Abstract
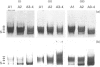
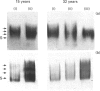
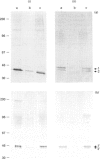
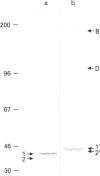
Immunological studies revealed the presence of several different forms of biglycan and decorin in human intervertebral-disc tissues (annulus fibrosus, nucleus pulposus and cartilage end-plate). In the young intervertebral disc, glycosaminoglycan-containing (glycanated) forms of both biglycan and decorin represented a greater proportion of the total proteoglycan population present in extracts of annulus fibrosus and cartilage end-plate compared with extracts of nucleus pulposus, in which they were barely detectable. In older discs the glycanated forms of biglycan and decorin represented only a small proportion of the total proteoglycan present. Immunochemical analyses with an antibody to chondroitin/dermatan sulphate isomers indicated differences in the glycosaminoglycans substituted on glycanated forms of small proteoglycans found in different disc tissues. Dermatan sulphate was the predominant glycosaminoglycan present on biglycan and decorin in annulus fibrosus extracts, whereas chondroitin 4-sulphate was present in both small proteoglycans isolated from cartilage end-plate. In addition, immunochemical analyses with antibodies against core protein epitopes identified two non-glycanated forms of both biglycan and decorin. These non-glycanated forms of the small proteoglycans were found in all three regions of the disc. The two nonglycanated forms of biglycan had estimated molecular masses of 37 and 41 kDa and those of decorin were 43 and 45 kDa, respectively. These non-glycanated forms of biglycan and decorin increased in proportion with aging. N-terminal sequence analysis indicated that the larger non-glycanated form of decorin was a degradation product of its glycanated precursor. However, no N-terminal sequence information was obtainable from the other non-glycanated form of decorin or the two non-glycanated forms of biglycan. These data are consistent with the hypothesis that some of the non-glycanated forms of decorin and biglycan are degradation products of native precursors. However, the possibility remains that several different post-translationally modified forms of decorin and biglycan are synthesized by intervertebral-disc tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco P., Fisher L. W., Young M. F., Termine J. D., Robey P. G. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990 Nov;38(11):1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- Brown D. C., Vogel K. G. Characteristics of the in vitro interaction of a small proteoglycan (PG II) of bovine tendon with type I collagen. Matrix. 1989;9(6):468–478. doi: 10.1016/s0934-8832(11)80016-8. [DOI] [PubMed] [Google Scholar]
- Brown T. A., Bouchard T., St John T., Wayner E., Carter W. G. Human keratinocytes express a new CD44 core protein (CD44E) as a heparan-sulfate intrinsic membrane proteoglycan with additional exons. J Cell Biol. 1991 Apr;113(1):207–221. doi: 10.1083/jcb.113.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney S. L., Bayliss M. T., Collier J. M., Muir H. Electrophoresis of 35S-labeled proteoglycans on polyacrylamide-agarose composite gels and their visualization by fluorography. Anal Biochem. 1986 Jul;156(1):38–44. doi: 10.1016/0003-2697(86)90150-8. [DOI] [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R., Couchman J. R. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc. 1985 Feb;44(2):386–393. [PubMed] [Google Scholar]
- Cole T. C., Burkhardt D., Frost L., Ghosh P. The proteoglycans of the canine intervertebral disc. Biochim Biophys Acta. 1985 Apr 17;839(2):127–138. doi: 10.1016/0304-4165(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Eyre D. R. Biochemistry of the intervertebral disc. Int Rev Connect Tissue Res. 1979;8:227–291. doi: 10.1016/b978-0-12-363708-6.50012-6. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Fisher L. W., MacDonald E. D., Jacobs L., Jr, Perlish J. S., Termine J. D. Decorin interacts with fibrillar collagen of embryonic and adult human skin. J Struct Biol. 1991 Feb;106(1):82–90. doi: 10.1016/1047-8477(91)90065-5. [DOI] [PubMed] [Google Scholar]
