Abstract
Objective
Survivors of pediatric brain tumors (SPBT) are at risk for social deficits, fewer friendships, and poor peer relations. SPBT also experience reduced brain connectivity via microstructural disruptions to white matter from neurological insults. Research with other populations implicates white matter connectivity as a key contributor to poor social functioning. This case-controlled diffusion-weighted imaging study evaluated structural connectivity in SPBT and typically developing controls (TDC) and associations between metrics of connectivity and social functioning.
Methods
Diffusion weighted-imaging results from 19 SPBT and 19 TDC were analyzed using probabilistic white matter tractography. Survivors were at least 5 years post-diagnosis and 2 years off treatment. Graph theory statistics measured group differences across several connectivity metrics, including average strength, global efficiency, assortativity, clustering coefficient, modularity, and betweenness centrality. Analyses also evaluated the effects of neurological risk on connectivity among SPBT. Correlational analyses evaluated associations between connectivity and indices of social behavior.
Results
SPBT demonstrated reduced global connectivity compared to TDC. Several medical factors (e.g., chemotherapy, recurrence, multimodal therapy) were related to decreased connectivity across metrics of integration (e.g., average strength, global efficiency) in SPBT. Connectivity metrics were related to peer relationship quality and social challenges in the SPBT group and to social challenges in the total sample.
Conclusions
Microstructural white matter connectivity is diminished in SPBT and related to neurological risk and peer relationship quality. Additional neuroimaging research is needed to evaluate associations between brain connectivity metrics and social functioning in SPBT.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11060-024-04724-0.
Keywords: Pediatric brain tumor, Diffusion imaging, White matter, Social competence
Improved survival for pediatric brain tumors has heightened the urgency of understanding and addressing disease- and treatment-related sequalae. Survivors of pediatric brain tumor (SPBT) experience late effects across many domains [1, 2] that pose challenges as they navigate their social milieu [3]. SPBT experience social connectedness difficulties, such as fewer friends and social interactions compared to siblings and other childhood cancer survivors [4, 5].
While the causes of these social difficulties are poorly understood, they likely relate to tumor- and treatment-driven changes in brain structure and function. Biopsychosocial models that are grounded in social cognitive neuroscience [6, 7] emphasize the connectivity of brain networks that support essential social information processes [8]. Higher-order cognitive functions are implemented by brain structures connected via white matter tracts that develop through early adulthood [9, 10]. Structural connectivity is vital to network function and various social information processes, including face processing [11, 12] and empathy [13]. Notably, white matter integrity appears related to connectedness within real-world social networks [14].
Disruptions to network connectivity in childhood may lead to difficulties with social information processing [15] with effects on later social connectedness. Among adults, white matter lesions have been linked to decreased theory of mind abilities [16]. Reduced white matter integrity and altered white matter microstructure have been linked to social challenges in pediatric traumatic brain injury (TBI) [17] and autism spectrum disorder (ASD) [18–20].
SPBT are at risk for disrupted structural connectivity secondary to tumor-directed treatments and tumor-related sequelae [21]. Generally, higher radiation therapy (RT) dose and volume are related to greater white matter injury, with initial evidence that proton RT reduces white matter disruption compared to photon RT [22]. Neurosurgery and chemotherapy also confer risk for white matter abnormalities but to a lesser degree than RT [23]. These factors impair myelination and alter the balance between gray and white matter [10]. Furthermore, focal damage may affect global network dynamics. Early neuroimaging research showed reduced white matter volume in SPBT treated with craniospinal RT, with younger age and hydrocephalus as risk factors [24].
Emerging diffusion-weighted imaging (DWI) research has shown diminished white matter integrity in SPBT compared to controls [25–29]. Graph theory methods quantify the efficiency of processing information from distributed brain regions (e.g., global efficiency) and the level of local processing (e.g., modularity). During typical development, brain networks move from random network configurations to networks that optimize the balance between global and local processing. In a graph theory-driven DWI study, adult SPBT exhibited reduced global efficiency and average clustering coefficients between brain areas [30], suggesting significant structural network disruptions.
Given associations between brain connectivity and social cognition [31], microstructural injury to white matter networks may lead to deficits in basic social-affective processes [32] and social function among SPBT [33]. However, little research has addressed the link between white matter and social functioning in SPBT directly. This study’s objectives were to: 1) compare DWI connectivity metrics of SPBT and typically developing controls (TDC); 2) evaluate associations between neurological risk and connectivity metrics; and 3) determine associations between DWI metrics and indices of social functioning among SPBT. We hypothesized that a) SPBT would have decreased whole-brain connectivity compared to TDC; b) increased neurological risk would be associated with diminished connectivity; and c) reduced connectivity would be associated with more social challenges among SPBT.
Methods
Participants
Participants consisted of 38 English-speaking youth (ages 8–17 years): SPBT (N = 19) and TDC (N = 19). See Table 1 for sample descriptives. Participants were 13.7 years old, approximately 58% female, and nearly 30% were non-White. Groups were matched in terms of age (t(36) = 0.723, p = 0.474) and IQ, as indexed by the Differential Abilities Scale, Second Edition (DAS-II) General Conceptual Ability Score (t(35) = -1.344, p = 0.188). Participant IQ was in the Average range (M = 104; SD = 13.9). Groups did not differ in terms of sex, Race, or Ethnicity.
