Highlights
-
•
Use of bacteriophages outside of compassionate access in paediatrics.
-
•
No adverse events observed through the delivery of bacteriophages via bronchoscopy and nebulisation.
-
•
No detection of Pseudomonas aeruginosa in sputum following bacteriophage therapy.
-
•
Improvement in lung function above standard therapies.
Keywords: Bacteriophage, Bronchoscopy, Nebulisation, Pseudomonas
Abstract
Introduction
Pseudomonas aeruginosa is an organism well known for causing significant morbidity and mortality in people living with chronic lung conditions such as cystic fibrosis. We describe the safety, tolerability, and potential efficacy of bronchoscopic and nebulised bacteriophage administration, offering insights into a potential breakthrough for the treatment of chronic infections particularly in children and adolescents.
Method
A 12-year-old female (F12) and a 17-year-old male (M17), both diagnosed with cystic fibrosis and chronic P. aeruginosa lung infection, underwent bacteriophage treatment (BT). The administration involved bronchoscopic instillation and subsequent nebulisation. This was performed concurrently with intravenous antibiotics and regular physiotherapy delivered in an in-patient setting for 14 days. Microbiological, clinical, and lung function assessments were conducted to assess this treatment modality.
Results
No adverse events (fever, localised reaction, wheeze or bronchospasm) occurred during BT. F12 demonstrated a 4% increase, while M17 showed a 5% improvement in FEV1% from their best FEV1% over the past three years following BT. A 12% (F12) and an 8% (M17) improvement from baseline FEV1% was observed. For F12 P. aeruginosa was not isolated from her sputum despite 12 previous hospitalisations for intravenous antibiotics.
Conclusion
Bronchoscopic and nebulised routes of bacteriophage administration were well-tolerated in these two adolescents. This early report underscores the potential of this treatment modality and encourages clinicians and researchers to actively explore this innovative approach.
1. Introduction
Pseudomonas aeruginosa is well known for causing significant morbidity and mortality in people living with chronic lung conditions such as cystic fibrosis (Milczewska et al., 2020; Emerson et al., 2002). Paired with an increase in antimicrobial resistance of this organism, treating P. aeruginosa continues to pose a challenge to clinicians and exposes patients to recurrent, prolonged antibiotic treatment that can lead to significant side effects (Malhotra et al., 2019; Jackson and Waters, 2021; Bonyadi et al., 2022).
While significant and promising short-term benefits have been demonstrated following the introduction of CF transmembrane conductance regulator (CFTR) modulators, concerns persist regarding the potential resurgence of pathogens, particularly in those with pre-existing lung damage (Taylor-Cousar et al., 2023; Hisert et al., 2023).
Novel therapies, such as bacteriophages, have shown promise but face regulatory challenges due to their high specificity and the variable nature of these viruses (Pelfrene et al., 2021). Here, we report on two adolescents receiving bacteriophage therapy (BT) through bronchoscopy and nebulisation, focusing on the tolerability, safety and clinical outcomes of this administration modality (Singh et al., 2023; Pirnay et al., 2022).
2. Clinical characteristics
A 12-year-old female (F12) and a 17-year-old male (M17) were both diagnosed with cystic fibrosis (CF) featuring homozygous F508del mutation and pancreatic insufficiency since birth.
In the case of F12, non-mucoid P. aeruginosa was initially identified in her sputum at the age of six, and three years later, a mucoid strain of P. aeruginosa was first isolated. Subsequently, mucoid P. aeruginosa consistently appeared in all sputum samples collected every 3–4 months (n = 17 samples). For M17, non-mucoid P. aeruginosa was initially detected in his sputum at the age of 11, and consistently appeared in all subsequent sputum samples collected every 3–4 months (n = 26 samples).
Both have been subjected to multiple courses of intravenous antibiotics (mainly courses of Piperacillin/Tazobactam and Tobramycin) following the first isolation of P. aeruginosa within their sputum (F12; 11 admissions, M17; 13 admissions). F12 commenced Elexacaftor-Tezacaftor-Ivacaftor (ETI) for 3 months, and M17 for 18 months before BT. Both participants have evidence of bronchiectasis on their chest computed tomography scan.
