Abstract
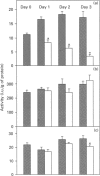
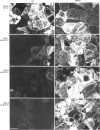
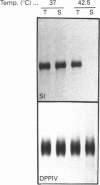
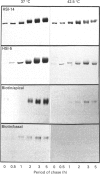
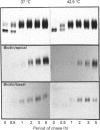
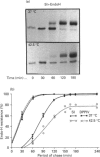
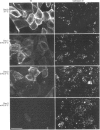
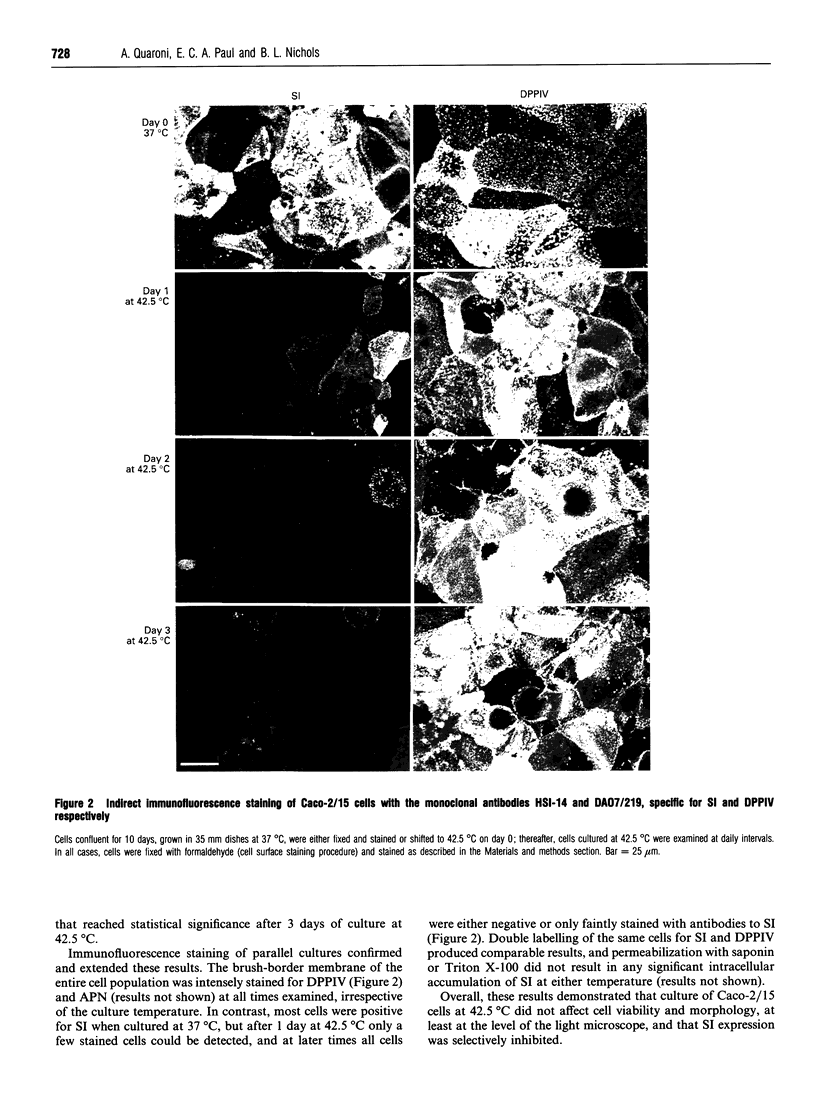
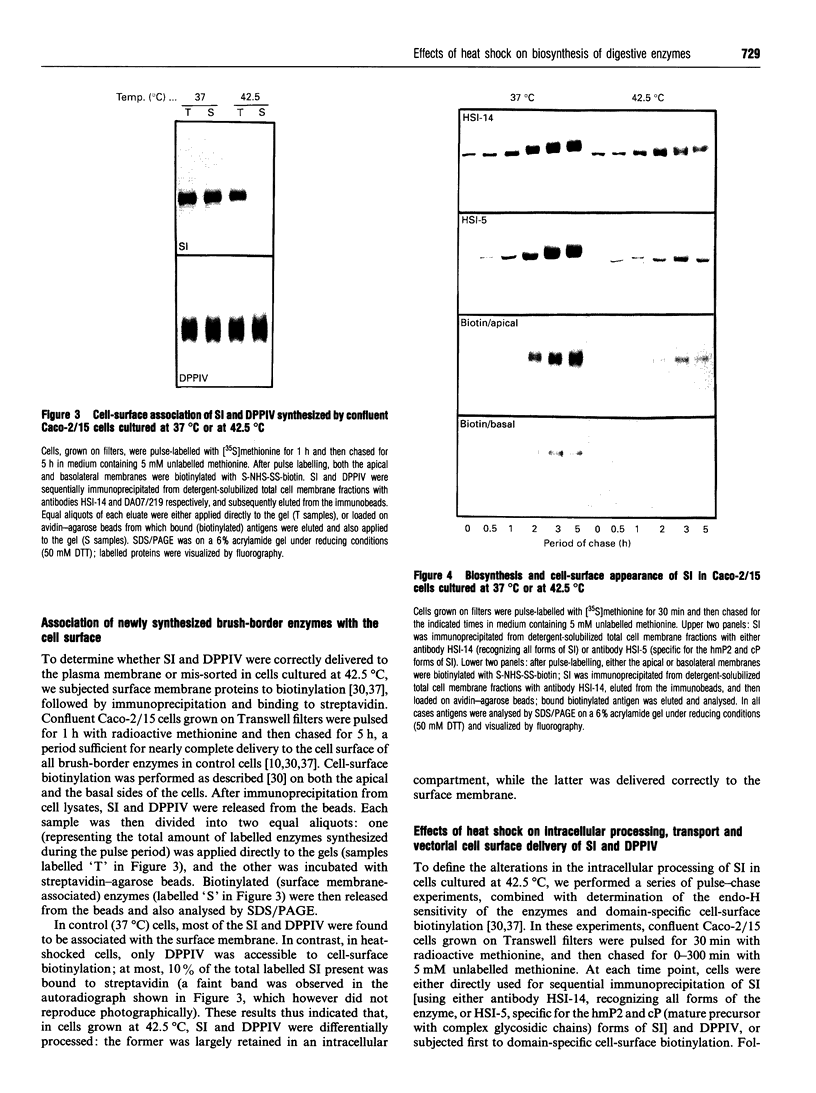
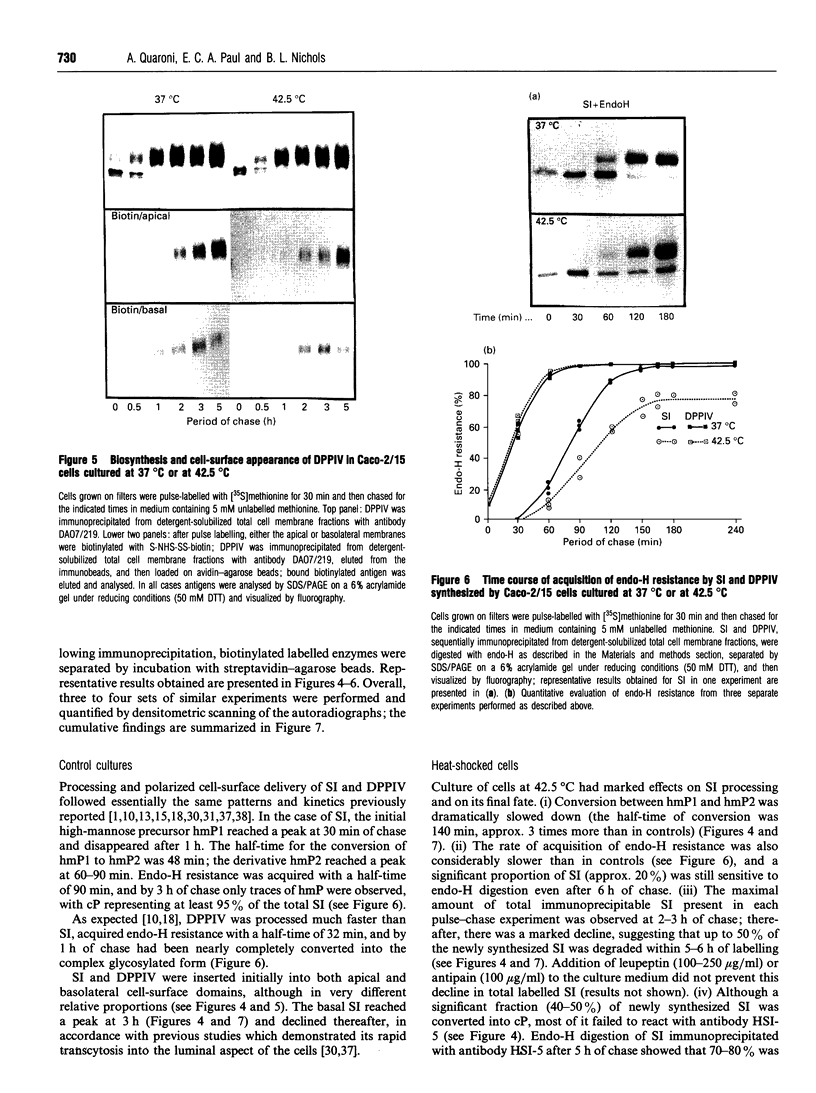
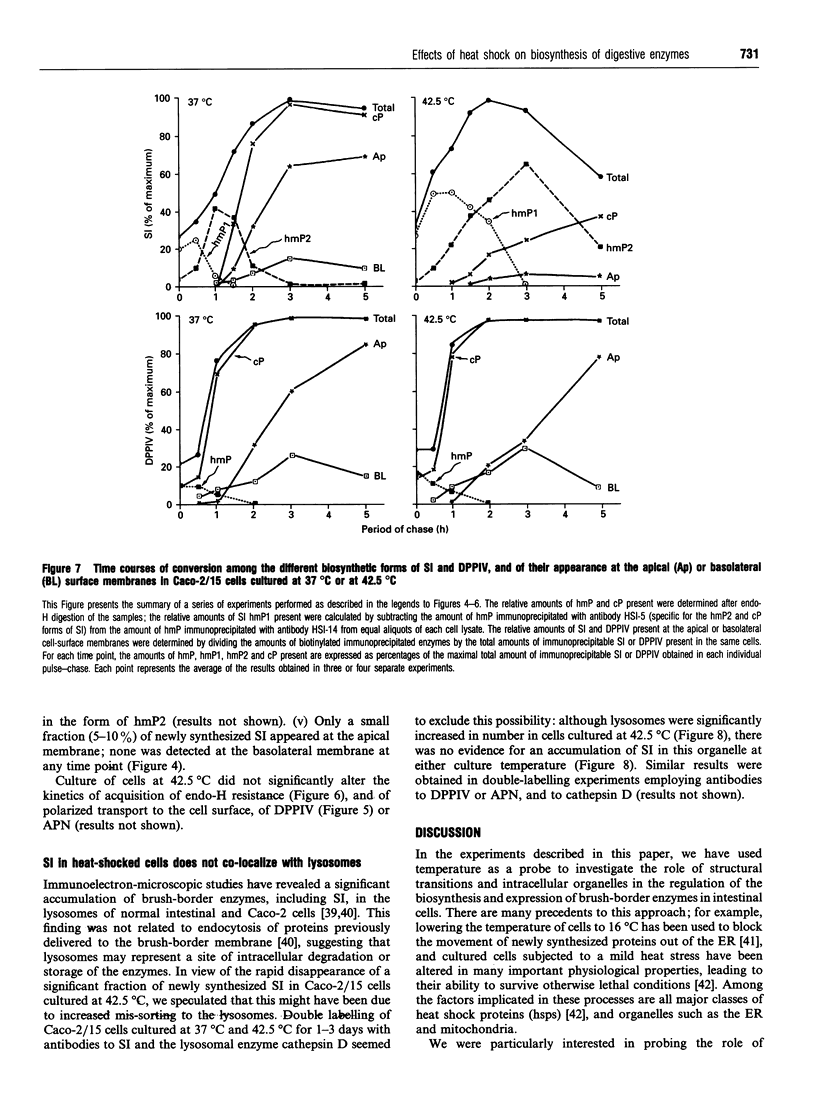
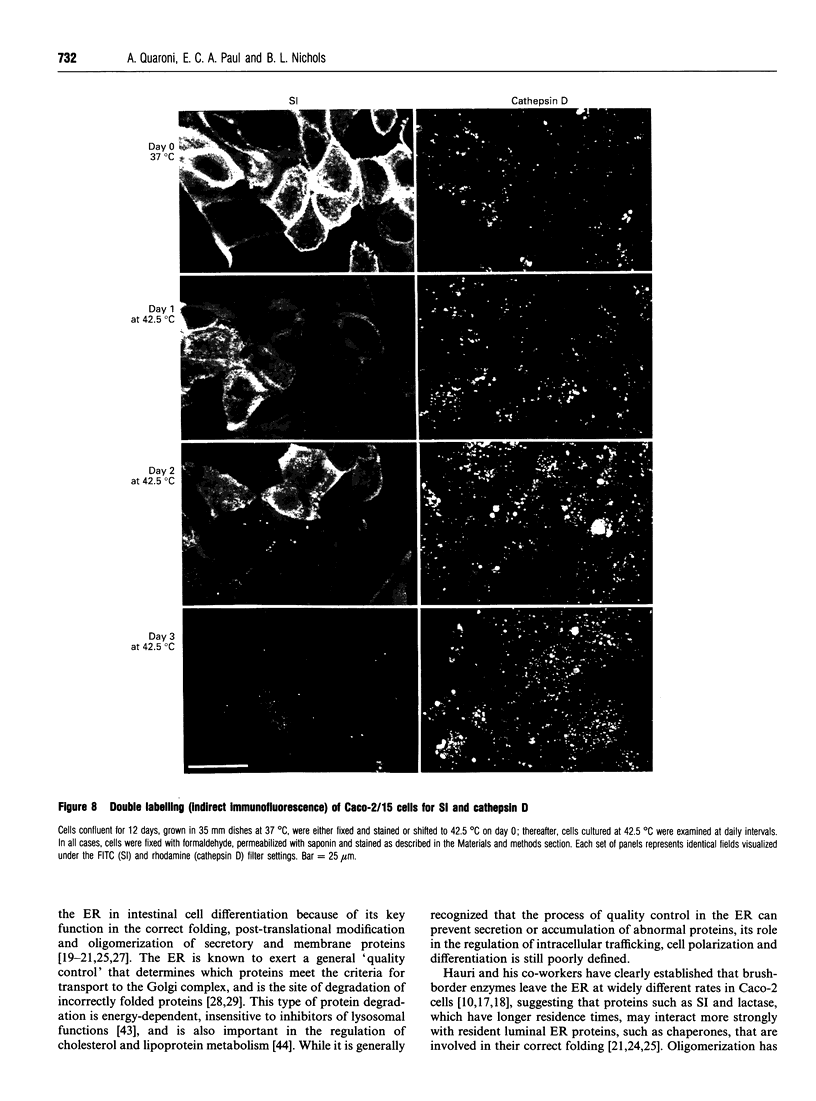
To investigate the role of post-translational events in intestinal cell differentiation we have studied the effects of heat shock on processing and cell surface delivery of sucrase-isomaltase (SI), dipeptidylpeptidase IV (DPPIV) and aminopeptidase N (APN) in Caco-2 cells. In cells cultured at 42.5 degrees C there was a rapid decline in sucrase activity, while DPPIV and APN were unaffected over a 3-day period. Immunofluorescence staining confirmed the selective disappearance of SI from the surface membrane after only 1 day of culture at 42.5 degrees C. Cell-surface biotinylation of cells metabolically labelled with [35S]methionine 4 h after a switch from 37 degrees C to 42.5 degrees C demonstrated that newly synthesized APN and DPPIV were associated with the surface membrane, while SI was almost completely retained intracellularly. Pulse-chase experiments confirmed that, in these cells, DPPIV and APN were normally processed and vectorially delivered to the cell surface; in contrast, conversion between the two conformationally distinct high-mannose precursor forms of SI (hmP1 and hmP2) was