Table 1.
Sample characteristics
| Variables | Brain Tumor (n = 19) n (%) or M ± SD |
Typically Developing (n = 19) n (%) or M ± SD |
Test of Statistical Difference (p-value) |
|---|---|---|---|
| Age in years | 14.05 ± 2.70 | 13.42 ± 2.63 |
t = 0.723 (p = .474) |
| Female sex | 11 (57.9%) | 11 (57.9%) |
Χ2 = 0 (p = 1.0) |
| Race |
Χ2 = 3.333 (p = .504) |
||
| Caucasian | 12 (63.2%) | 15 (78.9%) | |
| African-American | 4 (21.1%) | 2 (10.5%) | |
| Asian | 1 (5.3%) | 0 | |
| Multi-ethnic | 2 (10.5%) | 1 (5.3%) | |
| Other | 0 | 1 (5.3%) | |
| Hispanic/Latinx |
Χ2 = 0.892 (p = 0.345) |
||
| Hispanic/Latinx | 3 (15.8%) | 1 (5.3%) | |
| Not Hispanic/Latinx | 16 (84.2%) | 16 (84.2%) | |
| Unreported | 0 | 2 (10.5%) | |
| Income |
Χ2 = 5.855 (p = .054) |
||
| < $34,000 | 5 (26.3%) | 2 (10.5%) | |
| $34,000—$99,999 | 10 (52.6%) | 5 (26.3%) | |
| > 99,999 | 3 (15.8%) | 9 (47.4%) | |
| Highest Level of Maternal Education |
Χ2 = 4.100 (p = .129) |
||
| High school or less | 7 (36.8%) | 3 (15.8%) | |
| Some college | 5 (26.3%) | 3 (15.8%) | |
| College degree or more | 6 (31.6%) | 12 (63.2%) | |
| Treatment factors | |||
| Age at diagnosis | 5.65 ± 3.15 | ||
| Time since diagnosis | 8.45 ± 3.27 | ||
| Time since treatment completion | 6.76 ± 3.88 | ||
| Tumor types | |||
| Medulloblastoma | 4 (21.1%) | ||
| Ganglioglioma | 4 (21.1%) | ||
| Glioma | 4 (21.1%) | ||
| PNET | 1 (5.3%) | ||
| DNET | 1 (5.3%) | ||
| Pilocytic Astrocytoma | 5 (26.3%) | ||
| WHO grade | |||
| I | 13 (68.4%) | ||
| II | 1 (5.3%) | ||
| III | 0 | ||
| IV | 5 (26.3%) | ||
| Tumor Location | |||
| Supratentorial | 7 (36.8%) | ||
| Infratentorial | 12 (63.2%) | ||
| Treatment | |||
| Surgery only | 9 (47.4%) | ||
| Radiation only | 0 | ||
| Chemo only | 2 (10.5%) | ||
| Surgery + radiation | 1 (5.3%) | ||
| Surgery + chemo | 2 (10.5%) | ||
| Radiation + chemo | 0 | ||
| All three | 5 (26.3%) |
p-value < 0.05* p-value < 0.01** p-value < 0.001***
SPBT included those with any combination of resection, chemotherapy and/or cranial RT, diagnosed at least 5 years prior, and completed all tumor-directed treatments at least 2 years prior. Exclusion criteria for SPBT included any genetic condition affecting neurocognitive functioning (e.g., Neurofibromatosis), developmental delay prior to brain tumor diagnosis, and visual defects uncorrectable through lenses (e.g., field cuts). Per caregiver report, one participant had an anxiety disorder diagnosis and one had an ADHD diagnosis. Survivors’ family history included autism and Asperger's (N = 3), ADHD (N = 1), and depression or anxiety (N = 2).
Participants were 5.6 years old at diagnosis and completed tumor-directed therapy 6.8 years prior. 63% (N = 12) had an infratentorial tumor and the sample reflected both low- and high-grade pathologies. The majority underwent surgical resection (89.5%; 13 gross total, 4 subtotal), 47.4% (N = 9) received chemotherapy, 36.8% (N = 7) had RT, and 47.4% (N = 9) had multimodal therapy (e.g., some combination of treatment modalities). Of those treated with cranial RT, 4 had proton and 3 had photon. Furthermore, 4 underwent focal RT while 3 received craniospinal RT. Two had post-operative hydrocephalus requiring shunt placement, two had hemiparesis, one had posterior fossa syndrome, and one had ataxia.
TDC were selected from a pool of 67 who completed the same research protocol at the Center for Autism Research (CAR) at the Children’s Hospital of Philadelphia (CHOP), using the same MRI scanner. These data were collected between June 2010 and October 2012 (SPBT data were collected between August 2016 and March 2018). Participants were selected on a case–control basis to match the SPBT on age, IQ, and sex. Exclusion criteria for TDC included a) visual defects uncorrectable with lenses; b) a history of TBI or other neurological abnormality; c) autism-like impairments on screening by study personnel; d) a first- or second-degree relative with ASD; and e) a DSM-IV-TR Axis I disorder or significant symptoms of ADHD or mood, anxiety, substance-related, or conduct disorders.