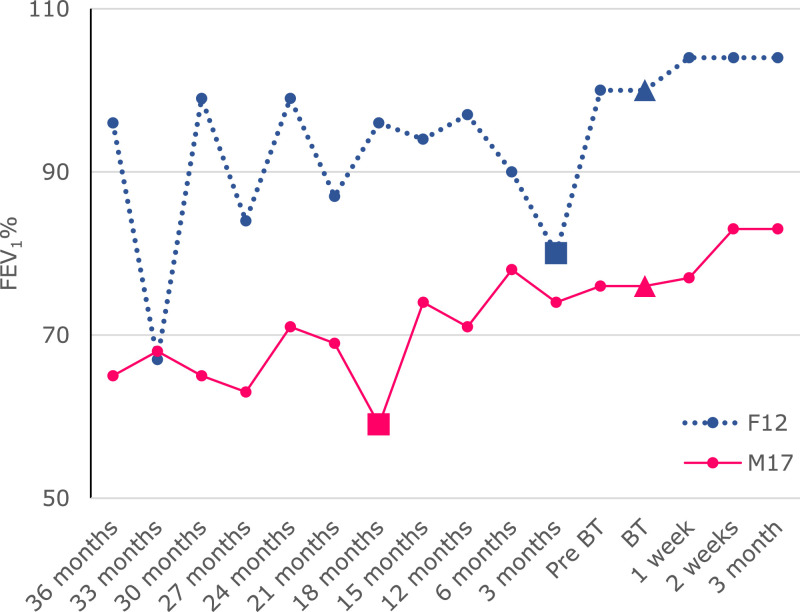
Both met the inclusion criteria which included; age ≥12 years and <18 years of age) with CF, positive sputum or bronchoalveolar lavage culture (P. aeruginosa) in more than 50% of sputum samples over the past year, continue to isolate P. aeruginosa in sputum despite undergoing eradication therapy using two antipseudomonal antibiotics, the latest clinical isolates of P. aeruginosa taken within 3 months of enrolment are susceptible to (demonstrates lytic activity) available anti-P. aeruginosa bacteriophage, and the ability to perform reliable spirometry (Singh et al., 2023). F12 had a baseline FEV1% (determined by the average of FEV1% performed in the past 12 months before BT) of 92% over the past 12 months and the best FEV1% of 100% over the past three years while M17 had a baseline FEV1% of 75% over the past 12 months and best FEV1% of 79% over the past three years (Fig. 1).
Fig. 1.
Spirometry trend over three years leading up to bacteriophage therapy. An increase in FEV1% was observed following the commencement of ETI in both F12 and M17, a further increase was observed in both following BT that resulted in the highest FEV1% over the past three years.  ; commencement of bacteriophage therapy,
; commencement of bacteriophage therapy,  ; commencement of ETI. ETI; Elexacaftor-Tezacaftor-Ivacaftor, BT; bacteriophage therapy.
; commencement of ETI. ETI; Elexacaftor-Tezacaftor-Ivacaftor, BT; bacteriophage therapy.
3. Phage administration
The methodology of the BT protocol (Sydney Children's Hospitals Network Human Research Ethics Committee ethics approval; 2022/ETH00241) has been previously published (Singh et al., 2023). Bacteriophage PBPA103 (Lyse N Tech, Heejoon Myung, South Korea) was selected for both participants as it provided the best lytic reaction for mucoid (F12) and non-mucoid (M17) strains of P. aeruginosa (LyseNTech 2009).
PBPA103 was instilled in all lobes of the lungs in equal aliquots. Intravenous Piperacillin/Tazobactam and Tobramycin were administered concurrently. Both had received this antibiotic combination in their previous admissions.
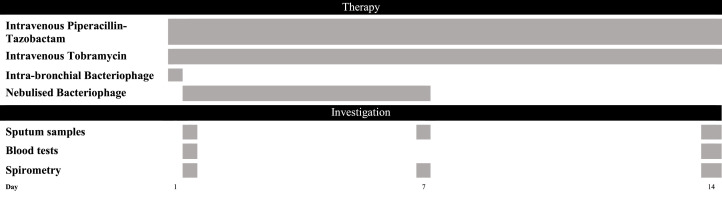
Both participants received nebulised bacteriophage on the following day, delivered through a non-vibrating mesh nebuliser over five minutes. PBPA103 was nebulised twice a day for the remaining seven days of BT. A summary of the treatment and investigations is illustrated in Fig. 2.
Fig. 2.
A summary of treatment and investigations that were performed on both F12 and M17. Both have received the same antibiotics in their previous admissions. Bacteriophage therapy was given for the first seven days of admission with the first dose of bacteriophage instilled into the airways through a bronchoscope followed by nebulised bacteriophages twice a day for the subsequent six days.
