Abstract
Study design
A feasibility pilot study.
Objective
To assess the feasibility a full-scale Randomized Controlled Trial aimed at assessing the beneficial effect of a Virtual Walking (VW)-based (Experimental intervention (EI)) on neuropathic pain and functionality in people with incomplete spinal cord injury (SCI).
Setting
A hospital service (Hospital Universitario y Politécnico La Fe) and disability associations (TetraSport, CODIFIVA and ASPAYM).
Methods
Twelve people with chronic incomplete SCI were randomized to EI (VW plus therapeutic exercise program (TE)) -or Control Intervention (CI (placebo VW and TE)) groups. A six-week intervention (3 sessions/week) was carried out. To assess feasibility, the following outcomes were used: level of restriction and validity of inclusion and exclusion criteria, participants’ compliance, accessibility and acceptability of the intervention for participants, adequate pre-training time of physiotherapists. To explore therapy effectiveness, pain severity, and interference, mean and maximum isometric strength, walking speed, and walking ability were assessed before (Time 1, T1) and after (Time 2, T2) the intervention.
Results
20% of the participants initially recruited did not meet inclusion criteria. In addition, all participants completed at least 80% of the intervention sessions and none of the participants dropped out before T2. No serious adverse event was found. Moreover, 91.67% of participants were willing to perform the intervention again and all therapists involved were adequately pre-trained. Finally, our preliminary results suggest that the proposed EI is effective.
Conclusion
A full-scale RCT is feasible and preliminary results suggest that VW with TE could have a beneficial impact on pain and functionality in this population.
Subject terms: Orthopaedics, Rehabilitation, Spinal cord diseases
Introduction
Previous studies have shown that the neuroplasticity of the residual corticospinal fibres, motor cortex and spinal neurons plays an important role in the spontaneous functional recovery of people with incomplete spinal cord injury (SCI) [1, 2]. However, recovery can be further promoted if the characteristic mechanisms of the neuroplasticity of these structures are correctly stimulated by techniques aimed at rehabilitating different deficits (for example, motor function or sensation). In general, intervention programs for improving neuroplasticity are usually carried out, either using external devices, such as exoskeletons [3] or, in most cases, using low-cost strategies such as therapeutic physical exercise programs [4]. Both the simple and the technologically advanced programs attempt to stimulate neuroplasticity through early, intensive and specific therapies [5] usually focused on the recovery of function through motor stimulation performing task-specific exercises (i.e., walking, balance, etc.).
Previous studies have shown that performing specific movements increases cortical representation of the muscles responsible for these movements [6, 7]. However, it has also been confirmed that nerve reorganization and/or functional recovery can also be influenced by the activity of mirror neurons, which are found both in motor and premotor areas as well as in other cortical and subcortical areas [8, 9]. These neurons report activation, not only upon execution of movement, but also when a motor action is carefully observed and examined with a specific purpose [10, 11].
This ability to activate through observation is especially relevant when a lesion of the Central Nervous System (CNS) occurs, given that the neuronal dysfunction does not allow the proper execution of certain motor tasks. For this reason, different studies have analysed the impact of therapy based on mirror neurons in populations with CNS involvement, such as stroke, head injuries, fragile elderly people or Alzheimer’s disease [12–15], thus showing promising results. Nevertheless, few studies have applied this type of therapy in people with complete SCI, especially the use of visual illusion systems in which the participants visually perceive executed motor actions. Moseley et al. [16] were the first to propose that a virtual gait system was able to reduce neuropathic pain in five people with complete SCI. This has been further supported in two broader studies carried out by the Guttmann Institut [17, 18], confirming that visual illusion therapy reduces neuropathic pain in people with complete SCI. However, the inclusion of people with complete SCI preventing analysing the impact of this therapy beyond pain, so more studies addressing its impact on other important variables related to functional recovery in people with incomplete SCI are needed.
Based on the foregoing, a feasibility study to ascertain if this type of therapy is suitable for people with incomplete SCI was called for. The research aims of this feasibility study was to explore the appropriate trial design of a final RCT to improve neuroplasticity mechanisms after an incomplete SCI, using mirror neuron stimulation by visual illusion therapy. Specifically, this RCT will assess the effectiveness of an experimental intervention (EI) based on Virtual Walking (VW) combined with a therapeutic exercise program (TE) compared against a control intervention (CI) based on Placebo VW and TE in terms of improving neuropathic pain and functionality in people with incomplete SCI.
The primary objectives of this pilot trial were as follows: i. To assess inclusion and exclusion criteria, ii. To determine the validity of eligibility criteria, and iii. To assess participant’s compliance with the protocol.
The secondary objectives were i. To assess intervention accessibility and acceptability for participants, ii. To assess adequate pre-training time of physical therapists, and iii. To explore the potential beneficial effects on the assessed variables.
