Abstract
Herbicide-resistant Conyza spp. are a threat to many crops. These widespread weeds are closely related species and often cooccur. To characterize the origins of their resistance and the mechanisms underlying their spread, we assessed the genomic variation in glyphosate-resistant Conyza spp. in Brazil. Twenty populations were sampled from soybean fields across four macroregions (MRSs). A genotyping-by-sequencing study resulted in 2,998 single-nucleotide polymorphisms (SNPs) obtained for C. bonariensis (L.) and the closely related C. sumatrensis (Retz) E. Walker. Higher genomic diversity (π) and heterozygosity (HO/HE) and lower inbreeding coefficient (FIS) values were detected in populations of Conyza spp. from MRS 1 (southern) than in those from other MRSs. Strong genomic structure clustered individuals into three groups (FST = 0.22; p value = 0.000) associated with the MRSs. Thus, resistance to glyphosate originated from independent selection in different MRSs across Brazil. Our dataset supports the occurrence of intraspecific gene flow in Brazil and identified individuals of C. bonariensis that did not group within species. These findings suggest that allelic introgressions within and among species have impacted the evolution and spread of resistance to glyphosate in Conyza spp. We discuss how to mitigate new resistance cases, particularly for the released stacked traits herbicide tolerance in soybeans.
Subject terms: Agricultural genetics, Evolutionary genetics
Introduction
Habitats and landscapes comprising extensive cropland present new challenges for ecologists and weed scientists every time novel technology emerges and is incorporated into a set of weed control tactics. The use of such technologies—such as no-tillage, herbicides, and herbicide-resistant crops—modifies cropland, allowing for the development of new weed problems1. In fact, changes in ecological dynamics (e.g., shifts in weed flora) and evolutionary adaptations (e.g., herbicide resistance) are mostly associated with farming practices2. These changes have been frequently detected in cropping systems in South America, where intensive and large-scale farming imposes fierce selection pressure on pests3. Although innovations have led to steadily improving weed management for crops such as soybeans, weed control failures are still more common than is desirable4,5.
Whether a weed population is capable of adapting in response to herbicides depends on whether that population contains the necessary genetic variation6,7. Population size, ploidy level, epigenetic regulation, gene flow, fitness costs, and selection play a role in the evolution of adaptive traits8–10. The use of herbicides decreases the genetic diversity of susceptible weed populations because of recurrent population bottlenecks and strong positive selection11. Each additional strong selection cycle will favor alleles that confer resistance to herbicides, if present in the population, reducing genetic variation as the frequency of resistant individuals increases. However, high genetic diversity may occur regardless of the resistance frequency because weed populations are also influenced by other environmental factors7. Herbicide rotations and mixtures, tillage, mowing, and pest and environmental pressures present variable selection pressures, which may reduce genetic diversity in all populations, making it difficult to detect reductions in diversity in populations with a high frequency of herbicide-resistant individuals7. The population genomics approach helps to obtain detailed molecular information on the diversity and structure of populations since next-generation sequencing has made it possible to identify thousands of single nucleotide polymorphism (SNP) markers that can be applied to evaluate the issues raised.
Currently, soybean is cultivated on approximately 45 million hectares in Brazil12 across five macroregions (MRSs) that differ in latitude and climatic conditions13. These production regions differ in terms of cropping systems and technological levels of soybean production, which influences weed flora and prevailing agricultural practices14. However, glyphosate-tolerant soybean varieties are widely adopted across Brazilian MRSs, and glyphosate has been used in approximately 98% of soybean fields in the last decade15. Cases of resistance to glyphosate have emerged exponentially across MRSs since 2005, and to date, glyphosate is the second most commonly applied herbicide in the number of resistant weed species4. Moreover, glyphosate is still the most commonly used herbicide in soybeans despite its ubiquitous resistance in weed populations, especially Conyza spp.14,16.
Conyza (Asteraceae: Astereae) is a New World genus that consists of as many as 150 dicot species17, among which there are some weed species among the most widespread throughout the world. C. bonariensis and the closely related C. sumatrensis are the main Conyza spp. weeds in soybean fields in Brazil, and most populations are glyphosate tolerant14,18. These weeds cooccur in similar environments because of shared traits and niches and commonly overlap, forming unique population structures across distinct regions of the globe. In fact, C. bonariensis and C. sumatrensis were recently speciated from a common ancestor, and therefore, there has been insufficient time for complete lineage sorting19. Although populations of these species have evolved herbicide resistance concomitantly, the processes governing the coexistence and population structuring of Conyza spp. are unknown.
In plants, the mating system is the most important life history trait influencing genomic structure since it determines the level of gene flow with other populations and even species20. Conyza spp. are primarily self-compatible through both intra- and inter-capitulum geitonogamy, but outcrossing frequently occurs within these species21,22. For instance, self- and cross-pollination of C. sumatrensis yielded 59 and 48 seeds per capitulum, respectively, due to the versatile mating system that allows intraspecific gene flow23. In addition, given that several weed species of this genus exhibit similar ploidy levels and numbers of chromosomes, some outcrossing can likely occur even among different species. When capitula of the diploids C. canadensis (L.) Cronq. and C. ramosissima Cronq. interacted freely, 3% of the ova were shown to be fertilized by the other species, generating interspecific hybrids22. However, little is known about interspecific gene flow between C. bonariensis and C. sumatrensis, which are hexaploid species with 54 chromosomes that interact freely in cropping systems worldwide.
Herein, it was hypothesized that gene flow within and among populations of closely related species may broaden herbicide resistance and govern population structuring across an agricultural landscape. Thus, we characterized the genomic variation in glyphosate-resistant populations of Conyza spp. across Brazil with genotyping-by-sequencing (GBS) to address the following questions: (i) did intraspecific gene flow of herbicide resistance alleles occur across the landscape? (ii) Is there interspecific gene flow and spread of resistance among polyploid Conyza spp.? We present the first study in which the genomic variation in mixed weed species that are closely related Conyza spp. and cooccur in soybean fields in Brazil and other regions of the globe. Our data allowed us to propose tactics to avoid the spread of resistance to other technologies, such as resistance to synthetic auxins, in the recently released 2,4-D- and dicamba-tolerant soybeans.
