Abstract
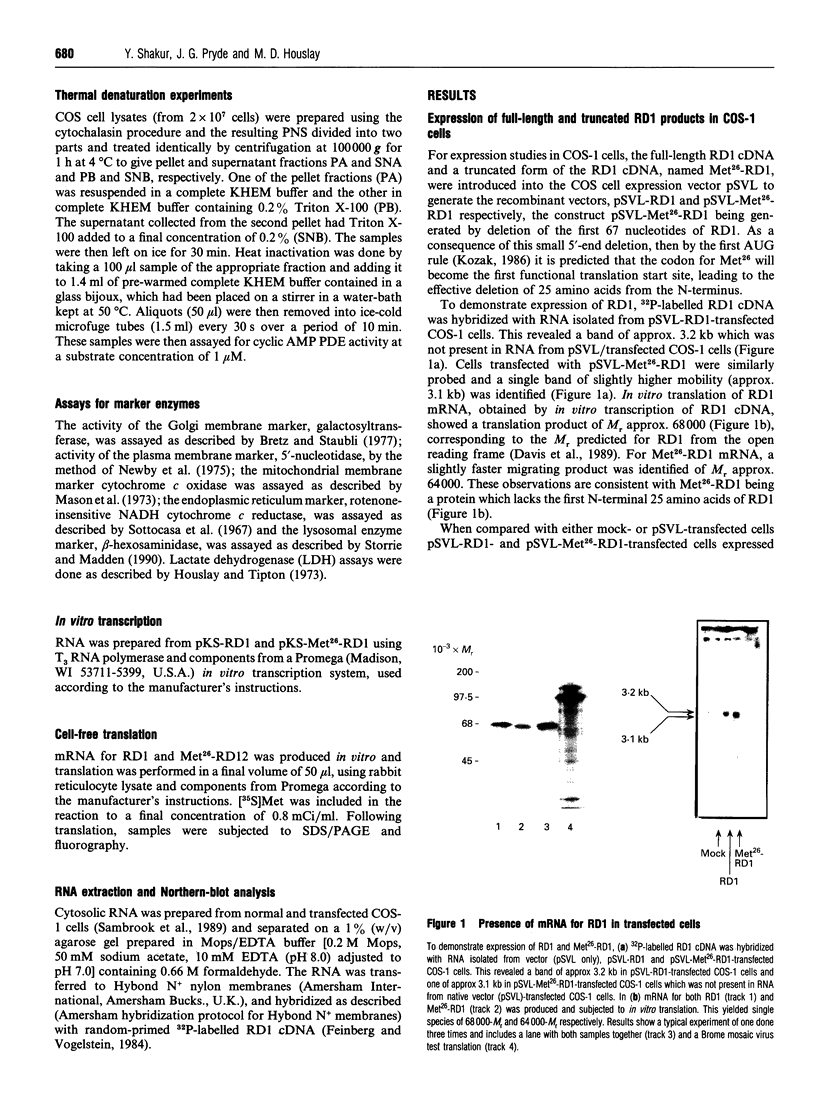
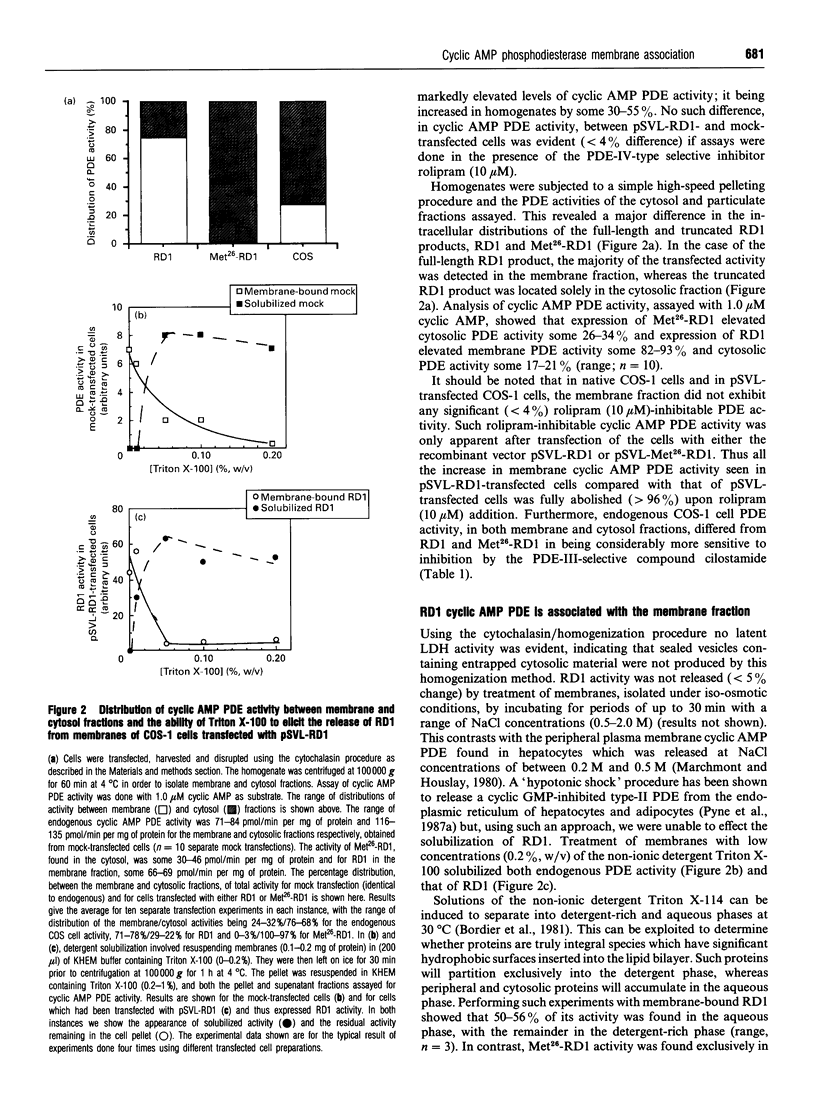
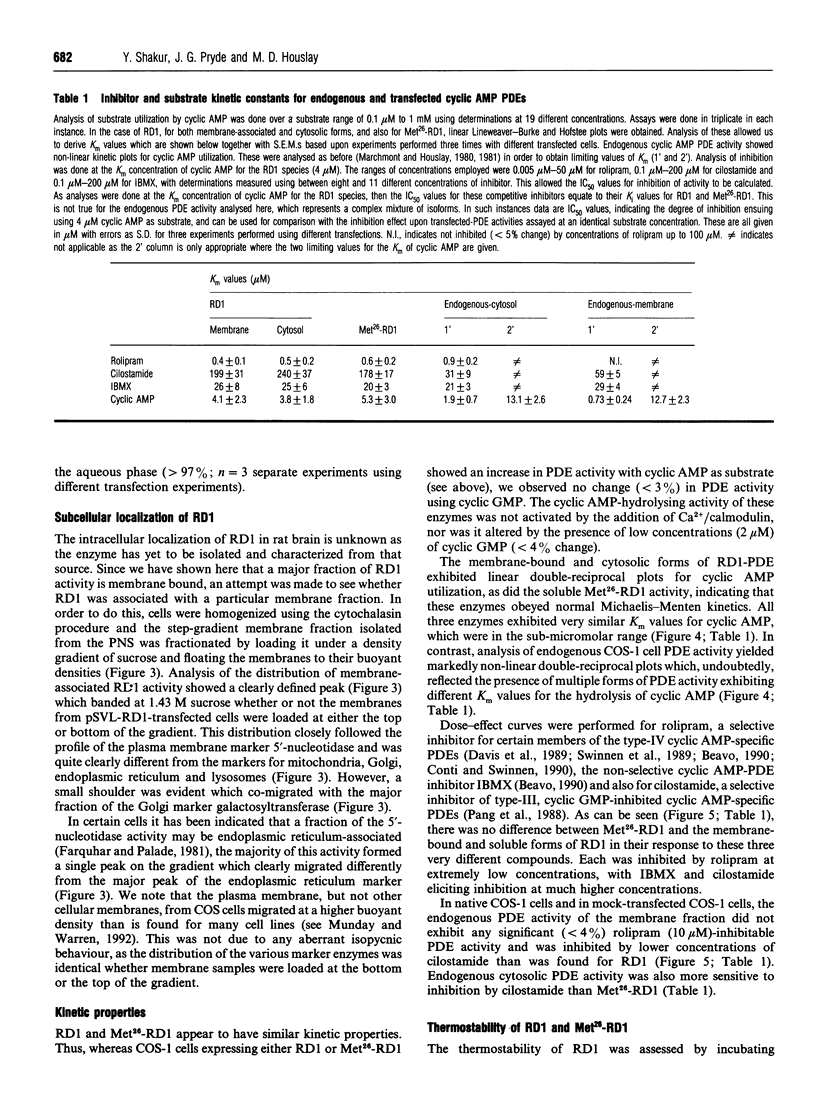
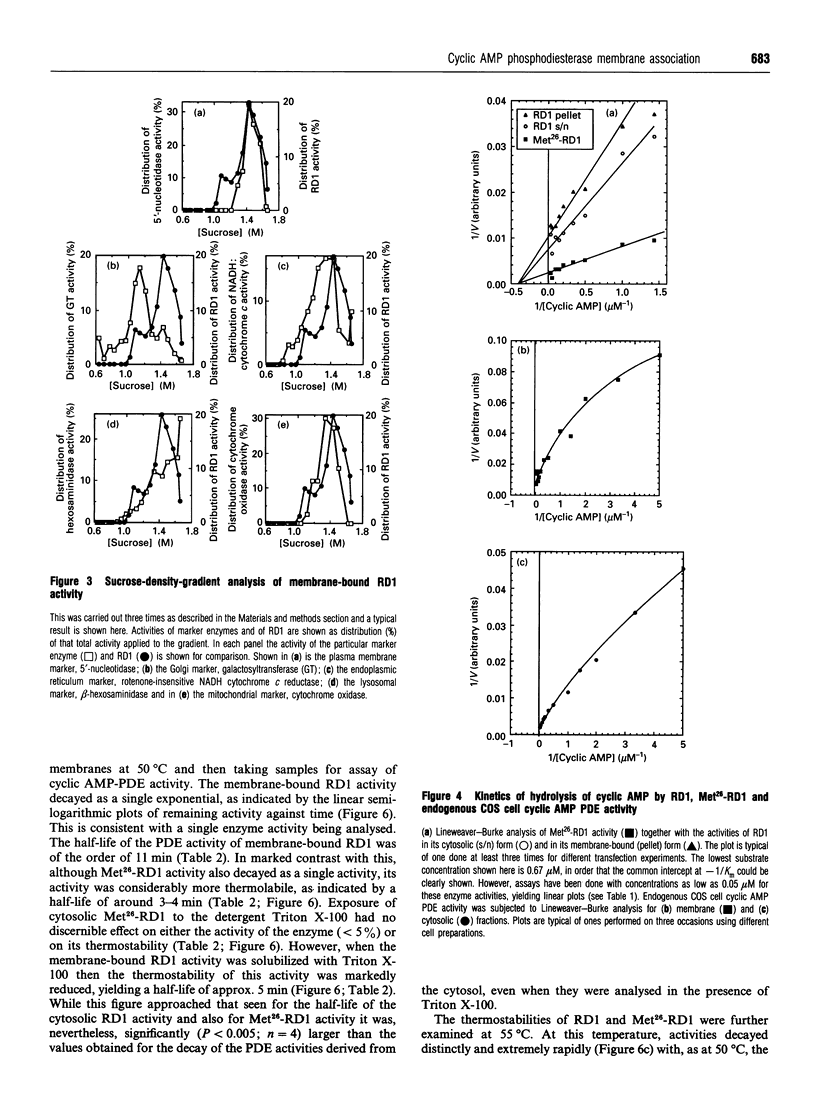
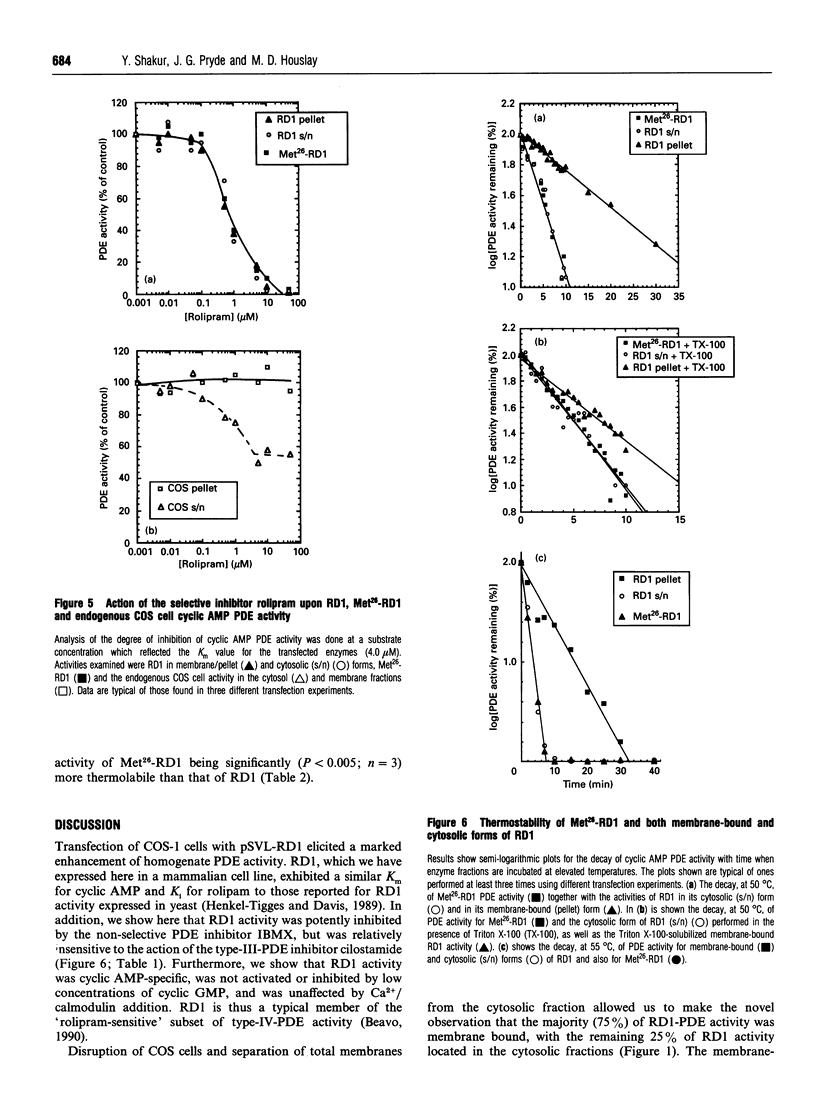
Full-length cDNA for the rat brain rolipram-sensitive cyclic AMP phosphodiesterase (PDE), RD1 was introduced into the expression vector pSVL. COS cells transfected with the recombinant vector pSVL-RD1 exhibited a 30-55% increase in homogenate PDE activity, which was abolished by rolipram (10 microM). Removal of the first 67 nucleotides of the RD1 cDNA yielded a truncated enzyme called Met26-RD1 which lacked the N-terminal first 25 amino acids. Whereas approx. 75% of RD1 activity was membrane-associated, Met26-RD1 activity was found exclusively in the cytosol fraction. Expression of RD1 nearly doubled membrane-associated PDE activity, while expression of Met26-RD1 increased cytosolic activity by approx. 30%. Membrane RD1 activity was found to be primarily associated with the plasma membrane, was not released by either high concentrations of NaCl or by a 'hypotonic shock' treatment, but was solubilized with low concentrations of Triton X-100. Phase separation of membrane components with Triton X-114 showed partition of RD1 into both the aqueous and detergent-rich phases, whereas Met26-RD1 partitioned exclusively into the aqueous phase. Both RD1 and Met26-RD1 specifically hydrolysed cyclic AMP; were unaffected by either Ca2+/calmodulin or by low cyclic GMP concentrations; exhibited linear Lineweaver-Burke plots with similar Km values for cyclic AMP (4 microM); both were potently and similarly inhibited by rolipram (Ki approx. 