Abstract
Both the brain-derived neurotrophic factor (BDNF) valine (Val)/methionine (Met) polymorphism and mismatch negativity (MMN) amplitude are reportedly linked to working memory impairments in schizophrenia. However, there is evident scarcity of research aimed at exploring the relationships among the three factors. In this secondary analysis of a randomized, controlled, double-blind trial, we investigated these relationships. The trial assessed the efficacy of transcranial direct current stimulation for enhancing working memory in clinically stable schizophrenia patients, who were randomly divided into three groups: dorsolateral prefrontal cortex stimulation, posterior parietal cortex stimulation, and sham stimulation groups. Transcranial direct current stimulation was administered concurrently with a working memory task over five days. We assessed the BDNF genotype, MMN amplitude, working memory capacity, and interference control subdomains. These assessments were conducted at baseline with 54 patients and followed up post-intervention with 48 patients. Compared to BDNF Met-carriers, Val homozygotes exhibited fewer positive and general symptoms and increased working memory capacity at baseline. A correlation between MMN amplitude and working memory capacity was noted only in BDNF Val homozygotes. The correlations were significantly different in the two BDNF genotype groups. Furthermore, in the intervention group that showed significant improvement in MMN amplitude, BDNF Val homozygotes exhibited greater enhancement in working memory capacity than Met-carriers. This study provides in vivo evidence for the interaction between MMN and BDNF Val/Met polymorphism for working memory capacity. As MMN has been considered a biomarker of N-methyl-D-aspartate receptor (NMDAR) function, these data shed light on the complex interactions between BDNF and NMDAR in terms of working memory in schizophrenia.
Subject terms: Schizophrenia, Genetics of the nervous system, Working memory
Introduction
Mismatch negativity (MMN) is an event-related potential that reflects the brain’s automatic attentional engagement in information processing. MMN is elicited by the presentation of infrequently occurring deviant stimuli interspersed within frequently occurring standard stimuli1. MMN is significantly impaired in schizophrenia, correlating closely with functional outcomes2–7, and it is considered one of the most mature biomarkers for schizophrenia8, providing a groundbreaking biological signpost for understanding and treating the disorder9. Additionally, MMN is a valid biomarker for N-methyl-D-aspartate receptor (NMDAR) function in schizophrenia10. Ketamine, an NMDAR antagonist, not only induces psychotic symptoms but also reduces MMN amplitude in healthy individuals11. Furthermore, MMN can serve as a biomarker for NMDAR-mediated improvements in negative symptoms12 and auditory plasticity13,14 in schizophrenia. Thus, it is crucial to understand the relationships of MMN amplitude with other behavioral phenotypes (e.g., cognitive functions) and influential factors, which may guide intervention trials for behavioral improvement.
Working memory serves as a fundamental element of executive function15 and is identified as a central cognitive dysfunction in schizophrenia16,17. A study suggested that MMN amplitude mediated the influence of the glutamine-to-glutamate ratio on working memory in schizophrenia18; however, some studies could not identify a link between MMN amplitude and working memory in schizophrenia19. These discrepancies may be partly attributed to variations in the working memory assessment tools used. For working memory assessments, previous research typically used classic neuropsychological tests like the digit sequencing task19. However, cognitive neuroscience approaches offer a more comprehensive assessment of working memory subdomains, thus facilitating a more lucid understanding of the intrinsic mechanisms20. The change detection task is a recommended cognitive neuroscience approach for evaluating working memory capacity21. Its modified version can be used for assessing both capacity and interference control subdomains of working memory22. Therefore, using the modified change detection task may provide vital insights into the relationships of MMN amplitude with working memory capacity and interference control.
Working memory deficits are closely tied to NMDAR-mediated gamma oscillation anomalies in schizophrenia16,23. The NMDAR function is regulated by brain-derived neurotrophic factor (BDNF)24, which is among the most extensively studied peripheral indicators associated with cognitive function in schizophrenia25. BDNF can increase the abundance of synaptic NMDAR26 and reduce extrasynaptic NMDAR death signaling27. The Val66Met polymorphism in the BDNF gene results in a substitution of methionine (Met) for valine (Val) at the 66th codon, resulting in insufficient BDNF secretion28. Thus, the BDNF Val66Met polymorphism could cause NMDAR dysfunction and working memory impairments in schizophrenia24,28. Carriers of the BDNF Met/Met genotype exhibit disrupted NMDAR-dependent long-term potentiation and depression compared to wildtype controls, particularly in brain regions such as the hippocampus29 and the medial prefrontal cortex30. In addition, research has shown that BDNF Val homozygotes have larger MMN amplitude than Met-carriers among musicians31. Thus far, there has been no investigation into the potential impact of the BDNF genotype on the correlation between working memory and MMN amplitude in patients with schizophrenia.
In a randomized, controlled, double-blind trial, we found that transcranial direct current stimulation (tDCS) targeting the posterior parietal cortex improved working memory and MMN amplitude in schizophrenia, compared to targeting the prefrontal cortex or sham stimulation32. In a secondary analysis of this trial, we analyzed data from baseline and post-intervention assessments to investigate the correlations of MMN amplitude with both working memory capacity and interference control in schizophrenia while considering the potential impact of the BDNF Val/Met polymorphism. Given that the BDNF Val/Met polymorphism may influence NMDAR-dependent long-term potentiation and depression29,30, which are foundational for memory33, we hypothesized that the relationship between MMN amplitude and working memory would be regulated by the BDNF Val/Met polymorphism.
