Abstract
Anaplastic thyroid carcinoma (ATC) is a highly aggressive human malignancy without effective treatment. Yes-associated protein (YAP) is a critical effector of the Hippo pathway, which is essential in thyroid carcinogenesis. However, the underlying mechanisms of aberrant YAP expression in ATC are not completely understood. Ubiquitylation-related enzyme siRNA screening identified the ubiquitin protein ligase E3 component n-recognin 1 (UBR1) as a stabilizer of YAP in ATC cells. UBR1 deficiency reduced YAP protein levels and its target gene expression. UBR1 directly interacted with YAP and promoted its monoubiquitylation, competitively suppressing its polyubiquitylation and resulting in extended protein half-life. UBR1 depletion reduced ATC cell proliferation and migration in vitro. Xenograft tumor studies also suggested that UBR1 knockdown suppressed ATC cell growth in vivo. Furthermore, exogenous YAP expression partially reversed the inhibitive effects of UBR1 depletion on ATC cell proliferation and migration. Our studies demonstrated that UBR1 directly interacts with YAP and stabilized it in a monoubiquitylation-dependent manner, consequently promoting ATC tumorigenesis, suggesting that UBR1 might be a potentially therapeutic target for ATC treatment.
Subject terms: Cancer, Ubiquitylation
Introduction
Over the last decade, the incident rates of thyroid cancer have grown rapidly, averaging 6.2% per year1. Thyroid cancer is typically classified into 2 primary categories based on its differentiation stages: differentiated thyroid carcinoma (DTC) and anaplastic thyroid carcinoma (ATC)2. DTC is the most prevalent form, accounting for more than 95% thyroid cancer cases, and most DTC patients have favorable prognosis, with a 5-year relative survival rate of approximately 98%3. However, although ATC is rare, it accounts for more than 50% of the mortality associated with thyroid cancer, characterized with high aggressiveness4,5. Unlike DTC, ATC cells do not retain follicular elements and thyroglobulin production, resulting in failure of conventional chemotherapy and radioiodine treatment4. Therefore, ATC presents important clinical challenges as there exist no effective therapeutic options.
The Hippo pathway was originally identified to modulate organ size in Drosophila, and its components were structurally and functionally conserved in evolution6. Accumulating studies revealed that the dysregulation of Hippo pathway was associated with the initiation and progression of numerous human malignancies7,8. Yes-associated protein (YAP) serves as an important downstream effector of the Hippo kinase cascades, functioning as a transcriptional co-activator to regulate the transcription of its target genes9,10. Previous researches reveal that YAP activation is widespread in malignance of multiple organs, including breast, colon, liver, and ovary8,11,12. YAP activity is predominantly governed by its phosphorylation downstream of the mammalian Ste20-like kinase (MST) 1/2- large tumor suppressor (LATS) 1/2 signaling cascade, subsequently resulting in cytoplasmic retention, interaction with 14-3-3 protein, or proteasomal degradation13,14. Without phosphorylation, YAP undergoes nuclear translocation and interacts with transcription factors, such as TEA domain transcription factor (TEAD). Their target genes are closely associated with several cell behaviors, such as cell proliferation, migration and invasion, rendering YAP a prospective therapeutic target for cancer therapy15. The expression levels and functions of YAP proteins are also subject to post-translational modifications, for instance, ubiquitination.
Ubiquitination regulates the degradation of various proteins and the maintenance of cellular homeostasis16. Ubiquitin can be attached to the substrates as a single ubiquitin (monoubiquitylation or multi-monoubiquitylation) or as polymeric ubiquitin chains (polyubiquitylation)17. Polyubiquitylation is associated with protein degradation, while increasing studies reveal that monoubiquitylation competes with polyubiquitylation for substrates, therefore improving protein stability18,19. Many ubiquitination-related enzymes, including Josephin domain-containing protein 2, parkin RBR E3 ubiquitin protein ligase (PARK2), beta-transducin repeat-containing protein (β-TrCP) and ubiquitin specific peptidase 10 (USP10) are involved in the regulation of Hippo signaling pathway20–24, and YAP was delicately regulated by both monoubiquitylation and polyubiquitylation25–27. The finely tunning of YAP ubiquitylation is still to be investigated in ATC.
In this work, the ubiquitin protein ligase E3 component n-recognin 1 (UBR1) was identified as a stabilizer for YAP proteins in ATC cells by a ubiquitylation-related enzyme screening. Consequent studies revealed that it stabilized YAP proteins in a post-transcriptional way. UBR1 directly interacted with YAP and modulated its protein stability via regulating its monoubiquitylation. Moreover, UBR1 knockdown suppressed ATC cell growth both in vitro and in vivo, and YAP restoration could reverse its inhibitive effects. Our researches suggested that targeting UBR1 might be a potential strategy for ATC therapeutic treatment.
Results
UBR1 deficiency downregulates YAP expression
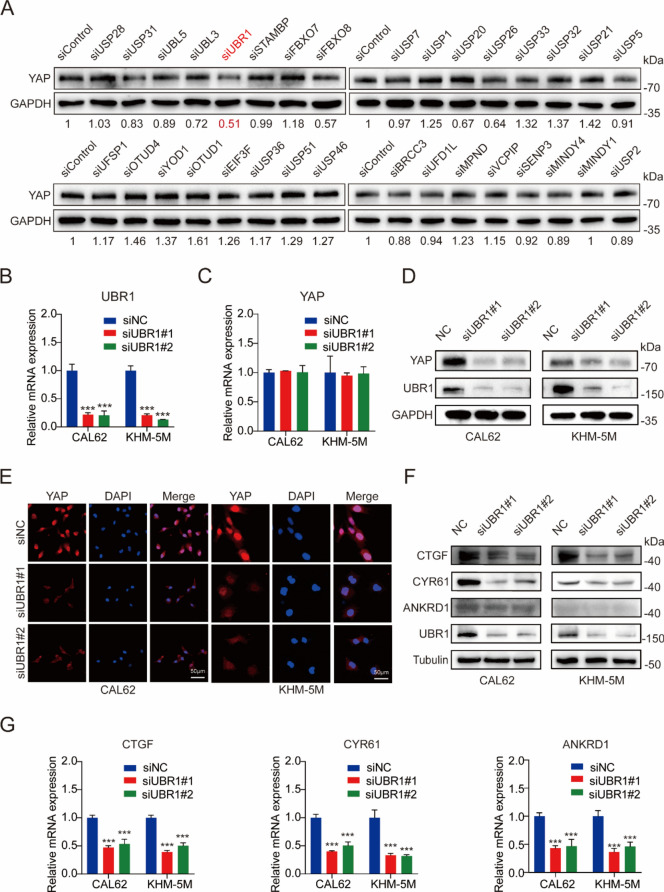
To explore the finely-tuning of Hippo signaling pathway by ubiquitylation in ATC, 32 randomly selected ubiquitylation-related enzymes were used for siRNA library screening of YAP protein levels in CAL62 cells. Among them, the E3 ubiquitin ligase UBR1 downregulation significantly reduces YAP protein levels (Fig. 1A), suggesting it as a potential stabilizer of YAP. While the mRNA levels of YAP were not affected, the protein levels of YAP were significantly decreased upon UBR1 deficiency in CAL62 and KHM-5M cells, suggesting the post-transcriptional regulation of YAP by UBR1 (Fig. 1B–D). Immunofluorescence also indicated decreased YAP protein levels in ATC cells (Fig. 1E). Moreover, the protein and mRNA levels of YAP target genes connective tissue growth factor (CTGF), cysteine-rich angiogenic inducer 61 (CYR61), and ankyrin repeat domain 1 (ANKRD1) were significantly reduced in UBR1-deficient CAL62 and KHM-5M cells (Fig. 1F, G). These results suggested UBR1 depletion downregulated YAP expression and its downstream pathways.