Measures
Cognitive function
The DAS-II measured general cognitive ability [34]. The DAS-II provides norm-referenced overall cognitive ability scores (M = 100, SD = 15) that correlate highly with other IQ tests [34].
Social behavior
The Social Responsiveness Scale, Second Edition (SRS-2); [35] is a 65-item informant-report that evaluates the frequency of reciprocal social behaviors, communication, and repetitive and stereotypic behaviors. It yields a sex-normed total T-score with higher scores representing greater social challenges (M = 50, SD = 10). It has high internal consistency, test–retest reliability and inter-rater reliability, and strong associations with other measures of social difficulties [35].
The Vineland Adaptive Behavior Scale-Second Edition: Parent Rating Form (Vineland-II) [36] is a highly reliable measure of adaptive behavior. The Socialization domain standard score (M = 100, SD = 15) evaluated participant social functioning with higher scores reflecting better functioning.
The Children’s Communication Checklist-2 (CCC-2) [37] is a reliable parent-report measure of structural (grammar, syntax) and pragmatic (social reciprocity, gesture use) components of social communication. The Social Relations score (M = 10, SD = 3) measures pragmatic language with others and was used in analyses. Higher scores indicate better pragmatic language.
The Patient-Reported Outcomes Measurement Information System (PROMIS) Pediatric Peer Relationships-Short Form is a validated self-report measure of peer relationships [38]. Participants rate eight statements (i.e., I felt accepted by other kids my age) over the past 7 days on a 0–4 scale (0 = never, 4 = almost always). The measure yields a total T-score, where higher scores indicate better peer relationships. The self-report measure is correlated with peer-reported social acceptance [38]. Only SPBT participants completed this measure.
The Neurological Predictor Scale (NPS) [39] is generated through medical chart review and integrates treatment variables (e.g., cranial radiation) and history of neurological complications (e.g., hydrocephalus, seizure medication) into a single score on a scale of 0–11, with higher scores indicating greater neurological risk. The NPS has been related to neurocognitive functioning [40] and DWI metrics [30].
Procedures
Study procedures were approved by the CHOP institutional review board. Potentially eligible SPBT were identified through electronic medical records and sent a letter describing the study. Families were contacted by phone to conduct a verbal screening to determine eligibility. Those meeting eligibility criteria and interested in participating were invited to a one-time, in-person evaluation. SPBT participants were given the option of completing a 1-h research MRI scan. Written informed consent was obtained from parents and child assent was obtained. The protocol with SPBT mirrored that used with TDC as part of separate studies measuring social function with a core set of measures. Parents completed informant reports while youth completed the assessment. SPBT and TDC data were combined for analyses. 207 SPBT were contacted and 97 were screened. Of those screened, 90 met criteria, 54 completed the cognitive assessment, and 23 completed neuroimaging. 4 SPBT had excessive movement during the scan, leaving a total of 19. There were no differences on main study measures (DAS-II GCA, SRS-2, CCC-2, Vineland Socialization) between SPBT who completed an MRI scan and those who did not.
MRI scanning
All scans were conducted on a Siemens Verio 3-Tesla. Diffusion-weighted imaging data consisted of a 30-direction sequence (80 axial slices, 2 mm isotropic voxels, b0 = 1000, TR/TE/Flip Angle = 11 s/76.4 ms/180 degrees). High-resolution structural data also were collected on all participants (TR/TE/Flip Angle/Voxel Size parameters of 1900 ms/ 2.54 ms/90 degrees/0.8x.8. × 9 mm), and used to register diffusion data into atlas space (see below).
Data processing
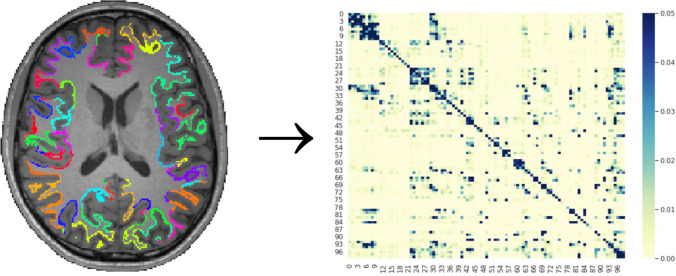
All data were corrected for motion and eddy currents using the program eddy_correct from the fMRIB Software Library (FSL) [41]. Connectivity data were estimated via probabilistic tractography, using the FSL programs bedpostx and probtrackx [42]. Probtrackx uses tensor information to develop probabilistic models of fiber pathways between seed and target areas. Bedpostx was configured up to two crossing fibers per voxel via a stick function deconvolution model. Our approach to identifying seed regions for probabilistic tractography follows established approaches [43]. Each participant's high-resolution structural MRI volume was segmented into gray and white matter using Freesurfer [44]. Using Freesurfer, we isolated portions of the Schaefer et al. parcellation [45] that overlap with their corresponding white matter boundaries (see Fig. 1). These boundaries were then transformed to each participant’s diffusion space using FSL’s program FLIRT [41]. All possible parcel pairings were included as seed and target areas, resulting in connection probability matrices for all parcels, for everyone in the sample. Because the Schaefer et al. template focuses on gray matter, the portions of each parcel on the white matter boundary (derived from per-participant Freesurfer segmentations [46]) were used as seed regions.
Fig. 1.