Methods
A two-arm randomized controlled pilot trial design was carried out. To reduce bias, both the physical therapist who performed the assessments and the statistician were unaware of group allocation. This study was approved by the Ethics Committee of the University of València (number: 1304149) and performed in accordance with the latest revision of the Declaration of Helsinki. All participants signed the informed consent to participate in the study. The authors have obtained written informed consent for publication of the images.
Participants
Volunteers with chronic incomplete SCI were recruited through a hospital service (Hospital Universitario y Politécnico La Fe) and disability associations (TetraSport, CODIFIVA and ASPAYM). The inclusion criteria were the following: i. Motor Incomplete SCI (ASIA impairment scale score (AIS) C or D); ii. Ability to walk with or without aids; iii. Chronicity for more than 1 year [1], and iv. Ability to understand instructions (Mini-Mental State Examination >23 points). Exclusion criteria were the following: i. Traumatic leg injury; ii. Other alterations of the CNS or peripheral nervous system; iii. Vestibular system disorders, and iv. Concomitant diseases. Compliance with the inclusion criteria was verified by the SCI specialist of the hospital unit or disability association.
Procedure
Before starting with the intervention procedure and the recruitment of participants, the therapists underwent two learning sessions where they acquired the necessary competences to apply the treatment described below.
In the first visit, eligible volunteers were informed about all study proceedings and an informed consent was signed. In this session, demographic data including age, sex, and time since SCI were obtained through a discharge report. If the volunteer met all the criteria to participate in the study, he/she was assigned to an intervention group with a blinded randomization method and was scheduled another day to perform the pre-treatment assessment.
Participants were divided into two intervention arms (i.e., EI and CI) using the simple randomization method with the Random Allocation Software carried out by an external assistant who was blinded to the study objectives [19]. Randomization and disclosure of group allocation took place after the first evaluation session and was carried out by a researcher not involved in data collection.
In the second visit, height and weight measurements and the clinical assessments (secondary outcomes described below) were carried out by a researcher blinded to group allocation (Time 1, T1). The same clinical assessment and feasibility assessment procedures were conducted by the same researcher at the end of the 6-week treatment (Time 2, T2).
Primary outcomes
The primary outcome of the study was the feasibility of a full-scale RCT. To assess level of restriction of inclusion and exclusion criteria, eligible participants (%) were computed. Moreover, to determine the validity of eligibility criteria, participants able to perform all procedures (%) were assessed.
An attendance list (%) was used to assess participant adherence. Participant drop-out events (%) were used to assess compliance. Moreover, all adverse events were reported.
Secondary outcomes
The secondary outcomes of the study were accessibility and acceptability for participants and pre-training of therapists. Moreover, effect size of the intervention was explored.
Regarding accessibility and acceptability of the intervention, the time required to perform all procedures (in hours) and the participants’ opinion on the intervention (through a semi-structured interview) were determined. Moreover, the physical therapists’ opinion (through a semi-structured interview) was used to assess the time needed for an adequate knowledge and pre-training of the physical therapists. This part was analysed qualitatively.
To explore the beneficial effects of the EI on the explored variables, the effect size achieved in the following tests and scales was used:
Brief Pain Inventory (BPI) [20] was used to explore the impact of the intervention on pain severity and pain interference in daily life activities.
Load cell (Chronojump) was used to assess isometric strength of the tibialis anterior and quadriceps, these being the most visible muscles in the VW protocol. Participants performed three 5-second maximum isometric contractions with a 5-second rest between repetitions using the least affected leg (assessed by ASIA impairment scale (AIS) score). For the tibialis anterior, participants lay in supine position with one foot off the stretcher, while to assess the quadriceps, participants lay in prone position with 90° knee flexion. Maximum and mean isometric strength (in Newtons) were obtained. For more detailed information, please see Supplementary Material 1 and 2.
The 10 m Walk Test (10MWT) [21] and Walking Index for Spinal Cord Injury (WISCI) [22] were used. 10MWT assesses the time required to cover 10 metres, while WISCI assess the amount of physical assistance needed, as well as devices required, for walking.
Specific details of primary and secondary outcomes and the thresholds for changing the final RCT are shown in Table 1.
Table 1.