Figure 1.
UBR1 deficiency downregulates YAP and its target genes. (A) Immunoblots of YAP upon ubiquitination-related enzyme knockdown by siRNAs in CAL62 cells. The numbers below images were the quantification of blots. (B) UBR1 mRNA levels were decreased by the siRNAs in both CAL62 and KHM-5M cells. (C) YAP mRNA levels were not affected by UBR1 depletion in both CAL62 and KHM-5M cells. (D) YAP protein levels were significantly decreased by UBR1 depletion in both CAL62 and KHM-5M cells. (E) Immunofluorescence demonstrated less YAP levels upon UBR1 deficiency in both CAL62 and KHM-5M cells. (F) UBR1 deficiency downregulated the protein levels of YAP target genes, CTGF, CYBR61 and ANKRD1, in both CAL62 and KHM-5M cells. (G) UBR1 deficiency downregulated the mRNA levels of CTGF, CYBR61 and ANKRD1 in both CAL62 and KHM-5M cells. n = 3; ***P < 0.001.
UBR1 interacts with YAP in the cytoplasm
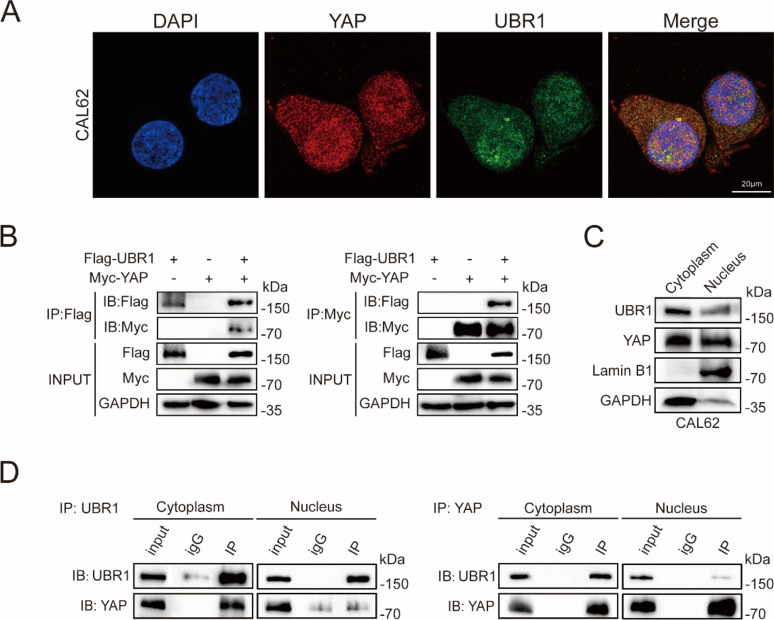
To further investigate the relationship between UBR1 and YAP, their interaction was next examined. Immunofluorescence demonstrated their colocalization in CAL62 cells (Fig. 2A). Immunoprecipitation assays revealed the direct interaction between UBR1 proteins with Flag tag (Flag-UBR1) and YAP proteins with Myc tag (Myc-YAP) in HEK 293T cells (Fig. 2B). Cell fraction separation assay revealed that YAP and UBR1 were distributed in both cytoplasm and nucleus of CAL62 cells (Fig. 2C). Co-immunoprecipitation assays in CAL62 cells suggested that UBR1 primarily interacted with YAP in the cytoplasm (Fig. 2D).
Figure 2.
UBR1 interacts with YAP. (A) Immunofluorescence demonstrated the colocalization of UBR1 and YAP in CAL62 cells. (B) Co-IP indicated the interaction between UBR1 proteins with Flag tag (Flag-UBR1) and YAP proteins with Myc tag (Myc-YAP) in HEK 293T cells. (C) Immunoblotting demonstrated expression of YAP and UBR1 in both the cytoplasm and nucleus of CAL62 cells. (D) Co-IP indicated the interaction between UBR1 and YAP in cytoplasm.
UBR1 improves the stability of YAP proteins via monoubiquitylation
UBR1 depletion significantly reduced YAP protein levels, but the proteasome inhibitor MG132 treatment could reverse this inhibition in ATC cells, suggesting that the downregulation of YAP protein levels by UBR1 depletion was proteasome-dependent (Fig. 3A). In presence of the protein syntheses inhibitor cycloheximide, UBR1 deficiency resulted in a significant reduction of the stability of YAP proteins (Fig. 3B, C), confirming that the regulation was post-transcriptional. Moreover, UBR1 depletion elevated the ubiquitylation levels of YAP proteins in CAL62 and KHM-5M cells (Fig. 3D). Further ubiquitylation assays indicated that UBR1 overexpression reduced the ubiquitylation levels of YAP proteins, especially the K48-linked polyubiquitylation, but increased the K0-linked monoubiquitylation in HEK 293T cells (Fig. 3E). These results suggested that UBR1 directly binds to YAP and improved its stability via monoubiquitylation.
Figure 3.
UBR1 improves the stability of YAP proteins through monoubiquitylation. (A) The proteasome inhibitor MG132 (10 μM, treated for 6 h) restored YAP protein levels upon UBR1 depletion in both CAL62 and KHM-5M cells. (B, C) UBR1 depletion decreased the stability of YAP proteins in both CAL62 and KHM-5M cells treated with cycloheximide (10 ug/ml). (D) UBR1 deficiency increased the polyubiquitination of YAP proteins in CAL62 cells. (E) UBR1 downregulated total ubiquitination and K48 polyubiquitination, but upregulated K0 monoubiquitination in HEK293 cells. n = 3; ***P < 0.001.