Connectivity Matrix Generation [Note. Illustration of connectivity matrix generation for one participant. The Schaefer et al. template was registered to each participant structural MRI. Using white matter segmentations and commands from Freesurfer, the portion of each parcellation overlapping with the white matter boundary was identified. These parcellation boundaries were then used as seed areas for probabilistic tractography using F ProbtrackX. An adjacency matrix was then generated for all possible pairs of areas. Graph theory metrics were then calculated for each participant’s adjacency matrix, which were used for group-level statistics.]
Statistical analyses
We evaluated hypotheses via graph theory analyses [47]. Graph theory provides several scalar metrics reflecting integration and segregation between networks and nodes (i.e., brain areas). Analyses focused on average connectivity strength (reflecting overall connectivity), global efficiency (a measure of network integration), assortativity (measure of the extent to which nodes in a sub-network associate with other nodes in the sub-network, and the resilience of the network against damage to main components), average clustering coefficient (measure of segregation, where high values indicate high levels of within-network, rather than global, connectivity), modularity (the degree to which nodes segregate into subnetworks), and betweenness centrality (the number of shortest paths between pairs of brain areas that also pass through any given area, averaged across nodes in a network). We predicted that these values would be decreased in the SPBT group, except for modularity and betweenness centrality (which typically increase when global connectivity decreases). All graph theory statistics were estimated using the Brain Connectivity Toolbox [47]. Binary input versions of graph theory formulas were used when estimating degrees and betweenness centrality; the rest used continuous input versions. T-tests compared imaging metrics between SPBT and TDC. The False Discovery Rate (FDR) correction controlled for family-wise error in analyses [48]. Effect sizes were estimated using Cohen's d, with scores of 0.2, 0.5, and 0.8 reflecting small, medium and large effects, respectively [49].
Evaluating brain connectivity among SPBT poses a dilemma – while global connectivity disruptions are of intrinsic importance, they would be expected based on the presence of tumors, resection, and associated neurosurgical procedures. We addressed this issue via a sensitivity analysis where we removed those SPBT with obvious morphological differences (i.e., readily identifiable from structural scans; n = 6), and re-running group comparisons. Pearson correlations evaluated associations between medical factors and imaging metrics within SPBT and associations between imaging metrics and indices of social function.
Results
Preliminary analyses
SPBT demonstrated higher levels of social challenges than TDC across all measures of social functioning, including the SRS-2 Total Score, t(35) = 4.366, p = < 0.001, the Vineland-2 Socialization Score, t(33) = -2.410, p = 0.029, and the CCC-2 Social Relations score t(32) = -3.862, p = < 0.001, with effect sizes ranging from medium to large. However, scores across measures for both groups were in the average range. See Table 2.
Table 2.
Differences in social functioning between SPBT and TDC
| Domain | SPBT Mean (SD) |
SPBT Range |
TDC Mean (SD) |
TDC Range |
T Value (1, 36) |
Cohen’s d (CI) |
|---|---|---|---|---|---|---|
| DAS-II IQ Score | 101.28 (12.51) | 74 — 124 | 107.37 (14.88) | 82 — 136 | - 1.344 |
- 0.442 (- 1.092, 0.214) |
| PROMIS Peer Relationships Score | 45.42 (7.79) | 29.24 — 56.82 | - | - | - | - |
| SRS-2 Total T Score | 52.17 (8.58) | 42 — 76 | 42.53 (4.26) | 38 — 53 | 4.293*** |
1.436 (0.701, 2.155) |
| Vineland Socialization Score | 101.18 (22.59) | 62 — 140 | 114.72 (7.47) | 101 — 129 | - 2.354* |
- 0.815 (- 1.501,—0.118) |
| CCC-2 Social Relations Score | 9.28 (2.89) | 4 — 13 | 12.13 (0.62) | 11 — 13 | - 4.081*** |
- 1.327 (- 2.066,—0.571) |
p-value < 0.05* p-value < 0.01** p-value < 0.001***
Within the full sample, average connectivity strength was associated with IQ (r = 0.366; p = 0.026). Among SPBT, average connectivity strength (r = 0.562; p = 0.015), global efficiency (r = 0.614; p = 0.007), and modularity (r = 0.692; p = 0.001) were significantly associated with IQ. Age at evaluation was positively associated with global efficiency (r = 0.458; p = 0.049) among SPBT (See Table 3).
Table 3.
Correlations between white matter connectivity and social functioning among SPBT
| Imaging Metrics | Age at Evaluation | NPS Scores | DAS-II IQ | PROMIS Peer Relationships Scale Self-Report | SRS-2 Total Score | Vineland-II Socialization Score | CCC2 Social Relationships Score |
|---|---|---|---|---|---|---|---|
| Average Connectivity Strength | .386 | -.541* | .562* | -.158 | -.022 | -.259 | -.324 |
| Global Efficiency | .458* | -.568* | .614** | -.147 | -.165 | -.187 | -.194 |
| Assortativity | .352 | .012 | -.039 | .477* | -.012 | .269 | -.209 |
| Clustering Coefficient | .010 | .133 | -.017 | -.136 | .518* | -.151 | -.407 |
| Modularity | .377 | -.028 | .692** | .015 | -.409 | .272 | -.212 |
| Betweenness Centrality | .258 | -.331 | .101 | -.166 | -.153 | .228 | .062 |
p-value < 0.05* p-value < 0.01** p-value < 0.001***
Diffusion weighted imaging by group
Table 4 presents imaging metrics by group. Compared to TDC, SPBT demonstrated decreased average connectivity strength, global efficiency, assortativity, and clustering coefficient (ps < 0.01) with large effect sizes. Modularity (p = 0.038) and betweenness centrality (p < 0.001) were significantly increased in SPBT with medium to large effect sizes.
