Abstract
Smartphone addiction, emerging from excessive use of smartphones, poses a challenge to inhibitory control functions within society. This research employed transcranial direct current stimulation (tDCS) as an intervention alongside the stop signal task (SST) to explore behavioral distinctions between individuals with smartphone addiction and a non-addicted control group, focusing on the efficacy of tDCS intervention. The participant cohort comprised 80 individuals, divided into an addiction group (39 participants, with 19 receiving active tDCS and 20 receiving sham tDCS) and a control group (41 participants, with 20 receiving active tDCS and 21 receiving sham tDCS), with anodal stimulation applied over the right dorsolateral prefrontal cortex (dlPFC) and cathodal placement over the left arm. The findings indicate that university students struggling with smartphone addiction exhibit reduced inhibitory control compared to their non-addicted peers, while maintaining similar levels of general cognitive control. Remarkably, tDCS interventions were observed to enhance inhibitory control in both groups. Although the improvement in the addiction group appeared more pronounced numerically than in the control group, no significant interaction with group was noted. However, a higher percentage of participants in the smartphone addiction (SA) group exhibited enhanced response inhibition under active tDCS. This study demonstrates the inhibitory control deficits in individuals addicted to smartphones and underscores the potential of tDCS in enhancing response inhibition. It provides a valuable reference for future tDCS research targeting smartphone addiction and highlights the importance of developing healthier smartphone usage habits.
Keywords: Smartphone addiction, tDCS, Inhibition behavior, College student
Subject terms: Neuroscience, Psychology, Health care
Introduction
In recent years, we have witnessed remarkable advancements in information technology and internet development, bringing unprecedented convenience to daily life. However, this digital revolution is not without its challenges. Mobile phones, as the foremost internet-access devices, have become a focal point of concern due to their contribution to the escalating issue of smartphone addiction (SA). Characterized by a low tolerance, the neglect of other activities, and a subjective sense of losing control, smartphone addiction represents a form of social dysfunction spurred by the excessive use of smartphones1,2. The repercussions of this addiction extend beyond mere inconvenience, posing significant threats to both physical and mental health, including bodily discomfort3, sleep disturbances4, and heightened depressive symptoms5.
In addressing the issue of smartphone addiction among college students, the academic community has developed a variety of assessment tools to accurately measure and diagnose the level of smartphone dependency within this group. Specifically, two widely recognized scales—The smartphone addiction scale–short version1 and the smartphone addiction scale for college students (SAS-C)6 play a crucial role in evaluating the extent of smartphone addiction among college students. The SAS-SV is a streamlined assessment tool designed to quickly identify an individual's dependency on smartphone use. It comprises a series of questions that cover potential social dysfunction, decreased tolerance, neglect of other activities, and a subjective sense of losing control due to excessive smartphone use. The brevity of SAS-SV makes it an effective tool for rapidly screening for tendencies towards smartphone addiction among college students. The SAS-C is tailored specifically for the college student demographic, taking into account their unique living environments and smartphone usage habits. SAS-C includes more detailed items, assessing not only the frequency and intensity of smartphone use but also considering the impact of excessive use on aspects such as learning, social interaction, and physical health. In this way, SAS-C provides researchers with a comprehensive tool to deeply understand the smartphone usage behavior of college students and its potential negative effects.
The application of these two scales allows researchers to understand the smartphone usage habits of college students from different perspectives and depths, identify potential addictive behaviors, and develop more effective intervention measures. Given the high dependency on smartphones among college students and the potential negative impacts of this dependency, the use of these specially designed diagnostic tools for assessment and intervention is particularly important. Through such measures, it is possible to help college students establish healthier smartphone usage habits, thereby promoting their overall well-being.
In exploring the mechanisms behind smartphone addiction, the concept of response inhibition is deemed crucial. Defined as the ability to suppress an action, response inhibition is key to understanding the cognitive mechanisms of smartphone addiction7. This cognitive process includes several sub-processes, such as cancellation and withholding (also known as restraint). Cancellation refers to stopping an action that has already commenced, typically assessed through the stop signal task (SST)8, while withholding involves preventing an anticipated but not yet initiated action. This study aims to delve deeply into these cognitive processes, elucidating their impact on smartphone addiction and exploring possible interventions. By understanding how individuals exercise response inhibition in the face of smartphone use temptations, we can gain a better understanding of the cognitive mechanisms of smartphone addiction and provide a theoretical basis for developing effective prevention and intervention strategies.
Furthermore, considering that individual differences in response inhibition capabilities may affect smartphone usage behavior and addiction tendencies, studying these differences and their specific impacts on smartphone addiction is particularly important. By assessing and enhancing individuals' response inhibition capabilities, it may be possible to reduce the risk of excessive smartphone use, thereby offering a potential intervention pathway to alleviate the issue of smartphone addiction.
In the context of smartphone addiction, research has consistently highlighted a significant relationship between impaired inhibitory control and excessive smartphone use9. From a neuropsychological standpoint, the right dorsolateral prefrontal cortex (dlPFC) emerges as a critical area for response inhibition. Aligning with these insights, our experiment specifically focuses on the right dlPFC10,11. This approach not only underscores the importance of inhibitory control in the context of smartphone addiction but also opens avenues for targeted interventions that could mitigate the adverse effects associated with excessive smartphone use.
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that delivers a constant, low electrical current to specific areas of the brain through electrodes placed on the scalp. This method has been increasingly used for various therapeutic and enhancement purposes in both clinical and research settings13–15. In recent years, tDCS has gained significant traction in addiction research. Studies have demonstrated that tDCS interventions can effectively reduce levels of addiction and cravings in contexts such as smartphone addiction12 and alcohol addiction13, Additionally, research has shown that anodic stimulation of the right dorsolateral prefrontal cortex (dlPFC) can enhance response inhibition capabilities11,14. To further investigate the effects of tDCS, numerous studies have adopted a comparative research methodology, contrasting active tDCS applications with sham controls15.
This study is centered around two principal inquiries. Firstly, it examines whether differences exist in response inhibition capabilities between individuals with smartphone addiction and those in the control group. Secondly, it investigates whether applying tDCS to the right dlPFC can enhance the response inhibition abilities of both the addicted individuals and the control group, and if there are notable distinctions in the degree of improvement across these groups. Stemming from these research foci, we have developed two experimental hypotheses:
Hypothesis 1 posits that, in the stop signal task (SST), the group with smartphone addiction will exhibit weaker response inhibition abilities in comparison to the control group.
Hypothesis 2 suggests that with the application of anodal tDCS to the right dlPFC, improvements in response inhibition abilities will be observed in both the smartphone addiction (SA) group and the control group under active tDCS conditions. Conversely, under sham tDCS conditions, neither group is expected to show enhancements in their response inhibition capabilities.