Results
Glyphosate resistance screening
Out of 314 plants from 20 soybean fields, 42 plants were taxonomically classified as C. bonariensis (identified in 8 of 20 fields), and 272 were identified as C. sumatrensis (identified in all 20 fields; see Online Supplementary Material, Table S1). Among them, 269 plants (86%) were screened as glyphosate resistant in the single-dose assay, comprising 30 plants of C. bonariensis and 233 of C. sumatrensis (Fig. 1). Glyphosate-resistant plants were then genotyped without distinction of weed species since the study aimed to assess the genomic variation as the populations of Conyza spp. cooccur in soybean fields.
Figure 1.
The frequencies of plant mortality (%) in response to glyphosate grouped into two clusters for 314 individuals of Conyza bonariensis (ERIBO) or C. sumatrensis (ERISU) sampled in 20 soybean fields across four cropping macroregions in 2021 in Brazil. The data were evaluated 42 days after treatment. Dashed lines correspond to the cluster limits. K-clustering was defined by the elbow criterion, and the limits among clusters were defined by the K-means method.
SNP discovery and data processing
A total of 430,431,768 raw reads were generated in the GBS analysis. After demultiplexing, quality control, and filtering to address bias corrections due to missing data, 2,998 high-quality nonduplicated SNPs were retained for population genomics analysis. Out of 269 plants, 67 were excluded for downstream bioinformatic processes due to low genome coverage (< 150,000 reads), excess missing data, and deviation of CG content, resulting in 202 plants being used in the population genomics analyses (Table 1).
Table 1.
Index of association of mixed populations of the closely related species Conyza bonariensis (ERIBO) and C. sumatrensis (ERISU) from four soybean macroregions (MRSs) in Brazil estimated from RADseq data (202 individuals and 2998 SNPs included).
| Macroregion | n | rd | p value |
|---|---|---|---|
| MRS 1 | 7 (26/54) | 0.308 | 0.001 |
| MRS 2 | 7 (02/80) | 0.009 | 0.001 |
| MRS 3 | 3 (02/18) | 0.027 | 0.001 |
| MRS 4 | 3 (00/20) | 0.040 | 0.001 |
n number of populations (number of ERIBO individuals/number of ERISU individuals), rd index of association.
Genomic diversity
For population genomics analyses, we did not discriminate SNPs into datasets that were putatively neutral or underselection to not favor either of the closely related species that were assessed concomitantly. The rd index was significant (p < 0.001) in all situations and revealed linkage disequilibrium between markers in Brazilian MRSs. In this test, the null hypothesis is that no linkage exists between markers, and the presence of disequilibrium indicates sexual reproduction in Conyza spp. (Table 1), even in MRSs with both C. bonariensis and C. sumatrensis (MRS 1, 2 and 3).
Greater variation in nucleotide diversity was observed throughout the landscape, which was greater in MRS 1 (π = 0.122) than in the other three MRSs (π ≤ 0.061). Notably, nucleotide diversity decreased as the number of C. bonariensis plants decreased in the MRSs (Tables 1 and 2), with the lowest π value identified in MRS 4 (0.042), where no C. bonariensis plants were identified. The same pattern was verified for the observed and expected heterozygosity. The inbreeding coefficient (FIS) also differed substantially among MRSs and ranged from 0.372 to 0.778, showing that the populations were not in Hardy–Weinberg equilibrium. In MRS 1, where there was the highest proportion of C. bonariensis/C. sumatrensis, the lowest but still highest FIS was found (0.372). As expected, MRS 4 had the highest inbreeding coefficient (0.778). It can therefore be concluded that both inbreeding and outbreeding influenced these estimates (Table 2). Therefore, our plant material exhibited variable genomic diversity through different populations and a hybrid mating system, consistent with recent population expansion due to gene flow within and among MRSs.
Table 2.
Genetic diversity statistics of mixed populations of the closely related species Conyza bonariensis (ERIBO) and C. sumatrensis (ERISU) from four soybean macroregions (MRSs) in Brazil estimated from RADseq data (202 individuals and 2998 SNPs included).
| Macroregion | n | HO | HE | FIS | π |
|---|---|---|---|---|---|
| MRS 1 | 7 (26/54) | 0.080 | 0.084 | 0.372 | 0.122 ± 0.003 |
| MRS 2 | 7 (02/80) | 0.036 | 0.040 | 0.567 | 0.061 ± 0.002 |
| MRS 3 | 3 (02/18) | 0.030 | 0.033 | 0.699 | 0.049 ± 0.002 |
| MRS 4 | 3 (00/20) | 0.028 | 0.029 | 0.778 | 0.042 ± 0.002 |
n number of populations (number of ERIBO individuals/number of ERISU individuals), H0 observed heterozygosity, HE expected heterozygosity FIS inbreeding coefficient, and π nucleotide diversity (mean ± SE).
