Abstract
Glutamate is involved in fundamental functions, including neuronal plasticity and memory. Astrocytes are integral elements involved in synaptic function, and the GLT–1 transporter possesses a critical role in glutamate uptake. Here, we study the role of GLT-1, specifically located in astrocytes, in the consolidation, expression, reconsolidation and persistence of spatial object recognition memory in rats. Administration of dihydrokainic acid (DHK), a selective GLT-1 inhibitor, into the dorsal hippocampus around a weak training which only induces short-term memory, promotes long-term memory formation. This promotion is prevented by hippocampal administration of protein-synthesis translation inhibitor, blockade of Activity-regulated cytoskeleton-associated protein (Arc) translation or Brain-Derived Neurotrophic Factor (BDNF) action, which are plasticity related proteins necessary for memory consolidation. However, DHK around a strong training, which induces long-term memory, does not affect memory consolidation. Administration of DHK before the test session impairs the expression of long-term memory, and this effect is dependent of Arc translation. Furthermore, DHK impairs reconsolidation if applied before a reactivation session, and this effect is independent of Arc translation. These findings reveal specific consequences on spatial memory stages developed under hippocampal GLT-1 blockade, shedding light on the intricate molecular mechanisms, governed in part for the action of glia.
Subject terms: Spatial memory, Long-term memory, Astrocyte
Modulation of glutamate uptake in the rat hippocampus via astroglial GLT-1 improves or impairs spatial objects memory depending on the intensity of learning session as well as the memory phase evaluated, being Arc and BDNF mediators of these effects.
Introduction
According to the tripartite synapse concept, astrocytes are integral elements involved in synaptic function and this makes them important players in brain function and animal behavior. Astrocytes establish bidirectional communication with neurons, respond to synaptically released neurotransmitters, reuptake them and, in turn, release gliotransmitters1. All these functions influence neuronal and synaptic activity, impacting information processing and memory formation and stabilization2.
One of the most important functions of astrocytes in the brain is their control of the recycling of neurotransmitters. In particular, the permanence of glutamate in the synaptic gap requires strict control to avoid excitotoxicity, and this control is exerted by the action of specific transporters expressed throughout the brain3. The EAAT1 and EAAT2 (GLT-1) reuptakers are located mainly in astrocytes, and GLT-1 is particularly abundant in the hippocampus and cortex3–7. Since signaling guided by the binding of glutamate to synaptic receptors is essential for memory processes8, the aim of this work is to study how interventions that affect the selective involvement of astrocytes in glutamate uptake impact memory processes.
The literature on the role of GLT-1 in memory is sparse and disparate. Its blockade in the central amygdala prior to cue fear training enhanced this memory9. However, when a GLT-1 blocker was intracerebrally administered in mice, the acquisition, consolidation and recall of object recognition memory was impaired10 as well as the expression of long-term memory in the Morris water maze test11. On the other hand, by blocking its action in the whole brain, the central amygdala or in the prefrontal cortex, anhedonia and/or anxiety were observed9,11,12
To investigate how astroglial glutamate uptake through GLT-1 impacts the dynamics of memory processes, we used a spatial object recognition (SOR) task in rats and administered a GLT-1 blocker, dihydrokainic acid (DHK), into the dorsal hippocampus (Hp). We found that GLT-1 blockade close to training sessions helps to consolidate spatial memories induced by weak training but does not alter consolidation mechanisms induced by strong training. However, the blockade of this transporter interferes with the expression of memory and with its reconsolidation. Furthermore, we observed opposite behavioral effects when we administered Ceftriaxone (CFT), an antibiotic that increases GLT-1 expression and its membrane integration. Our findings describe the full dynamics of a spatial memory formation and disruption under the hippocampal effects of astroglial glutamate uptake blockade. We further elucidate the underlying cellular mechanisms involving both the action of Arc and BDNF, which could represent possible sites of action for the development of new treatments for neuropsychiatric disorders and cognitive impairment.
Methods
Animals
Male and Female adult Wistar rats (Wistar IBCN) between 2 and 3 months of age (weight, 200–350 g) obtained from the Cellular Biology and Neurosciences Institute (IBCN) in the Faculty of Medicine of the University of Buenos Aires (Buenos Aires, Argentina) were used in this study. Animals were housed in groups of three with water and food ad libitum under a 12-h light/dark cycle at a constant temperature of 21–23°C. The behavioral testing took place during the light phase of the cycle. Rats were handled for 2 min for two consecutive days before each experiment to avoid unnecessary stress. During behavioral procedures, animals were individually moved from their home cages to the arena and returned immediately after each trial session. All animals were randomly assigned to the different experimental groups. All experiments were conducted in accordance with the National Institutes of Health Guides for Care and Use of Laboratory Animals (Publication No. 80–23, revised 1996) and approved by the Animal Care and Use Committee of the University of Buenos Aires, Buenos Aires, Argentina. We have complied with all relevant ethical regulations for animal use.
Surgery and drug infusion
For cannulae implantation, rats were deeply anesthetized (70 mg/kg ketamine and 7 mg/kg xylazine), and then 22-G cannulae were stereotaxically aimed to the dorsal hippocampus (Hp) at the following coordinates: A: −3.9 mm, L: ±3.0 mm, and D: −3.0 mm, from Bregma13. Then, they were cemented to the skull with dental acrylic. Animals received a subdermal application of analgesics and antibiotics during surgery (meloxicam 0.2 mg/kg, gentamicin 3 mg/kg). This analgesia and antibiotic protocol was maintained in the 2 days following the surgery. Animals were allowed to recover from surgery for at least five days. Drugs were infused using a 30-G needle with its tip protruding 1.0 mm beyond the guide. The infusion needles were linked by an acrylic tube to a Hamilton microsyringe and the entire bilateral infusion procedure lasted about 5 min. Needles were left in place for one additional minute after infusion to avoid back-flow.
Drugs
We administered dihydrokainic acid, a GLT-1 inhibitor (DHK, 12,5 nmol/1 µl per side), dissolved in a saline solution. The doses were chosen based on published studies11. DHK (Biosystems, Buenos Aires, Argentina) blocks the uptake of glutamate into astrocytes14,15, which leads to increased extrasynaptic glutamate16,17. Protein synthesis inhibitors were also used. In this case, Emetine (EME, 50 µg/0,8 µl per side) dissolved in saline solution. The effective dose and time of administration of EME were previously reported by us18. EME was purchased from Sigma (St. Louis, MO, USA). We also administered oligonucleotide pairs (ODNs, Genbiotech, S.R.L). They were prepared according to Guzowski et al.19. ODNs containing phosphorothioate linkages between the three bases on the 50 and 30 ends. Arc antisense ODN (Arc ASO) was directed against a 20-mer sequence (bases 209–228, GenBank accession number U19866) covering the Arc start site. Scrambled Arc ODN (Arc SCR) containing the same base composition in randomized order served as control. ODNs were delivered to the dorsal hippocampus via guide cannulae infusions (1 µg/1 µl per side). The infusion time was based on previous studies showing that ODN’s were maximally expressed in cells of the lateral amygdala at 3 h after infusion20 and also in our previous experience21. We also used function-blocking anti-BDNF antibodies (Chemicon, Temecula, CA; AB1513P) that were diluted to working concentration (0,5 µg/0,8 µl per side) with saline and administered 20 min before the time of interest18,22. Furthermore, we administered Ceftriaxone (CFT), a β-lactam antibiotic (dissolved in saline solution) intraperitoneally, (200 mg/kg/day) for 7 days23.
Histology
Histological examination of cannulae placements was performed after the end of the behavioral procedures by the infusion of 0.5 µl of 4% methylene blue in saline solution. Animals were sacrificed by decapitation 15 min after the infusion and their brains were sliced to verify the infusion area24. Only data from animals with correct cannulae implants were included in statistical analyses.