Materials and methods
Ethical approval
The prospective, parallel randomized controlled trial received approval from the Institutional Review Board of Sichuan Normal University (2023LS008). The research was conducted in strict adherence to the Declaration of Helsinki principles. All procedures were carried out in compliance with relevant guidelines and regulations. Informed consent was secured from all participants.
Participants
The study employed G*Power software to accurately estimate the required sample size for the experiment. By setting the effect size at the medium level (f = 0.25; η2 = 0.06)16 our calculations indicated that a total sample size of 78 participants (i.e. 39 per group) would be sufficient to detect the main group effect (70% statistical power) and interaction effects with group (85% statistical power) for repeated mesures ANOVA at a significance level of 0.05. To ensure sample adequacy, a total of 80 participants were enlisted from a university campus in Sichuan, consisting of 29 males and 51 females. The study employed the SAS-SV and SAS-C, as mentioned in the introduction, to divide participants into the smartphone addiction group and the control group. According to the SAS-SV, participants with scores above 31 (for males) and 33 (for females) were allocated to the smartphone addiction (SA) group, while those with lower scores were assigned to the control group. In the SAS-C, individuals scoring over 77 were categorized into the SA group, and those with scores under 65 were placed in the control group. Following the screening and categorization process, the final composition of the SA group included 39 participants (10 males and 29 females), and the control group comprised 41 participants (19 males and 22 females).
Experimental design
This study utilized the stop signal task (SST)17 as its experimental framework, combining psychological behavioral assessments with transcranial direct current stimulation (tDCS) interventions to investigate inhibitory control behaviors in college students with smartphone addiction and to assess the efficacy of tDCS interventions.
The initial phase of the experiment (pre-tDCS: T1) adopted a single-factor between-subjects design, identifying the group (SA group vs. control group) as the independent variable, with SST metrics such as stop signal reaction time (SSRT), go reaction time (Go RT), and probability of inhibition (P) serving as dependent variables. The subsequent phase (post-tDCS: T2) implemented a 2 (group: SA group vs. control group) × 2 (tDCS type: active tDCS vs. sham tDCS) between-subjects framework. The variables under investigation were the group and the type of tDCS, while the dependent variables remained the SST metrics including SSRT, Go RT, and P.
Transcranial direct current stimulation (tDCS)
Participants were randomly assigned to either the active or sham tDCS groups through random sampling. In the active tDCS group, participants received a consistent 2 mA current for 20 min. The tDCS electrodes were positioned on the scalp to target the dlPFC (F4), as per the international EEG 10/20 system, with the cathode positioned on the forearm18. In the sham tDCS condition, the electrode placement mirrored that of the active condition (F4 and forearm). However, the stimulator was only activated at 2 mA for the first and last 30 s of the 20-min session. This protocol was designed to mimic the initial sensation experienced in the active condition while avoiding continuous stimulation, a technique previously validated for its efficacy19.
In line with prior studies, tDCS commenced 5 min before the task and continued throughout the entire duration of the risk task, which lasted approximately 10 min20. Given that our stop signal task extended between 15 and 20 min, to maintain tDCS effectiveness from the onset, the stop signal task was conducted 10 min subsequent to tDCS initiation, allowing the remaining 10 min of stimulation to overlap with stop signal task execution.
Experimental tools
The measurement tools employed in this study are identical to those utilized in our prior research21, encompassing the smartphone addiction scale–short version (SAS-SV), the smartphone addiction scale for college students (SAS-C), the positive affect and negative affect scale (PANAS), the Barratt impulsiveness scale-11 (BIS-11), the beck depression inventory-II (BDI-II), and the beck anxiety inventory (BAI). Additionally, our previous studies have demonstrated the suitability and robust reliability of these instruments within the Chinese context21.
Experimental procedures
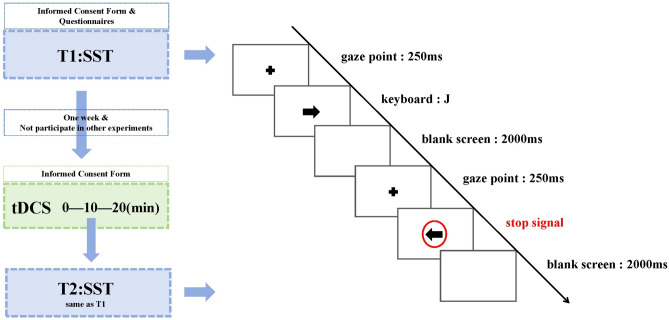
The study is structured into three distinct phases: the initial experiment (pre-tDCS: T1), the tDCS intervention phase, and the subsequent experiment (post-tDCS: T2). Both T1 and T2 were conducted in the laboratory setting. It is important to note that T1 and T2 were identical in design and procedure, with the only difference being the implementation of the tDCS intervention during T2. In line with earlier studies22,23, a 1-week interval separates T1 and T2 to minimize potential carryover effects. Before recruitment, candidates completed the SAS-SV and SAS-C to determine their grouping into either the smartphone addiction (SA) group or the control group. Upon arrival at the laboratory, participants completed an informed consent form followed by a series of questionnaires aimed at collecting demographic data and conducting psychological assessments, the latter of which was performed only during T1. This preparatory phase was subsequently followed by the main experimental session, which utilized the Stop Signal Task (SST) paradigm.
The experimental paradigm was constructed using E-Prime 3.0 software. Visual stimuli were displayed on a computer monitor with a resolution of 1920 × 1080 pixels. E-Prime 3.0 was also used to generate metrics for data analysis, ensuring a streamlined process from experiment execution to data collection.
The SST consisted of three blocks, each comprising 80 trials. Participants were allowed a 30-s rest period between blocks to prevent fatigue. Each block was structured to include both stop and go trials, maintaining a 1:3 ratio (20 stop signals and 60 reaction signals per block). This setup facilitated a balanced assessment of response inhibition capabilities. Initially, a fixation point is displayed for 250 ms, succeeded by an arrow pointing left or right, which vanishes upon the participant's key press (with the F/J keys corresponding to left/right arrows, respectively). Following a 2000 ms blank screen, a new trial commences with a 250 ms fixation point. It's crucial to note that the absence of a red circle around the arrow signifies a response signal; conversely, the appearance of a red circle around the arrow after a delay signals a stop signal, prompting the participant to suppress their initial response impulse. The entire behavioral experiment is completed within 15–20 min. The detailed procedure is illustrated in Fig. 1.
Figure 1.
The experimental task flowchart.
Questionnaire analysis for the analysis of questionnaire data, the variable "group" was utilized as the between-group factor, while the other questionnaire metrics served as dependent variables in independent samples t-tests. This approach was adopted to identify any disparities between the SA group and the control group.
Statistical analysis of the data collected from the stop signal task (SST) included several key performance indicators: stop signal reaction time (SSRT), go reaction time (Go RT), and the probability of inhibition (P). To assess differences in these SST performance metrics between the smartphone addiction (SA) group and the control group, an independent samples t-test was utilized, with "group" serving as the between-group factor.