Table 4.
Differences in white matter connectivity between SPBT and TDC
| Imaging Metrics | SPBT Mean (SD) | TDC Mean (SD) | T Value (1, 36) |
Cohen’s d (CI) |
|---|---|---|---|---|
| Average Connectivity Strength | 0.257 (0.036) | 0.324 (0.039) | -5.518*** |
- 1.790 (-2.539, -1.024) |
| Global Efficiency | 0.005 (> 0.001) | 0.006 (> 0.001) | -3.430** |
- 1.113 (-1.792, -0.421) |
| Assortativity | -0.050 (0.011) | -0.039 (0.008) | -3.562*** |
- 1.156 (-1.838, -0.459) |
| Clustering Coefficient | 0.0006 (< 0.001) | 0.0007 (< 0.001) | -5.385*** |
- 1.908 (-2.741, -1.052) |
| Modularity | 0.439 (0.023) | 0.424 (0.020) | 2.150* |
0.698 (0.037, 1.349) |
| Betweenness Centrality | 13.815 (4.278) | 8.143 (2.265) | 5.108*** |
1.657 (0.907, 2.390) |
p-value < 0.05* p-value < 0.01** p-value < 0.001***
Medical characteristics and imaging metrics
Average connectivity strength was related to recurrence (t = -3.276, p = 0.004), multimodal treatment (t = -2.958, p = 0.009), and chemotherapy (t = -2.741, p = 0.014). Global efficiency was related to recurrence (t = -2.599, p = 0.019) and multimodal treatment (t = -2.847, p = 0.011), but not chemotherapy (t = -2.112, p = 0.05). Higher NPS scores were related to reduced average strength and global efficiency. Each of these effects remained significant when applying family-wise error correction via FDR and when removing SPBT with gross morphological differences from resection. See Table 3. Age at diagnosis, time since diagnosis, and time since treatment completion were unrelated to imaging metrics.
DWI metrics and social functioning
Among the entire sample, increased social challenges on the SRS-2 were associated with reductions in average connectivity strength (r = -0.420; p = 0.010) and global efficiency (r = -0.379; p = 0.021). Additionally, higher levels of modularity were associated with worse social communication on the CCC2 (r = -0.365; p = 0.034) and higher levels of assortativity were associated with better social relations on the Vineland-II (r = 0.445, p = 0.007) (see Supplemental Table 2).
Among SPBT, increased assortativity was associated with better peer relationship scores on the PROMIS Pediatric Peer Relationships Scale (r = 0.477, p = 0.045). Furthermore, higher clustering coefficient was associated with greater social challenges on the SRS-2 (r = 0.518, p = 0.040). Among TDC, higher levels of assortativity were related to better Socialization scores on the Vineland (r = 0.475, p = 0.046). There were no other significant associations between imaging metrics and other primary outcomes among TDCs.
Discussion
Prior research has identified both disrupted white matter connectivity [21, 24–29] and social impairments [3, 4] in SPBT, yet few studies have attempted to connect these issues. Findings from this initial study of white matter connectivity and social functioning in SPBT offer preliminary support for this framework. Results suggest decreased global connectivity, as evidenced by global efficiency and average strength, reduced network resilience, as measured by assortativity, and increased within-network connectivity, as evidenced by increased modularity and betweenness centrality, in SPBT compared to age-, sex-, and IQ-matched TDC. Higher levels of neurological risk were associated with decreased connectivity. Further, higher assortativity was associated with better peer relationships among SPBT, suggesting that those with more resilient white matter networks characterized by stronger connections among nodes [47] experience greater social acceptance. Additionally, among SPBT higher within-network, compared to global, connectivity (clustering coefficient) was associated with more social challenges.
The pattern of connectivity seen in this study with school-age SPBT implies diminished global network organization and is consistent with a prior study employing graph theory approaches in adult SPBT [30]. Collectively, these findings suggest decreased processing efficiency across the brain and increased reliance on sub-network processing in SPBT. Further, connectivity metrics in both studies appear associated with neurological insults. Typical neurodevelopment involves progressing to networks that balance between integration and segregation between networks of brain areas [30], and disruptions in development from tumor and treatments likely contribute to the differences in SPBT connectivity. Longitudinal research is needed to document these neurodevelopmental processes in SPBT.
Study findings contribute to a growing body of research concerned with neurophysiological explanations underlying social behavior and social information processing among SPBT [50]. Greater connectivity between sub-network nodes, as measured by assortativity, was related to better self-reported peer relationships. However, other connectivity metrics were unrelated to measures of social behavior or relationships. These findings contrast somewhat with prior research linking white matter connectivity and social function in both typical and clinical populations [17–20]. Consistent with hypothesized models [33], network connectivity and reduced network segregation likely underlie the development of domains of social cognition, which in turn promote increased connections with others [31].