- Hedbom E., Heinegård D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem. 1989 Apr 25;264(12):6898–6905. [PubMed] [Google Scholar]
- Inerot S., Axelsson I. Structure and composition of proteoglycans from human annulus fibrosus. Connect Tissue Res. 1991;26(1-2):47–63. doi: 10.3109/03008209109152163. [DOI] [PubMed] [Google Scholar]
- Järveläinen H. T., Kinsella M. G., Wight T. N., Sandell L. J. Differential expression of small chondroitin/dermatan sulfate proteoglycans, PG-I/biglycan and PG-II/decorin, by vascular smooth muscle and endothelial cells in culture. J Biol Chem. 1991 Dec 5;266(34):23274–23281. [PubMed] [Google Scholar]
- Meek K. M., Scott J. E., Nave C. An X-ray diffraction analysis of rat tail tendons treated with Cupromeronic Blue. J Microsc. 1985 Aug;139(Pt 2):205–219. doi: 10.1111/j.1365-2818.1985.tb02637.x. [DOI] [PubMed] [Google Scholar]
- Neame P. J., Choi H. U., Rosenberg L. C. The primary structure of the core protein of the small, leucine-rich proteoglycan (PG I) from bovine articular cartilage. J Biol Chem. 1989 May 25;264(15):8653–8661. [PubMed] [Google Scholar]
- Orford C. R., Gardner D. L. Proteoglycan association with collagen d band in hyaline articular cartilage. Connect Tissue Res. 1984;12(3-4):345–348. doi: 10.3109/03008208409013696. [DOI] [PubMed] [Google Scholar]
- Quentin E., Gladen A., Rodén L., Kresse H. A genetic defect in the biosynthesis of dermatan sulfate proteoglycan: galactosyltransferase I deficiency in fibroblasts from a patient with a progeroid syndrome. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1342–1346. doi: 10.1073/pnas.87.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S., Menage J., Duance V., Wotton S., Ayad S. 1991 Volvo Award in basic sciences. Collagen types around the cells of the intervertebral disc and cartilage end plate: an immunolocalization study. Spine (Phila Pa 1976) 1991 Sep;16(9):1030–1038. [PubMed] [Google Scholar]
- Roberts S., Menage J., Urban J. P. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine (Phila Pa 1976) 1989 Feb;14(2):166–174. doi: 10.1097/00007632-198902000-00005. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Dermatan sulphate proteoglycans of human articular cartilage. The properties of dermatan sulphate proteoglycans I and II. Biochem J. 1989 Sep 15;262(3):823–827. doi: 10.1042/bj2620823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio L. de O., Bayliss M. T., Hardingham T. E., Muir H. Dermatan sulphate proteoglycan from human articular cartilage. Variation in its content with age and its structural comparison with a small chondroitin sulphate proteoglycan from pig laryngeal cartilage. Biochem J. 1988 Sep 15;254(3):757–764. doi: 10.1042/bj2540757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-collagen interactions in intervertebral disc. A chondroitin sulphate proteoglycan associates with collagen fibrils in rabbit annulus fibrosus at the d-e bands. Biosci Rep. 1986 Oct;6(10):879–888. doi: 10.1007/BF01116241. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. G., Nakano T., Dodd C. M., Pringle G. A., Kuc I. M. Proteoglycans of the articular disc of the bovine temporomandibular joint. II. Low molecular weight dermatan sulphate proteoglycan. Matrix. 1989 Aug;9(4):284–292. doi: 10.1016/s0934-8832(89)80004-6. [DOI] [PubMed] [Google Scholar]
- Stanescu V. The small proteoglycans of cartilage matrix. Semin Arthritis Rheum. 1990 Dec;20(3 Suppl 1):51–64. doi: 10.1016/0049-0172(90)90047-j. [DOI] [PubMed] [Google Scholar]
- Uldbjerg N., Danielsen C. C. A study of the interaction in vitro between type I collagen and a small dermatan sulphate proteoglycan. Biochem J. 1988 May 1;251(3):643–648. doi: 10.1042/bj2510643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel K. G., Koob T. J., Fisher L. W. Characterization and interactions of a fragment of the core protein of the small proteoglycan (PGII) from bovine tendon. Biochem Biophys Res Commun. 1987 Oct 29;148(2):658–663. doi: 10.1016/0006-291x(87)90927-2. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel K. G., Trotter J. A. The effect of proteoglycans on the morphology of collagen fibrils formed in vitro. Coll Relat Res. 1987 Jun;7(2):105–114. doi: 10.1016/s0174-173x(87)80002-x. [DOI] [PubMed] [Google Scholar]