Abstract
Oxidative stress (OS) is a major concern that impacts the overall health of chickens in modern production systems. It is characterized by an imbalance between antioxidant defence mechanisms and the production of reactive oxygen species (ROS). This literature review aims to provide a comprehensive overview of oxidative stress in poultry production, with an emphasis on its effects on growth performance, immune responses, and reproductive outcomes. This review highlights the intricate mechanisms underlying OS and discusses how various factors, including dietary components, genetic predispositions, and environmental stressors can exacerbate the production of ROS. Additionally, the impact of oxidative stress on the production performance and physiological systems of poultry is examined. The study also emphasizes the relationship between oxidative stress and poultry diseases, highlighting how impaired antioxidant defenses increase bird's susceptibility to infections. The review assesses the existing approaches to reducing oxidative stress in chickens in response to these challenges. This includes managing techniques to lower stress in the production environment, antioxidant supplements, and nutritional interventions. The effectiveness of naturally occurring antioxidants, including plant extracts, minerals, and vitamins to improve poultry resistance to oxidative damage is also examined. To improve the antioxidant defenses of poultry under stress conditions, the activation of cellular homeostatic networks termed vitagenes, such as Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) is necessary for the synthesis of protective factors that can counteract the increased production of ROS and RNS. Future studies into novel strategies for managing oxidative stress in chicken production would build on these research advances and the knowledge gaps identified in this review.
Key words: oxidative stress, poultry, climate change, performance, reactive oxygen species
INTRODUCTION
The agricultural industry has been adversely impacted by the recent notable rise in the average global temperature (Barrett et al., 2019; Uyanga et al., 2023), which has threatened the expansion of the sector. Poultry is a crucial sector in livestock industries with the quickest growth rate, which significantly improved nutrition and food security, and its products, which are eggs and meat, are consumed worldwide (Surai, 2016). One of the primary factors that adversely affect livestock production is oxidative stress (OS), which has been exacerbated by climate change (Vandana and Sejian, 2018; Oke et al., 2022, 2024). Chen et al. (2021) revealed that if global warming worsens, the detrimental effects of OS will become more evident. As shown in Table 1, numerous studies have found a connection between oxidant damage and the reduction of growth performance in birds under heat stress (Kikusato et al., 2021; Oni et al., 2023; Oni et al., 2024).
Table 1.
Growth performance as a result of oxidative stress in poultry.
| Reference | Oxidative stress on performance |
|---|---|
| Surai et al. (2019) | Lower growth rate |
| Mashkoor et al. (2023) | Higher FCR |
| Agrawal et al. (2023) | Muscle wasting and reduced protein synthesis |
| Zhang et al. (2018) | Reduced body weight |
Chicken meat has grown to be one of the most famous animal protein sources due to its high nutritional content and relative affordability (Petracci et al., 2015; Kpomasse et al., 2021; Oke et al., 2021a; Kpomasse et al., 2023a; Akosile et al., 2023a). To meet the ever-increasing meat demand, fast-growing broiler chickens are needed in poultry production. Therefore, to bridge the gap in the meat demand, poultry genes are always evolving. The selection of broiler chickens for fast growth has made them more vulnerable to rising temperatures than other poultry species, especially in the tropics (Oke et al., 2016). The lack of sweat glands, insulating feathers, and substantially higher mass-to-body surface area ratio also expose them to diverse environmental stressors (Bernabucci, 2019). A mature chicken's body temperature typically ranges from 40.6 to 41.7°C (Ranjan et al., 2019). The internal body temperature of the chicken increases when the outside temperature rises above 24°C, leading to some notable alterations in the physiology and metabolism of the birds (Cassuce et al., 2013). Adult chickens feel unperturbed when kept in an environment with ambient temperatures between 18 and 24°C, whereas chicks need a greater temperature during their first week of life about 32°C, which gradually drops as they grow (Scanes, 2015).
Management, microbiological, nutritional, and environmental factors contribute to stress in commercial poultry production, which has detrimental effects on the productivity and overall health of the birds (Estévez, 2015; Alo et al., 2024). Avian species have become more susceptible to oxidative stress (OS) due to harsh environmental conditions because of climate change (Gonzalez-Rivas et al., 2020). The thermoregulatory system of the birds has become more susceptible to harsh environmental conditions, which could be a deterrent to their production (Zaboli et al., 2019).
Stressful conditions predispose poultry species to produce more free radicals while antioxidant enzyme activity and the ability to scavenge free radicals decline (Miao et al., 2020). This can increase production costs and lower their meat quality. In poultry, a marked decrease in the growth of breast muscle has been reported in birds raised in hot climates, especially broilers, due to metabolic changes (Safdar and Maghami, 2014). The degree of stress affects the level of production of the birds as well as their effectiveness (Adu-Asiamah et al., 2021). Additionally, stressful conditions are primarily linked to decreased feed intake, increased water intake, stunted growth, elevated mortality, and altered meat quality in chicken production (Wasti et al., 2020). Cell damage causes an upregulation of HSP 70 expression, which is necessary for the cells to survive. Inducing HSP-70 could be a therapeutic target for a number of stressor-induced injuries. In chickens, stressful conditions have been connected to cellular OS and have been revealed to raise ROS production, which in turn stimulates the expression of HSP70 (Zheng et al., 2022). In view of the nexus between oxidative stress and poultry production, it is crucial for the industry to understand the interplay between them.
Oxidative Stress
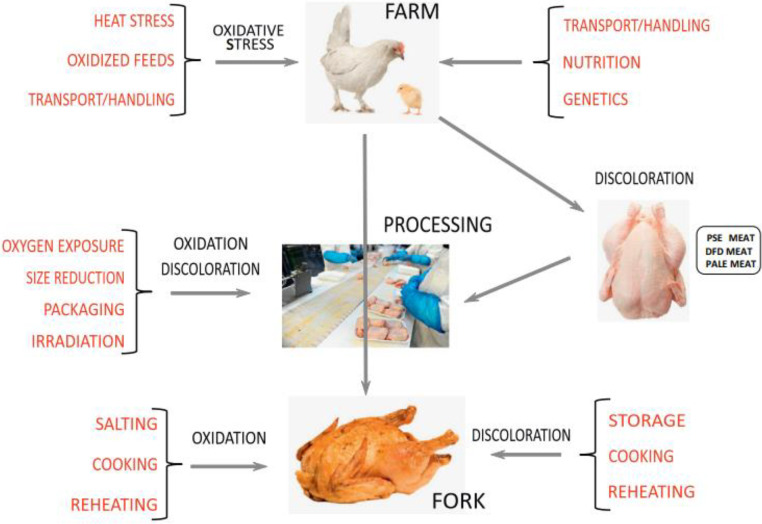
Oxidative Stress (OS) can be defined as an imbalance (in favour of the pro-oxidants) that exists between antioxidants and pro-oxidants. When reactive species (RS) are present in excess of what animal cells can accommodate as antioxidants, it leads to OS. As part of regular metabolism, the body constantly produces reactive oxygen species (ROS). This imbalance at the individual or cellular level results in OS Halliwell and Whiteman, 2004, Voljč et al., 2011. "Reactive oxygen/nitrogen/chlorine species" classifies several radicals and metabolic products harmful to the well-being of an animal (Halliwell and Whiteman, 2004). These compounds are highly reactive and can alter a variety of biologically significant macromolecules, like nucleic acids (RNA and DNA), proteins and lipids. These phenomena induce oxidative damage and OS, which lead to the development of various metabolic dysfunctions (Halliwell and Whiteman, 2004). ROS are produced more frequently in stressful environments, and this can result in oxidative damage and reduced antioxidant capacity (Mishra and Jha, 2019). OS is a crucial problem in the present-day poultry industry, and it can cause damage to biomacromolecules and, further dysfunction in cells and even injury to tissues (Cheng et al., 2017b; Gessner et al., 2017). OS is one of the main stresses that can adversely affect meat quality and chicken growth (Figure 1). Some factors related to the production of broiler breeders, such as spermatogenesis, egg production, the quality of stored eggs, and the livability of hatchlings, can be adversely impacted by OS (Lin et al., 2006).
Figure 1.
Sources of oxidative stress and discoloration to poultry and poultry meat (Carvalho et al., 2016).
Multiple antioxidants are present in living tissues to counteract oxidants. However, OS is indicated when the body's equilibrium between antioxidants and oxidants is disturbed and oxidants surpass a certain threshold. Living cells' mitochondria produce the majority of oxidants during cellular metabolism. ROS are generated not only by cellular metabolic activities but also by some external sources, such as feed that contains oxidized lipids and fats (Cadenas and Davies, 2000). The principal source of ROS is the release of electrons during oxidative phosphorylation from the mitochondrial respiratory chain (Mujahid et al., 2007). Since the electron transport chain is essential to the muscle's ability to produce energy, high environmental temperatures upregulate the generation of ROS. Decreased protein synthesis, accelerated muscle aging, and the inactivation of nuclear proteins are all possible consequences of increased ROS release (Celi and Gabai, 2015).
Elevated ROS concentrations rise in stressful environmental conditions, causing the chicken physiology to struggle to maintain thermal homeostasis (Sahin et al., 2016). Excessive production of ROS can cause poor nutrient absorption and digestion, which alter the redox status of the intestinal mucosa and cause the antioxidant system to malfunction, as was previously mentioned (Liu et al., 2014). Moreover, OS damages the mucosa of the intestines, impairs the effectiveness of digestion and nutrient absorption, and negatively affects the average growth of animals (Yara et al., 2013). Under OS, the body produces and releases heat shock proteins (HSP) in an attempt to protect itself from the harmful effects of ROS on cells (Archana et al., 2017). OS is often measured by the concentration of malondialdehyde (MDA) in the body (Cheng et al., 2017a).
OS has negative impacts on the quality of the meat in commercial poultry farming because oxidative reactions take place at every stage of the processing and production of poultry meat, from farm operations to the consumer (Estévez, 2015). Additionally, research indicates that, in comparison to other animals, poultry species are more vulnerable to OS and harsh environmental temperatures (Celi and Gabai, 2015; Estévez, 2015). Birds, such as broilers, layers, and turkeys, are especially vulnerable to oxygen deprivation due to breeding and choice of genes for fast development, greater nutrition utilization, and increased egg yields (Soleimani et al., 2011).
Reactive Oxygen Species
Reactive Oxygen Species (ROS) are chemically reactive and oxygen-containing molecules that have a number of advantageous functions in a living organism. The peroxisomes, plasma membrane, endoplasmic reticulum, and mitochondria are the main sources of ROS (Ayala et al., 2014). ROS are naturally produced as a consequence of oxygen metabolism and are crucial for homeostasis and cell signaling. However, during exposure to stressors, ROS levels can rise significantly, resulting in damage to cell structures. Excessive ROS can negatively impact the proteins, RNA, and DNA and may cause the death of cells (Xie et al., 2019). Some of the known reactive nitrogen species and reactive oxygen species are singlet oxygen (1O2), hydrogen peroxide (H2O2), hydroxyl radical (•OH) and superoxide anion (O2−), peroxynitrite anions (ONOO−), nitric oxide (NO) (Das and Roychoudhury, 2014; Ahmad et al., 2017). At low levels, ROS are crucial for cellular functions despite their destructive capacity. They can act assignaling molecules influencing processes like cell differentiation, defence against infectious agents, and apoptosis (Checa and Aran, 2020). However, a skewness between ROS production and the body's capacity to detoxify the radicals can lead to pathological conditions (Hernández-García et al., 2010).
Poultry oxidative damage disrupts normal metabolism, resulting in the formation of anomalies in the meat, like white striping and wooden breasts (Estévez, 2015; Carvalho et al., 2016). Chickens with reduced antioxidant capacity are more likely to have oxidative reactions in their meat after they are killed. It causes a pro-oxidant environment in muscle tissues, which eventually results in low-quality meat by oxidizing lipids, and proteins during the meat processing process, harming the meat's sensory qualities and nutritional makeup (Shakeri et al., 2018). Moreover, OS negatively affects the health and productivity of chickens by negatively affecting their gastrointestinal tract, a sensitive organ that is necessary for healthy digestion and absorption of nutrients because oxidative reactions generate free radicals, which in turn lower poultry productivity; they may cause damage to intestinal epithelia (Gonzalez-Rivas et al., 2020).
Sources of OS in Poultry
Micro-organisms
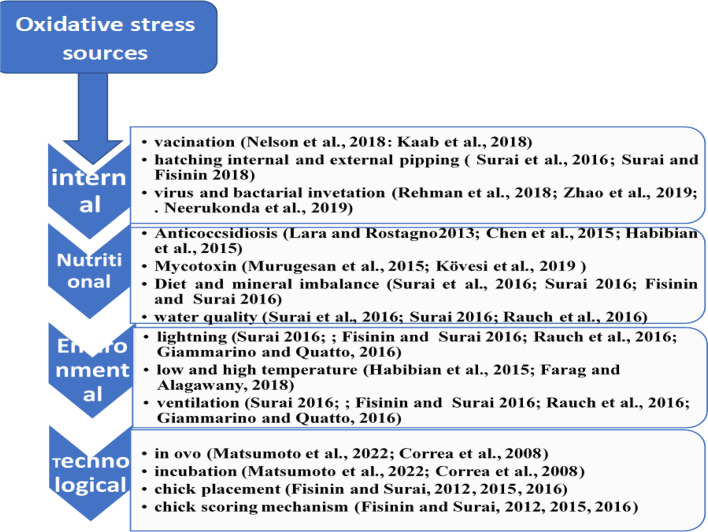
There are several ways in which microorganisms can cause oxidative injury in poultry (Figure 2). This is typically through the induction of the body's natural immune responses, causing the overproduction of ROS, or through the production of ROS as by-products of their metabolism (Mishra and Jha, 2019). When poultry is infected by a pathogenic micro-organism, the bird's immune system responds to clear the infection (Kogut et al., 2020). As part of this process, immune cells like macrophages and neutrophils generate ROS to kill the invading microorganisms, known as the oxidative burst (Slauch, 2011). While this is generally a good thing, under certain conditions, ROS production can exceed the capacity of the bird's antioxidant systems, leading to OS (Freitas et al., 2009).
Figure 2.
Main stressors in poultry production
Certain micro-organisms produce ROS as metabolic by-products. During aerobic respiration, bacteria can produce superoxide radicals and hydrogen peroxide, both of which are forms of ROS (Madkour, 2019). Some avian viral diseases can affect the production of ROS and increase OS. For instance, the Avian Influenza virus has been shown to cause OS by altered equilibrium in antioxidant defenses and ROS production in the host (Rehman et al., 2018). Microorganisms like bacteria, fungi, and protozoa are lined in the gastrointestinal tract of poultry birds, with the highest population at the distal end of the gastrointestinal tract (Gabriel et al., 2006). Studies have documented that the relationship between the internal lining and micro-organisms triggers OS (Naidoo et al., 2008).
Food Toxins
Food toxins, often referred to as mycotoxins, are toxic substances produced by certain types of fungi which can contaminate poultry feed (Da Silva et al., 2018). When ingested by poultry, these toxins can have several adverse effects, among which is the creation of OS (Filazi et al., 2017). Mycotoxins such as aflatoxins, ochratoxins, and fumonisins can cause a rise in the generation of ROS, like free radicals and peroxides (Omar, 2013). Normal metabolism and biochemical reactions in the body already generate some ROS. However, exposure to these toxins significantly increases ROS levels, overwhelming the birds' antioxidant defenses (Panda and Cherian, 2014). Antioxidants are molecules that can neutralize ROS and prevent them from causing damage. They are crucial in maintaining the balance between ROS production and elimination. Mycotoxins not only increase ROS generation but also inhibit the action or production of antioxidants, thereby amplifying the potential for OS (Birben et al., 2012; Liu et al., 2018). Mycotoxins also contribute to lipid peroxidation, a process in which ROS oxidize the fatty acids in cell membranes, causing cell damage and leading to many diseases. Lipid peroxidation under the influence of mycotoxins has been documented in several studies in poultry (Mavrommatis et al., 2021; Guerrini and Tedesco, 2023; Kulcsár et al., 2024; Okasha et al., 2024).