Genomic differentiation and structure
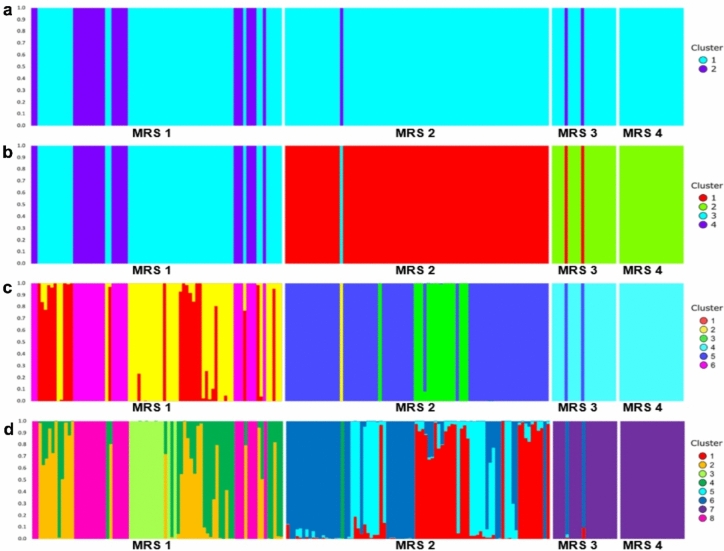
A significant genomic structure in Conyza spp. was identified via AMOVA (φST = 0.220, p < 0.001), where 78% of the variance was distributed within MRSs and 22% among MRSs (Table 3), which shows how internal factors affecting MRSs influence their genomic structuring. Considering the FST values of Weir and Cockerham populations24, these (mixed) populations had a considerable degree of differentiation, ranging from 0.084 to 0.274 (p < 0.001), mainly when the southern region (MRS 1) was compared with the other regions (Table 4). Thus, the greatest differentiation occurred between MRS 1 and MRS 4 (0.274), which comprise the southern and the Cerrado regions, respectively. As expected, a lower pairwise FST estimate was detected between MRS 3 and 4 (FST = 0.084), which comprise the populations of Conyza spp. sampled in the Cerrado Region in northern Brazil. Mantel’s test revealed a weak but significant correlation (r = 0.49; p = 0.048) between genomic variation (FST) and geographical distance (see Online Supplementary Material, Fig. S1 and Table S2). Despite indicating that genetic differences increase linearly with geographic distance, the weak correlation shows that there is some gene flow between populations that are geographically distant. In the discriminant analysis of principal components (DAPC), the lowest value found by Bayesian criterion information (BIC) was K = 8; however, decreases in this value were also considered, with observed values of K = 2, 4 and 6 (see Online Supplementary Material, Fig. S2; Fig. 2a–d). Consistent with the AMOVA results, the admixture analysis revealed strong structuration in Conyza spp. and populations within the MRSs, except for the C. sumatrensis samples from MRSs 3 and 4, which belong to the same ancestral group. The migration of a few individuals among the MRSs was noted. When K = 2 was used, migrations were observed between the four MRSs (Fig. 2a). However, when K = 4, 6 or 8 (Fig. 2b–d) were considered, migrations became less frequent, especially between MRS 1 and MRS 2. In addition, C. bonariensis plants differed from C. sumatrensis plants across all clusters but were not always genetically purebred or shared the same genomic background.
Table 3.
Hierarchical analysis of molecular variance (AMOVA) of mixed populations of the closely related species Conyza bonariensis (ERIBO) and C. sumatrensis (ERISU) from four soybean macroregions (MRSs) in Brazil estimated from RADseq data (202 individuals and 2998 SNPs included).
| Source of variance | Df | SS | VC | PV | ΦST | p value |
|---|---|---|---|---|---|---|
| Among MRSs | 3 | 4530 | 39 | 22 | 0.22 | 0.0001 |
| Among fields within MRSs | 201 | 27,826 | 138 | 78 | ||
| Total | 204 | 32,356 | 177 |
ϕST is the population differentiation statistic used to test hypotheses about population differentiation.
df degrees of freedom SS sum of squares, VC variance component, PV percentage of variation (%).
Table 4.
Pairwise FST for mixed populations of the closely related species Conyza bonariensis (ERIBO) and C. sumatrensis (ERISU) from four soybean macroregions (MRSs) in Brazil estimated from RADseq data (202 individuals and 2998 SNPs included).
| Macroregion | MRS 1 | MRS 2 | MRS 3 | MRS 4 |
|---|---|---|---|---|
| MRS 1 | – | 0.229 | 0.230 | 0.238 |
| MRS 2 | < 0.001 | – | 0.252 | 0.274 |
| MRS 3 | < 0.001 | < 0.001 | – | 0.084 |
| MRS 4 | < 0.001 | < 0.001 | < 0.001 | – |
Upper values, FST; bottom values, p values.
Figure 2.
Bar plots with the DAPC results for individuals of Conyza spp. sampled from 20 soybean fields across four cropping macroregions in 2021 in Brazil (202 individuals and 2998 SNPs included). Vertical bars represent individuals whose genotypes have been portioned into distinct clusters; (a) Bar plot of DAPC with ancestry coefficient for K = 2; (b) bar plot of K = 4; (c) bar plot of K = 6; and (d) bar plot of K = 8.
PCA (PC1 = 34.5%; PC2 = 3.8%) revealed two clusters for C. sumatrensis, separating individuals from southern (MRSs 1 and 2) and northern (MRSs 3 and 4; Fig. 3) Brazil. A third cluster composed of individuals of C. bonariensis was revealed in the analysis, although six individuals were grouped within the clusters of closely related C. sumatrensis.
Figure 3.
Principal component analysis (PCA) (202 individuals and 2,998 SNPs included). The separation between the Conyza spp. and soybean cropping macroregions in Brazil is shown. ERIBO refers to Conyza bonariensis, ERISU refers to Conyza sumatrensis, and MRS refers to the soybean cropping macroregion.
Dissimilarity analysis
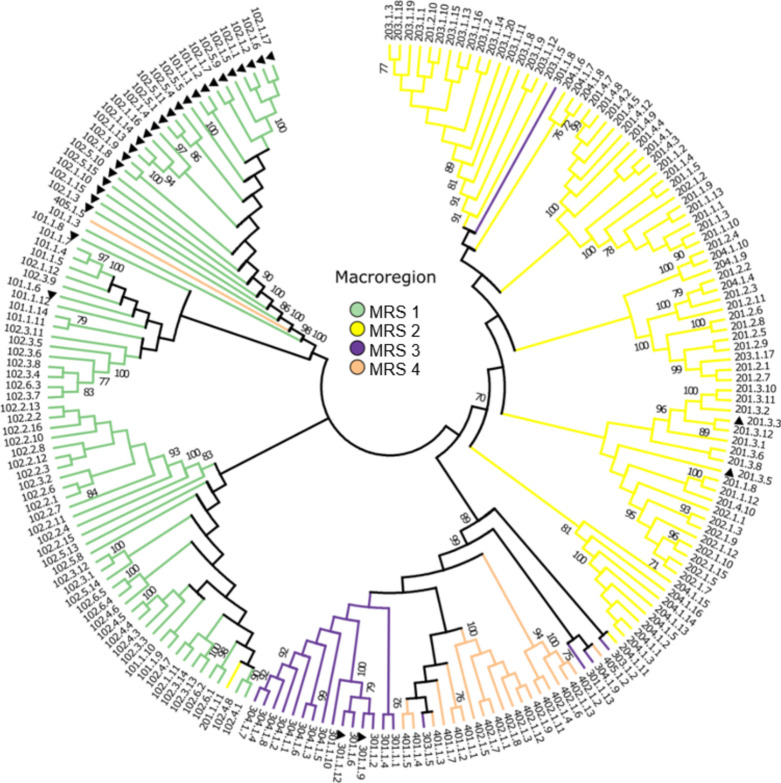
The dendrogram based on genomic similarity divided Conyza spp. collected from 20 populations and two species into four major clusters corresponding to the MRSs of Brazil with bootstrap support of at least 70% (Fig. 4). While plants from MRS 1 were divided into two clades mostly according to the weed species, those from MRS 2 had a specific clade, and those from MRSs 3 and 4 shared a clade. In addition, this analysis allowed for the identification of six C. bonariensis plants from different MRSs. PCA showed that these plants were not grouped within species: 101.1.8, 101.1.6 (MRS 1), 201.3.3, 201.3.5 (MRS 2), 301.1.9, and 301.1.12 (MRS 3; Fig. 4). The first four individuals were taxonomically classified as C. bonariensis var. bonariensis, while the last two individuals were classified as C. bonariensis var. angustifolia (see Online Supplementary Material, Table S1). Finally, seven individuals of C. sumatrensis that migrated among the MRSs were identified: 201.1.11, 301.1.8, 301.1.13, 303.1.2, 303.1.5, 304.1.9, and 405.1.5.
