Abstract
Macular edema is a known side effect of taxane-based anticancer drugs. We retrospectively investigated data from 11 centers between January 2016 and December 2021. Among 14,260 patients, 30 (0.21%) developed macular edema; from these, the number of cases associated with nab-paclitaxel was 16 (0.43%), significantly higher than the number of cases associated with paclitaxel or docetaxel (P < 0.01). Visual acuity (VA) and retinal choroidal change were examined in 27 patients, with a follow-up of at least 3 months. The patients’ mean age was 67.2 years; 14 (51.3%) were male and four (14.8%) had unilateral onset. The mean interval between anticancer drug initiation and the first ophthalmology visit was 290.1 days. Among the 20 patients who discontinued anticancer drugs, VA and edema significantly improved 2 months after discontinuation (LogMAR VA: 0.50 vs. 0.28, central retinal thickness: 472.7 µm vs. 282.5 µm, both P < 0.01). No significant changes were observed in the central choroidal thickness. A correlation was found between duration of taxane treatment and VA immediately before discontinuation of anticancer drugs (β = 0.00050; 95% confidence interval: 0.00036–0.00097; P < 0.05). Although taxane-induced macular edema is reversible, slower anticancer drug discontinuation worsened VA, highlighting the need for regular ophthalmologic evaluation during treatments.
Keywords: Taxane-based anticancer drugs, Macular edema, Incidence
Subject terms: Retinal diseases, Chemotherapy
Introduction
Taxane-based anticancer drugs share a common structure known as the taxane ring. Taxane-based drugs are derived from Taxus baccata1 and are globally utilized as anticancer drugs. The mechanism of taxane-based anticancer drugs may involve the inhibition of microtubules, which are necessary for cell division, thus preventing cancer cell division and ultimately leading to apoptosis and cancer cell death2. Currently, several taxane-based anticancer drugs are available; the most commonly used ones in Japan are docetaxel, paclitaxel, and nab-paclitaxel. Despite their efficacy and clinical benefits, taxanes may elicit various side effects, including leukemia, neutropenia, peripheral neuropathy, nausea, and vomiting. Ocular complications associated with taxane-based anticancer drugs include epiphora3, optic neuropathy4, and central macular edema (CME)5. Among these, CME is recognized as a serious complication because of its impact on vision loss and quality of life. However, most previous reports on taxane-based anticancer drugs have been case reports, and to our knowledge, there are no reports showing statistical analyses of CME characteristics and the prognosis of taxane-induced CME. Given the infrequent occurrence of taxane-induced CME, single-center case accumulation is difficult. Therefore, we conducted a retrospective study involving 11 centers to comprehensively investigate the pathogenesis and prognosis of taxane-induced CME.
Results
Baseline characteristics
A total of 14,206 patients received taxane-based anticancer therapy at 11 centers affiliated with the Japan Clinical REtina Study Group (J-CREST) between January 1, 2016 and December 31, 2021. Among them, 30 cases of taxane-induced CME were diagnosed1. Representative cases are illustrated in Fig. 1A–C. For instance, a man in his 70s presented to an ophthalmologist with decreased visual acuity in both eyes 4 months after initiation of paclitaxel treatment. Upon examination, he was diagnosed with CME after meeting all four diagnostic criteria. CME was considered a side effect of paclitaxel treatment. Patients treated with nab-paclitaxel showed a significantly higher incidence of CME than did those treated with docetaxel and paclitaxel (P < 0.01) (Table 1). The baseline characteristics of the 27 patients who could be followed for over 3 months are shown in Table 2. Most patients showed bilateral CME; only four showed monocular CME. Among monocular CME cases, eyes with macular edema tended to have decreased vision compared to that of eyes without macular edema, although the difference was not statistically significant (Fig. 2). On average, the interval between taxane initiation and the first ophthalmology appointment was 290.1 days.
Figure 1.