Behavioral procedures
SOR task
Animal memory performance was evaluated in the SOR task. SOR memory represents the ability to detect the spatial displacement of previously encountered objects. In this task, an animal reveals its learning of the spatial configuration of two identical objects, when it spends more time exploring the spatially displaced familiar object relative to a stationary familiar object in a test25. The SOR arena was a 60 cm wide × 40 cm long × 50 cm high acrylic box with different visual clues in its lateral white walls. The floor was white, the front wall was transparent and the back wall was hatched. All animals were habituated to the context. They were allowed to explore the arena without objects for a 20 min daily session for two consecutive days before the training day. In the training session, two identical plastic or glass objects were included in the arena in two adjacent corners and animals were left to explore it for 8 min in the case of a strong training (sSOR), and 4 min in a weak training (wSOR). In the test/reactivation session, one of the objects was displaced to a new position and animals were allowed to explore this context for 2 min. The exploration time for each object, defined as sniffing or touching it with the nose or forepaws, was measured using a hand stopwatch. Rats were excluded from the analysis when they explored one object more than 65% of the total object-exploration time during training sessions or when they did not reach 10 s in the total object-exploration time during the 2 min test session. Results are expressed as a preference index: [Exploration time of the object in a new location (Tn) – Exploration time of the object in the familiar location (Tf)]/[Tn + Tf]. A positive preference index in the test session, differing significantly from zero, indicates the presence of memory. A representative mean ± SEM of the total object-exploration time during a SOR training session and during a test session is shown in Supplementary Figs. 1, 2, for rats infused with DHK and CFT, respectively.
Open field task (OF)
The OF task consists of placing an animal within an arena to record its locomotor and exploratory behavior in this novel spatial context. The arena was a 50 cm wide × 50 cm long × 39 cm high square box, with black plywood walls and floor divided into nine squares by white lines. The number of line crossings and rearings was measured for 5 min under normal room lighting26.
Elevated Plus Maze
The Elevated Plus Maze test is used to measure anxiety-like behavior in rodents. It consists of four elevated arms which radiate from a central platform, forming a plus shape. Two of the opposed arms should be walled (apart from the ceiling, entrance and exit points) and the remaining two opposed arms should be open apart from the platform itself. In the test, a rat is placed in the central area and then it is left to explore the maze for a 5 min period. The amount of time spent in the walled arms is compared to the amount of time spent in the open arms as a measure of anxiety. The test is based on the natural tendencies of rodents to avoid open or elevated places counterbalanced with their innate curiosity to explore areas that are new to them. In theory, a rodent with a less anxious-like behavior will visit the open arms of the maze more frequently, whereas a rodent with elevated anxiety-like will tend to spend more time in the closed arms27.
Statistics and reproducibility
The sample size was determined based on previous studies. Experiments were conducted twice to ensure reproducibility. The experimenters were blinded to the experimental groups both during data collection and analysis. Numerical source data for all files in the manuscript can be found in the supplementary data file (Supplementary Data 1).
Results are expressed as preference index mean ± SEM. The bar graphs in the figures also show the individual data points. The index differences between groups were analyzed with unpaired Student’s t-test when comparing two groups, and a one-way ANOVA Test followed by Dunnett’s post-hoc test to compare each group against vehicle group or by Tukey’s posthoc comparison test to compare three or more groups between them. Differences were considered significant for α >0.05. If there were differences between SDs, the index differences between groups were analyzed with Welch’s ANOVA followed by Tamhane’s T2 multiple comparisons test. All tests were carried out after checking the normality of the data.
No software was used for data collection. Analyses were performed in GraphPad Prism version 8.00 (GraphPad Software, La Jolla, CA, USA). Effects were considered significant when P < 0.05. Also, to analyze animal locomotor activity and path traveled, we used Kinovea version 0.9.5.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Hippocampal GLT-1 inhibition promotes SOR-LTM from weak but not strong training
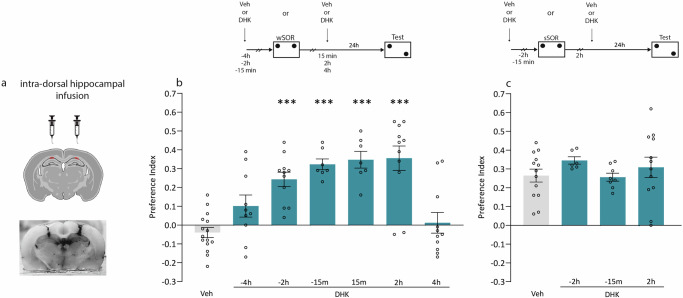
The objective of this work is to study the involvement of astroglial glutamate transporter GLT-1 in the specific processes of acquisition, consolidation, expression, reconsolidation, and persistence of SOR memory in rats. First, we administered vehicle solution (Veh) or the specific GLT-1 blocker DHK bilaterally in the dorsal Hp of rats (Fig. 1a) at different times either before or after (from −4 to 4 h) a weak SOR training (Fig. 1b), which consisted of a 4 min exploration session of a pair of identical objects. In the test session, 24 h later, memory was evidenced by the greater exploration towards the relocated object, and this is reflected in a preference index increment. As expected after weak training, Veh-infused animals did not show SOR-LTM (Fig. 1b, p > 0.05 vs. 0). In contrast, DHK infusion between −2 h to 2 h promoted SOR-LTM formation (p < 0.001 vs. Veh, Fig. 1b). However, the administration of DHK at times exceeding 3 h from the wSOR, failed to consolidate the LTM (p > 0.05 vs. Veh, Fig. 1b). These results suggest that the blockade of the astroglial specific glutamate transporter allowed establishing spatial memory induced by weak learning when given in a specific time window.
Fig. 1. Administration of DHK within a critical time window promotes SOR-LTM from weak but not strong training.
a Schematic representation of a coronal rat brain slice (adapted from “Mouse brain: coronal, thalamus and hippocampus”, by BioRender.com (2024). Retrieved from https://app.biorender.com/biorender-templates) showing the infusion area (top) and a picture of our dorsal Hp infusions (bottom). The top diagram shows the experimental design. Animals from individual groups received either weak or strong SOR training. b Animals subjected to weak training sessions were given intra-dorsal Hp infusions of DHK 4 h (n = 10), 2 h (n = 11), or 15 min (n = 7) before the training session; or 15 min (n = 7) 2 h (n = 11), or 4 h (n = 11) after that. LTM was tested 24 h after training. As Veh-infused rats did not show SOR-LTM regardless of the schedule of administration (−4, −2 h, −15, 15 min, 2 and 4 h from training session) a single representative Veh bar (n = 15) was plotted by randomly selecting animals from all groups that received Veh at every time point. Data are expressed as preference index mean ± SEM. ***p < 0,001 vs. Veh. Dunnett’s test after one-way ANOVA; F (6, 65) = 12.80. c Animals subjected to strong training sessions were given intra-dorsal Hp infusions of DHK 2 h (n = 6) or 15 min (n = 8) before, and 2 h (n = 12) after the training session. LTM was tested 24 h after training. As Veh-infused rats showed SOR-LTM at −2 h, −15 min, and 2 h from the training session, a single Veh representative bar (n = 13) was plotted by randomly selecting animals from those groups. Data are expressed as preference index mean ± SEM. p > 0,05 one-way ANOVA; F (3, 35) = 0.7613.
To investigate whether GLT-1 blockade modulates the consolidation of spatial memory induced by a strong training, we administered DHK into Hp of rats 2 h or 15 min before, or 2 h after a sSOR training session (Fig. 1c). SOR-LTM was similar between Veh and DHK-infused animals (Fig. 1c, p > 0.05 vs. Veh) suggesting that DHK does not modify memory consolidation induced by strong learning.
