Abstract
Aging-related biochemical changes in nerve cells lead to dysfunctional synapses and disrupted neuronal circuits, ultimately affecting vital processes such as brain plasticity, learning, and memory. The imbalance between excitation and inhibition in synaptic function during aging contributes to cognitive impairment, emphasizing the importance of compensatory mechanisms. Fear conditioning-related plasticity of the somatosensory barrel cortex, relying on the proper functioning and extensive up regulation of the GABAergic system, in particular interneurons containing somatostatin, is compromised in aging (one-year-old) mice. The present research explores two potential interventions, taurine supplementation, and environmental enrichment, revealing their effectiveness in supporting learning-induced plasticity in the aging mouse brain. They do not act through a mechanism normalizing the Glutamate/GABA balance that is disrupted in aging. Still, they allow for increased somatostatin levels, an effect observed in young animals after learning. These findings highlight the potential of lifestyle interventions and diet supplementation to mitigate age-related cognitive decline by promoting experience-dependent plasticity.
Subject terms: Learning and memory, Neural ageing, Somatosensory system, Synaptic plasticity, Neuroscience
Introduction
Aging-related biochemical alterations in nerve cells lead to malfunctioning synapses and, consequently, entire neuronal circuits, adversely affecting their functioning, including such vital processes as brain plasticity, learning, and memory. Along with the reduced release of neurotransmitters and decreased neuronal reactivity observed in aging, a decrease in the number of neurons and their synaptic contacts can cause a loss of balance between excitation and inhibition (E/I), leading to impaired plasticity. If compensatory mechanisms are ineffective, this can cause cognitive impairment and even activate biochemical pathways, leading to various pathological conditions. E/I imbalance is, however, also observed in physiological aging of the nervous system when it is associated with impaired plasticity and reduced cognitive potential. The direction of E/I changes may vary depending on the brain area studied. In the prefrontal cortex, available experimental data support an increase in inhibition with age, correlating with memory deficits1–5, while evidence of decreased inhibition was found in the sensory systems and hippocampus6. In the rat parietal cortex, an imbalance of arousal/inhibition in favor of inhibition correlated with cognitive impairment7. These data indicate that in aging, cognitive performance is affected by changes in the E/I balance at the synapse.
We have confirmed this hypothesis, showing a reduction in the glutamate/GABA ratio in the somatosensory cortex of aging mice, which was associated with impaired learning-dependent neuroplasticity8. Plasticity of cortical maps induced by fear-conditioning, in which a tactile stimulus applied to the vibrissae is paired with a mild electric aversive stimulus to the tail, requires a strong response from the GABAergic system9–12. The same conditioning pattern that expands the functional cortical representation of stimulated vibrissae in young mice becomes ineffective in aging animals8. Extension of the conditioning procedure from 3 to 7 days allowed to induce plastic change, which went hand in hand with an increase in GABA levels. This means that the decrease in plasticity in aging animals is due to the failure of the mechanisms that control plasticity, in this case, an insufficient, ineffective response of the GABAergic system to the increased demand for inhibition8. Maintaining a high plastic potential of the brain and engaging experience-dependent plasticity can offset the adverse effects of aging. This has been confirmed by several experiments demonstrating the beneficial effects of a stimulating environment (EE) and physical or cognitive training on brain function in rodents and humans [13–15, for review:16].
Two months of EE significantly improved the performance of hippocampal spatial memory tasks14,17,18 and caused an increase in, diminishing with age, hippocampal neurogenesis17,18. Long-lasting EE in one-year-old mice strongly increased mature BDNF levels and BDNF mRNA expression in the hippocampus19. The transcriptomics study of the hippocampus showed enhanced expression of a group of genes regulating dendritic spine plasticity14. Interestingly, the STED nanoscopy study revealed that following EE (although in young mice), the topography of the PSD95 nanoroganization was more dynamic, suggesting enhanced synaptic plasticity20. Synaptic plasticity in the hippocampus of aged mice appeared restored after a period of EE21,22, although these effects were found to vary with age, stimulation protocol, and duration of EE23.
The observed effects can be attributed to an interference with age-related compensatory/adaptive changes, which, unlike degenerative changes, are modifiable with various supporting strategies. The results of translational research have shown that certain lifestyle elements, so-called healthy habits, including physical activity, cognitive engagement, and an adequate diet, can promote successful aging24–26.
In this paper, two approaches were chosen that could help preserve plastic potential and improve cognitive abilities: taurine supplementation (TS) and enrichment of the animals' habitat environment (EE). Taurine is a sulfur-containing non-essential amino acid that plays many functions in the nervous system, including, among others, a role as a neurotransmitter, neurotrophic factor, neuromodulator, and neuroprotectant27. Taurine content declines with age, which in the brain was shown for the 27-month-old mouse hippocampus28 and cerebellum of 2-year-old rats29, and this has been correlated with cognitive deficits and age-associated diseases30. Taurine supplementation, on the other hand, ameliorates age-related cognitive deficits like time orientation or judgment and abstract thinking31, the performance of passive avoidance tasks, and cross-shaped water maze32. Because taurine is a modulator of the GABAergic system33,34, whose function is impaired in the aging brain, contributing to E/I imbalance, we tested its ability to support plasticity.
The second strategy—enriched environment—was selected due to its solidly documented proplastic effects, including neuroplasticity in old age [16,35,36 for review].
We demonstrated the effectiveness of both TS and EE strategies in supporting plasticity in the aging mouse brain. Each strategy affected the progression of plastic change in a slightly different way, and in neither case did the effect rely on restoring the balance between glutamate and GABA to the value observed in young animals.
Materials and methods
Animals
The experiments used 60 female C57BL/6 mice. In most experiments, two age groups, young (Y, 3-month-old, nY = 12) and aged (A, 12–14-month-old, nA = 42), were used. Only in an experiment investigating age-dependent taurine levels was an additional group of old animals (O, 22–24-month-old, nO = 6) included. All animals were housed in cages with nesting material, five per cage, unless otherwise declared, in a temperature- and humidity-controlled room (20–22 °C, 40–50% humidity) on a 12 h light/dark cycle (lights on at 7 am), with ad libitum access to food and water. All procedures followed the European Community Council Directive (2010/63/EU), were approved and carried out by the relevant guidelines and regulations of the First Local Ethical Committee for Animal Experiments in Warsaw, Poland, and were in accordance with the ARRIVE guidelines. Every effort was made to minimize the number of animals used and their suffering.
Experimental arrangement and training scheme
Fear conditioning procedure
Before the conditioning procedure, the animals were accustomed to being immobilized, 10 min a day for three weeks, in a restraining apparatus covering the neck of the mouse and allowing free head movements. After the adaptation period, the vibrissae in row B on one side of the muzzle were stimulated manually with a soft brush for 9 s with three uniform movements (conditioned stimulus, CS). In the last second of the third movement, the mice received a moderately strong electrical pulse in the tail (unconditioned stimulus, UCS, 0.5 mA, 0.5 s). The paired stimuli were repeated four times per minute for 10 min. The procedure was conducted for three days with one session per day, as described by37. This conditioning procedure results in plastic changes, including the extent of the functional cortical representation of the “trained” row of vibrissae. They do not occur in pseudo-conditioned animals and are eliminated by an extinction procedure37. All conditioning sessions were video recorded to register a conditioned response.
Conditioned response (CR) analysis
Behavioral verification of associative learning was performed as described previously38. The natural response of a mouse to the touch of vibrissae is to turn its head toward the stimulus, and during conditioning, this response is suppressed (mini freezing), which is considered a marker of acquiring a predictive value by the stimulus. We have shown that this CR was present 24 h after the training when only CS was presented in the same schedule as during the training38. The number of CSs accompanied by head movement was counted for each minute of the training (from 0 to 4 stimuli per minute), and then the counts were averaged for each session. A measure of the CR acquisition was a significant decrease in the number of CSs associated with head movement in session three compared to session one. The head movements associated with CS were presented as a % of all CSs applied during the session.