UBR1 knockdown reduces ATC cell proliferation and migration in vitro
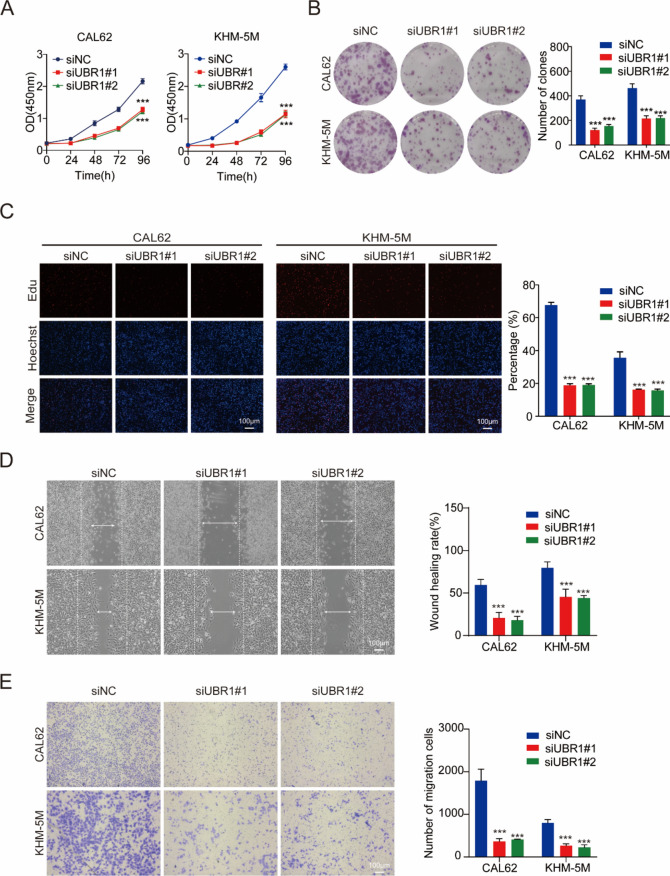
YAP signaling pathway is essential for cell proliferation and migration, we subsequently explored the regulative effects of UBR1 on ATC cell behaviors. The viability of CAL62 and KHM-5M cells was significantly reduced by UBR1 depletion (Fig. 4A). UBR1 downregulation also resulted in reduced colony formation of ATC cells (Fig. 4B). Consistently, UBR1 deficiency notably inhibited DNA synthesis in ATC cells as assessed by Edu staining (Fig. 4C). Further experiments revealed that UBR1 depletion reduced the migration and invasion ability of CAL62 and KHM-5M cells (Fig. 4D, E).
Figure 4.
UBR1 knockdown inhibits ATC cell proliferation and migration in vitro. (A) CCK8 assay indicated that UBR1 silencing decreased CAL62 and KHM-5M cell viability in vitro. (B) UBR1 depletion suppressed colony formation in both CAL62 and KHM-5M cells. (C) Edu staining demonstrated that UBR1 deficiency inhibited the proliferation of both CAL62 and KHM-5M cells. (D) Wound healing assays demonstrated UBR1 deficiency inhibited both CAL62 and KHM-5M cell migration. (E) Modified Boyden chamber assays demonstrated that UBR1 deficiency inhibited both CAL62 and KHM-5M cell invasion. n = 3; ***P < 0.001.
UBR1 overexpression improves ATC cell proliferation and migration in vitro
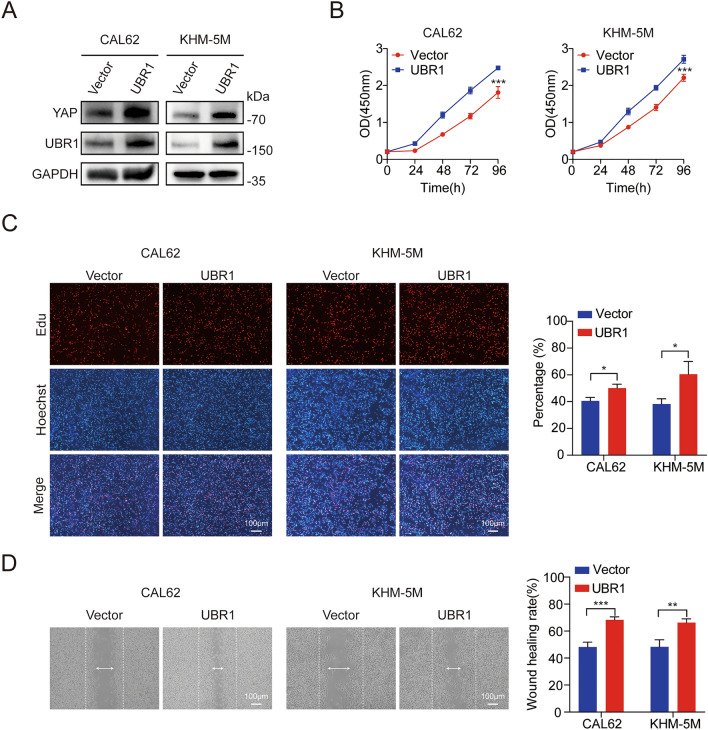
Subsequently, we examined the regulatory effects of UBR1 overexpression on ATC cell behaviors. Immunoblotting demonstrated significantly increased YAP protein levels in CAL62 and KHM-5M cells (Fig. 5A). CCK8 assay indicated that UBR1 overexpression increased ATC cell viability (Fig. 5B). EdU staining showed that DNA synthesis was significantly increased in UBR1-overexpressing CAL62 and KHM-5M cells (Fig. 5C). Wound healing assay revealed that UBR1 overexpression ATC cell migration (Fig. 5D). Our results suggested that UBR1 overexpression promoted ATC cell proliferation and migration in vitro.
Figure 5.
UBR1 overexpression improves ATC cell proliferation and migration in vitro. (A) YAP protein levels were increased by UBR1 overexpression in both CAL62 and KHM-5M cells. (B) CCK8 assay revealed that UBR1 overexpression increased CAL62 and KHM-5M cell viability in vitro. (C) Edu assays demonstrated that UBR1 overexpression increased the proliferation of both CAL62 and KHM-5M cells. (D) Wound healing assays demonstrated that UBR1 overexpression increased both CAL62 and KHM-5M cell migration. n = 3; *P < 0.05; **P < 0.01; ***P < 0.001.