Memory promotion by hippocampal GLT-1 inhibition depends on protein synthesis
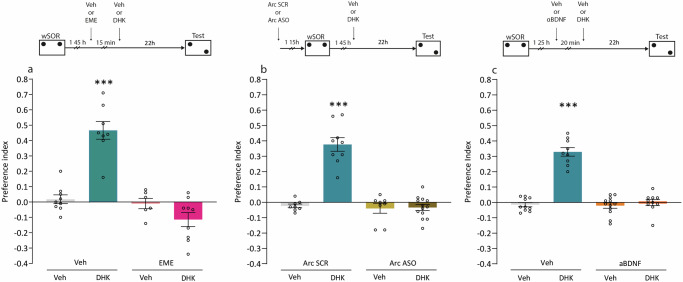
Since memory consolidation requires protein synthesis, our next objective was to study whether the promoting effect of DHK on SOR-LTM formation relied on this mechanism. For this, rats were trained in a wSOR and infused with Veh solution or a protein synthesis inhibitor (emetine, EME) and 15 min later were again infused with DHK or Veh as shown in Fig. 2a. As expected, Veh-Veh-infused rats did not show LTM 24 h after training, and Veh-DHK infused rats did (p < 0.001, Fig. 2a). In turn, EME administration prevented the promoting effect of DHK on LTM-SOR consolidation (p < 0.001, Fig. 2a), suggesting that the effect of DHK depends on protein synthesis. Then, we performed a similar experiment but administering a probe that prevents the translation of Arc (Arc ASO). In this case, Arc ASO was infused 3 h before DHK injection to allow it to exert its biological action (Fig. 2b). We also observed that the promoter effect of DHK was prevented by Arc ASO (p < 0.001, Fig. 2b), suggesting that GLT-1 blockade could induce Arc translation. Finally, we studied whether the action of DHK depended on the presence of brain-derived neurotrophic factor (BDNF). To do this, we infused an antibody that binds to BDNF (aBDNF), preventing its action on their receptors (Fig. 2c) We observed that the infusion of aBDNF 20 min prior to DHK injection induced LTM amnesia (p < 0.001, Fig. 2c). These results suggest that the promoting effect of DHK on SOR-LTM formation involves the action of BDNF in the dorsal Hp.
Fig. 2. Memory promotion through hippocampal GLT-1 inhibition is dependent on protein synthesis, the expression of the activity-regulated cytoskeletal protein and the activity of brain-derived neurotrophic factor.
The top diagram shows the experimental design. a Independent groups of animals were trained with a wSOR training session, 1 h 45 min later received an intra-dorsal Hp infusion of either Vehicle (Veh) or Emetine (EME), and 15 min later were injected with either Veh or DHK. LTM was tested for all groups (Veh-Veh n = 9; Veh-DHK n = 8; EME-Veh n = 6; EME-DHK n = 9) 24 h after training. Data are expressed as preference index mean ± SEM. ***p < 0,001 vs. all groups. Tukey’s multiple comparisons test after one-way ANOVA; F (3, 28) = 34.87. b Independent groups of animals received an intra-dorsal Hp infusion of either Arc SCR or Arc ASO, 1 h 15 min later were trained with a wSOR training session, and 1 h 45 min later were injected with either Veh or DHK. LTM was tested for all groups (Arc SCR-Veh n = 9; Arc SCR-DHK n = 8; Arc ASO-Veh n = 8; Arc ASO-DHK n = 13) 24 h after training. Data are expressed as preference index mean ± SEM. ***p < 0,001 vs. all groups. Tukey’s multiple comparisons test after one-way ANOVA; F (3, 34) = 49.27. c Independent groups of animals were trained with a wSOR training session, 1 h 25 min later received an intradorsal Hp infusion of either Veh or aBDNF and 20 min later received an injection of either Veh or DHK. LTM was tested for all groups (Veh-Veh n = 10; Veh-DHK n = 9; aBDNF-Veh n = 12; aBDNF-DHK n = 10) 24 h after training. Data are expressed as preference index mean ± SEM. ***p < 0.001 vs. all groups. Tukey’s multiple comparisons test after one-way ANOVA; F (3, 37) = 68.20.
Administration of DHK before a test session impairs memory expression
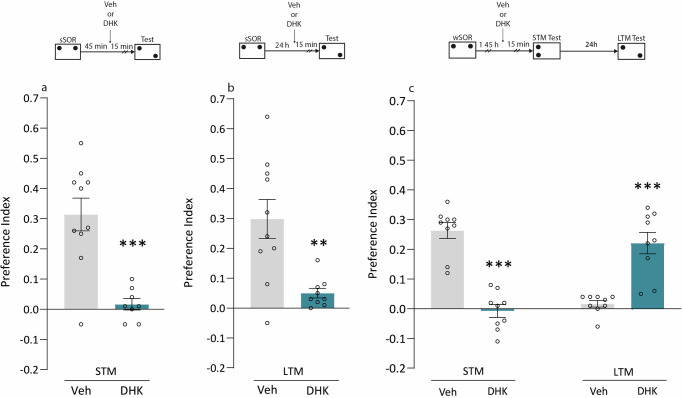
To study whether astroglial glutamate uptake through GLT-1 influences SOR memory expression, we administered DHK in Hp 15 min before the test session. In independent groups of rats, SOR memory was evaluated at 2 h (STM, Fig. 3a) or 24 h (LTM, Fig. 3b) after a sSOR training session. Animals infused with Veh solution expressed both types of memory, whereas rats infused with DHK expressed neither STM (p < 0.001, Fig. 3a) nor LTM (p < 0.01, Fig. 3b). This effect cannot be attributed to changes in locomotor activity or to anxiety-like states induced by DHK. This was evidenced by the administration of DHK into the Hp 15 min before an OF session, and by an Elevated Plus Maze session, which produced similar results to those of the Veh-infused rats (p > 0.05. Supplementary Fig. 3a–g).
Fig. 3. Administration of DHK 15 min before an STM test session impairs memory expression but promotes LTM formation.
The top diagram shows the experimental design. a Rats were trained with an sSOR session and received an intra-dorsal Hp infusion of either Veh (n = 10) or DHK (n = 8) 15 min before an STM test session. ***p < 0,001 Student’s t-test; t = 4.696. b Animals were trained with sSOR session and received an intra-dorsal Hp infusion of either Veh (n = 10) or DHK (n = 9) 15 min before an LTM test session. **p < 0,01 Student’s t-test; t = 3.503. c Animals were trained in a wSOR session, and 1 h 45 min later received an intra-dorsal Hp infusion of either Veh (n = 9) or DHK (n = 9). Memory was tested 15 min and 24 h after the injection. Data are expressed as preference index mean ± SEM.***p < 0,001 vs. Veh. Student’s t-test; t = 7.949 (left); t = 5.403 (right).
In Fig. 1b, we show that DHK administration 2 h after a wSOR promotes SOR-LTM, and in Fig. 3a, we see, in turn, that it acts by blocking STM expression after a sSOR. Then, to confirm that these effects occur simultaneously, we trained a group of male rats in a wSOR, and 1 h 45 min later, they were injected in Hp with Veh or DHK. STM was evaluated 2 h after training, and 24 h later, an additional test was performed to evaluate LTM in the same animals. As shown in Fig. 3c, DHK blocked wSOR-induced STM expression, observed in Veh animals (p < 0.001 vs. Veh, Fig. 3c), and at the same time promoted the SOR-LTM formation, which was absent in Veh animals (p < 0.001 vs. Veh, Fig. 3c). These results suggest that the negative effect of DHK on SOR-STM expression is reversible and is not observed in a long-term test session. Furthermore, they demonstrate that the action of DHK is not sex-specific, since both effects were observed either in females and males (Figs. 1b, 3c, respectively).
DHK-induced impairment of LTM expression depends on Arc but not on BDNF
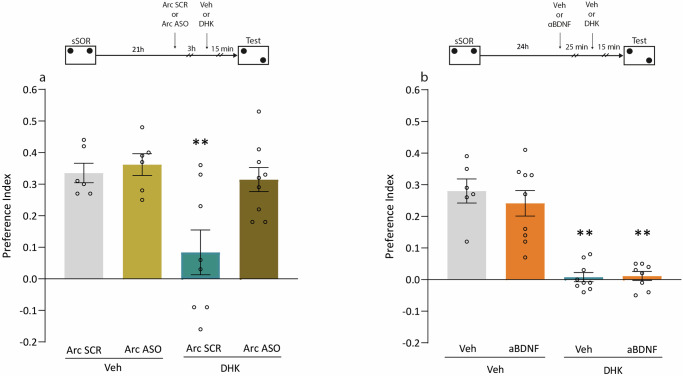
To determine whether the deleterious effect of DHK on SOR-LTM expression was dependent on Arc expression, we infused Arc ASO in the Hp of rats 3 h before DHK administration. The animals were trained in an sSOR session and, as expected, DHK infusion 15 min before the test session impaired LTM expression, and we found that the previously injected Arc ASO prevented this effect (p < 0.01 vs. all other groups, Fig. 4a). In contrast, aBDNF administration did not reverse the inhibitory effect of DHK on memory expression (p < 0.05 vs. Veh-DHK, Fig. 4b). Taken together, these results suggest that the impairment of SOR-LTM expression induced by DHK, administered minutes before the test session, is dependent on Arc translation but not on BDNF action.
