Abstract
Granulation is the critical process for the pharmaceutical development of poorly water-soluble drug products. Poorly formulated products have challenges in dissolution and bioequivalence studies. Rivaroxaban (RXB) is a poorly soluble drug and has 66% fasting bioavailability at a high strength of 20 mg. Establishing the bioequivalence between test and reference products for high strength requires comparative dissolution profiles and bioequivalence. Improper granulation products and the rest of the batches failed in virtual bioequivalence. The present study provided insight into the optimization of the wet granulation process for manufacturing RXB generic immediate-release tablets using PBPK modeling and simulations. Furthermore, PBPK models are not only useful for formulation optimization but also for process optimization during pharmaceutical product development.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40203-024-00249-6.
Keywords: Rivraoxban, Granulation, Dissolution, Bioequivalence, PBPK
Introduction
Tanisha: 0009-0008-5669-1038
Rivaroxaban (RXB) is a chemical carboxamide derivative and is widely used as a therapeutic agent in the treatment of venous thromboembolism (VTE) (Trujillo and Dobesh 2014). It is an orally active direct inhibitor of the activated serine protease Factor Xa. The drug is sold under the brand name Xarelto as a film-coated immediate-release (IR) tablet (FDA and CDER 2011). The reference-listed drug (RLD) product is available in multiple strengths such as 10, 15, and 20 mg. The highest strength of 20 mg possesses bioavailability issues. The fasting state bioavailability of 20 mg strength is 66% and 100% is in the fed state (Mueck et al. 2014). RXB exists in different polymorphic forms and form I is used in commercial manufacturing due to its thermodynamic stability (Public Assessment Report Scientific Discussion Rivaroxaban—Richter Film-Coated Tablets Rivaroxaban IS/H/0423/001-005/DC 2020), (Nadendla et al. 2021). Hence, monitoring the polymorphic form change during manufacturing plays an important role in the biopharmaceutical attributes of the dosage forms. Poorly water-soluble compounds, especially in high strength, have a potential risk of low and variable bioavailability caused by incomplete dissolution. Granulation is one of the key pharmaceutical processes, that impacts the disintegration and dissolution of finished products (Pandey et al. 2013). Granulation is the critical unit operation for tablet manufacturing, especially for low aqueous soluble drugs (Shanmugam 2015). The rate and extent of dissolution of the tablets are based on the choice of wet granulation equipment, solvent for granulation, and other process parameters (Shanmugam 2015). During the prototype development, it was observed that the dissolution of RXB tablets was greatly influenced by the granulation process. To understand the effect of wet granulation on tablet dissolution and virtual bioequivalence, different processes and process parameters were evaluated to optimize the granulation process for generic RXB immediate-release tablet manufacturing. The wet granulation process is widely used in the pharmaceutical industry to enhance particle properties for better process control and ease of manufacturing. High-shear wet granulation using a rapid mixture granulator is most commonly used for manufacturing granules (Holm et al. 2001). The advantages of this method include short processing time, uniformity of granules, better tablet properties, and reproducibility. However, high-density granules produced by this method may pose challenges in the de-aggregation and dissolution of the tablets. Fluid bed processing (FBP) is an expensive technique, that offers both high-shear granulation as well as low-shear granulation processes (Alshihabi et al. 2013). The FBP results in porous granules, which have higher surface areas for dissolution. However, low bulk density, irregular particle size, tedious optimization, and scale-up procedures limit its applicability in pharmaceutical manufacturing. Despite several disadvantages, the FBP technique is used in the pharmaceutical industry because of one-step granulation and drying, reduction in dust level, higher compaction, and better tablet properties (Giry et al. 2009). Product quality and manufacturing consistency play an important role not only in supply chain consistency but also in regulatory requirements and public health. While developing the prototype formulation, the granulation process was found to be a critical unit operation for manufacturing RXB immediate-release tablets, which impacted the dissolution behavior of the developed initial prototype formulations. The comparative multi-media dissolution studies between RLD and test products are an essential prerequisite for regulatory submission and biowaiver options. The present study aimed to investigate the effect of different granulating solvents on granule quality and dissolution behavior of the finished product. High shear granulation was performed using rapid mixture granulation (RMG) and low shear granulation using FBP. The effect of the granulation process on in silico bioequivalence is also explored in the present study.
Materials and methods
Materials
RXB (Batch No: 25515582, retesting date: 30.05.2022) was obtained from Mylan Laboratories, India. Lactose monohydrate was obtained from Kerry Ingredients India Pvt. Ltd, Vadodara, India (Batch No: 0003346391). Microcrystalline cellulose was obtained from JRS Pharma GmbH &Co, Rosenberg, Germany (Batch No: 6610174335). Hypromellose (HPMC-3 cps) was from Shin-Etsu, SE Tylose GmbH, Rheingausir, Germany (Batch No: DEAT363889). Sodium dodecyl sulfate was from BASF and manufactured from Obegi, Chemicals, Amman, Jordan (Lot No: 50253853). Croscarmellose sodium was from DFE pharma, DMV-Fonterra Excipients B.V, Foxhol, The Netherlands (Batch No: 0743737). Magnesium stearate was from Merck KGaA, Darmstadt, Germany (Batch No: K50105163). Opadry coating material was obtained from Colorcon India Ltd, Mumbai (Product code: 04F565019; Lot No: TKL 51331). All the chemicals used in the analysis were of analytical grade. RLD of RXB (Xarelto, Batch No: BXJJF71) 20 mg film-coated tablets were purchased from a local pharmacy.
Solubility studies
Saturation solubility studies were performed using a shake flask method. Each 100 mg of the drug was dispersed to 4 ml of 0.1 N HCl/buffers in 5 ml Eppendorf tubes (n = 6). The tubes were shaken at 200 rpm at 25 °C for 4 h using an orbital shaker (Eppendorf, Hamburg, Germany). The pH of the resulting suspensions was monitored at 4 h using a pH meter (Seven excellence S 400, Mettler-Toledo GmbH, Greifensee, Switzerland) and the suspensions were centrifuged for 10 min at 12,000 rpm at 37 °C (Centrifuge 5415R—Eppendorf, Hamburg, Germany). Each 1 ml of the supernatant solution was diluted with mobile phase and estimated for drug content using the validated HPLC method reported in the United States Pharmacopeia.
The saturation solubility studies of granules were performed at 37 °C with 200 rpm using a shake flask method using pH 6.8 phosphate buffer (Eppendorf, Hamburg, Germany). The coating of Xarelto 20 mg tablets was carefully removed and pre-digested with 2 ml of buffer for 10 min and 2 ml of buffer was added in the resulting suspension. The suspension was used for the saturation solubility of granules. Each 90 mg granule was dispersed to 4 ml of media in 5 ml Eppendorf tubes (n = 6). After 1 h, the suspensions were centrifuged for 10 min at 12,000 rpm at 37 °C (Centrifuge 5415R—Eppendorf, Hamburg, Germany). Each 1 ml of the supernatant solution was diluted with mobile phase and estimated for drug content using the validated HPLC method reported in pharmacopeia.
Quality target product profile (QTPP) synthesis
The quality Target Product Profile of the proposed generic product was synthesized from the summary basis of approval, public assessment report, orange book patents, nonorange book patents, pharmacopeia, and other literature. Furthermore, reverse engineering of RLD was performed using different orthogonal analytical techniques and has been reported in our previous publication (Administrable 2018; Jailani et al. 2023a, b; Lee et al. 2021; Metre et al. 2018; US Pharmacopeia 29, n.d., 2024).