Thermal Challenge and OS
Thermal challenges in poultry refer to the stress that birds experience when exposed to high ambient temperatures (Lara and Rostagno, 2013). Avian species do not have sweat glands and, therefore, cannot dissipate excess heat (Nawab et al., 2018). When exposed to temperatures beyond their thermoneutral zone (approximately 18–24°C for broilers), they can experience heat stress. This heat stress is particularly of concern in rapidly growing commercial broilers with high metabolic rates (Kumari and Nath, 2018). Heat stress in poultry leads to various physiological changes, including increased body temperature, rapid respiration (panting), and alterations in blood chemistry. Heat stress induces a redox imbalance in favour of prooxidants over antioxidants, which is one of the leading causes of systemic OS. One of the primary concerns is the OS induction (Fisinin and Kavtarashvili, 2015). Under the thermoneutral zones, the body has a sophisticated system, including enzymes such as GPX and SOD that neutralize ROS (Yang et al., 2010). However, during heat stress, the bird's metabolic rate may increase, leading to an overproduction of ROS. Additionally, heat stress may disrupt mitochondrial function, which can further enhance ROS production ((Algothmi et al., 2023). The OS resulting from heat stress can have widespread effects on the bird's health, including excess ROS and can lead to the peroxidation of lipids, altering the integrity and function of cell membranes and causing oxidative modification of proteins and nucleic acids (Emami et al., 2020). The adaptive and innate immune responses can be compromised, leading to higher disease susceptibility. As a result of increased temperature, OS can adversely affect growth performance, feed consumption, and overall meat quality (Zaboli et al., 2019; Gonzalez-Rivas et al., 2020; Valadez-García et al., 2021).
Antioxidant Defense Mechanism in Poultry
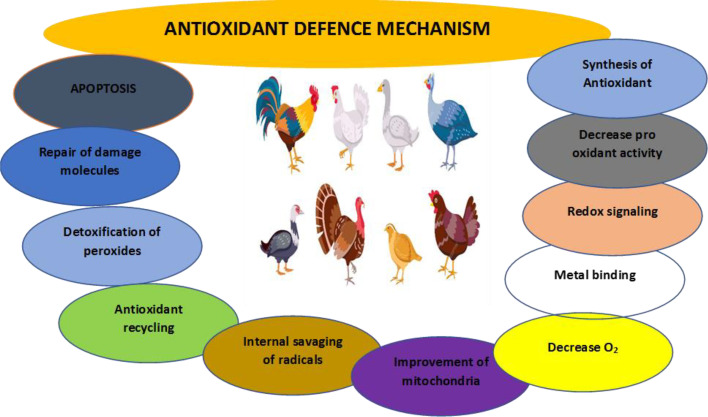
Antioxidant Defense Mechanism (ADM) plays a pivotal role in poultry to prevent the harmful effects of OS (Figure 3). These defence mechanisms include a complex array of nonenzymatic and enzymatic antioxidants, which work harmoniously to mitigate the damaging effects of ROS (Panda and Cherian, 2014; Surai et al., 2019), effectively balancing the formation process in living cells with detoxification of ROS in the body to maintain the level to avoid OS (He et al., 2017). Cells can withstand OS by using several antioxidants (Jîtcă et al., 2022). Excess ROS are damaging to the body; therefore, once the antioxidant defence mechanism reaches its limited ability, OS occurs, making polyunsaturated fatty acid, protein and DNA damage in the body, leading to detrimental health, low growth and death of the bird (Rehman et al., 2018). The antioxidant defence mechanism comprises different strategies, as shown in Figure 2.
Figure 3.
Antioxidant defense mechanism in poultry.
Antioxidant defence mechanisms attack free radical production in the body leading to decreased oxygen levels and reduced enzyme activity which produce ROS, thus letting off iron and copper glued to the protein and maintaining the capacity of the mitochondria, further preventing the formation of radicals in the body (Young and Woodside, 2001). Enzymatic antioxidants include glutathione peroxidase (GPX), catalase (CAT), superoxide dismutase (SOD), peroxiredoxins, thioredoxin reductase, etc. SOD is involved in the conversion of superoxide into H2O2, which is then neutralized by CAT and GPX to water and molecular oxygen, thereby reducing the levels of harmful ROS (Jeeva et al., 2015). Non-enzymatic antioxidants, which include glutathione, ascorbic acid, carotenoids, tocopherol, CoQ, carnitine, taurine, etc. can scavenge free radicals by giving off a hydrogen atom or electron. Both antioxidants are important in counteracting OS (McGraw, 2011; Panda and Cherian, 2014). The effectiveness of this antioxidant defense system is critical for the bird's ability to withstand conditions that induce OS (Surai et al., 2019). Any dysfunction in these antioxidants can disrupt the balance between ROS production and elimination, resulting in OS and potential damage to the bird's cells (Mishra and Jha, 2019).
Oxidative Stress and Cell Signaling Pathways
Oxidative stress plays a crucial role in modulating various cell signaling pathways, including Mitogen-Activated Protein Kinases (MAPKs), Nuclear Factor kappa B (NF-κB), Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2), and Phosphoinositide 3-Kinase/Protein Kinase B (PI3K/Akt), SCREB1, CREB, NOTCH, PPAR-γ, TP53, FoxO, AP1, etc. (Wang and Hai, 2016; Tu et al., 2019; Tonev and Momchilova, 2023). Over 500 distinct genes' expression has been linked to the activation of these transcription factors (Tu et al., 2019). Changes to the antioxidant and ROS-generating enzymes, which are vital for an animal's ability to respond to a variety of stressors, alters the network of antioxidant defense. These pathways influence key cellular processes, such as apoptosis, inflammation, and antioxidant defenses. The interplay between oxidative stress and cell signaling has been documented (Iqbal et al., 2024). Although the effects of oxidative stress have been extensively studied in poultry, the complex relationship with cell signaling pathways is poorly understood. This has, however, received attention in recent years.
Nrf2 is a master regulator of antioxidant defenses, maintaining redox equilibrium in cells and tissues by activating a variety of vitagenes and other protective molecules (Surai et al., 2019; Surai, 2020). Since superoxide is the main radical in the biological system, one of the main tasks of the first level of antioxidant defence is to effectively remove it. During OS, Nrf2 enters the nucleus and joins the MAF transcription factor (Maf) protein as a heterodimer, then binds to genes dependent on ARE to produce antioxidant effects (Lacher et al., 2018). Nrf2 deal with free radicals at their production sites and initiates the synthesis of the enzymes of the first line of the antioxidant defence, namely, catalase, GPx and SOD (Surai, 2016). Pan et al. (2021) observed that an OS caused by wooden breast myopathy elicited the activation of Nrf2 to protect chickens. In addition, Nrf2 directly modifies intermediate metabolism through metabolic reprogramming and upregulates genes that produce protective proteins that guide the breakdown and repair of affected macromolecules during stressful conditions (Zhou et al., 2014). Moreover, the finding of Rajput et al. (2018) revealed that AFB1 caused a notable decrease in the Nrf2 gene's mRNA and protein expression. Dietary inclusion of Antrodia cinnamomea powder at 0.1 to 0.4% for 35 d was shown to increase Nrf2 expression in the liver (Lee et al., 2018). This suggests that Nrf2 activation is one of the most significant mechanisms of phytogenics to protect poultry from OS. Additionally, the findings of Niu et al. (2019) revealed that chickens that were fed fermented ginkgo biloba leaf meal at 3.5 g/kg demonstrated enhanced growth performance and the small intestine showed an increase in Nrf2 expression. This suggests that phytogenics could be beneficial in ameliorating the detrimental impact of OS on the activities of Nrf2. Moreover, the recent findings of Gao et al. (2023) revealed the detrimental effect of T-2 toxin on oxidative damage and down-regulation of Nrf2 in broiler chickens. According to (Sun et al., 2020), quercetin alleviated intestinal oxidative stress generated by LPS in broiler chickens by activating the Nrf2 pathway. Both quercetin and resveratrol have been shown to activate the Nrf2 pathway and stimulate the production of ROS scavengers (Suraweera et al., 2020).
The transcription factors in the NF-κB family are known to bind to specific DNA sequences called κB sites, which are found in the enhancer and promoter regions of different genes. These domains are called Rel-homology domains (RHDs) (Kopitar-Jerala, 2015). It is known that NF-κB is found in the cytoplasm of dormant cells, where it is firmly linked to inhibitory IκB proteins to prevent binding to target sites. It has long been believed that NF-κB is a classic proinflammatory signaling pathway that activates the immune system in response to a variety of stimuli, such as oxidative stress. By causing apoptosis and aberrant autophagy via the ROS/NF-κB signaling pathway in the broilers, H2O2-induced OS dramatically reduced the quality of the meat. This suggests a crucial role and potential molecular mechanism for oxidative damage in the broiler thigh muscle (Yan et al., 2022). It has been proposed that NF-κB plays a key function in controlling mitochondrial respiration (Tornatore et al., 2012). As mitochondria are the primary generator of ROS, it is possible that NF-κB signaling controls the production of ROS, aids in detoxification, and preserves redox equilibrium (Surai et al., 2021). Existing studies suggest that Nrf2 and NF-κB oppose each other as a response to OS (Tkach et al., 2014; Zhang et al., 2019). A growing body of research suggests that different dietary antioxidants may regulate the activation of the transcription factors, such as downregulating NF-κB expression while concurrently upregulating Nrf2 expression. Several phytogenic compounds, primarily polyphenols, have been reported to boost Nrf2 expression/ activity and reduce NF-κB during oxidative stress. Some of the herbal-derived compounds are chiisanoside (Bian et al., 2019), rosmarinic acid, amygdalin (Tang et al., 2019), alpinetin (Liu et al., 2019), etc.
In thermally stressed birds, expression of NF-κB varies according to temperature, length of exposure, and age of the bird. When 25-week-old Roman egg-laying hens were exposed to thermal challenge (32 ± 1°C, 6 h daily for nine weeks), their serum levels of inflammatory cytokines (IL-1β, IL-6, and TNF-α) were found to be higher than those of control, nonstressed birds (Nawab et al., 2019). However, quails at 20 wk of age showed a significant decrease in NF-κB mRNA levels and a significant increase in liver IL-1β and TLR4 mRNA levels when they were heat-stressed (34°C for 4 h per day for 20 consecutive days) (Pu et al., 2020). One of the main nutritional stressors in the poultry industry is mycotoxins. The pathogenesis of mycotoxicosis is closely associated with oxidative stress in poultry production since these mycotoxins stimulate ribosomal stress that initiates several cellular cascades including MAPK and NF-κB signaling (Shah Alam et al., 2024). As such, feeding chickens a diet contaminated with AFB1 (74 μg/kg) increased the liver protein expressions of NF-κB p65 (Ma et al., 2015).
The members of MAPK cascades, c-Jun N-terminal kinase (JNK) and MAPK are triggered by oxidative stress and regulate intracellular redox state in animals (Watanabe et al., 2015). Because of its critical function in both cell survival and death, the MAPK pathway and OS are intricately connected (Kumar et al., 2021). Wang et al. (2020) investigated the protective effects of selenium yeast against cadmium (Cd)-induced necroptosis in chicken livers and found that while Cd could cause oxidative stress, activate the MAPK pathway, and cause necroptosis damage in chicken livers, selenium yeast had protective effects against this type of Cd-induced injury by down-regulating the MAPK pathway and inhibiting oxidative stress. Yang et al. (2019) observed that dietary supplementation with leonurine hydrochloride led to considerably lower protein expression levels of phosphorylated p38, ERK, and JNK in the jejunal mucosa when the broilers were challenged with lipopolysaccharide. It was suggested that the anti-inflammatory and antioxidant properties of leonurine hydrochloride could be linked to the inhibition of proinflammatory cytokine and enzyme expression that follows the suppression of the MAPK and NF-κB signaling pathways.
Gastrointestinal Tract and OS
The gastrointestinal tract (GIT) is an intricate and dynamic part of the digestive system essential for both immunological response and nutrient absorption (Lan et al., 2005). The lining of the intestine comprises connective tissues, epithelial cells, and diverse cell populations. It serves as a site for the digestion of chemical compounds present in feed. The digestive tissues are continuously exposed to a variety of potentially hazardous substances because they serve as a sort of filter separating the GIT's lumen surroundings from its tissues. The delicate harmony between the components of the chicken and the GIT can be adversely impacted by a variety of stressors, including feed toxins, which can ultimately have an effect on the overall performance of poultry. The accumulation of ROS in cells and tissues is caused by a contradiction between the generation of free radicals and the body's natural antioxidant defence.
A multitude of stresses related to surroundings, technology, diet, and biology and organs within are linked to commercial poultry production and are the cause of morbidity and reduced reproductive and productive performance (Surai and Fisinin, 2016). Stressors can potentially cause a range of changes in the gastrointestinal tract, including modifications to the protective microbiota and impaired integrity of the intestinal epithelium (Dinan and Cryan, 2012). In poultry, OS has a significant influence on the GIT, resulting in increased intestinal permeability and poor nutritional absorption (Kim et al., 2023). This leads to a low immune response and birds become more susceptible to infections. Extended exposure to OS frequently leads to chronic inflammation, which further impairs health and growth. Poultry's intestinal tract is home to a dynamic and complex microbial ecosystem known as the microbiome, which is influenced by a multitude of factors (Wei et al., 2013).
Impact of OS on Poultry Productivity
Performance of Poultry Birds
In poultry birds, OS can have a profound impact on various physiological parameters, including growth performance (Hafez et al., 2022). According to Etuah et al. (2020), the growth rate in poultry is a critical parameter for economic efficiency in poultry production. It is influenced by genetic, nutritional, and environmental factors. OS can negatively impact the growth rate through cell damage in poultry birds (Rehman et al., 2018). ROS can be detrimental to cellular components like DNA proteins and lipids. This damage can impair cell function and lead to apoptosis or necrosis, slowing down tissue growth, especially rapidly dividing ones like muscle (Davies, 2000; Slimen et al., 2014). OS can influence the immune system of birds. A compromised immune system requires energy that would otherwise be used for growth, thus diverting resources away from growth processes (Monaghan et al.,2009). Additionally, OS can interfere with hormonal balance. Hormones like growth hormones and thyroid hormones that are critical for growth may be affected, leading to reduced growth rates (Tarım, 2011).
Feed intake is another crucial factor for growth performance in poultry (Herd and Arthur, 2009). OS can influence feed intake by affecting the palatability and digestibility of feed as well as nutrient absorption in the gut, which can lead to decreased feed intake (Upadhaya and Kim, 2021). When birds experience OS, they may divert energy towards antioxidant production and repair mechanisms rather than towards growth, which can reduce their appetite (Arnold et al., 2015). OS leads to diseases like ascites or pulmonary hypertension in birds; these conditions can significantly reduce feed intake (Mohsen and Taimor, 2011).
The feed conversion ratio measures the quantity of feed needed to gain a unit of body weight. It is a critical measure of efficiency in poultry production (Fry et al., 2018). The impact of OS can be severe on feed conversion ratio (FCR) due to metabolic disturbances and gut damage, which make nutrient utilization less efficient (Celi et al., 2013; Yara et al., 2013). This means that more feed is required to produce the same amount of weight gain, leading to a higher FCR. Birds under OS may require more energy for maintenance functions such as detoxification and repair (Koivula and Eeva, 2010). This energy comes at the expense of growth, thus worsening the FCR. Resources may also be allocated to repairing oxidative damage rather than production, leading to a higher FCR (McGraw, 2011).
Meat Quality
In poultry, OS can significantly impact the quality of meat (Table 2), affecting various aspects of farm productivity (Fouad et al., 2016). Lipid oxidation is an intricate process that significantly impacts poultry meat quality, encompassing flavor, nutritional value, and shelf life (Domínguez et al., 2019). It is a primary cause of off-flavors and aroma alterations in poultry meat. According to Ayala et al. (2014), when lipids are exposed to OS, they break down into more minor compounds, such as aldehydes, ketones, and hydrocarbons. These compounds are often associated with rancid, metallic, or fishy odours and tastes, which are unappealing to consumers. The development of these flavors and aromas results from a complex series of reactions involving the unsaturated fatty acids present in the meat. The breakdown products from lipid oxidation can be so potent that they can affect the flavor profile of the meat at deficient concentrations, making it a critical quality control point for the poultry industry (Domínguez et al., 2019).
Table 2.