Materials and methods
Participants
All participants were from a double-blind, three-arm, randomized trial of which the primary objective was to compare the therapeutic differences among tDCS paradigms (targeting the prefrontal cortex vs. targeting the posterior parietal cortex vs. sham stimulation) synchronous with cognitive tasks for working memory in patients with schizophrenia32. Each participant provided formal informed consent before the trial commencement. This study received approval from the Ethics Committee of Beijing Anding Hospital (No. 2020-70), and was registered in the Chinese Clinical Trial Registry (ChiCTR2000038961); the information was synced with the World Health Organization international clinical trials registry platform.
The eligible participants were schizophrenia outpatients or community-dwelling schizophrenia patients with impaired working memory. High-definition tDCS was conducted using a DC-Stimulator Plus (NeuroConn, Germany) equipped with a 4 × 1 wire adaptor (Equalizer Box, NeuroConn, Germany). For the prefrontal cortex, the central electrode was positioned at F3, surrounded by four electrodes at F7, Fz, Fp1, and FC3. For the posterior parietal cortex, the central electrode was placed at P3 with four surrounding electrodes at P7, Pz, O1, and CP3. For the two real stimulation groups, the electrode intensity was set to 2 mA for a duration of 20 min, with a 40-s ramp-up and ramp-down period. The sham stimulation mirrored this setup, but the actual stimulation duration was limited to 40 s. High-definition tDCS with a concurrent N-back task, was administered twice daily for five consecutive days, and assessments were conducted at baseline, week 1, and week 2.
The primary outcome of the clinical trial was the change in spatial span test scores at week 1 from baseline. Secondary outcomes encompassed changes in scores of the change detection task and other cognitive assessments. Additionally, MMN was collected as a biomarker. Among the initial 60 participants, 54 underwent testing for single-nucleotide polymorphisms, the findings of which were employed in the baseline analysis of this report (Supplemental Fig. 1). Prior analysis revealed that compared to the prefrontal cortex stimulation group and the sham stimulation group, the posterior parietal cortex stimulation group had significant improvement in MMN amplitude at week 132. Consequently, the posterior parietal cortex stimulation group was considered as the group with notable MMN amplitude enhancement. The longitudinal data involving the change detection task served as the validation dataset in this study.
Clinical symptom and working memory assessments
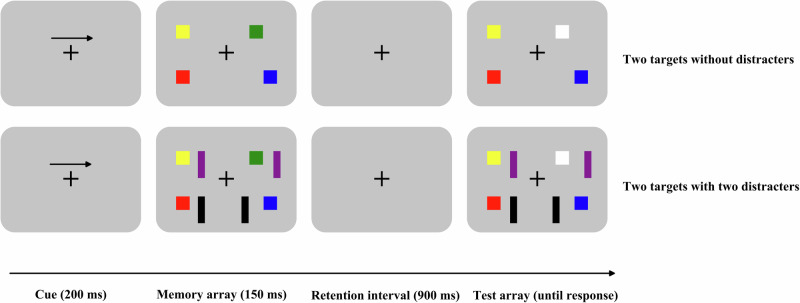
Clinical symptoms were assessed according to the positive and negative syndrome scale (PANSS), which took approximately 30 minutes. Capacity and interference control subdomains of working memory were evaluated utilizing a modified change detection task22, lasting about 40 min. In each trial, an arrow appeared initially, signaling participants to pay attention to the upcoming arrow-directed visual field. The task of the participants was to memorize the colors of all the squares (targets) within the arrow-directed field while ignoring the distracters and colors in the opposite field. After a retention interval, a test array prompted participants to recall if square colors had changed in the arrow-directed visual field. The task comprised four conditions: two targets without distracters, four targets without distracters, two targets with two distracters, and four targets with two distracters. Conditions were presented pseudo-randomly with two blocks for each condition, and each block comprised 80 trials. Changes in the test array occurred with a 50% probability (Fig. 1). To assess working memory capacity, Cowan’s K was calculated as follows: Cowan’s K = memory load × (the rate of change response when color change occurred - the rate of change response when no color change occurred)34. To assess interference control domain, filtering efficiency was computed as follows: filtering efficiency = Cowan’s K in the absence of distracters - Cowan’s K in the presence of distracters22.
Fig. 1.
Change detection task.
MMN paradigm
The MMN paradigm comprised 90% standard stimuli (50 ms, 675 trials) and 10% deviant stimuli (100 ms, 75 trials). MMN collection required approximately 12 min. Electroencephalographic recordings were obtained via a 128-channel high-density system from Electrical Geodesics, Inc., USA, operating at a 1000-Hz sampling rate and maintaining all electrode impedances under 50 kΩ. Participants focused on a “+” symbol while receiving auditory stimuli. Electroencephalograph data underwent high-pass filtering at 0.5 Hz, low-pass filtering at 30 Hz, and notch filtering between 48 Hz and 52 Hz to eliminate electrical interference. Continuous data were segmented into intervals from −100 ms to 500 ms, with the stimulus onset as the zero point. Replacement of bad electrodes and removal of bad segments were manually performed. Reference electrodes were set to the average reference, and artifacts were separated using independent component analysis. Segments exceeding ±100 μV in amplitude were removed. Electrode E6 (corresponding to the FCz in the 10–20 electroencephalograph system) was selected for analysis. The MMN waveform was obtained by deducting the waveform evoked by the standard stimuli from that evoked by the deviant stimuli. The amplitude minima between 140 ms and 240 ms were extracted as the MMN amplitude for each participant35. To ensure the stability of the findings, additional analyses were conducted based on two multi-site average clusters (electrodes 5, 6, 7, 12, 106; electrodes 5, 6, 7, 12, 13, 106, 112).