markedly inhibited, a significant fraction of newly synthesized enzyme was degraded, probably in the ER, and an immature form of complex-glycosylated SI precursor (cP) was produced and mostly retained intracellularly. Double labelling of Caco-2 cells for SI and cathepsin D excluded an accumulation of SI in the lysosomes, suggesting that this organelle was not involved in the degradation of SI. These results indicate that the ER may play an important role in intestinal cell differentiation by regulating the conformational maturation, degradation and eventual cellular localization of some digestive enzymes.
Full text
PDF

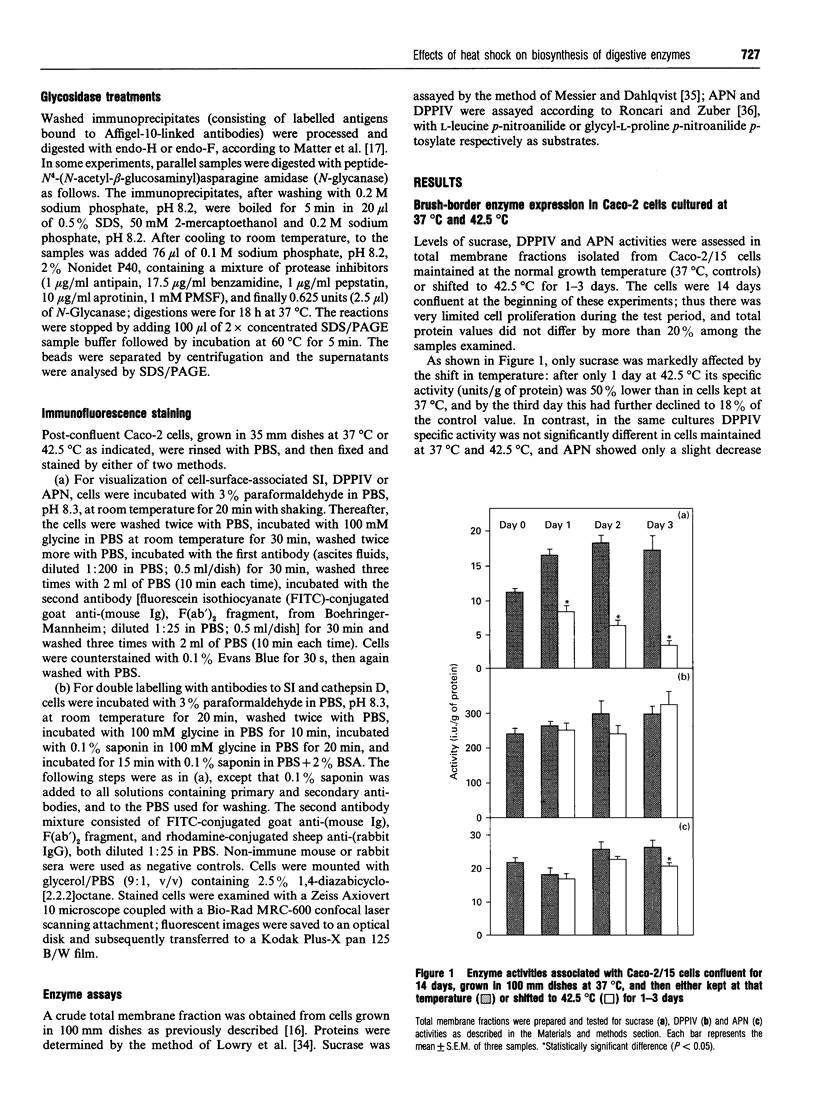


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaulieu J. F., Nichols B., Quaroni A. Posttranslational regulation of sucrase-isomaltase expression in intestinal crypt and villus cells. J Biol Chem. 1989 Nov 25;264(33):20000–20011. [PubMed] [Google Scholar]
- Beaulieu J. F., Quaroni A. Clonal analysis of sucrase-isomaltase expression in the human colon adenocarcinoma Caco-2 cells. Biochem J. 1991 Dec 15;280(Pt 3):599–608. doi: 10.1042/bj2800599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J. F., Weiser M. M., Herrera L., Quaroni A. Detection and characterization of sucrase-isomaltase in adult human colon and in colonic polyps. Gastroenterology. 1990 Jun;98(6):1467–1477. doi: 10.1016/0016-5085(90)91077-j. [DOI] [PubMed] [Google Scholar]
- Copeland C. S., Zimmer K. P., Wagner K. R., Healey G. A., Mellman I., Helenius A. Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell. 1988 Apr 22;53(2):197–209. doi: 10.1016/0092-8674(88)90381-9. [DOI] [PubMed] [Google Scholar]