Methodological strengths of this study include using a case-controlled design that matched SPBT to TDC in terms of age, IQ, and sex, and employing graph theory analyses for DWI data, which is relatively novel in research on SPBT. Limitations include a relatively small sample with complete imaging and social behavior data and a heterogenous sample of SPBT mixed in tumor type, location, and treatment combination. These issues reduced power to evaluate each of these factors independently. The TDC group also was relatively high functioning, which may have contributed to observed group differences.
This study offers an initial link between the neurophysiological changes seen in white matter in SPBT and their social difficulties. Additional studies with larger samples and longitudinal designs are needed to determine within-person associations between developmental (e.g., age at diagnosis) and medical (e.g., tumor location, radiation factors) variables, white matter network characteristics, and social information processing and social behavior in SPBT over time. Such research may identify the mechanistic factors that affect the variability in survivor social functioning and inform remediation interventions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported in part by the National Cancer Institute (K07CA178100, to MCH), National Institute of Mental Health (RC1MH08879, to RTS; K23MH086111 and R21MH092615 to BEY); the Intellectual and Developmental Disabilities Research Center funded by the National Institute of Child and Human Development (I5U54HD086984, to RTS; principal investigator, M. Robinson), the Pennsylvania Department of Health (SAP 4100042728 and SAP 4100047863, to RTS), Pfizer (to RTS); Robert Wood Johnson Foundation (6672, to RTS); and Shire Pharmaceuticals (to JDH). The authors thank all the participants who provided their time for this study. The authors have no conflicts of interest.
Author contributions
M.C.H. and J.D.H. wrote the main manuscript text. J.H.D. conducted the main imaging analyses. P.F. prepared all tables and conducted other analyses. All authors reviewed and helped revise the manuscript.
Funding
The authors have not disclosed any funding.
Data availability
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of the research participants.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Packer RJ, Gurney JG, Punyko JA, Donaldson SS, Inskip PD, Stovall M, Yasui Y, Mertens A, Sklar CA, Nicholson HS, Zeltzer LK, Neglia JP, Robison LL (2003) Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood Cancer Survivor Study. J Clin Oncol 21:3255–3261. 10.1200/JCO.2003.01.202 10.1200/JCO.2003.01.202 [DOI] [PubMed] [Google Scholar]
- 2.Robinson KE, Kuttesch JF, Champion JE, Andreotti CF, Hipp DW, Bettis A, Barnwell A, Compas BE (2010) A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer 55:525–531. 10.1002/pbc.22568 10.1002/pbc.22568 [DOI] [PubMed] [Google Scholar]
- 3.Hocking MC, McCurdy M, Turner E, Kazak AE, Noll RB, Barakat LP (2015) Social competence in pediatric brain tumor survivors: application of a model from social neuroscience and developmental psychology. Pediatr Blood Cancer 62:375–384. 10.1002/pbc.25300 10.1002/pbc.25300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulte F, Brinkman TM, Li C, Fay-McClymont T, Srivastava DK, Ness KK, Howell RM, Mueller S, Wells E, Strother D, Lafay-Cousin L, Leisenring W, Robison LL, Armstrong GT, Krull KR (2018) Social adjustment in adolescent survivors of pediatric central nervous system tumors: A report from the Childhood Cancer Survivor Study. Cancer 124:3596–3608. 10.1002/cncr31593 10.1002/cncr31593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamboli M, Means B, Jurbergs N, Conklin HM, Gajjar A, Willard VW (2024) Social participation of school-aged survivors of pediatric brain tumors: A daily diary report. Pediatr Blood Cancer 71:e30764. 10.1002/pbc.30764 10.1002/pbc.30764 [DOI] [PubMed] [Google Scholar]
- 6.Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, Taylor HG, Vannatta K (2007) Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychol Bull 133:535–556. 10.1037/0033-2909.133.3.535 10.1037/0033-2909.133.3.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beauchamp MH, Anderson V (2010) SOCIAL: An integrative framework for the development of social skills. Psychol Bull 136:39–64. 10.1037/a0017768 10.1037/a0017768 [DOI] [PubMed] [Google Scholar]
- 8.Johnson MH, Griffin R, Csibra G, Halit H, Farroni T, de Haan M, Tucker LA, Baron-Cohen S, Richards J (2005) The emergence of the social brain network: Evidence from typical and atypical development. Dev Psychol 17:599–619. 10.1017/S0954579405050297 10.1017/S0954579405050297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebel C, Beaulieu C (2011) Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 31:10937–10947. 10.1523/JNEUROSCI.5302-10.2011 10.1523/JNEUROSCI.5302-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore BD III (2005) Neurocognitive outcomes in survivors of childhood cancer. J Pediatr Psychol 30:51–63. 10.1093/jpepsy/jsi016 10.1093/jpepsy/jsi016 [DOI] [PubMed] [Google Scholar]