Genotyping
Whole blood was utilized for deoxyribonucleic acid extraction. The rs6265 locus sequence was detected for the BDNF Val66Met polymorphism. Genotype identification procedures involved polymerase chain reaction amplification and subsequent agarose gel electrophoresis. Among the 54 samples, 17 had BDNF Val/Val, 15 had BDNF Val/Met, and 22 had BDNF Met/Met. Participants with Val/Met and Met/Met were combined as Met-carriers for subsequent analysis.
Statistical analysis
Statistical analyses were performed utilizing the SPSS 20.0 software package (SPSS Inc., Chicago, IL, USA). The distribution of data normality was verified through histogram analysis and the Shapiro–Wilk test. To determine whether genotype distributions diverged from the Hardy–Weinberg equilibrium, the Chi-square test and Fisher’s exact test were employed. These tests, as well as the t-test, were applied to compare demographics, clinical characteristics, baseline working memory, and baseline MMN amplitude among genotype groups. Demographics and clinical characteristics that differed significantly among genotype groups were included as covariates in the covariance analysis. This controlled for potential influences on differences in baseline working memory and MMN amplitude across groups. Bonferroni corrections were implemented for multiple comparisons involving clinical symptoms, working memory capacity, and interference control.
Pearson correlation was used to assess the relationships of MMN amplitude with both working memory capacity and interference control in the entire sample and in specific genotype groups (BDNF Met-carriers and Val homozygotes) at baseline. Bonferroni corrections were adopted for capacity and interference control respectively. Additionally, the magnitude of these correlations was compared across genotype groups36. Furthermore, multiple linear regression was employed to examine the interaction effects between MMN amplitude and genotype in relation to working memory at baseline. In the multiple linear regression analysis, working memory served as the dependent variable, while the BDNF genotype, MMN amplitude, and their product were treated as independent variables. Gender, age, PANSS total score, and chlorpromazine equivalents were included as covariates. For working memory domains and genotypes with significant interaction results at baseline, the Fisher exact test and Welch analysis of variance were adopted to compare baseline characteristics and working memory enhancement across different genotype subgroups within the three intervention groups in the clinical trial. A P-value of less than 0.05 (two-sided) was set as the criterion for statistical significance.
Results
Clinical characteristics in genotype groups at baseline
The distribution of the BDNF genotype exhibited no departure from the Hardy–Weinberg equilibrium (χ²2 = 5.662, P = 0.059). Compared to BDNF Met-carriers, Val homozygotes showed significantly lower scores in PANSS positive symptoms (t51 = 2.839, P = 0.006), general symptoms (t51 = 3.325, P = 0.002), and total symptoms (t52 = 2.789, P = 0.007). These findings remained statistically significant even after Bonferroni corrections (P < 0.05). No significant disparity was observed in PANSS negative symptoms between the BDNF genotype groups (t52 = 1.105, P = 0.274) (Table 1).
Table 1.
Differences between BDNF Met-carriers and Val homozygotes at baseline.
| Met-carriers N = 37 |
Val homozygotes N = 17 |
||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Statistic | df | P | |
| Age | 34.19 | 7.52 | 32.06 | 7.54 | t = 0.967 | 52 | 0.338 |
| Education | 14.50 | 4.04 | 14.88 | 2.89 | t = −0.35 | 52 | 0.728 |
| Female/male | 22/15 | 9/8 | χ² = 0.202 | 1 | 0.653 | ||
| Han nationality/non | 33/4 | 16/1 | NA | NA | 1.000 | ||
| Employment/non | 15/22 | 8/9 | χ² = 0.202 | 1 | 0.653 | ||
| Marriage/non | 16/21 | 5/12 | χ² = 0.938 | 1 | 0.333 | ||
| Current smoker/non | 3/34 | 3/14 | NA | NA | 0.365 | ||
| Clozapine user/non | 8/29 | 1/16 | χ² = 1.099 | 1 | 0.295 | ||
| Chlorpromazine equivalents | 441.18 | 314.11 | 443.88 | 213.70 | t = −0.032 | 52 | 0.974 |
| Duration of illness | 12.14 | 8.51 | 9.48 | 7.09 | t = 1.124 | 52 | 0.266 |
| PANSS positive | 9.73 | 3.84 | 7.65 | 1.54 | t = 2.839 | 51 | 0.006 |
| PANSS negative | 12.86 | 4.60 | 11.47 | 3.56 | t = 1.105 | 52 | 0.274 |
| PANSS general | 22.11 | 4.86 | 18.76 | 2.51 | t = 3.325 | 51 | 0.002 |
| PANSS total | 44.70 | 9.14 | 37.88 | 6.19 | t = 2.789 | 52 | 0.007 |
| K_T2 (N: 34/17) | 1.36 | 0.47 | 1.52 | 0.34 | t = −1.235 | 49 | 0.223 |
| K_T4 (N: 34/17) | 1.59 | 0.80 | 2.11 | 0.59 | t = −2.356 | 49 | 0.023 |
| Filtering efficiency_T2 (N: 34/17) | 0.27 | 0.28 | 0.26 | 0.29 | t = 0.174 | 49 | 0.863 |
| Filtering efficiency_T4 (N: 34/17) | 0.70 | 0.43 | 0.84 | 0.44 | t = −1.014 | 49 | 0.315 |
| MMN amplitude (N: 36/17) | −2.83 | 1.64 | −3.02 | 1.65 | t = 0.377 | 51 | 0.708 |
BDNF, brain-derived neurotrophic factor, PANSS positive and negative syndrome scale, K_T2 Cowan’s K under only two targets condition of the change detection task, K_T4 Cowan’s K under only four targets condition of the change detection task, Filtering efficiency_T2 filtering efficiency for two targets, Filtering efficiency_T4 filtering efficiency for four targets, MMN mismatch negativity.