0.5 microM) and were similarly inhibited by cilostamide and 3-isobutyl-1-methylxanthine. Thermal inactivation, at 50 degrees C, showed that while the cytosolic-located fraction of RD1 (t0.5 approx. 3 min) and Met26-RD1 (t0.5 approx 3 min) were similarly thermolabile, membrane-bound RD1 was considerably more thermostable (t0.5 approx. 11 min). Treatment of both cytosolic RD1 and Met26-RD1 with Triton X-100 did not affect their thermostability, but solubilization of membrane RD1 activity with Triton X-100 markedly decreased its thermostability (t0.5 approx. 5 min). The N-terminal domain of RD1 appears not to influence either the substrate specificity or inhibitor sensitivity of this enzyme, but it does contain information which can allow RD1 to become plasma membrane-associated and thereby adopt a conformation which has enhanced thermostability.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bretz R., Stäubli W. Detergent influence on rat-liver galactosyltransferase activities towards different acceptors. Eur J Biochem. 1977 Jul 1;77(1):181–192. doi: 10.1111/j.1432-1033.1977.tb11656.x. [DOI] [PubMed] [Google Scholar]
- Brunton L. L., Hayes J. S., Mayer S. E. Functional compartmentation of cyclic AMP and protein kinase in heart. Adv Cyclic Nucleotide Res. 1981;14:391–397. [PubMed] [Google Scholar]
- Burke B., Gerace L. A cell free system to study reassembly of the nuclear envelope at the end of mitosis. Cell. 1986 Feb 28;44(4):639–652. doi: 10.1016/0092-8674(86)90273-4. [DOI] [PubMed] [Google Scholar]
- Chen C. N., Denome S., Davis R. L. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9313–9317. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicelli J., Birchmeier C., Michaeli T., O'Neill K., Riggs M., Wigler M. Isolation and characterization of a mammalian gene encoding a high-affinity cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1989 May;86(10):3599–3603. doi: 10.1073/pnas.86.