Abstract
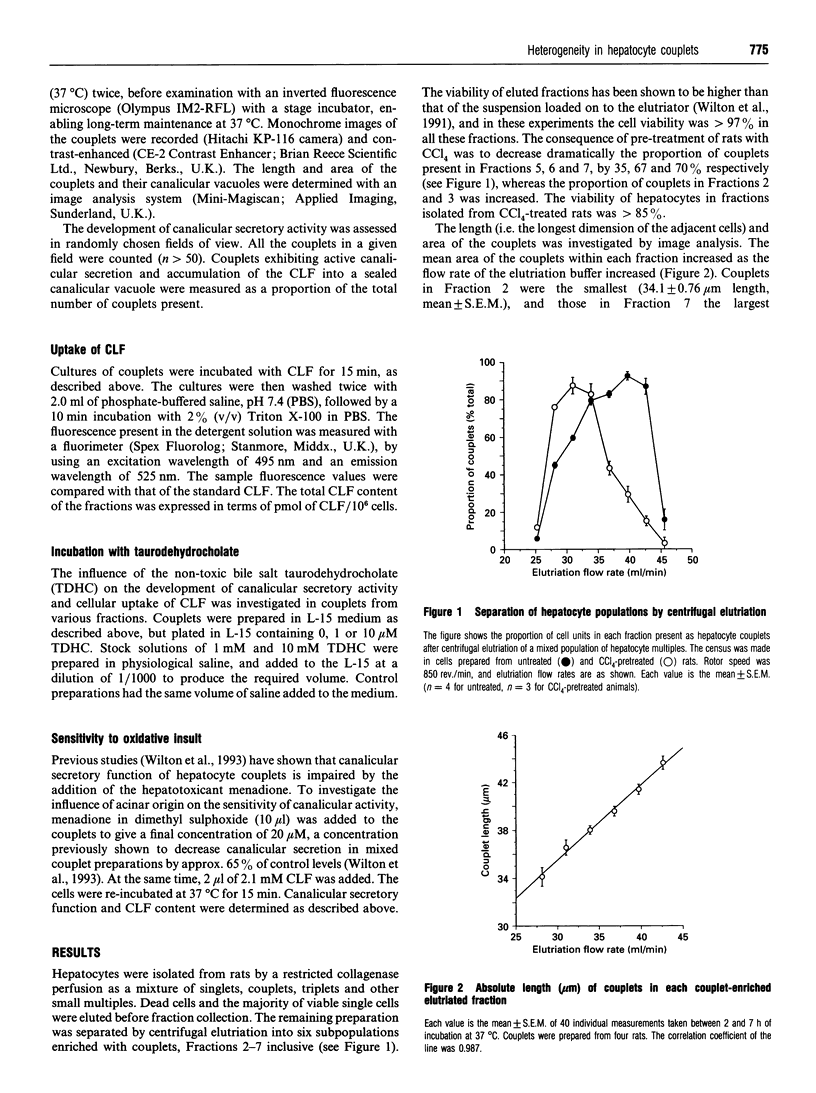
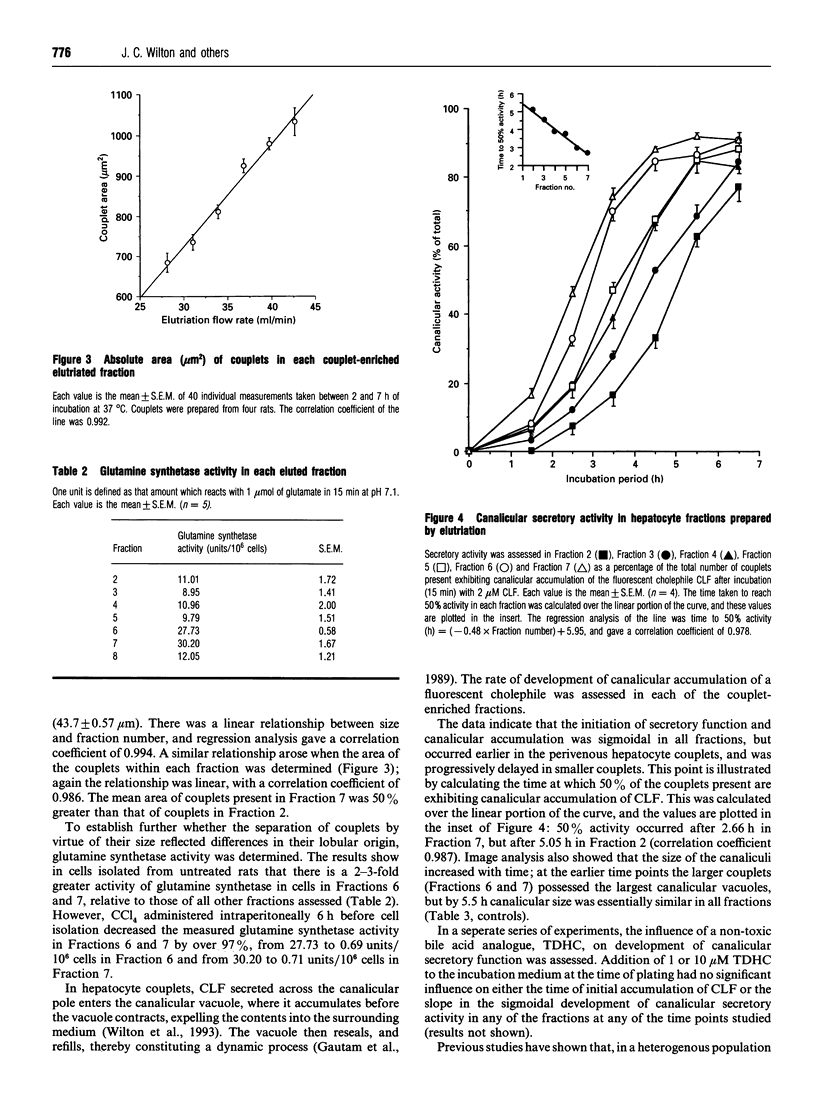
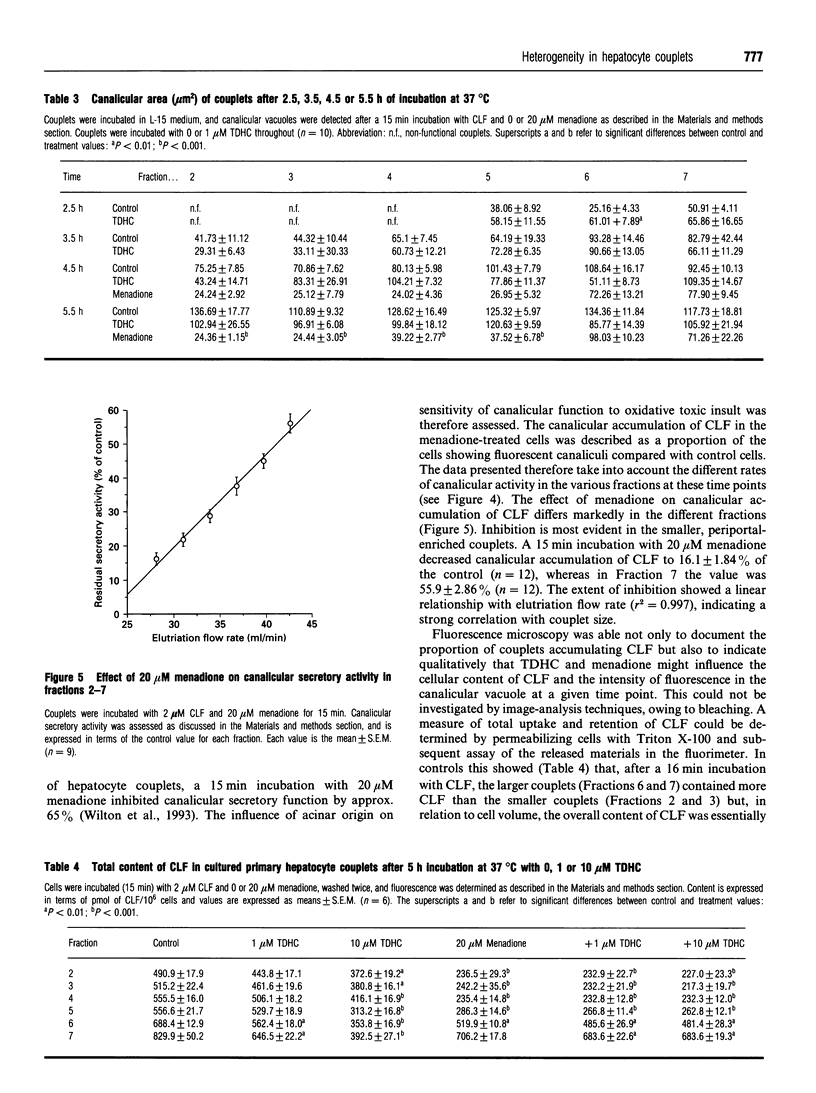
Unlike isolated single hepatocytes, hepatocyte couplets retain their apical polarity, and, during short-term culture form an enclosed canalicular space or vacuole between the two adjacent cells into which biliary secretion is initiated. Hepatocyte couplets were prepared after partial collagenase perfusion of rat liver. Centrifugal elutriation was used to fractionate the preparation into six couplet-containing suspensions. Image analysis was used to determine the size of cultured couplets. The size of the couplets ranged from 34.1 +/- 0.76 microns and 684 +/- 24.1 microns 2 (mean length and area respectively +/- S.E.M.) in Fraction 2, to 43.7 +/- 0.57 microns and 1033 +/- 33.8 microns 2 length and area respectively in Fraction 7. Glutamine synthetase activity was assessed in each freshly eluted fraction and was shown to be predominant in Fractions 6 and 7. Pretreatment of rats with CCl4, which selectively destroys perivenous hepatocytes, decreased the proportion of couplets in these fractions by over 67%, and their glutamine synthetase activity by over 97%. It was concluded that Fractions 2 and 3 contained predominantly couplets of Zone 1 (periportal) origin, Fractions 4 and 5 those from Zone 2, and Fractions 6 and 7 predominantly couplets of Zone 3 (perivenous) origin. The development of canalicular secretory activity was assessed in the couplets after a 15 min incubation with a fluorescent bile acid, cholyl-lysyl-fluorescein (CLF). This was sigmoidal in all fractions, but slower in the periportal couplets, taking 5.1 h for 50% to show secretory activity in Fraction 2, compared with 2.7 h for Fraction 7. Incubation of hepatocyte couplets with 1 or 10 microM taurodehydrocholate, a non-toxic bile acid analogue, did not influence the rate of development of accumulation of CLF by the couplets or the area of the canalicular vacuole in any fraction. However, it did decrease the CLF content of couplets incubated with CLF for 15 min to a greater extent in those of perivenous origin. After subjecting the couplets to oxidative stress by incubation with 20 microM menadione (2-methyl-1,4-naphthoquinone), it was evident that periportal couplets were less able to maintain canalicular secretory activity than perivenous couplets.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger H. J., Gebhardt R., Mayer C., Mecke D. Different capacities for amino acid transport in periportal and perivenous hepatocytes isolated by digitonin/collagenase perfusion. Hepatology. 1989 Jan;9(1):22–28. doi: 10.1002/hep.1840090105. [DOI] [PubMed] [Google Scholar]