MMN amplitude and working memory within BDNF genotypes at baseline
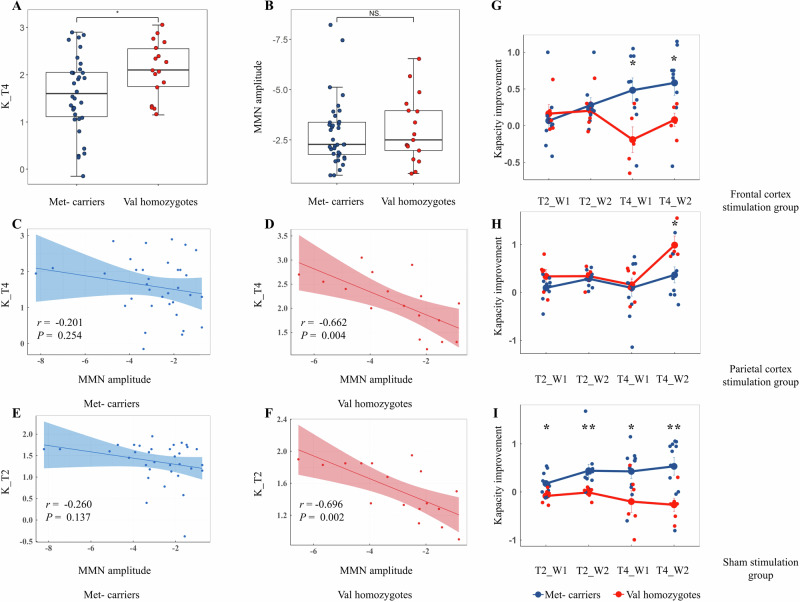
BDNF Val homozygotes demonstrated significantly higher working memory capacity under four target loads compared to Met-carriers (t49 = −2.356, P = 0.023), and this significance persisted after Bonferroni corrections (P < 0.05) (Fig. 2A). Significant differences were not observed between the BDNF genotype groups concerning interference control and MMN amplitude (Table 1) (Fig. 2B). The MMN results derived from two multi-site average clusters and single-site recordings were consistent. Using PANSS positive symptom, general symptom, and the total score as covariates, the covariance analysis revealed a significant difference in working memory capacity under four target loads between the groups (F1 = 5.153, P = 0.028), with borderline significance noted after adjustment (P = 0.056).
Fig. 2. MMN amplitude, BDNF genotypes and working memory.
Comparison of working memory capacity (A) and MMN amplitude (B) between the two genotype groups. C–F Correlations between working memory capacity and MMN amplitude across the genotype groups. Effects of BDNF genotypes on working memory capacity improvement in the prefrontal stimulation group (G), the parietal stimulation group (H) and the sham stimulation group (I). MMN, mismatch negativity; BDNF, brain-derived neurotrophic factor; K_T4, Cowan’s K under only four targets condition of the change detection task; K_T2, Cowan’s K under only two targets condition of the change detection task; T2_W1, only two targets condition of the change detection task at week 1; T2_W2, only two targets condition of the change detection task at week 2; T4_W1, only four targets condition of the change detection task at week 1; T4_W2, only four targets condition of the change detection task at week 2. *P < 0.05, ** P < 0.01, NS., not significant.
MMN amplitude, genotypes, and working memory at baseline
In the entire sample, MMN amplitude showed significant negative correlations with working memory capacity under two and four memory loads (r = −0.369, −0.316, adjusted P < 0.05). In BDNF Val homozygotes, significant negative associations were observed between MMN amplitude and working memory capacity under both two and four memory loads (r = −0.696, −0.662, adjusted P < 0.05); yet these relationships were not observed in BDNF Met-carriers (Fig. 2C–F). The correlations were significantly different in the two BDNF genotype groups (two memory loads, Z = 1.843, P = 0.033; four memory loads, Z = 1.840, P = 0.033). However, in the multiple linear regression analysis, there was no significant interaction between MMN amplitude and the BDNF genotype in relation to working memory capacity under either two or four memory loads, when controlling for covariates including gender, age, PANSS total score, and chlorpromazine equivalents. MMN amplitude did not correlate with interference control in the total sample, BDNF Val homozygotes, or BDNF Met-carriers (Table 2). The above MMN results obtained from both the two multi-site average clusters and single-site recordings exhibited consistency.
Table 2.