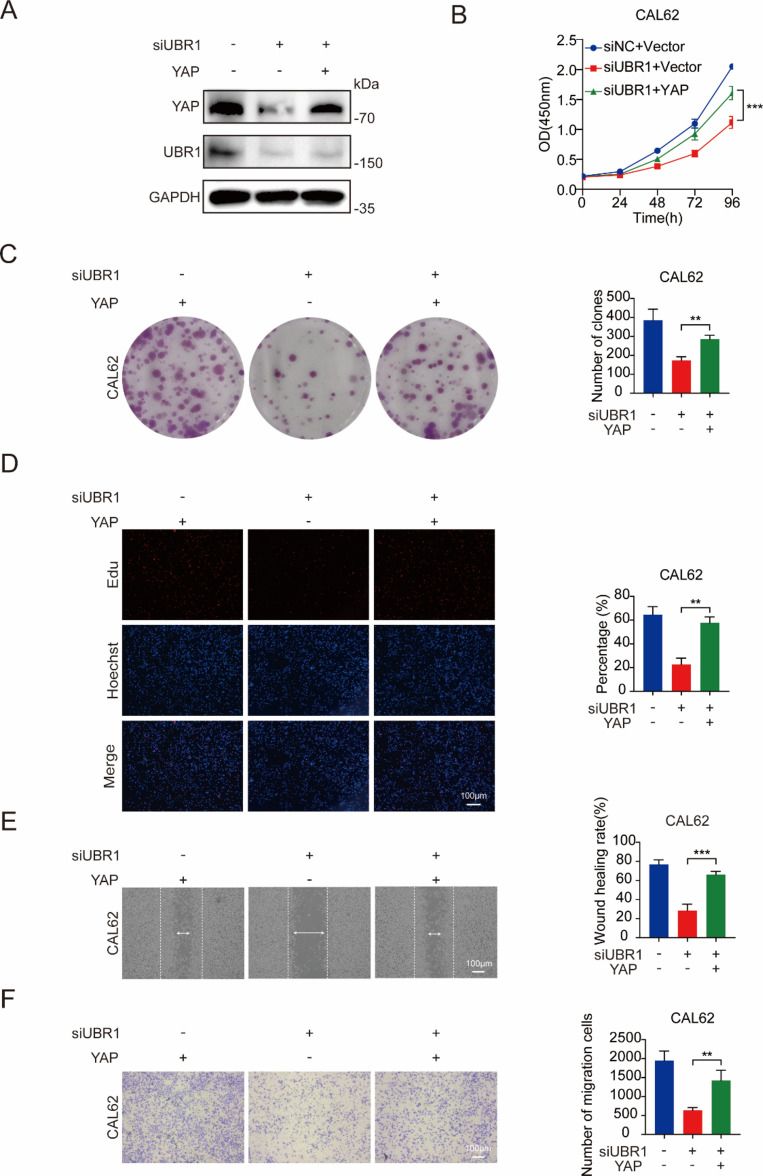
Exogenous YAP expression restored the suppressive effects of UBR1 deficiency
To confirm whether the regulation of ATC cell behaviors by UBR1 was dependent on YAP protein levels, we introduced exogenous YAP expression into UBR1-deficient ATC cells. Immunoblotting demonstrated the restoration of YAP proteins in CAL62 cells (Fig. 6A). CCK8 assays showed that YAP overexpression restored the deceleration of cell viability upon UBR1 knockdown in CAL62 cells (Fig. 6B). Moreover, exogenous YAP expression reversed the inhibition of colony formation by UBR1 deficiency in CAL62 cells (Fig. 6C). Immunofluorescence also demonstrated restored DNA synthesis upon YAP overexpression in UBR1-deficient CAL62 cells (Fig. 6D). Furthermore, exogenous YAP expression effectively counteracted the inhibitive effects of UBR1 knockdown on CAL62 cell migration and invasion (Fig. 6E, F). These findings confirmed that UBR1 regulated ATC cell behaviors via modulating YAP expression.
Figure 6.
Exogenous YAP expression restored the inhibitive effects of UBR1 deficiency on ATC cell behaviors. (A) Immunoblotting revealed that exogenous YAP expression restores YAP protein levels reduced by UBR1 deficiency in CAL62 cells. (B) Exogenous YAP expression restored CAL62 cell viability upon UBR1 deficiency. (C) Exogenous YAP expression restored colony formation upon UBR1 deficiency in CAL62 cells. (D) Immunofluorescence demonstrated that YAP restoration reversed the suppressive effects of UBR1 depletion on CAL62 cell proliferation. (E) Wound healing assays demonstrated that YAP restoration reversed the inhibitive effects of UBR1 depletion on CAL62 cell migration. (F) Modified Boyden chamber assays demonstrated that YAP restoration reversed the inhibitive effects of UBR1 depletion on CAL62 cell invasion. n = 3; **P < 0.01, ***P < 0.001.
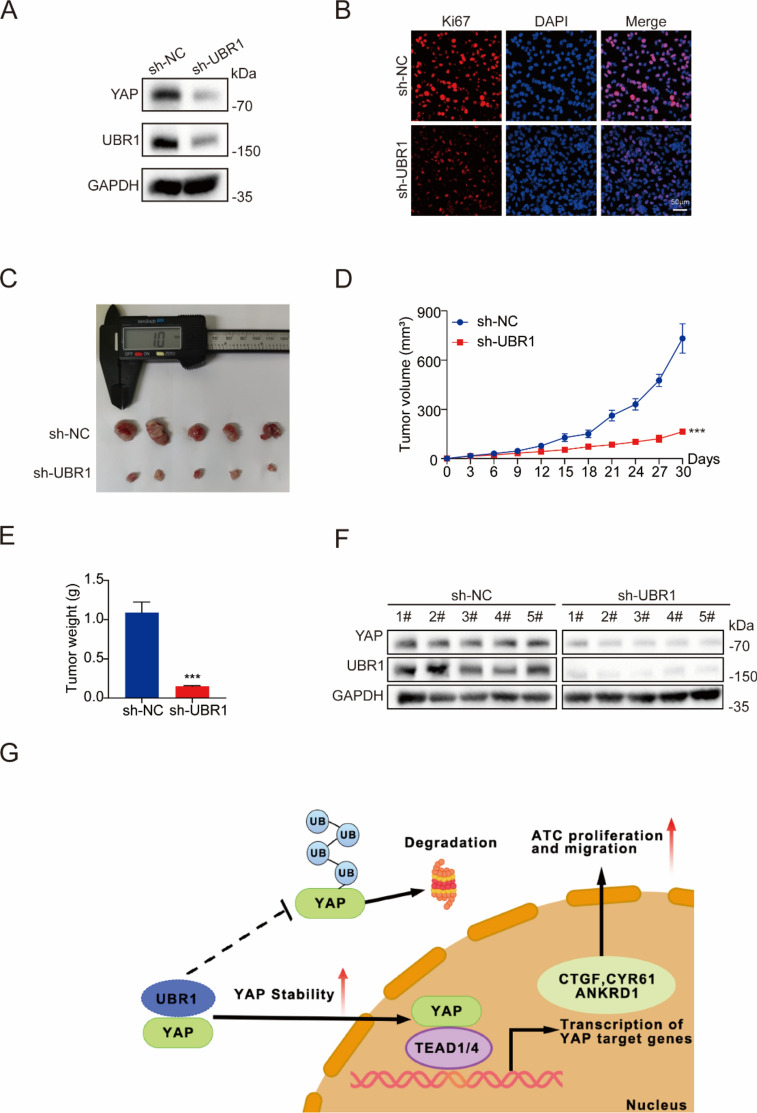
UBR1 regulates ATC cell growth in vivo
UBR1 was stably knocked down with lentiviruses in CAL61 cells (Fig. 7A). Ki67 staining revealed that proliferative activity was reduced in UBR1-deficient CAL62 cells (Fig. 7B). Subsequently, we explored the effects of UBR1 on ATC cell growth in vivo with a xenograft mouse model with subcutaneously injection of CAL62 cells. Tumor sizes were substantially decreased upon UBR1 deficiency vs. wild-type CAL62 cells in BALB/C-nude mice 30 days later (Fig. 7C). The tumor volumes and weighs were also significantly decreased by the lentivirus-mediated depletion of UBR1 (Fig. 7D, E). Immunoblotting revealed reduced YAP protein levels in the UBR1-deficient tumor tissues (Fig. 7F). Our results suggested that UBR1 depletion inhibited ATC cell growth in vivo.