Figure 4.
Neighbor-joining dendrograms based on Jaccard’s genetic similarity coefficient (202 individuals and 2998 SNPs included) showing potential interspecific hybridization. ERIBO refers to Conyza bonariensis, ERISU refers to Conyza sumatrensis, and MRS refers to the soybean cropping macroregion.
Discussion
C. bonariensis and C. sumatrensis are two of the most troublesome weeds in soybean cropping systems in Brazil and are exacerbated by the evolution of glyphosate-resistant populations. Currently, two hypotheses have been proposed to explain the rapid pace at which glyphosate-resistant Conyza spp. have evolved over extensive croplands. The first hypothesis suggests that resistance evolved once in the southern region, where it was first reported in Rio Grande do Sul State, and then spread northward. The second hypothesis suggests that intraspecific and even interspecific gene flow of herbicide resistance alleles has occurred across the landscape in Brazil. Our data support that multiple origins of resistance occurred in Brazil and that allelic introgressions within and among species have impacted the spread of resistance.
We wanted to determine whether intraspecific gene flow of herbicide resistance alleles (among populations) occurs across the landscape in Brazil. Overall, we found variable genomic diversity and significant differences in rd indexes through different populations associated with both inbreeding and crossbreeding behaviors, which enabled the occurrence of moderate intraspecific gene flow (Tables 1 and 2). According to AMOVA, only a quarter of the genomic variation occurred among the MSRs in Brazil, and Mantel’s test suggested that some gene flow occurred among MRSs (see Online Supplementary Material, Fig. S1; Tables 3 and 4). Finally, our findings support the idea that closely related Conyza spp. have formed unique structures of mixed but often well-differentiated populations that have experienced high admixture and local adaptation. Genomic structuring did not differ between MRSs 3 and 4, and there were differences among MRSs, which also reinforced the occurrence of regional gene flow (Figs. 2, 3 and 4). Thus, our dataset supports the evidence of intraspecific gene flow by pollen and seeds across the landscape in Brazil.
In C. sumatrensis, a microsatellites analysis revealed moderate gene flow (Nm = 0.5535) among six populations from MRS 2, suggesting outcrossing among plants from this region25. In another study, with the same species and analysis, moderate allele transfer (Nm = 0.3441) was suggested among 50 biotypes sampled from MRSs 1, 2, and 3 in Brazil26. This reduction in gene flow was similar to our population structuring results (FST and DAPC) found between MRS 2 and MRS 3. In C. bonariensis, multiple recombinant alleles were detected within 35 populations in California, United States, indicating gene flow of herbicide resistance genes27. These findings from different countries are consistent with those found in our study and confirm that C. bonariensis and C. sumatrensis display a mating system allowing gene flow.
Currently, populations of Conyza spp. resistant to glyphosate are widespread across most soybean cropping regions in Brazil, as reported in resistance surveys14,18. Our findings revealed that at least three genomic groups of Conyza spp. in Brazil rejected the possibility of a single founder effect that evolved and spread resistance (Fig. 3). In fact, resistance to herbicides may also evolve concomitantly in multiple locations, especially in cases where more than one resistance mechanism has evolved. In Brazil, resistance to glyphosate in Conyza spp. can be conferred by both target-site and nontarget site resistance mechanisms28–30. Resistance to glyphosate in C. bonariensis and C. canadensis in California, United States, was related to multiple independent origins according to multilocus data27,31.
Once resistance has evolved, the rate at which it evolves in a landscape with extensive areas of cultivation can be determined mainly by the degree and forms of gene flow to the surrounding regions. As discussed, our data revealed moderate gene flow among populations of Conyza spp. across MRSs and assisted in the spread of resistance through pollen, seeds, or vegetative propagation. In C. bonariensis, the analysis of shared multilocus genes indicated a greater influence of seeds than pollen on the spread of glyphosate resistance across a large landscape27. This phenomenon is consistent with the fact that Conyza spp. exhibit robust seed production, with an estimated 200,000 seeds per plant, and long-range seed dispersal is wind assisted32,33. Similarly, Conyza spp. seeds may have been transported with agricultural machinery between different soybean cropping regions because soybeans are sown in different locations and at different times, which leads to a rotation of available machinery between regions, which has been observed for Digitaria insularis (L.) Mez ex Ekman34.
We also wanted to determine whether there is interspecific gene flow (hybridization) and spread of resistance among polyploid Conyza spp. In our study, the observed and expected heterozygosity within populations varied from 0.028 to 0.084, with larger values related to a greater proportion of C. bonariensis individuals in the MRSs (Table 2). The inbreeding coefficients were positive and significant in all MRSs, ranging from 0.372 to 0.778, which emphasizes the possibility of crossbreeding between highly related species, especially in MRS 1 (Table 2). These findings suggest strong potential for interspecific gene flow between Conyza spp. and introgression of herbicide-resistant alleles, even in the absence of selection pressure. In fact, C. bonariensis plants did not cluster within species in three structural analyses (Figs. 2, 3 and 4), which is unusual considering the number of loci that were sequenced in the present study. We hypothesized at least three different reasons for these findings in the present study: misidentification of species, incomplete lineage sorting, and interspecific hybridization.