Assessment of primary and secondary outcomes.
| Primary outcomes | How | When | Decisions for the final RCT |
|---|---|---|---|
| 1. To assess the level of restriction of inclusion/exclusion criteria |
QT % Of eligible participants |
T2 |
% >80% To keep the inclusion/exclusion criteria % <80% To modify (enlarge) |
| 2. To determine the validity of eligibility criteria. |
QT % Of participants that could perform the entire protocol |
T2 |
% >80% To keep eligibility criteria % <80% To modify (restrictions about ASIAs level or type of injury) |
| 3. To assess participant’s compliance with the protocol |
QT a. Session attendance registration (%) b. Dropouts (%) c. Adverse event (n) |
T2 |
a. Attendance to sessions <80%, revise duration of the protocol and frequency and duration of the sessions b. If dropouts >20%, revise protocol based on drop-out reasons. c. In case of any serious adverse event, revise the protocol base on this adverse event. |
| Secondary outcomes | How | When | Decisions for the final RCT |
| 1. To assess intervention acceptability and accessibility for the participants |
QL a. Participant’s opinion about time required to perform all procedures (interview). b. Participant’s opinion on the protocol (% of participants that would repeat the experience) |
T2 |
a. More time than conventional therapies, revise protocol b. % <80% to be modified (include more motivation elements) |
| 2. To assess adequate pre-training time of physical therapists. |
QL Adequate knowledge (therapist interviews) |
T2 | If therapist believe that more pre-training is needed, include adequate knowledge at full-scale RCT |
| 3. To analyse potential effectiveness of the protocol in improving the explored variables |
QT Effect Size of the intervention |
T1 and T2 | This outcome does not affect a full-scale RCT because of the small sample size. |
QT quantitative method, QL qualitative method.
Interventions
Both interventions (EI and CI) lasted 6 weeks (3 days a week), and the sessions were carried out in groups of 3 people. In each session, researchers recorded if any type of adverse effect occurred.
EI consisted in a visual illusion therapy intervention based on virtual walking (VW) and a therapeutic exercise program (TE), whilst CI included placebo VW and the same TE program. For the VW, the patient was facing a mirror (from the waist up), and a standing frame set-up provided support for the lower body (with an standing ad-hoc designed help system). For the lower body, a screen (from the waist down) where a video of legs walking on a treadmill was projected (Fig. 1). The projected legs were adapted to each subject according to their height and weight. This program lasted 10 min.
Fig. 1.
Set-up protocol.
For the placebo VW, the set-up and duration were the same as in the experimental VW, but videos of landscapes without featuring any type of human or animal movement were projected. Finally, TE was divided into two parts, as shown in Table 2: i) gait technique training (i.e. coordination exercises), and ii) multicomponent training (i.e. strength, balance and stretching exercises). The total duration was 35 min.
Table 2.
TE program.
| Part | Exercise | Series | Repetitions | Time |
|---|---|---|---|---|
| Coordination exercises | Normal walking with obstacles | 3 | 3 | 35 min |
| Lateral walking with obstacles | 3 | 3 | ||
| Backward walking | 3 | 3 | ||
| Strength exercises | Squat | 3 | 10 | |
| Ankle flexion-extension with body weight | 3 | 10 | ||
| Hip abduction | 3 per leg | 10 | ||
| Knee flexion in prone position with weights | 3 | 10 | ||
| Balance exercise | Monopodal balance | 3 per leg | 1 (1 min) | |
| Stretching | Lower limb passive stretching | 1 | 1 |
Statistical analysis
Statistical data analysis was conducted using SPSS v26 (Inc. IBM., Chicago, IL, USA). For the clinical variables (secondary outcome measures) a mixed ANOVA was used to explore the size effect of the impact, with a between-subject factor “group” (i.e., EI and CI) and a within-subject factor “time” (i.e., T1 and T2). Mean and standard deviation (SD) were used for calculating the effect size (Cohen’s d).
For post-hoc comparisons between assessments, the Šidák correction was used.
Results
Between September 2020 and July 2021, 15 people agreed to participate in this study.
Primary outcomes
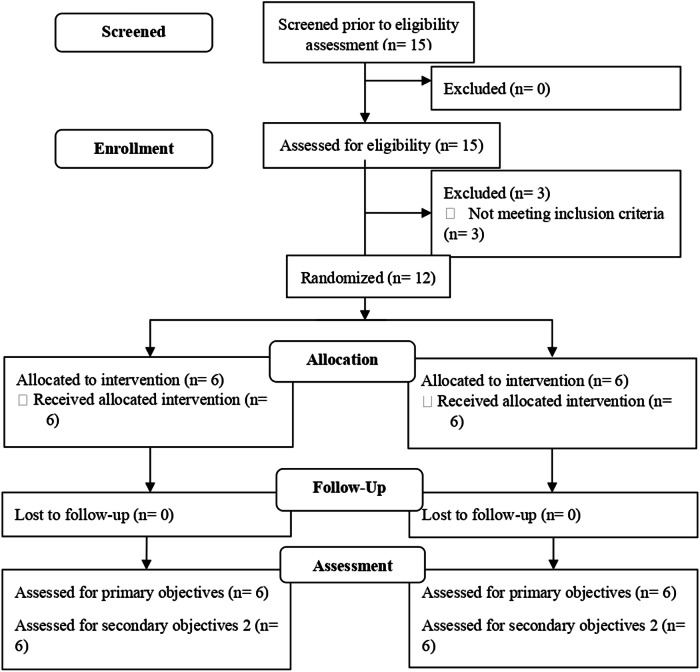
When the level of restriction was assessed, 20% of exclusions was reached after telephone screening. In total, three participants were discarded because they failed to meet the inclusion criteria, since they had complete SCI (Fig. 2).