- Cross H. S., Quaroni A. Inhibition of sucrose-isomaltase expression by EGF in the human colon adenocarcinoma cells Caco-2. Am J Physiol. 1991 Dec;261(6 Pt 1):C1173–C1183. doi: 10.1152/ajpcell.1991.261.6.C1173. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A., AURICCHIO S., SEMENZA G., PRADER A. Human intestinal disaccharidases and hereditary disaccharide intolerance. The hydrolysis of sucrose, isomaltose, palatinose (isomaltulose), and a 1,6-alpha-oligosaccharide (isomalto-oligosaccharide) preparation. J Clin Invest. 1963 Apr;42:556–562. doi: 10.1172/JCI104744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M. Folding of intestinal brush border enzymes. Evidence that high-mannose glycosylation is an essential early event. Biochemistry. 1992 Mar 3;31(8):2266–2272. doi: 10.1021/bi00123a008. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M. Perturbation of intestinal microvillar enzyme biosynthesis by amino acid analogs. Evidence that dimerization is required for the transport of aminopeptidase N out of the endoplasmic reticulum. J Biol Chem. 1990 Aug 25;265(24):14566–14571. [PubMed] [Google Scholar]
- Doms R. W., Ruusala A., Machamer C., Helenius J., Helenius A., Rose J. K. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988 Jul;107(1):89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M. A., Nichols B. L., Rosenberger J., Perkinson J. S., Reeds P. J. Feeding status affects in vivo prosucrase.isomaltase processing in rat jejunum. J Nutr. 1992 Mar;122(3):528–534. doi: 10.1093/jn/122.3.528. [DOI] [PubMed] [Google Scholar]