4. Clinical and microbiological outcomes
Following bronchoscopic and nebulised administration, vital signs taken every 15 minutes for one hour post-administration remained normal. No wheeze or respiratory distress was observed at one hour post-administration. Four-hourly vital signs remained within the normal range throughout BT. Spirometry performed before and after nebulisation of PBPA103 did not show any bronchospasm.
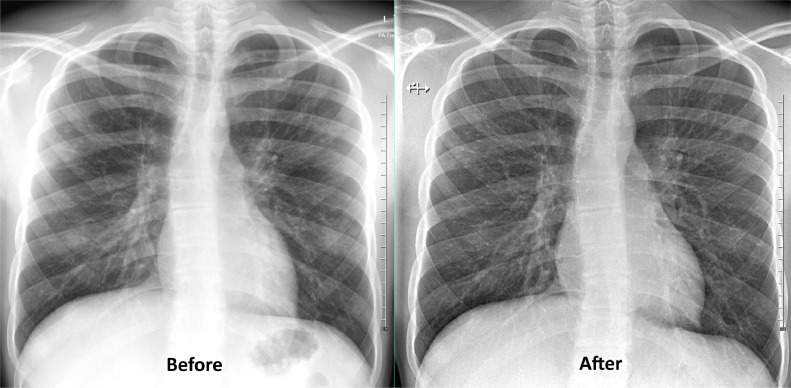
In terms of clinical outcomes, decreased cough and sputum expectoration were reported by both participants and the CF physiotherapist (performing twice-daily supervised physiotherapy). At the end of the BT, F12 demonstrated an increase of FEV1% by 4%, while M17 demonstrated an increase of FEV1% by 5% from their best FEV1% in the past three years. F12 demonstrated an increase of 12% and M17 demonstrated an improvement of 8% in terms of improvement from baseline FEV1%. An increase in markers such as white blood cells, liver transaminases and interleukin-6 were observed without any discernible clinical effects (Table 1). The serial chest x-ray of M17 before and after BT shown in Fig. 3 demonstrated improvement in the perihilar and right lower zone markings
Table 1.
Blood investigations performed to monitor the effects of bacteriophage therapy. BT; bacteriophage therapy, Ig; immunoglobulin.
| Baseline before BT | Day 2 following BT | Day 14 following BT | |
|---|---|---|---|
| Haemoglobin (115–160 g/L) | |||
| F12 | 103 | 122 | 113 |
| M17 | 121 | 130 | 139 |
| White blood cell count (4.5- 13.5 × 109/L) | |||
| F12 | 6.6 | 12.6 | 6.2 |
| M17 | 5.1 | 5.9 | 4.2 |
| Neutrophils (1.5–8 × 109/L) | |||
| F12 | 2.9 | 10.3 | 4 |
| M17 | 2.8 | 4.5 | 2.6 |
| Lymphocytes (1.5–7 × 109/L) | |||
| F12 | 3.1 | 1.5 | 1.6 |
| M17 | 1.5 | 0.6 | 1.1 |
| Eosinophil (0–1.1 × 109/L) | |||
| F12 | 0.1 | 0 | 0.1 |
| M17 | 0.1 | 0.1 | 0.1 |
| Monocytes (0.2–1 × 109/L) | |||
| F12 | 0.5 | 0.7 | 0.5 |
| M17 | 0.6 | 0.6 | 0.4 |
| Basophils (0–0.2 × 109/L) | |||
| F12 | 0.1 | 0 | 0 |
| M17 | 0 | 0 | 0 |
| C- Reactive protein (0–10 mg/L) | |||
| F12 | <0.3 | <0.3 | <0.3 |
| M17 | 0.9 | 4.5 | 0.5 |
| Aspartate aminotransferase (U/L) | |||
| F12 (0–41 U/L) | 32 | 91 | 81 |
| M17 (0–49 U/L) | 27 | 83 | ⁎⁎ |
| Alanine transaminase (U/L) | |||
| F12 (0–36 U/L) | 25 | 34 | 86 |
| M17 (0–39 U/L) | 31 | 103 | ⁎⁎ |
| Bilirubin (0–10 umol/L) | |||
| F12 | <2 | 11 | 6 |
| M17 | <2 | 2 | ⁎⁎ |
| Urea (2–6.8 mmol/L) | |||
| F12 | 1.8 | 3.9 | 2.8 |
| M17 | 4.4 | 5 | ⁎⁎ |
| Creatinine (35–74 umol/L) | |||
| F12 | 26 | 35 | 38 |
| M17 | 57 | 64 | ⁎⁎ |
| IgG (6.24–14.4 g/L) | |||
| F12 | 6.99 | 9.18 | 9.87 |
| M17 | 9.17 | 10.2 | 12.2 |
| IgA (0.74–2.28 g/L) | |||
| F12 | 0.6 | 0.84 | 0.83 |
| M17 | 1.25 | 1.44 | 1.7 |
| IgM (0.48–2.57 g/L) | |||
| F12 | 1.25 | 1.53 | 2.03 |
| M17 | 1.33 | 1.48 | 1.9 |
| Tumour necrosis factor-α (<0.067 pg/mL) | |||
| F12 | <0.067 | <0.067 | 0.26 * |
| M17 | 0.39 | 0.67 | 1.23 |
| Interleukin-6 (< 5.22 pg/mL) | |||
| F12 | 3.84 | 5.72 | 9.77 * |
| M17 | 0.72 | 13.85 | 1.78 |
A repeat blood test performed 6 weeks post-BT showed a decline of tumour necrosis factor α to <0.067 pg/mL and Interleukin-6 to 6.77 pg/mL.
