of the entire population) underwent planned percutaneous coronary artery revascularization. The remaining patients, whose lesions were deemed subcritical by FFR during angiography, received conservative treatment. A high Agatston score (> 100) was observed in 29.5% of the patients, with 5 patients exceeding a score of 1000. After the diagnosis of coronary artery disease, all patients had their antiplatelet and lipid-lowering therapies optimized. Syntax score ranges between 0 and 24 (mean 3.46 ± 6.0), only 3 patients have > 22. Correlations between LVEF, GLS, Agatson score and Syntax score were presented in Table 3.
Table 3. Correlations between LVEF, GLS, Agatson score e Syntax score.
| LVEF | GLS | AGATSON SCORE | SYNTAX SCORE | |
| *P < 0.05; **P < 0.01. | ||||
| LVEF | 1 | -0.671** | -0.163 | -0.120 |
| GLS | - | 1 | 0.271* | 0.235 |
| AGATSON SCORE | - | - | 1 | 0.536** |
| SYNTAX SCORE | - | - | - | 1 |
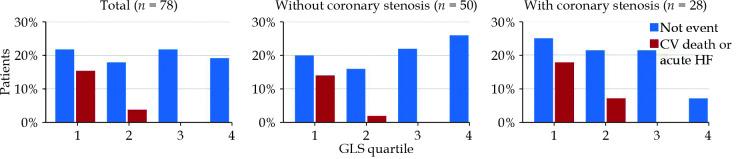
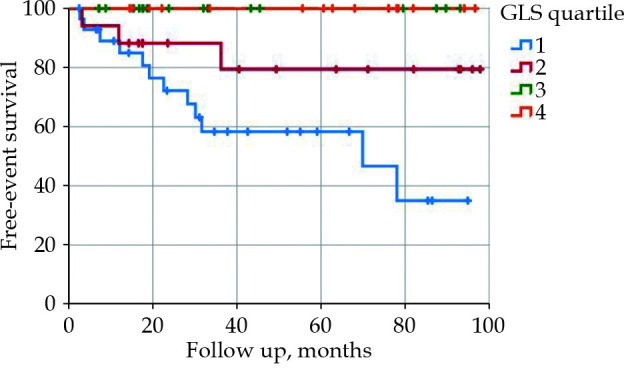
Significant associations were found between the primary target (CV death or development of acute HF) and several factors: LVEF (P = 0.001), diastolic dysfunction grade ≥ 2 (P = 0.02), GLS (P < 0.001), multiple coronary stenosis (P = 0.04), Agatston score (P = 0.05), and Syntax score (P < 0.001). After adjusting for confounders, multivariate Cox models confirmed the relationships with EF (R2 = 0.89, IQR = 0.83–0.95, P < 0.001), diastolic dysfunction (R2 = 3.30, IQR = 1.05–10.43, P = 0.04), GLS (R2 = 1.43, IQR = 1.22–1.68, P < 0.001), Agatston score (R2 = 1.01, IQR = 1.00–1.02, P = 0.05), and Syntax score (R2 = 1.16, IQR = 1.06–1.28, P = 0.001). Comparing together these parameters, impaired GLS emerged as the best predictor of the development of acute HF (R2 = 1.29, IQR = 1.03–1.63, P = 0.03). When the population was divided according the GLS quartile (Q1 < -13.7, Q2 -13.7–16.8, Q3: -16.8–18.9, Q4: >-18.9), only patients with a cut-off value above the median (-16.8) exhibited a risk of developing HF (Figure 1-2, P < 0.001). Interestingly, the prognostic role of GLS appeared to be more significant in patients without coronary stenosis, even after adjusting for confounders (R2 = 1.67, IQR = 1.25–2.24, P < 0.001 vs. R2 = 1.32; IQR = 0.95–1.83, P = 0.09) (Table 2, Figure 1). The use of a cut-off value for GLS (-16.8%) doesn’t appear to be significant (ROC area = 0.20). It should be noted that the presence of at least three risk factors was associated with an increased prevalence of coronary stenosis (58.8% vs.18.2%, P < 0.001) and an Agatston score > 100 (82.6% vs. 27.2%, P < 0.001).
Figure 1.
HF-free survival in patients with LBBB splitting by GLS quartile (Q1: < -13.7, Q2: -13.7 to -16.8, Q3: -16.8 to 18.9, Q4: > -18.9).
Kaplan-Meier analysis.
Figure 2.
Hospitalization for HF in LBBB patients divided for quartile of GLS.
DISCUSSION
This analysis investigated the utility of GLS and CTA in evaluating patients with LBBB, particularly for identifying those at high risk of adverse clinical cardiovascular outcomes (cv death/developing of HF).