Meat quality as a result of oxidative stress in poultry.
| Reference | Effect of oxidative stress |
|---|---|
| He et al., 2019 | Increased water holding capacity |
| Chen et al., 2022 | Decreased in meat quality parameters |
| (Zhang et al., 2012) | Lower redness |
| Wang et al., 2009) | Negatively affected water holding capacity |
| (Jongberg et al., 2014) | Increased toughness of the skin |
| Utrera et al. (2014) | Texture Hardness increased |
| (Archile-Contreras and Purslow, 2011) | Texture Hardness increased |
| (Min and Ahn, 2005) | Decreased flavor of the meat |
The rate of lipid oxidation in poultry meat directly correlates with its shelf life (Gray et al., 1996). Oxidative changes can lead to spoilage even before there are visible signs, such as colour changes or microbial growth (Ercolini et al., 2006). This means that poultry products can become unsellable due to flavor and aroma changes before they would otherwise be considered spoiled by appearance. Protein oxidation in poultry meat is a detrimental process that has significant implications for the product's sensory and visual appeal (Zhang et al., 2013). The proteins in muscle fibres are responsible for the meat's texture, including tenderness and juiciness (Listrat et al., 2016). OS can lead to the oxidation of these proteins, particularly myofibrillar proteins, which are crucial for muscle contraction and structure (Steinberg, 2012). When these proteins oxidize, they can cross-link and form aggregates, making the meat tougher and decreasing its water-holding capacity. According to Cheng and Sun (2008), this loss of water-holding capacity means that the meat can become dry and less juicy, significantly reducing its palatability.
The poultry meat colour is primarily determined by myoglobin's content and chemical state, a pigment-protein that binds oxygen (Fletcher, 1999). OS can alter the redox state of myoglobin, leading to the formation of metmyoglobin, which has a dull brownish colour (Suman and Joseph, 2013). This change can make fresh meat appear older and less fresh than it is, which can be particularly problematic for final consumers, where visual appeal is paramount. Consumers often associate bright red or pink hues with freshness and quality; therefore, meat that has undergone protein oxidation may be perceived as less desirable or spoiled (McMillin, 2008). Additionally, texture is a key quality trait that consumers use to judge the quality of the meat, and thus, oxidative changes to proteins can lead to a less desirable product (Nawaz et al., 2022. The connection between texture and colour alterations due to protein oxidation is a concern for consumer perception and the overall quality and value of poultry products. Tougher, discoloured meat will likely be rejected by consumers, leading to increased wastage and economic losses for producers.
Egg Production and Quality
OS plays a significant function in the reproductive performance of poultry, especially impacting egg production and quality (Fouad et al., 2016). The excessive accumulation of ROS can induce oxidative damage to various cellular components, with far-reaching consequences for poultry reproduction (Pamplona and Costantini, 2011). The ova, or egg cells, are particularly vulnerable to oxidative damage due to their high lipid content and the presence of polyunsaturated fatty acids (Dunning et al., 2014). High levels of ROS can lead to lipid peroxidation, protein denaturation, and DNA damage within the ova (Wang et al., 2018). This oxidative damage can compromise the integrity and viability of the ova, resulting in poor egg quality. Eggs may exhibit various deficiencies such as weak eggshells, which are more susceptible to breakage and microbial invasion, poor yolk quality with potential implications for embryonic development, and altered egg white consistency that may affect the egg's structural integrity and its functionality for industrial processing (Jin et al., 2022).
Eggshell formation is an intricate process that depends on the balanced interaction of hormones, minerals, and proteins (Nys et al., 2022). According to Ermak and Davies (2002), calcium plays a pivotal role in this process, and OS has been shown to disrupt calcium metabolism (Chen et al., 2022). This disruption can lead to suboptimal deposition of calcium carbonate in the eggshell matrix, resulting in thinner or weaker shells (Batres, 2022). Such shells not only compromise the physical protection of the developing embryo but also affect the productivity of the eggs. Moreover, the eggshell's quality is an important determinant of the egg's gas exchange capabilities and its microbial defence; thus, any impairment in shell quality can have serious repercussions for embryo survival (McClelland et al., 2023).
Reproduction and Fertility
During lipid peroxidation, oxidative degradation of lipids causes free radicals to "steal" electrons from the lipids in cell membranes, thereby damaging the cells (Kong and Lin, 2010). This process is particularly detrimental to the yolk, which is rich in lipids that provide essential nutrients for the developing embryo. OS-induced lipid peroxidation can lead to the formation of MDA and other toxic byproducts that not only degrade the nutritional quality of the yolk but may also exert teratogenic effects or cause developmental anomalies in the embryo (Ahmed and Rahman, 2015). The oxidative stability of the yolk is, therefore, critical for maintaining its role as a nutrient reservoir for embryogenesis.
The sperm quality and fertility can be adversely impacted by OS in poultry, affecting the fundamental aspects of sperm function and integrity (Khan, 2011). Reactive oxygen species can induce DNA fragmentation in spermatozoa, which is a critical factor leading to reduced fertility rates (Bui et al., 2018). The integrity of sperm DNA is crucial for correctly transferring genetic information to the offspring. When ROS levels exceed the antioxidant defences of the sperm, they can cause breaks in the DNA strand (Bisht and Dada, 2017). This damage can result in decreased fertility, increased rates of miscarriage, and even the potential transmission of genetic defects to the progeny (Alahmar, 2019). The stability of the genetic material within the sperm is, therefore, paramount for ensuring successful reproduction and the health of future generations (Smith and Spadafora, 2005).
Sperm motility is crucial for fertilization as it enables the sperm to travel through the female reproductive tract to reach and fertilize the eggs (Suarez and Pacey, 2006). High ROS levels negatively affect this motility; therefore, OS can lead to a reduction in ATP production, which is the energy source for sperm movement and can also cause damage to the axoneme, the structure inside the sperm's tail that is responsible for its whip-like motion (Zhu et al., 2019). As a result, sperm with poor motility may not be able to reach the egg, or they may arrive in a suboptimal state, unable to fertilize the egg successfully. Furthermore, the sperm membrane plays an essential role in protecting the cell's interior and mediating the fusion with the egg during fertilization (Glabe et al., 2019). This membrane is rich in polyunsaturated fatty acids and is particularly susceptible to oxidative damage through lipid peroxidation. OS can cause a loss of membrane integrity and fluidity, impairing the sperm's ability to interact with the egg's outer layers (Saraswat et al., 2012). Moreover, lipid peroxidation can disrupt membrane receptors and channels, impairing sperm function and signaling pathways necessary for successful fertilization (Aitken, 2017).
Hormonal Balance and OS
Steroids are synthesized from cholesterol and transformed into other steroid hormones (Miller and Auchus, 2011). These steroid hormones, such as estrogen and progesterone in hens and testosterone in roosters, are essential for regulating reproductive functions, including sexual maturation, gametogenesis, and reproductive cycles (Morohashi et al., 2012). According to Oruç (2010), OS can interfere with the enzymes responsible for steroid hormone synthesis. This leads to imbalances that may manifest as irregularities in egg-laying cycles, reduced fertility, and suboptimal sexual behavior. For instance, oxidative damage to the mitochondria, where steroidogenesis initiates, can result in insufficient production of sex hormones, thereby affecting reproductive efficiency (Chainy and Sahoo, 2020).
Chronic OS is known to elevate levels of corticosterone, the primary stress hormone in birds (Vagasi et al., 2018). Elevated corticosterone can have a catabolic effect on the body, diverting energy away from reproductive processes to support the fight-or-flight response (Gangloff and Greenberg, 2023). It can inhibit gonadal function, leading to decreased production of sex steroids, suppressing mating behavior, and declining fertility (Archibong et al., 2018). Prolonged exposure to high corticosterone levels due to OS may result in long-term negative impacts on the reproductive health of poultry (Hedlund et al., 2019).
Immune Response and OS
Various events might trigger a physiological response that aims to restore homeostasis and regulate the internal constant milieu, hence initiating an inflammatory process (Table 3). The innate and adaptive immune response pathways are interrelated and work together to safeguard the organism from pathogens or other stresses that have the potential to trigger an immune response (Ciliberti et al., 2020). Antioxidant systems and reactive oxygen species must be in balance for T cells to function properly under normal conditions. Changes in or an accumulation of reactive nitrogen species and extracellular ROS can modify immunological responses, which can lead to a systemic inflammatory state mostly caused by oxidative stress (Colitti et al., 2019). The immune system's ability to function effectively is closely linked to its oxidative status, which can trigger systemic inflammation, negatively impacting reproductive organs (Dutta et al., 2021). Practically, the body's immune responses are compromised under OS. For example, oviduct inflammation could lead to impaired egg passage, abnormal egg formation, or increased susceptibility to infections that can further disrupt reproductive functions (Weiss et al., 2009). Chronic inflammation mediated by OS may also cause tissue damage and scarring, which can negatively affect fertility (Dutta et al., 2021).
Table 3.
Immune function as a consequence of oxidative stress in poultry.
| Reference | Tissue | Marker of oxidative stress | Immunostimulation method | Signed Pearson result |
|---|---|---|---|---|
| Koinarski et al. (2005) | Red blood cell | CAT/ MDA/SOD | Eimeria acervulina oocysts | P > 0.05 |
| Georgieva et al. (2006) | Red blood cell | CAT/ MDA/SOD | Eimeria tenella oocysts | P < 0.05 |
| Cohen et al. (2007) | Blood (serum/plasma | micromolecular antioxidants (Trolox equivalents)/ Plasma micromolecular antioxidants/ Serum micromolecular antioxidants | Lipopolysaccharide | P < 0.05 |
CAT: catalsae; MDA: malondialdehyde; SOD: superoxide dismutase.
Behavior and Reproduction
In poultry, OS may affect physiological health and has significant implications for behavior, a critical indicator of animal welfare (Altan et al., 2003). For instance, studies have documented that poultry experiencing OS may show a reduction in feed intake (Surai, 2016). This behavior is thought to be a consequence of the energy deficit created by the body's attempts to neutralize and repair the damage caused by ROS. The resulting negative energy balance can exacerbate the stress condition, potentially creating a vicious cycle where the bird's ability to cope with environmental demands is continually compromised. OS can also manifest in altered social interactions within a flock. Birds under OS may become more aggressive or more withdrawn (Milewski et al., 2022). This change in social dynamics can be attributed to the physiological burden of OS, which may affect the birds' social cognition and behavior (Hoogenboom et al., 2012). The competition for resources may become fiercer if the poultry birds perceive their survival as threatened, leading to increased aggression or social disruption.
According to Speakman and Garratt (2014), reproduction is an energetically costly process that can be severely affected by OS. Studies have revealed that OS can lead to reduced reproductive performance in poultry, which is evident through decreased mating behaviors, poor egg quality, and lower fertility rates (Alagawany et al., 2017; Ding et al., 2022). The exact mechanisms may involve oxidative damage to reproductive tissues or hormones that regulate reproductive behaviors. Chronic exposure to OS has been linked with cognitive deficits in poultry, impacting their learning and memory (Hossain et al., 2016; Mohammed et al., 2021). This can have practical implications for the birds' ability to adapt to their environment, learn from past experiences, and avoid harmful stimuli. Cognitive impairment due to OS may lead to decreased efficiency in foraging and altered responses to environmental challenges (Mahmoudi et al., 2021).
Mitigating Strategies of OS in Poultry
As shown in Table 4, different strategies are used in alleviating the impacts of oxidative stress in poultry. Different strategies, including environmental approaches such as controlled housing, management practices, thermal conditioning, genetic options like the use of selected breeds of chickens and marker-assisted selected breeding; different dietary approaches and phytogenics with their mechanism of actions are summarized.
Table 4.
Different strategies used in alleviating the effects of oxidative stress in poultry.
| Strategies | Method | Mechanisms of action | References |
|---|---|---|---|
| Environmental approach | |||
| Controlled housing | Use of cooling equipment. E.g: fan, Ventilator, sprinkler system | Reduces the heat load on the body of the birds | Daghir, 2008 |
| Management | Reduced stocking rate | Helps to reduce heat buildup and ammonia concentration in the pen | Saeed et al., 2018 |
| Thermal conditioning | Embryonic thermal conditioning | Helps to inhibit the generation of uncoupled proteins and by increasing HSP70 synthesis thereby enhancing the bird's adaptive capacity | Oke et al., 2020; Liew et al., 2003, Loyau et al., 2016; Meteyake et al., 2020 |
| Genetic approach | |||
| By using selected breeds of chicken | - naked neck genes | the reduced plumage helps in dissipating heat | Patra et al., 2002; Desta, 2021; Fernandes et al., 2023 |
| Frizzle gene | Helps to decrease feather intensity leading to an increase in the ability of the birds to dissipate heat -It also increases heat loss from the body |
Fathi et al., 2019; Wasti et al., 2020, | |
| Marker-assisted selected breeding | Use of candidate gene | of HSP90 and HSP70 gene expression helps to improve the thermotolerance of birds | Mahmoud et al., 2003 |
| Dietary approach | |||
| Feeding method | Feed restriction | Restricting feed during high ambient temperatures helps to reduce thermal load caused by digestion | Syafwan et al., 2011 |
| Withdrawal of feed | It minimizes heat load accumulation caused by metabolic heat produced during the processes of digestion, absorption, assimilation, and excretion | Syafwan et al., 2011 | |
| Wet feeding | Helps to promote water intake thereby relieving the birds from heat | Syafwan et al., 2011 | |
| Use of selected feedstuffs | Supplementation with fat and oil | Oils and fats contain high energy value which helps to promote feed consumption, reduces heat load, and improves performance | Attia et al., 2021 |
| Supplementation with dried plum | Dried plum helps to increase the HSP-related gene expression (HSP90, HSP70, HSF3, and HSF1) and antioxidant-related genes (GPX, GPX, and SOD) | Wasti et al., 2021 | |
| Amino acids | Supply of essential amino acids like lysine and arginine is advantageous to minimize the impacts of heat stress. Also, supplementation with Methionine reduced muscle oxidation and improve antioxidant status in thermally-challenged chickens Sulfur amino acids supplementation also helps in reducing chronic heat stress by increasing antioxidant production and also protects the intestinal permeability of broiler chickens |
Saeed et al., 2018; Zeitz et al., 2020; Ajayi et al.; 2022; Sarsour et al., 2022 | |
| Use of non-limiting amino acids and their derivatives (betaine taurine, L-citrulline and L-theanine,) | They have bioactive compounds that have properties that make them act as antioxidants, immunomodulators, anti-stressors, gut stimulants and anti-inflammatory when fed to heat-stressed birds | Uyanga et al., 2022 | |
| Vitamins | Vitamins A, B, D, E, and C are involved in upregulating immunocompetence and antioxidant defense in heat- stressed broilers | Niu et al., 2009; Khna et al., 2011; Calik et al., 2022 | |
| Minerals | Offering potassium chloride to heat-stressed birds reduced the blood pH there by enhancing the thermotolerance of the birds. Zinc is a cofactor for several enzymes and its addition to feed brings about a decrease in plasma corticosterone levels Selenium helped to increase the heat resistance of heat-stressed birds Manganese helps to increase the secretion of heat shock protein and also promotes antioxidants expression Chromium helps to improve oxidative stability and blood biochemical indices |
Ahmad et al., 2008; Toghyani et al 2012; Khan et al., 2014; Habibian et al 2015 | |
| Phytogenics | Resveratrol, ginkgo, cinnamon, licorice, moringa, rosemary, thyme, hot red pepper, sweet wormwood, turmeric, black cumin and ginger | Bioactive agents including quercetin resveratrol, and curcumin have been shown to activate vitagenes which helps to regulate the antioxidant defense system effectively. They help in scavenging free radicals, enhancing the immune system, decreasing corticosterone release, controlling heat shock response, improving nutrient digestibility, protecting intestinal health, exerting antimicrobial effects, decreasing lipid peroxidation, promoting the antioxidant defense system and regulating blood biochemical properties. It should be noted that the phytogenic active compounds for example, polyphenols may contain anti-nutritional factors or may be poorly absorbed and in some cases may not be detected in target tissues. |
Nawab et al., 2018, 2019; Madkour et al., 2022; Oyelola et al., 2024; Ding et al., 2023, Akosile et al., 2023b; Kpomasse et al., 2023b; Oke et al., 2017 ; Onagbesan et al., 2023; Tokofai et al., 2023; Oke, 2018; Oke et al., 2021b |
Factors Influencing OS in Poultry
Genetics and Breed-Specific Variations
The oxidative status of poultry has been shown to depend on their genotypes (Surai, 2016). Domestic birds, such as turkeys, broilers and layers are more vulnerable to oxidative stress due to genetic selection for quick development, better feed conversion, and high egg production rates (Soleimani et al., 2011). Certain breeds possess adaptive mechanisms that aid in the mitigation of oxidative challenges, namely by augmenting the production of antioxidant enzymes. Nevertheless, contemporary broiler chickens, owing to their expedited growth and heightened metabolic rate, exhibit a greater susceptibility to oxidative harm (Kochish et al., 2023). Conversely, meat-type chickens, characterized by a more gradual growth trajectory, manifest a diminished susceptibility to oxidative stress. Furthermore, in comparison to broilers, layer hens experience elevated levels of oxidative stress due to the necessity of allocating resources not only for growth and maintenance but also for egg production (Surai et al., 2019).