Plastic change detection
[14C]-2-deoxyglucose autoradiography (2-DG)
To visualize conditioning-induced plastic changes in the barrel cortex, we used [14C]-2-deoxyglucose autoradiography, which allows for studying brain metabolic activity in vivo39. The cortical representation of the vibrissae row B stimulated during conditioning, and the control row B on the other side of the muzzle was labeled with 2-DG autoradiography 24 h after the last conditioning session37. Each mouse was placed in a restraining device, and all vibrissae, except the B rows to be imaged, were removed. A single dose (10 μCi per animal) of [14C]-2-deoxy-D-glucose (American Radiolabeled Chemicals Inc., specific activity 53 mCi/mmol) was injected intramuscularly, and row B of vibrissae on both sides of the snout was stimulated manually for 30 min at 2 Hz. Immediately after stimulation, mice were perfused with 4% PFA in PBS; the cortex was isolated and flattened between two microscopic slides separated by 2 mm separators to obtain tangential sections and then frozen at −80 °C. Next, the cortex was cut using a cryostat into 30—35 μm thick slices. Subsequent slices were collected on coverslips and immediately dried on a heating plate. The slides were then exposed for two weeks against mammographic film (Kodak) in the presence of 14C radioactivity standards. The films were developed and fixed using Kodak X-ray photography reagents, then rinsed and dried. To quantify the extent of the plastic change, the width of the row B functional representation labeling was measured using a previously established criterion40, considering as activated regions in which the labeling, measured by the degree of 2-DG incorporation, was 15% greater than in adjacent areas of the cortex. We have established previously that the 15% difference corresponds to two standard deviations above the mean value of labeling in the surrounding cortex40.
The barrel cortex of the contralateral hemisphere is used as an internal control in studying plastic changes
The possibility of treating the somatosensory cortex contralateral to the experimental one as an internal control for young and aging animals was confirmed by the following experiments, and by our previous studies40. Plasticity studies: (i) the width of labeling of the representation of row B of vibrissae in the control hemisphere in conditioned animals was not significantly different from that in naive animals of the same age group; (ii) there was also no change in the width of labeling in the control hemisphere between young (572 ± 51 µm, n = 3, but see also40,41 and aged animals (562 ± 45 µm, n = 3, see also10; (iii) there were no differences in the width of labeling in the control hemisphere of animals subjected to TS (553.3 ± 41 µm, n = 4) or EE (554.1 ± 8.5 µm, n = 4) when compared to the age-matched naive animals.
Strategies supporting plasticity
Enriched environment (EE) exploration in aged mice
Selected groups of aged mice were exposed to an enriched environment (A_EE). It consisted of a larger-than-standard cage measuring 60 × 40 × 30 cm, inside which houses (shelter), small objects of different textures, small mazes, swings, and nesting material were placed. All accessories were changed weekly to stimulate the mice to explore their new environment. The mice spent six weeks in EE and were used in further experiments.
Taurine supplementation (TS) in aged mice
For six weeks, selected groups of aged mice received 5% aqueous taurine solution (Sigma; T0625) ad libitum instead of water (A_TS). Animals were housed in home cages, five animals per cage. The water requirement of an adult mouse is approximately 7 ml per day. During the experiment, the animals were not restricted in their access to the taurine solution. The water was changed once a week, and the volume of the remaining solution was checked. During the experiment, no significant changes were observed in the amount of taurine solution consumed, nor were there differences in the consumption of taurine solution compared to water. Studies of the potential long-term toxicity of taurine in Wistar rats showed that a diet of 0.5 or 5% taurine for 18 months did not cause any clinical, behavioral, or hematological effects. No adverse histological effects were observed in the brain, cerebellum, myocardium, spleen, pancreas, gastrointestinal tract walls, or testes42. After this time, the animals were used in subsequent procedures. The dose of taurine administered to the animals was determined according to the “GRAS exemption claim for taurine for use in enhanced water beverages—2015”42 and based on a study by Hwang and colleagues43.
Biochemical analyses
Tissue sample collection for HPLC and ELISA analyses
To collect tissue samples, animals were sacrificed by severing the spinal cord to avoid the possible effects of anesthetics on the measurements taken. For analyses of aging-related changes, tissue fragments from both hemispheres were combined, while for analyses of changes after induced plasticity, tissue fragments from each hemisphere—control and experimental—were analyzed separately.
The brains were removed from the skull, the cerebral cortex was separated on ice, flattened, and then somatosensory cortex fragments containing the barrel cortex were taken. The somatosensory cortex was dissected using a 2 mm diameter template according to the method described by Strominger and Woolsey44.
Estimation of amino acid levels using the HPLC method
To determine the amino acid content, tissue samples were homogenized in ten volumes of ice-cold 2% perchloric acid, and the supernatants were collected and filtered through a 0.45 μm filter (Millipore) after centrifugation. HPLC analysis of amino acids was performed using a Luna C18 (250 × 5 mm) 5 μm reverse phase column. Compounds were eluted isocratically with the mobile phase delivered at 0.7 ml/min using a Shimadzu Class LC-10ADvp pump. An electrochemical detector with a flow-through cell (Intro-Antec Leyden) linked to the Shimadzu Class VP Integrator SCL-10 Avp was used. A high-density glassy carbon-working electrode (Antec) was operated at + 0.85 V. Samples were injected through a Rheodyne 7725i dispensing valve with a 20 μl loop. Preparation of the mobile phase and the derivatizing agents was based on the method of Rowley et al.45, with some modifications. The mobile phase consisted of 45 mM disodium phosphate and 0.15 mM EDTA with 24% methanol (v/v) water, adjusted to pH 3.9 with 0.2 M citric acid. It was then filtered through 0.45-μm filters and degassed for 15 min. Stock solutions (0.01 M) of amino acid standards were prepared in double deionized water and maintained at four °C for five days. To prevent adhesion to glass, amino acid (especially GABA) standards were prepared in polyethylene vials. Working solutions were prepared daily by dilution of the stock solutions. Derivatization was performed at room temperature for 8 min. The derivatization reagent was obtained by dissolving 22 mg of OPA (phthalaldehyde) in 0.5 ml of 1 M sodium sulfite, 0.5 ml of methanol, 0.9 ml of 0.1 M tetraborate buffer, brought to pH 10.4 with 5 M NaOH. Shimadzu's Class-VP computer program was used to record and process the chromatograms. The concentration of amino acids in the sample was calculated from a standard curve prepared in the concentration range of 0.05–8 μM, then calculated as μM/g tissue.
Somatostatin (SOM) level analysis with enzyme-linked immunosorbent assay ELISA
Somatosensory cortex tissue for ELISA analysis was collected 24 h after the last conditioning session. Tissue was dissected, and samples were homogenized in sterile PBS containing a protease inhibitor mixture (87786, Thermo Scientific) and centrifuged at 11,000 rpm for 20 min at 4 °C. The supernatant was then collected, and protein concentrations were measured using a BCA protein assay kit. Protein levels were analyzed using the MBS701139 Mouse somatostatin ELISA Kit, MyBioSource Inc., according to the manufacturer's instructions.
Statistical analyses
Data are presented as mean ± S.D., unless otherwise stated. All the collected material was coded for the unbiased primary data assessment. The statistical analysis began with the decoding of the primary data. The normality of the intragroup distribution was assessed using the Kolmogorov–Smirnov test. Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software) or R environment statistical and graphical packages. A paired Student's t-test (significance level p < 0.05) was used to compare the results from the experimental and control hemispheres. In contrast, a one-way analysis of variance (ANOVA) with Dunett's test as post hoc was used for the analysis between age groups. Spearman's rank correlation was used to examine the age-related changes in taurine.
For CR analysis, the number of head movements in response to the CS was expressed as a percentage for each minute of conditioning during each training day. The results from a given day were then averaged, and differences between consecutive days were analyzed using one-way ANOVA and Tukey's post hoc test. The significance of the glutamate/GABA ratio after TS and EE was determined using an unpaired Student's t-test.
Results
Taurine level in the mouse somatosensory cortex decreases with age
The HPLC analysis revealed that the taurine levels in the mouse somatosensory cortex decrease with age. This discovery, supported by Spearman's rho analysis (rs = −0.76, p < 0.001) (Fig. 1A), is particularly noteworthy as it demonstrates a clear negative correlation between taurine levels and age. Notably, a statistically significant decrease was observed only in old (O; 2-year-old mice), despite the downward trend already visible between young (Y) and aged animals (A) (one-way ANOVA: F(15,2) = 19.25, p < 0.001; Tukey’s post hoc test: Y vs. O: p < 0.001; A vs. O p < 0.01) (Fig. 1B).