Figure 7.
UBR1 regulates ATC cell proliferation in vivo. (A) YAP protein levels were reduced in CAL62 cells with UBR1 stable knockdown. (B) Immunofluorescence demonstrated less ki67 levels upon UBR1 deficiency in CAL62 cells. (C–E) UBR1 depletion suppressed CAL62 cell growth in vivo. (F) YAP protein levels were lower in tumor tissues of the sh-UBR1 group compared to sh-NC group. (G) Mechanism diagram. n ≥ 3; ***P < 0.001.
Discussion
ATC is a rare but exceedingly aggressive malignance that traditional treatments fail to extend the survival of its patients. Therefore, exploring the molecular mechanisms of ATC development and progression is an urgent problem. Previous researches revealed that YAP activation is common in ATC28, and clinical studies suggested close relationship of chemoresistance with YAP expression and activation, implicating the critical role of the Hippo pathway in ATC carcinogenesis26,27,29. Furthermore, ATC cell behaviors, including proliferation and migration, are significantly regulated by YAP expression, and its protein levels were finely tuned by both transcriptional and post-transcriptional manners25. Targeting YAP is expected to be a promising therapeutic approach to treat ATC patients.
As an ultimate transducer effector of the Hippo pathway, YAP expression is correlated with cell proliferation, tumor metastasis, poor prognosis, and recurrence risk, making it a crucial target for cancer therapy8. The delicate regulation of YAP is essential for maintaining normal cellular function. In addition to phosphorylation and subcellular translocation, YAP is also subject to other post-translational modification, such as ubiquitination. The stability of YAP is mainly regulated by ubiquitin-mediated proteasomal degradation30,31. For instance, RNF187 inhibits the growth and migration of breast cancer cells through the promotion of YAP protein degradation32. In triple-negative breast cancer, RNF31 suppresses tumor growth and sensitize it to immunotherapy via repressing PD-L1 expression and facilitating YAP degradation through K76-linked polyubiquitylation33. In breast cancer and pancreatic cancer, β-TRCP is recruited by S384/S387-phosphorylated-YAP, and subsequently catalyzes YAP ubiquitination and degradation23,24,34. Its expression levels vary in ATC and normal thyroid epithelial cells (Supplemental Fig. 1), suggesting its potential roles in ATC development. In addition, in esophageal squamous cell carcinoma, PARK2 functions as an E3 ubiquitin ligase to promote K48-linked polyubiquitylation and degradation of YAP21. Although the majority of E3 ubiquitin ligases accelerate protein degradation and induce YAP stability, several ones including TRIM11 increases YAP protein levels via promoting its monoubiquitylation25.
Our studies identified another E3 ubiquitin ligase UBR1 as a stabilizer of YAP in ATC cells. Its absence reduced YAP protein levels in ATC cells. UBR1 directly interacts with YAP, and the protein stability of YAP was reduced in UBR1-deficient ATC cells. Further studies indicated that UBR1 promotes YAP monoubiquitylation while simultaneously inhibiting its K48-linked polyubiquitylation and proteasomal degradation. The relationship between UBR1 and other E3 ubiquitin ligases including β-TRCP and PARK1 is to be clarified by further investigation of YAP protein levels upon their simultaneous presence and their targeting amino acid residues on YAP proteins in future studies.
UBR1 is a RING-type E3 ubiquitin ligase with a distinctive UBR-box that participates in N-end rule protein degradation35,36. UBR1 gene mutation or deletion has a significant impact on the development of Johanson-Blizzard syndrome, and UBR1 depletion causes pancreatic dysfunction and malformations37,38. Several studies have shown that the functions of UBR1 are not limited to the pancreas, it also plays crucial roles in tumor cells39–41. UBR1 depletion and TLX1/HOX11 overexpression synergize to increase chromosome losses and accelerate B-cell lymphomagenesis42. In hepatocellular carcinoma cells, downregulation of UBR1 resulted in decreased cell proliferation and migration, increased apoptosis and enhanced chemosensitivity43,44. UBR1 inhibitors in combination with chemotherapeutic drugs showed combined anti-tumor effects in colon cancer mouse models43. In this work, our results indicated that the absence of UBR1 dramatically reduced ATC cell proliferation and migration. Furthermore, the restoration of YAP expression eliminated the impact caused by UBR1 deletion, suggesting that UBR1 functions in a YAP-dependent manner.
Taken together, our results revealed that UBR1 was an effective E3 ubiquitin enzyme for the regulation of YAP, and that UBR1 promoted ATC cell proliferation and migration via increasing YAP monoubiquitylation and stabilizing its protein. Our studies offer a novel perspective on the impact of UBR1 in the Hippo/YAP axis, and suggested that modulation of UBR1 activity might be a potential strategy for the therapy of ATC.
Material and methods
Cells
The ATC cell lines, CAL62 and KHM-5M, were purchased from Procell (Wuhan, China), and HEK293T cells from the Chinese Academy of Sciences (Shanghai, China). Short tandem repeat profiling was used to authenticate all the cells. CAL62 and HEK293T were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, USA), and KHM-5M cells were cultured with RPMI1640 medium (Gibco, USA). All cells were maintained with 10% fetal bovine serum (FBS, Gibco, USA) and supplemented with 1% penicillin–streptomycin (Procell, Wuhan, China), cultured in an atmosphere of 5% CO2 at 37 °C. Medium was changed once other day during culture and long-term experiments.