Results not available due to sample haemolysis.
Fig. 3.
Chest X-rays of M17 taken 6 months before bacteriophage therapy (Before) and 6 months after completion of bacteriophage therapy (After) that demonstrated an improvement in the perihilar and right lower zone.
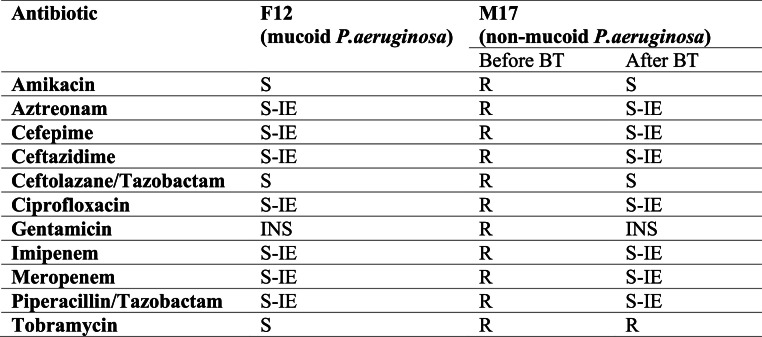
For F12, no P. aeruginosa species was isolated from induced sputum samples taken on days three, seven and 14, three, six- and nine-months post-BT. M17 continued to isolate non-mucoid P. aeruginosa from samples taken during the same intervals as was performed on F12 (Fig. 4). The P. aeruginosa isolate demonstrated a more sensitive organism towards antibiotics following BT. PBPA103 continued to cause lysis when tested on the bacterial lawn of his P. aeruginosa species isolated during and after BT.
Fig. 4.
The antibiotic susceptibility profile of P. aeruginosa of F12 and M17. The changes of antibiotic sensitivity was observed in isolates from M17S: Sensitive; R: Resistant; S-IE: Susceptible with increased exposure; INS: Insufficient evidence; BT: Bacteriophage therapy
5. Discussion
The absence of fever, bronchospasm (no wheezing or cough and, stable spirometry), and localised reactions during and after BT in these two adolescents have been promising. There was an improvement in cough and sputum production. Furthermore, we found that both demonstrated a rise of FEV1% above their best FEV1% in the past three years and the improvement exceeded what was achieved in previous admissions without BT. The improvement in lung function demonstrates a higher average of increase attributable to ETI (Middleton et al., 2019). In terms of the effect of ETI on FEV1%, studies have demonstrated that improvements of ppFEV1% of about 10% in lung function are seen in the first few weeks of commencing ETI, which plateaus thereafter (Middleton et al., 2019; He et al., 2024). In our cohort, a similar pattern was observed following the initial initiation of ETI which then plateaued. A further increase in FEV1% was then observed following BT, which is outside of the typical time and magnitude observed from ETI alone.