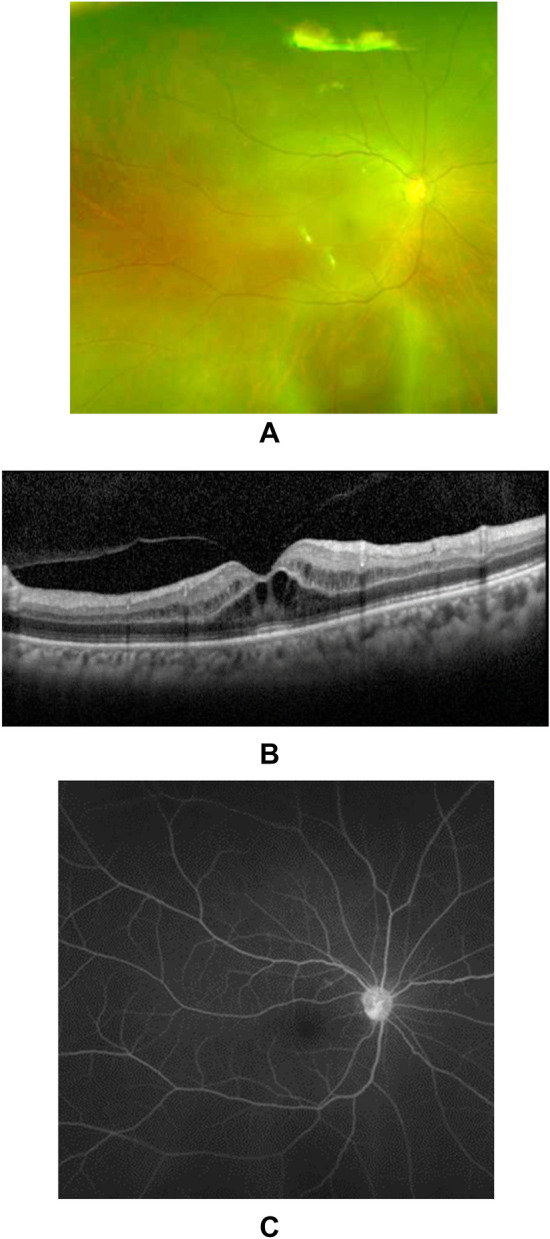
Typical OCT image of CME caused by taxane-based anticancer drugs. The patient, a man in his 70s, complained of vision loss and was referred to an ophthalmologist 4 months after the initiation of paclitaxel treatment for esophageal cancer. Fundus photography did not reveal any obvious cause for the vision loss (A). OCT imaging revealed CME mainly in the outer retina (B). Fluorescein angiography did not show any fluorescein leakage in the macular region (C). OCT optical coherence tomography, CME central macular edema.
Table 1.
CME induced by different taxanes.
| Nab-Paclitaxel | Paclitaxel | Docetaxel | Total | |
|---|---|---|---|---|
| Number of CME (%) | 16 (0.434) | 7 (0.133) | 7 (0.133) | 30 (0.211) |
| P-value | < 0.01 | < 0.01 | ||
| Total patients’ number | 3683 | 5260 | 5263 | 14,206 |
For CME incidence, calculations were performed using Fisher's exact test with Bonferroni correction against Nab-Paclitaxel group.
CME central macular edema.
Table 2.
Baseline characteristics of the 27 patients included in the study.
| Characteristics | All = 27 |
|---|---|
| Age, mean years, (SD) | 67.2 (8.5) |
| Sex (men), number (%) | 14 (51.9) |
| Number of taxanes (nab-paclitaxel/paclitaxel/docetaxel) | 13/8/6 |
| Bilateral (%) | 23 (85.2) |
| Number of days from the initial taxane therapy to the ophthalmology visit, days (SD) | 290.1 (267.4) |
| BCVA at the initial visit, OD/OS, mean logMAR units (SD) | 0.28 (0.23)/0.41 (0.29) |
| CMT, OD/OS, mean (SD) μm | 422.8 (108.6)/464.4 (144.6) |
| CCT, OD/OS, mean (SD) μm | 282.1 (71.8)/288.2 (81.6) |
SD standard deviation, BCVA best-corrected visual acuity, CMT central macular thickness, CCT central choroidal thickness, OD oculus dexter, OS oculus sinister.
Figure 2.
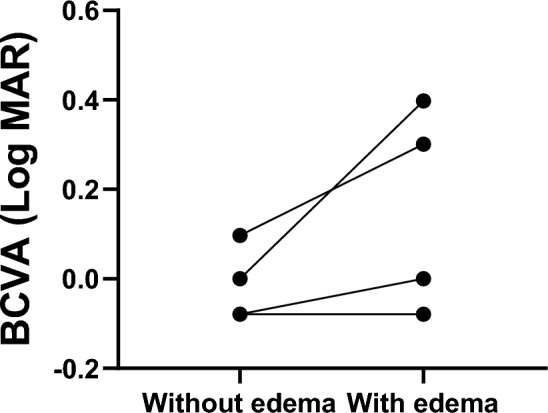
Comparison of BCVA between eyes with CME and those without CME. Comparisons were performed using the Wilcoxon matched-pairs signed rank test. There was no statistically significant difference because of the small number of cases (P = 0.125), although the group with CME tended to have poorer BCVA. CME central macular edema.
