Abstract
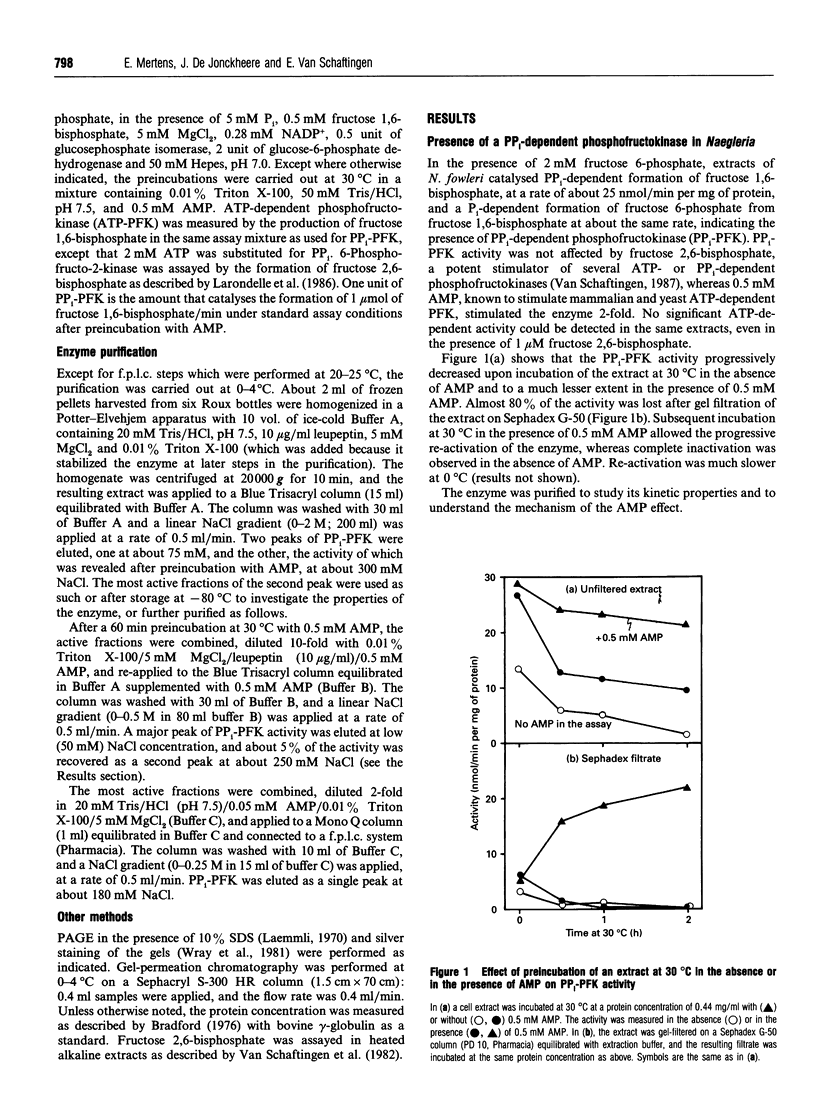
PPi-dependent phosphofructokinase (PPi-PFK) was detected in extracts of the amoeba Naegleria fowleri, with a specific activity of about 15-30 nmol/min per mg of protein, which was increased about 2-fold by 0.5 mM AMP. PPi-PFK was inactivated upon gel filtration and could be re-activated by incubation at 30 degrees C in the presence of AMP. N. fowleri PPi-PFK was purified more than 1100-fold to near homogeneity with a yield of about 25%. The pure enzyme had a specific activity of 65 mumol/min per mg of protein, and SDS/PAGE analysis showed a single band, of 51 kDa. Size-exclusion chromatography revealed the existence of two forms: a large one (approximately 180 kDa), presumably a tetramer, which was active, and a smaller one (approximately 45 kDa), presumably the monomer, which was inactive, but could be re-activated and converted into the large form by incubation at 30 degrees C in the presence of 0.5 mM AMP. Reactivation was also observed at 30 degrees C in the absence of AMP, particularly at higher enzyme concentration or in the presence of poly(ethylene glycol). Inactivation of the tetrameric enzyme was promoted by 0.25 M potassium thiocyanate. The enzyme displayed Km values of 10 and 15 microM for fructose 6-phosphate and PPi, respectively, in the forward reaction, and of 35 and 590 microM for fructose 1,6-bisphosphate and Pi in the backward reaction. The activity was dependent on the presence of Mg2+. AMP increased Vmax. about 2-fold without changing the affinity for the substrates; its half-maximal effect was observed at 2 microM.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clark C. G. Genome structure and evolution of Naegleria and its relatives. J Protozool. 1990 Jul-Aug;37(4):2S–6S. doi: 10.1111/j.1550-7408.1990.tb01138.x. [DOI] [PubMed] [Google Scholar]
- De Jonckheere J. Use of an axenic medium for differentiation between pathogenic and nonpathogenic Naegleria fowleri isolates. Appl Environ Microbiol. 1977 Apr;33(4):751–757. doi: 10.1128/aem.33.4.751-757.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deville-Bonne D., Le Bras G., Teschner W., Garel J. R. Ordered disruption of subunit interfaces during the stepwise reversible dissociation of Escherichia coli phosphofructokinase with KSCN. Biochemistry. 1989 Feb 21;28(4):1917–1922. doi: 10.1021/bi00430a073. [DOI] [PubMed] [Google Scholar]
- Fulton C., Webster C., Wu J. S. Chemically defined media for cultivation of Naegleria gruberi. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2406–2410. doi: 10.1073/pnas.81.8.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham K. C. Precipitation of proteins with polyethylene glycol. Methods Enzymol. 1990;182:301–306. doi: 10.1016/0076-6879(90)82025-w. [DOI] [PubMed] [Google Scholar]
- John D. T. Primary amebic meningoencephalitis and the biology of Naegleria fowleri. Annu Rev Microbiol. 1982;36:101–123. doi: 10.1146/annurev.mi.36.100182.000533. [DOI] [PubMed] [Google Scholar]
- Kruger N. J., Hammond J. B. Molecular Comparison of Pyrophosphate- and ATP-Dependent Fructose 6-Phosphate 1-Phosphotransferases from Potato Tuber. Plant Physiol. 1988 Mar;86(3):645–648. doi: 10.1104/pp.86.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larondelle Y., Mertens E., Van Schaftingen E., Hers H. G. Purification and properties of spinach leaf phosphofructokinase 2/fructose 2,6-bisphosphatase. Eur J Biochem. 1986 Dec 1;161(2):351–357. doi: 10.1111/j.1432-1033.1986.tb10454.x. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F. Biology of Naegleria spp. Microbiol Rev. 1988 Mar;52(1):114–133. doi: 10.1128/mr.52.1.114-133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens E. Occurrence of pyrophosphate:fructose 6-phosphate 1-phosphotransferase in Giardia lamblia trophozoites. Mol Biochem Parasitol. 1990 Apr;40(1):147–149. doi: 10.1016/0166-6851(90)90088-4. [DOI] [PubMed] [Google Scholar]