Fig. 2.
Flow diagram.
The twelve participants recruited completed the six-week intervention to which they were assigned (100%). Demographic data and injury characteristics are shown in Table 3.
Table 3.
Demographic data and injury characteristics.
| ID | Age (years) | Group | Level of injury | AIS | Cause of Injury | Time since injury (years) |
|---|---|---|---|---|---|---|
| 01 | 64 | EI | C4-C7 | D | Trauma | 6 |
| 02 | 73 | EI | L5-S1 | D | Trauma | 11 |
| 03 | 49 | EI | L5-S1 | C | Spinal cord swelling | 5 |
| 04 | 39 | EI | L5-S1 | D | Trauma | 5 |
| 05 | 41 | EI | C5-D1 | D | Surgery | 5 |
| 06 | 40 | EI | L3 | C | Trauma | 4 |
| 07 | 35 | CI | C3-C6 | D | Trauma | 2 |
| 08 | 65 | CI | C2-C5 | D | Trauma | 2 |
| 09 | 68 | CI | D8 | D | Surgery | 2 |
| 10 | 42 | CI | D12 | C | Spinal cord swelling | 8 |
| 11 | 38 | CI | S1 | D | Spinal cord swelling | 10 |
| 12 | 70 | CI | C4 | D | Trauma | 3 |
AIS ASIA impairment scale score, CI Control Intervention, EI Experimental Intervention.
Regarding protocol adherence and compliance, all participants completed at least 80% of the intervention sessions and none of the participants (0%) dropped out before T2.
With regard to unwanted effects, all participants reported fatigue at the end of each session. Moreover, ID10 suffered dizziness while viewing placebo VW video on the second session of the intervention. Researcher proceeded to lay him on a stretcher with his feet up while his recovery was monitored using a sphygmomanometer.
Secondary outcomes
After the intervention, the participants considered that the time required to participate in the study (18 intervention sessions (13.5 h) and 2 assessment sessions (2 h)) was considerable, but they all acknowledged that this was necessary and none of them felt that the time burden was excessive. Indeed, 100% of participants from both groups were not about to change any part of the intervention protocol and believed the intervention was useful. 91.67% of them would be prepared to perform the intervention again.
Regarding physical therapists’ opinion about their pre-training time, since they had worked with this type of population previously, 30 min of pre-study training was found appropriate.
Finally, Table 4 shows mean, SD and the effect size of the explored variables in the two assessments sessions (i.e., T1, T2,). We found significant improvement in the EI group for tibialis anterior strength, 10MWT and WISCI, while no differences were found between assessments in the CI group.
Table 4.
Preliminary results of clinical trial outcomes.
| Outcome measure/group | T1 | T2 | Comparison T1-T2 | |
|---|---|---|---|---|
| 95% IC | Cohen’s d | |||
| Pain Severity (BPI, points) | ||||
| - EI | 3.58 (2.29) | 2.83 (2.94) | −0.45 to 1.95 | 0.28 |
| - CI | 3.75 (2.32) | 3.88 (2.14) | −1.08 to 1.33 | −0.06 |
| Pain Interference (BPI, points) | ||||
| - EI | 4.14 (3.31) | 3.60 (3.83) | −0.98 to 2.07 | 0.15 |
| - CI | 4.24 (2.36) | 3.83 (2.23) | −1.12 to 1.93 | 0.17 |
| Tibialis anterior Mean Strength (Newtons) | ||||
| - EI | 38.91 (42.98) | 68.06 (69.34) | −56.29 to −2.02 | −0.51 |
| - CI | 43.95 (37.71) | 48.98 (49.66) | −32.168 to 22.11 | −0.11 |
| Quadriceps Mean Strength (Newtons) | ||||
| - EI | 90.17 (43.46) | 104.70 (65.03) | −55.202 to 26.15 | −0.26 |
| - CI | 77.91 (41.44) | 71.97 (43.97) | −34.73 to 46.62 | 0.14 |
| Tibialis anterior Maximum Strength (Newtons) | ||||
| - EI | 39.76 (42.77) | 71.54 (69.60) | −60.67 to −2.90 | -0.18 |
| - CI | 47.05 (39.00) | 53.53 (53.02) | −35.36 to 22.41 | −0.14 |
| Quadriceps Maximum Strength (Newtons) | ||||
| - EI | 94.45 (44.08) | 111.39 (62.98) | −58.78 to 24.89 | −0.31 |
| - CI | 84.87 (42.91) | 78.46 (46.24) | −35.42 to 48.24 | 0.14 |
| Walking speed (10MWT, s) | ||||
| - EI | 14.33 (7.75) | 10.88 (5.44) | 0.90 to 6.00 | 0.52 |
| - CI | 11.97 (4.35) | 9.71 (4.12) | −0.294 to 4.80 | 0.53 |
| Walking ability (WISCI, points) | ||||
| - EI | 17.5 (2.67) | 18.83 (1.83) | −2.51 to −0.159 | 0.13 |
| - CI | 16.67 (5.93) | 16.33 (6.71) | −0.84 to 1.51 | 0.05 |
Data are expressed in mean (SD).