Manufacturing of generic product
Wet granulation process
High shear granulation was carried out using rapid mixer granulator (RMG) and top spray FBP for low shear granulation. Water, ethanol, and hydroalcoholic solutions were used as granulation fluid. High shear granulation trials were performed with a batch size of 180 g. In brief, RXB, lactose monohydrate, microcrystalline cellulose, and croscarmellose sodium (part-1) were individually sifted through a 500-micron sifter sieve. The sifted materials were taken in a poly bag and mixed for 10 min manually. Sodium lauryl sulfate was added to granulation fluid (purified water or mixture of 50:50 purified water: absolute ethanol or absolute ethanol) under mixing to get a clear solution. HPMC was slowly added through the vortex and mixed well for 30 min, then the solution was allowed to stand for 20 min. Dry mixed materials were transferred into the rapid mixer granulator (RMG-1L, KEL, India). Slow addition of granulation fluid was performed by mixing with an impeller and a chopper was on at a slow speed to break down the lumps. The obtained wet mass was passed through a # 10 mesh S.S screen and loaded into a fluid bed dryer (FBD) (Nanofluid, Hugopharm, India)and dried at 60 °C until with targeted loss on drying of 2.0–2.5%. The dried granules were milled through a 1.0 mm screen using a Multi mill (Lab Unimill, Hugopharm, India). Extra granular materials microcrystalline cellulose and croscarmellose sodium (part-2) passed through a 500-micron sieve. The low shear granulation trials were conducted using a top spray fluid bed processor (Aeromatic fielder Fluid Bed Processor, Unifluid 1.1, Hugopharm Technologies, India). Table 1 provides the batch description of the wet granulation process. Tables 1 and 2 in Supplement 1 provide the wet granulation process parameters. The milled granules obtained from the granulation process are blended with extra-granular material for 10 min in a bin blender (Rimek 1L, KEL, India) to get the pre-lubricated blend. Magnesium stearate was passed through a 250-micron sieve and blended with the pre-lubricated blend for 5 min in the bin blender.
Table 1.
Batch description for the wet granulation process
| Wet granulation | ||||||
|---|---|---|---|---|---|---|
| Granulation fluid | Pure water | Pure water | Hydro-alcoholic | Pure alcoholic | Hydro-alcoholic | Pure alcoholic |
| Batch no. | HS-AQ | LS-AQ | HS-HA | HS-AH | LS-HA | LS-AH |
| Type of granulation | High shear | Low shear | High shear | High shear | Low shear | Low shear |
| RMG | FBP | RMG | RMG | FBP | FBP | |
| Granulation type | Aqueous | Aqueous | Hydroalcoholic | Hydroalcoholic | Alcoholic | Alcoholic |
| Batch size (g) | 180 | 360 | 180 | 180 | 360 | 360 |
| Batch size (tablets) | 2000 | 4000 | 2000 | 2000 | 4000 | 4000 |
| Intra granular load (Qty/batch) | ||||||
| Drymix load (g) | 153 | 306 | 153 | 153 | 306 | 306 |
| Binder solution quantity (Qty/batch) | ||||||
| Absolute ethanol (g) | – | 120 | 30 | 60 | 60 | 120 |
| Purified water (g) | 40 | – | 30 | – | 60 | – |
| Binder solution |
SLS + HPMC 3 cps |
SLS + HPMC 3 cps |
SLS + HPMC 3 cps |
SLS + HPMC 3 cps |
SLS + HPMC 3 cps |
SLS + HPMC 3 cps |
| Appearance | Clear | Clear | Clear | Dispersion | Clear | Dispersion |
| Dry mixing | ||||||
| Dry mixing | 10* | 10 | 10* | 10* | 10 | 10 |
*Impeller: 80 ± 5 RPM. Mixing time:10 min; chopper: off
Characterization of lubricated blend and compressed tablets
The lubricated blend was characterized for bulk density (bulk density tester, EV02s, Electrolab Mumbai, India), tapped density (Tap density apparatus, TD 1025, LabIndia Analytical, Mumbai, India), compressibility index, and Hausner’s ratio and granule size distribution Electromagnetic Sieve Shaker EMS-08, Electrolab Mumbai, India) as per standard operating procedures. Compressed tablets were characterized for weight variation (Tablet tester, EBT-2PL, Electrolab Mumbai, India), and disintegration test (Tablet Disintegration Tester DT 1000, LabIndia Analytical, Mumbai, India) using the method described in pharmacopeia (US Pharmacopeia 29, n.d., 2024). The other pharmaceutical characteristics of tablets such as thickness, hardness, and friability were evaluated for their large-scale manufacturing, packaging, and shipping feasibilities.
Compression of tablets
Initial feasibility assessment trials were taken for the compression of tablets. Based on the trial outcomes the compression parameters including punch selection, production speed, and other parameters were optimized. The lubricated blend was charged into the hopper of the tablet compression machine (Minipress II-MT, KEL, India). The gravitational feeder was used to feed the powder blend on the turret. Four sets of B tooling punch and die sets (Parle Elizabeth Tools Pvt. Ltd, India) were diagonally fixed in the machine. 6 mm shallow concave punches were used for compression of tablets. The production speed of 2000 tablets/h was optimized to achieve better tablet properties and manufacturing ease. The rest of the set parameters for tablet compression were selected based on the granules. Qualitative and quantitative composition including the technical grade and vendor of the inactive ingredients are the same for all test formulations. The composition of the test products is shown in Table 2.
Table 2.
Composition of the test formulations
| Batch no. | HS-AQ | LS-AQ | HS-HA | HS-AH | LS-HA | LS-AH |
|---|---|---|---|---|---|---|
| Intra-granular (mg) | ||||||
| Rivaroxaban | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Lactose monohydrate | 21.76 | 21.76 | 21.76 | 21.76 | 21.76 | 21.76 |
| Microcrystalline cellulose | 28.64 | 28.64 | 28.64 | 28.64 | 28.64 | 28.64 |
| Hypromellose-3cps (hydroxypropyl methyl cellulose) | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 |
| Sodium lauryl sulphate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Croscarmellose sodium | 3.30 | 3.30 | 3.30 | 3.30 | 3.30 | 3.30 |
| Absolute ethanol | – | 30 | 15 | 30 | 15 | 30 |
| Purified water | 20 | – | 15 | – | 15 | – |
| Extra granular (mg) | ||||||
| Microcrystalline Cellulose | 10 | 10 | 10 | 10 | 10 | 10 |
| Croscarmellose sodium | 2 | 2 | 2 | 2 | 2 | 2 |
| Lubrication (mg) | ||||||
| Magnesium stearate | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Core total tablet weight (mg) | 90.00 | 90.00 | 90.00 | 90.00 | 90.00 | 90.00 |
Nonfunctional film-coating of tablets
Aqueous film coating was carried out using 15% w/w Opadry dispersion in a lab conventional pan coater (The pharma R&D coater, ICPL, India) with a 6″ pan with 3 baffles. 15 g of Opadry ready mix was dispersed in 100 ml of water and homogenized at 1000 rpm for 45 min and the obtained dispersion was used as a coating solution. The process operations conditions are inlet temperature (55 ± 3 °C), bed temperature (42 ± 3 °C), pan speed (4–6 rpm), spray rate (1.1 g/min), pan load (180 g), operation time (30 min) and curing time (10 min). The curing of the coated tablets was performed with a pan speed of 1 rpm. The targeted weight gain of the tablet after coating was set as 2.2% w/w.