Additionally, Pearson correlation analysis was conducted to explore the linear relationships between the scores on the smartphone addiction scale for college students (SAS-C) and the SST metrics (SSRT, Go RT, and P).
For T1 and T2 data analysis, given the assumption that the Successful Inhibition Rate (P) is around 50%, a 2 (group: SA group vs. control group) × 2 (time: T1 vs. T2) × 2 (tDCS type: active tDCS vs. sham tDCS) repeated measures ANOVA was utilized to analyze both SSRT and Go RT. In this model, "time" is treated as the within-subject factor, whereas "group" and "tDCS type" are the between-subject factors.
Further detailed analyses were conducted using paired samples t-tests and independent samples t-tests, with "group" as the factor for between-subject comparisons, to provide a deeper exploration of the data. These analyses were particularly critical in understanding the inter-individual variability observed in tDCS effects, which is an important aspect of the study given the personalized nature of brain stimulation responses.
Results
Demographic characteristics and scale outcomes
The demographic data and outcomes from each scale were examined through independent samples t-tests across both groups, with the results detailed in Table 1. Notable disparities were found in the scores for the smartphone addiction scale–short version (SAS-SV), the smartphone addiction scale for college students (SAS-C), the positive and negative affect scale (PANAS) negative affect scores, impulsivity along with its three subscale scores, and anxiety levels. In these measures, the smartphone addiction (SA) group exhibited significantly higher scores compared to the control group. Conversely, no significant differences were identified in the scores for positive affect and depression between the SA and control groups.
Table 1.
Demographic information and related scale scores.
| Control group (M ± SD) | SA group (M ± SD) | t | P | |
|---|---|---|---|---|
| Sample size | 41 | 39 | ||
| Age | 19.76 ± 1.39 | 20.36 ± 2.02 | 1.547 | 0.127 |
| Gender (F/M) | 1.54 ± 0.50 | 1.74 ± 0.44 | 1.953 | 0.054 |
| SAS-SV | 46.83 ± 9.20 | 80.33 ± 12.49 | 13.709 | 0.000 |
| SAS-C | 24.73 ± 5.71 | 45.97 ± 7.23 | 14.612 | 0.000 |
| PANAS: positive | 28.29 ± 5.51 | 28.87 ± 7.04 | 0.411 | 0.682 |
| PANAS: negative | 14.78 ± 4.03 | 17.77 ± 5.67 | 2.705 | 0.009 |
| BIS: attentional impulsivity | 12.34 ± 1.88 | 15.54 ± 2.49 | 6.502 | 0.000 |
| BIS: movement impulsivity | 20.02 ± 3.24 | 22.28 ± 4.35 | 2.644 | 0.010 |
| BIS: unplanned impulsivity | 22.24 ± 3.48 | 25.31 ± 3.58 | 3.880 | 0.000 |
| BIS | 54.61 ± 6.05 | 63.13 ± 8.27 | 5.236 | 0.000 |
| BDI | 7.70 ± 8.89 | 11.23 ± 9.20 | 1.742 | 0.085 |
| BAI | 29.66 ± 5.16 | 36.00 ± 11.30 | 3.203 | 0.002 |
SAS-SV smartphone addiction scale-short version, SAS-C smartphone addiction scale for college students, PANAS positive and negative affect scale, BIS Barratt impulsiveness scale-11, BDI beck depression inventory-II, BAI beck anxiety inventory.
Table 2 presents the correlation matrix for the variables examined in this study. The analysis reveals significant positive correlations between smartphone addiction, as measured by the smartphone addiction scale–short version (SAS-SV), and several psychological dimensions. Specifically, smartphone addiction is positively associated with negative emotions (r = 0.284, p < 0.05), attentional impulsivity (r = 0.596, p < 0.01), motor impulsivity (r = 0.356, p < 0.01), non-planning impulsivity (r = 0.381, p < 0.01), overall impulsivity as assessed by the Barratt impulsiveness scale (BIS) (r = 0.537, p < 0.01), depression as measured by the beck depression inventory (BDI) (r = 0.297, p < 0.01), and anxiety levels according to the beck anxiety inventory (BAI) (r = 0.405, p < 0.01).
Table 2.
Correlation matrix of each variable.
| Correlation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | SAS-SV | SA-SC | PANAS: positive | PANAS: negative | BIS: attentional impulsivity | BIS: movement impulsivity | BIS: unplanned impulsivity | BIS | BDI | |
| SAS-SV | 0.098 | |||||||||
| SAS-C | 0.119 | 0.907** | ||||||||
| PANAS: positive | − 0.231* | 0.032 | 0.037 | |||||||
| PANAS: negative | 0.009 | 0.284* | 0.296** | – 0.141 | ||||||
| BIS: attentional impulsivity | − 0.002 | 0.596** | 0.608** | − 0.119 | 0.323** | |||||
| BIS: movement impulsivity | 0.038 | 0.356** | 0.288** | 0.113 | 0.172 | 0.311** | ||||
| BIS: unplanned impulsivity | − 0.047 | 0.381** | 0.324** | − 0.225* | 0.208 | 0.558** | 0.455** | |||
| BIS | − 0.004 | 0.537** | 0.483** | − 0.088 | 0.282* | 0.728** | 0.784** | 0.855** | ||
| BDI | 0.138 | 0.297** | 0.237* | − 0.454** | 0.497** | 0.242* | 0.042 | 0.208 | 0.194 | |
| BAI | 0.102 | 0.405** | 0.343** | − 0.120 | 0.608** | 0.371** | 0.135 | 0.186 | 0.269* | 0.576** |
SAS-SV smartphone addiction scale-short version, SAS-C smartphone addiction scale for college students, BIS Barratt impulsiveness scale-11, BDI beck depression inventory-II, BAI beck anxiety inventory.
*At the 0.05 level (two tailed), the correlation is significant.
**At the 0.01 level (two tailed), the correlation is significant.
Additionally, smartphone addiction, as quantified by the smartphone addiction scale for college students (SAS-C), shows significant positive correlations with negative emotions (r = 0.296, p < 0.01) and the composite score for positive and negative emotions as determined by the positive affect and negative affect scale (PANAS) (r = 0.232, p < 0.05). It also correlates positively with attentional impulsivity (r = 0.608, p < 0.01), motor impulsivity (r = 0.288, p < 0.01), non-planning impulsivity (r = 0.324, p < 0.01), total impulsivity (BIS) (r = 0.483, p < 0.01), depression (BDI) (r = 0.237, p < 0.05), and anxiety (BAI) (r = 0.343, p < 0.01).
T1 experiment results
In the stop signal task, the success inhibition rates (P) for the two groups were recorded at 0.523 and 0.506, respectively. These rates approximate 50% of the correct response rate, which is in line with the expectations of this experimental paradigm. Subsequent analyses focused on the stop signal reaction time (SSRT) and the go reaction time (Go RT).