In our clinical practice, we have emphasized the diagnostic and prognostic significance of CTA and GLS in outpatients with complete LBBB who present with atypical chest pain. We detected coronary stenosis greater than 50% in 35.9% of these patients. Over a median follow-up period of 33 months, 17.9% of patients required urgent outpatient visits for acute decompensated heart failure (HF), with 15.4% subsequently hospitalized for this condition. Cardiovascular causes accounted for the death of only one patient (1.5%).
The high prevalence of coronary artery disease (CAD) in our cohort aligns with literature reports, such as the Framingham Heart Study, which also indicates a higher mortality rate.[16,17] Notably, the use of CTA, a highly specific method, suggested a greater prevalence of CAD compared to earlier data that relied on less precise CAD markers, such as symptoms or functional tests for inducible ischemia—tests whose accuracy is compromised by the presence of LBBB.[18]
In our experience, coronary stenosis did not correlate with the combined endpoint of acute HF or cardiovascular death, which may be due to the concurrent presence of non-ischemic cardiomyopathy. The Agatston score emerged as a superior predictor of clinical events in our patient population. The favorable cardiovascular outcomes observed may be attributable to the implementation of specific secondary coronary prevention therapies once coronary anatomy was established.[19,20] These findings advocate for rigorous clinical follow-up in heart failure clinics to potentially enhance cardiovascular prognosis in LBBB patients with demonstrated coronary disease, regardless of successful coronary revascularization.
GLS has also proven to be a valuable tool for accurately identifying patients at high risk of adverse clinical outcomes. Research has shown that left ventricular GLS is a strong prognostic indicator, more closely associated with poor outcomes than other echocardiographic parameters capable of detecting subtle left ventricular dysfunction.[21] GLS was particularly reliable in patients with left ventricular dysfunction, outperforming LVEF in predicting cardiovascular events.[22] Moreover, in HF patients with severe left ventricular dysfunction, a GLS below -10%, CTA was able to identify patients who experienced ventricular arrhythmias (specificity of 90%).[23] Intriguingly, Stokke, et al.[24] demonstrated through a combined mathematical and echocardiographic study that in patients with preserved LVEF, GLS more accurately reflects systolic function than LVEF due to geometric confounders.
Impaired GLS, rather than diastolic dysfunction or LVEF, appears to be a reliable predictor of the risk of developing heart failure, with or without coronary stenosis. These findings underlined the importance of GLS in risk stratification management for patients with complete LBBB, where normal left ventricular systolic function will not precisely predict future HF risk. Additionally, when stratifying the population by GLS quartiles, only patients with a GLS greater than -16.8 faced a risk of developing HF. CTA's role is crucial in excluding CAD and, if present, initiating appropriate medical therapy. The prognostic value of GLS is particularly pronounced in patients without coronary stenosis, highlighting the potential progression to cardiomyopathy in those with complete LBBB but no CAD.
Our study has several limitations, including its retrospective nature, the single-center sample size for patients undergoing CTA, and the absence of a control group. Our goal was to investigate the long-term prognostic implications of LBBB in the general population. We also aimed to minimize potential historical bias that could arise from including a control group at a later date than the LBBB patients (enrolled from 2011 onwards), as technological advancements and increased operator experience could influence outcomes. Additionally, our sample may be subject to referral bias, as high-risk symptomatic patients might have been directly referred for coronary angiography.
In conclusion, the prognostic implications of a first diagnosis of LBBB remain a debated issue. However, our study has confirmed the association between complete LBBB and adverse clinical outcomes during mid-term follow-up. For patients presenting with atypical chest pain and complete LBBB, incorporating GLS measurements alongside CTA aids in identifying individuals at high risk. We advocate for the integration of this approach into routine clinical practice to enhance the diagnostic and prognostic classification of patients with an initial diagnosis of LBBB. While CTA enables the identification of patients with CAD, providing an opportunity for primary prevention through optimal medical therapy before the development of ischemic events, our findings are particularly insightful for patients with compromised absolute GLS values, regardless of left ventricular systolic or diastolic function. Patients with complete LBBB and impaired GLS, independently by the presence of CAD, should be actively monitored due to their increased risk of hospitalization for heart failure over the long term being the first clinical manifestation of cardiomyopathy.
Conflict of Interest
None.
References
- 1.Kumar V, Venkataraman R, Aljaroudi W, et al Implications of left bundle branch block in patient treatment. Am J Cardiol. 2013;111:291–300. doi: 10.1016/j.amjcard.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 2.Francia P, Balla C, Paneni F, Volpe M. Left bundle-branch block--pathophysiology, prognosis, and clinical management. Clin Cardiol 2007; 30: 110-115.
- 3.Zhang Z, Rautaharju PM, Soliman EZ, et al. Mortality risk associated with bundle branch blocks and related repolarization abnormalities (from the Women’s Health Initiative [WHI]). Am J Cardiol 2012; 110: 1489-1495.