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Sompayrac L. M. Efficient infection of monkey cells with SV40 DNA. II. Use of low-molecular-weight DEAE-dextran for large-scale experiments. J Virol Methods. 1982 Dec;5(5-6):335–341. doi: 10.1016/0166-0934(82)90025-8. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Kauvar L. M. Drosophila cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:393–402. [PubMed] [Google Scholar]
- Davis R. L., Takayasu H., Eberwine M., Myres J. Cloning and characterization of mammalian homologs of the Drosophila dunce+ gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3604–3608. doi: 10.1073/pnas.86.10.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Henkel-Tigges J., Davis R. L. Rat homologs of the Drosophila dunce gene code for cyclic AMP phosphodiesterases sensitive to rolipram and RO 20-1724. Mol Pharmacol. 1990 Jan;37(1):7–10. [PubMed] [Google Scholar]
- Houslay M. D. 'Crosstalk': a pivotal role for protein kinase C in modulating relationships between signal transduction pathways. Eur J Biochem. 1991 Jan 1;195(1):9–27. doi: 10.1111/j.1432-1033.1991.tb15671.x. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Marchmont R. J. The insulin-stimulated cyclic AMP phosphodiesterase binds to a single class of protein sites on the liver plasma membrane. Biochem J. 1981 Sep 15;198(3):703–706. doi: 10.1042/bj1980703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D., Tipton K. F. The nature of the electrophoretically separable multiple forms of rat liver monoamine oxidase. Biochem J. 1973 Sep;135(1):173–186. doi: 10.1042/bj1350173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgour E., Anderson N. G., Houslay M. D. Activation and phosphorylation of the 'dense-vesicle' high-affinity cyclic AMP phosphodiesterase by cyclic AMP-dependent protein kinase. Biochem J. 1989 May 15;260(1):27–36. doi: 10.1042/bj2600027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lavan B. E., Lakey T., Houslay M. D. Resolution of soluble cyclic nucleotide phosphodiesterase isoenzymes, from liver and hepatocytes, identifies a novel IBMX-insensitive form. Biochem Pharmacol. 1989 Nov 15;38(22):4123–4136. doi: 10.1016/0006-2952(89)90694-1. [DOI] [PubMed] [Google Scholar]
- Livi G. P., Kmetz P., McHale M. M., Cieslinski L. B., Sathe G. M., Taylor D. P., Davis R. L., Torphy T. J., Balcarek J. M. Cloning and expression of cDNA for a human low-Km, rolipram-sensitive cyclic AMP phosphodiesterase. Mol Cell Biol. 1990 Jun;10(6):2678–2686. doi: 10.1128/mcb.10.6.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J. 1980 May 1;187(2):381–392. doi: 10.1042/bj1870381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. Characterization of the phosphorylated form of the insulin-stimulated cyclic AMP phosphodiesterase from rat liver plasma membranes. Biochem J. 1981 Jun 1;195(3):653–660. doi: 10.1042/bj1950653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T. L., Poyton R. O., Wharton D. C., Schatz G. Cytochrome c oxidase from bakers' yeast. I. Isolation and properties. J Biol Chem. 1973 Feb 25;248(4):1346–1354. [PubMed] [Google Scholar]
- McHale M. M., Cieslinski L. B., Eng W. K., Johnson R. K., Torphy T. J., Livi G. P. Expression of human recombinant cAMP phosphodiesterase isozyme IV reverses growth arrest phenotypes in phosphodiesterase-deficient yeast. Mol Pharmacol. 1991 Feb;39(2):109–113. [PubMed] [Google Scholar]