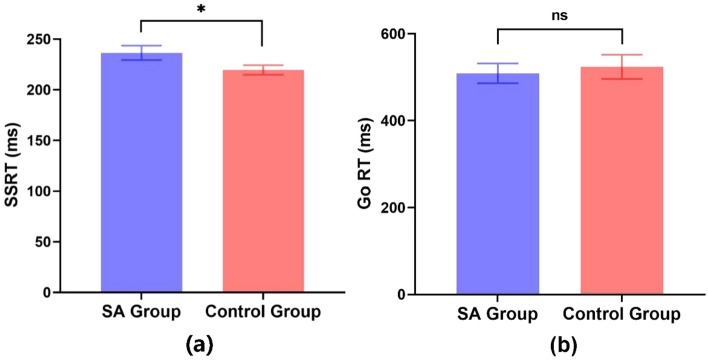
An independent samples t-test was conducted to compare the SSRT and Go RT scores between the SA (addiction group) and CG (control group) groups without tDCS intervention. The analysis revealed a significant difference in SSRT scores (t(79) = 2.008, p < 0.05, Cohen’s d = 0.45). Specifically, the control group exhibited significantly lower SSRT scores compared to the SA group. In contrast, the Go RT scores did not show a significant difference between the two groups (t(79) = − 0.423, p > 0.05, Cohen’s d = 0.09), see Fig. 2.
Figure 2.
Comparison of SSRT (a) and Go RT (b) between the smartphone addiction (SA) group (n = 39) and the control group (n = 41) at T1 (error bars represent standard errors; ns: p > 0.05; *p < 0.05).
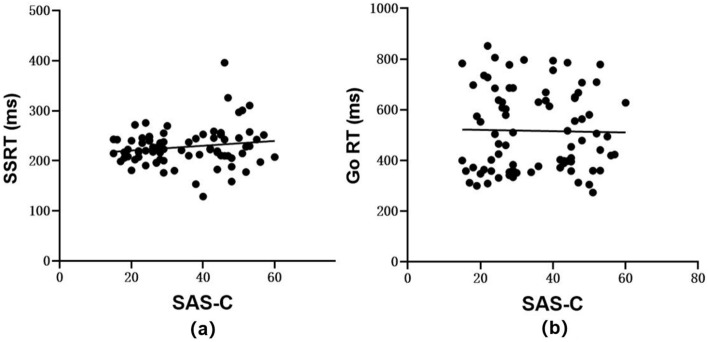
Ultimately, a Pearson correlation analysis was performed revealing that no linear correlation exists between the SAS-C scores and both SSRT (p > 0.05, r = 0.157) and Go RT (p > 0.05, r = − 0.020), as illustrated in Fig. 3 panels a and b.
Figure 3.
Scatter plots of correlation analyses (n = 80): (a) between SAS-C and SSRT, and (b) between SAS-C and Go Reaction Time (Go RT).
Results from the T1 compared to T2 experiments with tDCS (active and sham) intervention
For the T1 experiment, the success inhibition rates (P) were observed at 0.523 and 0.506 for the SA and control groups, respectively. During the T2 experiment, these rates slightly decreased to 0.483 for the SA group and 0.471 for the control group, with an overall average P of 0.477. Subsequent analyses focused on the Stop Signal Reaction Time (SSRT) and Go Reaction Time (Go RT).
To evaluate the impact of active tDCS stimulation on pre-posttest, independent sample t-tests were performed for both participant groups at two distinct time points, T1 and T2. A marginally significant difference in SSRT was noted between the two groups at T1 (t(38) = 1.891, p = 0.066, Cohen's d = 0.61). By T2, no significant difference in SSRT between two groups (t(38) = 1.186, p = 0.243, Cohen's d = 0.38), referred Table 3.
Table 3.
Intergroup differences in SSRT at T1 and T2 time period during active tDCS stimulation.
| Pre-posttest | SA group (M ± SE) | Control group (M ± SE) | t | p |
|---|---|---|---|---|
| Sample size | 19 | 20 | ||
| T1 | 249.68 ± 11.46 | 225.18 ± 6.42 | 1.891 | 0.066 |
| T2 | 222.85 ± 5.81 | 211.92 ± 7.08 | 1.186 | 0.243 |
SA smartphone addiction, T1 pre-tDCS, T2 post-tDCS.
Subsequently, to assess whether both the SA group and the control group exhibited improvements in response inhibition ability under active tDCS conditions, paired sample t-tests were employed for T1 vs. T2. Significant reductions in SSRT from T1 to T2 were observed in both the SA group (t(18) = 2.201, p < 0.05, Cohen's d = 0.68) and the control group (t(19) = 0.319, p < 0.05, Cohen's d = 0.44), referred Table 4.
Table 4.
Comparison of SSRT differences at T1 and T2 during active tDCS stimulation in SA and control groups.
| Group | Sample size | T1 (M ± SE) | T2 (M ± SE) | t | p |
|---|---|---|---|---|---|
| SA | 19 | 249.68 ± 11.46 | 222.85 ± 5.81 | 2.201 | 0.041 |
| Control | 20 | 225.18 ± 6.42 | 211.91 ± 7.08 | 2.319 | 0.032 |
SA smartphone addiction, T1 pre-tDCS, T2 post-tDCS.
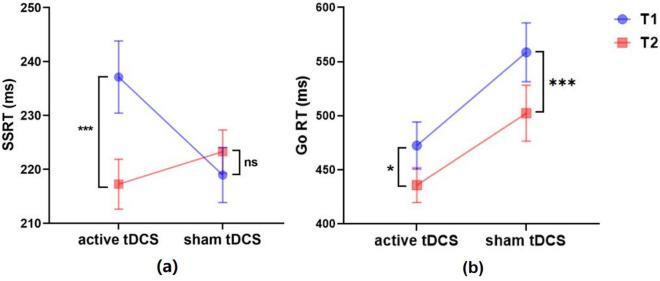
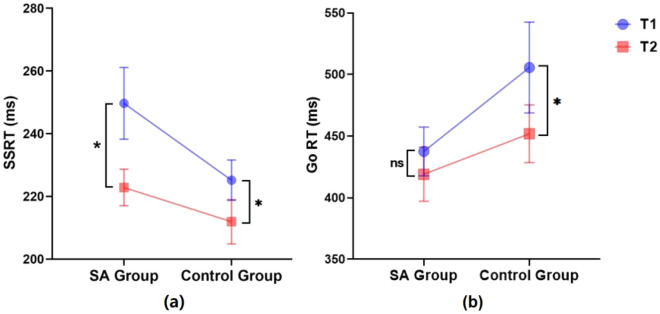
Further analysis, the SSRT and Go RT were analyzed using a 2 (group: SA vs. group) × 2 (pre-post tDCS: T1 vs. T2) × 2 (tDCS stimulus type: active vs. sham) repeated measures ANOVA. This analysis revealed a significant interaction between time and tDCS type (F(1,76) = 9.356, p < 0.01, partial η2 = 0.110) in SSRT. Further simple effect analysis indicated a marked reduction in SSRT from T1 to T2 under active tDCS conditions (p < 0.001, Cohen's d = 0.55). Conversely, no significant change was observed under sham tDCS conditions (p > 0.05, Cohen's d = 0.15), as depicted in Fig. 4a. Furthermore, the results showed no significant main effect of time (F(1,76) = 3.918, p > 0.05, partial η2 = 0.049). The interaction between time and group was not significant (F(1,76) = 1.085, p > 0.05, partial η2 = 0.014), and the triple interaction involving time, tDCS type, and group also failed to reach statistical significance (F(1,76) = 0.441, p > 0.05, partial η2 = 0.006). In case of Go RT, the results showed significant main effect of time (F(1,76) = 15.632, p < 0.001, partial η2 = 0.171), T1 was significantly higher than T2 (p < 0.001, Cohen's d = 0.31). Specifically, under both active stimulation (p < 0.05, Cohen's d = 0.31) and sham stimulation (p < 0.001, Cohen's d = 0.33) conditions, the Go RT at T1 was significantly higher than at T2 (Fig. 4b). There was no significant interaction between time and tDCS type (F(1,76) = 0.742, p > 0.05, partial η2 = 0.010). Moreover, the interaction between time and group was not significant (F(1,76) = 0.464, p > 0.05, partial η2 = 0.006), and the triple interaction involving time, tDCS type, and group also failed to reach statistical significance (F(1,76) = 0.684, p > 0.05, partial η2 = 0.009).