Environment
Environmental stresses particularly harmful to animal agriculture include heat stress (Nienaber and Hahn, 2007). Heat stress is caused by the interaction of airspeed, radiant heat, humidity and ambient temperature; high environmental temperature has a major detrimental effect on these factors (Kpomasse et al., 2023a). A type of environmental stress known as heat stress results from temperatures and humidity levels rising above an animal's threshold for thermotolerance. Poultry birds are sensitive to external temperatures and have a restricted range of thermoregulatory thresholds, which can be stressful. The size and muscular development of poultry birds have rapidly improved over time due to genetic selection, but the physiological improvement of the heat resistance is not commensurate. In the world's tropical and dry regions, thermal stress is hazardous and can cause large economic losses in the livestock industry. Additionally, intestinal inflammation during thermal challenge reduces the absorption of nutrients, which in turn reduces weight gain (Zhao et al., 2017). Elevated environmental temperature has been recognized to cause OS, leading to oxidative damage to broiler liver tissues, further disrupting the metabolism of lipids (Mujahid et al., 2005; Emami et al., 2020). All of these stresseors lead to OS (Estevez, 2015). Increased production of prooxidants and OS are caused by high ambient temperature, which also leads to prooxidant imbalance (Gonzalez-Rivas et al., 2020).
Nutrition and Dietary Components
Toxins from the environment, including bacterial and fungal toxins that have been linked to gut health, are often present in poultry diets and feed ingredients. The tight junction proteins and the intestinal luminal epithelial cells that link 2 adjacent epithelial cells prevent the absorption of toxins from the surrounding environment. In addition to changing cellular processes, OS also alters the function of the digestive barrier. Many fungi, but primarily moulds, can produce metabolites known as mycotoxins unique to their strain. When these toxins (e.g., fumonisin, ochratoxin, zearalenone) come into contact with the epithelial cells or the GIT, they cause OS, which has a significant impact on absorption. In numerous chicken body tissues, prolonged or severe arsenic exposure causes lipid peroxidation, lowers antioxidant levels, and ultimately causes apoptosis (Zhao et al., 2017). The intestinal mucosa becomes inflamed and is destroyed when copper and arsenic are combined (Wang et al., 2018).
Management Practices
Different management practices can influence the oxidative status of poultry birds (Oke et al., 2021b). Changes in metabolism elicited by high stocking density may result in a surplus ROS, which can oxidatively damage biological molecules (Fang et al., 2002). The report of Simsek et al. (2009) indicated that high stocking density reduced the serum GSH-Px but raised the serum MDA. Similarly, the findings of Jobe et al. (2019) revealed that elevated stocking density raised the concentration of blood MDA. It has also been shown that high stocking density raised plasma MDA, GPx activity, and total antioxidant capacity (Miao et al., 2021). Investigation on the impacts of rearing systems with the use of perforated plastic slate rearing systems, litter rearing systems and cage rearing systems on broiler chickens revealed that superoxide dismutase increased cage and litter rearing systems, and lipid peroxidation decreased in the birds in litter and cage rearing system (Abo Ghanima et al., 2020). Additionally, it has been shown that monochromatic blue light attenuated heat stress-induced oxidative stress (Abdo et al., 2017). The authors attributed this improvement to the decrease in MDA levels, increase in catalase expression and SOD activity, and enhanced expression of the HSF1, HSP90 and HSP70 genes. In the breast and thigh muscles, blue light dramatically decreased MDA levels while increasing SOD, GPx, and overall antioxidant capacity activities (Ke et al., 2011).
Infection and Disease
Infected cells and tissues can become inflamed and destroyed by pathogenic bacteria (Zahlten et al., 2015). Unfolded protein response (UPR) and bacterial pathogens are related, which suggests that pathogenic bacteria cause OS in the endoplasmic reticulum. This implies that this is the path for obtaining nutrients from the host and avoiding internalization or causing irreversible cell damage (Baruch et al., 2014). Different cells and tissues experience damage and hypoxic conditions due to OS (O'Neill et al., 2015). Numerous diseases, such as cancer, and chronic obstructive pulmonary disease atherosclerosis, have been linked to OS. These findings have uncovered the various ways in which oxidants cause cellular damage (Valko et al., 2007). However, because the effect of OS on disease pathophysiology varies widely, there may be a limit to how effective boosting antioxidant defence can be.
Measurement of OS in Poultry Species
Direct Measurement of Reactive Oxygen Species
The primary molecules accountable for the harmful consequences of OS are ROS. Determining the conditions of OS can thus be done through direct measurement of their cellular levels. Fluorogenic probes are one method of measuring the amounts of ROS within cells (Cohen et al., 2016).
Assessment of Oxidative Damage
ROS levels are difficult to directly measure with high accuracy and precision because of their short half-life and quick reactivity with components that regulate redox states. A practical substitute method for determining OS in clinical samples is the indirect measurement of ROS by looking at the oxidative damage these radicals cause to the lipids, proteins, and nucleic acids of the cells (Gáspár, 2011).
Protein Damage
One frequently used indicator of the oxidative modification of proteins is the protein carbonyl content (PC), which provides strong evidence of OS in clinical samples. PCs are produced when ROS molecules oxidize the backbones of proteins and amino acid residues, including proline, arginine, lysine, and threonine. The 2,4-dinitrophenylhydrazine method, which was simplified by Mesquita et al. (2014), can be used to measure the oxidized proteins.
Lipid Damage
Lipid peroxidation is a widely used marker for ROS-induced cellular membrane damage. One of the most well-studied by-products of polyunsaturated fatty acid peroxidation in clinical samples is MDA, which is widely used to evaluate the degree of OS. Thiobarbituric acid reactive substances (TBARS) can be used to measure MDA levels (Woźniak et al., 2012). However, despite its popularity, it is noteworthy to state that there is a limitation of non-specificity and comparatively low sensitivity of this method as several organic compounds containing carbonyl that are chemically reactive, such as those originating from oxidized biomolecules other than lipids, can react with TBA and are therefore classified as TBARS (Tsikas, 2017).
DNA Damage
OS circumstances can also result in single- or double-stranded breaks in the DNA. The comet assay, which is based on the ability of cleaved DNA fragments to migrate out of the nucleus when an electric field is applied, can be used to identify these oxidative DNA lesions. Undamaged DNA migrates more slowly and stays within the nucleoid. Thus, DNA damage within cells can be assessed by analyzing the shape of the DNA “comet” tail and its migration pattern (Lorenzo et al., 2013). Gas chromatography-mass spectrometry with selected ion monitoring can also, more broadly, be used to measure different modified DNA bases in clinical samples (Dizdaroglu et al., 2001; Jaruga et al., 2008). Utilizing their stable isotope-labelled analogues as internal standards, isotope-dilution mass spectrometry is used in this instance to quantify these products.
Assessment of Antioxidant Status
To balance out the harmful effects of oxidative free radicals, the body has an antioxidant system. OS may arise from a disruption in the equilibrium between antioxidants and reactive oxygen species, also known as redox homeostasis. Increasing the production of free radicals, inactivating antioxidant enzymes, or consuming too many antioxidants can all upset the balance between prooxidants and antioxidants. Therefore, the degree of OS in clinical samples can be examined with the assessment of antioxidant status. Antioxidant components in diets lower intestinal free radical levels and support intestinal mucosa maintenance. According to a number of studies, OS predisposes birds to a variety of pathological and welfare situations. Thus, developing a financially sensible plan to reduce OS is crucial.
Enzymatic and nonenzymatic low molecular compounds comprise the 2 arms of the antioxidant machinery that control redox homeostasis:
Enzymatic Antioxidants
Organisms use enzymatic and nonenzymatic antioxidant systems to protect against ROS and subsequent damage to membranes and macromolecules. These important systems are responsible for genomic integrity and homeostasis. Hydroxyl radical production is inhibited by the enzymatic antioxidant systems that scavenge free radicals escaping from the machinery that produces mitochondrial energy. They are:
Superoxide Dismutase: Superoxide dismutase (SOD) is a family of antioxidant enzymes that catalyzes the reduction of superoxide to hydrogen peroxide and molecular oxygen, thereby regulating the levels of reactive oxygen species (ROS) (Younus, 2018).
Catalase: The antioxidant enzyme catalase is widely expressed and is in charge of converting hydrogen peroxide into oxygen and water (Djordjevic, 2004).
Glutathione Peroxidase: Another antioxidant enzyme called glutathione peroxidase (GPx) catalyzes the conversion of lipid peroxides and hydrogen peroxides to water and the corresponding lipid alcohols by oxidizing reduced glutathione (GSH) to glutathione disulfide (GSSG) (Arthur, 2000).
Nonenzymatic Antioxidants
The nonenzymatic system comprises a myriad of antioxidant molecules, including:
Glutathione. Free glutathione can exist in the cell in both the reduced GSH and oxidized GSSG forms, but glutathione reductase mainly keeps it in the reduced form (Lu, 2009). The most prevalent low-molecular-weight thiol inside cells, GSH is essential for several metabolic defence processes, such as eliminating hydroperoxide, detoxifying xenobiotics, and scavenging free radicals (Wu et al., 2004).
Vitamin C: Ascorbic acid, or water-soluble vitamin C, is mostly present in the cytosol and extracellular fluid, where it interacts with reactive oxygen species (ROS) molecules like aqueous peroxyl radicals to prevent oxidative damage (Suntres, 2011; Birben et al., 2012)
Vitamin E: As a lipid-soluble vitamin that scavenges lipid peroxyl radicals, vitamin E (α-tocopherol) inhibits the formation of lipid peroxidation chain reactions in cell membranes (Birben et al., 2012).
Total Antioxidant Capacity: The levels or activities of each antioxidant have been measured independently in order to investigate the status of antioxidants. Nonetheless, because different antioxidants interact in different ways, measuring the total impact of antioxidants can be highly helpful in determining the oxidative state of the organism. Enzymatic and nonenzymatic antioxidants work together to form antioxidant defence systems in living things, which control the concentrations of these free radicals (Phaniendra et al., 2015). The primary factor influencing how cells and bodies adapt to different stress conditions that are relevant to the commercial world is SOD, an essential antioxidant (Surai, 2016). Since superoxide radicals are the primary free radicals generated by cells under physiological conditions, SOD is thought to be the essential component of the cell's initial line of defence against oxidants (Surai, 2016).
Protective Effects of Dietary Antioxidants
Dietary antioxidants are essential for safeguarding the body from OS. Supplementing the diet with nutrients rich in antioxidants reduces intestinal free radicals and helps protect the intestine's mucosal lining. As a result, creating a budget-friendly, healthy diet that includes antioxidants is essential for reducing OS. Different antioxidants, such as vitamins C and E, polyphenols, carotenoids, etc., aid in scavenging harmful free radicals that can disrupt DNA and cells. Through the enhancement of the body's natural defence and the promotion of cellular health, an antioxidant-rich diet can help lower the risk of various health problems (Oke et al., 2021b; Calik et al., 2022; Ding et al., 2023; Oni et al., 2024). As shown in Table 4, the protective effects of several antioxidants have been documented. A recent study by Oni et al. (2024) revealed that supplemental vitamin E and selenium upregulated the antioxidant defence of broiler chickens during heat stress. The antioxidant defence ability of vitamins C and E is due to their strong reactivity as electron (vitamin C) or hydrogen (vitamin E) donors to free radical oxidants, which avert oxidative damage to cells and tissues (Zwolak, 2020). Several studies have documented the roles of different dietary vitamins, such as A, B, D, E, and C, in modulating the antioxidant defence in heat-stressed chickens (Akinyemi and Adewole, 2021; Calik et al., 2022). Under summer conditions, dietary supplementation with vitamin C led to a higher antioxidant defence and a lower mRNA expression of HSP70 and pro-inflammatory cytokines (Jang et al., 2014).
In addition, some nutrient and non-nutrient components including amino acids, minerals, enzymes, prebiotics, probiotics, synbiotics, pigments, medicinal herbs, plant extracts, organic acids, essential oils, etc., may exert antioxidatnt properties once incorporated into poultry diets. These are considered nutraceuticals given their nutritional, therapeutic, and pharmaceutical importance by preventing diseases and exerting immunomodulating properties, thus providing potential health benefits (Alagawany, et al., 2020). Among others, phytogenic feed additives have also been receiving attention as sources of antioxidants to ameliorate the impacts of OS in recent years. Different phytogenic additives, such as resveratrol, ginkgo, cinnamon, liquorice, moringa, rosemary, thyme, hot red pepper, sweet wormwood, turmeric, black cumin, ginger, etc., have been reported to be beneficial in improving the antioxidant status of birds during oxidative stress (Madkour et al., 2022; Ding et al., 2023; Akosile et al., 2023a, Akosile et al., 2023b; Oyelola et al., 2024). Due to their highly conjugated system, numerous hydroxyl groups, and aromatic structural features, dietary polyphenols, also known as flavonoids, are effective scavengers of ROS and free radicals (Salisbury and Bronas, 2015). Their ability to neutralize ROS or inhibit cellular OS prevents oxidative damage to biomolecules (DNA, lipids, and proteins), reducing tissue inflammation (Checa and Aran, 2020). A recent study reported a beneficial antioxidant defence through dietary supplementation of polyphenolic antioxidants (rapeseed, quercetin, and flaxseed oil) in chickens (Sierżant et al., 2023). Altogether, a systematic review of the literature over the last decades (2000–2020) has confirmed the beneficial effects of phytogenic additives on oxidative stability, organoleptic characteristics, and fatty acids profile of poultry, with potent effects similar to vitamin E, a well-established antioxidant in poultry nutrition (Pitino et al., 2021).
Challenges and Future Directions
Current Challenges in Studying and Managing Oxidative Stress in Poultry
The significant rise in the average global temperature has been negatively affecting the poultry business, posing a danger to the sector's growth (Nyoni et al., 2019). One of the main factors negatively affecting poultry productivity is oxidative stress, which is being exacerbated by the increase in temperatures (Lara and Rostagno, 2013). As a result of rising temperatures brought by climate change, there are more cases of oxidative stress or hyperthermia in poultry. If global warming persists, this condition may get worse, making it more challenging to control.
Virus infections in poultry can cause oxidative stress by aggravating intestinal oxidative stress and tissue damage in chickens that result from the Newcastle disease virus, even when vitamin E is supplemented (Akinyemi and Adewole, 2021). This shows how difficult it is to manage oxidative stress in disease conditions.
Since mycotoxins can lead to oxidative stress among other problems, there is a need for an ongoing creation of fresh approaches to mitigate their consequences (Haque et al., 2020; Desbruslais and Wealleans, 2022). These difficulties emphasise the necessity of continuing research work to create efficient measures and strategies against oxidative stress, which is vital because of its effects on the overall health and productivity of poultry, an industry that makes a substantial contribution to global food security and nutrition.
Potential Areas for Future Research and Development
There are research areas that could contribute to improving poultry welfare and productivity by addressing the multifaceted challenges of oxidative stress under the current and future climate scenarios. Future research and development in the context of oxidative stress in poultry should include:
Photoperiod Optimization: Investigating the influence of light sources and photoperiod on broiler chickens' growth, health, and oxidative stress levels, as different lighting programs may mitigate stress responses (Jiang et al., 2023; Wilcox et al., 2023) .
Environmental Stressors: Research on the management responses to thermal challenges, particularly how to best condition and support poultry during periods of heat stress, which can cause oxidative stress.
Antioxidants Utilization: Further exploration into the role of vitamins and other antioxidants in counteracting the oxidative stress and toxicity is needed.