Fig. 4. DHK-induced impairment of memory expression is dependent on Arc expression but not on BDNF activity.
The top diagram shows the experimental design. All animals were trained with sSOR. a The following day, independent groups of animals received an intra-dorsal Hp infusion of either Arc SCR or Arc ASO, 3 h later, they were injected with either Veh or DHK and 15 min later, LTM was tested for all groups (Arc SCR-Veh n = 6; Arc ASO-Veh n = 6; Arc SCR-DHK n = 8; Arc ASO-DHK n = 9). **p < 0.01 vs. all groups. Tukey’s multiple comparisons test after one-way ANOVA; F (3, 25) = 6.853. b Independent groups of animals received an intra-dorsal Hp infusion of either Veh or aBDNF, 25 min later received an intra-dorsal hippocampal infusion of either Veh or DHK, and 15 min later LTM was tested for all groups (Veh–Veh n = 6; aBDNF–Veh n = 9; Veh–DHK n = 9; aBDNF–DHK n = 8). **p < 0.01 vs. Veh–Veh and aBNDF–Veh. Tamhane’s T2 multiple comparisons after Welch’s ANOVA; W (3.000, 16.55) = 24.14.
DHK does not induce a drug-state-dependent effect
We studied the possibility that the animals require the administration of DHK prior to the sSOR training and prior to the SOR test to express the SOR-LTM. So, we performed such an experiment to study if there is a state dependency with DHK on SOR memory. As expected, the group of animals infused with DHK pretraining and with Veh pretest (DHK-Veh), as well as the Veh–Veh group showed SOR-LTM (Fig. 5). Also, we confirmed that the administration of DHK pretest (Veh–DHK) prevented the expression of SOR-LTM (p < 0.01 vs. Veh–Veh, Fig. 5) but an additional administration of DHK prior to training (DHK–DHK) did not prevent this SOR-LTM amnesia (p < 0.001 vs. Veh–Veh, Fig. 5). These results rule out the possibility that DHK infusion generates state dependence on SOR memory.
Fig. 5. DHK does not induce a drug-state-dependent effect.

The top diagram shows the experimental design. Independent groups of animals received a first intra-dorsal Hp infusion of either Veh or DHK, 15 min later experienced an sSOR training session, and a second infusion of either Veh or DHK was administered 15 min before the test session (Veh–Veh, n = 6, DHK–Veh, n = 7, Veh–DHK, n = 6, DHK–DHK, n = 7). In all groups, LTM was tested 24 h after the training session. **p < 0,01 ***p < 0,001 vs. Veh–Veh and DHK–Veh. Tukey’s multiple comparisons test after one-way ANOVA; F (3, 22) = 15.09.
DHK-induced impairment of LTM persistence depends on Arc expression
In previous figures, we showed that the infusion of DHK prevented SOR memory expression through a mechanism dependent on Arc expression. We have recently shown that SOR-LTM expression is required for wSOR retraining to prolong SOR-LTM persistence at least for a week28. Thus, we evaluated the persistence of SOR memory after administering DHK in the presence of Arc SCR or Arc ASO prior to retraining. When sSOR was followed 24 h later by a wSOR, the promotion of SOR-LTM persistence was observed both in the groups that received Arc SCR-Veh and Arc ASO-Veh (p < 0.001 vs. 0 Fig. 6). However, the administration of Arc SCR followed by DHK 15 min prior to the wSOR retraining session, prevented the persistence of the memory (p < 0.001 vs. all groups Fig. 6). As expected, the administration of Arc ASO before DHK, prevented the amnesia (p < 0.001 vs. 0 Fig. 6). These results suggest that DHK acting on the SOR-LTM expression mechanisms, prevented memory from persisting over time and that this mechanism depends on Arc expression.
Fig. 6. DHK-induced impairment of LTM persistence promoted by a retraining protocol is dependent on Arc expression.

The top diagram shows the experimental design. Independent groups of animals experienced a sSOR training session, 21 h later received a first intra-dorsal Hp infusion of Arc SCR or Arc ASO, 3 h later received a second infusion of Veh or DHK, and 15 min later experienced a wSOR training session. All groups (Arc SCR-Veh n = 8; Arc ASO-Veh n = 8; Arc SCR-DHK n = 11; Arc ASO-DHK n = 10) were tested 7 days later. Data are expressed as preference index mean ± SEM.***p < 0,001 vs. all groups. Tukey’s multiple comparisons test after one-way ANOVA; F (3, 33) = 10.39.
DHK-induced impairment of memory reconsolidation is Arc expression-independent
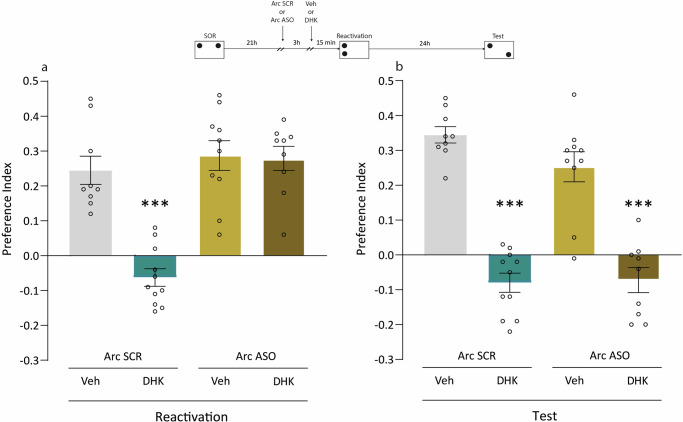
Our next objective was to study whether the blockade of glutamate reuptake by astroglial GLT-1 affects the SOR reconsolidation process. Some reminders induce memory reconsolidation, which is a process that allows memory to be sustained and even updated with new information29. We used the experimental protocol consisting of a sSOR session followed 24 h later by a SOR test session, a reminder event that functions as an inducer of the reconsolidation process, which we will refer to as a reactivation session30. The infusion of Veh or DHK solution into the Hp was performed 15 min before the reactivation session, and memory was tested the following day. In the case that the drug blocks the reconsolidation process, SOR-LTM will not be observed in the test session carried out 48 h after sSOR. As expected, Fig. 7a shows that DHK administration before reactivation impaired the expression of SOR-LTM evaluated in this session (p < 0.001 vs. Arc SCR-Veh, Fig. 7a), and this effect was blocked by Arc ASO (p < 0.001 vs. Arc SCR-DHK, Fig. 7a). The SOR amnesia induced by Arc SCR-DHK pre reactivation was irreversible since it was also observed in the test session carried out 48 h post sSOR (p < 0.001 vs. Arc SCR-Veh, Fig. 7b). This means that DHK, administered before reactivation impaired the process of reconsolidation, and the same result was observed even when Arc ASO administration did not prevent memory expression at the time of the reactivation session (p > 0.05, Arc SCR-DHK vs. Arc ASO-DHK Fig. 7b). Taken together, these results suggest that Arc ASO infusion prevented the negative effects of DHK on SOR memory expression, but not those acting on the process of SOR memory reconsolidation. We also observed that DHK impaired the SOR memory reconsolidation even when it was administered after the reactivation session (Supplementary Fig. 4). In this case, a non-reactivated group was included to confirm that the amnesic effect of DHK was indeed dependent on the SOR memory reactivation process. These results suggest that the infusion of DHK, peri-reactivation of a spatial memory trace, acted negatively on the reconsolidation process and prevented the persistence of SOR memory.
Fig. 7. DHK-induced impairment of memory reconsolidation does not depend on Arc expression.
The top diagram shows the experimental design. Independent groups of animals experienced a sSOR training session, 21 h later received a first intra-dorsal Hp infusion of Arc SCR or Arc ASO, 3 h later received a second infusion of Veh or DHK, and 15 min later experienced a 2 min reactivation session (Arc SCR-Veh n = 9; Arc SCR-DHK n = 11; Arc ASO-Veh n = 10; Arc ASO-DHK n = 9). a All groups were tested during the reactivation session, 24 h after the training session. ***p < 0.001 vs. all groups. Tukey’s multiple comparisons test after one-way ANOVA; F (3, 35) = 23.78. b All groups were tested 48 h after the training session. Data are expressed as preference index mean ± SEM. ***p < 0,001 vs. Arc SCR-Veh and Arc ASO-Veh. Tukey’s multiple comparisons test after one-way ANOVA; F (3, 35) = 42.34.