Correlations between working memory and MMN amplitude in BDNF Met-carriers, Val homozygotes and total participants at baseline.
| Met-carriers N = 34 |
Val homozygotes N = 17 |
Total participants N = 51 |
||
|---|---|---|---|---|
| MMN amplitude | MMN amplitude | MMN amplitude | ||
| K_T2 | r | −0.260 | −0.696 | −0.369 |
| P | 0.137 | 0.002 | 0.008 | |
| K_T4 | r | −0.201 | −0.662 | −0.316 |
| P | 0.254 | 0.004 | 0.024 | |
| Filtering efficiency_T2 | r | −0.014 | −0.115 | −0.047 |
| P | 0.936 | 0.660 | 0.745 | |
| Filtering efficiency_T4 | r | −0.128 | 0.016 | −0.086 |
| P | 0.469 | 0.950 | 0.549 |
MMN mismatch negativity, BDNF brain-derived neurotrophic factor, K_T2 Cowan’s K under only two targets condition of the change detection task, K_T4 Cowan’s K under only four targets condition of the change detection task, Filtering efficiency_T2 filtering efficiency for two targets, Filtering efficiency_T4 filtering efficiency for four targets.
MMN amplitude improvement, BDNF genotype, and working memory improvement
All genotype subgroups in the three intervention groups showed balanced baseline characteristics except for the PANSS general symptom (F5,19 = 3.726, P = 0.016) (Supplemental Table 1). In the parietal stimulation group, BDNF Val homozygotes exhibited significantly greater improvements in working memory capacity under four memory loads at week 2 compared to Met-carriers (F1, 8 = 6.091, P = 0.040) (Fig. 2H). In contrast, in the prefrontal stimulation group, BDNF Val homozygotes showed significantly lesser enhancements in working memory capacity under four memory loads at both week 1 and week 2 compared to Met-carriers (all P < 0.05) (Fig. 2G). In the sham stimulation group, BDNF Val homozygotes had significantly lesser augmentations in working memory capacity under both two and four memory loads at week 1 and week 2 compared to Met-carriers (all P < 0.05) (Table 3) (Fig. 2I).
Table 3.
BDNF genotypes and working memory improvement across the three intervention groups.
| Group | Working memory improvement | Met-carriers | Val homozygotes | F | df | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |||||
| Frontal cortex stimulation group | K_T2 at week 1 | 9 | 0.07 | 0.40 | 5 | 0.17 | 0.28 | 0.291 | 1, 11 | 0.600 |
| K_T2 at week 2 | 9 | 0.28 | 0.30 | 5 | 0.21 | 0.28 | 0.219 | 1, 9 | 0.651 | |
| K_T4 at week 1 | 9 | 0.48 | 0.50 | 5 | −0.19 | 0.39 | 7.735 | 1, 10 | 0.019 | |
| K_T4 at week 2 | 9 | 0.58 | 0.51 | 5 | 0.08 | 0.20 | 6.853 | 1, 11 | 0.023 | |
| Parietal cortex stimulation group | K_T2 at week 1 | 11 | 0.10 | 0.23 | 6 | 0.33 | 0.35 | 2.282 | 1, 7 | 0.172 |
| K_T2 at week 2 | 9 | 0.28 | 0.17 | 4 | 0.34 | 0.24 | 0.171 | 1, 4 | 0.698 | |
| K_T4 at week 1 | 11 | 0.10 | 0.57 | 6 | 0.16 | 0.35 | 0.079 | 1, 15 | 0.782 | |
| K_T4 at week 2 | 9 | 0.37 | 0.50 | 4 | 0.99 | 0.38 | 6.091 | 1, 8 | 0.040 | |
| Sham stimulation group | K_T2 at week 1 | 11 | 0.17 | 0.22 | 6 | −0.08 | 0.14 | 8.371 | 1, 14 | 0.012 |
| K_T2 at week 2 | 11 | 0.44 | 0.44 | 6 | −0.01 | 0.12 | 10.082 | 1, 12 | 0.008 | |
| K_T4 at week 1 | 11 | 0.43 | 0.48 | 6 | −0.20 | 0.55 | 5.415 | 1, 9 | 0.044 | |
| K_T4 at week 2 | 11 | 0.53 | 0.60 | 6 | −0.27 | 0.34 | 12.218 | 1, 15 | 0.003 | |
BDNF brain-derived neurotrophic factor, K_T2 Cowan’s K under only two targets condition of the change detection task, K_T4 Cowan’s K under only four targets condition of the change detection task, Filtering efficiency_T2 filtering efficiency for two targets, Filtering efficiency_T4 filtering efficiency for four targets.
Discussion
This study represents the first exploration into the complex interaction between the BDNF genotype and MMN amplitude in relation to working memory in schizophrenia. The findings indicated that BDNF Val homozygotes exhibited fewer positive, general symptoms and larger working memory capacity compared to Met-carriers at baseline. A correlation existed between MMN amplitude and working memory capacity within BDNF Val homozygotes. The correlations between MMN amplitude and working memory capacity were different in the two BDNF genotype groups. However, the multiple linear regression model did not reveal a significant interaction between the BDNF genotype and MMN amplitude. This lack of significance might be attributed to the limited sample size, potentially reducing the power to test for multivariate relationships. The longitudinal data further underscored that only in the intervention group with notable MMN amplitude enhancement did BDNF Val homozygotes outperform Met-carriers in terms of improvement in working memory capacity.
BDNF Val homozygotes presented with fewer positive and general symptoms compared to Met-carriers, aligning with previous literature37–39. Given that patients in this study were clinically stable, the findings were less affected by symptom or medication fluctuations, thus reinforcing the effect of BDNF Val/Met polymorphism in schizophrenia symptomatology variation28. This study also found that BDNF Val homozygotes had greater working memory capacity than Met-carriers. However, the two groups showed no difference in interference control or MMN amplitude. Similarly, ref. 40. reported that schizophrenia patients with BDNF Val/Val performed better during an N-back task. Nevertheless, disparate findings by refs. 41,42. suggested no connection between the BDNF Val/Met polymorphism and working memory in schizophrenia, using the digit span test and letter-number sequencing test. The differences in findings may be attributed to inconsistencies in assessment tools. Moreover, this study indicated that the effect of the BDNF genotype on working memory in schizophrenia may be domain-specific and limited to the capacity aspect.