Dietary Strategies: Formulating diets supplemented with specific nutrients that support vitagenes, a group of genes crucial for preserving cellular homeostasis during stressful conditions, could provide another layer of defence against oxidative stress.
Mycotoxin Control: Development of more effective strategies to neutralize or minimize the presence of mycotoxins in feed, which leads to oxidative stress and other health issues.
Modified Atmosphere Packaging: For poultry meat preservation, expanding knowledge on modified atmosphere packaging could improve how oxidative stress affects meat quality.
Resilience Breeding: Examining the genetics behind oxidative stress resistance and selecting traits that help poultry cope better with stressors might create breeds with enhanced resilience.
Disease Management: Since viral infections can exacerbate oxidative stress, studying the interaction between disease and oxidative stress in poultry and developing management practices or treatments to mitigate these effects could be beneficial.
Technology and precision farming are essential for tackling the challenges associated with oxidative stress in chickens (Schillings et al., 2021). Data from precision livestock farming may be used to improve the living conditions for poultry. For instance, reducing the danger of heat stress which, as was previously mentioned, leads to oxidative stress can be achieved by using automated systems with sensor integration to adjust the temperature within chicken houses. With the use of precision feeding technology, feed is given to chickens based on their individual needs. This might entail keeping an eye on the amount of nutrients taken in order to reduce oxidative stress. Furthermore, careful feed design might enhance the benefits of antioxidants in the diet.
Technologies that continually monitor health indicators, such as heart rate and activity levels, can identify early symptoms of stress in animals. Examples of these technologies include remote sensing and wearable sensors. The massive volumes of data produced by precision agricultural technologies may be processed by machine learning and big data analytics to anticipate and reduce stress episodes before they happen. Farmers can prevent oxidative stress by, for example, examining trends in environmental data (such as temperature and humidity) and the responses of birds to these trends. By monitoring crop conditions and preventing the growth of fungi that create mycotoxins, precision agriculture technology can ensure the quality of feed components and lessen oxidative stress brought on by contaminated feed. Improved natural defences against oxidative stress can be identified in breeds thanks to advanced genetic methods. Genomic data may be used to guide selective breeding programmes that aim to improve stress resistance features in chicken populations.
CONCLUSIONS
To conclude, this review has offered a thorough analysis of the complex effects of oxidative stress on poultry production. The complex interactions between oxidative stress and poultry health have been extensively studied, from clarifying the underlying pathways to investigating viable mitigation measures. Researchers must work together to further our understanding of oxidative stress since it remains a major threat to the poultry business. Poultry welfare and production may be increased by implementing new ideas, such as food changes, antioxidant supplementation, and better management techniques. In order to address the intricacies of oxidative stress in poultry going forward and provide robust and sustainable production systems that benefit both producers, an all-encompassing and multidisciplinary strategy is required. Enhancing poultry's antioxidant defences under stress involves a novel approach that encompasses restoration of cellular homeostasis (through Nrf2-related mechanisms such as thioredoxin, glutathione, heme oxygenase-1, superoxide dismutase or other mechanisms like HSP, sirtuins, etc.) to reestablish the internal antioxidant protection and maintain redox balance.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.104003.
Appendix. Supplementary materials
REFERENCES
- Abdo S.E., El-Kassas S., El-Nahas A.F., Mahmoud S. Modulatory effect of monochromatic blue light on heat stress response in commercial broilers. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/1351945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu-Asiamah P., Zhang Y., Amoah K., Leng Q.Y., Zheng J.H., Yang H., Zhang L. Evaluation of physiological and molecular responses to acute heat stress in two chicken breeds. Anim. 2021;15 doi: 10.1016/j.animal.2020.100106. [DOI] [PubMed] [Google Scholar]
- Ahmad G., Almasry M., Dhillon A.S., Abuayyash M.M., Kothandaraman N. a Complicated Phenomenon. Springer; New York City: 2017. Overview and sources of reactive oxygen species (ROS) in the reproductive system. Pages 1–16 in Oxidative Stress in Human Reproduction: Shedding Light on. [DOI] [Google Scholar]
- Ahmad T., Khalid T., Mushtaq T., Mirza M.A., Nadeem A., Babar M.E., Ahmad G. Effect of potassium chloride supplementation in drinking water on broiler performance under heat stress conditions. Poult. Sci. 2008;87:1276–1280. doi: 10.3382/ps.2007-00299. [DOI] [PubMed] [Google Scholar]
- Ahmed T.H., Rahman M.M. An overview of oxidative stress and its effect on fetal development and organogenesis. Am. J. Biopharm. Biochem. Life Sci. 2015;4:1–8. [Google Scholar]
- Aitken R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017;84:1039–1052. doi: 10.1002/mrd.22871. [DOI] [PubMed] [Google Scholar]
- Ajayi O.I., Smith O.F., Oso A.O., Oke O.E. Evaluation of in ovo feeding of low or high mixtures of cysteine and lysine on performance, intestinal morphology and physiological responses of thermal-challenged broiler embryos. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.972041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi F., Adewole D. Environmental stress in chickens and the potential effectiveness of dietary vitamin supplementation. Front. Anim. Sci. 2021;2 [Google Scholar]
- Alagawany M., Farag M.R., Abd El-Hack M.E., Patra A. Heat stress: effects on productive and reproductive performance of quail. World's Poult. Sci. J. 2017;73:747–756. [Google Scholar]
- Akosile O.A., Kehinde F.O., Oni A.I., Oke O.E. Potential implication of in ovo feeding of Phytogenics in poultry production. Transl. Anim. Sci. 2023;7:txad094. doi: 10.1093/tas/txad094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akosile O.A., Sogunle O.M., Majekodunmi B.C., Oke O.E. In ovo Injection of Cinnamon or Clove alters the Physiology and Growth of Broilers in a Hot-Tropical Environment. Transl. Anim. Sci. 2023;7 doi: 10.1093/tas/txad036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R., Tiwari R., Yatoo M.I., Karthik K., Michalak I., Dhama K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health - a comprehensive review. The vet. Quart. 2020;41:1–29. doi: 10.1080/01652176.2020.1857887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmar A.T. Role of oxidative stress in male infertility: an updated review. J. Hum. Reprod. Sci. 2019;12:4. doi: 10.4103/jhrs.JHRS_150_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algothmi K.M., Mahasneh Z.M., Abdelnour S.A., Khalaf Q.A., Noreldin A.E., Barkat R.A., Khalifa N.E., Khafaga A.F., Tellez-Isaias G., Alqhtani A.H., Swelum A.A., Abd El-Hack M.E. Protective impacts of mitochondria enhancers against thermal stress in poultry. Poult. Sci. 2023;103:103218. doi: 10.1016/j.psj.2023.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alo E.T., Daramola J.O., Wheto M., Oke O.E. Impact of broiler breeder hens’ age and egg storage on egg quality, embryonic development, and hatching traits of FUNAAB-alpha chickens. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altan Ö., Pabuçcuoğlu A., Altan A., Konyalioğlu S., Bayraktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003;44:545–550. doi: 10.1080/00071660310001618334. [DOI] [PubMed] [Google Scholar]
- Archana P.R., Aleena J., Pragna P., Vidya M.K., Abdul Niyas, Bagath M., Krishnan G., Manimaran A., Beena V., Kurien E.K., Sejian V., Bhatta R. Role of heat shock proteins in livestock adaptation to heat stress. J. Dairy Vet. Anim. Res. 2017:13–19. [Google Scholar]
- Archibong A.E., Rideout M.L., Harris K.J., Ramesh A. Oxidative stress in reproductive toxicology. Curr. Opin. Toxicol. 2018;7:95–101. doi: 10.1016/j.cotox.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archile-Contreras A.C., Purslow P.P. Oxidative stress may affect meat quality by interfering with collagen turnover by muscle fibroblasts. Food Res. Int. 2011;44:582–588. [Google Scholar]
- Arnold K.E., Herborn K.A., Adam A., Alexander L. Individual variation in the oxidative costs of personality traits. Funct. Ecol. 2015;29:522–530. [Google Scholar]
- Arthur J.R. The glutathione peroxidase. Cell Mol. Life Sci. 2000;57:1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia Y.A., Al-Harthi M.A., Hassan S.S. Responses of broiler chicken to different oil levels within constant energy levels from 20 to 40 days of age under hot weather conditions. Ital. J. Anim. Sci. 2021;20:664–676. [Google Scholar]
- Agrawal S., Chakole S., Shetty N., Prasad R., Lohakare T., Wanjari M. Exploring the role of oxidative stress in skeletal muscle atrophy: mechanisms and implications. Cureus. 2023;15:1–9. doi: 10.7759/cureus.42178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med. Cell Longev. 2014;2014:1–31. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batres D. Food and Nutritional Science University of Alberta; Canada: 2022. Evaluation of dietary calcium level effects on the productivity, eggshell quality, and bone traits of laying hens. A Thesis of the Department of Agricultural. [Google Scholar]
- Barrett N.W., Rowland K., Schmidt C.J., Lamont S.J., Rothschild M.F., Ashwell C.M., Persia M.E. Effects of acute and chronic heat stress on the performance, egg quality, body temperature, and blood gas parameters of laying hens. Poult. Sci. 2019;98:6684–6692. doi: 10.3382/ps/pez541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch M., Hertzog B.B., Ravins M., Anand A., Youting C., Biswas D., Tirosh B., Hanski E. Induction of endoplasmic reticulum stress and unfolded protein response constitutes a pathogenic strategy of group A streptococcus. Front. Cell. Infect. Microbiol. 2014;4:105. doi: 10.3389/fcimb.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabucci U. Climate change: Impact on livestock and how can we adapt. Anim. Front. 2019;9:3–5. doi: 10.1093/af/vfy039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian X., Liu X., Liu J., Zhao Y., Li H., Zhang L., Li P., Gao Y. Hepatoprotective effect of chiisanoside from Acanthopanax sessiliflorus against LPS/D-GalN-induced acute liver injury by inhibiting NF-κB and activating Nrf2/HO-1 signaling pathways. J. Sci. Food Agric. 2019;99:3283–3290. doi: 10.1002/jsfa.9541. [DOI] [PubMed] [Google Scholar]
- Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht S., Dada R. Oxidative stress: Major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front. Biosci. (Schol Ed). 2017;9:420–447. doi: 10.2741/s495. [DOI] [PubMed] [Google Scholar]
- Bui A.D., Sharma R., Henkel R., Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. 2018;50:e13012. doi: 10.1111/and.13012. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Davies K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Calik A., Emami N.K., Schyns G., White M.B., Walsh M.C., Romero L.F., Dalloul R.A. Influence of dietary vitamin E and selenium supplementation on broilers subjected to heat stress, Part II: oxidative stress, immune response, gut integrity, and intestinal microbiota. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho R., Shimokomaki M. and Estévez M., Poultry meat color and oxidation, Pages 133–157 in Poultry Quality Evaluation, 2016, 10.1016/B978-0-08-100763-1.00006-4 [DOI]
- Cassuce D.C., Tinôco I.D.F., Baêta F.C., Zolnier S., Cecon P.R., Vieira M.D.F. Thermal comfort temperature update for broiler chickens up to 21 days of age. Eng. Agríc. 2013;33:28–36. [Google Scholar]
- Celi P., Gabai G. Oxidant/antioxidant balance in animal nutrition and health: the role of protein oxidation. Front. Vet. Sci. 2015;2:48. doi: 10.3389/fvets.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celi P., Selle P.H., Cowieson A.J. Effects of organic selenium supplementation on growth performance, nutrient utilisation, oxidative stress and selenium tissue concentrations in broiler chickens. Anim. Prod. Sci. 2013;54:966–971. [Google Scholar]
- Chainy G.B., Sahoo D.K. Hormones and oxidative stress: an overview. Free Radic. Res. 2020;54:1–26. doi: 10.1080/10715762.2019.1702656. [DOI] [PubMed] [Google Scholar]
- Chen S., Yong Y., Ju X. Effect of heat stress on growth and production performance of livestock and poultry: mechanism to prevention. J. Therm. Biol. 2021;99 doi: 10.1016/j.jtherbio.2021.103019. [DOI] [PubMed] [Google Scholar]
- Checa J.J., Aran M. Reactive oxygen species: drivers of physiological and pathological processes. J. Inflamm. Res. 2020;13:1057–1073. doi: 10.2147/JIR.S275595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Xing T., Li L., Zhang J., Jiang Y., Gao F. Oxidative stress impairs the meat quality of broiler by damaging mitochondrial function, affecting calcium metabolism and leading to ferroptosis. Anim. Biosci. 2022;35:1616. doi: 10.5713/ab.22.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K., Song Z.H., Zheng X.C., Zhang H., Zhang J.F., Zhang L.L., Zhou Y.M., Wang T. Effects of dietary vitamin E type on the growth performance and antioxidant capacity in cyclophosphamide immunosuppressed broilers. Poult. Sci. 2017;96:1159–1166. doi: 10.3382/ps/pew336. [DOI] [PubMed] [Google Scholar]
- Cheng Q.D., Sun W. Factors affecting the water holding capacity of red meat products: a review of recent research advances. Crit. Rev. Food Sci. Nutr. 2008;48:137–159. doi: 10.1080/10408390601177647. [DOI] [PubMed] [Google Scholar]
- Cheng K., Zhang M., Huang X., Zheng X., Song Z., Zhang L., Wang T. An evaluation of natural and synthetic vitamin E supplementation on growth performance and antioxidant capacity of broilers in early age. Can. J. Anim. Sci. 2017;98:187–193. [Google Scholar]
- Ciliberti M.G., Albenzio M., Palo P.D., Santillo A., Caroprese M. Nexus between immune responses and oxidative stress: the role of dietary hydrolyzed lignin in ex vivo bovine peripheral blood mononuclear cell response. Front. Vet. Sci. 2020, 1 -12;7 doi: 10.3389/fvets.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Klasing K., Ricklefs R. Measuring circulating antioxidants in wild birds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007;147:110–121. doi: 10.1016/j.cbpb.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Cohen S., Mehrabi S., Yao X., Millingen S., Aikhionbare F.O. Reactive oxygen species and serous epithelial ovarian adenocarcinoma. Cancer Res. J. 2016;4:106–114. doi: 10.11648/j.crj.20160406.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colitti M., Stefanon B., Gabai G., Gelain M.E., Bonsembiante F. Oxidative stress and nutraceuticals in the modulation of the immune function: current knowledge in animals of veterinary interest. Antioxidants. 2019;8:28. doi: 10.3390/antiox8010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva E.O., Bracarense A.P.F.L., Oswald I.P. Mycotoxins and oxidative stress: where are we? World Mycotoxin J. 2018;11:113–134. [Google Scholar]
- Daghir, N. J. ed. 2008. Poultry production in hot climates. CABI.