Figure 4.
Interaction effects of time and tDCS modality for SSRT (a) and Go RT (b) (active tDCS: n = 39; sham tDCS: n = 41; error bars represent standard errors; ns: p > 0.05; *p < 0.05; ***p < 0.001).
On another note, for participants assigned to active tDCS, a significant decrease in SSRT from T1 to T2 was observed in both SA group (t(18) = 2.201, p < 0.05, Cohen's d = 0.68) and the control group (t(19) = 2.319, p < 0.05, Cohen's d = 0.44) (refer to Fig. 5a). The analysis of Go RT revealed no significant difference between T1 and T2 in the SA group (t(18) = 1.683, p > 0.05, Cohen's d = 0.20); however, in the control group, the Go RT at T1 was significantly higher than at T2 (t(19) = 2.102, p < 0.05, Cohen’s d = 0.39). Refer to Fig. 5b.
Figure 5.
T1 and T2 for SA and control participants assigned to active tDCS (SA group: n = 19; control group: n = 20; error bars represent standard errors; ns: p > 0.05; *p < 0.05).
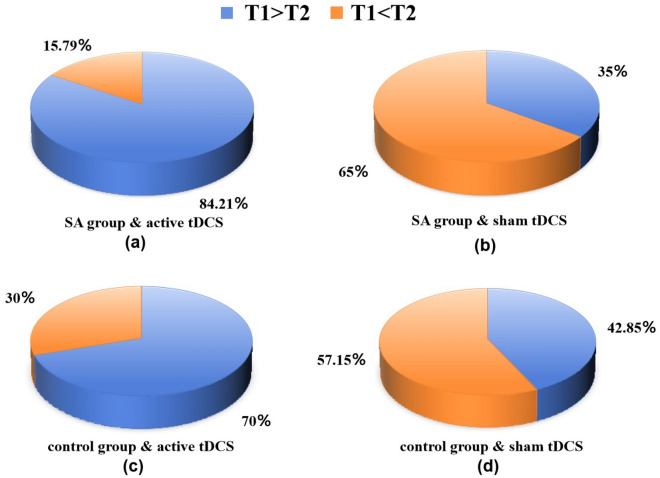
Around 84.21% of participants in the SA group and 70% in the control group exhibited enhanced response inhibition under active tDCS. In contrast, only 35% of SA participants and approximately 42.85% of control participants showed improvement under sham tDCS conditions (refer to Fig. 6).
Figure 6.
Comparative analysis and proportional improvement of SSRT from T1 to T2 under active and sham tDCS for both the SA group and the control group: (a) SA group with active tDCS; (b) SA group with sham tDCS; (c) control group with active tDCS; (d) control group with sham tDCS).
Discussion
We conducted two stop signal tasks (SSTs) to examine differences in response inhibition abilities between the smartphone addiction (SA) group and the control group, and to assess the impact of transcranial direct current stimulation (tDCS) on enhancing these abilities. The findings revealed that the SA group exhibited weaker response inhibition compared to the control group. Notably, tDCS proved effective in enhancing response inhibition abilities across both groups.
Our questionnaire analysis, consistent with our previous research21, revealing that college students grappling with smartphone addiction experience heightened negative emotions, increased impulsivity, and more pronounced anxiety levels compared to their non-addicted peers. This finding corroborates existing literature that establishes a significant link between smartphone addiction, anxiety, and impulsivity24,25.
Pera et al. have highlighted the close connection between excessive smartphone usage and psychological states such as stress, anxiety, and impulsivity. These emotional conditions may prompt individuals to seek an escape in the digital world, thereby intensifying their reliance on smartphones. Furthermore, the study suggests that heavy smartphone usage could detrimentally affect social engagement, with a scarcity of social interactions potentially diminishing the individual's capacity for enjoyment and, in turn, exacerbating smartphone dependency26. It is thus conceivable that individuals heavily engaged with their devices, coupled with insufficient real-world social interactions, may find it increasingly challenging to detach from their smartphones, potentially deepening the addiction.
In examining T1, we observed that the response inhibition capability in the smartphone addiction (SA) group was inferior to that of the control group. However, no significant difference was detected in the Go Reaction Time (Go RT), indicating that the impairment in smartphone addicts was specific to response inhibition, without affecting overall cognitive control functions. This outcome aligns with the hypothesis proposed in study 1.
Given the detrimental impacts of smartphone addiction on physical, psychological, and social well-being, it has emerged as a leading non-substance addiction today. Similar to substance, internet, and other addictions, smartphone addiction is characterized by typical withdrawal symptoms, including tolerance, mood fluctuations, and relapse. such as tolerance, mood changes, and relapse27,28. The finding echoed in studies of substance use disorders29, gambling disorders30, and excessive internet use31. Although research into response inhibition in smartphone addiction is nascent, the parallels in symptomatic presentation suggest that individuals with smartphone addiction likely experience compromised response inhibition to some degree.
Impulsivity, marked by an inability to restrain certain behaviors and deficits in executive function—especially in inhibitory control—has been identified as a behavioral hallmark of anxiety32,33. Kraan et al. have specifically noted the association between impaired response inhibition and anxiety34. Moreover, links between depression and response inhibition have been established35,36, with Liang et al. suggesting that individuals with subclinical depression (SD) may exhibit response inhibition deficits in implicit emotional processing, potentially indicating a predisposition to depression37. Our questionnaire results indicate elevated levels of impulsivity, anxiety, and depression among smartphone addicts, suggesting compromised inhibitory control and, consequently, diminished response inhibition compared to controls. This lack of inhibitory control can, in turn, precipitate impulsive behavior32. We also found a positive correlation between smartphone addiction scores and levels of impulsivity, depression, and anxiety, indicating that more severe addiction is likely associated with greater psychological distress and impaired inhibitory control. These insights underscore the complexity and gravity of inhibitory control impairment in individuals with smartphone addiction, warranting significant attention.