In the total sample, MMN amplitude was negatively correlated with working memory capacity. Similarly, most studies in a systematic review19 consistently reported a correlation between MMN amplitude and working memory in schizophrenia. Although the current study substantiated a connection between MMN amplitude and capacity subdomain, it could not identify a correlation between MMN amplitude and interference control subdomain. This discrepancy could be attributed to working memory capacity being intertwined with theta oscillations, whereas interference control is closely linked to alpha oscillations43,44. Notably, MMN primarily reflects theta band activity45,46.
The correlation between MMN amplitude and working memory was particularly observed in BDNF Val homozygotes, suggesting that the Val/Val genotype bolsters the relationship between the NMDAR function and working memory. This may be due to the association of BDNF Val/Val genotype with elevated NMDAR-dependent long-term potentiation and depression29, which are foundational for memory33. This study provides clinical evidence for the BDNF Val/Met polymorphism modulating NMDAR-mediated synaptic plasticity in schizophrenia. In addition, the findings suggest that BDNF Val homozygotes more readily experience NMDAR-mediated improvements in working memory. In line with this inference, some studies have reported greater cortical responses in BDNF Val homozygotes than in Met-carriers after neural modulations in a healthy population47,48. Su et al49. also found that schizophrenia patients with BDNF Val homozygotes exhibited enhanced immediate memory after repetitive transcranial magnetic stimulation, a response not observed in Met-carriers.
Participants with BDNF Val/Val genotype showed superior enhancements in working memory capacity compared to Met-carriers, but only when MMN amplitude was improved. This further substantiates the interactive influence of MMN amplitude and the BDNF genotype in relation to working memory capacity in schizophrenia. This phenomenon may be attributed to the increase in glutamatergic activity caused by the improved NMDAR function, offering a broader range of synaptic plasticity and an elevation in BDNF release50. This improved the visibility of the effects of the BDNF genotype on NMDAR-dependent synaptic plasticity. The findings of other groups in this study can be interpreted as a decrease in the homeostatic level of the excitatory–inhibitory balance when the NMDAR function is not improved, leading to a deterioration of the self-regulation ability to cope with large-scale network activities51 and impaired or even disrupted activity-dependent regulatory role of BDNF. These results highlight the significance of the interaction between the enhanced NMDAR function and the BDNF Val/Met polymorphism for working memory improvements in schizophrenia.
This study, which utilized a modified change detection task, is the first to report an association between MMN amplitude and working memory capacity in schizophrenia. Furthermore, our research provides data from both baseline and post-intervention assessments in a clinical trial, indicating an interaction between BDNF genotype and MMN amplitude in relation to working memory capacity in schizophrenia. All participants in this study were in a clinically stable phase, reducing potential confounding impacts on the findings. However, this study also has some limitations. First, the sample size in this study was relatively small, necessitating further validation using larger sample studies. Second, this study lacked a healthy control group. Therefore, it remains unknown whether similar results occur in healthy populations, making it a challenge to discern whether the findings are specific to the state of schizophrenia.
Conclusion
In summary, this research revealed a noteworthy interaction between MMN and BDNF Val/Met polymorphism in relation to working memory capacity. The results contribute to our current understanding of the pathological mechanisms underlying the deficits in working memory capacity. Additionally, they provide valuable guidance for future intervention studies, particularly those mediated by NMDAR, aimed at addressing working memory deficits in schizophrenia.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81971250, 82171501), the Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (No. ZLRK202335), the Beijing Anding Hospital, Capital Medical University (No. YJ2022-04) and the Early Psychosis Cohort Program of Beijing Anding Hospital (No. ADDL-03).
Author contributions
The project was designed by W.H., X.C., X..L., F.Z., and C.W. Data collection was carried out by H.L., Q.W., Y.D., and R.W. Data analysis was performed by W.H., X.Q., and F.D. The manuscript was written and revised by W.H., Q.B., X.L., F.Z., and C.W.
Data availability
The data that support the findings of this study are available on request from the corresponding author (C.Y.W).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xianbin Li, Fuchun Zhou, Chuanyue Wang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41537-024-00493-x.