Plasmids, siRNAs and reagents
The plasmids of UBR1 gene with Flag tag (Flag-UBR1) and YAP gene with Myc tag (Myc-YAP) were acquired from HANBIO Biological (Shanghai, China). The plasmids of HA-Ub, HA-K0, HA-K6, HA-K11, HA-K27, HA-K29, HA-K33, HA-K48 and HA-K63 were obtained from Addgene (USA). Lipofectamine 2000 (Invitrogen, USA) was used for plasmid transfection. A siRNA library with 32 randomly selected ubiquitination-related enzymes was purchased from Dharmacon (USA). UBR1-targeting siRNAs were synthesized by Genepharma (Shanghai, China): siRNA#1, 5’-GGACAAAUUGGGAAGAGUATT-3’; siRNA#2, 5’-GGAUGAAUAUGGAGAAACATT-3’. Rfect siRNA Transfection Reagent (BaiDai biotechnology, Changzhou, China) was used for siRNA transfection.
Lentivirus production and infection
HEK293T cells were transfected with pCDH-UBR1-Puro lentiviral vector containing UBR1-shRNA sequence 5’-GGATGAATATGGAGAAACA-3’ or pCDH-NC-Puro vector containing control sequence 5’-TTCTCCGAACGTGTCACGT-3’, as well as package components psPAX2 and pMD2.G using lipofectamine 3000 (Invitrogen, USA). The medium was collected at 48 and 72 h later. CAL62 cells were incubated with the supernatants and polybrene (8 μg/ml) overnight at 37 °C, and stable transfected cells were selected with puromycin (2 μg/ml) for 3 days.
RNA extraction and RT-qPCR analysis
The total RNA extraction from ATC cells was accomplished by the HiPure Total RNA Mini Kit (Magen, Guangzhou, China). Reverse transcription was conducted with the Hiscript III Reverse Transcriptase (Vazyme, Nanjing, China). RT-qPCR was conducted utilizing the SYBR green mix (Vazyme, Nanjing, China) in conjunction with the QuantStudio™ Real-time PCR system (Thermo Fisher, USA). The relative gene expression levels were determined by the 2−ΔΔCt method. GAPDH was utilized as an internal control.
Cell viability assay
ATC cells were digested and seeded into 96-well plates (3000 cells/well), and the Cell Counting Kit-8 (CCK8, Meilunbio, Dalian, China) was used to assess cell viability. Each well was treated with 10 μl of CCK8 solution reagent and incubated at 37 °C for 2 h. The absorbance was detected with a spectrophotometer at a wavelength of 450 nm.
Cell proliferation assay
ATC cells were digested and seeded into 96-well plates (1 × 105 cells/well). After 24 h, each well was incubated with 5-ethyl-2′-deoxyuridine (EdU, 50 μM, Ribobio, Guangzhou, China) for 2 h. After fixation with 4% formaldehyde for 15 min, the cells were subjected to Triton X-100 (0.5%) for 20 min, and subsequently Apollo staining solution (100 μl) for 30 min. After incubated with Hoechst 33,342 (100 μl) for another 30 min, and images were obtained with a fluorescence microscope.
Colony formation assay
ATC cells are digested and seeded into 6-well plates (1000 cells/well). After culture for 14 days, the cells were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet for visualization. ImageJ (NIH, USA) was used to quantify the images.
Wound healing and modified Boyden chamber assays
ATC cells were cultured in 6-well plates to full confluency. The cell monolayer was mechanically disrupted using a sterile tip and washed with phosphate buffered saline (PBS, Gibco, USA). The cells were then cultured in a serum-free medium, and images were captured at 0 and 24 h. For modified Boyden chamber assay, the cells were subjected to serum deprivation for 24 h, and then seeded into the upper chamber inserts (pore size of 8.0 µm, Corning, USA) of 24-well plates (5 × 105 cells/well), maintained in a serum-free medium. The complete medium containing 10% FBS was added into the lower chambers, and the cells were cultured for 24 h. After fixation with 4% paraformaldehyde for 30 min, the cells were then stained with 1% crystal violet. Images were captured using microscopy.
Immunofluorescence
ATC cells were cultured onto the sterile glass coverslips in the 24-well plates. After rinsed with cold PBS, the cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.2% Triton X-100 for 5 min at room temperature. After blocked with 5% BSA for 1 h, the cells were incubated with primary antibodies against YAP (Proteintech, 66900-1-Ig), UBR1 (Affinity, DF4049) or Ki67 (Abmart, TW0001) overnight at 4 °C. The secondary antibodies, Cy3-labeled goat anti-mouse IgG, Cy3-labeled goat anti-rabbit IgG or FITC-labeled goat anti-rabbit IgG were purchased from Beyotime (Shanghai, China). Images were captured with a confocal laser-scanning microscope (NIKON80i).
Xenograft tumor mouse model
BALB/c-nude mice (5-week-old) were purchased from Hubei Beiente Biotechnology (Wuhan, Chian). CAL62 cells with or without stable UBR1 knockdown (1 × 107 cells/mouse) were subcutaneously injected into the right back of mice. Tumor sizes was recorded every 3 days, and the tumor volume (mm3) was calculated as long axis × short axis2/2. After 30 days or the tumor size reached 1000 mm3, mice were euthanized using isoflurane anesthesia followed by manual cervical dislocation, and the tumors were photographed and weighed. Animal experiments in this study have been approved by the Ethics Committee of the Zhongnan Hospital of Wuhan University (ZN2021215) and animal experiments procedures were conducted in accordance with relevant guidelines and regulations. All methods were carried out in accordance with ARRIVE guidelines.
Co-immunoprecipitation (co-IP)
The cells were lysed with Western and IP lysis buffer (Beyotime, Shanghai, China) supplemented with the protease inhibitor cocktail (MedChemExpress, Shanghai, China). After pre-clearing with rabbit IgG (Cell signaling Technology, #2729) or mouse IgG (Proteintech, B900620) for 2 h at 4 °C, the lysates were incubated with protein A/G magnetic beads (MedChemExpress, Shanghai, China) conjugated with specified Myc (Proteintech, 60003-2-Ig) or Flag (Proteintech, 20543-1-AP) antibodies at 4 °C for 2 h. Immuno-complexes were washed with PBS, and bound proteins were redissolved in lysis buffer.