- Di Monte D., Ross D., Bellomo G., Eklöw L., Orrenius S. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch Biochem Biophys. 1984 Dec;235(2):334–342. doi: 10.1016/0003-9861(84)90206-6. [DOI] [PubMed] [Google Scholar]
- Gautam A., Ng O. C., Boyer J. L. Isolated rat hepatocyte couplets in short-term culture: structural characteristics and plasma membrane reorganization. Hepatology. 1987 Mar-Apr;7(2):216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- Gautam A., Ng O. C., Strazzabosco M., Boyer J. L. Quantitative assessment of canalicular bile formation in isolated hepatocyte couplets using microscopic optical planimetry. J Clin Invest. 1989 Feb;83(2):565–573. doi: 10.1172/JCI113919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt R., Burger H. J., Heini H., Schreiber K. L., Mecke D. Alterations of hepatic enzyme levels and of the acinar distribution of glutamine synthetase in response to experimental liver injury in the rat. Hepatology. 1988 Jul-Aug;8(4):822–830. doi: 10.1002/hep.1840080421. [DOI] [PubMed] [Google Scholar]
- Gebhardt R., Mecke D. Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. EMBO J. 1983;2(4):567–570. doi: 10.1002/j.1460-2075.1983.tb01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gores G. J., Nieminen A. L., Fleishman K. E., Dawson T. L., Herman B., Lemasters J. J. Extracellular acidosis delays onset of cell death in ATP-depleted hepatocytes. Am J Physiol. 1988 Sep;255(3 Pt 1):C315–C322. doi: 10.1152/ajpcell.1988.255.3.C315. [DOI] [PubMed] [Google Scholar]
- Gumucio J. J., May M., Dvorak C., Chianale J., Massey V. The isolation of functionally heterogeneous hepatocytes of the proximal and distal half of the liver acinus in the rat. Hepatology. 1986 Sep-Oct;6(5):932–944. doi: 10.1002/hep.1840060521. [DOI] [PubMed] [Google Scholar]
- Haüssinger D. Nitrogen metabolism in liver: structural and functional organization and physiological relevance. Biochem J. 1990 Apr 15;267(2):281–290. doi: 10.1042/bj2670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D. Hepatocyte heterogeneity in glutamine and ammonia metabolism and the role of an intercellular glutamine cycle during ureogenesis in perfused rat liver. Eur J Biochem. 1983 Jun 15;133(2):269–275. doi: 10.1111/j.1432-1033.1983.tb07458.x. [DOI] [PubMed] [Google Scholar]