Changes in best-corrected visual acuity (BCVA), central macular thickness (CMT), and central choroidal thickness (CCT)
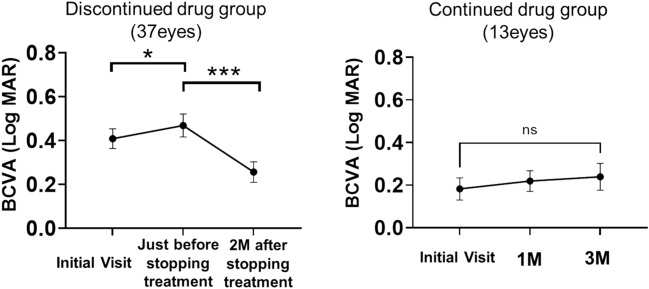
The 27 patients were divided into two groups: those who discontinued taxane therapy (the “discontinued” group, n = 20) and those who continued taxane therapy (the “continued” group, n = 7) during the study period. Figure 3 illustrates the changes in BCVA in each group. In the discontinued group, compared with that at the first visit, BCVA was significantly decreased before the discontinuation of anticancer drugs (P < 0.05). However, 2 months after discontinuation, BCVA significantly improved relative to that before discontinuation (P < 0.01).
Figure 3.
Changes in BCVA. Changes in BCVA were analyzed using the Wilcoxon matched-pairs signed-rank test (*P < 0.05, ***P < 0.001). BCVA best-corrected visual acuity.
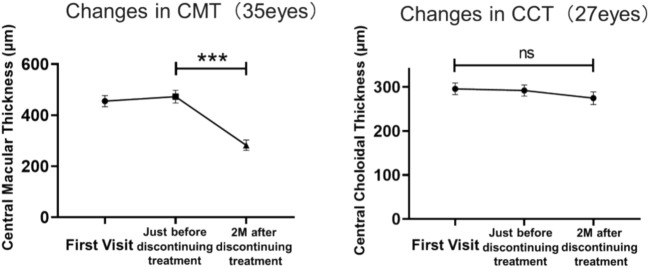
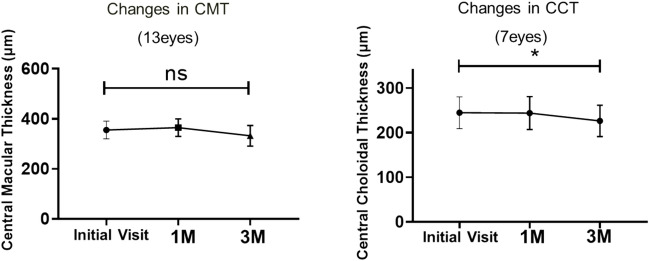
In the continued group, no significant differences were found between BCVA at the initial visit and that at 1 and 3 months after the initial visit. In the discontinued group, discontinuation of taxanes significantly reduced CMT, whereas there was no significant change in CCT (Fig. 4). In the continued group, CMT did not change significantly at the three time points, whereas CCT was significantly reduced at 3 months relative to that at the first visit (P < 0.05) (Fig. 5).
Figure 4.
Changes in CMT and CCT in the group that discontinued taxane treatment. Changes in CMT and CCT were analyzed using the Wilcoxon matched-pairs signed-rank test (***P < 0.001). CMT central macular thickness, CCT central choroidal thickness.
Figure 5.
Changes in CMT and CCT in the group that continued taxane treatment. Changes in CMT and CCT were analyzed using the Wilcoxon matched-pairs signed rank test (*P < 0.05). CMT central macular thickness, CCT central choroidal thickness.
Correlation between duration of taxane treatment and BCVA
Simple linear regression analysis revealed a positive correlation between duration of taxane treatment and BCVA at the first ophthalmology visit (regression coefficient = 0.00050, 95% confidence interval 0.00036–0.00097, P < 0.05) (Fig. 6A). The correlation between CMT and the duration of taxane treatment was not significant (regression coefficient = − 0.07054, 95% confidence interval − 0.2088 to 0.06776; Fig. 6B).