In-vitro release studies
The dissolution tests were performed using a USP type II dissolution testing apparatus to compare the release profile of the manufactured generic products and RLD. The optimization of surfactant and its concentration in dissolution media is essential not only to provide the maximum extent of drug release for quality control testing but also to mimic the in vivo behavior of the dosage forms. 0.4% w/v sodium dodecyl sulfate (SDS) was selected as a surfactant for dissolution studies. The dissolution was performed in three different pH media using 0.1 N HCl, 4.5 acetate buffer, and 6.8 phosphate buffer. The standard media volume of 900 ml was chosen to perform the dissolution studies at the temperature of 37 °C ± 0.5 °C. The apparatus was operated at a rotation speed of 75 rpm. 5 ml were withdrawn at predetermined time intervals (10, 15, 20, 30, and 45 min) and immediately replenished with an equal volume of freshly prepared buffer to maintain sink conditions. The samples were then filtered through 0.45 µm filters and the drug content was determined using the HPLC method described in the USP monograph using an HPLC coupled with a UV detector at a wavelength of 250 nm (USP Rivaroxaban Tablets Monograph Dissolution Test 2 2023). All the dissolution experiments were conducted using six tablets as a sample size.
Physiological-based pharmacokinetic (PBPK) modeling
The PBPK model was developed for establishing virtual bioequivalence using commercially available GastroPlusversion 9.8.3 (Simulation Plus Inc.), with the Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) predictor module, Advanced Compartmental Absorption Transit (ACAT) module, and the PBPK Plus module. The physicochemical properties of the drug substance and clinical PK data were collected from literature using search engines such as Google, Web of Science, and PubMed and the keywords used are “Rivaroxaban”, “Biopharmaceutics”, “physicochemical properties”, “pharmacokinetics”, “PBPK”, and “Clinical pharmacokinetics”. The in-silico physicochemical properties of the drug were predicted using the ADMET Predictor module of GastroPlus. Clinical PK study data were digitized using WebPlotDigitizer version 4.6.
RXB is a carboxamide derivate and has a molecular mass of 435.8. It is classified as a Class II compound (low solubility and high permeability) under the Biopharmaceutical Classification System. RXB has pH-independent low solubility in aqueous buffers (5–7 mg/L; pH 1–9). The apparent Caco2 permeability of the drug at concentrations of 1–100 M was approximately 8 × 10–6 cm/s. RXB has moderate lipophilicity with a log P value of 1.5. RXB is a direct, specific Factor Xa inhibitor. The oral absorption of RXB was rapid with a Tmax of 2–4 h. The oral bioavailability of RXB 10 mg strength was reported as 80–100% and there is no food effect on oral absorption. However, higher dose of 20 mg, the reported fasting and fed oral bioavailability were 66% and 100% respectively. Thus, in fed condition dose-proportional pharmacokinetic linearity was observed across the available clinical doses (Stampfuss et al. 2013). The reported plasma protein binding for RXB is 92–95% using in vitro model. The volume of distribution at steady state is approximately 0.62 l/kg and it reveals that the drug has lower affinity with peripheral tissues. The drug is majorly metabolized by the CYP enzyme and 28% of the dose was excreted via feces. The drug undergoes renal excretion as well. 30% of the drug and 36% of metabolites were excreted in urine. The drug has a high affinity with P-glycoprotein as well as breast cancer-resistant protein, which involves active renal secretion of the drug (Stampfuss et al. 2013). RXB has age-dependent renal clearance and hence, the half-life of the drug depends on age. Young males have a drug half-life of 5–9 h. The drug has moderate inter-individual pharmacokinetic variability of 30–40% (Stampfuss et al. 2013). Biopharmaceutics and pharmacokinetic parameters of RXB are shown in Table 3 of supplement 1.
Initially, the PBPK Plus model was explored to develop whole-body PBPK model development, however, prediction errors were high and hence it was not decided to go with a whole-body PBPK model. The semi-mechanistic PBPK model was developed using the literature pharmacokinetic data. The model consists of an ACAT model for the prediction of gastrointestinal absorption and a best-fit compartmental model for the distribution and elimination process. Seventeen sets of pharmacokinetic data were obtained from six different studies and were used to build the pharmacokinetic models (Table 4 in Supplement 1). Demographic information such as age, body weight, sampling times, and methodology was obtained from the literature (Mueck et al. 2014; Stampfuss et al. 2013).
Intravenous (IV) pharmacokinetic data was used to understand the distribution and elimination parameters. IV PK data were used to select suitable PK and PBPK models using Gastroplus 9.8.3 software. Based on best fit and prediction errors, the optimized model was selected. The selected model was then used for the development of the oral absorption model. The influence of tablet disintegration on pharmacokinetics was evaluated using the oral suspension pharmacokinetic model. Food effect was computed with fasting and fed study data of oral tablets. Dose linear prediction in pharmacokinetics was assessed using dose-ranging studies data of oral tablets. The developed model was validated retrospectively for their suitability to conduct virtual BE trials of the developed test products using different granulation techniques.
Virtual bioequivalence (VBE) trials to investigate the effect of granulation process
The developed and retrospectively validated PBPK model was used for VBE trials of the proposed test formulations (20 mg strength) using Xarelto 20 mg IR tablets as an RLD. The dissolution data generated for all formulations were generated using test and RLD products. The presence of a high concentration of surfactant in dissolution media resulted in faster release profiles. The generated dissolution profiles did not show fasting bio-relevancy due to high surfactant concentration. Moreover, building the model using particle size data and solubility data for IR products results in better predictability. Saturation solubility data of granules were integrated with oral PBPK model data and used to build the PBBM model for test products. Moreover, The patient demographics and other input parameters for these models were taken from the published literature (Stampfuss et al. 2013; Kubitza et al. 2007). The developed model was then validated by using PK data of Xarelto 20 mg IR tablets. Seven VBE trials were conducted to estimate the in vivo PK and BE of test formulations. Each trial was designed in two periods, two sequences, two treatments, randomized, single-dose, cross-over, VBE simulations with PK endpoints in healthy male subjects under fasting conditions. The sensitivity for determining the formulation differences was accessed by comparing the PK parameters such as Cmax, AUC0–t, and AUCi. The 90% confidence intervals for geometric mean ratios of test and reference are between 80 and 125%. Table 3 summarizes the details of the test product.
Table 3.