A temporary rise in white blood cell count, tumour necrosis factor α (TNF-α), and interleukin-6 (IL-6) levels was observed. While TNF-α and IL-6 increased in both F12 and M17, an increase in white blood cells was only observed in F12. A review of her blood results from previous admissions showed a similar trend, possibly related to inherent factors. An alternative explanation that favours BT could be that the administration of BT may improve phagocytic activity, thereby enhancing the intracellular killing of chronic P. aeruginosa which will show a neutrophilic reaction (Roach et al., 2017; Górski et al., 2018). Given the successful eradication of the organism from her sputum, this rise in neutrophils could be a hallmark of neutrophil-phage synergy, signalling effective phage therapy (Roach et al., 2017). The changes observed in the TNF-α and IL-6 is in contrast to several studies performed in-vitro and in animal models that showed either no change or a reduction in the expression of these cytokines (Górski et al., 2023; Al-Ishaq et al., 2020). However, our findings align with Van Belleghem et al., who described an increase in these cytokines that were clear, reproducible and, independent of the level of endotoxins within the bacteriophage preparation (Van Belleghem et al., 2017). From this, we postulate that the expression of these cytokines in our cohort is; 1) due to the interaction between the bacteriophage and bacterial host and not due to the bacteriophage alone; 2) while bacteriophages share similar properties (e.g., capsid protein folds) (Fokine and Rossmann, 2014), a single-point mutation can alter the serotype and the subsequent immune response towards the bacteriophage and therefore is strain specific; (Van Belleghem et al., 2017) 3) the modality of administration of our bacteriophage, particularly through bronchoscopy, may induce a more significant innate immune response that commences at the level of the respiratory epithelium. This transient upregulation in cytokines following BT will need to be further studied as it will add to our understanding of the effects of bacteriophages in the immune system and cannot be concluded from our findings alone. A two-fold rise in their liver transaminases could also be attributed to factors such as β-lactams administration will require further monitoring.
This is the first time P. aeruginosa was not found in the sputum sample of F12 over six years, despite repeated hospitalisations requiring intravenous antibiotics and the introduction of ETI. P. aeruginosa has not been detected within her sputum samples nine months after BT. An important aspect of this report that needs to be considered is the confounding role that ETI may have in our findings. Studies have demonstrated that the introduction of ETI has been shown to cause a decline in density of P. aeruginosa. These studies have shown that while ETI results in a decline in P. aeruginosa, the decline in bacterial density usually stabilises within the first three months following initiation of ETI, after which BT was commenced in both our participants (Nichols et al., 2023; Schaupp et al., 2023).
This report outlines the innovative application of bacteriophages delivered bronchoscopically and through nebulisation in treating challenging infections in children. While presenting two cases, it is important to acknowledge a significant limitation in the form of a small participant pool undergoing this treatment. The success in eradicating chronic infection warrants further microbiological and clinical surveillance over six and 12 months for a more conclusive assessment. However, the success in achieving eradication, following repeated courses of intravenous antibiotics over the past six years for chronic mucoid P. aeruginosa, cannot be understated.
Commencing BT poses multiple challenges, notwithstanding a decade of compassionate access treatments. The establishment of robust clinical trials, particularly in the context of our experience in conducting the first personalised BT as part of a clinical trial in children through bronchoscopy and nebulisation, faced formidable yet conquerable hurdles in ethics, governance, regulation, and production. This inaugural report emphatically underscores the feasibility of conducting clinical trials beyond the conventional bounds of compassionate access. Consequently, it serves as a compelling call to action for clinicians and researchers to actively explore and embrace this innovative approach.
Funding/Support
We appreciate the scholarship and bacteriophage production funding provided by The Cure4CF Foundation and The Team Simon Foundation for Cystic Fibrosis for this study.
CRediT authorship contribution statement
Jagdev Singh: Writing – original draft, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Stephanie Lynch: Writing – review & editing, Resources, Methodology, Investigation. Jonathan Iredell: Writing – review & editing, Validation, Supervision, Resources. Hiran Selvadurai: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The data that has been used is confidential.