- Fransen J. A., Ginsel L. A., Hauri H. P., Sterchi E., Blok J. Immuno-electronmicroscopical localization of a microvillus membrane disaccharidase in the human small-intestinal epithelium with monoclonal antibodies. Eur J Cell Biol. 1985 Jul;38(1):6–15. [PubMed] [Google Scholar]
- Fransen J. A., Hauri H. P., Ginsel L. A., Naim H. Y. Naturally occurring mutations in intestinal sucrase-isomaltase provide evidence for the existence of an intracellular sorting signal in the isomaltase subunit. J Cell Biol. 1991 Oct;115(1):45–57. doi: 10.1083/jcb.115.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gilbert T., Le Bivic A., Quaroni A., Rodriguez-Boulan E. Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. J Cell Biol. 1991 Apr;113(2):275–288. doi: 10.1083/jcb.113.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel J. P., Ferrero A., Chambraud L., Rigal A., Bonicel J., Maroux S. Expression of sucrase-isomaltase and dipeptidylpeptidase IV in human small intestine and colon. Gastroenterology. 1991 Sep;101(3):618–625. doi: 10.1016/0016-5085(91)90517-o. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Sterchi E. E., Bienz D., Fransen J. A., Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985 Sep;101(3):838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Spiess M., Semenza G., Lodish H. F. The sucrase-isomaltase complex: primary structure, membrane-orientation, and evolution of a stalked, intrinsic brush border protein. Cell. 1986 Jul 18;46(2):227–234. doi: 10.1016/0092-8674(86)90739-7. [DOI] [PubMed] [Google Scholar]