Details of test products for VBE trials
| Trial ID | Batch no. | Granulation type | Granulation fluid |
|---|---|---|---|
| 1 | BXJJF71 | Xarelto IR | – |
| 2 | LS-AH | Low shear | Alcoholic |
| 3 | HS-AH | High shear | Alcoholic |
| 4 | LS-HA | Low shear | Hydroalcoholic |
| 5 | LS-AQ | Low shear | Aqueous |
Male subjects; crossover design; n = 23
Results and discussion
Solubility studies
Table 4 shows the results of RXB solubility studies at 25 °C. The % relative standard deviation of each buffer was found to be < 10%. The pH of the buffers was monitored before and after the solubility experiments. The reported solubility of RXB is 7–9 µg/ml in the pH range of 1–9 (Mueck et al. 2014). The solubility values obtained from the literature are similar to those of the values reported in the literature, except 0.1 N HCl. In silico pKa values of RXB are − 1.6 (base) and 13.1 (acid). The probable reason could be the partial ionization of the molecule at pH 1.2 and subsequent solubilization of the drug. However, based on the biopharmaceutical classification system, the highest strength of 20 mg required a drug solubility of 80 µg/ml to qualify for high solubility criteria. The obtained solubility values are lower than the cut-off value, hence the drug is classified as a low-soluble drug based on the biopharmaceutical classification system. The drug bioavailability of low-soluble drugs is based on the particle, size, surface area, and polymorphic behaviors, hence optimizing and monitoring these parameters are critical to attain the drug product quality.
Table 4.
pH-dependent solubility data of RXB
| Solvent | pH -initial | pH-end | Solubility (µg/ml)* |
|---|---|---|---|
| 0.1N HCl | 1.23 | 1.24 | 18.54 |
| pH 4.50 acetate buffer | 4.50 | 4.55 | 9.74 |
| Water | 6.75 | 5.72 | 7.52 |
| pH 6.80 phosphate buffer | 6.80 | 6.85 | 8.21 |
| pH 7.40 phosphate buffer | 7.41 | 7.43 | 8.42 |
*Mean of n = 6; %RSD < 6.0%
Four batches of granules (LS-AH, HS-AH, LS-HA, and LS-AQ) and reference formulation were selected for saturation solubility studies. Saturation solubility studies were carried out in pH 6.8 phosphate buffer to understand the influence of the granulation process on the rate and extent of solubilization of RXB. The highest solubility of 2.09 ± 0.11 µg/ml was observed with LS-AH batch, whereas the solubilities for HS-AH, LS-HA, and LS-AQ batches were 1.54 ± 0.07, 1.46 ± 0.07, and 1.05 ± 0.07 µg/ml, respectively. The solubility of RLD granules was observed as 2.21 ± 0.18 µg/ml. The bulk density of the granules could be correlated with fine particle size and solubility. The highest solubility of the LS-AH batch was well correlated with the lowest bulk density of 0.33 g/ml and 40.2% of fine particles. Similarly, the relatively high bulk density (0.55 g/ml) and 18.9% fine particles of the LS-AQ batch resulted in the lowest solubility of 1.05 ± 0.07 µg/ml. However, the results obtained from granule characterization studies have not revealed any firm conclusion for selecting granulation methodology for further development. Hence, all these four batches were compressed as tablets to understand the impact of granulation on dissolution behavior.
Quality target product profile (QTPP) synthesis
QTPP was synthesized for screening and selection of proof of concept batches. In addition, scaleup linearity assessments were done based on the QTTP metrics. Table 5 in Supplement 1 enumerates the QTPP elements and targets for the RXB test product.
Manufacturing of the generic product
Wet granulation process and granule characterization
Table 5 represents the characterization of granules. Wet granulation was carried out using high-shear granulation and low-shear granulation techniques. The process parameters were kept constant, to understand the effect of granulation fluid on granules properties as well as dissolution behavior. The physical, mechanical, and dissolution profiles of the granules were based on the choice of binder, solvents, and granulation process (Holm et al. 2001; Alshihabi et al. 2013). RXB has poor solubility in both water and ethanol and hence the solubilization of the drug and formation of in-situ solid dispersion with HPMC is quite impossible, during the granulation process (data on file). Moreover, the solid content of HPMC in the matrix is 2%, which is inadequate for solubility enhancement. High shear aqueous granulation (Batch No: HS-AQ) resulted in hard granules with high bulk density as well as high particle size. This batch resulted in a higher disintegration time of 14.45 min, hence it was not chosen for further development. The relatively higher particle size of the granules reduces the dissolution of RXB due to slow disintegration, poor wettability, and low porosity. In contrast, low-shear aqueous granulation (Batch No: LS-AQ) resulted in high bulk density and 10% more fine particles (45.4% vs 35.8% of D250µ). This could be linked to low-level granulation efficiency and saturation of granulation fluid, which is a higher level with high shear granulation. In the case of hydroalcoholic granulation, the effect of the granulation process (high shear vs low shear) has minimal impact on granulation parameters such as particle size, bulk density, flow properties, and compressibility. Low-shear alcoholic granulation resulted in finer particles (92.4% of D75µ) and also provided similar granule parameters. RXB exhibits pH-independent poor solubility across the physiological pH range and hence the intestinal re-precipitation of the drug has no impact on the bioavailability. However, finer granule size reduces the time for de-aggregation and enhances the drug dissolution (Thapa et al. 2019). It is therefore to be concluded that low-shear alcoholic granules may provide better drug dissolution when compared with the rest of the granulation batches.
Table 5.
Characterization of granules
| Parameters | HS-AQ | LS-AQ | HS-HA | LS-HA | HS-AH | LS-AH |
|---|---|---|---|---|---|---|
| Size distribution (µ) | Dried granules: cumulative % retained (n = 1) | |||||
| 500 | 1.8 | 1.4 | 1.4 | 1.3 | 0.8 | 0.2 |
| 250 | 45.4 | 35.8 | 35.4 | 29.8 | 28.4 | 9.5 |
| 150 | 70.2 | 67.0 | 65.2 | 61.9 | 55.2 | 52.2 |
| 75 | 89.1 | 86.1 | 88.1 | 81.5 | 78.1 | 92.4 |
| Bottom pan | ~ 100 | ~ 100 | ~ 100 | ~ 100 | ~ 100 | ~ 100 |
| Lubricated granules: average values (n = 3); %RSD < 2 | ||||||
| Bulk density (g/ml) | 0.59 | 0.55 | 0.56 | 0.49 | 0.51 | 0.33 |
| Tapped density (g/ml) | 0.72 | 0.66 | 0.69 | 0.61 | 0.63 | 0.40 |
| Hausner ratio | 1.22 | 1.20 | 1.23 | 1.24 | 1.23 | 1.21 |
| Flow | Fair | Fair | Fair | Fair | Fair | Fair |
| Carr’s index | 15.7 | 16.66 | 18.84 | 19.67 | 19.05 | 17.5 |
| Compressibility | Good | Fair | Fair | Fair | Fair | Fair |
Characterization of uncoated tablets and coated tablets
The average weight of the tablets was found between 90.4 to 91.1 mg. All the manufactured batches passed the weight variation test with the limit of 90.0 mg ± 10.0%. The flow properties of the granules were fair with all the manufactured granules and hence there were no issues found with weight variation. The hardness of the tablets was found in the range of 2.06–5.30 Kp which was acceptable and comparable with RLD. The disintegration time of all the manufactured batches was well within the limit of 15 min. High shear aqueous granulation (Batch No: HS-AQ) resulted in hard granules and excellent compressibility. However, the disintegration time was 14.45 min, which has a high risk of failure during commercial batch manufacturing. Hence, this batch is excluded from further characterizations. All batches exhibited excellent friability that can withstand the abrasions. In-process quality control parameters of uncoated tablets are presented in Table 6. The pharmaceutical properties of manufactured tablets were well within the limit of regulatory requirements and hence could be subjected to dissolution studies.
Table 6.