Chronic systemic administration of Ceftriaxone impairs LTM formation
We were interested in evaluating the effect that the gain of function of GLT-1 may exert on SOR memory, hypothesizing that it would be opposite to what we observed with GLT-1 blocker administration. To do this, we used the antibiotic Ceftriaxone (CFT), a β-lactam antibiotic, which, administered sub-chronically, induces an upregulation in GLT-1 in Hp and other brain regions23,31. We performed systemic administration of either CFT or Veh once daily for 7 days before the sSOR training session and studied its impact on SOR memory acquisition, consolidation, and expression. Figure 8 shows that the group of animals treated with Veh expressed both SOR-STM and SOR-LTM. CFT-injected animals showed SOR-STM (p > 0.05 vs. Veh) but did not show SOR-LTM (p < 0.001 vs. Veh), suggesting that they were able to acquire the information but not consolidate the memory trace. We ruled out that the effects of CFT on SOR memory are due to changes in the exploratory activity of the rats or to an anxiolytic-like state, since the parameters recorded during an OF session followed by an Elevated Plus Maze were not different from those of the Veh-injected animals (Supplementary Fig. 5). These results support the hypothesis that the effects of CFT are opposite to those observed after DHK administration, characterized by the reversible blockade of SOR-STM expression (Fig. 3b) and the absence of effects on consolidation triggered by sSOR (Fig. 1c). These results suggest that if training is strong enough to induce LTM, the transient glutamate increase in the synaptic cleft, due to loss of GLT-1 function, does not alter the process of memory consolidation. However, the gain of GLT-1 function, provoked by chronic administration of CFT, prevents consolidation probably by decreasing the glutamate level in the cleft. These suggest that a sufficient level of glutamate at the cleft is needed at training to form memories and that GLT-1 plays a fundamental role in this balance.
Fig. 8. Chronic systemic administration of Ceftriaxone (CFT) does not affect acquisition and STM expression but impairs LTM formation.

The top diagram shows the experimental design. All groups were trained with a sSOR session. All animals received intraperitoneal injections of either Veh (n = 9) or CFT (n = 10) for 7 days. STM was tested (left) 1 h after training. Data are expressed as preference index mean ± SEM. p > 0.05 vs. Veh STM Student’s t-test; t = 0.6798. The same groups of rats were tested 24 h after the STM test (right). Data are expressed as preference index mean ± SEM. ***p < 0.001 vs. Veh LTM. Student’s t-test; t = 6.449.
Discussion
Glutamate uptake is necessary to end the action of this neurotransmitter in the synaptic cleft, and astrocytes are essential players in this function. Here, we studied how alterations in glutamate homeostasis in rat Hp modulated spatial learning memory. Our results demonstrate that the regulation of GLT-1 function (decreasing it with DHK or increasing it with CFT administrations, respectively), differentially affects the processes of formation, expression and/or reconsolidation of spatial memory in rodents. Therefore, physiological mechanisms that alter the expression or stabilization of GLT-1 on the cell surface will affect memory processes. In this sense, glutamate transporters posttranslational modifications, such as sumoylation, palmitoylation, nitrosylation, or ubiquitination, would contribute to such regulation32,33. Furthermore, it has been demonstrated the role of PKC in cellular localization and regulation of GLT-134. Also, neuronal activity can regulate astrocytic glutamate transporters via signaling through soluble factors35. Other factors, such as estrogen levels or chronic restraint stress, would contribute to an increase in the expression of GLT-136–38.
Here, we found that blockading the hippocampal astroglial GLT-1 glutamate transporter by the administration of DHK promoted SOR-LTM formation induced by weak training without affecting the consolidation process induced by strong training. On the other hand, the blockade of astroglial GLT-1 hindered the expression of STM and LTM in the SOR task, also affecting the process of memory reconsolidation and memory persistence induced by retraining. In contrast, treatment with CFT, a β-lactam antibiotic that increases GLT-1 expression, did not interfere with SOR acquisition and STM expression, but impaired memory consolidation. In other words, the role of astrocytes in glutamate neurotransmission affects the information being processed, impacting the formation, expression, and persistence of SOR memory.
This is the first work that characterizes the effects on memory processes induced by an acute infusion of DHK into the rodent dorsal Hp. Our results suggest that the administration of 12,5 nmol/side of DHK into the Hp did not cause excitotoxicity. Instead, it promoted the formation of LTM in a time-dependent manner or caused a reversible impairment of memory expression when administered before the STM test, without affecting LTM the following day. In turn, DHK did not alter the locomotor activity or the anxious-like state of the rat, similar to what was previously reported after icv administration by Bechtholt-Gompf et al.11. However, Tian et al.10 trained mice in a novel object recognition task and found that the icv administration of DHK blocked the acquisition, consolidation and expression of this memory and also induced locomotor and exploratory deficits. The discrepancy in these results may depend on the learning task, species and/or route of administration used.
It is known that protein synthesis is necessary to consolidate LTM39,40. In that sense, it is considered that wSOR does not form LTM because it does not induce enough plasticity-related proteins (PRPs). Therefore, by associating wSOR with a PRP provider event (e.g. novel open field, elevated platform, spaced training, etc.) it is possible to consolidate the SOR-LTM. In fact, by inhibiting the protein translation induced by such associated events, the promoting effect on the formation of SOR-LTM disappears18,41–43. These works also showed that the promoter effect occurs only if the associated event is experienced in a critical time window around the wSOR. The mechanisms responsible for this phenomenon are characterized not only in spatial tasks but also in aversive ones44–48 and are part of the behavioral tagging hypothesis, postulated by our group 15 years ago26. Interestingly, here we observed that the promoting effect of the DHK infusion on the formation of SOR-LTM induced by a wSOR was similar to that described for the promoting effect induced by OF, occurring at −2 and +2 h of the wSOR, but being ineffective at more distant times (Ballarini et al.41 and Fig. 1a). Furthermore, both the effects of OF and DHK were shown to be dependent on protein translation (Ballarini et al.41 and Fig. 2a). For all these reasons, we propose that the acute administration of DHK into the Hp could facilitate LTM consolidation induced by weak training through the transient increase in glutamate levels, which can activate their synaptic receptors contributing to the cellular signaling involved in protein synthesis. We also observed that administration of DHK around the training session did not modify the SOR-LTM induced by sSOR (Fig. 1b). This suggests that when the learning is strong enough to induce the PRPs synthesis, additional help is not needed to consolidate the LTM. In addition, these results indicate that DHK did not impair SOR memory acquisition.
Our work also demonstrates that infusing DHK into the rat Hp affects LTM consolidation (Figs. 1b, 2) and expression (Figs. 3, 4), and these processes are Arc and/or BDNF dependent, revealing, to the best of our knowledge, novel drug action mechanisms. Some previous reports suggest that GLT-1 function may regulate the expression of these proteins. The icv administration of DHK increased the number of cfos-positive cells in the rat dorsal dentate gyrus11, and it is known that the cFos pathway increases BDNF expression49. It was also reported that the downregulation of GLT-1 increased BDNF levels in rat striatum50. In turn, BDNF can induce Arc gene transcription51. Thus, all these findings, together with the involvement of Arc and BDNF in the establishment of synaptic plasticity and memory consolidation52,53 are in agreement with our results showing the dependence of these proteins in promoting SOR-LTM formation induced by DHK.
Previous work demonstrated that icv administration of DHK impaired LTM expression in the Morris water maze and Novel Object Recognition tasks10,11. Our results describe that blocking the GLT-1 transporter in dorsal Hp is sufficient to prevent the expression of both SOR-STM and SOR-LTM (Fig. 3a, b). In addition, these effects depend on Arc translation (Fig. 4a). Although the molecular mechanism of memory expression remains incompletely described, we know that different types of learning require kinase activity54,55 and AMPA receptor trafficking to the membrane, which depends on online protein synthesis56. Indeed, rapamycin or anisomycin infusion prior to memory test sessions for various learning tasks, including SOR, has been shown to impair memory expression56–58. Ongoing protein synthesis is necessary to maintain stable GluA1 levels in the postsynaptic density, which is a requirement for successful memory retrieval56,59. Thus, Bast et al.60 describe that retrieval of a place memory depends on fast excitatory hippocampal transmission that is mediated by AMPA receptors.