BPI Brief Pain Inventory, CI Control Intervention, EI Experimental Intervention, WISCI Walking Index for Spinal Cord Injury, 10MWT 10 m walking test.
*p < 0.05 in bold.
Discusion
Previous studies have assessed effectiveness of bodily illusion therapies on pain in people with complete SCI [16–18]. Nevertheless, given that it is possible to stimulate neuroplasticity [1, 2] of the residual motor neuron fibres in people with incomplete SCI, it becomes necessary to explore the effects of a bodily illusion therapy on functional outcomes in this population. To the best of our knowledge, this is the first study that aims to assess the feasibility of a full-scale RCT for researching the effectiveness of an intervention based on VW combined with TE for improving motor function and pain in people with incomplete SCI.
Due to the specific characteristics of people with incomplete SCI, it is difficult to recruit the sample [23], so it is important to establish the level of the restriction and validity of the inclusion and exclusion criteria of the protocol designed for a possible full-scale RCT, while determining if it is possible to achieve an adequate sample size. Moreover, due to the comorbidities associated with SCI (i.e., psychological disorders [24], arthritis, hypertension, hypotension, hyperlipidaemia, obesity, diabetes, etc. [25–27]) that could affect the adherence and compliance of the intervention, it is important to ensure the feasibility of a full-scale RCT and to explore the difficulties and limitations of the designed protocol. In addition, assessing the accessibility and acceptability of the intervention for participants and the required pre-training time of physical therapists is essential to warrant a feasible full-scale RCT.
Primary outcomes
We found that 80% of people initially contacted were ultimately recruited, which suggests that inclusion and exclusion criteria are valid and with an adequate level of restriction to ensure that around 80% of the preliminary participants would be eligible for the study. Besides, compliance with the study was high (100% of participants performed T2 assessment), as well as the adherence rate (all of them attended >80% of the sessions). These results suggest that a full-scale RCT with these conditions is feasible. However, it is worth noting that these high rates were likely obtained thanks to schedule adjustments to ensure participation, since some sessions conflicted with medical appointments or holidays. Rescheduling the sessions should be considered in the full-scale RCT protocol.
In terms of adverse events, fatigue was the most common complaint reported by participants. Although fatigue has been described as a common secondary condition that occurs in over 50% of people with SCI living in the community [28, 29] and thus can be classified as a mild adverse event, the full-scale RCT should include a 10-minute resting period after each session to ensure appropriate rest and recovery. Another not so common adverse event was dizziness produced by orthostatic hypotension [30]. In able-bodied individuals, arterial tension changes are maintained through a rapid and effective reflex adjustment of the autonomic nervous system and slower humoral compensatory changes to counteract the gravitational forces on blood. Failure of these mechanisms may lead to orthostatic hypotension, which is very common among people with SCI [25]. When this event occurred, researchers lay the participant on a stretcher with his feet up while his recovery was monitored using a sphygmomanometer [31]. Both procedures to attend adverse events should be reflected in the protocol of the full-scale RCT.
Secondary outcomes
The participants of this pilot study reported that the time required to participate in the study (around 20 h of treatment and assessment plus the travelling to the laboratory) was significant, but they all understood that such time was necessary and 91.67% of them would be willing to undergo the intervention again. However, because of their mobility difficulties and the time spent on trips, patients believe that moving the protocol to different sites would improve patient recruitment and adherence. Therefore, including different venues to perform the intervention would be useful to improve adherence and compliance in a full-scale RCT. Using a multicentre design, researchers may ensure a large number of participants from different geographic regions and the ability to compare results from all of them [32, 33].
With regard to the physical therapists’ views on the pre-study training, they stated that 30 min were enough, since they had worked with this population previously. However, it would be important to extend pre-study training if therapists are not used to working with SCI patients.