The incorrect identification of C. bonariensis is unlikely since individuals differ as the plant matures, comprising a well-defined plant morphotype identified according to Pruski & Sancho35. Additionally, twelve voucher specimens representing the species and varieties found in the study were deposited in the Irina Delanova Gemtchújnicov Herbarium/Botu of UNESP (BOTU 34833-34844; see Online Supplementary Material, Table S1), where plant species identification was confirmed. Incomplete lineage sorting is also unlikely even though C. bonariensis is paraphyletic to C. sumatrensis because of recent speciation from a common ancestor19. In addition, the number of loci studied was sufficient to detect genomic differences among individuals once we observed variable genomic diversity, with higher values in MRS 1, where there was a greater mixture of species (Tables 1 and 2). Thus, the C. bonariensis plants that did not group within species are possibly interspecific Conyza hybrids generated from individuals who interact freely in soybean fields across Brazil. Interestingly, hybridization occurred in the macroregions regardless of C. bonariensis abundance, reinforcing random encounters. It is also possible that these Conyza hybrids are indeed genomically closer to C. sumatrensis, which is why they are grouped together. A similar event has been identified in other plant species, such as Quercus spp., where the parental species did not contribute equally to generating hybrids36.
According to the samples and results obtained in our study, C. bonariensis and C. sumatrensis are genetically compatible, capable of transferring alleles, and capable of producing vigorous and fertile interspecific hybrids. These weeds exhibit similar phenology, inflorescence traits, pollen/ovule ratios37, and shared pollinators23, which assist in pollen transfer between the two species. In addition, C. bonariensis and C. sumatrensis feature a common hexaploidy structure of 2n = 54 that allows for successful chromosome pairing during cell meiosis38. Both weed species are sibling species, as determined by different DNA barcode gene regions in which they commonly cluster within a monophyletic clade19. This phylogenetic analysis revealed a recent speciation event between C. bonariensis and C. sumatrensis and provided further support for our hypothesis of genetic compatibility between them.
C. bonariensis and C. sumatrensis are closely related weed species that cooccur because of shared traits and niches and overlap in soybean fields, forming unique, multispecies populations. In addition, these weeds show extensive phenotypic plasticity for vegetative traits, as shown by observations of variable morphotypes in the same field. In the present study, we adopted a multispecies approach, and glyphosate-resistant individuals were genotyped without distinguishing between the species. While it is opportune to capture the intrabreed effects between the species, this approach makes it difficult to interpret the causes of the observed genetic variation. The interspecific hybridization between C. bonariensis and C. sumatrensis evidenced in our study warrant further species-level sampling and investigation.
In the Conyza genus, evidence of interspecific hybridization is currently restricted to Europe and the United States, mainly for diploid species such as C. canadensis22,38. To our knowledge, there is a unique report of hybrids between C. bonariensis and C. sumatrensis, namely, C. daveauana Sennen in France, with unknown fertility39. In cropped areas, C. bonariensis and C. canadensis have crossed and generated hybrid progenies, which likely reduced fitness due to differences in ploidy level27,31. If mutations for herbicide resistance occur frequently in both Conyza spp., interspecific gene flow may not be a major factor contributing to the evolution and spread of resistance. Otherwise, introgression from other species might be a source of alleles and may have had an impact on the evolution of resistance in one or both Conyza spp.27.
At least three well-defined genetic backgrounds of mixed populations of Conyza spp. in MRS 1, MRS 2 and combined MRS 3 and 4 in Brazil were found in the present research. These data should be considered for the development and using of new herbicide-tolerant technologies, such as in the recently released 2,4-D- and dicamba-tolerant soybeans. For example, weed control strategies should ideally be tested in these three distinct scenarios, and their technical positioning and stewardship should be customized by MRS. Weed shifts and resistance evolution in Conyza spp. are expected to occur first in MRSs 1 and 2, where cultivation has been ongoing for several years, while adaptations to cropping practices are expected to occur slowly in MRSs 3 and 4, where there are intense planting and more production cycles. As resistance originates from multiple selections and then spreads through gene flow in Conyza spp., future technologies should be based on reducing selection pressure from herbicides. Thus, recently released stacked trait 2,4-D- or dicamba-tolerant soybeans should diversify weed management practices to maintain the resistance of Conyza spp. and other weed species under control. Ultimately, integrated weed management approaches incorporating safer alternative herbicides and nonchemical methods applied at the regional scale are needed to reduce the selection pressure and evolution of herbicide-resistant populations.
Conclusion
C. bonariensis and closely related C. sumatrensis have formed unique structures of mixed but often well-differentiated populations that have experienced high admixture and local adaptation. Three genetic backgrounds of mixed populations were noted in MRS 1, MRS 2 and the combination of MRSs 3 and 4 of Brazil, but gene flow and even migration were detected among the four MRSs. The origin of the resistance to glyphosate was related to multiple independent selections that influenced the different genetic backgrounds found in the current mixed populations of Conyza spp. After resistance evolved and was detected, evidence suggested the spread of resistance within MRSs through gene flow among populations and species (interspecific hybridization).
Materials and methods
Plant material
A total of 314 Conyza spp. plants were sampled from 20 soybean fields across four MRSs in Brazil in the 2021 growth season: 7 in MRS 1, 7 in MRS 2, 3 in MRS 3, and 3 in MRS 4 (see Online Supplementary Material, Fig. S3 and Table S1). The fields were selected based on reports from farmers and consultants about the occurrence of mature plants of Conyza spp. that have escaped control by glyphosate. In each field, at least 15 plants were sampled and treated as an individual of Conyza spp., and sampling was not repeated on the same individual (at least 50 m apart) to explore the diversity within the fields. Seeds from mature inflorescences were sampled and placed in a paper bag (single-plant sample), labeled with their geographic origins, and stored at − 2 °C until plant material sowing. As the collection of plant material occurred only from nonnative species of Brazil38, no permissions were required according to the Brazilian Normative Instruction no. 42 of 5 July 2002. The collection was performed according to the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora, although C. bonariensis and C. sumatrensis were not listed on the IUCN Red List of Threatened Species in 2018.