References
- 1.Näätänen, R., Paavilainen, P., Rinne, T. & Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin. Neurophysiol.118, 2544–2590 (2007). 10.1016/j.clinph.2007.04.026 [DOI] [PubMed] [Google Scholar]
- 2.Erickson, M. A., Ruffle, A. & Gold, J. M. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biol. Psychiatry79, 980–987 (2016). 10.1016/j.biopsych.2015.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koshiyama, D. et al. Hierarchical pathways from sensory processing to cognitive, clinical, and functional impairments in schizophrenia. Schizophr. Bull.47, 373–385 (2021). 10.1093/schbul/sbaa116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avissar, M. et al. Meta-analysis of mismatch negativity to simple versus complex deviants in schizophrenia. Schizophr. Res.191, 25–34 (2018). 10.1016/j.schres.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haigh, S. M., Coffman, B. A. & Salisbury, D. F. Mismatch negativity in first-episode schizophrenia: a meta-analysis. Clin. EEG Neurosci.48, 3–10 (2017). 10.1177/1550059416645980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koshiyama, D. et al. Unique contributions of sensory discrimination and gamma synchronization deficits to cognitive, clinical, and psychosocial functional impairments in schizophrenia. Schizophr. Res.228, 280–287 (2021). 10.1016/j.schres.2020.12.042 [DOI] [PubMed] [Google Scholar]
- 7.Loiodice, S. et al. Mismatch negativity as EEG biomarker supporting CNS drug development: a transnosographic and translational study. Transl. Psychiatry11, 253 (2021). 10.1038/s41398-021-01371-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler, P. D. et al. Perceptual measurement in schizophrenia: promising electrophysiology and neuroimaging paradigms from CNTRICS. Schizophr. Bull.38, 81–91 (2012). 10.1093/schbul/sbr106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Light, G. A. & Näätänen, R. Mismatch negativity is a breakthrough biomarker for understanding and treating psychotic disorders. Proc. Natl Acad. Sci. USA110, 15175–15176 (2013). 10.1073/pnas.1313287110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avissar, M. & Javitt, D. Mismatch negativity: a simple and useful biomarker of N-methyl-d-aspartate receptor (NMDAR)-type glutamate dysfunction in schizophrenia. Schizophr. Res.191, 1–4 (2018). 10.1016/j.schres.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 11.Rosburg, T. & Kreitschmann-Andermahr, I. The effects of ketamine on the mismatch negativity (MMN) in humans - a meta-analysis. Clin. Neurophysiol.127, 1387–1394 (2016). 10.1016/j.clinph.2015.10.062 [DOI] [PubMed] [Google Scholar]
- 12.Greenwood, L. M. et al. The effects of glycine on auditory mismatch negativity in schizophrenia. Schizophr. Res.191, 61–69 (2018). 10.1016/j.schres.2017.05.031 [DOI] [PubMed] [Google Scholar]
- 13.Kantrowitz, J. T. N-methyl-d-aspartate-type glutamate receptor modulators and related medications for the enhancement of auditory system plasticity in schizophrenia. Schizophr. Res.207, 70–79 (2019). 10.1016/j.schres.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 14.Sehatpour, P. et al. Dose-dependent augmentation of neuroplasticity-based auditory learning in schizophrenia: a double-blind, placebo-controlled, randomized, target engagement clinical trial of the NMDA glutamate receptor agonist D-serine. Biol. Psychiatry94, 164–173 (2023). 10.1016/j.biopsych.2023.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond, A. Executive functions. Annu. Rev. Psychol.64, 135–168 (2013). 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lett, T. A., Voineskos, A. N., Kennedy, J. L., Levine, B. & Daskalakis, Z. J. Treating working memory deficits in schizophrenia: a review of the neurobiology. Biol. Psychiatry75, 361–370 (2014). 10.1016/j.biopsych.2013.07.026 [DOI] [PubMed] [Google Scholar]
- 17.Luck, S. J., Hahn, B., Leonard, C. J. & Gold, J. M. The hyperfocusing hypothesis: a new account of cognitive dysfunction in schizophrenia. Schizophr. Bull.45, 991–1000 (2019). 10.1093/schbul/sbz063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowland, L. M. et al. Frontal glutamate and gamma-aminobutyric acid levels and their associations with mismatch negativity and digit sequencing task performance in schizophrenia. Jama Psychiatry73, 166–174 (2016). 10.1001/jamapsychiatry.2015.2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrottelli, A. et al. Unveiling the associations between EEG indices and cognitive deficits in schizophrenia-spectrum disorders: a systematic review. Diagnostics12, 2193 (2022). 10.3390/diagnostics12092193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter, C. S. & Barch, D. M. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr. Bull.33, 1131–1137 (2007). 10.1093/schbul/sbm081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barch, D. M., Moore, H., Nee, D. E., Manoach, D. S. & Luck, S. J. CNTRICS imaging biomarkers selection: working memory. Schizophr. Bull.38, 43–52 (2012). 10.1093/schbul/sbr160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spronk, M., Vogel, E. K. & Jonkman, L. M. No behavioral or ERP evidence for a developmental lag in visual working memory capacity or filtering in adolescents and adults with ADHD. Plos ONE8, e62673 (2013). 10.1371/journal.pone.0062673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh, F. et al. Modulation of frontal gamma oscillations improves working memory in schizophrenia. Neuroimage Clin.27, 102339 (2020). 10.1016/j.nicl.2020.102339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notaras, M., Hill, R. & van den Buuse, M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol. Psychiatry20, 916–930 (2015). 10.1038/mp.2015.27 [DOI] [PubMed] [Google Scholar]
- 25.Bora, E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: a meta-analysis. Psychol. Med.49, 1971–1979 (2019). 10.1017/S0033291719001685 [DOI] [PubMed] [Google Scholar]
- 26.Afonso, P., et al. BDNF increases synaptic NMDA receptor abundance by enhancing the local translation of Pyk2 in cultured hippocampal neurons. Sci. Signal. 12, eaav3577 (2019). [DOI] [PubMed]
- 27.Lau, D., Bengtson, C. P., Buchthal, B. & Bading, H. BDNF reduces toxic extrasynaptic NMDA receptor signaling via synaptic NMDA receptors and nuclear-calcium-induced transcription of inhba/activin A. Cell Rep.12, 1353–1366 (2015). 10.1016/j.celrep.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 28.Notaras, M., Hill, R. & van den Buuse, M. A role for the BDNF gene Val66Met polymorphism in schizophrenia? A comprehensive review. Neurosci. Biobehav. Rev.51, 15–30 (2015). 10.1016/j.neubiorev.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 29.Ninan, I. et al. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J. Neurosci.30, 8866–8870 (2010). 10.1523/JNEUROSCI.1405-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pattwell, S. S. et al. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J. Neurosci.32, 2410–2421 (2012). 10.1523/JNEUROSCI.5205-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonetti, L. et al. Moderate associations between BDNF Val66Met gene polymorphism, musical expertise, and mismatch negativity. Heliyon9, e15600 (2023). 10.1016/j.heliyon.2023.e15600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou, W. et al. Effect of transcranial direct current stimulation with concurrent cognitive performance targeting posterior parietal cortex vs prefrontal cortex on working memory in schizophrenia: a randomized clinical trial. Transl. Psychiatry14, 279 (2024). 10.1038/s41398-024-02994-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nabavi, S. et al. Engineering a memory with LTD and LTP. Nature511, 348–352 (2014). 10.1038/nature13294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowan, N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav. Brain Sci.24, 87–114 (2001). 114-185. 10.1017/S0140525X01003922 [DOI] [PubMed] [Google Scholar]
- 35.Duncan, C. C. et al. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol.120, 1883–1908 (2009). 10.1016/j.clinph.2009.07.045 [DOI] [PubMed] [Google Scholar]
- 36.Lenhard, W., Lenhard, A. Hypothesis tests for comparing correlations. Psychometrica., https://www.psychometrica.de/correlation.html (2014).