Figure 1.
Changes in taurine levels induced by aging in the somatosensory cortex. (A) Taurine level is negatively correlated with age. Spearman’s rho analysis. (B) Taurine level in young, aged, and old mice. One-way ANOVA, Tukey’s post hoc, **p < 0.01, ***p < 0.001; mean ± s.d. Y—young (3-month-old), A—aged (1-year-old), O—old (2-year-old), nY = 6, nA = 6, nO = 6.
Both TS and EE are effective in supporting experience-dependent plasticity in aged animals
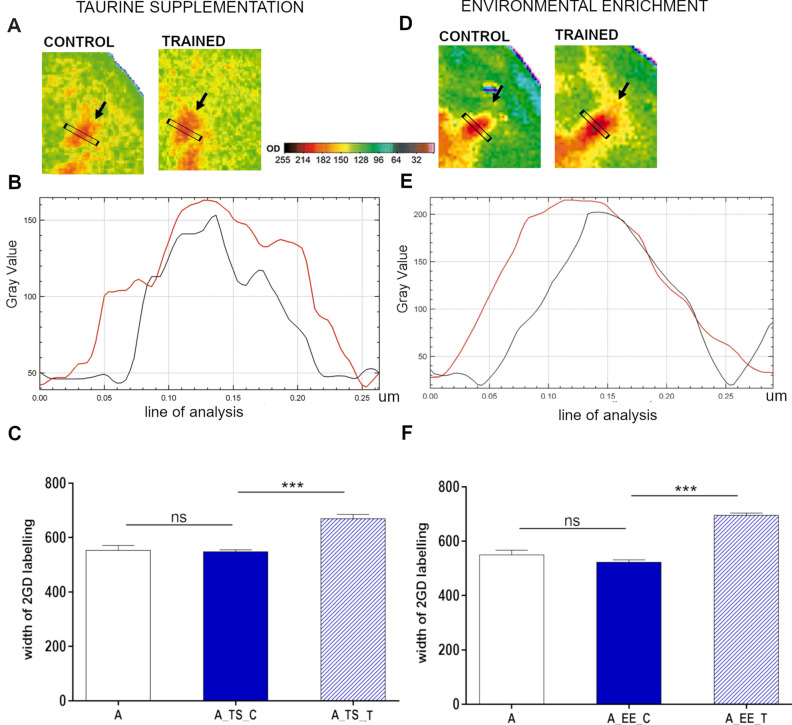
Analysis of autoradiograms showed that both TS and EE enabled the induction of plastic change as a result of the short (3-day lasting) conditioning regimen, which, as we have shown earlier, was not possible in aged mice10. In both strategies, we confirmed a significant enlargement of trained row representation in the aged group following TS and EE when compared to control hemisphere (one-way ANOVA, F(2,14) = 47.05, p < 0.0001, Dunett's post hoc test, p < 0.0001 for TS and F(2,14) = 168.3, p < 0.0001, Dunett's post hoc test p < 0.0001 for EE) (Fig. 2A–C). The between-group analysis showed also that the labeling width of row B of vibrissae representation in the control hemisphere was not significantly different from that measured in the control age-matched mice, which were not subjected to the conditioning procedure (549.0 ± 14.6 μm vs. 544.3 ± 16.68 μm for TS, one-way ANOVA, F(2,14) = 47.05, p < 0.0001, Dunett's post hoc test p = 0.9; and 523.0 ± 18.7 μm vs. 551.3 ± 15.6 μm for EE; one-way ANOVA, F(2,14) = 168.3, p < 0.0001, Dunett's post hoc test p = 0.3).
Figure 2.
Plastic change analysis in aged mice barrel cortex after TS (A, B, C) and EE (D, E, F). (A,D) Examples of autoradiograms of layer IV barrel cortex tangential sections, shown in pseudocolors, presenting the pattern of 2-DG incorporation during conditioning. The most active areas are shown in red and the least in blue. Arrows indicate representations of row B vibrissae (control and trained). (B,E) labeling intensity profiles of the regions the black rectangle indicates. Black line—control row of vibrissae, red line—trained row of vibrissae. (C,F) the width of labeling of row B representation of aged naïve mice (A), control row of vibrissae representation of aged conditioned mice (A_TS_C and A_EE_C), and trained row representation of aged conditioned mice (A_TS_T and A_EE_T). One-way ANOVA, Dunnett’s post hoc test; data represent mean ± s.d.; ***p < 0.001; nA = 5, nA _TS = 6 and nA_EE = 6.
Following TS, the width of the trained row of vibrissae representation labeling was, on average, 18.2% greater than the width of the representation of control row of vibrissae (contralateral control hemisphere) (671.0 ± 34.9 μm, trained row representation vs. 549.0 ± 14.6 μm control row representation; paired Student's t-test, p < 0.005). As for the EE strategy, the width of the labeling of the trained row of vibrissae representation in layer IV of the barrel cortex of aged mice was, on average, 24.79% larger than the control row representation (696.5 ± 17.4 μm vs. 523.0 ± 18.7 μm; paired Student's t-test, p < 0.005) (Fig. 2D–F).
The CR development course and dynamics differ for TS and EE
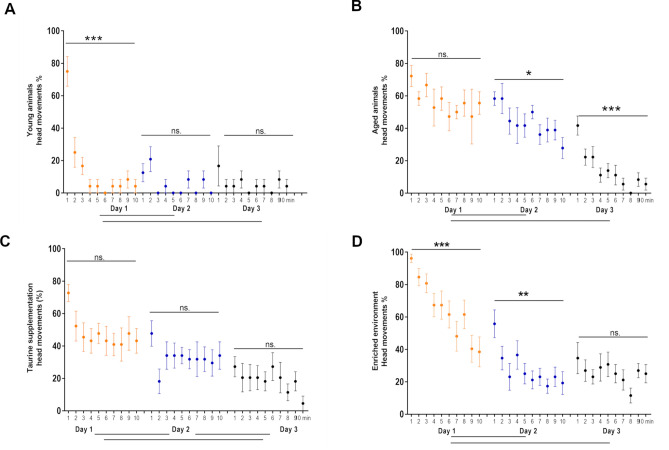
Similarly to young animals (repeated measures ANOVA; F(5,45) = 19.98; Tukey’s post hoc, day 1 vs. day 2; p < 0.001) (Fig. 3a), aged mice present a CR in the course of 3-day conditioning paradigm (repeated measures ANOVA with; F(6,72) = 7.9; Tukey’s post hoc, day 1 vs. day 2; p < 0.05 and day 2 vs. day 3 p < 0.001) (Fig. 3b), even though they do not develop the plastic change in the form of broadening the trained row of vibrissae representation. Our previous publications have described these results10–12. In the current publication, we have replicated and included them in this section for comparative purposes.
Figure 3.
Dynamics of the acquisition of the conditioned response (CR) in young (A) and aged mice (B, C, D) after classical conditioning (B); classical conditioning in aged mice, following taurine supplementation (TS) (C) or environmental enrichment (EE) (D). The graphs show the percentage of trials in successive minutes of conditioning involving the head turn toward the CS. (A) Young mice develop the CR very dynamically. A significant change is evident as early as the first minutes of the first session and persists until the end of training (from 14.6 ± 2.8% during the first session to 5.4 ± 3.9% during the last one). (B) In aged mice, a significant decrease in CS stimuli associated with head movement is seen only on the second conditioning day. During the third session, further development of the CR (decrease in head movements in response to CS) is observed. (C) In aged mice, CR following TS develops slowly. There is no significant decrease in CS stimuli associated with head movement within sessions, but the differences occur between averaged values from individual sessions. (D) Following EE in conditioned aged mice, CR developed more slowly than in young animals, but with similar dynamics. A significant decrease in head movement-associated CS stimuli is evident in the course of the first (red) and second (blue) sessions and remains low during session 3 (black). Repeated measures ANOVA, mean ± s.e.m.; *p < 0.05, **p < 0.01, ***p < 0.001; young mice, nY = 6; aged mice, nA = 9; aged mice, TS, nA_TS = 11; aged mice, EE, nA_EE = 13. Differences between successive days of conditioning are shown below the graphs.