Figure 6.
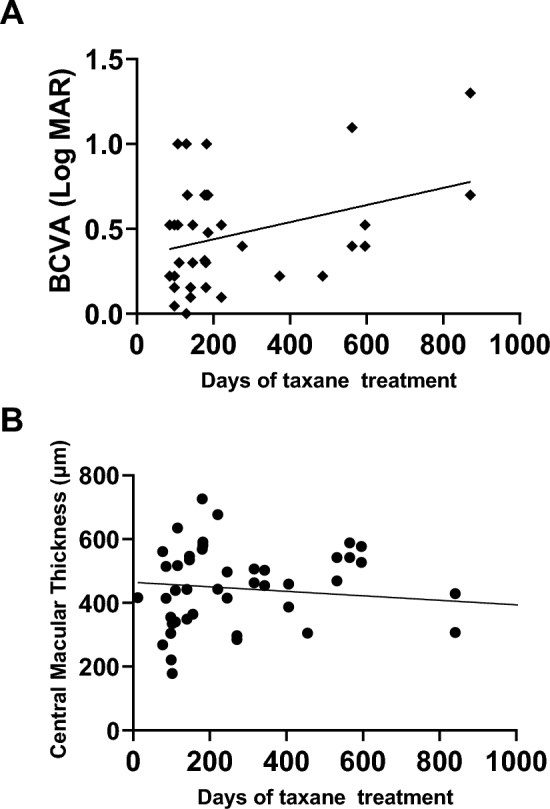
Correlation between the duration of taxane treatment and BCVA. A simple linear analysis was performed between the duration of taxane treatment in days and BCVA (regression coefficient = 0.00050, 95% confidence interval 0.00036–0.0097, P < 0.05; A). The correlation between CMT and the duration of taxane treatment was not significant (regression coefficient = − 0.07054, 95% confidence interval: − 0.2088 to 0.06776; B). BCVA best-corrected visual acuity.
Discussion
To the best of our knowledge, this is the first study to observe the clinical features and subsequent development of macular edema as a side effect of taxane-based anticancer drugs. Macular edema has long been recognized as a potential side effect of taxane-based therapy6,7. However, most reports on taxane-induced ocular complications have been limited to case reports, with no comprehensive statistical analysis of the characteristics and prognosis of cases. Notably, Sodhi et al. reported a higher risk of developing CME, lacrimation, and optic neuropathy among patients with breast cancer treated with taxane than among those treated with tamoxifen, emphasizing the importance of monitoring ocular complications. However, none of these studies investigated the specific macular condition or its progression in these patients8.
The present study revealed five significant findings. First, among the taxane-based anticancer drugs, nab-paclitaxel caused the highest incidence of CME. Given that nab-paclitaxel does not require pre-dosing with steroids to prevent hypersensitivity reactions, it was anticipated to reduce side effects and patient burden compared to those with paclitaxel and docetaxel. However, Li et al. suggested that nab-paclitaxel treatment causes more skin toxicity than does paclitaxel9. Moreover, nab-paclitaxel is more likely to induce peripheral neuropathy than is docetaxel10. While paclitaxel is dissolved in alcohol and oil, nab-paclitaxel is a drug in which paclitaxel is bound and dissolved in human serum albumin. Consequently, it has been speculated that the difference in solvents may be the reason for differences in the immune response and cellular uptake9,10.
Behar et al. reported that taxane-based anticancer drugs often cause fluid retention, a reduction in colloid osmotic pressure, and capillary protein leakage, leading to intratissue edema, and that steroid pre-medication might be useful for preventing docetaxel-induced fluid retention11. If macular edema is considered a type of intratissue edema, then administration of steroids may have a preventive effect against macular edema. However, the effect of pre-steroid treatment on the incidence of CME remains unclear. Further studies are required to understand the differences in the incidence of CME.
Second, bilateral CME was observed in most cases; only four cases (14.8%) showed monocular CME. Although there are no diagnostic criteria for taxane-induced macular edema, bilateral macular edema has been described as a characteristic of this condition12. Nevertheless, our study suggests that clinicians should also consider the possibility of unilateral macular edema being a side effect of taxane-based anticancer drugs.