- Jascur T., Matter K., Hauri H. P. Oligomerization and intracellular protein transport: dimerization of intestinal dipeptidylpeptidase IV occurs in the Golgi apparatus. Biochemistry. 1991 Feb 19;30(7):1908–1915. doi: 10.1021/bi00221a025. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Klausner R. D. Sorting and traffic in the central vacuolar system. Cell. 1989 Jun 2;57(5):703–706. doi: 10.1016/0092-8674(89)90783-6. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Bivic A., Quaroni A., Nichols B., Rodriguez-Boulan E. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J Cell Biol. 1990 Oct;111(4):1351–1361. doi: 10.1083/jcb.111.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Bonifacino J. S., Yuan L. C., Klausner R. D. Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell. 1988 Jul 15;54(2):209–220. doi: 10.1016/0092-8674(88)90553-3. [DOI] [PubMed] [Google Scholar]
- Lloyd M. L., Olsen W. A. A study of the molecular pathology of sucrase-isomaltase deficiency. A defect in the intracellular processing of the enzyme. N Engl J Med. 1987 Feb 19;316(8):438–442. doi: 10.1056/NEJM198702193160804. [DOI] [PubMed] [Google Scholar]
- Matter K., Brauchbar M., Bucher K., Hauri H. P. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2). Cell. 1990 Feb 9;60(3):429–437. doi: 10.1016/0092-8674(90)90594-5. [DOI] [PubMed] [Google Scholar]
- Matter K., Bucher K., Hauri H. P. Microtubule perturbation retards both the direct and the indirect apical pathway but does not affect sorting of plasma membrane proteins in intestinal epithelial cells (Caco-2). EMBO J. 1990 Oct;9(10):3163–3170. doi: 10.1002/j.1460-2075.1990.tb07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Hauri H. P. Intracellular transport and conformational maturation of intestinal brush border hydrolases. Biochemistry. 