When the preliminary results were explored, we found no significant pain decrease after any of the interventions. This contrasts with the results obtained by Moseley et al. [16] (who included a similar sample size) in which a pain decrease was achieved in the VW group when compared to their placebo group. However, their sample consisted of people with complete SCI, so there was no exercise- based intervention, which has been posited as the gold standard among rehabilitation-based therapeutic approaches in this population [4]. According to our study, the pain severity decrease was slightly higher in the EI group (−0.75 points) than its increase in the CI group (0.13 points), but such difference did not reach the level of significance (p > 0.05). Further, both groups obtained a similar non-significant decrease of pain interference (−0.54 points in EI and −0.41 points in CI). Therefore, an appropriate sample size calculation is necessary to improve the power of the statistical analysis in the full-scale RCT to conclude an effect of this intervention on perceived pain.
When motor function was assessed, interestingly most outcomes experienced a larger improvement in EI than in CI. There was a significantly higher mean increase and maximum strength gain of the tibialis anterior in EI (31.78 N and 29.15 N, respectively, p < 0.05), while CI did not experience any improvement after the intervention (p > 0.05). Regarding quadriceps strength, higher mean increase and maximum strength gain was present in EI compared to CI, but no significant differences were found (p > 0.05). Briefly, three mechanisms are involved in strength gain: intermuscular coordination, intramuscular coordination and muscle hypertrophy. In contrast to intramuscular coordination and muscle hypertrophy, intermuscular coordination depends strictly on learning the technique of motor patterns [34, 35]. Therefore, visualizing the normal gait pattern could help to improve intermuscular coordination and thus, increase strength in SCI patients.
In terms of functional outcomes, walking speed as measured with 10MWT and walking ability as measured with WISCI showed significant differences between T1 and T2 in the EI group (p < 0.05) with a difference of 3.45 s and 1.33 points, respectively. On the contrary, the CI group did not show significant differences in any of the two functional variables between assessments (p > 0.05). The 10MWT outcome achieved the highest effect size (d = 0.52) and exceeded the minimal detectable change (MDC = 1.3 s) [36], while the WISCI also exceeded the MDC [37] (MDC = 1) but with a lower effect size (d = 0.13).
The different clinical outcome measures used to explore the effect size show significant improvements for the EI group, but not for the CI group. TE can improve functional parameters in people with SCI affording to the nervous system a specific experience associated with the goal [4]. However, this mechanism could be further enhanced by VW, which aims to modulate cortical sensorimotor integration [38].
Although the results of this pilot study suggest that VW combined with TE may have a positive impact on function and neuropathic pain, the considerations explained above should be considered before a larger RCT is initiated.
Conclusion
A full-scale RCT analysing the beneficial effects of an intervention program based on VW combined with TE on pain and motor function in people with chronic incomplete SCI is feasible. Moreover, our preliminary results suggest that VW therapy combined with TE could have a beneficial impact on functionality in people with chronic incomplete SCI.
Supplementary information
Acknowledgements
To the language translation service of Il·lustre Col·legi Official de Fisioterapeutes de la Comunitat Valenciana (SPAIN). To the Hospital Universitari i Politècnic La Fe (Spain) and Carmen Grao Castellote. To the disability associations (TetraSport, CODIFIVA and ASPAYM), Spain.
Author contributions
SM-C, EM-G, MA-R, PS-A, MI, NM-S and NS-R participated in the conception, design, data acquisition; SM-C and PS-A have performed statistical analyses and data interpretation. All authors were involved in the interpretation of data and drafting, revision of the manuscript and approval of the final version of the manuscript and agreed to be accountable for all aspects of the work.
Funding
This work was supported by Grants from the Spanish Government [PID2021-125694OB-I00; co-financed by EU FEDER funds]; Generalitat Valenciana, Conselleria d’Innovació, Universitats, Ciència i Societat [CIAICO/2021/215[; and from Universitat de València [INV19-01-13-07].
Data availability
Datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval
The institutional review board approved this study. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41394-024-00675-w.