- James R., Desmond P., Küpfer A., Schenker S., Branch R. A. The differential localization of various drug metabolizing systems within the rat liver lobule as determined by the hepatotoxins allyl alcohol, carbon tetrachloride and bromobenzene. J Pharmacol Exp Ther. 1981 Apr;217(1):127–132. [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989 Jul;69(3):708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Kanai K., Kanamura S., Watanabe J. Peri- and postnatal development of heterogeneity in the amounts of endoplasmic reticulum in mouse hepatocytes. Am J Anat. 1986 Apr;175(4):471–480. doi: 10.1002/aja.1001750406. [DOI] [PubMed] [Google Scholar]
- Kawahara H., Marceau N., French S. W. Effect of agents which rearrange the cytoskeleton in vitro on the structure and function of hepatocytic canaliculi. Lab Invest. 1989 May;60(5):692–704. [PubMed] [Google Scholar]
- Kera Y., Sippel H. W., Penttilä K. E., Lindros K. O. Acinar distribution of glutathione-dependent detoxifying enzymes. Low glutathione peroxidase activity in perivenous hepatocytes. Biochem Pharmacol. 1987 Jun 15;36(12):2003–2006. doi: 10.1016/0006-2952(87)90500-4. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Brauneis U., Gatmaitan Z., Arias I. M. Extracellular ATP, intracellular calcium and canalicular contraction in rat hepatocyte doublets. Hepatology. 1991 Oct;14(4 Pt 1):640–647. doi: 10.1016/0270-9139(91)90051-v. [DOI] [PubMed] [Google Scholar]
- Kuo F. C., Darnell J. E., Jr Evidence that interaction of hepatocytes with the collecting (hepatic) veins triggers position-specific transcription of the glutamine synthetase and ornithine aminotransferase genes in the mouse liver. Mol Cell Biol. 1991 Dec;11(12):6050–6058. doi: 10.1128/mcb.11.12.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindros K. O., Penttilä K. E. Digitonin-collagenase perfusion for efficient separation of periportal or perivenous hepatocytes. Biochem J. 1985 Jun 15;228(3):757–760. doi: 10.1042/bj2280757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loud A. V. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol. 1968 Apr;37(1):27–46. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T., Yoshihara H., Jeffs R., Takei Y., Nukina S., Hijioka T., Evans R. K., Kauffman F. C., Thurman R. G. Hormones increase oxygen uptake in periportal and pericentral regions of the liver lobule. Am J Physiol. 1992 Apr;262(4 Pt 1):G645–G650. doi: 10.1152/ajpgi.1992.262.4.G645. [DOI] [PubMed] [Google Scholar]
- Mills C. O., Rahman K., Coleman R., Elias E. Cholyl-lysylfluorescein: synthesis, biliary excretion in vivo and during single-pass perfusion of isolated perfused rat liver. Biochim Biophys Acta. 1991 Dec 6;1115(2):151–156. doi: 10.1016/0304-4165(91)90024-b. [DOI] [PubMed] [Google Scholar]