Immunoblotting
After washing with cold PBS, the cells were lysed in radio immunoprecipitation assay (RIPA) buffer (Beyotime, Shanghai, China). Total proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Millipore, USA). The primary antibodies were YAP (Proteintech, 13584-1-AP), UBR1 (Santa Cruz, sc-515753), GAPDH (Proteintech, 60004-1-Ig), HA (Proteintech, 51064-2-AP), Myc (Proteintech, 60003-2-Ig), Flag (Proteintech, 20543-1-AP), CTGF (Wanleibio, WL02602), CYR61 (UpingBio, YP-Ab-06868), ANKRD1 (UpingBio, YP-Ab-01588), Lamin B1 (UpingBio, YP-Ab-00568) and Tubulin (Abmart, M20005F). The secondary antibodies were HRP-labeled Goat Anti-Rabbit IgG(H + L) ((Beyotime, Shanghai, China), HRP-labeled Goat Anti-Mouse IgG(H + L) ((Beyotime, Shanghai, China).
Statistical analysis
All data were analyzed using Prism 7.0 software (GraphPad, USA) and shown as mean ± standard deviation (SD). Student's t-test was used for differences between 2 independent groups, while one-way ANOVA was employed for comparisons between multiple groups. A p-value less than 0.05 was considered as statistically significant.
Supplementary Information
Author contributions
M.X., C.L., Y.G. and C.X. conceived and designed the experiments; M.X., C.L., Y.Y. and J.L. performed the experiments; M.X., C.L., Y.Z. and J.T. interpreted the results; M.X., J.T., M.Z., and Z.J. finished the data analysis and statistical analysis; M.X. and C.L. prepared the manuscript; Y.G. and C.X. edited and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (81800429 and 81972852), Key Research & Development Project of Hubei Province (2020BCA069), Health Commission of Hubei Province Medical Leading Talent Project, Young and Middle-Aged Medical Backbone Talents of Wuhan (WHQG201902).
Data availability
All data generated in this study that support our findings are included in this paper and its supplemental data file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Min Xia and Chen Liang.
Contributor Information
Yan Gong, Email: yan.gong@whu.edu.cn.
Conghua Xie, Email: chxie_65@whu.edu.cn.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-70458-8.
References
- 1.Rossi, E. D., Pantanowitz, L. & Hornick, J. L. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol.9, 193–194. 10.1016/S2213-8587(21)00049-8 (2021). 10.1016/S2213-8587(21)00049-8 [DOI] [PubMed] [Google Scholar]
- 2.Asa, S. L. The current histologic classification of thyroid cancer. Endocrinol. Metab. Clin. North Am.48, 1–22. 10.1016/j.ecl.2018.10.001 (2019). 10.1016/j.ecl.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 3.Saini, S., Tulla, K., Maker, A. V., Burman, K. D. & Prabhakar, B. S. Therapeutic advances in anaplastic thyroid cancer: A current perspective. Mol. Cancer17, 154. 10.1186/s12943-018-0903-0 (2018). 10.1186/s12943-018-0903-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allegri, L., Capriglione, F., Maggisano, V., Damante, G. & Baldan, F. Effects of dihydrotanshinone I on proliferation and invasiveness of paclitaxel-resistant anaplastic thyroid cancer cells. Int. J. Mol. Sci.10.3390/ijms22158083 (2021). 10.3390/ijms22158083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molteni, E., Baldan, F., Damante, G. & Allegri, L. GSK2801 reverses paclitaxel resistance in anaplastic thyroid cancer cell lines through MYCN downregulation. Int. J. Mol. Sci.10.3390/ijms24065993 (2023). 10.3390/ijms24065993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu, Z. & Guan, K. L. Hippo signaling in embryogenesis and development. Trends Biochem. Sci.46, 51–63. 10.1016/j.tibs.2020.08.008 (2021). 10.1016/j.tibs.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccolo, S., Panciera, T., Contessotto, P. & Cordenonsi, M. YAP/TAZ as master regulators in cancer: Modulation, function and therapeutic approaches. Nat. Cancer4, 9–26. 10.1038/s43018-022-00473-z (2023). 10.1038/s43018-022-00473-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanconato, F., Cordenonsi, M. & Piccolo, S. YAP/TAZ at the roots of cancer. Cancer Cell29, 783–803. 10.1016/j.ccell.2016.05.005 (2016). 10.1016/j.ccell.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen, C. G., Moroishi, T. & Guan, K. L. YAP and TAZ: A nexus for Hippo signaling and beyond. Trends Cell Biol.25, 499–513. 10.1016/j.tcb.2015.05.002 (2015). 10.1016/j.tcb.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu, F. X., Zhao, B. & Guan, K. L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell163, 811–828. 10.1016/j.cell.2015.10.044 (2015). 10.1016/j.cell.2015.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overholtzer, M. et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. U.S.A.103, 12405–12410. 10.1073/pnas.0605579103 (2006). 10.1073/pnas.0605579103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel, S. H., Camargo, F. D. & Yimlamai, D. Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology152, 533–545. 10.1053/j.gastro.2016.10.047 (2017). 10.1053/j.gastro.2016.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng, Z., Moroishi, T. & Guan, K. L. Mechanisms of Hippo pathway regulation. Genes Dev.30, 1–17. 10.1101/gad.274027.115 (2016). 10.1101/gad.274027.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rausch, V. & Hansen, C. G. The Hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol.30, 32–48. 10.1016/j.tcb.2019.10.005 (2020). 10.1016/j.tcb.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 15.Harvey, K. F., Zhang, X. & Thomas, D. M. The Hippo pathway and human cancer. Nat. Rev. Cancer13, 246–257. 10.1038/nrc3458 (2013). 10.1038/nrc3458 [DOI] [PubMed] [Google Scholar]
- 16.Swatek, K. N. & Komander, D. Ubiquitin modifications. Cell Res.26, 399–422. 10.1038/cr.2016.39 (2016). 10.1038/cr.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh, E., Akopian, D. & Rape, M. Principles of ubiquitin-dependent signaling. Annu. Rev. Cell. Dev. Biol.34, 137–162. 10.1146/annurev-cellbio-100617-062802 (2018). 10.1146/annurev-cellbio-100617-062802 [DOI] [PubMed] [Google Scholar]