Analysis of the CR acquired by the aged animals during fear conditioning (mini freezing) following TS and EE showed that the number of head movements toward the CS decreased during the 3-day conditioning in both groups. In the case of TS, the average percentage of CSs associated with head movement was 47.73 ± 4.43% on the first day of conditioning and significantly decreased to 32.73 ± 4.11% on day 2 (repeated measures ANOVA; F(10,90) = 26.85; Tukey’s post hoc, day 1 vs. day 2; p < 0.01), and on day three to 18.86 ± 2.91% (repeated measures ANOVA; F(10,90) = 26.85; Tukey’s post hoc, day 2 vs. day 3; p < 0.01). Thus, a 28.86% reduction in the number of head movements was observed throughout conditioning (repeated measures ANOVA; F(10,90) = 26.85; Tukey’s post hoc, day 1 vs. day 3; p < 0.001). Aged mice after TS presented a specific course of the acquisition of the CR, different from young (Fig. 3A) or aged mice housed in the standard conditions (Fig. 3B). No significant differences were observed in successive minutes during a single session. In contrast, on each successive day of conditioning, the level of head movements in the first minute of the session was similar to the number of head movements from the last minute of the previous day's session (Fig. 3C).
Aged mice housed in EE also developed a typical CR during the 3-day conditioning session. The reduction in the number of CSs (expressed as %) followed by head movement ranged from 64.62 ± 5.31% on the first day to 27.88 ± 4.41% on the second day (repeated measures ANOVA; F(12,108) = 97.43; Tukey's post hoc, day 1 vs. day 2; p < 0.001) to 25.38 ± 4.43% on day 3 (repeated measures ANOVA; F(12,108) = 97.43, Tukey’s post hoc, day 2 vs. day 3; p = ns). A 39.24% reduction in the number of head movements was recorded throughout conditioning (repeated measures ANOVA; F(12,108) = 97.43; Tukey’s post hoc ; p < 0.001). In this group of animals, a different course of acquisition of the CR was observed than in the TS, young, or aged group. It was found that during the first two sessions, mice significantly reduced the number of head movements (one-way ANOVA, 1 min vs. 10 min—day 1; p < 0.001, 1 min vs. 10 min—day 2; p < 0.005). In addition, it was noted that in the first minute of subsequent conditioning sessions, animals reacted to more CSs with head movement than in the last minute of the previous session (Fig. 3D).
Neither TS nor EE restores glutamate/GABA ratio values to those observed in young animals
Our previous studies have shown an imbalance between the levels of two primary neurotransmitters, glutamate, and GABA, in the somatosensory cortex of aging mice as measured by the glutamate/GABA ratio (5.11 ± 0.13 in young and 4.38 ± 0.05 in aged animals; [see 8].
Here, HPLC analysis showed that in aged animals following TS, the glutamate/GABA ratio in the somatosensory cortex remained significantly lower (4.93 ± 0.04) than in young (5.75 ± 0.13) animals (p < 0.001). A similar situation was observed following EE in aged mice. It did not restore the balance between the two major cortical neurotransmitters with a glutamate/GABA ratio of 5.23 ± 0.06 in aged animals p < 0.01) (Fig. 4).
Figure 4.
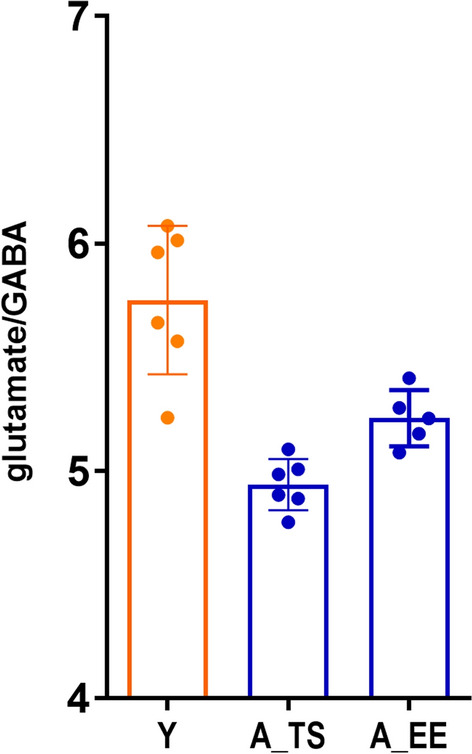
Neither taurine supplementation (A_TS) nor environmental enrichment (A_EE) restores the glutamate/GABA balance in the somatosensory cortex of aged animals. It remains significantly lower than in young animals (Y). Unpaired Student’s t-test, mean ± s.d., nY = 6, nA_TS = 6; nA_EE = 6.
TS and EE differently influence conditioning-induced levels of SOM
As shown previously, the subclass of GABAergic interneurons involved in conditioning-induced plasticity in young animals are somatostatin-containing cells (SOM INs) [see 11]. A significant increase in SOM level can be seen in the somatosensory cortex of the experimental hemisphere in comparison to the control hemisphere with the experimental/control hemisphere ratio of 1.34 ± 0.1 (paired Student’s t-test, p = 0.002). It was significantly higher than in the aged group (one-way ANOVA, F(3,20) = 5.42, p = 0.04), which did not present the up regulation of SOM in the experimental hemisphere. Both TS and EE induced significant SOM levels increase in the aged somatosensory cortex after training [experimental control hemisphere ratios: 1.186 ± 0.04 (paired Student's t-test, p = 0.003) and 1.44 ± 0.13 (paired Student’s t-test, p = 0.002), respectively]. However, in comparison to the aged conditioned animals ratio, only EE induced a significantly higher increase (one-way ANOVA, F(3,20) = 5.42, p = 0.006; Tukey’s post hoc; p < 0.05, p < 0.01) (Fig. 5).
Figure 5.
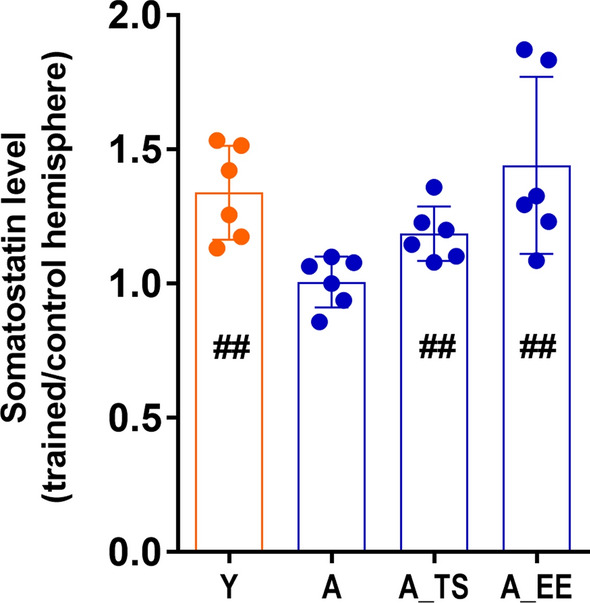
Differences in somatostatin (SOM) levels in the somatosensory cortex induced by fear conditioning are shown as the ratio of SOM levels in the experimental hemisphere relative to the control hemisphere. #—a significant increase of SOM levels in experimental hemispheres (paired Student’s t-test; ##p < 0.01). *significant differences between different experimental groups (one-way ANOVA, *p < 0.05, **p < 0.01). Mean ± s.d., n = 6 for each group: Y—young animals, A—aged animals, A_TS—taurine supplementation, aged animals, A_EE—enriched environment, aged animals.