- 11.Gschwind M, Pourtois G, Schwartz S, Van De Ville D, Vuilleumier P (2012) White-matter connectivity between face-responsive regions in the human brain. Cereb Cortex 22:1564–1576. 10.1093/cercor/bhr226 10.1093/cercor/bhr226 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Metoki A, Alm KH, Olson IR (2018) White matter pathways and social cognition. Neurosci Biobehav Rev 90:350–370. 10.1016/j.neubiorev.2018.04.015 [DOI] [PMC free article] [PubMed]
- 13.Parkinson C, Wheatley T (2014) Relating anatomical and social connectivity: White matter microstructure predicts emotional empathy. Cereb Cortex 24:614–625. 10.1093/cercor/bhs347 10.1093/cercor/bhs347 [DOI] [PubMed] [Google Scholar]
- 14.Hyon R, Chavez RS, Chwe JAH, Wheatley T, Kleinbaum AM, Parkinson C (2022) White matter connectivity in brain networks supporting social and affective processing predicts real-world social network characteristics. Comm Biol 5:1048. 10.1038/s42003-022-03655-8 10.1038/s42003-022-03655-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris SE, Cuthbert BN (2012) Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci 14:29–37. 10.31887/DCNS.2012.14.1/smorris 10.31887/DCNS.2012.14.1/smorris [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlton RA, Barrick TR, Markus HS, Morris RG (2009) Theory of mind associations with other cognitive functions and brain imaging in normal aging. Psychol Aging 24:338–348. 10.1037/a0015225 10.1037/a0015225 [DOI] [PubMed] [Google Scholar]
- 17.Tasker RC, Westland AG, White DK, Williams GB (2010) Corpus callosum and inferior forebrain white matter microstructure are related to functional outcome from raised intracranial pressure in child traumatic brain injury. Dev Neurosci 32:374–384. 10.1159/000316806 10.1159/000316806 [DOI] [PubMed] [Google Scholar]
- 18.Ameis SH, Catani M (2015) Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex 62:158–181. 10.1016/j.cortex.2014.10.014 10.1016/j.cortex.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 19.Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TK, Ho TP, McAlonan GM (2009) White matter fractional anisotropy differences and correlates of diagnostic symptoms in autism. J Child Psychol Psychiatry 50:1102–1112. 10.1111/j.1469-7610.2009.02086.x 10.1111/j.1469-7610.2009.02086.x [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald J, Gallagher L, McGrath J (2016) Widespread disrupted white matter microstructure in autism spectrum disorders. J Autism Dev Disord 49:2664–2674. 10.1007/s10803-016-2803-8 10.1007/s10803-016-2803-8 [DOI] [PubMed] [Google Scholar]
- 21.Reddick WE, White HA, Glass JO, Wheeler GC, Thompson SJ, Gajjar A, Leigh L, Mulhern RK (2003) Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer 97:2512–2519. 10.1002/cncr.11355 10.1002/cncr.11355 [DOI] [PubMed] [Google Scholar]
- 22.Moxon-Emre I, Bouffet E, Taylor MD, Laperriere N, Sharpe MB, Laughlin S, Bartels U, Scantleberry N, Law N, Malkin D, Skocic J, Richard L, Mabbott DJ (2016) Vulnerabiity of white matter to insult during childhood: evidence from patients treated for medulloblastoma. J Neurosurg Pediatr 18:29–40. 10.3171/2016.1.PEDS15580 10.3171/2016.1.PEDS15580 [DOI] [PubMed] [Google Scholar]
- 23.Mash LE, Kahalley LS, Raghubar KP, Goodrich-Hunsaker NJ, Abiildskov TJ, De Leon LA, MacLeod M, Stancel HH, Parsons K, Biekman B, Desai NK, Grosshans D, Paulino AC, Chu ZD, Whitehead WE, Okcu MF, Chintagumpala M, Wilde EA (2023) Cognitive Sparing in Proton versus Photon Radiotherapy for Pediatric Brain Tumor Is Associated with White Matter Integrity: An Exploratory Study. Cancers 15:1844. 10.3390/cancers15061844 10.3390/cancers15061844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddick WE, Glass JO, Palmer SL, Wu S, Gajjar A, Langston JW, Kun LE, Xiong X, Mulhern RK (2005) Atypical white matter volume development in children following craniospinal irradiation. Neuro Oncol 7:12–19. 10.1215/S1152851704000079 10.1215/S1152851704000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scantlebury N, Bouffet E, Laughlin S, Strother D, McConnell D, Hukin J, Fryer C, Laperriere N, Montour-Proulx I, Keene D, Fleming A, Jabado N, Liu F, Riggs L, Law N, Mabbott DJ (2016) White matter and information processing speed following treatment with cranial-spinal radiation for pediatric brain tumor. Neuropsychology 30:425–438. 10.1037/neu0000258 10.1037/neu0000258 [DOI] [PubMed] [Google Scholar]