- 4.Haataja P, Nikus K, Kähönen M, et al Prevalence of ventricular conduction blocks in the resting electrocardiogram in a general population: the Health 2000 Survey. Int J Cardiol. 2013;167:1953–1960. doi: 10.1016/j.ijcard.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Fahy GJ, Pinski SL, Miller DP, et al Natural history of isolated bundle branch block. Am J Cardiol. 1996;77:1185–1190. doi: 10.1016/S0002-9149(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 6.Imanishi R, Seto S, Ichimaru S, Nakashima E, Yano K, Akahoshi M Prognostic significance of incident complete left bundle branch block observed over a 40-year period. Am J Cardiol. 2006;98:644–648. doi: 10.1016/j.amjcard.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 7.Knuuti J, Wijns W, Saraste A, et al 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 8.Omar AMS, Bansal M, Sengupta PP Advances in Echocardiographic Imaging in Heart Failure With Reduced and Preserved Ejection Fraction. Circ Res. 2016;119:357–374. doi: 10.1161/CIRCRESAHA.116.309128. [DOI] [PubMed] [Google Scholar]
- 9.Lejeune S, Roy C, Ciocea V, et al. Right Ventricular Global Longitudinal Strain and Outcomes in Heart Failure with Preserved Ejection Fraction. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 2020; 33: 973-984.e2.
- 10.Witt CM, Wu G, Yang D, et al Outcomes With Left Bundle Branch Block and Mildly to Moderately Reduced Left Ventricular Function. JACC Heart Fail. 2016;4:897–903. doi: 10.1016/j.jchf.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Koepfli P, Wyss CA, Gaemperli O, et al Left bundle branch block causes relative but not absolute septal underperfusion during exercise. Eur Heart J. 2009;30:2993–2999. doi: 10.1093/eurheartj/ehp372. [DOI] [PubMed] [Google Scholar]
- 12.Clerc OF, Possner M, Maire R, et al Association of left bundle branch block with obstructive coronary artery disease on coronary CT angiography: a case–control study. Eur Hear J - Cardiovasc Imaging. 2016;17:765–771. doi: 10.1093/ehjci/jev202. [DOI] [PubMed] [Google Scholar]
- 13.Ghostine S, Caussin C, Daoud B, et al Non-invasive detection of coronary artery disease in patients with left bundle branch block using 64-slice computed tomography. J Am Coll Cardiol. 2006;48:1929–1934. doi: 10.1016/j.jacc.2006.04.103. [DOI] [PubMed] [Google Scholar]
- 14.SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J 2021; 42: 2439-2454.
- 15.Leipsic J, Abbara S, Achenbach S, et al SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:342–358. doi: 10.1016/j.jcct.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Schneider JF, Thomas HEJ, Kreger BE, McNamara PM, Kannel WB Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med. 1979;90:303–310. doi: 10.7326/0003-4819-90-3-303. [DOI] [PubMed] [Google Scholar]
- 17.Maron DJ, Hochman JS, Reynolds HR, et al Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395–1407. doi: 10.1056/NEJMoa1915922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahsepar AA, Arbab-Zadeh A. Cardiac CT vs. Stress Testing in Patients with Suspected Coronary Artery Disease: Review and Expert Recommendations. Curr Cardiovasc Imaging Rep 2015; 8: 29.
- 19.Min JK, Dunning A, Lin FY, et al Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23, 854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–860. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 20.Hlatky MA, Shilane D, Hachamovitch R, Dicarli MF Economic outcomes in the Study of Myocardial Perfusion and Coronary Anatomy Imaging Roles in Coronary Artery Disease registry: the SPARC Study. J Am Coll Cardiol. 2014;63:1002–1008. doi: 10.1016/j.jacc.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 21.Hwang IC, Cho GY, Yoon YE, Park JJ. Association Between Global Longitudinal Strain and Cardiovascular Events in Patients With Left Bundle Branch Block Assessed Using Two-Dimensional Speckle-Tracking Echocardiography. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 2018; 31: 52-63. e6.
- 22.Mignot A, Donal E, Zaroui A, et al Global longitudinalò strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr. 2021;23:1019–1024. doi: 10.1016/j.echo.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Nikko MH, Naeemi R, Moaref A, Attar A Global longitudinal strain for prediction of ventricular arrhytmia in patients with heart failure. ESC Heart Fail. 2020;7:2956–2961. doi: 10.1002/ehf2.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokke TM, Hasselberg NE, Smedsrud MK, et al Geometry as a confounder when assessing ventricular systolic function Comparison between ejection fraction and strain. J Am Coll Cardiol. 2017;70:942–954. doi: 10.1016/j.jacc.2017.06.046. [DOI] [PubMed] [Google Scholar]