- McIlhinney R. A., Chadwick J. K., Pelly S. J. Studies on the cellular location, physical properties and endogenously attached lipids of acylated proteins in human squamous-carcinoma cell lines. Biochem J. 1987 May 15;244(1):109–115. doi: 10.1042/bj2440109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy D. I., Warren G. Mitosis and inhibition of intracellular transport stimulate palmitoylation of a 62-kD protein. J Cell Biol. 1992 Jan;116(1):135–146. doi: 10.1083/jcb.116.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C., Luzio J. P., Hales C. N. The properties and extracellular location of 5'-nucleotidase of the rat fat-cell plasma membrane. Biochem J. 1975 Mar;146(3):625–633. doi: 10.1042/bj1460625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N. Modification of proteins with covalent lipids. Prog Lipid Res. 1988;27(3):177–197. doi: 10.1016/0163-7827(88)90012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pyne N. J., Anderson N., Lavan B. E., Milligan G., Nimmo H. G., Houslay M. D. Specific antibodies and the selective inhibitor ICI 118233 demonstrate that the hormonally stimulated 'dense-vesicle' and peripheral-plasma-membrane cyclic AMP phosphodiesterases display distinct tissue distributions in the rat. Biochem J. 1987 Dec 15;248(3):897–901. doi: 10.1042/bj2480897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne N. J., Cooper M. E., Houslay M. D. Identification and characterization of both the cytosolic and particulate forms of cyclic GMP-stimulated cyclic AMP phosphodiesterase from rat liver. Biochem J. 1986 Mar 1;234(2):325–334. doi: 10.1042/bj2340325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne N. J., Cooper M. E., Houslay M. D. The