- 26.King TZ, Wang L, Mao H (2015) Disruption of White Matter Integrity in Adult Survivors of Childhood Brain Tumors: Correlates with Long-Term Intellectual Outcomes. PLoS ONE 10:e0131744. 10.1371/journal.pone.0131744 10.1371/journal.pone.0131744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makola M, Ris MD, Mahone EM, Yeates KO, Cecil KM (2017) Long-term effects of radiation therapy on white matter of the corpus callosum: a diffusion tensor imaging study in children. Pediatr Radiol. 10.1007/s00247-017-3955-1 10.1007/s00247-017-3955-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron Nelson M, Compton P, Macey PM, Patel SK, Jacob E, O’Neil S, Ogren J, Finlay JL, Harper RM (2016) Diffusion tensor imaging and neurobehavioral outcome in children with brain tumors treated with chemotherapy. J Pediatr Oncol Nurs 33:119–128. 10.1177/1043454215590104 10.1177/1043454215590104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozyurt J, Muller HL, Warmuth-Metz M, Thiel CM (2017) Hypothalamic tumors impact gray and white matter volumes in fronto-limbic brain areas. Cortex 89:98–110. 10.1016/j.cortex.2017.01.017 10.1016/j.cortex.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 30.Na S, Li L, Crosson B, Dotson V, MacDonald TJ, Mao H, King TZ (2018) White matter network topology relates to cognitive flexibility and cumulative neurological risk in adult survivors of pediatric brain tumors. NeuroImage: Clin 20:485–497. 10.1016/j.nicl.2018.08.015 10.1016/j.nicl.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filley CM (2020) Social cognition and white matter: Connectivity and cooperation. Cogn Behav Neurol 33:67–75. 10.1097/WNN.0000000000000223 10.1097/WNN.0000000000000223 [DOI] [PubMed] [Google Scholar]
- 32.Moxon-Emre I, Taylor MJ, Farb NAS, Oyefiade AA, Taylor MD, Bouffet E, Laughlin S, Skocic J, de Medeiros CB, Mabbott DJ (2020) Eye movements and white matter are associated with emotional control in children treated for brain tumors. J Int Neuropsychol Soc 26:978–992. 10.1017/S1355617720000491 10.1017/S1355617720000491 [DOI] [PubMed] [Google Scholar]
- 33.Igoshina E, Wu LC, Moxon-Emre I, Mabbott DJ (2023) Social affective outcomes and brain injury in children and adolescents treated for brain tumours. Lancet Child Adolesc Health. 10.1016/S2352-4642(23)00079-2 10.1016/S2352-4642(23)00079-2 [DOI] [PubMed] [Google Scholar]
- 34.Elliott CD (2007) Differential Ability Scales - Second edition (DAS-II). Harcourt Assessment, San Antonio, TX
- 35.Constantino JN, Gruber CP (2012) (SRSTM -2) Social Responsiveness ScaleTM, Second Edition. Western Psychological Services, Torrance, CA
- 36.Sparrow SS, Cicchetti DV, Balla DA (2005) Vineland Adaptive Behavior Scales - Second Edition. AGS, Circle Pines, MN
- 37.Bishop D (2006) Children's Communication Checklist-2. Pearson, San Antonio, TX
- 38.Devine KA, Willard VW, Hocking MC, Stapleton JL, Rotter D, Bukowski WM, Noll RB (2018) PROMIS Peer Relationships Short Form: How well does self-report correlate with data from peers? J Pediatr Psychol 43:1059–1067. 10.1093/jpepsy/jsy038 10.1093/jpepsy/jsy038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Micklewright JL, King TZ, Morris RD, Krawiecki N (2008) Quantifying pediatric neuro-oncology risk factors: Development of the Neurological Predictor Scale. J Child Neurol 23:455–458. 10.1177/0883073807309241 10.1177/0883073807309241 [DOI] [PubMed] [Google Scholar]
- 40.Srsich AR, McCurdy MD, Fantozzi PM, Hocking MC (2024) Predicting neuropsychological late effects in pediatric brain tumor survivors using the Neurological Predictor Scale and the Pediatric Neuro-Oncology Rating of Treatment Intensity. J Int Neuropsychol Soc 30:380–388. 10.1017/S1355617723000589 10.1017/S1355617723000589 [DOI] [PubMed] [Google Scholar]
- 41.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219. 10.1016/j.neuroimage.2004.07.051 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 42.Behrens TEJ, Johansen-Berg H, Jbadi S, Rushworth MFS, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations. What can we gain? Neuroimage 23:144–155. 10.1016/j.neuroimage.2006.09.018 10.1016/j.neuroimage.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ingalhalikar M, Smith AE, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R (2014) Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A 111:823–828. 10.1073/pnas.1316909110 10.1073/pnas.1316909110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischl B (2012) FreeSurfer. NeuroImage 62:774–781. 10.1016/j.neuroimage.2012.01.021 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, Eickhoff SB, Yeo BTT (2018) Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex 28:3095–3114. 10.1093/cercor/bhx179 10.1093/cercor/bhx179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Destrieux C, Fischl B, Dale A, Halgren E (2010) Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53:1–15. 10.1016/j.neuroimage.2010.06.010 10.1016/j.neuroimage.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. 10.1016/j.neuroimage.2009.10.003 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 48.Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practica and powerful approach to multiple testing. J Roy Stat Soc B 57:289–300. 10.1111/j.2517-6161.1995.tb02031.x 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 49.Cohen J (1992) A power primer. Psychol Bull 112:155–159. 10.1037/0033-2909.112.1.155 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 50.Hocking MC, Schultz RT, Minturn JE, Brodsky C, Albee M, Herrington JD (2022) Reduced fusiform gyrus activation during face processing in pediatric brain tumor survivors. J Int Neuropsychol Soc 28:937–946. 10.1017/S135561772100117X 10.1017/S135561772100117X [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of the research participants.