Following the application of active tDCS, a significant reduction in SSRT was observed in both groups, with no notable changes following sham tDCS. This outcome validates the effectiveness of our experimental approach and supports our second hypothesis. The enhancement in response inhibition abilities post-active stimulation across both groups suggests a beneficial impact of tDCS on inhibitory control.
Additionally, we observed that the Go RT for participants were faster at T2 compared to T1. Given the 7–10 day interval between T1 and T2, the potential for practice effects was substantially minimized22,23. However, due to neural plasticity, improvements in working memory and task performance with practice are still possible38,39. Consequently, the experimental conditions at T2 might have been slightly influenced by the prior experience during T1, leading to increased familiarity with the task procedures and button operations, and thus resulting in faster reaction times.
The findings of our investigation lend further support to existing research. Scholars have previously identified that impaired response inhibition is linked to reduced activation of the dorsolateral prefrontal cortex (dlPFC), and that anodal transcranial direct current stimulation (tDCS) can enhance cortical excitability, thereby improving response inhibition capabilities40. Friehs and colleagues, through their research conducted in 201811 and 201914, explored the effects of both anodal and cathodal tDCS on the right dlPFC. Their findings underscored that response inhibition is intricately connected to the neural dynamics of the right dlPFC, where activation of this region correlates with superior performance on the stop signal task (SST), as evidenced by shorter stop signal reaction times (SSRT). Thus, the application of anodal stimulation to the right dlPFC as a means to bolster response inhibition abilities emerges as a judicious and empirically grounded approach. Our study's outcomes further underscore the pivotal role of the right dlPFC in overarching goal-directed cognitive control, particularly highlighting the link between its heightened activation and improved SST performance10. Moreover, our findings align with the viewpoint posited by Friehs et al. that inhibitory control is not a static resource but varies with the functional state of the prefrontal cortex (PFC)11.
In assessing sustained effects and potential clinical applications of tDCS, a more cautious approach is warranted. Transcranial magnetic stimulation (TMS) exhibits a stronger stimulatory impact, and the scholarly consensus recommends prudence regarding its long-term effectiveness41. This cautionary stance is similarly applicable to tDCS. For example, in a study where researchers administered tDCS over a period of five days, they observed that the effects persisted during follow-up sessions at one and two months post-intervention42. Furthermore, Ljubisavljevic et al. demonstrated that repeated tDCS applications to the right dorsolateral prefrontal cortex (dlPFC) could prolong the duration of effects, detectable up to 30 days following stimulation43. Although our investigation was limited to a single experiment, by integrating our results with prior research, we find compelling evidence supporting the efficacy of tDCS. Additionally, our analysis leads us to hypothesize that multiple tDCS sessions could potentially sustain long-term effects. Overall, our findings further validate the potential of tDCS in modulating cognitive behaviors among individuals with smartphone addiction. This opens up exciting prospects for leveraging tDCS to influence negative behavioral patterns in smartphone addicts through extensive application of this technology.
It is crucial to note that the majority of tDCS studies typically position the anode and cathode electrodes on the scalp44, or alternatively, one on the forehead and the other on the right supra-orbital area45. This experiment, however, employed a less conventional approach by placing the anode on the right dlPFC and the cathode on the contralateral arm. Despite the relative scarcity of research utilizing this configuration, our study demonstrates its viability, offering novel insights for future electrode placement strategies in tDCS studies.
In conclusion, this study sheds light on the impact of smartphone addiction on inhibitory control, thereby broadening our comprehension of the negative repercussions associated with excessive smartphone use. Additionally, it underscores the potential of transcranial direct current stimulation (tDCS) as a therapeutic intervention for modifying the cognitive behaviors of individuals afflicted with smartphone addiction. The implications of this research are manifold, encompassing the promotion of healthier patterns of smartphone usage, informing clinical interventions, and contributing to the ongoing refinement of tDCS methodologies.
Nevertheless, it is critical to acknowledge certain limitations inherent in our study. Primarily, the application of anodal stimulation was confined to the right dlPFC, without exploring the effects of bilateral brain stimulation. Previous research has suggested that bilateral tDCS might exert a more pronounced effect compared to unilateral stimulation15,46,47. Furthermore, our study was characterized by a relatively small sample size, which limits the generalizability of the findings. In addition, the participant pool consisted exclusively of university students, and the tDCS intervention was administered only once, without assessing its long-term effects. Additionally, the study did not ensure a balanced gender distribution.
Another crucial point to note is that in the repeated measures ANOVA conducted on SSRT, we did not find a significant interaction between time and group, which indicates that the SA group did not exhibit greater improvement in response inhibition than the control group. While we infer a larger improvement in response inhibition for the SA group after active tDCS based on the percentage improvement in both groups, the comparison of SSRT between the two groups under active tDCS at T1 and T2, and the SSRT reduction interval shown in Fig. 5a, this inference lacks statistical rigor. We earnestly hope to expand the sample size in future investigations to statistically demonstrate that tDCS can better enhance response inhibition in the SA group.
Thirdly, our experimental design was single-blind rather than double-blind, which may introduce some experimenter bias. Fourthly, challenges arose from using the Brain Premier direct current stimulator as the tDCS device due to its instability. High-definition transcranial direct current stimulation (HD-tDCS) could potentially address the limitations of traditional tDCS. With its higher spatial resolution and focal precision, HD-tDCS allows for more targeted stimulation of cortical regions compared to traditional tDCS48. This could enhance the efficacy and reproducibility of the results in future studies. Future research would benefit from utilizing more reliable HD-tDCS equipment, potentially incorporating bilateral cortical stimulation and exploring the long-term effects of such interventions. Additionally, it is important to note that our experimental task utilized only visual cues as signals. Research has shown that different stop signal modalities (auditory, tactile, visual) can influence response inhibition49, and the integration of cross-modal stop signals has been demonstrated to enhance reactive response inhibition50. Given the prevalent use of tactile and auditory signals in smartphone applications for notifications, our future research will include elements of smartphone usage to investigate cross-modal stop signals. Our aim is to develop a more sophisticated experimental paradigm that allows researchers to delve deeper into this area of study.
To encapsulate, the empirical findings from our research indicate that college students grappling with smartphone addiction exhibit diminished response inhibition capabilities relative to their non-addicted counterparts. Notably, the deficiencies observed in the addicted group are specific to response inhibition, without extending to broader cognitive control functions. Importantly, tDCS interventions prove beneficial across the board, enhancing response inhibition abilities in both groups.