Growing conditions and species identification
Seeds from single plant samples were germinated in Petri dishes in growth chambers at 22 ± 1 °C with 10 h of light, after which one seedling was transplanted to a 1 dm−3 pot filled with soil potting mix. Plants were grown in open greenhouses under natural temperature (25 ± 3 °C), humidity (65 ± 13%) and photoperiod (11.2 ± 0.4 h) conditions and received 2 mm sprinkler irrigation four times a day (see Online Supplementary Material, Fig. S4). The Conyza species were taxonomically classified by the dichotomous key of Pruski & Sancho35, in which the main morphological traits were involucre, capitula and inflorescence types. In addition, specimens of each species found were sent to an independent taxonomist to confirm the identification (Identifier: Angelo A. Schneider). The vouchers with the species and varieties found in the research were deposited in the public Herbarium Irina Delanova Gemtchújnicov – BOTU of the State University of São Paulo (Voucher specimens: BOTU 34833-34844; Identifier: Ana P. F. Perez).
Whole-plant assay to screen for glyphosate resistance
Conyza spp. (n = 314) plants were screened for glyphosate at 1200 g ha−1 when the plants reached the 4- to 6-leaf stage according to Mendes et al.18 In addition, four reference populations of C. sumatrensis characterized as putative resistant (n = 2) or susceptible (n = 2) in previous studies were included as standards for comparison. The level of plant mortality (0–100%) was visually rated 42 days after glyphosate treatment, and the data were subjected to hierarchical cluster analysis to distinguish herbicide-resistant individuals. An individual was screened as glyphosate resistant when it was grouped in Cluster 1, and its level of mortality was significantly lower than that of the susceptible reference population of the assay.
DNA isolation and genomic library preparation
Leaf tissue (100 mg) from the fourth leaf was sampled, packed in gauze tissue, and then freeze-dried for 72 h in a bench lyophilizer. Subsequently, the plant material was ground into a powder to extract genomic DNA using a DNeasy® Plant Mini Kit (Qiagen) following the manufacturer's protocol. DNA quality and quantity were assessed by electrophoresis on agarose gels (1% w v-1) stained with SYBR Safe DNA Gel Stain and by visual comparison with phage lambda DNA (Invitrogen). DNA samples were resuspended in 100 µl of water and then diluted to 30 ng µl−1, after which the DNA samples were stored at ‑ 20 °C until the sequence-based genotyping step.
The GBS libraries were prepared using PstI and MseI restriction enzymes according to the protocol of Poland et al.40 The digested DNA sequences were subsequently amplified via polymerase chain reaction (PCR). The sequences were amplified for 18 cycles consisting of 95 °C (30 s), 62 °C (30 s), 68 °C (30 s). Sequencing was performed on an Illumina NextSeq2000 platform with single reads 101 nt in length, and the libraries were quantified using a KAPA Library Quantification Kit (KAPA Biosystems; see Online Supplementary Material, Fig. S5). The Illumina-generated reads from the Conyza spp. samples investigated in the present study were submitted to the NCBI Sequence Read Archive (SRA; NCBI Number: PRJNA971662).
Genotyping and loci filtering
Samples were demultiplexed with the process_radtag module from STACKS v. 1.4241. Only samples with at least 150,000 sequencing reads were retained. The raw reads were trimmed to 70 bp, and shorter reads were discarded with CUTADAPT42. Then, the reads were aligned to the reference genome of C. canadensis (NCBI Accession GCF 010,389,155.1; see Laforest et al.43) using the BWA-MEN default parameters44. Aligned reads were processed with STACKS, in which a minimum stack depth of three (M = 3) and a maximum of four mismatches (m = 4) were allowed to generate the RADloci45. The population module filtered the data to limit missing loci to a maximum of 50% within fields and 50% among fields, as well as a minor allele frequency of at least 5% (min-maf = 0.05; Roesti et al.46). The outputs were saved in genepop (.gen), vcf (.vcf), and structure (.str) formats for downstream analyses.
Population genomics analysis
The genomic diversity of Conyza spp. estimated for each MRS in Brazil included the observed heterozygosity (HO), expected heterozygosity (HE), nucleotide diversity (π), and inbreeding coefficient (FIS). Similarly, we tested the standardized index of association, rBarD47, and genomic differentiation through pairwise FST24, HO, HE, π, and FIS estimates were determined using the R package adegenet48, while for rBarD, the R package poppr was used with 1,000 permutations49. A Mantel test with 9,999 permutations was performed between the matrix of FST described above and a matrix of geographic distances using the library ecodist50 of R. We also performed a hierarchical analysis of molecular variance (AMOVA) in GenAlEx software51 to examine the distribution of genomic variation within and among MRSs.
The genomic structure of Conyza spp. was examined using discriminant analysis of principal components (DAPC) with the R package adegenet48. DAPC provides a visual assessment of the organization of genomic diversity, maximizing the differences between groups of samples while decreasing the correlation between original variables, which makes it an especially interesting analysis for large sets of SNP markers52. The K-means method was used to define genomic groups, testing for the existence of K = 1 to 30 groups, using the find.clusters function. The definition of the genomic groups was based on the inflection point of the Bayesian criterion information (BIC)52, but to identify gene flow, more than one value identified by the BIC was used. Moreover, DAPC was carried out after the optimization procedure with the optim.a.score() function to retain the ideal number of principal components (PCs) and reduce the overfitting of the DAPC membership coefficients52. With these results, bar plots were generated. Finally, the genomic structure was also examined through principal component analysis (PCA) using the adegenet48 package to compare the clustering patterns within and among species and populations.
Dissimilarity analysis
A dendrogram was constructed to cluster the genotypes based on their similarity and identify potential interspecific hybridization when individuals of one species grouped in the cluster of another species. Thus, the neighbor-joining method based on the Jaccard similarity index in the DARwin program was used53. Clade support within the neighbor-joining trees was assessed by a 10,000-replicate bootstrap test, and dendrograms were built using the GTR model in MEGA v. 754.