- Misra U. K., Yamanaka H., Kizaki Z., Kauffman F. C., Thurman R. G. A new method for the isolation of fresh hepatocytes from periportal and pericentral regions of the liver lobule. Biochem Biophys Res Commun. 1988 Aug 30;155(1):455–462. doi: 10.1016/s0006-291x(88)81108-2. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Nickola I., Frimmer M. Preservation of cellular polarity in isolated hepatocytes. Visualization of cytoskeletal structures by indirect immunofluorescence and fluorescent staining with tetramethylrhodaminyl-phalloidin. Cell Tissue Res. 1986;243(2):437–440. doi: 10.1007/BF00251061. [DOI] [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989 Jul;10(7):281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Oshio C., Phillips M. J. Contractility of bile canaliculi: implications for liver function. Science. 1981 May 29;212(4498):1041–1042. doi: 10.1126/science.7015506. [DOI] [PubMed] [Google Scholar]
- Phillips M. J., Oshio C., Miyairi M., Katz H., Smith C. R. A study of bile canalicular contractions in isolated hepatocytes. Hepatology. 1982 Nov-Dec;2(6):763–768. doi: 10.1002/hep.1840020603. [DOI] [PubMed] [Google Scholar]
- Quistorff B. Gluconeogenesis in periportal and perivenous hepatocytes of rat liver, isolated by a new high-yield digitonin/collagenase perfusion technique. Biochem J. 1985 Jul 1;229(1):221–226. doi: 10.1042/bj2290221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redick J. A., Jakoby W. B., Baron J. Immunohistochemical localization of glutathione S-transferases in livers of untreated rats. J Biol Chem. 1982 Dec 25;257(24):15200–15203. [PubMed] [Google Scholar]
- Reverdin E. C., Weingart R. Electrical properties of the gap junctional membrane studied in rat liver cell pairs. Am J Physiol. 1988 Feb;254(2 Pt 1):C226–C234. doi: 10.1152/ajpcell.1988.254.2.C226. [DOI] [PubMed] [Google Scholar]
- Ross D., Thor H., Orrenius S., Moldeus P. Interaction of menadione (2-methyl-1,4-naphthoquinone) with glutathione. Chem Biol Interact. 1985 Oct;55(1-2):177–184. doi: 10.1016/s0009-2797(85)80126-5. [DOI] [PubMed] [Google Scholar]
- Schmucker D. L., Mooney J. S., Jones A. L. Stereological analysis of hepatic fine structure in the Fischer 344 rat. Influence of sublobular location and animal age. J Cell Biol. 1978 Aug;78(2):319–337. doi: 10.1083/jcb.78.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert B., Oesch F., Steinberg P. Distribution and induction of cytochrome P-450 and two cytochrome P-450-dependent monooxygenase activities in rat liver parenchymal cell subpopulations separated by centrifugal elutriation. Arch Toxicol. 1989;63(1):18–22. doi: 10.1007/BF00334628. [DOI] [PubMed] [Google Scholar]