Author contributions
X.B. and X.L conceived the experiment's concept. X.B. and H.L. were responsible for data analysis and drafting the manuscript. L.X. and X.L. undertook subsequent revisions and modifications to the manuscript. Both X.B. and X.L. contributed to the design of the study's algorithm. Data collection was carried out by X.B., H.L., and T.L. All authors have reviewed and approved the final version of the manuscript for publication.
Funding
This research was funded by two grants from the Natural Science Foundation of China (32271142), Sichuan Applied Psychology Research Center (CSXL-22204), and Natural Science Foundation of Sichuan Province (2022NSFSC1631).
Data availability
The datasets generated during and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xue Bai and Huafang Liu.
Contributor Information
Lei Xu, Email: xulei@sicnu.edu.cn.
Xiaolong Liu, Email: xiaolongliu@sicnu.edu.cn.
References
- 1.Kwon, M., Kim, D. J., Cho, H. & Yang, S. The smartphone addiction scale: Development and validation of a short version for adolescents. PLoS One8(12), e83558 (2013). 10.1371/journal.pone.0083558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon, M. et al. Development and validation of a smartphone addiction scale (SAS). PLoS One8(2), e56936 (2013). 10.1371/journal.pone.0056936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi, Y. S. A study on mobile phone addiction and physical pain based on characteristics of mobile phone usage. J. Med. Imaging Health Inf.9(6), 1191–1195 (2019). 10.1166/jmihi.2019.2716 [DOI] [Google Scholar]
- 4.Mei, S. et al. Health risks of mobile phone addiction among college students in China. Int. J. Mental Health Addict.21(4), 2650–2665 (2023). 10.1007/s11469-021-00744-3 [DOI] [Google Scholar]
- 5.Yuan, G., Elhai, J. D. & Hall, B. J. The influence of depressive symptoms and fear of missing out on severity of problematic smartphone use and Internet gaming disorder among Chinese young adults: A three-wave mediation model. Addict. Behav.112, 106648 (2021). 10.1016/j.addbeh.2020.106648 [DOI] [PubMed] [Google Scholar]
- 6.Su, S. et al. Development of the smartphone addiction scale for college students. Chin. Mental Health J.28(5), 392–397 (2014). [Google Scholar]
- 7.Wright, L., Lipszyc, J., Dupuis, A., Thayapararajah, S. W. & Schachar, R. Response inhibition and psychopathology: A meta-analysis of go/no-go task performance. J. Abnorm. Psychol.123(2), 429–439 (2014). 10.1037/a0036295 [DOI] [PubMed] [Google Scholar]
- 8.Kooijmans, R., Scheres, A. & Oosterlaan, J. Response inhibition and measures of psychopathology: A dimensional analysis. Child Neuropsychol.6(3), 175–184 (2000). 10.1076/chin.6.3.175.3154 [DOI] [PubMed] [Google Scholar]
- 9.Chen, J., Liang, Y., Mai, C., Zhong, X. & Qu, C. General deficit in inhibitory control of excessive smartphone users: Evidence from an event-related potential study. Front. Psychol.7, 511 (2016). 10.3389/fpsyg.2016.00511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, T. et al. Transcranial direct current stimulation of the right dorsolateral prefrontal cortex improves response inhibition. Int. J. Psychophysiol.162, 34–39 (2021). 10.1016/j.ijpsycho.2021.01.014 [DOI] [PubMed] [Google Scholar]
- 11.Friehs, M. A. & Frings, C. Pimping inhibition: Anodal tDCS enhances stop-signal reaction time. J. Exp. Psychol. Hum. Percept. Perform.44(12), 1933–1945 (2018). 10.1037/xhp0000579 [DOI] [PubMed] [Google Scholar]
- 12.Lee, E. The effect of transcranial direct current stimulation on smartphone addiction and stress: A randomized controlled study. Phys. Ther. Rehabilit. Sci.10(1), 76–81 (2021). 10.14474/ptrs.2021.10.1.76 [DOI] [Google Scholar]
- 13.Boggio, P. et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study. Drug Alcohol Depend.92(1–3), 55–60 (2008). 10.1016/j.drugalcdep.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 14.Friehs, M. A. & Frings, C. Cathodal tDCS increases stop-signal reaction time. Cogn. Affect. Behav. Neurosci.19(5), 1129–1142 (2019). 10.3758/s13415-019-00740-0 [DOI] [PubMed] [Google Scholar]
- 15.Fecteau, S. et al. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J. Neurosci.27(23), 6212–6218 (2007). 10.1523/JNEUROSCI.0314-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen, J. Statistical Power Analysis for the Behavioral Sciences (Routledge, 1988). [Google Scholar]
- 17.Aksiotis, V., Myachykov, A. & Tumyalis, A. Stop-signal delay reflects response selection duration in stop-signal task. Atten. Percept. Psychophys.85(6), 1976–1989 (2023). 10.3758/s13414-023-02752-y [DOI] [PubMed] [Google Scholar]
- 18.Khedr, E. M. et al. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain Stimul.10(5), 893–901 (2017). 10.1016/j.brs.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 19.Gandiga, P. C., Hummel, F. C. & Cohen, L. G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol.117(4), 845–850 (2006). 10.1016/j.clinph.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 20.Boggio, P. S. et al. Modulation of decision-making in a gambling task in older adults with transcranial direct current stimulation. Eur. J. Neurosci.31(3), 593–597 (2010). 10.1111/j.1460-9568.2010.07080.x [DOI] [PubMed] [Google Scholar]
- 21.Liu, X., Tian, R., Liu, H., Bai, X. & Lei, Y. Exploring the impact of smartphone addiction on risk decision-making behavior among college students based on fNIRS technology. Brain Sci.13(9), 1330 (2023). 10.3390/brainsci13091330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorini, A., Lucchiari, C., Russell-Edu, W. & Pravettoni, G. Modulation of risky choices in recently abstinent dependent cocaine users: A transcranial direct-current stimulation study. Front. Hum. Neurosci.8, 661 (2014). 10.3389/fnhum.2014.00661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu, L. L. et al. A role for the right dorsolateral prefrontal cortex in enhancing regulation of both craving and negative emotions in internet gaming disorder: A randomized trial. Eur. Neuropsychopharmacol.36, 29–37 (2020). 10.1016/j.euroneuro.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rho, M. J. et al. Types of problematic smartphone use based on psychiatric symptoms. Psychiatry Res.275, 46–52 (2019). 10.1016/j.psychres.2019.02.071 [DOI] [PubMed] [Google Scholar]