1991 Feb 19;30(7):1916–1923. doi: 10.1021/bi00221a026. [DOI] [PubMed] [Google Scholar]
- Matter K., McDowell W., Schwartz R. T., Hauri H. P. Asynchronous transport to the cell surface of intestinal brush border hydrolases is not due to differential trimming of N-linked oligosaccharides. J Biol Chem. 1989 Aug 5;264(22):13131–13139. [PubMed] [Google Scholar]
- Matter K., Stieger B., Klumperman J., Ginsel L., Hauri H. P. Endocytosis, recycling, and lysosomal delivery of brush border hydrolases in cultured human intestinal epithelial cells (Caco-2). J Biol Chem. 1990 Feb 25;265(6):3503–3512. [PubMed] [Google Scholar]
- Messer M., Dahlqvist A. A one-step ultramicro method for the assay of intestinal disaccharidases. Anal Biochem. 1966 Mar;14(3):376–392. doi: 10.1016/0003-2697(66)90280-6. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Naim H. Y., Roth J., Sterchi E. E., Lentze M., Milla P., Schmitz J., Hauri H. P. Sucrase-isomaltase deficiency in humans. Different mutations disrupt intracellular transport, processing, and function of an intestinal brush border enzyme. J Clin Invest. 1988 Aug;82(2):667–679. doi: 10.1172/JCI113646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A. Crypt cell development in newborn rat small intestine. J Cell Biol. 1985 May;100(5):1601–1610. doi: 10.1083/jcb.100.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K. J. Study of intestinal cell differentiation with monoclonal antibodies to intestinal cell surface components. Dev Biol. 1985 Oct;111(2):267–279. doi: 10.1016/0012-1606(85)90482-8. [DOI] [PubMed] [Google Scholar]