- Mertens E. Pyrophosphate-dependent phosphofructokinase, an anaerobic glycolytic enzyme? FEBS Lett. 1991 Jul 8;285(1):1–5. doi: 10.1016/0014-5793(91)80711-b. [DOI] [PubMed] [Google Scholar]
- Mertens E., Van Schaftingen E., Müller M. Presence of a fructose-2,6-bisphosphate-insensitive pyrophosphate: fructose-6-phosphate phosphotransferase in the anaerobic protozoa Tritrichomonas foetus, Trichomonas vaginalis and Isotricha prostoma. Mol Biochem Parasitol. 1989 Dec;37(2):183–190. doi: 10.1016/0166-6851(89)90150-3. [DOI] [PubMed] [Google Scholar]
- Nerad T. A., Visvesvara G., Daggett P. M. Chemically defined media for the cultivation of Naegleria: pathogenic and high temperature tolerant species. J Protozool. 1983 May;30(2):383–387. doi: 10.1111/j.1550-7408.1983.tb02935.x. [DOI] [PubMed] [Google Scholar]
- O'Brien W. E., Bowien S., Wood H. G. Isolation and characterization of a pyrophosphate-dependent phosphofructokinase from Propionibacterium shermanii. J Biol Chem. 1975 Nov 25;250(22):8690–8695. [PubMed] [Google Scholar]
- Peng Z. Y., Mansour T. E. Purification and properties of a pyrophosphate-dependent phosphofructokinase from Toxoplasma gondii. Mol Biochem Parasitol. 1992 Sep;54(2):223–230. doi: 10.1016/0166-6851(92)90114-y. [DOI] [PubMed] [Google Scholar]
- Pernin P., Cariou M. L., Jacquier A. Biochemical identification and phylogenetic relationships in free-living amoebas of the genus Naegleria. J Protozool. 1985 Nov;32(4):592–603. doi: 10.1111/j.1550-7408.1985.tb03085.x. [DOI] [PubMed] [Google Scholar]
- Reeves R. E., South D. J., Blytt H. J., Warren L. G. Pyrophosphate:D-fructose 6-phosphate 1-phosphotransferase. A new enzyme with the glycolytic function of 6-phosphofructokinase. J Biol Chem. 1974 Dec 25;249(24):7737–7741. [PubMed] [Google Scholar]
- Sabularse D. C., Anderson R. L. D-Fructose 2,6-bisphosphate: a naturally occurring activator for inorganic pyrophosphate:D-fructose-6-phosphate 1-phosphotransferase in plants. Biochem Biophys Res Commun. 1981 Dec 15;103(3):848–855. doi: 10.1016/0006-291x(81)90888-3. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E. Fructose 2,6-bisphosphate. Adv Enzymol Relat Areas Mol Biol. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Visvesvara G. S., Stehr-Green J. K. Epidemiology of free-living ameba infections. J Protozool. 1990 Jul-Aug;37(4):25S–33S. doi: 10.1111/j.1550-7408.1990.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Weik R. R., John D. T. Cell and mitochondria respiration of Naegleria fowleri. J Parasitol. 1979 Oct;65(5):700–708. [PubMed] [Google Scholar]
- Weik R. R., John D. T. Cell size, macromolecular composition, and O2 consumption during agitated cultivation of Naegleria gruberi. J Protozool. 1977 Feb;24(1):196–200. doi: 10.1111/j.1550-7408.1977.tb05306.x. [DOI] [PubMed] [Google Scholar]
- Wood H. G., O'brien W. E., Micheales G. Properties of carboxytransphosphorylase; pyruvate, phosphate dikinase; pyrophosphate-phosphofructikinase and pyrophosphate-acetate kinase and their roles in the metabolism of inorganic pyrophosphate. Adv Enzymol Relat Areas Mol Biol. 1977;45:85–155. doi: 10.1002/9780470122907.ch2. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yan T. F., Tao M. Multiple forms of pyrophosphate:D-fructose-6-phosphate 1-phosphotransferase from wheat seedlings. Regulation by fructose 2,6-bisphosphate. J Biol Chem. 1984 Apr 25;259(8):5087–5092. [PubMed] [Google Scholar]