In-process quality control parameters of the uncoated tablets and coated tablets
| Tablet parameters | Target* | HS-AQ | LS-AQ | HS-HA | LS-HA | HS-AH | LS-AH |
|---|---|---|---|---|---|---|---|
| Uncoated tablets | |||||||
| Average weight (mg) | 90.0 ± 2.7 | 90.6 | 90.4 | 91.1 | 90.9 | 91.0 | 90.5 |
| % weight variation | 90 ± 10.0 | 88.9–94.2 | 89.8–93.2 | 89.3–94.1 | 89.0–94.2 | 89.1–92.8 | 89.3–93.6 |
| Hardness (Kp) | 2–6 | 2.11–4.90 | 2.06–5.30 | 2.28–3.19 | 2.15–3.86 | 2.08–3.90 | 2.06–4.31 |
| Thickness (mm) | 3.0–3.6 | 3.40–3.50 | 3.42–3.56 | 3.10–3.49 | 3.38–3.50 | 3.33–3.46 | 3.33–3.47 |
| DT (min)** | NMT 15 | 14.45 | 12.00 | 8.30 | 7.15 | 4.00 | 1.0 |
| Friability (%) | NMT 1.0 | 0.00 | 0.00 | 0.05 | 0.01 | 0.01 | 0.01 |
| Coated tablets | |||||||
| Average weight (mg) | 92.0 ± 2.76 | 91.8 | 92.4 | 90.8 | 93.0 | 92.8 | 90.7 |
| % weight variation | 90 ± 10.0 | 86.4–96.1 | 90.8–95.5 | 86.4–91.9 | 91.0–96.2 | 91.2–93.7 | 88.4–91.3 |
| Hardness (Kp)*** | – | NA | NA | NA | NA | NA | NA |
| Thickness (mm) | 3.0–3.6 | 3.46–3.52 | 3.51–3.62 | 3.07–3.38 | 3.34–3.55 | 3.27–3.40 | 3.29–3.42 |
| DT (min)** | NMT 15 | 14.45 | 12.00 | 8.30 | 7.15 | 4.00 | 1.0 |
| Friability (%)*** | – | NA | NA | NA | NA | NA | NA |
HS-AQ, high shear, water granulation; LS-AQ, low shear, water granulation; HS-HA, high shear, hydroalcoholic granulation; LS-HA, low shear, hydroalcoholic granulation; HS-AH, high shear, alcoholic granulation; LS-AH, low shear, alcoholic granulation
*Sample size and statistical parameters are based on regulatory requirements and are well within the limit
**Coated tablets
***Not applicable
In-vitro release studies of coated tablets
RXB tablets are official in the United States Pharmacopeia (US Pharmacopeia 29, n.d., 2024). The compendia dissolution method consists of sodium dodecyl sulfate (SDS) as a surfactant to maintain the sink condition. 0.2% SDS is used as a wetting agent and solubilizer for lower-strength tablets (5 mg and 10 mg), whereas 0.4% for higher-strength tablets (15 mg and 20 mg). This is due to maintaining the sink conditions for a complete release profile. Dissolution studies were carried out at different pH such as 1.2 (0.1 N HCl), 4.5 (acetate buffer 50 mM), and 6.8 pH (phosphate buffer 50 mM). As stated in the pharmacopeia, 0.4% of SDS was used as a surfactant. Based on the primary pharmaco-technical parameters four test batches (such as LS-AH, HS-AH, LS-HA, and LS-AQ) were selected for comparative dissolution studies. Xarelto IR tablets 20 mg (Batch No: BXJJF71) was chosen as a reference product. The specified dissolution (Q%) point in pharmacopeia is 15 min for 20 mg strength and the % drug release was > 85% (Q + 5%). The dissolution efficacy at 15 min for the reference product was > 85% in all three pH media. Hence, the reference product complies with pharmacopeial requirements. Except for the LS-AH batch, all the batches failed to meet the dissolution criteria. The f2 metric is not required for the very rapid dissolution profiles (both test and reference product dissolution > 85% in < 15 min). The comparison of the f2 metric value might be useful to understand the sameness between the test and reference product. However, three test batches failed to meet the criteria of rapid dissolution profile, and hence f2 metric was applied for three test batches (HS-AH, LS-HA, and LS-AQ). All three test products (HS-AH, LS-HA, and LS-AQ) failed to meet the criteria of f2 metrics. Interestingly, the results obtained from saturation solubility studies of granules were well correlated with the dissolution behavior of the formulations. Virtual BE studies were carried out to understand the influence of solubility and dissolution behavior of the test formulation on pharmacokinetics.
PBPK modeling
PBPK modeling for RXB is widely reported in the literature. The objectives of these developed models are drug-drug interaction, drug-disease interaction, optimization of dose for renal and hepatic impairments, P-Glycoprotein efflux transport, pediatric dosing regimen, pediatric formulation development, and dose titrations in clinical settings (Cheong et al. 2022; Ngo et al. 2022; Terrier et al. 2023; Willmann et al. 2021, 2022). Most of the developed PBPK models are complex and are whole-body PBPK models. Semi-mechanistic PBPK models are easy to develop and validate and could be useful for formulation development, process optimizations, scale-up, and post-approval changes. The Advanced Compartmental Absorption Transit (ACAT) model is a widely used mechanistic gastrointestinal model, which is frequently used for predicting the absorption behavior of drug substances and drug products. The distribution and elimination kinetics could be derived from the compartmental pharmacokinetic models. The combination of mechanistic absorption models (e.g. ACTA) and empirical disposition models (e.g. compartmental PK models), also known as semi-mechanistic models, shall be useful for the development of dosage forms and prediction of pharmacokinetic parameters. For instance, a semi-mechanistic model for the prediction of the food effect of RXB is reported in the literature. However, this model utilized an immediate-release oral solution dosage form (10 mg) for predicting the disposition rate constants. RXB is a poorly soluble drug, when administered in solution dosage form, it may have a chance of gastrointestinal precipitation. The absorption process of RXB may influence the disposition kinetics, and hence, the present study aimed to attempt the development of a disposition compartment model using IV pharmacokinetic data.