Supplementary Information
Acknowledgements
The authors are thankful for the support from several field agronomists, field scientists and technical assistants of Corteva AgriscienceTM who collected and submitted seed samples. We herein confirm that the study was performed in accordance with relevant institutional, national, and international guidelines and legislation relative to the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Author contributions
A.K., E.D.V., A.M.J and C.A.C did the initial conceptualization and planning of the experiments; A.K., E.D.V., A.M.J and C.A.C developed the methodology; A.K. screened for resistance; A.K did the genotype by sequencing library; A.K., E.D.V., A.M.J and C.A.C did the data analysis; A.K. did the literature research; A.K. wrote —original draft preparation; A.K., E.D.V., A.M.J and C.A.C wrote —reviewed and edited the manuscript; C.A.C did the supervision; C.A.C. did the project administration; C.A.C. had the funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available in the NCBI Sequence Read Archive (SRA) repository, with the accession number and direct link (PRJNA971662; https://www.ncbi.nlm.nih.gov/bioproject/PRJNA971662/).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-70153-8.
References
- 1.Chauhan, B. S., Singh, R. G. & Mahajan, G. Ecology and management of weeds under conservation agriculture: A review. Crop Prot.38, 57–65 (2012). 10.1016/j.cropro.2012.03.010 [DOI] [Google Scholar]
- 2.Radosevich, S. R., Holt, J. S. & Ghersa, C. M. Ecology of Weeds and Invasive Plants: Relationship to Agriculture and Natural Resource Management (Wiley, 2007). [Google Scholar]
- 3.Zucchi, M. I. et al. Patterns of genome-wide variation, population differentiation and SNP discovery of the red banded stink bug (Piezodorus guildinii). Sci. Rep.9, 1–11 (2019). 10.1038/s41598-019-50999-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heap, I. International survey of herbicide-resistant weeds. http://www.weedscience.org (2022).
- 5.Heap, I. & Duke, S. O. Overview of glyphosate-resistant weeds worldwide. Pest Manag. Sci.74, 1040–1049 (2018). 10.1002/ps.4760 [DOI] [PubMed] [Google Scholar]
- 6.Délye, C., Jasieniuk, M. & Le Corre, V. Deciphering the evolution of herbicide resistance in weeds. Trends Genet.29, 649–658 (2013). 10.1016/j.tig.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 7.Karn, E. & Jasieniuk, M. Genetic diversity and structure of Loliumperenne ssp. multiflorum in California vineyards and orchards indicate potential for spread of herbicide resistance via gene flow. Evol. Appl.10, 616–629 (2017). 10.1111/eva.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leimu, R., Mutikainen, P., Koricheva, J. & Fischer, M. How general are positive relationships between plant population size, fitness and genetic variation?. J. Ecol.94, 942–952 (2006). 10.1111/j.1365-2745.2006.01150.x [DOI] [Google Scholar]
- 9.Markus, C., Pecinka, A., Karan, R., Barney, J. N. & Merotto, A. Epigenetic regulation—Contribution to herbicide resistance in weeds?. Pest Manag. Sci.74, 275–281 (2018). 10.1002/ps.4727 [DOI] [PubMed] [Google Scholar]
- 10.Smith, A. L. et al. Global gene flow releases invasive plants from environmental constraints on genetic diversity. Proc. Natl. Acad. Sci. U. S. A.117, 4218–4227 (2020). 10.1073/pnas.1915848117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neve, P., Vila-Aiub, M. & Roux, F. Evolutionary-thinking in agricultural weed management. New Phytol.184, 783–793 (2009). 10.1111/j.1469-8137.2009.03034.x [DOI] [PubMed] [Google Scholar]
- 12.USDA. Foreign agricultural service. https://fas.usda.gov/ (2022).
- 13.Kaster, M. & Farias, J. R. B. Regionalização dos testes de Valor de Cultivo e Uso e da indicação de cultivares de soja - Terceira Aproximação Milton. https://www.embrapa.br/busca-de-publicacoes/-/publicacao/917252/regionalizacao-dos-testes-de-valor-de-cultivo-e-uso-e-da-indicacao-de-cultivares-de-soja---terceira-aproximacao (2012).
- 14.Lucio, F. R. et al. Dispersal and frequency of glyphosate-resistant and glyphosate-tolerant weeds in soybean-producing edaphoclimatic microregions in Brazil. Weed Technol.33, 217–231 (2019). 10.1017/wet.2018.97 [DOI] [Google Scholar]
- 15.Spark. BIP SOYBEAN – 2021/2022. http://spark-ie.com.br/ (2022).
- 16.Silva, A. F. d. et al. Monitoramento de Plantas Daninhas Resistentes a Glifosato no Brasil. (2021).
- 17.CWG. Global Compositae Database (Publication no. 10.14284/411). (2022).
- 18.Mendes, R. R. et al. Monitoring glyphosate-and chlorimuron-resistant Conyza spp. populations in Brazil. An. Acad. Bras. Cienc.93, 1–14 (2021). 10.1590/0001-3765202120190425 [DOI] [PubMed] [Google Scholar]
- 19.Alpen, K., Gopurenko, D., Wu, H., Lepschi, B. J. & Weston, L. A. The development of a DNA barcode system for species identification of Conyza spp. (fleabane). Ninet. Australas. Weeds Conf. 401–404 (2014).
- 20.Duminil, J., Hardy, O. J. & Petit, R. J. Plant traits correlated with generation time directly affect inbreeding depression and mating system and indirectly genetic structure. BMC Evol. Biol.9, 1–14 (2009). 10.1186/1471-2148-9-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry, R. S., Davis, V. M. & Johnson, W. G. Open-pollinated transfer of Glyphosate resistance in horseweed (Conyza canadensis) in greenhouse isolation. Dep. Bot. Plant Pathol. Purdue Univ. 2018 Feb 22]. 1 (2008).