- 37.Han, D. H. et al. Effects of brain-derived neurotrophic factor-catecholamine-o-methyltransferase gene interaction on schizophrenic symptoms. Neuroreport19, 1155–1158 (2008). 10.1097/WNR.0b013e32830867ad [DOI] [PubMed] [Google Scholar]
- 38.Zhai, J. et al. Association of the brain-derived neurotrophic factor gene G196A rs6265 polymorphisms and the cognitive function and clinical symptoms of schizophrenia. Int. J. Clin. Exp. Pathol.6, 1617–1623 (2013). [PMC free article] [PubMed] [Google Scholar]
- 39.Suchanek, R. et al. BDNF Val66Met polymorphism is associated with age at onset and intensity of symptoms of paranoid schizophrenia in a Polish population. J. Neuropsychiatry Clin. Neurosci.25, 88–94 (2013). 10.1176/appi.neuropsych.11100234 [DOI] [PubMed] [Google Scholar]
- 40.Rybakowski, J. K. et al. Prefrontal cognition in schizophrenia and bipolar illness in relation to Val66Met polymorphism of the brain-derived neurotrophic factor gene. Psychiatry Clin. Neurosci.60, 70–76 (2006). 10.1111/j.1440-1819.2006.01462.x [DOI] [PubMed] [Google Scholar]
- 41.Mezquida, G. et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with negative symptoms severity, but not cognitive function, in first-episode schizophrenia spectrum disorders. Eur. Psychiatry38, 61–69 (2016). 10.1016/j.eurpsy.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 42.Martinho, E. J. et al. BDNF gene polymorphism, cognition and symptom severity in a Brazilian population-based sample of first-episode psychosis subjects. Braz. J. Psychiatry34, S219–S225 (2012). 10.1016/j.rbp.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 43.Riddle, J., Scimeca, J. M., Cellier, D., Dhanani, S. & D’Esposito, M. Causal evidence for a role of theta and alpha oscillations in the control of working memory. Curr. Biol.30, 1748–1754 (2020). 10.1016/j.cub.2020.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Vries, I., Slagter, H. A. & Olivers, C. Oscillatory control over representational states in working memory. Trends Cognit. Sci.24, 150–162 (2020). 10.1016/j.tics.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 45.Javitt, D. C., Lee, M., Kantrowitz, J. T. & Martinez, A. Mismatch negativity as a biomarker of theta band oscillatory dysfunction in schizophrenia. Schizophr. Res.191, 51–60 (2018). 10.1016/j.schres.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 46.Valt, C. et al. Reduced magnetic mismatch negativity: a shared deficit in psychosis and related risk. Psychol. Med.53, 6037–6045 (2023). 10.1017/S003329172200321X [DOI] [PubMed] [Google Scholar]
- 47.Sasaki, R., Kojima, S. & Onishi, H. Do brain-derived neurotrophic factor genetic polymorphisms modulate the efficacy of motor cortex plasticity induced by non-invasive brain stimulation? A systematic review. Front. Hum. Neurosci.15, 742373 (2021). 10.3389/fnhum.2021.742373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riddle, J. et al. Brain-derived neurotrophic factor (BDNF) polymorphism may influence the efficacy of tACS to modulate neural oscillations. Brain Stimul.13, 998–999 (2020). 10.1016/j.brs.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su, X. et al. Neuronavigated repetitive transcranial stimulation improves neurocognitive functioning in veterans with schizophrenia: a possible role of BDNF polymorphism. Curr. Neuropharmacol.21, 142–150 (2023). 10.2174/1570159X20666220803154820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lituma, P. J., Kwon, H. B., Alviña, K., Luján, R., Castillo, P. E. Presynaptic NMDA receptors facilitate short-term plasticity and BDNF release at hippocampal mossy fiber synapses. Elife. 10, e66612 (2021). [DOI] [PMC free article] [PubMed]
- 51.Lewis, D. A., Curley, A. A., Glausier, J. R. & Volk, D. W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci.35, 57–67 (2012). 10.1016/j.tins.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (C.Y.W).