- Roncari G., Zuber H. Thermophilic aminopeptidases from Bacillus stearothermophilus. I. Isolation, specificity, and general properties of the thermostable aminopeptidase I. Int J Protein Res. 1969;1(1):45–61. doi: 10.1111/j.1399-3011.1969.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Saraste J., Palade G. E., Farquhar M. G. Temperature-sensitive steps in the transport of secretory proteins through the Golgi complex in exocrine pancreatic cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6425–6429. doi: 10.1073/pnas.83.17.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annu Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- Sitia R., Meldolesi J. Endoplasmic reticulum: a dynamic patchwork of specialized subregions. Mol Biol Cell. 1992 Oct;3(10):1067–1072. doi: 10.1091/mbc.3.10.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford F. J., Bonifacino J. S. A permeabilized cell system identifies the endoplasmic reticulum as a site of protein degradation. J Cell Biol. 1991 Dec;115(5):1225–1236. doi: 10.1083/jcb.115.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterchi E. E., Mills P. R., Fransen J. A., Hauri H. P., Lentze M. J., Naim H. Y., Ginsel L., Bond J. Biogenesis of intestinal lactase-phlorizin hydrolase in adults with lactose intolerance. Evidence for reduced biosynthesis and slowed-down maturation in enterocytes. J Clin Invest. 