A striking fact is that AMPAR removal from the postsynaptic membrane involves the Arc protein, through its high binding affinity to unphosphorylated CaMKIIβ, which is prevalent at inactive synapses61,62. CaMKII, including the CaMKIIβ subunit, is the most abundant protein in excitatory synapses and is central to synaptic plasticity, learning, and memory63. We propose that prior to the test session, the synapses would be mostly inactivated, and therefore the nonphosphorylated form of CaMKIIβ would prevail. Considering the background previously described the mechanisms of memory retrieval, the blockage of LTM expression caused by the pre-test administration of DHK could involve the expression of Arc, which binds to nonphosphorylated CaMKIIβ, signaling the mechanism for AMPA receptor endocytosis. In accordance with this, we found that DHK memory expression impairment is dependent on Arc translation, but not on the action of BDNF (Fig. 4b).
It may seem contradictory that we find Arc involved both in impairing SOR-LTM expression and in improving SOR consolidation induced by weak learning (Fig. 2b). What is the mechanism by which Arc triggers the promnesic effect? As a result of diverse learning experiences, consisting of either aversive or spatial training sessions, CaMKII is activated and autophosphorylated64,65. The administration of hippocampal DHK around training could supply neurons with Arc which takes part in the signaling triggered by activated CaMKII, favoring mechanisms tending to mature and strengthen spine structural plasticity66,67. Thereby, we propose that depending on the biochemical synaptic environment (e.g., CaMKII inactivated or activated), Arc would be involved in weakening synapses and preventing LTM expression or in stabilizing them, allowing memory consolidation. Our proposal is in accordance with the hypothesis of bidirectional regulation of synaptic plasticity based on the Arc oligomeric state. Arc has distinct states in the neuron, where Arc dimer regulates actin cytoskeleton dynamics and a tetramer that facilitates AMPAR endocytosis. It is relevant that regulation of the oligomeric state would occur by post-translational modifications, such as CaMKIIα-dependent phosphorylation, which blocks the generation of Arc large oligomers68.
Another question to solve was whether the SOR-LTM amnesia induced by DHK administration before the test session relied on a drug state-dependence phenomenon. In other words, do animals need to have the same brain state before training and before test sessions to express LTM? There are reports of memories being retrieved more easily when the drug-induced brain state during recall is similar to the drug-induced brain state when learning occurred69,70. Our results showed that amnesia induced by pretest DHK infusion occurred regardless of whether or not the animals received a dose of DHK prior to sSOR training (Fig. 5). Thus, DHK does not exhibit state dependence; instead, DHK might be activating a mechanism that prevents memory expression.
We have recently shown that the cellular mechanism involved in LTM expression is required to promote the persistence of the memory trace by spaced training28. The experiments showed that a single sSOR did not induce memory expression a week later. However, when a subsequent wSOR trial was applied one day after the original training, the persistence of SOR-LTM was promoted. This phenomenon was blocked by disrupting the molecular mechanism of memory expression at the time of retraining28. Here, we showed that DHK completely impaired the memory persistence promoted by spaced training, confirming the deleterious role of DHK in SOR memory expression, which involved Arc expression (Fig. 6).
Another strategy to maintain the memory trace includes exposing the animal to a reminding event like a test session. It was described that a test session given one day after sSOR-induced memory reconsolidation30, which refers to the process of destabilization/restabilization of a memory after its activation71. DHK administered prior to the reactivation session, conducted 24 h after the sSOR, induced both SOR-LTM lack of expression and amnesia evaluated in the subsequent test performed the following day (Fig. 7). These findings evidence its negative effect on the reconsolidation process. However, DHK’s detrimental effect on the reconsolidation process was not prevented by Hp Arc ASO administration, suggesting that Arc was not involved in this mechanism (Fig. 7b). Also, we found that GLT-1 blockade immediately after recall prevented reconsolidation (Supplementary Fig. 4). This result is in agreement with previous works studying the role of astrocyte-derived lactate on memory conditioning to cocaine-induced place preference72,73. Authors showed long-term amnesia induced by glycogenolysis inhibition into the basolateral amygdala after the reactivation session, evidencing the action of glia on the reconsolidation process. Our work is the first to show that GLT-1 blockade in Hp prevents memory reconsolidation, placing astrocytes as crucial players in memory trace restabilization.
The other side of the coin was to study SOR memory processes by upregulating astroglial GLT-1. It has been shown that the chronic and systemic administration of Ceftraixone increases GLT-1 protein levels in the membrane. This increase is more sensitive and earlier in the Hp of the animals than in other brain regions31. We trained rats after one week of treatment with CFT and observed that they expressed SOR-STM, but not SOR-LTM, demonstrating that the animals were able to acquire the information, but they could not consolidate it (Fig. 8). Furthermore, animals that underwent chronic administration of CFT display memory impairments in novel object recognition, when compared to control rats74 with no behavioral abnormalities observed in the OF test or Morris water maze test75. We did not observe changes in the OF exploration, nor in the Elevated Plus Maze rat performance after systemic administration of CFT, suggesting that SOR-LTM amnesia induced by CFT was not due to changes in locomotor activity or in the anxiety-like state of rats.
Conclusion
We conclude that glutamate uptake modulation through astroglial GLT-1 affects spatial memory processes. The effect of this modulation depends on the intensity of the spatial learning, as well as the phase of memory evaluated. We demonstrated that, under hippocampal GLT-1 blockade conditions, rats were able to acquire spatial learning and even consolidate the memory induced by weak training. Also, GLT-1 blockade around strong training did not modify spatial memory consolidation. However, rats were unable to express or reconsolidate the trace while GLT-1 was blocked. We also revealed that hippocampal GLT-1 blockade activated a mechanism that depends on Arc translation and/or BDNF action. In contrast, GLT-1 upregulation impaired spatial memory consolidation, but did not prevent learning acquisition or expression. In conclusion, here we described the critical involvement of hippocampal glutamate uptake from astroglia in the processes of formation, persistence, and access to spatial memories. Our findings highlight possible sites of action for the development of new treatments for neuropsychiatric disorders such as specific phobias, posttraumatic stress disorder, and cognitive impairment.
Supplementary information
Acknowledgements
This work was supported by funding from grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PICT 2019-00967), Argentina, to Haydee Viola.
Author contributions
J.G.R.: Conceptualization; methodology; formal analysis; investigation; writing— original draft preparation; writing—review & editing. J.C.: Conceptualization; investigation; writing—review & editing. M.M.R.: Investigation; writing—review & editing. R.T.: Investigation; writing—review & editing. H.V.: Conceptualization; methodology; formal analysis; investigation; writing—original draft preparation; writing—review & editing; project administration; funding acquisition.
Peer review
Peer review information
Communications Biology thanks Tomonori Takeuchi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Joao Valente.
Data availability
Numerical source data for all files in the manuscript can be found in supplementary data file at 10.17632/72zh75ks9t.1 (Supplementary Data 1). None of the experiments were preregistered.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-06586-8.