- Das K., Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014;2:53. [Google Scholar]
- Davies K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- Desbruslais A., Wealleans A.L. Oxidation in poultry feed: impact on the bird and the efficacy of dietary antioxidant mitigation strategies. Poult. 2022;1:246–277. [Google Scholar]
- Desta T.T. The genetic basis and robustness of naked neck mutation in chicken. Trop. Anim. Health Prod. 2021;53:95. doi: 10.1007/s11250-020-02505-1. [DOI] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J.F. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Ding K.N., Lu M.H., Guo Y.N., Liang S.S., Mou R.W., He Y.M., Tang L.P. Resveratrol relieves chronic heat stress-induced liver oxidative damage in broilers by activating the Nrf2-Keap1 signaling pathway. Ecotoxicol. Environ. Saf. 2023;249 doi: 10.1016/j.ecoenv.2022.114411. [DOI] [PubMed] [Google Scholar]
- Ding X., Cai C., Jia R., Bai S., Zeng Q., Mao X., Xu S., Zhang K., Wang J. Dietary resveratrol improved production performance, egg quality, and intestinal health of laying hens under oxidative stress. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M., Jaruga P., Rodriguez H. Measurement of 8-hydroxy-2′-deoxyguanosine in DNA by high-performance liquid chromatography-mass spectrometry: comparison with measurement by gas chromatography-mass spectrometry. Nucleic Acids Res. 2001;29:e12. doi: 10.1093/nar/29.3.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic V.B. Free radicals in cell biology. Int. Rev. Cytol. 2004;237:57–89. doi: 10.1016/S0074-7696(04)37002-6. [DOI] [PubMed] [Google Scholar]
- Domínguez R., Pateiro M., Gagaoua M., Barba F.J., Zhang W., Lorenzo J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants. 2019;8:429. doi: 10.3390/antiox8100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning K.R., Russell D.L., Robker R.L. Lipids and oocyte developmental competence: the role of fatty acids and b-oxidation. Reprod. 2014;148:R15–R27. doi: 10.1530/REP-13-0251. [DOI] [PubMed] [Google Scholar]
- Dutta S., Sengupta P., Slama P., Roychoudhury S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int. J. Mol. Sci. 2021;22:10043. doi: 10.3390/ijms221810043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Jung U., Voy B., Dridi S. Radical response: effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants. 2020;10:35. doi: 10.3390/antiox10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolini D., Russo F., Torrieri E., Masi P., Villani F. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl. Envir. Microbiol. 2006;72:4663–4671. doi: 10.1128/AEM.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermak G., Davies K.J. Calcium and oxidative stress: from cell signaling to cell death. Mol. Immunol. 2002;38:713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- Estévez M. Oxidative damage to poultry: from farm to fork. Poult. Sci. 2015;94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Etuah S., Ohene-Yankyera K., Liu Z., Mensah J.O., Lan J. Determinants of cost inefficiency in poultry production: Evidence from small-scale broiler farms in the Ashanti region of Ghana. Trop. Anim. Health Prod. 2020;52:1149–1159. doi: 10.1007/s11250-019-02115-6. [DOI] [PubMed] [Google Scholar]
- Fang Y.Z., Yang S., Wu G. Free radicals, antioxidants, and nutrition. Nutr. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Fathi M., Al-Homidan I., Rayan G., El-Safty S., Ebeid T., Abou-Emera O. Laying performance, immune response and antioxidant properties of hens segregating for naked neck and frizzle genes under low ambient temperature. Czech J. Anim. Sci. 2019;64:216–225. [Google Scholar]
- Fernandes E., Raymundo A., Martins L.L., Lordelo M., de Almeida A.M. The naked neck gene in the domestic chicken: a genetic strategy to mitigate the impact of heat stress in poultry production—a review. Animals. 2023;13:1007. doi: 10.3390/ani13061007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filazi A., Yurdakok-Dikmen B., Kuzukiran O., Sireli U.T. Mycotoxins in poultry. Poult. Sci. 2017;96:73–92. [Google Scholar]
- Fisinin V.I., Kavtarashvili A.S. Heat stress in poultry. II. Methods and techniques for prevention and alleviation. Sel'skokhozyaistvennaya Biologiya. 2015;4:431–443. [Google Scholar]
- Fletcher D.L. Poultry Meat Sci. CABI Publishing; NY: 1999. Poultry meat colour; pp. 159–176. [Google Scholar]
- Fouad A.M., Chen W., Ruan D., Wang S., Xia W.G., Zheng C.T. Impact of heat stress on meat, egg quality, immunity and fertility in poultry and nutritional factors that overcome these effects: a review. Int. J. Poult. Sci. 2016;15:81. [Google Scholar]
- Freitas M., Lima J.L., Fernandes E. Optical probes for detection and quantification of neutrophils’ oxidative burst. A review. Anal. Chim. Acta. 2009;649:8–23. doi: 10.1016/j.aca.2009.06.063. [DOI] [PubMed] [Google Scholar]
- Fry J.P., Mailloux N.A., Love D.C., Milli M.C., Cao L. Feed conversion efficiency in aquaculture: do we measure it correctly? Environ. Res. Lett. 2018;13 [Google Scholar]
- Gabriel I., Lessire M., Mallet S., Guillot J.F. Microflora of the digestive tract: critical factors and consequences for poultry. World's. Poult. Sci. J. 2006;62:499–511. [Google Scholar]
- Gangloff E.J., Greenberg N. Springer International Publishing; Cham: 2023. Biology of stress. Pages 93-142 in Health and Welfare of Captive Reptiles. [Google Scholar]
- Gao S., Wang K., Xiong K., Xiao S., Wu C., Zhou M., Li L., Yuan G., Jiang L., Xiong Q., et al. Unraveling the Nrf2-ARE signaling pathway in the DF-1 chicken fibroblast cell line: insights into T-2 toxin-induced oxidative stress regulation. Toxins. 2023;15:627. doi: 10.3390/toxins15110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gáspár S. Pages 289-309 in Oxidative Stress: Diagnostics, Prevention, and Therapy. ACS Publications; USA: 2011. Detection of superoxide and hydrogen peroxide from living cells using electrochemical sensors. [Google Scholar]
- Georgieva N.V., Koinarski V., Gadjeva V. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet. J. 2006;172:488–492. doi: 10.1016/j.tvjl.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Gessner D.K., Ringseis R., Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. (Berl). 2017;101:605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- Ghanima M.M.A., Abd El-Hack M.E., Othman S.I., Taha A.E., Allam A.A., Abdel-Moneim A.M.E. Impact of different rearing systems on growth, carcass traits, oxidative stress biomarkers, and humoral immunity of broilers exposed to heat stress. Poult. Sci. 2020;99:3070–3078. doi: 10.1016/j.psj.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C.G., Hong K., Vacquier V.D. Membrane Fusion. CRC Press; Florida: 2019. Fusion of sperm and egg plasma membranes during fertilization; pp. 627–646. [Google Scholar]
- Gonzalez-Rivas P.A., Chauhan S.S., Ha M., Fegan N., Dunshea F.R., Warner R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020;162 doi: 10.1016/j.meatsci.2019.108025. [DOI] [PubMed] [Google Scholar]
- Gray J.I., Gomaa E.A., Buckley D.J. Oxidative quality and shelf life of meats. Meat Sci. 1996;43:111–123. doi: 10.1016/0309-1740(96)00059-9. [DOI] [PubMed] [Google Scholar]
- Guerrini A., Tedesco D.E.A. Restoring activity of milk thistle (Silybum marianum L.) on serum biochemical parameters, oxidative status, immunity, and performance in poultry and other animal species, poisoned by mycotoxins: a review. Animals. 2023;13:330. doi: 10.3390/ani13030330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibian M., Sadeghi G., Ghazi S., Moeini M.M. Selenium as a feed supplement for heat-stressed poultry: a review. Biol. Trace Elem. Res. 2015;165:183–193. doi: 10.1007/s12011-015-0275-x. [DOI] [PubMed] [Google Scholar]
- Hafez M.H., El-Kazaz S.E., Alharthi B., Ghamry H.I., Alshehri M.A., Sayed S., Shukry M., El-Sayed Y.S. The impact of curcumin on growth performance, growth-related gene expression, oxidative stress, and immunological biomarkers in broiler chickens at different stocking densities. Animals. 2022;12:958. doi: 10.3390/ani12080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M.A., Wang Y., Shen Z., Li X., Saleemi M.K., He C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: a review. Microb. Pathog. 2020;142 doi: 10.1016/j.micpath.2020.104095. [DOI] [PubMed] [Google Scholar]
- He J., Xia C., He Y., Pan D., Cao J., Sun Y., Zeng X. Proteomic responses to oxidative damage in meat from ducks exposed to heat stress. Food Chem. 2019;295:129–137. doi: 10.1016/j.foodchem.2019.05.073. [DOI] [PubMed] [Google Scholar]
- He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- Hedlund L., Whittle R., Jensen P. Effects of commercial hatchery processing on short-and long-term stress responses in laying hens. Sci. Rep. 2019;9:2367. doi: 10.1038/s41598-019-38817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd R.M., Arthur P.F. Physiological basis for residual feed intake. J. Anim. Sci. 2009;87(suppl_14):E64–E71. doi: 10.2527/jas.2008-1345. [DOI] [PubMed] [Google Scholar]
- Hernández-García D., Wood C.D., Castro-Obregón S., Covarrubias L. Reactive oxygen species: a radical role in development? Free Radic. Biol. Med. 2010;49:130–143. doi: 10.1016/j.freeradbiomed.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Hoogenboom M.O., Metcalfe N.B., Groothuis T.G., de Vries B., Costantini D. Relationship between oxidative stress and circulating testosterone and cortisol in pre-spawning female brown trout. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012;163:379–387. doi: 10.1016/j.cbpa.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Hossain S., Bhowmick S., Jahan S., Rozario L., Sarkar M., Islam S., Basunia M.A., Rahman A., Choudhury B.K., Shahjalal H. Maternal lead exposure decreases the levels of brain development and cognition-related proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology. 2016;56:150–158. doi: 10.1016/j.neuro.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Iqbal M.J., Kabeer A., Abbas Z. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024;22:1–16. doi: 10.1186/s12964-023-01398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I., Ko Y., Moon Y., Sohn S. Effects of vitamin C or E on the pro-inflammatory cytokines, heat shock protein 70 and antioxidant status in broiler chicks under summer conditions. Asian-Australas. J. Anim. Sci. 2014;27:749–756. doi: 10.5713/ajas.2013.13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaruga P., Kirkali G., Dizdaroglu M. Measurement of formamidopyrimidines in DNA. Free Radic. Biol. Med. 2008;45:1601–1609. doi: 10.1016/j.freeradbiomed.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Jeeva J.S., Sunitha J., Ananthalakshmi R., Rajkumari S., Ramesh M., Krishnan R. Enzymatic antioxidants and its role in oral diseases. J. Pharm. Bioallied Sci. 2015;7(Suppl 2):S331. doi: 10.4103/0975-7406.163438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Fu Y., Cheng H. Daylight exposure and circadian clocks in broilers: Part I—photoperiod effect on broiler behavior, skeletal health, and fear response. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.103162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Zhou Q., Lan F., Li J., Yang N., Sun C. Microbial composition of egg component and its association with hatchability of laying hens. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.943097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jîtcă G., Ősz B.E., Tero-Vescan A., Miklos A.P., Rusz C.M., Bătrînu M.G., Vari C.E. Positive aspects of oxidative stress at different levels of the human body: a review. Antioxidants. 2022;11:572. doi: 10.3390/antiox11030572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe M.C., Ncobela C.N., Kunene N.W., Opoku A.R. Effects of Cassia abbreviata extract and stocking density on growth performance, oxidative stress and liver function of indigenous chickens. Trop. Anim. Health Prod. 2019;51:2567–2574. doi: 10.1007/s11250-019-01979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongberg S., Jinzhu W., Torngren M.A., Lund M.N. Effect of highoxygen atmosphere packaging on oxidative stability and sensory quality of two chicken muscles during chill storage. Food Pack. Shelf Life. 2014;1:38–48. [Google Scholar]
- Ke Y.Y., Liu W.J., Wang Z.X., Chen Y.X. Effects of monochromatic light on quality properties and antioxidation of meat in broilers. Poult. Sci. 2011;90:2632–2637. doi: 10.3382/ps.2011-01523. [DOI] [PubMed] [Google Scholar]
- Khan R.U. Antioxidants and poultry semen quality. World's Poult. Sci. J. 2011;67:297–308. [Google Scholar]
- Khan R.U., Shabana N., Dhama K. Chromium: pharmacological applications in heat-stressed poultry. Int. J. Pharmacol. 2014;10:213–217. [Google Scholar]
- Kikusato M., Xue G., Pastor A., Niewold T.A., Toyomizu M. Effects of plant-derived isoquinoline alkaloids on growth performance and intestinal function of broiler chickens under heat stress. Poult. Sci. 2021;100:957–963. doi: 10.1016/j.psj.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.W., Lee S.Y., Hur S.J., Kil D.Y., Kim J.H. Effects of functional nutrients on chicken intestinal epithelial cells induced with oxidative stress. J. Anim. Sci. Technol. 2023;65:1040. doi: 10.5187/jast.2023.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochish I.I., Titov V.Y., Nikonov I.N., Brazhnik E.A., Vorobyov N.I., Korenyuga M.V., Myasnikova O.V., Dolgorukova A.M., Griffin D.K., Romanov M.N. Unraveling signatures of chicken genetic diversity and divergent selection in breed-specific patterns of early myogenesis, nitric oxide metabolism and post-hatch growth. Front. Genet. 2023;13 doi: 10.3389/fgene.2022.1092242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H., Lee A., Santin E. Microbiome and pathogen interaction with the immune system. Poult. Sci. 2020;99:1906–1913. doi: 10.1016/j.psj.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinarski V., Georgieva N., Gadjeva V., Petkov P. Antioxidant status of broiler chickens, infected with Eimeria acervulina. Rev. Med. Vet. 2005;156:498. [Google Scholar]
- Koivula M.J., Eeva T. Metal-related oxidative stress in birds. Environ. Pollut. 2010;158:2359–2370. doi: 10.1016/j.envpol.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Kong Q., Lin G. Oxidative damage to RNA: Mechanisms, consequences, and diseases. CMLS. 2010;67:1817–1829. doi: 10.1007/s00018-010-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitar-Jerala N. innate immune response in brain, NF-Kappa B signaling and cystatins. Front. Mol. Neurosci. 2015;8:73. doi: 10.3389/fnmol.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kpomasse C.C., Oso O.M., Lawal K.O., Oke O.E. Juvenile growth, thermotolerance and gut histomorphology of broiler chickens fed curcuma longa under hot-humid environments. Heliyon. 2023;9:e13060. doi: 10.1016/j.heliyon.2023.e13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kpomasse C.C., Kouame Y.A.E., N'nanle O., Houndonougbo F.M., Tona K., Oke O.E. The productivity and resilience of the indigenous chickens in the tropical environments: improvement and future perspectives. J. Appl. Anim. Res. 2023;51:456–469. [Google Scholar]
- Kpomasse C.C., Oke O.E., Houndonougbo F.M., Tona K. Broiler production challenges in the tropics: a review. Vet. Med. Sci. 2021;7:831–842. doi: 10.1002/vms3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcsár S., Turbók J., Kövér G., Balogh K., Zándoki E., Ali O., Mézes M. Exposure to a combination of fusarium mycotoxins leads to lipid peroxidation and influences antioxidant defenses, fatty acid composition of phospholipids, and renal histology in laying hens. Toxins. 2024;16:226. doi: 10.3390/toxins16050226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Singh S.K., Rana B., Rana A. The regulatory function of mixed lineage kinase 3 in tumor and host immunity. Pharmacol. Ther. 2021;219:107704. doi: 10.1016/j.pharmthera.2020.107704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari K.N.R., Nath D.N. Ameliorative measures to counter heat stress in poultry. World's Poult. Sci. J. 2018;74:117–130. [Google Scholar]
- Lacher S.E., Levings D.C., Freeman S., Slattery M. Identification of a functional antioxidant response element at the hif1a locus. Redox Biol. 2018;19:401–411. doi: 10.1016/j.redox.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y.M.W.A., Verstegen M.W.A., Tamminga S., Williams B.A. The role of the commensal gut microbial community in broiler chickens. Worlds Poult. Sci. J. 2005;61:95–104. [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.T., Lin W.C., Wang S.Y., Lin L.J., Yu B., Lee T.T. Evaluation of potential antioxidant and anti-inflammatory effects of Antrodia cinnamomea powder and the underlying molecular mechanisms via Nrf2- and NF-κB-dominated pathways in broiler chickens. Poult. Sci. 2018;97:2419–2434. doi: 10.3382/ps/pey076. [DOI] [PubMed] [Google Scholar]