Discussion
Several studies have shown an association between lifestyle factors and the structure and function of the aging brain, mainly cognitive functions25. Dietary approaches like caloric restriction and intermittent fasting or pharmacological interventions involving antioxidants, telomerase, and probiotics seem to have the potential to decelerate or reverse aging-related processes. The plasticity of somatosensory cortical representations induced by classical conditioning, involving unilateral tactile stimulation of vibrissae as a conditioned stimulus and an aversive stimulus administered to the tail, is compromised in aging but can be stimulated with an extended conditioning paradigm10. The prerequisite for this type of plastic change is the proper functioning of the GABAergic system12. Furthermore, it is accompanied by an extensive mobilization of the GABAergic system, as indicated by an increase in the level and expression of several GABAergic markers, including GAD mRNA and protein level, mRNA for α1 subunit of the GABAA receptor, GABA level, density of GABA-immunoreactive cells in layer IV cortical representation of trained row of vibrissae, number of inhibitory synapses on dendritic spines, increase in tonic inhibition8,10,13,46–49.
Here, we have shown the effectiveness of two approaches supporting plasticity in aging animals' somatosensory cortex. The first one was taurine supplementation. Taurine is a biogenic amino acid found in various organs, including the brain, heart, and kidneys50. It has declined with age in the hippocampus, striatum, and cerebellum29,51. We confirmed a decrease of taurine levels in the somatosensory cortex, but only in 2-year-old animals. This means that at the time of plasticity investigation (1-year-old mice), the taurine level in the somatosensory cortex was not significantly altered.
Among other effects, taurine affects the function of the GABAergic system. It has been shown to act as a GABAA receptor agonist, a structural analog of glycine and GABA33,52. Thus, taurine supplementation might positively modulate the GABAergic system, offering hope that it can effectively counteract the changes observed in aging and stimulate the neuroplastic potential. Indeed, six weeks of TS (5% aqueous solution) enabled the initiation of the plastic change in the somatosensory cortex of aged mice with a plasticity extent similar to the young animals. Chronic supplementation with low doses of taurine was shown to improve learning in old mice and performance on a spatial task in a water pool53 and in the Y maze test30. It also allowed restoration of plasticity damaged after alcohol administration and in a mouse model of Alzheimer’s disease, in addition to improving visual discrimination impaired in old mice54,55.
Moreover, taurine was shown to potentiate synaptic transmission due to increased presynaptic axon excitability and synaptic efficacy56,57. The results of further studies lead to the conclusion that taurine uptake is required to induce a late phase of LTP58,59.
The present paper shows, for the first time, the positive influence of TS on experience-dependent learning-associated plasticity in the somatosensory cortex. Thus, taurine appears to support neuroplasticity potential in various brain areas significantly. In addition to its direct action on the GABAergic system, the beneficial effects of taurine in the aging nervous system may be related to its multiple actions in various cellular processes, including the maintenance of calcium homeostasis60–62, protection against glutamate excitotoxicity, apoptosis62,63, inflammation54, telomerase deficiency, DNA damage, mitochondrial dysfunction30 and oxidative stress64. These processes are essential in aging and may also affect plasticity induction. Taurine was shown to potentiate synaptic transmission due to increased presynaptic axon excitability and synaptic efficacy56,57. The results of further studies lead to the conclusion that taurine uptake is required to induce a late phase of LTP58,59.
The second strategy was an exposition of aged animals for environmental enrichment, whose positive effects on the phenomenon of neuronal plasticity have been widely verified. Several experimental studies have demonstrated that EE mitigates age-related alterations in the brain, including cortical thickness, dendritic branching, spine density, neurogenesis, and gliogenesis. These effects consistently correlate with enhanced performance in diverse learning tasks among older animals65,66. Environmental enrichment has also been shown to reduce cognitive deficits and Aβ levels in animal models of AD67. In animal studies, EE that provides cognitive stimulation was associated with improved cognitive functioning across the lifespan68, as well as increased neurogenesis, positive regulation of neuronal growth factors, and increased synapse density69–71.
Moreover, several studies used EE to counteract the effects of aging on synaptic plasticity. Kumar et al. found that in old mice’s hippocampus, EE decreased after hyperpolarisation, thus augmenting neuronal excitability21. Enhanced short-term potentiation and LTP and decreased LTD in aged rodents after EE were also shown22,72,73.
One of the critical parameters for synaptic plasticity and cognitive functions such as learning and memory is the balance between excitation and inhibition, and we have shown that it was disturbed in the somatosensory cortex of aging animals10. The link between the reduced ability to induce plastic change in the somatosensory cortex and the glutamate/GABA imbalance in aged animals, coupled with the lack of GABAergic system mobilization following conditioning, allowed us to speculate that one of the reasons for the reduced plastic potential in aging somatosensory cortex may be dysregulation of neurotransmission. Both TS and EE, by affecting elements of the GABAergic or glutamatergic system, or both, can affect the balance between them. Chronic supplementation of taurine in mice resulted in increased levels of GABA and glutamate33 and up regulation of GAD levels in the cortex and hippocampus74. After EE, increased levels of glutamate and GABA were observed in the hippocampus of old rats17. Still, there were also reports of reduced GABA release and reduced inhibition in the visual system of mice and humans after EE, which was associated with improved plasticity75,76. This indicates that this strategy may work differently depending on the area studied.
Nevertheless, in our experiments, neither TS nor EE restored the glutamate/GABA balance in the aged somatosensory cortex as their ratio remained reduced compared to young animals due to a decrease in glutamate level. This result does not allow us to conclude unquestionably that there is no effect of the applied strategies on the homeostasis between excitation and inhibition since it is determined not only by the neurotransmitter levels but also by receptors functioning and activation of relevant signaling pathways. The parameters mentioned above can be influenced by both of the strategies we used. Taurine is an agonist of GABAA and glycine receptors and a weak agonist of presynaptic NMDA receptors, which alters their affinity for glycine77. EE resulted in increased expression of the GLUR1 subunit gene of the AMPA receptor in the hippocampus78. In rats, Glutamate metabotropic receptor levels in the prefrontal cortex, striatum79, and hippocampus73 were also altered. Still, the direction of the changes depended on the subunit and area studied.
Our previous research showed that a specific subclass of GABAergic neurons containing the somatostatin peptide plays a pivotal role in forming the conditioning-induced plastic change in the mice's somatosensory cortex. There was a significant increase in the number of SOM-containing GABAergic interneurons in the representation of the conditioned vibrissae 11. Moreover, chemogenetic silencing of somatostatin interneurons in the barrel cortex prevented fear conditioning-induced plasticity in this area80. Here, we demonstrated an increase in the level of SOM in the somatosensory cortex of the experimental barrel cortex of young mice, which could not be seen in aged, conditioned animals. It was, however, observed in the experimental barrel cortex of aged animals subjected to both TS and EE. Long-term administration of low doses of taurine increased the level of SOM in the brain74 and the number of SOM neurons in the cortex and hippocampus32, which, given the involvement of a subset of somatostatin-containing interneurons in the plasticity we studied, could have induced the desired effects of enabling the plastic change of cortical representation. This is most likely related to the involvement of SOM INs, particularly those located in cortical layer IV, in the disinhibitory loop on excitatory neurons. One of the functions of SOM INs is to regulate the activity of parvalbumin-containing neurons (PV INs), which are activated by tactile stimuli and, in turn, exert a strong inhibitory effect on excitatory cells81,82. This effect can be weakened by the inhibitory action of SOM INs on the PV INs in layer IV of SI. In this way, SOM INs boost the information from the thalamus, leading to plastic change induced by associative learning83,84.
An essential aspect of conditioning is acquiring a conditioned response (CR), indicating the association between CS and UCS. During the fear conditioning, we observe so-called “minifreezing,” understood as refraining from the animal's natural reflex of turning its head toward a tactile stimulus (CS). In young conditioned animals, the course of CR acquisition is quite repetitive. It is characterized by a significant reduction in CS-associated head movements in the first 2–3 min of the first conditioning session. This effect persists for subsequent sessions11. Aged mice presented the correct CR at the end of the conditioning even though they did not develop the plastic change. However, it developed with different dynamics than in the young group. That means that they developed an association between CS and UCS, but this did not translate into a plastic change expressed as a broadening of the cortical representation of the trained row of vibrissae.