References
- 1.Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. 10.1038/sj.sc.3102007 [DOI] [PubMed] [Google Scholar]
- 2.de Araújo AVL, Ribeiro FPG, Massetti T, Potter-Baker KA, Cortes M, Plow EB, et al. Effectiveness of anodal transcranial direct current stimulation to improve muscle strength and motor functionality after incomplete spinal cord injury: a systematic review and meta-analysis. Spinal Cord. 2020. 10.1038/s41393-020-0438-2. [DOI] [PubMed]
- 3.Roosink M, Mercier C. Virtual feedback for motor and pain rehabilitation after spinal cord injury. Spinal Cord. 2014;52:860–6. 10.1038/sc.2014.160 [DOI] [PubMed] [Google Scholar]
- 4.Hornby TG, Reisman DS, Ward IG, Scheets PL, Miller A, Haddad D, et al. Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury. J Neurologic Phys Ther. 2020;44:49–100. 10.1097/NPT.0000000000000303 [DOI] [PubMed] [Google Scholar]
- 5.Alashram AR, Padua E, Hammash AK, Lombardo M, Annino G. Effectiveness of virtual reality on balance ability in individuals with incomplete spinal cord injury: A systematic review. J Clin Neurosci. 2020;72:322–7. 10.1016/j.jocn.2020.01.037 [DOI] [PubMed] [Google Scholar]
- 6.Houlé JD, Côté MP. Axon regeneration and exercise-dependent plasticity after spinal cord injury. Ann N Y Acad Sci. 2013;1279:154–63. 10.1111/nyas.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nardone R, Höller Y, Brigo F, Seidl M, Christova M, Bergmann J, et al. Functional brain reorganization after spinal cord injury: Systematic review of animal and human studies. Brain Res. 2013;1504:58–73. 10.1016/j.brainres.2012.12.034 [DOI] [PubMed] [Google Scholar]
- 8.Dushanova J, Donoghue J. Neurons in primary motor cortex engaged during action observation. Eur J Neurosci. 2010;31:386–98. 10.1111/j.1460-9568.2009.07067.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigneswaran G, Philipp R, Lemon RN, Kraskov A. M1 corticospinal mirror neurons and their role in movement suppression during action observation. Curr Biol. 2013;23:236–43. 10.1016/j.cub.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraskov A, Philipp R, Waldert S, Vigneswaran G, Quallo MM, Lemon RN. Corticospinal mirror neurons. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130174–20130174. 10.1098/rstb.2013.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan H, Luo Z. Functional integration of mirror neuron system and sensorimotor cortex under virtual self-actions visual perception. Behav Brain Res. 2022;423:113784. 10.1016/j.bbr.2022.113784 [DOI] [PubMed] [Google Scholar]
- 12.Gandhi D, Sterba A, Khatter H, Pandian J. Mirror Therapy in Stroke Rehabilitation: Current Perspectives. Therapeutics Clin Risk Manag. 2020;16:75–85. 10.2147/TCRM.S206883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggio MG, Torrisi M, Buda A, De Luca R, Piazzitta D, Cannavò A, et al. Effects of robotic neurorehabilitation through lokomat plus virtual reality on cognitive function in patients with traumatic brain injury: A retrospective case-control study. Int J Neurosci. 2020;130:117–23. 10.1080/00207454.2019.1664519 [DOI] [PubMed] [Google Scholar]
- 14.Mollà-Casanova S, Muñoz-Gómez E, Sempere-Rubio N, Inglés M, Aguilar-Rodríguez M, Page Á, et al. Effect of virtual running with exercise on functionality in pre-frail and frail elderly people: randomized clinical trial. Aging Clin Exp Res. 2023;35:1459–67. [DOI] [PMC free article] [PubMed]
- 15.Mollà-Casanova S, Page Á, López-Pascual J, Inglés M, Sempere-Rubio N, Aguilar-Rodríguez M, et al. Effects of mirror neuron activation therapies on functionality in older adults: Systematic review and meta-analysis. Geriatr Nurs. 2024;56:115–23. 10.1016/j.gerinurse.2024.02.006 [DOI] [PubMed] [Google Scholar]
- 16.Moseley GL. Using visual illusion to reduce at-level neuropathic pain in paraplegia. Pain. 2007;130:294–8. 10.1016/j.pain.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 17.Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, Fregni F, et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain. 2010;133:2565–77. 10.1093/brain/awq184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumru H, Soler D, Vidal J, Navarro X, Tormos JM, Pascual-Leone A, et al. The effects of transcranial direct current stimulation with visual illusion in neuropathic pain due to spinal cord injury: an evoked potentials and quantitative thermal testing study. Eur J Pain. 2013;17:55–66. 10.1002/j.1532-2149.2012.00167.x [DOI] [PubMed] [Google Scholar]