- 25.Kim, Y. J., Jang, H. M., Lee, Y., Lee, D. & Kim, D. J. Effects of internet and smartphone addictions on depression and anxiety based on propensity score matching analysis. Int. J. Environ. Res. Public Health15(5), 859 (2018). 10.3390/ijerph15050859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pera, A. The psychology of addictive smartphone behavior in young adults: Problematic use, social anxiety, and depressive stress. Front. Psychiatry11, 573473 (2020). 10.3389/fpsyt.2020.573473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yen, C. F. et al. Symptoms of problematic cellular phone use, functional impairment and its association with depression among adolescents in Southern Taiwan. J. Adolesc.32(4), 863–873 (2009). 10.1016/j.adolescence.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 28.Chen, J. et al. Impulsivity and response inhibition related brain networks in adolescents with internet gaming disorder: A preliminary study utilizing resting-state fMRI. Front. Psychiatry11, 618319 (2020). 10.3389/fpsyt.2020.618319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, J. L., Mattick, R. P., Jamadar, S. D. & Iredale, J. M. Deficits in behavioural inhibition in substance abuse and addiction: A meta-analysis. Drug Alcohol Depend.145, 1–33 (2014). 10.1016/j.drugalcdep.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 30.Billieux, J. et al. Investigation of impulsivity in a sample of treatment-seeking pathological gamblers: A multidimensional perspective. Psychiatry Res.198(2), 291–296 (2012). 10.1016/j.psychres.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 31.Dong, G., Devito, E. E., Du, X. & Cui, Z. Impaired inhibitory control in “internet addiction disorder”: A functional magnetic resonance imaging study. Psychiatry Res.203(2–3), 153–158 (2012). 10.1016/j.pscychresns.2012.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bari, A. & Robbins, T. W. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol.108, 44–79 (2013). 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 33.Leshem, R. & Yefet, M. Does impulsivity converge distinctively with inhibitory control? Disentangling the cold and hot aspects of inhibitory control. Pers. Individ. differ.145, 44–51 (2019). 10.1016/j.paid.2019.03.003 [DOI] [Google Scholar]
- 34.Kraan, C. M. et al. Impaired response inhibition is associated with self-reported symptoms of depression, anxiety, and ADHD in female FMR1 premutation carriers. Am. J. Med. Genet. B Neuropsychiatr. Genet.165b(1), 41–51 (2014). 10.1002/ajmg.b.32203 [DOI] [PubMed] [Google Scholar]
- 35.Asgharian Asl, F. & Vaghef, L. The effectiveness of high-frequency left DLPFC-rTMS on depression, response inhibition, and cognitive flexibility in female subjects with major depressive disorder. J. Psychiatr. Res.149, 287–292 (2022). 10.1016/j.jpsychires.2022.01.025 [DOI] [PubMed] [Google Scholar]
- 36.Sjoerds, Z., Van Den Brink, W., Beekman, A. T., Penninx, B. W. & Veltman, D. J. Response inhibition in alcohol-dependent patients and patients with depression/anxiety: A functional magnetic resonance imaging study. Psychol. Med.44(8), 1713–1725 (2014). 10.1017/S0033291713002274 [DOI] [PubMed] [Google Scholar]
- 37.Liang, J. N. et al. Impairment of response inhibition to emotional face stimuli in individuals with subclinical depression. Psych J.11(3), 327–334 (2022). 10.1002/pchj.548 [DOI] [PubMed] [Google Scholar]
- 38.Choudhury, S. & McKinney, K. A. Digital media, the developing brain and the interpretive plasticity of neuroplasticity. Transcult. Psychiatry50(2), 192–215 (2013). 10.1177/1363461512474623 [DOI] [PubMed] [Google Scholar]
- 39.Jak, A. J. The impact of physical and mental activity on cognitive aging. In Behavioral Neurobiology of Aging (eds Pardon, M.-C. & Bondi, M. W.) 273–291 (Springer Berlin Heidelberg, 2011). [DOI] [PubMed] [Google Scholar]
- 40.Max, S. M., Plewnia, C., Zipfel, S., Giel, K. E. & Schag, K. Combined antisaccade task and transcranial direct current stimulation to increase response inhibition in binge eating disorder. Eur. Arch. Psychiatry Clin. Neurosci.271(1), 17–28 (2021). 10.1007/s00406-020-01164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He, Q., Geißler, C. F., Ferrante, M., Hartwigsen, G. & Friehs, M. A. Effects of transcranial magnetic stimulation on reactive response inhibition. Neurosci. Biobehav. Rev.157, 105532 (2024). 10.1016/j.neubiorev.2023.105532 [DOI] [PubMed] [Google Scholar]
- 42.Gilmore, C. S., Dickmann, P. J., Nelson, B. G., Lamberty, G. J. & Lim, K. O. Transcranial direct current stimulation (tDCS) paired with a decision-making task reduces risk-taking in a clinically impulsive sample. Brain Stimul.11(2), 302–309 (2018). 10.1016/j.brs.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 43.Ljubisavljevic, M., Maxood, K., Bjekic, J., Oommen, J. & Nagelkerke, N. Long-term effects of repeated prefrontal cortex transcranial direct current stimulation (tDCS) on food craving in normal and overweight young adults. Brain Stimul.9(6), 826–833 (2016). 10.1016/j.brs.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 44.Weber, M. J., Messing, S. B., Rao, H., Detre, J. A. & Thompson-Schill, S. L. Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: A tDCS-fMRI study. Hum. Brain Mapp.35(8), 3673–3686 (2014). 10.1002/hbm.22429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allenby, C. et al. Transcranial direct current brain stimulation decreases impulsivity in ADHD. Brain Stimul.11(5), 974–981 (2018). 10.1016/j.brs.2018.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo, H., Zhang, Z., Da, S., Sheng, X. & Zhang, X. High-definition transcranial direct current stimulation (HD-tDCS) of left dorsolateral prefrontal cortex affects performance in balloon analogue risk task (BART). Brain Behav.8(2), e00884 (2018). 10.1002/brb3.884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu, H. et al. Increased interbrain synchronization and neural efficiency of the frontal cortex to enhance human coordinative behavior: A combined hyper-tES and fNIRS study. Neuroimage282, 120385 (2023). 10.1016/j.neuroimage.2023.120385 [DOI] [PubMed] [Google Scholar]
- 48.Alam, M., Truong, D. Q., Khadka, N. & Bikson, M. Spatial and polarity precision of concentric high-definition transcranial direct current stimulation (HD-tDCS). Phys. Med. Biol.61(12), 4506–4521 (2016). 10.1088/0031-9155/61/12/4506 [DOI] [PubMed] [Google Scholar]
- 49.Markiewicz, N., Russa, M., Fokkens, A., Dechant, M. & Friehs, M. A. You got it in your hands: Stop-signal modality influences on reactive response inhibition with gaming controls. Int. J. Hum. Comput. Int.10.1080/10447318.2023.2285624 (2023). 10.1080/10447318.2023.2285624 [DOI] [Google Scholar]
- 50.Friehs, M. A. et al. A touching advantage: Cross-modal stop-signals improve reactive response inhibition. Exp. Brain Res.242(3), 599–618 (2024). 10.1007/s00221-023-06767-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study available from the corresponding author on reasonable request.