References
- 1.Durkee, C. A. & Araque, A. Diversity and specificity of Astrocyte–neuron communication. Neuroscience396, 73–78 (2019). 10.1016/j.neuroscience.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamsky, A. & Goshen, I. Astrocytes in memory function: pioneering findings and future directions. Neuroscience370, 14–26 (2018). 10.1016/j.neuroscience.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 3.Furuta, A., Rothstein, J. D. & Martin, J. L. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J. Neurosci.17, 8363–8375 (1997). 10.1523/JNEUROSCI.17-21-08363.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothstein, J. D. et al. Localization of neuronal and glial glutamate transporters. Neuron13, 713–725 (1994). 10.1016/0896-6273(94)90038-8 [DOI] [PubMed] [Google Scholar]
- 5.Chaudhry, F. A. et al. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron15, 711–720 (1995). 10.1016/0896-6273(95)90158-2 [DOI] [PubMed] [Google Scholar]
- 6.Lehre, K. P., Levy, L. M., Ottersen, O. P., Storm-Mathisen, J. & Danbolt, N. C. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J. Neurosci.15, 1835–1853 (1995). 10.1523/JNEUROSCI.15-03-01835.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt, A., Asan, E., Püschel, B. & Kugler, P. Cellular and regional distribution of the glutamate transporter GLAST in the CNS of rats: nonradioactive in situ hybridization and comparative immunocytochemistry. J. Neurosci.17, 1–10 (1997). 10.1523/JNEUROSCI.17-01-00001.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riedel, G., Platt, B. & Micheau, J. Glutamate receptor function in learning and memory. Behav. Brain Res.140, 1–47 (2003). 10.1016/S0166-4328(02)00272-3 [DOI] [PubMed] [Google Scholar]
- 9.John, C. S. et al. Blockade of the GLT-1 transporter in the central nucleus of the amygdala induces both anxiety and depressive-like symptoms. Neuropsychopharmacology40, 1700–1708 (2015). 10.1038/npp.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian, S. W., Yu, X. D., Cen, L. & Xiao, Z. Y. Glutamate transporter GLT1 inhibitor dihydrokainic acid impairs novel object recognition memory performance in mice. Physiol. Behav.199, 28–32 (2019). 10.1016/j.physbeh.2018.10.019 [DOI] [PubMed] [Google Scholar]
- 11.Bechtholt-Gompf, A. J. et al. Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology35, 2049–2059 (2010). 10.1038/npp.2010.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John, C. S. et al. Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology37, 2467–2475 (2012). 10.1038/npp.2012.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates 6th edn (Academic Press, 2006).
- 14.Anderson, C. M. & Swanson, R. A. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia32, 1–14 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Arriza, J. L. et al. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci.14, 5559–5569 (1994). 10.1523/JNEUROSCI.14-09-05559.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallgren, Å. B. & Paulsen, R. E. A microdialysis study in rat brain of dihydrokainate, a glutamate uptake inhibitor. Neurochem. Res.21, 19–25 (1996). 10.1007/BF02527667 [DOI] [PubMed] [Google Scholar]
- 17.Robinson, M. B., Hunter-Ensor, M. & Sinor, J. Pharmacologically distinct sodiumdependentl-[3H]glutamate transport processes in rat brain. Brain Res.544, 196–202 (1991). 10.1016/0006-8993(91)90054-Y [DOI] [PubMed] [Google Scholar]
- 18.Lopes da Cunha, P., Villar, M. E., Ballarini, F., Tintorelli, R. & Ana María Viola, H. Spatial object recognition memory formation under acute stress. Hippocampus29, 491–499 (2019). 10.1002/hipo.23037 [DOI] [PubMed] [Google Scholar]
- 19.Guzowski, J. F. et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci.20, 3993–4001 (2000). 10.1523/JNEUROSCI.20-11-03993.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ploski, J. E. et al. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of Pavlovian fear conditioning in the lateral amygdala. J. Neurosci.28, 12383–12395 (2008). 10.1523/JNEUROSCI.1662-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez, M. C., Alen, N., Ballarini, F., Moncada, D. & Viola, H. Memory traces compete under regimes of limited Arc protein synthesis: implications for memory interference. Neurobiol. Learn. Mem.98, 165–173 (2012). 10.1016/j.nlm.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 22.Slipczuk, L. et al. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS ONE4, e6007 (2009). 10.1371/journal.pone.0006007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamidi, N., Nozad, A., Sheikhkanloui Mila, H., Salari, A. A. & Amani, M. Effect of ceftriaxone on paired-pulse response and long-term potentiation of hippocampal dentate gyrus neurons in rats with Alzheimer-like disease. Life Sci.238, 116969 (2019). 10.1016/j.lfs.2019.116969 [DOI] [PubMed] [Google Scholar]
- 24.Villar, M. E., Martinez, M. C., Lopes da Cunha, P., Ballarini, F. & Viola, H. Memory consolidation and expression of object recognition are susceptible to retroactive interference. Neurobiol. Learn. Mem.138, 198–205 (2017). 10.1016/j.nlm.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 25.Dere, E., Huston, J. P. & De Souza Silva, M. A. Integrated memory for objects, places, and temporal order: evidence for episodic-like memory in mice. Neurobiol. Learn. Mem.84, 214–221 (2005). 10.1016/j.nlm.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 26.Moncada, D. & Viola, H. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J. Neurosci.27, 7476–7481 (2007). 10.1523/JNEUROSCI.1083-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraeuter, A. K., Guest, P. C. & Sarnyai, Z. The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods Mol. Biol.1916, 69–74 (2019). 10.1007/978-1-4939-8994-2_4 [DOI] [PubMed] [Google Scholar]
- 28.Correa, J., Tintorelli, R., Budriesi, P. & Viola, H. Persistence of spatial memory induced by spaced training involves a behavioral-tagging process. Neuroscience497, 215–227 (2022). 10.1016/j.neuroscience.2022.02.032 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Ortiz, C. J. & Bermúdez-Rattoni, F. Determinants to trigger memory reconsolidation: the role of retrieval and updating information. Neurobiol. Learn. Mem.142, 4–12 (2017). 10.1016/j.nlm.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 30.Orlandi, I. R. et al. Behavioral tagging underlies memory reconsolidation. Proc. Natl Acad. Sci. USA117, 18029–18036 (2020). 10.1073/pnas.2009517117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smaga, I., Fierro, D., Mesa, J., Filip, M. & Knackstedt, L. A. Molecular changes evoked by the beta-lactam antibiotic ceftriaxone across rodent models of substance use disorder and neurological disease. Neurosci. Biobehav. Rev.115, 116–130 (2020). 10.1016/j.neubiorev.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-Villarreal, J., García Tardón, N., Ibáñez, I., Giménez, C. & Zafra, F. Cell surface turnover of the glutamate transporter GLT-1 is mediated by ubiquitination/deubiquitination. Glia60, 1356–1365 (2012). 10.1002/glia.22354 [DOI] [PubMed] [Google Scholar]
- 33.Peterson, A. R. & Binder, D. K. Post-translational regulation of GLT-1 in neurological diseases and its potential as an effective therapeutic target. Front. Mol. Neurosci.12, 164 (2019). 10.3389/fnmol.2019.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalandadze, A., Wu, Y. & Robinson, M. B. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J. Biol. Chem.277, 45741–45750 (2002). 10.1074/jbc.M203771200 [DOI] [PubMed] [Google Scholar]
- 35.Perego, C. et al. The GLT-1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. J. Neurochem.75, 1076–1084 (2000). 10.1046/j.1471-4159.2000.0751076.x [DOI] [PubMed] [Google Scholar]
- 36.Pawlak, J., Brito, V., Küppers, E. & Beyer, C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res. Mol. Brain Res.138, 1–7 (2005). 10.1016/j.molbrainres.2004.10.043 [DOI] [PubMed] [Google Scholar]
- 37.Reagan, L. P. et al. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc. Natl Acad. Sci. USA101, 2179–2184 (2004). 10.1073/pnas.0307294101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zschocke, J. et al. Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. J. Biol. Chem.280, 34924–34932 (2005). 10.1074/jbc.M502581200 [DOI] [PubMed] [Google Scholar]
- 39.McGaugh, J. L. Memory—a century of consolidation. Science287, 248–251 (2000). 10.1126/science.287.5451.248 [DOI] [PubMed] [Google Scholar]