- Liew P.K., Zulkifli I., Hair-Bejo M., Omar A.R., Israf D.A. Effects of early age feed restriction and heat conditioning on heat shock protein 70 expression, resistance to infectious bursal disease, and growth in male broiler chickens subjected to heat stress. Poult. Sci. 2003;82:1879–1885. doi: 10.1093/ps/82.12.1879. [DOI] [PubMed] [Google Scholar]
- Lin H., Jiao H.C., Buyse J., Decuypere E. Strategies for preventing heat stress in poultry. Worlds Poult. Sci. J. 2006;62:71–86. [Google Scholar]
- Listrat A., Lebret B., Louveau I., Astruc T., Bonnet M., Lefaucheur L., Bugeon J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016:3182746. doi: 10.1155/2016/3182746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Gao R., Meng Q., Zhang Y., Bi C., Shan A. Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.G., Sha K.H., Zhang L.G., Liu X.X., Yang F., Cheng J.Y. Protective effects of alpinetin on lipopolysaccharide/d-Galactosamine-induced liver injury through inhibiting inflammatory and oxidative responses. Microb. Pathog. 2019;126:239–244. doi: 10.1016/j.micpath.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Liu Z., Ren Z., Zhang J., Chuang C.C., Kandaswamy E., Zhou T., Zuo L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018;9:477. doi: 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo Y., Costa S., Collins A.R., Azqueta A. The comet assay, DNA damage, DNA repair and cytotoxicity: Hedgehogs are not always dead. Mutagenesis. 2013;28:427–432. doi: 10.1093/mutage/get018. [DOI] [PubMed] [Google Scholar]
- Loyau T., Hennequet-Antier C., Coustham V., Berri C., Leduc M., Crochet S., Collin A. Thermal manipulation of the chicken embryo triggers differential gene expression in response to a later heat challenge. BMC Genomics. 2016;17:1–15. doi: 10.1186/s12864-016-2661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Li Y., Fan Y., Zhao L., Wei H., Ji C., Zhang J. Molecular mechanisms of lipoic acid protection against aflatoxin b₁-induced liver oxidative damage and inflammatory responses in broilers. Toxins. 2015;7:5435–5447. doi: 10.3390/toxins7124879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madkour L.H. Function of reactive oxygen species (ROS) inside the living organisms and sources of oxidants. Pharm. Sci. Anal. Res. J. 2019;2 [Google Scholar]
- Madkour M., Salman F.M., El-Wardany I., Abdel-Fattah S.A., Alagawany M., Hashem N.M., Dhama K. Mitigating the detrimental effects of heat stress in poultry through thermal conditioning and nutritional manipulation. J. Therm. Biol. 2022;103 doi: 10.1016/j.jtherbio.2021.103169. [DOI] [PubMed] [Google Scholar]
- Mahmoud K.Z., Edens F.W., Eisen E.J., Havenstein G.B. Effect of ascorbic acid and acute heat exposure on heat shock protein 70 expression by young white Leghorn chickens. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003;136:329–335. doi: 10.1016/j.cca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Mahmoudi J., Hosseini L., Sadigh-Eteghad S., Farajdokht F., Vatandoust S.M., Ziaee M. Sericin alleviates thermal stress induced anxiety-like behavior and cognitive impairment through regulation of oxidative stress, apoptosis, and heat-shock protein-70 in the hippocampus. Neurochem. Res. 2021;46:2307–2316. doi: 10.1007/s11064-021-03370-6. [DOI] [PubMed] [Google Scholar]
- Mashkoor J., Al-Saeed F.A., Guangbin Z., Alsayeqh A.F., Gul S.T., Hussain R., Khan A. Oxidative stress and toxicity produced by arsenic and chromium in broiler chicks and application of vitamin E and bentonite as ameliorating agents. Front. Vet. Sci. 2023;10 doi: 10.3389/fvets.2023.1128522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrommatis A., Giamouri E., Tavrizelou S., Zacharioudaki M., Danezis G., Simitzis P.E., Feggeros K. Impact of mycotoxins on animals’ oxidative status. Antioxidants. 2021;10:214. doi: 10.3390/antiox10020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland S.C., Attard G., Bowen J., Horrocks C., Jamie G.A., Dixit T., Spottiswoode C.N., Portugal S.J. Eggshell composition and surface properties of avian brood-parasitic species compared with non-parasitic species. R Soc. Open Sci. 2023;10 doi: 10.1098/rsos.221023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw K.J. In: Studies on Veterinary Medicine. Oxidative Stress in Applied Basic Research and Clinical Practice. Mandelker L., Vajdovich P., editors. Humana Press; Totowa, NJ: 1911. Avian Antioxidants and Oxidative Stress: Highlights from Studies of Food, Physiology, and Feathers. [DOI] [Google Scholar]
- McMillin K.W. Where is MAP going? A review and future potential of modified atmosphere packaging for meat. Meat Sci. 2008;80:43–65. doi: 10.1016/j.meatsci.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Mesquita C.S., Oliveira R., Bento F., Geraldo D., Rodrigues J.V., Marcos J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014;458:69–71. doi: 10.1016/j.ab.2014.04.034. [DOI] [PubMed] [Google Scholar]
- Meteyake H.T., Bilalissi A., Oke O.E., Voemesse K., Tona K. Effect of thermal manipulation during incubation and heat challenge during the early juvenile stage on production parameters of broilers reared under a tropical climate. Eur. Poult. Sci. 2020;84:1–16. [Google Scholar]
- Miao Q., Si X., Xie Y., Chen L., Liu Z., Liu L., Zhang H. Effects of acute heat stress at different ambient temperature on hepatic redox status in broilers. Poult. Sci. 2020;99:4113–4122. doi: 10.1016/j.psj.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z.Q., Dong Y.Y., Qin X., Yuan J.M., Han M.M., Zhang K.K. Dietary supplementation of methionine mitigates oxidative stress in broilers under high stocking density. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewski T.M., Lee W., Champagne F.A., Curley J.P. Behavioural and physiological plasticity in social hierarchies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022;377 doi: 10.1098/rstb.2020.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.L., Auchus R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B., Ahn D.U. Mechanism of lipid peroxidation in meat and meat products-a review. Food Sci. Biotechnol. 2005;14:152–163. [Google Scholar]
- Mishra B., Jha R. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019;6:60. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A., Mahmoud M., Murugesan R., Cheng H.W. Effect of a synbiotic supplement on fear response and memory assessment of broiler chickens subjected to heat stress. Animals. 2021;11:427. doi: 10.3390/ani11020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsen D., Taimor T. The role of oxidative stress in the development of congestive heart failure (CHF) in broilers with pulmonary hypertension syndrome (PHS) J. Cell Anim. Biol. 2011;5:176–181. [Google Scholar]
- Monaghan P., Metcalfe N.B., Torres R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements, and interpretation. Ecol. Lett. 2009;12:75–92. doi: 10.1111/j.1461-0248.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Morohashi K., Baba T., Tanaka M. Steroid hormones and the development of reproductive organs. Sex. Dev. 2012;7:61–79. doi: 10.1159/000342272. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Akiba Y., Warden C.H., Toyomizu M. Sequential changes in superoxide production, anion carriers and substrate oxidation in skeletal muscle mitochondria of heat-stressed chickens. FEBS Lett. 2007;581:3461–3467. doi: 10.1016/j.febslet.2007.06.051. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Yoshiki Y., Akiba Y., Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 2005;84:307–314. doi: 10.1093/ps/84.2.307. [DOI] [PubMed] [Google Scholar]
- Naidoo V., McGaw L.J., Bisschop S.P.R., Duncan N., Eloff J.N. The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Vet. Parasitol. 2008;153:214–219. doi: 10.1016/j.vetpar.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Nawab A., Ibtisham F., Li G., Kieser B., Wu J., Liu W., An L. Heat stress in poultry production: mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018;78:131–139. doi: 10.1016/j.jtherbio.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Nawab A., Li G., An L., Wu J., Chao L., Xiao M., Zhao Y., Birmani M.W., Ghani M.W. Effect of curcumin supplementation on TLR4 mediated non-specific immune responses in liver of laying hens under high-temperature conditions. J. Therm. Biol. 2019;84:384–397. doi: 10.1016/j.jtherbio.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Nawaz A., Irshad S., Khan I.A., Khalifa I., Walayat N., Aadil R.M., Lorenzo V. Protein oxidation in muscle-based products: Effects on physicochemical properties, quality concerns, and challenges to the food industry. Food Res. Int. 2022;157 doi: 10.1016/j.foodres.2022.111322. [DOI] [PubMed] [Google Scholar]
- Nienaber J.A., Hahn G.L. Livestock production system management responses to thermal challenges. Int. J. Biometeorol. 2007;52:149–157. doi: 10.1007/s00484-007-0103-x. [DOI] [PubMed] [Google Scholar]
- Niu Y., Zhang J.F., Wan X.L., Huang Q., He J.T., Zhang X.H., Zhao L.G., Zhang L.L., Wang T. Effect of fermented Ginkgo biloba leaves on nutrient utilisation, intestinal digestive function and antioxidant capacity in broilers. Br. Poult. Sci. 2019;60:47–55. doi: 10.1080/00071668.2018.1535166. [DOI] [PubMed] [Google Scholar]
- Niu Z.Y., Liu F.Z., Yan Q.L., Li W.C. Effects of different levels of vitamin E on the growth performance and immune responses of broilers under heat stress. Poult. Sci. 2009;88:2101–2107. doi: 10.3382/ps.2009-00220. [DOI] [PubMed] [Google Scholar]
- Nyoni N.M.B., Grab S., Archer E.R. Heat stress and chickens: climate risk effects on rural poultry farming in low-income countries. Clim. Dev. 2019;11:83–90. [Google Scholar]
- Nys Y., Gautron J., Rodriguez-Navarro A.B., Hincke M. Pages 833-879 in Sturkie's Avian Physiology. Academic Press; USA: 2022. Mechanisms and hormonal regulation of shell formation: supply of ionic and organic precursors, shell mineralization. [Google Scholar]
- Okasha H., Song B., Song Z. Hidden Hazards revealed: mycotoxins and their masked forms in poultry. Toxins. 2024;16:137. doi: 10.3390/toxins16030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke O.E., Emeshili U.K., Iyasere O.S., Abioja M.O., Daramola J.O., Ladokun A.O., Abiona J.A., Williams T.J., Rahman S.A., Rotimi S.O., Balogun S.I., Adejuyigbe A.E. Physiological responses and performance of broiler chickens offered olive leaf extract under hot humid tropical climate. J. Appl. Poult. Res. 2017;26:376–382. [Google Scholar]
- Oke O.E. Evaluation of physiological response and performance by supplementation of Curcuma longa in broiler feed under hot humid tropical climate. Trop. Anim. Hlth Prod. 2018;50:1071–1077. doi: 10.1007/s11250-018-1532-8. [DOI] [PubMed] [Google Scholar]
- Oke O.E., Alo E.T., Oke F.O., Oyebamiji Y.A., Ijaiya M.A., Odefemi M.A., Kazeem R.Y., Soyode A.A., Aruwajoye O.M., Ojo R.T., Adeosun S.M., Onagbesan O.M. Early age thermal manipulation on the performance and physiological response of broiler chickens under hot humid tropical climate. J. Thermal Biol. 2020;88 doi: 10.1016/j.jtherbio.2020.102517. [DOI] [PubMed] [Google Scholar]
- Oke O.E., Akosile O.A., Uyanga V.A., Oke F.O., Oni A.I., Tona K., Onagbesan O.M. Climate change and broiler production. Vet. Med. and Sci. 2024;10:e1416. doi: 10.1002/vms3.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke O.E., Oso O.M., Logunleko M., Uyanga V., Akinyemi F., Okeniyi F., Akosile O., Baloyi J., Onagbesan O. Adaptation of the white fulani cattle to the tropical environment. J. Therm. Biol. 2022;110:103372. doi: 10.1016/j.jtherbio.2022.103372. [DOI] [PubMed] [Google Scholar]
- Oke O.E., Oyelola O.B., Iyasere O.S., Njoku C.P., Oso A.O., Oso O.M., Fatoki S.T., Bankole K.O., Jimoh I.O., Sybill N.I., Awodipe H.O., Adegbite H.O., Rahman S.A., Daramola J.O. In ovo injection of Black Cumin (Nigella Sativa) extract on hatching and post hatch Performance of thermally challenged broiler chickens during incubation. Poult. Sci. 2021 doi: 10.1016/j.psj.2020.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke O.E., Sorungbe F.O., Abioja M.O., Oyetunji O., Onabajo A.O. Effect of different levels of honey on physiological, growth and carcass traits of broiler chickens during dry season. Acta Argic. Slovenica. 2016;108:45–53. [Google Scholar]
- Oke O.E., Uyanga V.A., Iyasere O.S., Oke F.O., Majokdunmi B.C., Logunleko M.O., Abiona J.A., Nwosu E.U., Abioja M.O., Daramola J.A., Onagbesan O.M. Environmental Stress and Livestock Productivity under Hot-Humid Tropics: Alleviation and future perspectives. J. Therm. Biol. 2021;100 doi: 10.1016/j.jtherbio.2021.103077. [DOI] [PubMed] [Google Scholar]
- Omar H.E.-D. Mycotoxins-Induced Oxidative Stress and Disease. InTech. 2013 doi: 10.5772/51806. [DOI] [Google Scholar]
- Onagbesan O., Uyanga V.A., Oso O., Tona K., Oke O.E. Alleviating heat stress effects in poultry: updates on methods and mechanisms of actions. Front Vet. Sci. 2023;10:1255520. doi: 10.3389/fvets.2023.1255520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S., Brault J., Stasi M.J., Knaus U.G. Genetic disorders coupled to ROS deficiency. Redox Biol. 2015;6:135–156. doi: 10.1016/j.redox.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oni A.I., Abiona J.A., Fafiolu A.O., Oke O.E. Early-age thermal manipulation and supplemental antioxidants on physiological, biochemical and productive performance of broiler chickens in hot-tropical environments. Stress. 2024;27 doi: 10.1080/10253890.2024.2319803. [DOI] [PubMed] [Google Scholar]
- Oni A.I., Adeleye O.O., Adebowale T.O., Oke O.E. The role of phytogenic feed additives in stress mitigation in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2023;108:81–98. doi: 10.1111/jpn.13869. [DOI] [PubMed] [Google Scholar]
- Oruç E.Ö. Oxidative stress, steroid hormone concentrations, and acetylcholinesterase activity in Oreochromis niloticus exposed to chlorpyrifos. Pestic. Biochem. Physiol. 2010;96:160–166. [Google Scholar]
- Oyelola O.B., Iyasere O.S., Adeleye O.O., Oke O.E. The influence of in ovo feeding of black cumin extract on the physiological responses of broilers under hot tropical environments. Acta Sci. Anim. Sci. 2024;46:e62653. [Google Scholar]
- Pamplona R., Costantini D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R843–R863. doi: 10.1152/ajpregu.00034.2011. [DOI] [PubMed] [Google Scholar]
- Pan X., Zhang L., Xing T., Li J., Gao F. The impaired redox status and activated nuclear factor-erythroid 2-related factor 2/antioxidant response element pathway in wooden breast myopathy in broiler chickens. Anim. Biosci. 2021;34:652–661. doi: 10.5713/ajas.19.0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A.K., Cherian G. Role of vitamin E in counteracting oxidative stress in poultry. J. Poult. Sci. 2014;51:109–117. [Google Scholar]
- Patra B.N., Bais R.K.S., Prasad R.B., Singh B.P. Performance of naked neck versus normally feathered coloured broilers for growth, carcass traits and blood biochemical parameters in tropical climate. Asian-Australas. J. Anim. Sci. 2002;15:1776–1783. [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. World's Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitino R., De M. Marchi C.L.M., Johnson M., Simoni M., Righi F., Tsiplakou E. Plant feed additives as natural alternatives to the use of synthetic antioxidant vitamins on yield, quality, and oxidative status of poultry products: a review of the literature of the last 20 years. Antioxidants. 2021;10:757. doi: 10.3390/antiox10050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu S., Usuda K., Nagaoka K., Gore A., Crews D., Watanabe G. The relation between liver damage and reproduction in female Japanese quail (Coturnix japonica) exposed to high ambient temperature. Poult. Sci. 2020;99:4586–4597. doi: 10.1016/j.psj.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput S.A., Sun L., Zhang Y., Khalil M.M., Ling Z., Chong L., Wang S., Rajput I.R., Bloch D.M., Khan F.A., Shaukat A., Qi D. Grape seed proanthocyanidin extract alleviates aflatoxinB1-induced immunotoxicity and oxidative stress via modulation of NF-κB and Nrf2 signaling pathways in broilers. Toxins. 2018;11 doi: 10.3390/toxins11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan A., Sinha R., Devi I., Abdul Rahim4, Tiwari S. Effect of heat stress on poultry production and their managemental approaches. Int. J. Curr. Microbiol. App. Sci. 2019;8:1548–1555. [Google Scholar]