Third, macular edema resolved within 2 months of discontinuation of taxane-based anticancer drugs in all cases. Most case reports also indicated an improvement within 2 months of discontinuation of taxane-based anticancer drugs13–16; however, Matsuoka et al. reported that macular edema did not improve for over 11 months, even after the discontinuation of the taxane drug and sub-Tenon injection of triamcinolone acetonide17. In other words, discontinuation of taxane-based anticancer drugs should result in a decrease in CMT and an improvement in VA within 2 months; if there is no improvement within this time, some form of treatment may be necessary. Currently, there are no established treatments, although sub-Tenon injections of triamcinolone acetonide17, intravitreal bevacizumab injections18 and dorzolamide eye drops19 have been utilized. Further studies are required to establish effective treatments for taxane-induced CME. Importantly, although discontinuation of taxane-based anticancer drugs is the best treatment for CME, collaboration with oncologists is crucial before discontinuation of taxane drugs, considering that treatment discontinuation may shorten the patient’s life.
Fourth, the continued use of taxane-based anticancer drugs may contribute to choroidal thinning. In other words, taxane-based anticancer drugs may affect not only the retina but also the choroid. Odrobina et al. reported choroidal thinning in cases of acute CME after cataract surgery20 and suggested that the choroidal blood flow in the choriocapillaris might be decreased in eyes with CME. Although the mechanism of taxane-induced macular edema remains unclear, choroidal blood flow changes may be implicated.
Finally, a positive correlation was observed between the duration of taxane treatment and BCVA. Cumulative administration of taxanes may adversely affect BCVA. Although it is desirable to elucidate the precise interval between the day of initiation of taxane-based anticancer drugs and the onset of CME, there is no way to identify the precise time of CME onset. Accordingly, we postulated two hypotheses for the reason behind the positive correlation between the duration of treatment with taxane-based anticancer drugs and BCVA. First, the precise time of onset of CME varies widely among individuals. Although the number of days between CME onset and the first ophthalmology visit appears to be similar, patients with a longer interval show more severe CME. Second, although the number of days until the onset of CME is almost the same, there are differences in the number of days between CME onset and the first ophthalmology visit; this can result in differences in VA. Although we have not tested these two hypotheses in the present study, the correct hypothesis can be confirmed through regular optical coherence tomography (OCT) screening examinations. Despite BCVA restoration after discontinuation of taxane-based anticancer treatment, the reduction in the quality of vision during taxane treatment also warrants ophthalmic screening using OCT after treatment initiation.
Our study has a few limitations. First, the study focused on patients undergoing anticancer treatment who visited the ophthalmology department. In reality, some patients may have developed macular edema due to the treatment but may not have visited an ophthalmologist. Second, this study did not investigate differences in the incidence of macular edema according to the frequency or dosage of taxane-based anticancer drugs. Third, because our patient population was relatively older, the influence of cataracts on changes in BCVA cannot be completely ruled out. Fourth, it is important to be aware of risk factors for taxane-induced CME. But in our study, since only the total number of patients in the non-CME group was examined, patient demographics such as age and sex are unknown and could not be compared. Therefore, risk factors for the development of CME are unknown from our study. Finally, all the participants in this study were Asian; therefore, the results may differ for other racial groups.
Future prospective studies may need to examine the usefulness of screening for macular edema using OCT for the prevention of vision loss and the effectiveness of pre-medication with steroids as a preventive measure for macular edema in several racial groups.
Methods
Study design
This retrospective study was performed by investigating the medical records of the participants.
The study was approved by the Ethics Committee of Nara Medical University (Approval Code: 13-032) and the ethics committees of the other participating facilities: Yamaguchi University, Fukui University, Hyogo Medical University, Tokushima University, Shinshu University, Kurume University, Mie University, Tachikawa Hospital, Shiga University of Medical Science, and Osaka Medical and Pharmaceutical University. The study was adhered to the Declaration of Helsinki, and the need for informed consent was waived by the all-ethics committees described above.
Participants
A flow diagram of the study design is presented in Fig. 7. Among 14,206 patients receiving taxane anticancer drug therapy at 11 centers affiliated with J-CREST between January 1, 2016 and December 31, 2021, the medical records of patients with taxane-induced CME were selected. There were 30 cases of taxane-induced CME. Analysis was conducted for 27 patients who underwent at least 3 months of follow-up. CMT was measured in 23 patients and CCT was measured in 11. The 27 patients were divided into two groups: those who discontinued taxane therapy (n = 20) and those who continued taxane therapy (n = 7). None of the patients received treatment for macular edema other than discontinuation of taxane-based anticancer drugs. The mean time from initial diagnosis of taxane-induced CME to discontinuation of taxane drugs was 0.85 ± 0.87(Standard deviation) months.