Interestingly, the dynamics of the CR development in the aged animals following TS or EE differed from each other and other groups investigated. The described differences in the behavioral response of animals to conditioning suggest that there may be differences in the mechanisms of the learning process in each particular group. These differences do not seem to be linked to the SOM level in the cortex, which was augmented every time the plasticity was present. Other types of interneurons may also be involved here. In preliminary studies, in the somatosensory cortex of the experimental hemisphere, we found a decrease in Neuropeptide Y mRNA in young animals (data not shown). Neuropeptide Y in the cerebral cortex often co-occurs with SOM and lowers the excitability of neuronal circuits. It can have both stimulatory and impairing effects on memory, depending on the type of memory, the brain area studied, and the receptor subtypes activated85. Intrathecal administration of NPY before fear conditioning induces an anti-anxiety effect and is associated with impaired memory acquisition86,87.
On the other hand, deleting the Npy gene or its Y1 receptor enhanced fear response acquisition88. Thus, the down regulation of Npy we observed in the experimental barrel cortex in young animals may enhance the UCS effect and support conditioning. This hypothesis requires further investigation.
Our findings demonstrated that promoting brain plasticity through specific dietary patterns or environmental reinforcement can effectively offset the adverse effects of aging on learning-induced experience-dependent plasticity. This plasticity is crucial in the aging brain, enabling individuals to continually adapt to new information and environments and fostering cognitive resilience. Its effective stimulation by forming and strengthening neural connections can counteract age-related decline and promote cognitive flexibility, contributing to overall well-being in aging.
Acknowledgements
The work was supported by a National Science Centre Grant (2013/09/B/NZ3/00540) to MLL. Some of the results presented in this publication were obtained using equipment supported by a project financed by the Polish Ministry of Science and Higher Education: Polish Euro-BioImaging Node “Advanced Light Microscopy Node Poland, No 2022/WK/05.
Author contributions
AG, RZ, AS, AC carried out the experiments and analyzed the results; ACK and MK contributed to the interpretation of the results; ACK, MK and MLL wrote the manuscript with input from all authors; MLL supervised the project, designed the figures and took the lead in writing the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Potier, B., Jouvenceau, A., Epelbaum, J. & Dutar, P. Age-related alterations of Gabaergic input to Ca1 pyramidal neurons and its control by nicotinic acetylcholine receptors in rat hippocampus. Neuroscience142, 187–201 (2006). 10.1016/j.neuroscience.2006.06.040 [DOI] [PubMed] [Google Scholar]
- 2.Stanley, E. M., Fadel, J. R. & Mott, D. D. Interneuron loss reduces dendritic inhibition and Gaba release in hippocampus of aged rats. Neurobiol. Aging33(431), e1-13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bories, C., Husson, Z., Guitton, M. J. & De Koninck, Y. Differential balance of prefrontal synaptic activity in successful versus unsuccessful cognitive aging. J. Neurosci.33, 1344–1356 (2013). 10.1523/JNEUROSCI.3258-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bañuelos, C. et al. Prefrontal cortical Gabaergic dysfunction contributes to age-related working memory impairment. J. Neurosci.34, 3457–3466 (2014). 10.1523/JNEUROSCI.5192-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox, L. A. et al. Integrated multi-omics analysis of brain aging in female nonhuman primates reveals altered signaling pathways relevant to age-related disorders. Neurobiol. Aging132, 109–119 (2023). 10.1016/j.neurobiolaging.2023.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu, Z. Y., Wang, W., Fritschy, J. M., Witte, O. W. & Redecker, C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res.1099, 73–81 (2006). 10.1016/j.brainres.2006.04.118 [DOI] [PubMed] [Google Scholar]
- 7.Wong, T. P. et al. Imbalance towards inhibition as a substrate of aging-associated cognitive impairment. Neurosci. Lett.397, 64–68 (2006). 10.1016/j.neulet.2005.11.055 [DOI] [PubMed] [Google Scholar]
- 8.Liguz-Lecznar, M. et al. Altered Glutamate/Gaba equilibrium in aged mice cortex influences cortical plasticity. Brain Struct. Funct.220, 1681–1693 (2015). 10.1007/s00429-014-0752-6 [DOI] [PubMed] [Google Scholar]
- 9.Siucinska, E. Gad67-positive puncta: Contributors to learning-dependent plasticity in the barrel cortex of adult mice. Brain Res.1106, 52–62 (2006). 10.1016/j.brainres.2006.05.061 [DOI] [PubMed] [Google Scholar]
- 10.Liguz-Lecznar, M., Siucinska, E., Zakrzewska, R. & Kossut, M. Impairment of experience-dependent cortical plasticity in aged mice. Neurobiol. Aging32, 1896–1905 (2011). 10.1016/j.neurobiolaging.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 11.Cybulska-Klosowicz, A. et al. Interneurons containing somatostatin are affected by learning-induced cortical plasticity. Neuroscience254, 18–25 (2013). 10.1016/j.neuroscience.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 12.Posluszny, A. et al. Learning-dependent plasticity of the barrel cortex is impaired by restricting Gaba-ergic transmission. PLoS ONE10, e0144415 (2015). 10.1371/journal.pone.0144415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahncke, H. W. et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: A randomized, controlled study. Proc. Natl. Acad. Sci. USA103(33), 12523–12528 (2006). 10.1073/pnas.0605194103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt, S. et al. Restoring age-related cognitive decline through environmental enrichment: A transcriptomic approach. Cells11, 3864 (2022). 10.3390/cells11233864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaushik, M., Kaushik, P., Panwar, S., Joshi, S. D. & Parvez, S. Environment enrichment facilitates long-term memory consolidation through behavioral tagging. eNeuro10, ENEURO.0365-22.2023 (2023). 10.1523/ENEURO.0365-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sale, A., Berardi, N. & Maffei, L. Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiol. Rev.94, 189–234 (2014). 10.1152/physrev.00036.2012 [DOI] [PubMed] [Google Scholar]
- 17.Segovia, G., Yague, A. G., Garcia-Verdugo, J. M. & Mora, F. Environmental enrichment promotes neurogenesis and changes the extracellular concentrations of glutamate and GABA in the hippocampus of aged rats. Brain Res. Bull.70, 8–14 (2006). 10.1016/j.brainresbull.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 18.Speisman, R. B. et al. Environmental enrichment restores neurogenesis and rapid acquisition in aged rats. Neurobiol. Aging34, 263–274 (2013). 10.1016/j.neurobiolaging.2012.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obiang, P. et al. Enriched housing reverses age-associated impairment of cognitive functions and tPA-dependent maturation of BDNF. Neurobiol. Learn. Mem.96(2), 121–129 (2011). 10.1016/j.nlm.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 20.Wegner, W., Steffens, H., Gregor, C., Wolf, F. & Willig, K. I. Environmental enrichment enhances patterning and remodeling of synaptic nanoarchitecture as revealed by STED nanoscopy. Elife11, e73603 (2022). 10.7554/eLife.73603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar, A., Rani, A., Tchigranova, O., Lee, W. H. & Foster, T. C. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol. Aging33(4), 828.e1–17 (2012). 10.1016/j.neurobiolaging.2011.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein, L. R., O’Dell, K. A., Funatsu, M., Zorumski, C. F. & Izumi, Y. Short-term environmental enrichment enhances synaptic plasticity in hippocampal slices from aged rats. Neuroscience329, 294–305 (2016). 10.1016/j.neuroscience.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarif, H., Nicolas, S., Petit-Paitel, A., Chabry, J. & Guyon, A. How does enriched environment impact hippocampus brain plasticity? In The Hippocampus—Plasticity and Functions (ed. Stuchlik, A.) (InTech, 2018). [Google Scholar]