- 19.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. 10.1186/1471-2288-4-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badia X, Muriel C, Gracia A, Manuel Núñez-Olarte J, Perulero N, Gálvez R, et al. Validación española del cuestionario Brief Pain Inventory en pacientes con dolor de causa neoplásica. Med Clínica. 2003;120:52–9. 10.1016/S0025-7753(03)73601-X [DOI] [PubMed] [Google Scholar]
- 21.Olmos LE, Freixes O, Gatti MA, Cozzo DA, Fernandez SA, Vila CJ, et al. Comparison of gait performance on different environmental settings for patients with chronic spinal cord injury. Spinal Cord. 2008;46:331–4. 10.1038/sj.sc.3102132 [DOI] [PubMed] [Google Scholar]
- 22.Dittuno P, Dittuno J Jr. Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39:654–6. 10.1038/sj.sc.3101223 [DOI] [PubMed] [Google Scholar]
- 23.Engel-Haber E, Botticello A, Snider B, Kirshblum S. Incomplete Spinal Cord Syndromes: Current Incidence and Quantifiable Criteria for Classification. J Neurotrauma. 2022. 10.1089/neu.2022.0196. [DOI] [PubMed]
- 24.Craig A, Nicholson Perry K, Guest R, Tran Y, Dezarnaulds A, Hales A, et al. Prospective Study of the Occurrence of Psychological Disorders and Comorbidities After Spinal Cord Injury. Arch Phys Med Rehabilit. 2015;96:1426–34. 10.1016/j.apmr.2015.02.027 [DOI] [PubMed] [Google Scholar]
- 25.Krassioukov A, Eng JJ, Warburton DE, Teasell R. A Systematic Review of the Management of Orthostatic Hypotension After Spinal Cord Injury. Arch Phys Med Rehabilit. 2009;90:876–85. 10.1016/j.apmr.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tallqvist S, Kauppila AM, Vainionpää A, Koskinen E, Bergman P, Anttila H, et al. Prevalence of comorbidities and secondary health conditions among the Finnish population with spinal cord injury. Spinal Cord. 2022;60:618–27. 10.1038/s41393-021-00704-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guest J, Datta N, Jimsheleishvili G, Gater DR. Pathophysiology, Classification and Comorbidities after Traumatic Spinal Cord Injury. J Pers Med. 2022;12:1126. 10.3390/jpm12071126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anton HA, Miller WC, Townson AF, Imam B, Silverberg N, Forwell S. The course of fatigue after acute spinal cord injury. Spinal Cord. 2017;55:94–7. 10.1038/sc.2016.102 [DOI] [PubMed] [Google Scholar]
- 29.Onate-Figuérez A, Avendaño-Coy J, Fernández-Canosa S, Soto-León V, López-Molina MI, Oliviero A. Factors Associated With Fatigue in People With Spinal Cord Injury: A Systematic Review and Meta-analysis. Arch Phys Med Rehabil. 2023;104:132–42. 10.1016/j.apmr.2022.07.017 [DOI] [PubMed] [Google Scholar]
- 30.Vaccaro DH, Weir JP, Noonavath M, Bryce TN, Escalon MX, Huang V, et al. Orthostatic systemic and cerebral hemodynamics in newly injured patients with spinal cord injury. Auton Neurosci. 2022;240:102973. 10.1016/j.autneu.2022.102973 [DOI] [PubMed] [Google Scholar]
- 31.Jordan J. New trends in the treatment of orthostatic hypotension. Curr Hypertens Rep. 2001;3:216–26. 10.1007/s11906-001-0041-7 [DOI] [PubMed] [Google Scholar]
- 32.Chung KC, Song JW. A Guide on Organizing a Multicenter Clinical Trial: the WRIST study group. Plast Reconstr Surg. 2010;126:515–23. 10.1097/PRS.0b013e3181df64fa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan H, Guo Q, Zhang Z, Ou L, Wang H, Yu H, et al. Sex, age, role and geographic differences in traumatic spinal fractures caused by motor vehicle collisions: a multicentre retrospective study. Sci Rep. 2023;13:3712. 10.1038/s41598-023-30982-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moritani T. Motor Unit and Motoneurone Excitability during Explosive Movement. In book: Strength and Power in Sport, Second Edition. 2008;27–49. 10.1002/9780470757215.ch3.
- 35.Houston M, Seo G, Fang F, Park JH, Park HS, Roh J, et al. Modulating Inter-Muscular Coordination Patterns in the Upper Extremity Induces Changes to Inter-Muscular, Cortico-Muscular, and Cortico-Cortical Connectivity. IEEE J Biomed Health Inform. 2024; 10.1109/JBHI.2024.3413080. [DOI] [PubMed]
- 36.Lam T, Noonan VK, Eng JJ, SCIRE Research Team. A systematic review of functional ambulation outcome measures in spinal cord injury. Spinal Cord. 2008;46:246–54. 10.1038/sj.sc.3102134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns AS, Delparte JJ, Patrick M, Marino RJ, Ditunno JF. The Reproducibility and Convergent Validity of the Walking Index for Spinal Cord Injury (WISCI) in Chronic Spinal Cord Injury. Neurorehabil Neural Repair. 2011;25:149–57. 10.1177/1545968310376756 [DOI] [PubMed] [Google Scholar]
- 38.Jeannerod M. Neural Simulation of Action: A Unifying Mechanism for Motor Cognition. NeuroImage. 2001;14:S103–9. 10.1006/nimg.2001.0832 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.