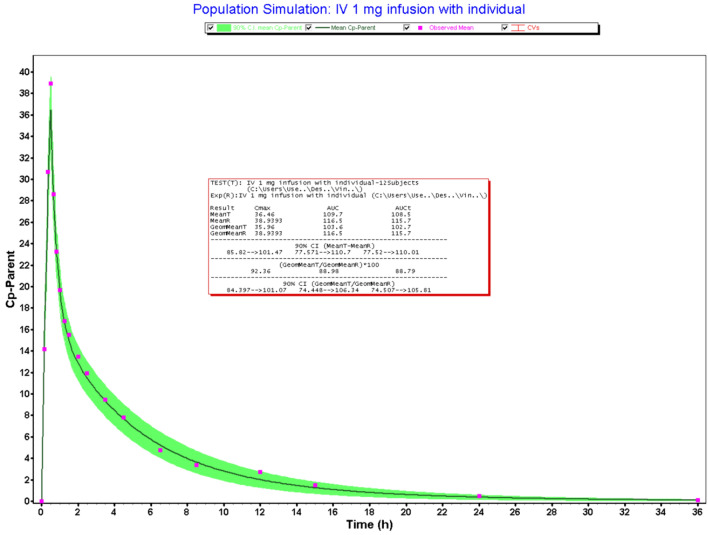
Based on the literature search, no attempt has been made to develop the semi-mechanistic model using IV pharmacokinetic data (Stampfuss et al. 2013a; Kubitza et al. 2007). Hence, it was decided to build the model using IV pharmacokinetic data. IV infusion data was obtained from the literature and used to build the compartmental pharmacokinetic model. The three-compartment pharmacokinetic model was found to be the best-fit model for RXB IV pharmacokinetics and hence it was used for population simulations using 12 subjects. The demographic parameters of the subjects for population simulations were kept similar to those of the published literature. The power of the study was not reported in the literature. The PK parameters of three compartmental model fittings for RXB IV infusion are 8.72 l/h (CL), 0.218 l/kg (Vc), 1.55 l/h (K12), 1.6 l/h (K21), 0.211 l/kg (V2), 0.31 l/h (K13), 0.30 l/h (K13), and 0.23 l/kg (V3). The 26% CV for Cmax and 34% for AUCi were observed after 30-min IV infusion administration of RXB in 12 healthy male subjects (Stampfuss et al. 2013). The predicted r square for the three-compartmental model was 0.9927 with Akaike information criterion (AIC) value of 90.86. This is due to the higher % CV and inadequate sample size. The prediction errors for Cmax and AUC0–i were − 21.6% and − 19.0%. Although prediction errors fall outside the regulatory requirements. Moreover, plot-digitized data from individual graphs resulted in the % prediction errors of less than 10%, which was well within the limit. Based on the %CV and sample size, this study might be underpowered to estimate the true mean values for pharmacokinetic parameters such as Cmax and AUC0-i. Hence, the developed and validated IV pharmacokinetic model was further used to build the oral pharmacokinetic model. Figure 1 represents the plasma drug concentration–time profile of the IV infusion population pharmacokinetics. Model validation and prediction errors are shown in Table 7.
Fig. 1.
Population simulation of IV infusion pharmacokinetics
Table 7.
Population simulation of RXB pharmacokinetic data
| PK metrics | Sample size (n) | Dose: 1 mg; IV infusion 30 min | Dose: 5 mg; oral | Dose: 10 mg; oral tablet | Dose: 20 mg; oral tablet | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Pred | % PE | Obs | Pred | % PE | Obs | Pred | % PE | Obs | Pred | % PE | ||
| Cmax (µg/ml) | 12 | 29.5 | 35.9 | − 21.6 | |||||||||
| 23 | 80 | 74.2 | 7.5 | 136 | 117 | 13.8 | 160 | 126 | 21.4 | ||||
| 6 | 72 | 74 | 2.8 | 141 | 132 | 6.6 | 173 | 142 | 17.9 | ||||
| 24/10* | 184 | 121 | 34.4 | 158 | 126 | 20.1 | |||||||
| 24 | 105 | 119 | − 13.3 | 136 | 124 | 21.5 | |||||||
| 24$ | 93 | 105 | − 12.9 | 111 | 133 | − 20.9 | |||||||
| 111**/97* | 128 | 118 | 7.8 | 146 | 130 | 10.7 | |||||||
| AUC0–i (µg.h/ml) | 12 | 87 | 103.6 | − 19.0 | |||||||||
| 23 | 520 | 561 | − 7.9 | 1223 | 1053 | 13.9 | 1477 | 1629 | − 10.2 | ||||
| 6 | 466 | 520 | − 10.4 | 1020 | 1184 | − 16.0 | 1612 | 1700 | − 5.5 | ||||
| 24/10* | 1234 | 1077 | 12.7 | 1629 | 1661 | 0.5 | |||||||
| 24 | 954 | 1136 | − 16.0 | 1223 | 1616 | − 32.1 | |||||||
| 24$ | 883 | 1077 | − 18.0 | 1313 | 1613 | − 22.8 | |||||||
| 111**/97* | 1053 | 1104 | − 4.8 | 1442 | 1643 | − 13.9 | |||||||
*n = 10 for 20 mg
$Oral suspension; the mean age and body weight of the above groups are 31.5–43.0 years and 78.6–84.9 kg and hence, insignificant impact on PK outputs
**Pooled data
The oral PBPK model was developed using three different doses such as 5 mg, 10 mg, and 20 mg (Fig. 5–7 in supplement 1). One of the challenges in oral pharmacokinetic model development is inter-study variability. The study to study variability in weight-normalized pharmacokinetic data in men resulted in prediction errors (Stampfuss et al. 2013a). The oral PBPK model was developed using a validated IV pharmacokinetic model. In-vivo drug absorption was computed using both dissolution data and saturation solubility data of lubricated granules. The dissolution media consists of 0.4% SDS and hence it was not able to get the desired reliable model. Hence, saturation solubility data obtained from the granules was computed to build the model. The population simulations pharmacokinetic studies were carried out for 5 mg, 10 mg, and 20 mg tablets. As expected, the prediction errors of the developed models were not well within the limit. However, the pooled pharmacokinetic data analysis (n = 111 for 10 mg and 97 for 20 mg) revealed that the developed model has acceptable prediction errors for both Cmax and AUCi. There is a strong correlation with regression of 0.989 between dissolution data and saturation solubility data obtained from 0.1 N HCl with 0.5% SLS and hence, multi-media dissolution data has an in-vitro in-vivo relationship, but not exact correlations. However, the impact of the granulation process on bioavailability could be predicted using this model. Hence, VBE studies were conducted to understand the impact of the granulation process on the bioavailability of RXB. Table 7 summarizes the population simulation results of RXB.