- Rehman Z.U., Meng C., Sun Y., Safdar A., Pasha R.H., Munir M., Ding C. Oxidative stress in poultry: lessons from the viral infections. Oxidative Med. Cell. Long. 2018:5123147. doi: 10.1155/2018/5123147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M., Yatao X., Hassan F-u, Arain M., Abd El-Hack M., Noreldin A., Sun C. Influence of graded levels of l-theanine dietary supplementation on growth performance, carcass traits, meat quality, organs histomorphometry, blood chemistry and immune response of broiler chickens. IJMS. 2018;19(2):462. doi: 10.3390/ijms19020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A.H., Maghami S.P. Heat stress in poultry: practical tipsEur. J. Exp. Biol. 2014;4:625–631. [Google Scholar]
- Sahin K., Orhan C., Tuzcu M., Sahin N., Hayirli A., Bilgili S., Kucuk O. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult. Sci. 2016;95:1088–1095. doi: 10.3382/ps/pew012. [DOI] [PubMed] [Google Scholar]
- Salisbury D., Bronas U. Reactive oxygen and nitrogen species: impact on endothelial dysfunction. Nurs.Res. 2015;64:53–66. doi: 10.1097/NNR.0000000000000068. [DOI] [PubMed] [Google Scholar]
- Saraswat S., Jindal S.K., Priyadharsini R., Ramachandran N., Yadav S. The effect of antioxidants supplementation to cryopreservation protocol on seminal attributes and sperm membrane characteristics in Sirohi goat. J. Physiol. Pharm. Adv. 2012;2(1):49–58. [Google Scholar]
- Schillings J., Bennett R., Rose D.C. Animal welfare and other ethical implications of Precision Livestock Farming technology. CABI Agric. Biosci. 2021;2:17. [Google Scholar]
- Shah Alam M., Maowa Z., Subarna S.D., Hoque M.N. Mycotoxicosis and oxidative stress in poultry: pathogenesis and therapeutic insights. World’s Poult. Sci. J. 2024:1–30. [Google Scholar]
- Shakeri M., Cottrell J., Wilkinson S., Ringuet M., Furness J., Dunshea F. Betaine and antioxidants improve growth performance, breast muscle development and ameliorate thermoregulatory responses to cyclic heat exposure in broiler chickens. Animals. 2018;8:162. doi: 10.3390/ani8100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierżant K., Piksa E., Konkol D., Lewandowska K., Asghar M.U. Performance and antioxidant traits of broiler chickens fed with diets containing rapeseed or flaxseed oil and optimized quercetin. Sci. Rep. 2023;13:1–13. doi: 10.1038/s41598-023-41282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek U.G., Dalkilic B., Ciftci M., Yuce A. The influences of different stocking densities on some welfare indicators, lipid peroxidation (MDA) and antioxidant enzyme activities (GSH, GSH-Px, CAT) in broiler chickens. J. Anim. Vet. Adv. 2009;8:1568–1572. [Google Scholar]
- Slauch J.M. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011;80:580–583. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimen I.B., Najar T., Ghram A., Dabbebi H., Ben Mrad M., Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperthermia. 2014;30:513–523. doi: 10.3109/02656736.2014.971446. [DOI] [PubMed] [Google Scholar]
- Soleimani A.F., Zulkifli I., Omar A.R., Raha A.R. Physiological responses of 3 chicken breeds to acute heat stress. Poult. Sci. 2011;90:1435–1440. doi: 10.3382/ps.2011-01381. [DOI] [PubMed] [Google Scholar]
- Speakman J.R., Garratt M. Oxidative stress as a cost of reproduction: beyond the simplistic trade-off model. BioEssays. 2014;36:93–106. doi: 10.1002/bies.201300108. [DOI] [PubMed] [Google Scholar]
- Steinberg S.F. Oxidative stress and sarcomeric proteins. Circ. Res. 2012;112:393. doi: 10.1161/CIRCRESAHA.111.300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez S.S., Pacey A.A. Sperm transport in the female reproductive tract. Human Reprod. Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- Suman S.P., Joseph P. Myoglobin chemistry and meat color. Annual Rev. Food Sci. Tech. 2013;4:79–99. doi: 10.1146/annurev-food-030212-182623. [DOI] [PubMed] [Google Scholar]
- Sun L., Xu G., Dong Y., Li M., Yang L., Lu W. Quercetin protects against lipopolysaccharide-induced intestinal oxidative stress in broiler chickens through activation of Nrf2 pathway. Molecules. 2020;25 doi: 10.3390/molecules25051053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntres Z.E. Liposomal antioxidants for protection against oxidant-induced damage. J. Toxicol. 2011;2011:152474. doi: 10.1155/2011/152474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F. Antioxidant systems in poultry biology: Superoxide dismutase. J. Anim. Res. Nutr. 2016;1:1–8. [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T.. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants (Basel) 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraweera T.L., Vasantha Rupasinghe, Dellaire G., Xu Z. Regulation of Nrf2/ARE pathway by dietary flavonoids: a friend or foe for cancer management? Antioxidants. 2020;9:873. doi: 10.3390/antiox9100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syafwan S., Kwakkel R.P., Verstegen M.W.A. Heat stress and feeding strategies in meat-type chickens. World’s Poult J. Sci. 2011;67:653–674. [Google Scholar]
- Tang F., Fan K., Wang K., Bian C. Amygdalin attenuates acute liver injury induced by D-galactosamine and lipopolysaccharide by regulating the NLRP3, NF-κB and Nrf2/NQO1 signalling pathways. Biomed. Pharmacother. 2019;111:527–536. doi: 10.1016/j.biopha.2018.12.096. [DOI] [PubMed] [Google Scholar]
- Tarım Ö. Thyroid Hormones and Growth in Health and Disease. J. Clinical Res. Ped. Endocrinol. 2011;3:51–55. doi: 10.4274/jcrpe.v3i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach K.E., Oyler J.E., Altan-Bonnet G. Cracking the NF-κB code. Sci. Signal. 2014;7:pe5. doi: 10.1126/scisignal.2005108. [DOI] [PubMed] [Google Scholar]
- Toghyani M., Toghyani M., Shivazad M., Gheisari A., Bahadoran R. Chromium supplementation can alleviate the negative effects of heat stress on growth performance, carcass traits, and meat lipid oxidation of broiler chicks without any adverse impacts on blood constituents. Biol. Trace Elem. Res. 2012;146:171–180. doi: 10.1007/s12011-011-9234-3. [DOI] [PubMed] [Google Scholar]
- Tokofai B.M., Orounladji B.M., Idoh K., Oke O.E., Agbonon A. Effect of Vernonia amygdalina on intestinal mucosa's digestive enzymes, absorption capacity, and immunity in broiler chickens. J. Appl. Anim. Nutr. 2023;610:3920. [Google Scholar]
- Tonev D., Momchilova A. Oxidative stress and the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway in multiple sclerosis: Focus on certain exogenous and endogenous Nrf2 activators and therapeutic plasma exchange modulation. Int. J. Mol. Sci. 2023;24:1–23. doi: 10.3390/ijms242417223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore L., Thotakura A.K., Bennett J., Moretti M., Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22:557–566. doi: 10.1016/j.tcb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal. Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Tu W., Wang H., Li S., Liu Q., Sha H. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019;10:637–651. doi: 10.14336/AD.2018.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhaya S.D., Kim I.H. The impact of weaning stress on gut health and the mechanistic aspects of several feed additives contributing to improved gut health function in weanling piglets—a review. Animals. 2021;11:2418. doi: 10.3390/ani11082418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utrera M., Parra V., Estévez M. Protein oxidation during frozen storage and subsequent processing of different beef muscles. Meat Sci. 2014;96:812–820. doi: 10.1016/j.meatsci.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Uyanga V.A., Musa T.H., Oke O.E., Zhao J., Wang X., Jiao H., Lin H. Global trends and research frontiers on heat stress in poultry from 2000 to 2021: a bibliometric analysis. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1123582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyanga V.A., Oke O.E., Amevor F.K., Zhao J., Wang X., Jiao H., Lin H. Functional roles of taurine, L-theanine, L-citrulline, and betaine during heat stress in poultry. J. Anim. Sci. Biotechnol. 2022;131:1–20. doi: 10.1186/s40104-022-00675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagasi C.I., Pătraș L., Pap P.L., Vincze O., Mureșan C., Nemeth J., Lendvai A.Z. Experimental increase in baseline corticosterone level reduces oxidative damage and enhances innate immune response. PLoS One. 2018;132 doi: 10.1371/journal.pone.0192701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadez-García K.M., Avendaño-Reyes L., Díaz-Molina R., Mellado M., Meza-Herrera C.A., Correa-Calderón A., Macías-Cruz U. Free ferulic acid supplementation of heat-stressed hair ewe lambs: oxidative status, feedlot performance, carcass traits and meat quality. Meat Sci. 2021;173 doi: 10.1016/j.meatsci.2020.108395. [DOI] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;391:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vandana G.D., Sejian V. Towards identifying climate-resilient poultry birds. J. Dairy Vet. Anim. Res. 2018;73:84–85. [Google Scholar]
- Voljč M., Frankič T., Levart A., Nemec M., Salobir J. Evaluation of different vitamin E recommendations and bioactivity of α-tocopherol isomers in broiler nutrition by measuring oxidative stress in vivo and the oxidative stability of meat. Poult. Sci. 2011;907:1478–1488. doi: 10.3382/ps.2010-01223. [DOI] [PubMed] [Google Scholar]
- Wang R.R., Pan X.J., Peng Z.Q. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult. Sci. 2009;885:1078–1084. doi: 10.3382/ps.2008-00094. [DOI] [PubMed] [Google Scholar]
- Wang X., Hai C. Novel insights into redox system and the mechanism of redox regulation. Mol. Biol. Rep. 2016;43:607–628. doi: 10.1007/s11033-016-4022-y. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen H., Chang W., Chen R., Xu S., Tao D. Protective effects of selenium yeast against cadmium-induced necroptosis via inhibition of oxidative stress and MAPK pathway in chicken liver. Ecotoxicol. Environ. Saf. 2020;206 doi: 10.1016/j.ecoenv.2020.111329. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao H., Liu J., Shao Y., Li J., Luo L., Xing M. Copper and arsenic-induced oxidative stress and immune imbalance are associated with activation of heat shock proteins in chicken intestines. Int. Immunopharmacol. 2018;60:64–75. doi: 10.1016/j.intimp.2018.04.038. [DOI] [PubMed] [Google Scholar]
- Wasti S., Sah N., Mishra B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals. 2020;108:1266. doi: 10.3390/ani10081266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasti S., Sah N., Singh A.K., Lee C.N., Jha R., Mishra B. Dietary supplementation of dried plum: a novel strategy to mitigate heat stress in broiler chickens. J. Anim. Sci. Biotechnol. 2021;12:1258. doi: 10.1186/s40104-021-00571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Sekine S., Naguro I., Sekine Y., Ichijo H. Apoptosis signal-regulating kinase 1 (ASK1)-p38 pathway-dependent cytoplasmic translocation of the orphan nuclear receptor NR4A2 is required for oxidative stress-induced necrosis. J. Biol. Chem. 2015;290:10791–10803. doi: 10.1074/jbc.M114.623280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;923:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Weiss G., Goldsmith L.T., Taylor R.N., Bellet D., Taylor H.S. Inflammation in reproductive disorders. Reprod. Sci. 2009;16:216–229. doi: 10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C.H., Sandilands V., Mayasari N., Asmara I.Y., Anang A. A literature review of broiler chicken welfare, husbandry, and assessment. World's Poult. Sci. J. 2023;80:3–32. [Google Scholar]
- Woźniak A., Masiak R., Szpinda M., Mila-Kierzenkowska C., Woźniak B., Makarewicz R., Szpinda A. Oxidative stress markers in prostate cancer patients after HDR brachytherapy combined with external beam radiation. Oxid Med. Cell Longev. 2012;2012:789870. doi: 10.1155/2012/789870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Lupton J.R., Turner N.D., Fang Y., Yang S. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Xie X., He Z., Chen N., Tang Z., Wang Q., Cai Y. The roles of environmental factors in the regulation of oxidative stress in plants. Biomed. Res. Int. 2019:9732325. doi: 10.1155/2019/9732325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Chen X., Huang J., Huan C., Li C. H2O2-induced oxidative stress impairs meat quality by inducing apoptosis and autophagy via ROS/NF-κB signaling pathway in broiler thigh muscle. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Liu G., Lian K., Qiao Y., Zhang B., Zhu X., Luo Y., Shang Y., Gu X.L. Dietary leonurine hydrochloride supplementation attenuates lipopolysaccharide challenge-induced intestinal inflammation and barrier dysfunction by inhibiting the NF-κB/MAPK signaling pathway in broilers. J. Anim. Sci. 2019;97:1679–1692. doi: 10.1093/jas/skz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Tan G.Y., Fu Y.Q., Feng J.H., Zhang M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010;1512:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Yara S., Lavoie J.C., Beaulieu J.F., Delvin E., Amre D., Marcil V., Levy E. Iron-ascorbate-mediated lipid peroxidation causes epigenetic changes in the antioxidant defense in intestinal epithelial cells: impact on inflammation. PLoS One. 2013;85:e63456. doi: 10.1371/journal.pone.0063456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I.S., Woodside J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001;543:176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younus H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018;123:88–93. [PMC free article] [PubMed] [Google Scholar]
- Zaboli G., Huang X., Feng X., Ahn D.U. How can heat stress affect chicken meat quality?–a review. Poult. Sci. 2019;983:1551–1556. doi: 10.3382/ps/pey399. [DOI] [PubMed] [Google Scholar]
- Zahlten J., Kim Y.J., Doehn J.M., Pribyl T., Hocke A.C., García P., Hübner R.H. Streptococcus pneumoniae–induced oxidative stress in lung epithelial cells depends on pneumococcal autolysis and is reversible by resveratrol. J. Infect. Dis. 2015;21111:1822–1830. doi: 10.1093/infdis/jiu806. [DOI] [PubMed] [Google Scholar]
- Zeitz J.O., Fleischmann A., Ehbrecht T., Most E., Friedrichs S., Whelan R., Eder K. Effects of supplementation of DL-methionine on tissue and plasma antioxidant status during heat-induced oxidative stress in broilers. Poult. Sci. 2020;9912:6837–6847. doi: 10.1016/j.psj.2020.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Xiao X., Arnold P.R., Li X.C. Transcriptional and epigenetic regulation of immune tolerance: Roles of the NF-κB family members. Cell. Mol. Immunol. 2019;16:315–323. doi: 10.1038/s41423-019-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.F., Bai K.W., Su W.P., Wang A.A., Zhang L.L., Huang K.H., Wang T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poul. Sci. 2018;97:1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Jia G.Q., Zuo J.J., Zhang Y., Lei J., Ren L., Feng D.Y. Effects ofconstant and cyclic heat stress on muscle metabolism and meat quality of broilerbreast fillet and thigh meat. Poult. Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]
- Zhang W., Xiao S., Ahn D.U. Protein oxidation: basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013;5311:1191–1201. doi: 10.1080/10408398.2011.577540. [DOI] [PubMed] [Google Scholar]
- Zhao H., He Y., Li S., Sun X., Wang Y., Shao Y., Xing M. Subchronic arsenism-induced oxidative stress and inflammation contribute to apoptosis through mitochondrial and death receptor-dependent pathways in chicken immune organs. Oncotarget. 2017;825:40327. doi: 10.18632/oncotarget.16960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Ruan L., Kline J.T., Omkar S., Sikora J., Torres M.T., Wang Y., Takakuwa J.E., Huguet R., Klemm C., Segarra V.A., Winters M.J., Pryciak P.M., Thorpe P.H., Tatebayashi K., Li R., Fornelli L., Truman A.W. Comprehensive characterization of the Hsp70 interactome reveals novel client proteins and interactions mediated by posttranslational modifications. PLOS Biol. 2022;20 doi: 10.1371/journal.pbio.3001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Sun W., Zhang Z., Zheng Y. The role of Nrf2-mediated pathway in cardiac remodelling and heart failure. Oxid. Med. Cell. Longev. 2014;2014 doi: 10.1155/2014/260429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Kawai T., Umehara T., Hoque S.M., Zeng W., Shimada M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic. Biol. Med. 2019;141:159–171. doi: 10.1016/j.freeradbiomed.2019.06.018. [DOI] [PubMed] [Google Scholar]
- Zwolak I. Protective effects of dietary antioxidants against vanadium-induced toxicity: a review. Oxid. Med. Cell. Longev. 2020:1–14. doi: 10.1155/2020/1490316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.