- 40.Costa-Mattioli, M. & Sonenberg, N. Translational control of gene expression: a molecular switch for memory storage. Prog. Brain Res.169, 81–95 (2008). 10.1016/S0079-6123(07)00005-2 [DOI] [PubMed] [Google Scholar]
- 41.Ballarini, F., Moncada, D., Martinez, M. C., Alen, N. & Viola, H. Behavioral tagging is a general mechanism of long-term memory formation. Proc. Natl Acad. Sci. USA106, 14599–14604 (2009). 10.1073/pnas.0907078106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cassini, L. F. et al. Memory reconsolidation allows the consolidation of a concomitant weak learning through a synaptic tagging and capture mechanism. Hippocampus23, 931–941 (2013). 10.1002/hipo.22149 [DOI] [PubMed] [Google Scholar]
- 43.Tintorelli, R. et al. Spatial-memory formation after spaced learning involves ERKs1/2 activation through a behavioral-tagging process. Sci. Rep.10, 98 (2020). 10.1038/s41598-019-57007-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moncada, D., Ballarini, F., Martinez, M. C., Frey, J. U. & Viola, H. Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc. Natl Acad. Sci. USA108, 12931–12936 (2011). 10.1073/pnas.1104495108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okuda, K., Højgaard, K., Privitera, L., Bayraktar, G. & Takeuchi, T. Initial memory consolidation and the synaptic tagging and capture hypothesis. Eur. J. Neurosci.54, 6826–6849 (2021). 10.1111/ejn.14902 [DOI] [PubMed] [Google Scholar]
- 46.Redondo, R. L. & Morris, R. G. M. Making memories last: the synaptic tagging and capture hypothesis. Nat. Rev. Neurosci.12, 17–30 (2011). 10.1038/nrn2963 [DOI] [PubMed] [Google Scholar]
- 47.Vishnoi, S., Raisuddin, S. & Parvez, S. Behavioral tagging: A novel model for studying long-term memory. Neurosci. Biobehav. Rev.68, 361–369 (2016). 10.1016/j.neubiorev.2016.05.017 [DOI] [PubMed] [Google Scholar]
- 48.Budriesi, P. et al. A behavioral tagging account of kinase contribution to memory formation after spaced aversive training. iScience26, 107278 (2023). 10.1016/j.isci.2023.107278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramírez, D. et al. Melanocortin 4 receptor activates ERK-cFos pathway to increase brain-derived neurotrophic factor expression in rat astrocytes and hypothalamus. Mol. Cell. Endocrinol.411, 28–37 (2015). 10.1016/j.mce.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 50.Alasmari, F. et al. E-cigarette aerosols containing nicotine modulate nicotinic acetylcholine receptors and astroglial glutamate transporters in mesocorticolimbic brain regions of chronically exposed mice. Chem. Biol. Interact.333, 109308 (2021). 10.1016/j.cbi.2020.109308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kikuchi, K. et al. Involvement of SRF coactivator MKL2 in BDNF-mediated activation of the synaptic activity-responsive element in the Arc gene. J. Neurochem.148, 204–218 (2019). 10.1111/jnc.14596 [DOI] [PubMed] [Google Scholar]
- 52.Bramham, C. R. et al. The Arc of synaptic memory. Exp. Brain Res.200, 125–140 (2010). 10.1007/s00221-009-1959-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyler, W. J., Alonso, M., Bramham, C. R. & Pozzo-Miller, L. D. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal dependent learning. Learn. Mem.9, 224–237 (2002). 10.1101/lm.51202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szapiro, G. et al. Participation of hippocampal metabotropic glutamate receptors, protein kinase A and mitogen-activated protein kinases in memory retrieval. Neuroscience99, 1–5 (2000). 10.1016/S0306-4522(00)00236-0 [DOI] [PubMed] [Google Scholar]
- 55.Szapiro, G. et al. Molecular mechanisms of memory retrieval. Neurochem. Res.27, 1491–1498 (2002). 10.1023/A:1021648405461 [DOI] [PubMed] [Google Scholar]
- 56.Lopez, J., Gamache, K., Schneider, R. & Nader, K. Memory retrieval requires ongoing protein synthesis and NMDA receptor activity-mediated AMPA receptor trafficking. J. Neurosci.35, 2465–2475 (2015). 10.1523/JNEUROSCI.0735-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pereyra, M., Katche, C., De Landeta, A. B. & Medina, J. H. mTORC1 controls long-term memory retrieval. Sci. Rep.8, 8759 (2018). 10.1038/s41598-018-27053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez-Ortiz, C. J., Garcia-DeLaTorre, P., Benavidez, E., Ballesteros, M. A. & Bermudez-Rattoni, F. Intrahippocampal anisomycin infusions disrupt previously consolidated spatial memory only when memory is updated. Neurobiol. Learn. Mem.89, 352–359 (2008). 10.1016/j.nlm.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 59.Pereyra, M., de Landeta, A. B., Dalto, J. F., Katche, C. & Medina, J. H. AMPA receptor expression requirement during long-term memory retrieval and its association with mTORC1 signaling. Mol. Neurobiol.58, 1711–1722 (2021). 10.1007/s12035-020-02215-7 [DOI] [PubMed] [Google Scholar]
- 60.Bast, T., Da Silva, B. M. & Morris, R. G. M. Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J. Neurosci.25, 5845–5856 (2005). 10.1523/JNEUROSCI.0698-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okuno, H. et al. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIβ. Cell149, 886–898 (2012). 10.1016/j.cell.2012.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okuno, H., Minatohara, K. & Bito, H. Inverse synaptic tagging: an inactive synapse specific mechanism to capture activity-induced Arc/arg3.1 and to locally regulate spatial distribution of synaptic weights. Semin. Cell Dev. Biol.77, 43–50 (2018). 10.1016/j.semcdb.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 63.Yasuda, R., Hayashi, Y. & Hell, J. W. CaMKII: a central molecular organizer of synaptic plasticity, learning and memory. Nat. Rev. Neurosci.23, 666–682 (2022). 10.1038/s41583-022-00624-2 [DOI] [PubMed] [Google Scholar]
- 64.Cammarota, M. et al. Participation of CaMKII in neuronal plasticity and memory formation. Cell. Mol. Neurobiol.22, 259–267 (2002). 10.1023/A:1020763716886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lisman, J., Schulman, H. & Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci.3, 175–190 (2002). 10.1038/nrn753 [DOI] [PubMed] [Google Scholar]
- 66.Newpher, T. M., Harris, S., Pringle, J., Hamilton, C. & Soderling, S. Regulation of spine structural plasticity by Arc/Arg3.1. Semin. Cell Dev. Biol.77, 25–32 (2018). 10.1016/j.semcdb.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 67.Zhang, H. & Bramham, C. R. Arc/Arg3.1 function in long-term synaptic plasticity: emerging mechanisms and unresolved issues. Eur. J. Neurosci.54, 6696–6712 (2021). 10.1111/ejn.14958 [DOI] [PubMed] [Google Scholar]
- 68.Eriksen, M. S. & Bramham, C. R. Molecular physiology of Arc/Arg3.1: the oligomeric state hypothesis of synaptic plasticity. Acta Physiol.236, e13886 (2022). 10.1111/apha.13886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jovasevic, V. et al. GABAergic mechanisms regulated by miR-33 encode statedependent fear. Nat. Neurosci.18, 1265–1271 (2015). 10.1038/nn.4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osorio-Gómez, D., Saldivar-Mares, K. S., Perera-López, A., McGaugh, J. L. & Bermúdez-Rattoni, F. Early memory consolidation window enables drug induced statedependent memory. Neuropharmacology146, 84–94 (2019). 10.1016/j.neuropharm.2018.11.033 [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez-Ortiz, C. J., Balderas, I., Garcia-DeLaTorre, P. & Bermudez-Rattoni, F. Taste aversion memory reconsolidation is independent of its retrieval. Neurobiol. Learn. Mem.98, 215–219 (2012). 10.1016/j.nlm.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 72.Boury-Jamot, B. et al. Disrupting astrocyte–neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol. Psychiatry21, 1070–1076 (2016). 10.1038/mp.2015.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, Y. et al. Inhibition of lactate transport erases drug memory and prevents drug relapse. Biol. Psychiatry79, 928–939 (2016). 10.1016/j.biopsych.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 74.Matos-Ocasio, F., Hernández-López, A. & Thompson, K. J. Ceftriaxone, a GLT-1 transporter activator, disrupts hippocampal learning in rats. Pharmacol. Biochem. Behav.122, 118–121 (2014). 10.1016/j.pbb.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Postnikova, T. Y. et al. Ceftriaxone treatment weakens long-term synaptic potentiation in the hippocampus of young rats. Int. J. Mol. Sci.22, 8417 (2021). 10.3390/ijms22168417 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Numerical source data for all files in the manuscript can be found in supplementary data file at 10.17632/72zh75ks9t.1 (Supplementary Data 1). None of the experiments were preregistered.