Virtual bioequivalence trials
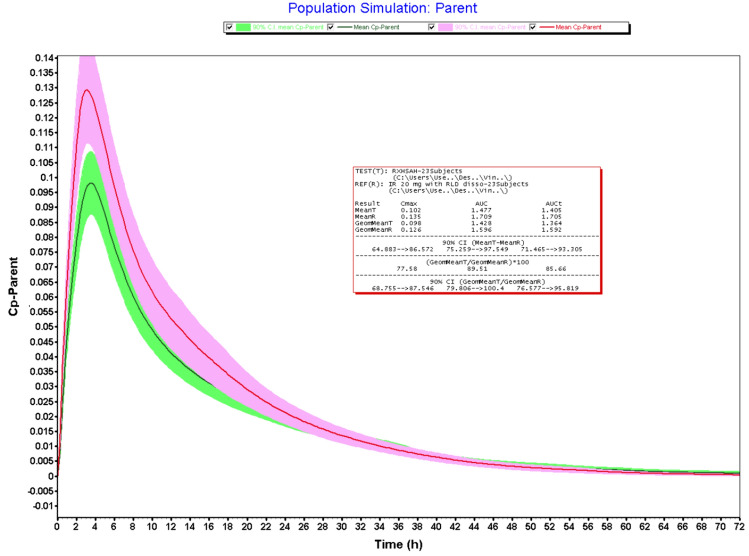
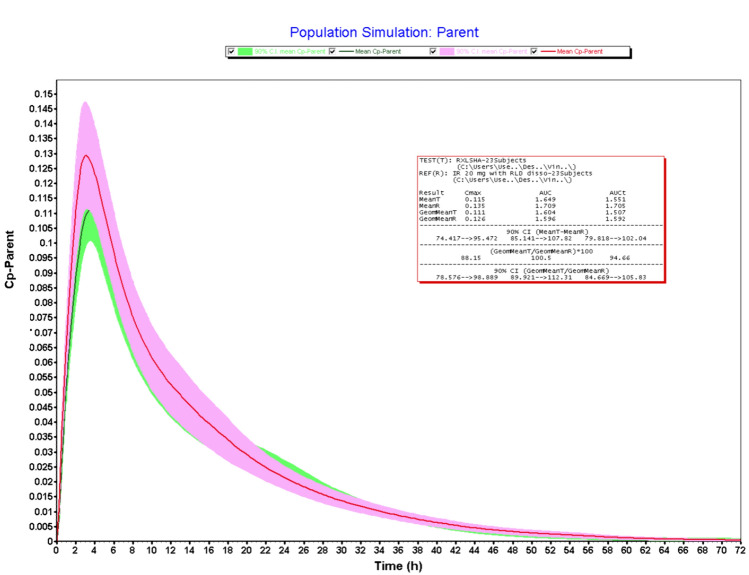
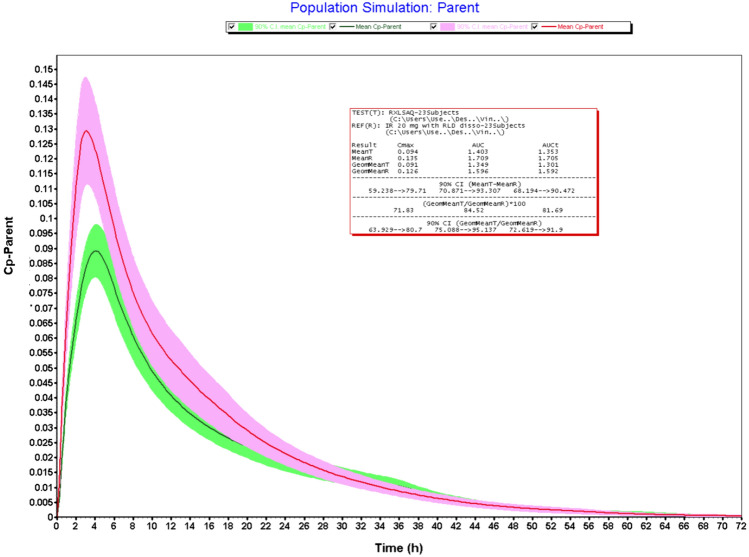
Figures 2, 3, 4 and 5 illustrates the results obtained from VBE trials. The bioequivalence studies were conducted using four test formulations (20 mg IR tablets) such as LS-AH, HS-AH, LS-HA, and LS-AQ against Xarelto IR tablets 20 mg (Batch No: BXJJF71) as reference product. Validated IV and oral pharmacokinetic models were used to conduct the VBE studies. The submission of abbreviated new drug applications requires bioequivalence studies using RLD as a reference product. Granulation is a critical process, that impacts the dissolution and bioequivalence of RXB tablets. To mitigate the bioequivalence study failure, saturation solubility of granules and dissolution studies of the finished dosage forms were performed. Both studies indicated the impact of granulation on the biopharmaceutical performance of drug products. These studies could be useful for process discrimination and shall be used for process optimization trials to establish the similarity between test and RLD products. However, the in-vivo relevance of these studies to predict the pharmacokinetic profile of the product remains unanswered. The objective of the VBE trials is to establish the bio-relevance of the saturation solubility data and dissolution data. Dissolution study results revealed that > 85% of the drug was released within 15 min. However, the absolute bioavailability of RXB 20 mg tablets was reported as 66% and is due to the presence of a high concentration of the surfactant in dissolution media. Hence, logically it is not right to use the dissolution study data for computing the VBE trials. Therefore VBE trials were conducted using saturation solubility data obtained from granules.
Fig. 2.
VBE of test (LS-AH) vs RLD (n = 23)
Fig. 3.
VBE of test (HS-AH) vs RLD (n = 23)
Fig. 4.
VBE of test (LS-HA) vs RLD (n = 23)
Fig. 5.
VBE of test (LS-AQ) vs RLD (n = 23)
The geometric mean T/R ratios of the LS-AH batch were found to be 100.7% and 100.5% for Cmax and AUCi, respectively. The 90% confidence intervals were well within the limit of 80–125%. Hence this batch meets the bioequivalence criteria with reference products. The LS-AH batch also meets the criteria for dissolution studies and the pharmaceutical properties of this batch were well within the regulatory limit and could be dosed for clinical bioequivalence study. The LS-AH batch failed in Cmax with a lower confidence interval of 78.56%. The VBE trials were carried out using 23 subjects, if the study powered with adequate sample size, this batch may qualify for bioequivalence criteria. However, the batches such as LS-AQ and HS-AH failed in both Cmax and AUCi. The results obtained from VBE trials indicated that saturation solubility of granules is useful for predicting the bio-equivalence of the RXB test formulations and could be useful for granulation process optimization, submission, and post-approval life cycle management. Dissolution data could be useful as a bio-discriminatory method because there is a correlation between saturation solubility data and dissolution data. The present exercise of VBE trials helped not only with granulation process optimization but also derisked the bioequivalence failure. Moreover, the VBE trial outcomes were useful for the rationale selection of the investigation product for dosing bioequivalence trials, minimized the clinical failure rate, and reduced the cost of generic product development. In addition, VBE trials provided the guidelines for subject selection, sample size, and clinical protocol synthesis.
Conclusion
The present study aimed to optimize the wet granulation process for the development of a generic RXB 20 mg tablet using the PBPK model. The wet granulation process was found to be a critical unit operation for establishing the dissolution similarity and bioequivalence with RLD. The granulation techniques such as high-shear granulation (RMG) and low-shear (FBP) were used to understand the influence of the granulation process on solubility and dissolution behavior. Three different solvent systems such as water, ethanol, and hydroalcoholic solution were selected for the granulation trials. The prototype screening resulted in four optimized test batches such as LS-AH, HS-AH, LS-HA, and LS-AQ. These batches were subjected to saturation solubility studies, dissolution studies, and VBE studies. The VBE results indicated that the LS-AH batch was found to be bioequivalent with RLD. Formulation discriminatory dissolution studies were performed to understand the impact of granulation on the rate and extent of drug dissolution. However, the extent of dissolution was lower than the reported absolute bioavailability of RXB tablets. Hence, the developed model using dissolution data underpredicted the PK parameters. Saturation solubility studies of granules with a 1 h sampling point provided a better correlation. Moreover, the model built with saturation solubility data also provided the in vivo bile effect on the absorption of RXB. The use of drug solubility data for developing the PBPK model has already been reported in the literature. However, the use of solubility data from tablets for predicting PK parameters has already been reported in a few publications. The present investigation attempted to develop the PBPK model using saturation solubility of granules and the results were encouraging to use these data for building PBPK models. The proposed exercise was useful for finding the right bio-relevant tool for generic product development, selecting the appropriate batch for bioequivalence studies, and minimizing the risk of clinical failures.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the support of the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India.
Author contributions
Jailani. S: formulation development and analytical characterization; Tanisha: drafting and PBPK model building; Sonam Sharma: literature search, PBPK model validation; Kishor Chakraborty: supervision and formulation strategies; C.K. Dhanapal: conceptualization and designing of the project; Noohu Abdulla Khan: clinical data analytics; Rajkumar Malayandi: design and supervision of biopharmaceutics and PBPK model, writing original draft preparation, and editing.