1990 Oct;86(4):1329–1337. doi: 10.1172/JCI114842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger B., Matter K., Baur B., Bucher K., Höchli M., Hauri H. P. Dissection of the asynchronous transport of intestinal microvillar hydrolases to the cell surface. J Cell Biol. 1988 Jun;106(6):1853–1861. doi: 10.1083/jcb.106.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki C. K., Bonifacino J. S., Lin A. Y., Davis M. M., Klausner R. D. Regulating the retention of T-cell receptor alpha chain variants within the endoplasmic reticulum: Ca(2+)-dependent association with BiP. J Cell Biol. 1991 Jul;114(2):189–205. doi: 10.1083/jcb.114.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trugnan G., Ogier-Denis E., Sapin C., Darmoul D., Bauvy C., Aubery M., Codogno P. The N-glycan processing in HT-29 cells is a function of their state of enterocytic differentiation. Evidence for an atypical traffic associated with change in polypeptide stability in undifferentiated HT-29 cells. J Biol Chem. 1991 Nov 5;266(31):20849–20855. [PubMed] [Google Scholar]
- Trugnan G., Rousset M., Chantret I., Barbat A., Zweibaum A. The posttranslational processing of sucrase-isomaltase in HT-29 cells is a function of their state of enterocytic differentiation. J Cell Biol. 1987 May;104(5):1199–1205. doi: 10.1083/jcb.104.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva A. M., Balch W. E., Helenius A. Quality control in the endoplasmic reticulum: folding and misfolding of vesicular stomatitis virus G protein in cells and in vitro. J Cell Biol. 1990 Sep;111(3):857–866. doi: 10.1083/jcb.111.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]