- 24.Moore, K., Hughes, C. F., Ward, M., Hoey, L. & McNulty, H. Diet, nutrition and the ageing brain: Current evidence and new directions. Proc. Nutr. Soc.77, 152–163 (2018). 10.1017/S0029665117004177 [DOI] [PubMed] [Google Scholar]
- 25.Krivanek, T. J., Gale, S. A., McFeeley, B. M., Nicastri, C. M. & Daffner, K. R. Promoting successful cognitive aging: A ten-year update. J. Alzheimers Dis.81, 871–920 (2021). 10.3233/JAD-201462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weuve, J. et al. Physical activity, including walking, and cognitive function in older women. JAMA292, 1454–1461 (2004). 10.1001/jama.292.12.1454 [DOI] [PubMed] [Google Scholar]
- 27.Wu, J. Y. & Prentice, H. Role of taurine in the central nervous system. J. Biomed. Sci.17(1), S1 (2010). 10.1186/1423-0127-17-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu, M., Akimana, C., Wang, E. & Ng, C. K. 1H-MRS quantitation of age-dependent taurine changes in mouse brain. Mol. Imaging Biol.21, 812–817 (2019). 10.1007/s11307-019-01333-6 [DOI] [PubMed] [Google Scholar]
- 29.Suárez, L. M., Muñoz, M. D., del Río, R. M. & Solís, J. M. Taurine content in different brain structures during ageing: Effect on hippocampal synaptic plasticity. Amino Acids48, 1199–1208 (2016). 10.1007/s00726-015-2155-2 [DOI] [PubMed] [Google Scholar]
- 30.Singh, P. et al. Taurine deficiency as a driver of aging. Science380(6649), eabn9257 (2023). 10.1126/science.abn9257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae, M. A., Gao, R., Kim, S. H. & Chang, K. J. Past taurine intake has a positive effect on present cognitive function in the elderly. Adv. Exp. Med. Biol.975(Pt 1), 67–77 (2017). 10.1007/978-94-024-1079-2_6 [DOI] [PubMed] [Google Scholar]
- 32.El Idrissi, A., Shen, C. H. & L’amoreaux, W. J. Neuroprotective role of taurine during aging. Amino Acids45, 735–750 (2013). 10.1007/s00726-013-1544-7 [DOI] [PubMed] [Google Scholar]
- 33.El Idrissi, A. & Trenkner, E. Taurine as a modulator of excitatory and inhibitory neurotransmission. Neurochem. Res.29, 189–197 (2004). 10.1023/B:NERE.0000010448.17740.6e [DOI] [PubMed] [Google Scholar]
- 34.Chen, C. et al. Roles of taurine in cognitive function of physiology, pathologies and toxication. Life Sci.231, 116584 (2019). 10.1016/j.lfs.2019.116584 [DOI] [PubMed] [Google Scholar]
- 35.Gelfo, F., Mandolesi, L., Serra, L., Sorrentino, G. & Caltagirone, C. The neuroprotective effects of experience on cognitive functions: Evidence from animal studies on the neurobiological bases of brain reserve. Neuroscience370, 218–235 (2018). 10.1016/j.neuroscience.2017.07.065 [DOI] [PubMed] [Google Scholar]
- 36.Mora, F. Successful brain aging: plasticity, environmental enrichment, and lifestyle. Dialog. Clin. Neurosci.15, 45–52 (2013). 10.31887/DCNS.2013.15.1/fmora [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siucinska, E. & Kossut, M. Short-lasting classical conditioning induces reversible changes of representational maps of vibrissae in mouse SI cortex—A 2DG study. Cereb. Cortex6, 506–513 (1996). 10.1093/cercor/6.3.506 [DOI] [PubMed] [Google Scholar]
- 38.Cybulska-Klosowicz, A., Zakrzewska, R. & Kossut, M. Brain activation patterns during classical conditioning with appetitive or aversive UCS. Behav. Brain Res.204, 102–111 (2009). 10.1016/j.bbr.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 39.Sokoloff, L. et al. The [14C] Deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem.28, 897–916 (1977). 10.1111/j.1471-4159.1977.tb10649.x [DOI] [PubMed] [Google Scholar]
- 40.Kossut, M., Hand, P. J., Greenberg, J. & Hand, C. L. Single vibrissal cortical column in SI cortex of rat and its alterations in neonatal and adult vibrissa-deafferented animals: A quantitative 2DG study. J. Neurophysiol.60, 829–852 (1988). 10.1152/jn.1988.60.2.829 [DOI] [PubMed] [Google Scholar]
- 41.Jablonska, B., Gierdalski, M., Kossut, M. & Skangiel-Kramska, J. Partial blocking of NMDA receptors reduces plastic changes induced by short-lasting classical conditioning in the SI barrel cortex of adult mice. Cereb. Cortex9, 222–231 (1999). 10.1093/cercor/9.3.222 [DOI] [PubMed] [Google Scholar]
- 42.GRAS Exemption Claim for Taurine for Use in Enhanced Water Beverages GRAS Notice (GRN), vol. 2023, No. 822. edn. U.S. Food & Drug Administration. GRAS Notice Inventory. https://www.fda.gov/media/93642/download (2015).
- 43.Hwang, D. F., Hour, J. L. & Cheng, H. M. Effect of taurine on toxicity of oxidized fish oil in rats. Food Chem. Toxicol.38, 585–591 (2000). 10.1016/S0278-6915(00)00052-1 [DOI] [PubMed] [Google Scholar]
- 44.Strominger, R. N. & Woolsey, T. A. Templates for locating the whisker area in fresh flattened mouse and rat cortex. J. Neurosci. Methods22, 113–118 (1987). 10.1016/0165-0270(87)90004-5 [DOI] [PubMed] [Google Scholar]
- 45.Rowley, H. L., Martin, K. F. & Marsden, C. A. Determination of in vivo amino acid neurotransmitters by high-performance liquid chromatography with O-phthalaldehyde-sulphite derivatisation. J. Neurosci. Methods57, 93–99 (1995). 10.1016/0165-0270(94)00132-Z [DOI] [PubMed] [Google Scholar]
- 46.Siucinska, E., Kossut, M. & Stewart, M. G. Gaba immunoreactivity in mouse barrel field after aversive and appetitive classical conditioning training involving facial vibrissae. Brain Res.843, 62–70 (1999). 10.1016/S0006-8993(99)01881-8 [DOI] [PubMed] [Google Scholar]
- 47.Gierdalski, M. et al. Rapid regulation of Gad67 mRNA and protein level in cortical neurons after sensory learning. Cereb. Cortex11, 806–815 (2001). 10.1093/cercor/11.9.806 [DOI] [PubMed] [Google Scholar]
- 48.Jasinska, M. et al. Rapid, learning-induced inhibitory synaptogenesis in murine barrel field. J. Neurosci.30, 1176–1184 (2010). 10.1523/JNEUROSCI.2970-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urban-Ciecko, J., Kossut, M. & Mozrzymas, J. W. Sensory learning differentially affects GABAergic tonic currents in excitatory neurons and fast spiking interneurons in layer 4 of mouse barrel cortex. J. Neurophysiol.104, 746–754 (2010). 10.1152/jn.00988.2009 [DOI] [PubMed] [Google Scholar]
- 50.Aerts, L. & Van Assche, F. A. Taurine and taurine-deficiency in the perinatal period. J. Perinat. Med.30, 281–286 (2002). 10.1515/JPM.2002.040 [DOI] [PubMed] [Google Scholar]
- 51.Dawson, R. Jr., Pelleymounter, M. A., Cullen, M. J., Gollub, M. & Liu, S. An age-related decline in striatal taurine is correlated with a loss of dopaminergic markers. Brain Res. Bull.48, 319–324 (1999). 10.1016/S0361-9230(99)00003-9 [DOI] [PubMed] [Google Scholar]
- 52.El Idrissi, A., Messing, J., Scalia, J. & Trenkner, E. Prevention of epileptic seizures by taurine. Adv. Exp. Med. Biol.526, 515–525 (2003). 10.1007/978-1-4615-0077-3_62 [DOI] [PubMed] [Google Scholar]
- 53.Trenkner, E., El Idrissi, A., Dumas, R. & Rabe, A. Functional consequences of calcium uptake modulation by taurine in vivo and in vitro. Adv. Exp. Med. Biol.442, 277–284 (1998). 10.1007/978-1-4899-0117-0_35 [DOI] [PubMed] [Google Scholar]
- 54.Kim, C. & Cha, Y. N. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids46, 89–100 (2014). 10.1007/s00726-013-1545-6 [DOI] [PubMed] [Google Scholar]