References
- Al-Ishaq R.K., Skariah S., Büsselberg D. Bacteriophage treatment: critical evaluation of its application on world health organization priority pathogens. Viruses. 2020;13(1) doi: 10.3390/v13010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonyadi P., Saleh N.T., Dehghani M., Yamini M., Amini K.J.M.P. Prevalence of antibiotic resistance of Pseudomonas aeruginosa in cystic fibrosis infection: a systematic review and meta-analysis. Microb. Pathogen. 2022;165 doi: 10.1016/j.micpath.2022.105461. [DOI] [PubMed] [Google Scholar]
- Emerson J., Rosenfeld M., McNamara S., Ramsey B., Gibson RLJPp. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- Fokine A., Rossmann M.G. Molecular architecture of tailed double-stranded DNA phages. Bacteriophage. 2014;4(1):e28281. doi: 10.4161/bact.28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górski A., Międzybrodzki R., Jończyk-Matysiak E., Kniotek M., Letkiewicz S. Therapeutic phages as modulators of the immune response: practical implications. Clin. Infect. Dis. 2023;77(Supplement_5):S433–S439. doi: 10.1093/cid/ciad483. [DOI] [PubMed] [Google Scholar]
- Górski A., Międzybrodzki R., Łobocka M., et al. Phage therapy: what have we learned? Viruses. 2018;10(6) doi: 10.3390/v10060288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R., Lin F., Deng Z., Yu B. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with Phe508del mutation: evidence from randomized controlled trials. SAGE Open Med. 2024;12 doi: 10.1177/20503121231225874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisert K.B., Birket S.E., Clancy J.P., et al. Understanding and addressing the needs of people with cystic fibrosis in the era of CFTR modulator therapy. Lancet 2023;11(10):916–931. [DOI] [PubMed]
- Jackson L., Waters V. Factors influencing the acquisition and eradication of early Pseudomonas aeruginosa infection in cystic fibrosis. J. Cystic Fibrosis. 2021;20(1):8–16. doi: 10.1016/j.jcf.2020.10.008. [DOI] [PubMed] [Google Scholar]
- LyseNTech. Bacteriophage bank listing. 2009; http://www.phagebank.or.kr/phage/eng_bp_list.jsp.
- Malhotra S., Hayes D., Wozniak D.J. Cystic Fibrosis and pseudomonas aeruginosa: the host-microbe interface. Clin. Microbiol. Rev. 2019;32(3) doi: 10.1128/CMR.00138-18. 10.1128/cmr.00138-00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton P.G., Mall M.A., Dřevínek P., et al. Elexacaftor–Tezacaftor–Ivacaftor for cystic fibrosis with a single Phe508del allele. N. Engl. J. Med. 2019;381(19):1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milczewska J., Wołkowicz T., Zacharczuk K., et al. Clinical outcomes for cystic fibrosis patients with Pseudomonas aeruginosa cross-infections. Pediatr. Pulmonol. 2020;55(1):161–168. doi: 10.1002/ppul.24535. [DOI] [PubMed] [Google Scholar]
- Nichols D.P., Morgan S.J., Skalland M., et al. Pharmacologic improvement of CFTR function rapidly decreases sputum pathogen density, but lung infections generally persist. J. Clin. Invest. 2023;133(10) doi: 10.1172/JCI167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelfrene E., Sebris Z., Cavaleri M.J.B.B., Technology Therapy. Regulatory aspects of the therapeutic use of bacteriophages. Europe. 2021:1165–1177. [Google Scholar]
- Pirnay J.-P., Ferry T., Resch G. Recent progress toward the implementation of phage therapy in Western medicine. FEMS Microbiol. Rev. 2022;46(1):fuab040. doi: 10.1093/femsre/fuab040. [DOI] [PubMed] [Google Scholar]
- Roach D.R., Leung C.Y., Henry M., et al. Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe. 2017;22(1):38–47. doi: 10.1016/j.chom.2017.06.018. e34. [DOI] [PubMed] [Google Scholar]
- Schaupp L., Addante A., Völler M., et al. Longitudinal effects of elexacaftor/tezacaftor/ivacaftor on sputum viscoelastic properties, airway infection and inflammation in patients with cystic fibrosis. Eur. Respir. J. 2023;62(2) doi: 10.1183/13993003.02153-2022. [DOI] [PubMed] [Google Scholar]
- Singh J., Fitzgerald D.A., Jaffe A., et al. Single-arm, open-labelled, safety and tolerability of intrabronchial and nebulised bacteriophage treatment in children with cystic fibrosis and <em>Pseudomonas aeruginosa</em>. BMJ Open Respir. Res. 2023;10(1) doi: 10.1136/bmjresp-2022-001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Cousar J.L., Robinson P.D., Shteinberg M., Downey DGJTL. CFTR modulator therapy: transforming the landscape of clinical care in cystic fibrosis. Lancet. 2023;402(10408):1171–1184. doi: 10.1016/S0140-6736(23)01609-4. [DOI] [PubMed] [Google Scholar]
- Van Belleghem J.D., Clement F., Merabishvili M., Lavigne R., Vaneechoutte M. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci. Rep. 2017;7(1):8004. doi: 10.1038/s41598-017-08336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.