Figure 7.
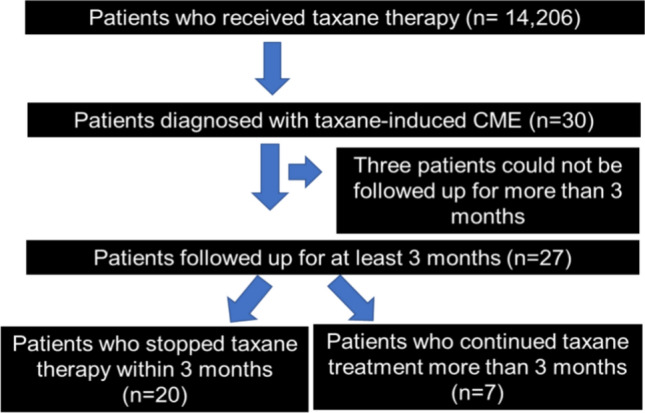
Study flowchart. A total of 14,206 patients who received taxane therapy were enrolled in our study.
Definition of taxane-induced CME
No precise definition has been established for taxane-induced CME. However, the following criteria have been defined based on previous reports21: (1) CME in the outer retina; (2) minimal or no leakage on fluorescein fundus angiography; (3) recovery from CME after the discontinuation of taxane therapy; and (4) exclusion of other causes of macular edema, such as retinal vein occlusion, age-related macular degeneration, diabetic macular edema, and macular telangiectasia.
Other measurements
All patients with CME underwent slit-lamp examinations. OCT (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany) images were obtained and analyzed by a retinal specialist. The following information was acquired from medical records: age, sex, duration of taxane treatment, date of the first ophthalmology visit, types of taxane-based anticancer drugs used, and BCVA.
Statistical analysis
Fisher’s exact test with Bonferroni correction was used to examine differences in CME incidence among different taxane anticancer drugs. All the variables (age, interval between the initiation of taxane therapy to the first ophthalmology visit, BCVA, CMT, and CCT) were continuous and normally distributed; they are expressed as means and standard deviations. To examine the changes in BCVA, CMT, and CCT, the Wilcoxon matched-pairs signed-rank test was used to compare values at different time points. We investigated the association between the duration of taxane treatment and BCVA using a simple linear regression model. Statistical significance was set at P < 0.05. All analyses were performed using GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA).
Author contributions
Conceptualization, H.T.; methodology, S.I., and T.U.; investigation, S.I., M.W., Y.Y., T.S., F.M., T.H., S.Y., K.K., T.K., M.S., R.M., T.I., and G.I.; validation, H.T.; formal analysis, S.I. and T.U.; writing-original draft preparation, H.T.; writing-review and editing, N.O.; visualization, H.T. and S.I.; resources: H.T.; supervision, T.U. and N.O.; project administration, H.T.; funding acquisition, H.T. All authors have read and agreed to the published version of the manuscript.
Data availability
The data are not publicly available due to privacy or ethical restrictions. The datasets that support the findings of current study are available on reasonable request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wani, M. C., Taylor, H. L., Wall, M. E., Coggon, P. & McPhail, A. T. Plant antitumor agents. VI. The isolation and structure of Taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc.93, 2325–2327 (1971). 10.1021/ja00738a045 [DOI] [PubMed] [Google Scholar]
- 2.Dumontet, C. & Sikic, B. I. Mechanisms of action of and resistance to antitubulin agents: Microtubule dynamics, drug transport, and cell death. J. Clin. Oncol.17, 1061–1070 (1999). 10.1200/JCO.1999.17.3.1061 [DOI] [PubMed] [Google Scholar]
- 3.Noguchi, Y. et al. Risk factors for eye disorders caused by paclitaxel: A retrospective study. Biol. Pharm. Bull.41, 1694–1700 (2018). 10.1248/bpb.b18-00444 [DOI] [PubMed] [Google Scholar]
- 4.Moloney, T. P. et al. Toxic optic neuropathy in the setting of docetaxel chemotherapy: A case report. BMC Ophthalmol.14, 18 (2014). 10.1186/1471-2415-14-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanakis, M., Georgalas, I., Makatsoris, T. & Pharmakakis, N. Taxane induced cystoid macular edema: Case report and integrated pathogenic theory. Curr. Drug Saf.14, 43–47 (2019). 10.2174/1574886313666180828163016 [DOI] [PubMed] [Google Scholar]