insulin- and glucagon-stimulated 'dense-vesicle' high-affinity cyclic AMP phosphodiesterase from rat liver. Purification, characterization and inhibitor sensitivity. Biochem J. 1987 Feb 15;242(1):33–42. doi: 10.1042/bj2420033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne N. J., Cushley W., Nimmo H. G., Houslay M. D. Insulin stimulates the tyrosyl phosphorylation and activation of the 52 kDa peripheral plasma-membrane cyclic AMP phosphodiesterase in intact hepatocytes. Biochem J. 1989 Aug 1;261(3):897–904. doi: 10.1042/bj2610897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H. H., Schmiechen R., Brezinski M., Seidler J. Stereospecific binding of the antidepressant rolipram to brain protein structures. Eur J Pharmacol. 1986 Aug 7;127(1-2):105–115. doi: 10.1016/0014-2999(86)90210-4. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A. M., Backlund P. S., Jr, Butrynski J. E., Jones T. L., Simonds W. F. The G protein connection: molecular basis of membrane association. Trends Biochem Sci. 1991 Sep;16(9):338–341. doi: 10.1016/0968-0004(91)90139-m. [DOI] [PubMed] [Google Scholar]
- Storrie B., Madden E. A. Isolation of subcellular organelles. Methods Enzymol. 1990;182:203–225. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- Swinnen J. V., Joseph D. R., Conti M. Molecular cloning of rat homologues of the Drosophila melanogaster dunce cAMP phosphodiesterase: evidence for a family of genes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5325–5329. doi: 10.1073/pnas.86.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- de Mazancourt P., Giudicelli Y. Brain low-Km cAMP phosphodiesterase. Methods Enzymol. 1988;159:766–772. doi: 10.1016/0076-6879(88)59073-0. [DOI] [PubMed] [Google Scholar]