Funding
The author (s) reported there is no funding associated with the work featured in this article.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Administrable O (2018) (12) United States Patent (45) Date of Patent: 2(12)
- Alshihabi F, Vandamme T, Betz G (2013) Focused beam reflectance method as an innovative (PAT) tool to monitor in-line granulation process in fluidized bed. Pharm Dev Technol 18(1):73–84. 10.3109/10837450.2011.627868 10.3109/10837450.2011.627868 [DOI] [PubMed] [Google Scholar]
- Cheong EJY, Ng DZW, Chin SY, Wang Z, Chan ECY (2022) Application of a physiologically based pharmacokinetic model of rivaroxaban to prospective simulations of drug–drug–disease interactions with protein kinase inhibitors in cancer-associated venous thromboembolism. Br J Clin Pharmacol 88(5):2267–2283. 10.1111/bcp.15158 10.1111/bcp.15158 [DOI] [PubMed] [Google Scholar]
- FDA & CDER (2011) Highlights of prescribing information. http://www.fda.gov/medwatch
- Giry K, Viana M, Genty M, Louvet F, Wthrich P, Chulia D (2009) Comparison of single pot and multiphase granulation. Part 2: effect of the drying process on granules manufactured in a single pot granulator and dried either in situ or in a fluid bed dryer. Pharm Dev Technol 14(2):149–158. 10.1080/10837450802588942 10.1080/10837450802588942 [DOI] [PubMed] [Google Scholar]
- Holm P, Schaefer T, Larsen C (2001) End-point detection in a wet granulation process. Pharm Dev Technol 6(2):181–192. 10.1081/PDT-100000739 10.1081/PDT-100000739 [DOI] [PubMed] [Google Scholar]
- Jailani S, Dhanapal CK, Khan NA (2023a) Decoding of API particle size in reference market product for bio-equivalent and cost effective generic product development. J Popul Ther Clin Pharmacol 30(15):144–151. 10.47750/JPTCP.2023.30.15.016 10.47750/JPTCP.2023.30.15.016 [DOI] [Google Scholar]
- Jailani S, Dhanapal CK, Khan NA (2023b) Comparative characterization of reference market product and generic product developed using reverse engineering methodology. Int J Pharm Qual Assur 14(4):987–992. 10.25258/ijpqa.14.4.26 10.25258/ijpqa.14.4.26 [DOI] [Google Scholar]
- Kubitza D, Becka M, Mueck W, Zuehlsdorf M (2007) Rivaroxaban (BAY 59–7939)—an oral, direct factor Xa inhibitor—has no clinically relevant interaction with naproxen. Br J Clin Pharmacol 63(4):469–476. 10.1111/j.1365-2125.2006.02776.x 10.1111/j.1365-2125.2006.02776.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Jeong HS, Jeong J, Koo T, Kim D, Cho YH, Lee GW (2021) The development and optimization of hot-melt extruded amorphous solid dispersions containing rivaroxaban in combination with polymers [DOI] [PMC free article] [PubMed]
- Metre S, Mukesh S, Samal SK, Chand M, Sangamwar AT (2018) Enhanced biopharmaceutical performance of rivaroxaban through polymeric amorphous solid dispersion. 10.1021/acs.molpharmaceut.7b01027 [DOI] [PubMed]
- Mueck W, Stampfuss J, Kubitza D, Becka M (2014) Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet 53(1):1–16. 10.1007/s40262-013-0100-7 10.1007/s40262-013-0100-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadendla RR, Juluri S, Doppalapudi SSS, Pyda VH, Tadiboina A, Addanki V, Kalluri S (2021) Physico-chemical characterization of rivaroxaban and compatibility studies with its pharmaceutical excipients. Int J Life Sci Pharma Res 11(4):25–32. 10.22376/ijpbs/lpr.2021.11.3.p25-p32 10.22376/ijpbs/lpr.2021.11.3.p25-p32 [DOI] [Google Scholar]
- Ngo LT, Yang S, Yang S, Shin S, Cao DT, Van Nguyen H, Jung S, Lee JY, Lee JH, Yun H, Yun H, Chae JW (2022) Application of physiologically-based pharmacokinetic model approach to predict pharmacokinetics and drug–drug interaction of rivaroxaban: a case study of rivaroxaban and carbamazepine. CPT Pharmacometr Syst Pharmacol 11(11):1430–1442. 10.1002/psp4.12844 10.1002/psp4.12844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, Tao J, Chaudhury A, Ramachandran R, Gao JZ, Bindra DS (2013) combined experimental and modeling approach to study the effects of high-shear wet granulation process parameters on granule characteristics. 18(May 2012):210–224. 10.3109/10837450.2012.700933 [DOI] [PubMed]
- Public Assessment Report Scientific discussion Rivaroxaban (2020) Richter film-coated tablets rivaroxaban IS/H/0423/001–005/DC. August, 1–8
- Shanmugam S (2015) Granulation techniques and technologies: recent progresses. BioImpacts 5(1):55. 10.15171/BI.2015.04 10.15171/BI.2015.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfuss J, Kubitza D, Becka M, Mueck W (2013) The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther 51(7):549–561. 10.5414/CP201812 10.5414/CP201812 [DOI] [PubMed] [Google Scholar]
- Terrier J, Gaspar F, Gosselin P, Raboud O, Lenoir C, Rollason V, Csajka C, Samer C, Fontana P, Daali Y, Reny JL (2023) Apixaban and rivaroxaban’s physiologically-based pharmacokinetic model validation in hospitalized patients: a first step for larger use of a priori modeling approach at bed side. CPT Pharmacometr Syst Pharmacol 12(12):1872–1883. 10.1002/psp4.13036 10.1002/psp4.13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa P, Choi DH, Kim MS, Jeong SH (2019) Effects of granulation process variables on the physical properties of dosage forms by combination of experimental design and principal component analysis. Asian J Pharm Sci 14(3):287. 10.1016/J.AJPS.2018.08.006 10.1016/J.AJPS.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo T, Dobesh PP (2014) Clinical use of rivaroxaban : pharmacokinetic and pharmacodynamic rationale for dosing regimens in different indications factor Xa factor IIa factor III factor IIIa fibrinogen fibrin. 1587–1603. 10.1007/s40265-014-0278-5 [DOI] [PMC free article] [PubMed]
- US Pharmacopeia 29. (n.d.)
- USP Rivaroxaban Tablets Monograph Dissolution Test 2 (2023) https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/rivaroxaban-tabs-pmp-ncr-20230929.pdf
- Willmann S, Coboeken K, Zhang Y, Mayer H, Ince I, Mesic E, Thelen K, Kubitza D, Lensing AWA, Yang H, Zhu P, Mück W, Drenth HJ, Lippert J (2021) Population pharmacokinetic analysis of rivaroxaban in children and comparison to prospective physiologically-based pharmacokinetic predictions. CPT Pharmacometr Syst Pharmacol 10(10):1195–1207. 10.1002/psp4.12688 10.1002/psp4.12688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann S, Ince I, Ahsman M, Coboeken K, Zhang Y, Thelen K, Kubitza D, Zannikos P, Zhou W, Pina LM, Post T, Lippert J (2022) Model-informed bridging of rivaroxaban doses for thromboprophylaxis in pediatric patients aged 9 years and older with congenital heart disease. CPT Pharmacometr Syst Pharmacol 11(8):1111–1121. 10.1002/psp4.12830 10.1002/psp4.12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.