- 6.Hofstra, L. S., de Vries, E. G. & Willemse, P. H. Ophthalmic toxicity following paclitaxel infusion. Ann. Oncol.8, 1053 (1997). 10.1023/A:1008249230056 [DOI] [PubMed] [Google Scholar]
- 7.Rotsos, T. G. & Moschos, M. M. Cystoid macular edema. Clin. Ophthalmol.2, 919–930 (2008). 10.2147/OPTH.S4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sodhi, M., Yeung, S. N., Maberley, D., Mikelberg, F. & Etminan, M. Risk of ocular adverse events with taxane-based chemotherapy. JAMA Ophthalmol.140, 880–884 (2022). 10.1001/jamaophthalmol.2022.3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, H. B., Wang, W. H. & Wang, Z. Y. A meta-analysis of the incidence and risk of skin toxicity with nab-paclitaxel and paclitaxel in cancer treatment. Am. J. Transl. Res.15, 4279–4290 (2023). [PMC free article] [PubMed] [Google Scholar]
- 10.Guo, X. et al. Does nab-paclitaxel have a higher incidence of peripheral neuropathy than solvent-based paclitaxel? Evidence from a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol.139, 16–23 (2019). 10.1016/j.critrevonc.2019.04.021 [DOI] [PubMed] [Google Scholar]
- 11.Béhar, A. et al. The pathophysiological mechanism of fluid retention in advanced cancer patients treated with docetaxel, but not receiving corticosteroid comedication. Br. J. Clin. Pharmacol.43, 653–658 (1997). 10.1046/j.1365-2125.1997.00613.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoe, T. et al. Cystoid macular edema during treatment with paclitaxel and bevacizumab in a patient with metastatic breast cancer: A case report and literature review. Case Rep. Oncol.10, 605–612 (2017). 10.1159/000477897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haider, A., Bababeygy, S. R. & Lu, S. Y. Cystoid macular edema and macular pigmentation associated with nab-paclitaxel therapy. Retin. Cases Brief Rep.9, 220–222 (2015). 10.1097/ICB.0000000000000143 [DOI] [PubMed] [Google Scholar]
- 14.Lee, J., Ra, H. & Baek, J. Ultra-widefield angiographic imaging of albumin-bound paclitaxel-induced cystoid macular edema. Indian J. Ophthalmol.67, 2058–2059 (2019). 10.4103/ijo.IJO_734_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka, Y. et al. Cystoid macular edema induced by nab-paclitaxel. Breast Cancer22, 324–326 (2015). 10.1007/s12282-012-0373-y [DOI] [PubMed] [Google Scholar]
- 16.Smith, S., Benz, M. & Brown, D. Cystoid macular edema secondary to albumin-bound paclitaxel therapy. Arch. Ophthalmol.126, 1605–1606 (2008). 10.1001/archopht.126.11.1605 [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka, N., Hasebe, H., Mayama, T. & Fukuchi, T. Sub-tenon injections of triamcinolone acetonide had limited effect on cystoid macular edema secondary to nanoparticle albumin-bound-paclitaxel (Abraxane). Case Rep. Ophthalmol. Med.2015, 181269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman, H. T., Yeh, S. & Bergstrom, C. S. Cystoid macular edema without leakage secondary to nab-paclitaxel (Abraxane): Clinical experience with intravitreal bevacizumab. J. Ocul. Pharmacol. Ther.29, 360–362 (2013). 10.1089/jop.2011.0178 [DOI] [PubMed] [Google Scholar]
- 19.Ehlers, J. P., Rayess, H. & Steinle, N. Topical dorzolamide therapy for taxane-related macular oedema. Eye (Lond.)27, 102–104 (2013). 10.1038/eye.2012.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odrobina, D. & LaudaŃska-Olszewska, I. Choroidal thickness in clinically significant pseudophakic cystoid macular edema. Retina35, 136–140 (2015). 10.1097/IAE.0000000000000273 [DOI] [PubMed] [Google Scholar]
- 21.Di Pippo, M., Di Staso, F., De Ponte, C., Fragiotta, S. & Abdolrahimzadeh, S. Nab-paclitaxel related cystoid macular edema. Clin. Ter.173, 377–383 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions. The datasets that support the findings of current study are available on reasonable request from the corresponding author.