- 55.Paulucio, D. et al. Acute effect of ethanol and taurine on frontal cortex absolute beta power before and after exercise. PLoS ONE13, e0194264 (2018). 10.1371/journal.pone.0194264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galarreta, M., Bustamante, J., Martin del Río, R. & Solís, J. M. Taurine induces a long-lasting increase of synaptic efficacy and axon excitability in the hippocampus. J. Neurosci.16(1), 92–102 (1996). 10.1523/JNEUROSCI.16-01-00092.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.del Olmo, N. et al. Taurine-induced synaptic potentiation and the late phase of long-term potentiation are related mechanistically. Neuropharmacology.44(1), 26–39 (2003). 10.1016/S0028-3908(02)00310-6 [DOI] [PubMed] [Google Scholar]
- 58.del Olmo, N. et al. Role of taurine uptake on the induction of long-term synaptic potentiation. Eur. J. Neurosci.19(7), 1875–1886 (2004). 10.1111/j.1460-9568.2004.03309.x [DOI] [PubMed] [Google Scholar]
- 59.Suárez, L. M., Bustamante, J., Orensanz, L. M., del Río, R. M. & Solís, J. M. Cooperation of taurine uptake and dopamine D1 receptor activation facilitates the induction of protein synthesis-dependent late LTP. Neuropharmacology79, 101–111 (2014). 10.1016/j.neuropharm.2013.10.035 [DOI] [PubMed] [Google Scholar]
- 60.El Idrissi, A. Taurine improves learning and retention in aged mice. Neurosci. Lett.436, 19–22 (2008). 10.1016/j.neulet.2008.02.070 [DOI] [PubMed] [Google Scholar]
- 61.Wu, H. et al. Mode of action of taurine as a neuroprotector. Brain Res.1038, 123–131 (2005). 10.1016/j.brainres.2005.01.058 [DOI] [PubMed] [Google Scholar]
- 62.Foos, T. M. & Wu, J. Y. The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem. Res.27, 21–26 (2002). 10.1023/A:1014890219513 [DOI] [PubMed] [Google Scholar]
- 63.Galindo-Leon, E. E., Lin, F. G. & Liu, R. C. Inhibitory plasticity in a lateral band improves cortical detection of natural vocalizations. Neuron.62, 705–716 (2009). 10.1016/j.neuron.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menzie, J., Prentice, H. & Wu, J. Y. Neuroprotective mechanisms of taurine against ischemic stroke. Brain Sci.3, 877–907 (2013). 10.3390/brainsci3020877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mora, F., Segovia, G. & del Arco, A. Aging, plasticity and environmental enrichment: Structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res. Rev.55, 78–88 (2007). 10.1016/j.brainresrev.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 66.Kolb, B., Gibb, R. & Gorny, G. Experience-dependent changes in dendritic arbour and spine density in neocortex vary qualitatively with age and sex. Neurobiol. Learn. Mem.79, 1–10 (2003). 10.1016/S1074-7427(02)00021-7 [DOI] [PubMed] [Google Scholar]
- 67.Lazarov, O. et al. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell120, 701–713 (2005). 10.1016/j.cell.2005.01.015 [DOI] [PubMed] [Google Scholar]
- 68.Sampedro-Piquero, P. & Begega, A. Environmental enrichment as a positive behavioral intervention across the lifespan. Curr. Neuropharmacol.15, 459–470 (2017). 10.2174/1570159X14666160325115909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Praag, H., Kempermann, G. & Gage, F. H. Neural consequences of environmental enrichment. Nat. Rev. Neurosci.1, 191–198 (2000). 10.1038/35044558 [DOI] [PubMed] [Google Scholar]
- 70.Pham, T. M., Winblad, B., Granholm, A. C. & Mohammed, A. H. Environmental influences on brain neurotrophins in rats. Pharmacol. Biochem. Behav.73, 167–175 (2002). 10.1016/S0091-3057(02)00783-9 [DOI] [PubMed] [Google Scholar]
- 71.Cotman, C. W. & Berchtold, N. C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci.25, 295–301 (2002). 10.1016/S0166-2236(02)02143-4 [DOI] [PubMed] [Google Scholar]
- 72.Buschler, A. & Manahan-Vaughan, D. Metabotropic glutamate receptor, mGlu5, mediates enhancements of hippocampal long-term potentiation after environmental enrichment in young and old mice. Neuropharmacology115, 42–50 (2017). 10.1016/j.neuropharm.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 73.Cortese, G. P., Olin, A., O’Riordan, K., Hullinger, R. & Burger, C. Environmental enrichment improves hippocampal function in aged rats by enhancing learning and memory, LTP, and mGluR5-Homer1c activity. Neurobiol. Aging63, 1–11 (2018). 10.1016/j.neurobiolaging.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El Idrissi, A. et al. Decreased GABA(a) receptor expression in the seizure-prone fragile X mouse. Neurosci. Lett.377, 141–146 (2005). 10.1016/j.neulet.2004.11.087 [DOI] [PubMed] [Google Scholar]
- 75.Sale, A. et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat. Neurosci.10, 679–681 (2007). 10.1038/nn1899 [DOI] [PubMed] [Google Scholar]
- 76.Begenisic, T. et al. Environmental enrichment decreases GABAergic inhibition and improves cognitive abilities, synaptic plasticity, and visual functions in a mouse model of down syndrome. Front. Cell Neurosci.5, 29 (2011). 10.3389/fncel.2011.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suárez, L. M. & Solís, J. M. Taurine potentiates presynaptic NMDA receptors in hippocampal schaffer collateral axons. Eur. J. Neurosci.24, 405–418 (2006). 10.1111/j.1460-9568.2006.04911.x [DOI] [PubMed] [Google Scholar]
- 78.Mlynarik, M., Johansson, B. B. & Jezova, D. Enriched environment influences adrenocortical response to immune challenge and glutamate receptor gene expression in rat hippocampus. Ann. N. Y. Acad. Sci.1018, 273–280 (2004). 10.1196/annals.1296.032 [DOI] [PubMed] [Google Scholar]
- 79.Melendez, R. I., Gregory, M. L., Bardo, M. T. & Kalivas, P. W. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology29, 1980–1987 (2004). 10.1038/sj.npp.1300507 [DOI] [PubMed] [Google Scholar]
- 80.Dobrzanski, G. et al. Learning-induced plasticity in the barrel cortex is disrupted by inhibition of layer 4 somatostatin-containing interneurons. Biochim. Biophys. Acta Mol. Cell. Res.1869, 119146 (2022). 10.1016/j.bbamcr.2021.119146 [DOI] [PubMed] [Google Scholar]
- 81.O’Connor, D. H. et al. Neural coding during active somatosensation revealed using illusory touch. Nat. Neurosci.16, 958–965 (2013). 10.1038/nn.3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scala, F. et al. Layer 4 of mouse neocortex differs in cell types and circuit organization between sensory areas. Nat. Commun.10, 4174 (2019). 10.1038/s41467-019-12058-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Letzkus, J. J., Wolff, S. B. & Lüthi, A. Disinhibition, a circuit mechanism for associative learning and memory. Neuron88, 264–276 (2015). 10.1016/j.neuron.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 84.Liguz-Lecznar, M., Urban-Ciecko, J. & Kossut, M. Somatostatin and somatostatin-containing neurons in shaping neuronal activity and plasticity. Front. Neural Circuits10, 48 (2016). 10.3389/fncir.2016.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beck, B. & Pourié, G. Ghrelin, neuropeptide Y, and other feeding-regulatory peptides active in the hippocampus: role in learning and memory. Nutr. Rev.71, 541–561 (2013). 10.1111/nure.12045 [DOI] [PubMed] [Google Scholar]
- 86.Karlsson, R. M., Holmes, A., Heilig, M. & Crawley, J. N. Anxiolytic-like actions of centrally-administered neuropeptide Y, but not galanin, in C57bl/6j mice. Pharmacol. Biochem. Behav.80, 427–436 (2005). 10.1016/j.pbb.2004.12.009 [DOI] [PubMed] [Google Scholar]
- 87.Gøtzsche, C. R. & Woldbye, D. P. The role of Npy in learning and memory. Neuropeptides55, 79–89 (2016). 10.1016/j.npep.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 88.Verma, D., Tasan, R. O., Herzog, H. & Sperk, G. Npy controls fear conditioning and fear extinction by combined action on Y1 and Y2 receptors. Br. J. Pharmacol.166, 461–473 (2012). 10.1111/j.1476-5381.2012.01872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.