- 18.Eakin, C. M., Maccoss, M. J., Finney, G. L. & Klevit, R. E. Estrogen receptor alpha is a putative substrate for the BRCA1 ubiquitin ligase. Proc. Natl. Acad. Sci. U.S.A.104, 5794–5799. 10.1073/pnas.0610887104 (2007). 10.1073/pnas.0610887104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang, J. et al. TRIM11 promotes breast cancer cell proliferation by stabilizing estrogen receptor alpha. Neoplasia22, 343–351. 10.1016/j.neo.2020.06.003 (2020). 10.1016/j.neo.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian, M. et al. Deubiquitinase JOSD2 stabilizes YAP/TAZ to promote cholangiocarcinoma progression. Acta Pharm. Sin. B11, 4008–4019. 10.1016/j.apsb.2021.04.003 (2021). 10.1016/j.apsb.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou, X. et al. Regulation of Hippo/YAP signaling and esophageal squamous carcinoma progression by an E3 ubiquitin ligase PARK2. Theranostics10, 9443–9457. 10.7150/thno.46078 (2020). 10.7150/thno.46078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu, H. et al. USP10 promotes proliferation of hepatocellular carcinoma by deubiquitinating and stabilizing YAP/TAZ. Cancer Res.80, 2204–2216. 10.1158/0008-5472.CAN-19-2388 (2020). 10.1158/0008-5472.CAN-19-2388 [DOI] [PubMed] [Google Scholar]
- 23.Liu, J. et al. SDCBP promotes pancreatic cancer progression by preventing YAP1 from beta-TrCP-mediated proteasomal degradation. Gut72, 1722–1737. 10.1136/gutjnl-2022-327492 (2023). 10.1136/gutjnl-2022-327492 [DOI] [PubMed] [Google Scholar]
- 24.Yuan, B. et al. HERC3 promotes YAP/TAZ stability and tumorigenesis independently of its ubiquitin ligase activity. EMBO J.42, 111549. 10.15252/embj.2022111549 (2023). 10.15252/embj.2022111549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang, J., Tian, Z., Liao, X. & Wu, G. SOX13/TRIM11/YAP axis promotes the proliferation, migration and chemoresistance of anaplastic thyroid cancer. Int. J. Biol. Sci.17, 417–429. 10.7150/ijbs.54194 (2021). 10.7150/ijbs.54194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang, J. et al. The deubiquitinating enzyme UCHL3 promotes anaplastic thyroid cancer progression and metastasis through Hippo signaling pathway. Cell Death Differ.30, 1247–1259. 10.1038/s41418-023-01134-z (2023). 10.1038/s41418-023-01134-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ugolini, C. et al. Role of YAP-1 in thyroid tumor progression and outcome. Appl. Immunohistochem. Mol. Morphol.25, 581–585. 10.1097/PAI.0000000000000344 (2017). 10.1097/PAI.0000000000000344 [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Rendueles, M. E. R. et al. Yap governs a lineage-specific neuregulin1 pathway-driven adaptive resistance to RAF kinase inhibitors. Mol. Cancer21, 213. 10.1186/s12943-022-01676-9 (2022). 10.1186/s12943-022-01676-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celano, M. et al. Expression of YAP1 in aggressive thyroid cancer. Endocrine59, 209–212. 10.1007/s12020-017-1240-6 (2018). 10.1007/s12020-017-1240-6 [DOI] [PubMed] [Google Scholar]
- 30.Song, L. & Luo, Z. Q. Post-translational regulation of ubiquitin signaling. J. Cell Biol.218, 1776–1786. 10.1083/jcb.201902074 (2019). 10.1083/jcb.201902074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mussell, A., Frangou, C. & Zhang, J. Regulation of the Hippo signaling pathway by deubiquitinating enzymes in cancer. Genes Dis.6, 335–341. 10.1016/j.gendis.2019.06.004 (2019). 10.1016/j.gendis.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, Z. et al. Regulation of Hippo signaling and triple negative breast cancer progression by an ubiquitin ligase RNF187. Oncogenesis9, 36. 10.1038/s41389-020-0220-5 (2020). 10.1038/s41389-020-0220-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang, H. et al. RNF31 represses cell progression and immune evasion via YAP/PD-L1 suppression in triple negative breast cancer. J. Exp. Clin. Cancer Res.41, 364. 10.1186/s13046-022-02576-y (2022). 10.1186/s13046-022-02576-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao, B., Li, L., Tumaneng, K., Wang, C. Y. & Guan, K. L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev.24, 72–85. 10.1101/gad.1843810 (2010). 10.1101/gad.1843810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang, C. S., Shemorry, A., Auerbach, D. & Varshavsky, A. The N-end rule pathway is mediated by a complex of the RING-type Ubr1 and HECT-type Ufd4 ubiquitin ligases. Nat. Cell Biol.12, 1177–1185. 10.1038/ncb2121 (2010). 10.1038/ncb2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan, M. et al. Structural insights into Ubr1-mediated N-degron polyubiquitination. Nature600, 334–338. 10.1038/s41586-021-04097-8 (2021). 10.1038/s41586-021-04097-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallahi, G. H. et al. Novel UBR1 gene mutation in a patient with typical phenotype of Johanson-Blizzard syndrome. Eur. J. Pediatr.170, 233–235. 10.1007/s00431-010-1239-y (2011). 10.1007/s00431-010-1239-y [DOI] [PubMed] [Google Scholar]
- 38.Zenker, M. et al. Deficiency of UBR1, a ubiquitin ligase of the N-end rule pathway, causes pancreatic dysfunction, malformations and mental retardation (Johanson-Blizzard syndrome). Nat. Genet.37, 1345–1350. 10.1038/ng1681 (2005). 10.1038/ng1681 [DOI] [PubMed] [Google Scholar]
- 39.An, J. Y. et al. Impaired neurogenesis and cardiovascular development in mice lacking the E3 ubiquitin ligases UBR1 and UBR2 of the N-end rule pathway. Proc. Natl. Acad. Sci. U.S.A.103, 6212–6217. 10.1073/pnas.0601700103 (2006). 10.1073/pnas.0601700103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eldeeb, M. A., Fahlman, R. P., Esmaili, M. & Ragheb, M. A. Regulating apoptosis by degradation: The N-End Rule-mediated regulation of apoptotic proteolytic fragments in mammalian cells. Int. J. Mol. Sci.10.3390/ijms19113414 (2018). 10.3390/ijms19113414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, W. et al. Identification of Ubr1 as an amino acid sensor of steatosis in liver and muscle. J. Cachexia Sarcopenia Muscle14, 1454–1467. 10.1002/jcsm.13233 (2023). 10.1002/jcsm.13233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen, E., Kwon, Y. T., Lim, M. S., Dube, I. D. & Hough, M. R. Loss of Ubr1 promotes aneuploidy and accelerates B-cell lymphomagenesis in TLX1/HOX11-transgenic mice. Oncogene25, 5752–5763. 10.1038/sj.onc.1209573 (2006). 10.1038/sj.onc.1209573 [DOI] [PubMed] [Google Scholar]
- 43.Leboeuf, D. et al. Downregulation of the Arg/N-degron pathway sensitizes cancer cells to chemotherapy in vivo. Mol. Ther.28, 1092–1104. 10.1016/j.ymthe.2020.01.021 (2020). 10.1016/j.ymthe.2020.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pore, S. K. et al. N-end rule pathway inhibitor sensitizes cancer cells to antineoplastic agents by regulating XIAP and RAD21 protein expression. J. Cell Biochem.121, 804–815. 10.1002/jcb.29326 (2020). 10.1002/jcb.29326 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this study that support our findings are included in this paper and its supplemental data file.