- 22.Zelaya, I. A., Owen, M. D. K. & VanGessel, M. J. Transfer of glyphosate resistance: Evidence of hybridization in Conyza (Asteraceae). Am. J. Bot.94, 660–673 (2007). 10.3732/ajb.94.4.660 [DOI] [PubMed] [Google Scholar]
- 23.Hao, J. H., Qiang, S., Liu, Q. Q. & Cao, F. Reproductive traits associated with invasiveness in Conyza sumatrensis. J. Syst. Evol.47, 245–254 (2009). 10.1111/j.1759-6831.2009.00019.x [DOI] [Google Scholar]
- 24.Weir, B. & Clark Cockerham, C. Estimating F-statistics for the analysis of population structure. Evolution (N. Y.)38, 1358–1370 (1984). [DOI] [PubMed] [Google Scholar]
- 25.Marochio, C. A. et al. Mistura genética em espécies de Conyza (Asteraceae) reveladas por marcadores microsatélites. Acta Sci. Agron.39, 437–445 (2017). 10.4025/actasciagron.v39i4.32947 [DOI] [Google Scholar]
- 26.Ruiz, M. R. et al. Mechanisms that may lead to high genetic divergence and to the invasive success of tall fleabane (Conyza sumatrensis; Asteraceae). Weed Sci.70, 64–78 (2022). 10.1017/wsc.2021.59 [DOI] [Google Scholar]
- 27.Okada, M. et al. Evolution and spread of glyphosate resistance in Conyza bonariensis in California and a comparison with closely related Conyza canadensis. Weed Res.55, 173–184 (2015). 10.1111/wre.12131 [DOI] [Google Scholar]
- 28.Ferreira, E. A. et al. Glyphosate translocation in hairy fleabane (Conyza bonariensis) biotypes. Planta Daninha26, 637–643 (2008). 10.1590/S0100-83582008000300020 [DOI] [Google Scholar]
- 29.Kaspary, T. E. et al. Investigation of the mechanism of resistance to glyphosate herbicide in hairy fleabane. Planta Daninha34, 555–564 (2016). 10.1590/s0100-83582016340300016 [DOI] [Google Scholar]
- 30.Piasecki, C. et al. Oxidative stress and differential antioxidant enzyme activity in glyphosate-resistant and sensitive hairy fleabane in response to glyphosate treatment. Bragantia78, 379–396 (2019). 10.1590/1678-4499.20180289 [DOI] [Google Scholar]
- 31.Okada, M. et al. Evolution and spread of glyphosate resistance in Conyza canadensis in California. Evol. Appl.6, 761–777 (2013). 10.1111/eva.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang, H. et al. Wind-mediated horseweed (Conyza canadensis) gene flow: Pollen emission, dispersion, and deposition. Ecol. Evol.5, 2646–2658 (2015). 10.1002/ece3.1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye, R. et al. Field studies on dynamic pollen production, deposition, and dispersion of glyphosate-resistant horseweed (Conyza canadensis). Weed Sci.64, 101–111 (2016). 10.1614/WS-D-15-00073.1 [DOI] [Google Scholar]
- 34.Gonçalves Netto, A. et al. Population genomics of Digitaria insularis from soybean areas in Brazil. Pest Manag. Sci.77, 5375–5381 (2021). 10.1002/ps.6577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pruski, J. F. & Sancho, G. Conyzasumatrensis var. leiotheca (Compositae: Astereae), a new combination for a common neotropical weed. Novon16, 96–101 (2006). 10.3417/1055-3177(2006)16[96:CSVLCA]2.0.CO;2 [DOI] [Google Scholar]
- 36.Li, X., Wei, G., El-Kassaby, Y. A. & Fang, Y. Hybridization and introgression in sympatric and allopatric populations of four oak species. BMC Plant Biol.21, 1–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sancho, G. Flora Vascular de La República Argentina 7(1): Dicotyledoneae Asteraceae (Anthemideae a Gnaphalieae). In: Zuloaga, O., Belgrano, M. J. & Anton, & A. M. (eds) Tribu Astereae. 38–246 (2014).
- 38.Thebaud, C. & Abbott, R. J. Characterization of invasive Conyza species (Asteraceae) in Europe: Quantitative trait and isozyme analysis. Am. J. Bot.82, 360–368 (1995). 10.1002/j.1537-2197.1995.tb12640.x [DOI] [Google Scholar]
- 39.McClintock, D. & Marshall, J. B. On Conyza sumatrensis (Retz.) E. Walker and certain hybrids in the genus. Watsonia (1988).
- 40.Poland, J. A., Brown, P. J., Sorrells, M. E. & Jannink, J. Development of high-density genetic maps for barley and wheat using a novel two-enzyme Genotyping-by-Sequencing approach. PLoS One7, e32253 (2012). 10.1371/journal.pone.0032253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catchen, J., Hohenlohe, P. A., Bassham, S. & Amores, A. Stacks: An analysis tool set for population genomics. Mol. Ecol.22, 3124–3140 (2013). 10.1111/mec.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J.17, 10–12 (2011). 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- 43.Laforest, M. et al. A chromosome-scale draft sequence of the Canada fleabane genome. Pest Manag. Sci.76, 2158–2169 (2020). 10.1002/ps.5753 [DOI] [PubMed] [Google Scholar]
- 44.Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. 00, 1–3 (2013).
- 45.Ilut, D. C., Nydam, M. L. & Hare, M. P. Defining loci in restriction-based reduced representation genomic data from nonmodel species: Sources of bias and diagnostics for optimal clustering. Biomed Res. Int.2014, 1–9 (2014). 10.1155/2014/675158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roesti, M., Salzburger, W. & Berner, D. Uninformative polymorphisms bias genome scans for signatures of selection. BMC Evol. Biol.10.1186/1471-2148-12-94 (2012). 10.1186/1471-2148-12-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agapow, P. M. & Burt, A. Indices of multilocus linkage disequilibrium. Mol. Ecol. Notes1, 101–102 (2001). 10.1046/j.1471-8278.2000.00014.x [DOI] [Google Scholar]
- 48.Jombart, T., Lyon, D. & Biome, L. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics24, 1403–1405 (2008). 10.1093/bioinformatics/btn129 [DOI] [PubMed] [Google Scholar]
- 49.Kamvar, Z. N., Tabima, J. F. & Grünwald, N. J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ2, e281 (2014). 10.7717/peerj.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goslee, S. C. & Urban, D. L. The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw.22, 1–19 (2007). 10.18637/jss.v022.i07 [DOI] [Google Scholar]
- 51.Smouse, R. P. P. & Peakall, R. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics28, 2537–2539 (2012). 10.1093/bioinformatics/bts460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jombart, T. et al. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet.11, 94 (2010). 10.1186/1471-2156-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perrier, X. & Jacquemoud-Collet, J. P. DARwin software: Dissimilarity analysis and representation for windows. Website http//darwin. cirad. fr/darwin [Accessed 1 March 2013] (2006).
- 54.Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol.33, 1870–1874 (2016). 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the NCBI Sequence Read Archive (SRA) repository, with the accession number and direct link (PRJNA971662; https://www.ncbi.nlm.nih.gov/bioproject/PRJNA971662/).