- Sesardic D., Rich K. J., Edwards R. J., Davies D. S., Boobis A. R. Selective destruction of cytochrome P-450d and associated monooxygenase activity by carbon tetrachloride in the rat. Xenobiotica. 1989 Jul;19(7):795–811. doi: 10.3109/00498258909042316. [DOI] [PubMed] [Google Scholar]
- Sigal S. H., Brill S., Fiorino A. S., Reid L. M. The liver as a stem cell and lineage system. Am J Physiol. 1992 Aug;263(2 Pt 1):G139–G148. doi: 10.1152/ajpgi.1992.263.2.G139. [DOI] [PubMed] [Google Scholar]
- Smith M. T., Loveridge N., Wills E. D., Chayen J. The distribution of glutathione in the rat liver lobule. Biochem J. 1979 Jul 15;182(1):103–108. doi: 10.1042/bj1820103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Ginzberg R. D., Morales E. A., Gatmaitan Z., Arias I. M. Electrophysiological properties of gap junctions between dissociated pairs of rat hepatocytes. J Cell Biol. 1986 Jul;103(1):135–144. doi: 10.1083/jcb.103.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubberfield C. R., Cohen G. M. NAD+ depletion and cytotoxicity in isolated hepatocytes. Biochem Pharmacol. 1988 Oct 15;37(20):3967–3974. doi: 10.1016/0006-2952(88)90081-0. [DOI] [PubMed] [Google Scholar]
- Traber P. G., Chianale J., Gumucio J. J. Physiologic significance and regulation of hepatocellular heterogeneity. Gastroenterology. 1988 Oct;95(4):1130–1143. doi: 10.1016/0016-5085(88)90194-1. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Phillips M. J. Ca2+ causes active contraction of bile canaliculi: direct evidence from microinjection studies. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6164–6168. doi: 10.1073/pnas.81.19.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson R. A., Liem H. H., Miyai K., Muller-Eberhard U. Heterogeneous distribution of drug metabolism in elutriated rat hepatocytes. Biochem Pharmacol. 1985 May 1;34(9):1463–1470. doi: 10.1016/0006-2952(85)90685-9. [DOI] [PubMed] [Google Scholar]
- Wilton J. C., Williams D. E., Strain A. J., Parslow R. A., Chipman J. K., Coleman R. Purification of hepatocyte couplets by centrifugal elutriation. Hepatology. 1991 Jul;14(1):180–183. doi: 10.1002/hep.1840140129. [DOI] [PubMed] [Google Scholar]
- el Mouelhi M., Kauffman F. C. Sublobular distribution of transferases and hydrolases associated with glucuronide, sulfate and glutathione conjugation in human liver. Hepatology. 1986 May-Jun;6(3):450–456. doi: 10.1002/hep.1840060322. [DOI] [PubMed] [Google Scholar]


