Abstract
Soluble decoy receptors (DR) are circulating proteins that act as molecular traps for ligands that modulate various signalling pathways. These proteins can be exploited as biomarkers and, in some cases, as drugs in various disease contexts. Inflammation is a key area where DRs have shown significant potential. By binding to pro-inflammatory cytokines, inflammatory DRs, such as soluble tumour necrosis factor receptors (sTNFRs), can inhibit downstream inflammatory signalling. This modulation of the inflammatory response holds promise for therapeutic interventions in various inflammatory conditions, including cardiovascular and chronic kidney diseases. Soluble DRs for advanced glycation end products (sRAGE) bind to advanced glycation end products (AGEs), reducing their detrimental effects on vascular function and atherosclerosis. High circulating sRAGE levels are associated with a lower risk for CV events, highlighting the potential of these soluble receptors for assessing the role of AGEs in CV diseases and managing the attendant risk. DRs may serve as biomarkers and therapeutic agents to advance our understanding of disease mechanisms and improve patients' outcomes. Their ability to modulate signalling pathways in a controlled manner opens up new opportunities for therapeutic interventions in various diseases, ranging from inflammation to cardiovascular and renal disorders.
Keywords: biomarkers, CKD, decoy receptors, epidemiology, inflammation
Decoy receptors (DRs), a class of proteins with wide-ranging effects, have emerged as significant regulators of cellular signalling pathways, particularly inflammation signalling [1], with implications for a broad spectrum of biological processes and diseases.
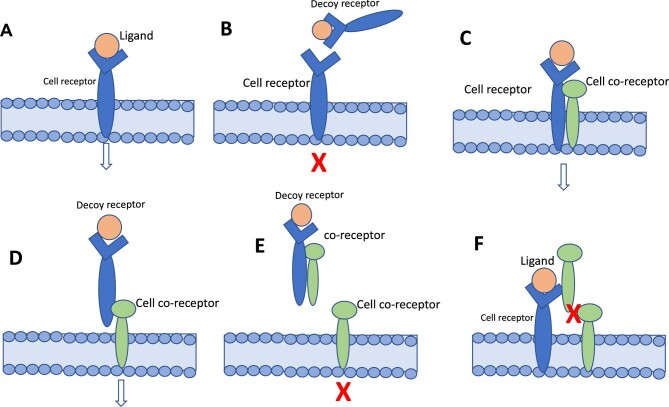
Beyond the classical concept of the ligand as a soluble molecule and the receptor as a membrane-bound protein, different forms of ligands and receptors exist on the cell surface and in the extracellular space [2]. These include membrane receptors and soluble receptors, both in ligand-bound and ligand-free form. Ligands can be soluble, unassociated, bound to membrane receptors or soluble receptors in the extracellular space or circulation. Soluble receptors primarily bind to their ligands and neutralize them. The term ‘decoy receptor’ originates from the concept of a decoy, which is something used to distract or mislead. In the context of receptors, a DR functions by binding to ligands and preventing them from interacting with functional receptors, thereby diverting, or neutralizing their effects. This mechanism helps regulate the signalling pathways activated by the ligands, preventing them from interacting with membrane-bound receptors and influencing cellular responses (Fig. 1B). Some receptors need a membrane co-receptor to signal (Fig. 1D). Several examples of these co-receptors exist. For example, CD40 is a co-receptor that interacts with DRs TNFα-R1 and R2. DRs ‘TNFα-related apoptosis-inducing ligands’ (TRAIL)-R1 and TRAIL-R2 are co-receptors that interact to regulate the apoptotic signalling of TNF ligands. IL-1R1 is a co-receptor that interacts with the DR IL-1R2 to modulate the signalling of IL-1β (IL-1). VEGFR-2 is a co-receptor that interacts with DR VEGFR-1 to modulate the signalling of vascular endothelial growth factor (VEGF) and regulate angiogenesis and tumour growth. Like membrane receptors, co-receptors can also be released in soluble forms and bind to receptors or ligands on the cell surface or in the extracellular space.
Figure 1:
(A) Cell receptor and its ligand at the cell surface. The link of the ligand to the corresponding receptor activates cell signalling. (B) A DR ‘captures’ the ligand in the circulation and prevents it from activating the cell receptor. (C) Some receptors need to receptors to activate cell signalling. (D) Without a cell receptor, the DR–ligand complex can still activate cell signalling by linking to a co-receptor. (E) If a circulating co-receptor binds to the DR–ligand complex in the circulation, it may prevent this complex from activating cell signalling via the cell co-receptor. (F) The soluble, circulating co-receptor, can bind to the cell receptor, and this link may prevent cell signalling if the link with a cell co-receptor is needed to activate cell signalling. The arrow indicates normal cell signalling, and the red X indicates the cell signalling block.
Soluble receptors, derived from membrane receptors, are generated through (i) proteolytic cleavage, (ii) alternative mRNA splicing, or (iii) release in extracellular vehicles such as exosomes [1]. The first mechanism is realized via enzymes belonging to the family of A Disintegrin and Metalloproteases (ADAMS), ADAM17 (or TACE—TNF-alpha converting enzyme), and ADAM10 being the most relevant among these proteolytic enzymes. The second mechanism involves alternative splicing of a membrane receptor mRNA that omits the receptor's transmembrane domain. Like other secreted proteins, the resultant soluble receptor is secreted from the cell into the extracellular space. As to the third mechanism, cytokines and growth factor receptors have been identified in extracellular vesicles (EVs), such as exosomes, maintaining full-length receptors on the vesicle membrane. These released receptors, including TNFR1 and IL-6R, can bind ligands to modulate signalling properties. TNFR1 EVs lack an active signalling complex but can bind exogenous TNF. Functional proteases in exosomes suggest the potential shedding of receptor parts, although cytokine receptor shedding from exosomes has not been demonstrated yet.
The binding properties of ligands, receptors, co-receptors, and adhesion molecules determine the signalling outcome. SDRs can also interact with other cell membrane proteins, adding to their complexity (Table 1). In this regard, soluble adhesion molecules modulate inflammation by regulating the recruitment and activation of immune cells. Indeed, selectins, integrins, and immunoglobulin superfamily members are released into the bloodstream in response to inflammation and can either promote or inhibit the inflammatory response [3]. For almost every membrane receptor of cytokines, growth factors, and cell adhesins, soluble versions are naturally produced by cells. Moreover, viruses hijacked these same DRs during their co-evolution with their hosts [4].
Table 1:
This structured summary provides an overview of the cell membrane proteins that DRs can interact with, the nature of their interactions, and the resulting biological effects.
| Cell membrane proteins | Interaction with decoy receptors | Biological effects |
|---|---|---|
| Receptor Tyrosine Kinases (RTKs) | Competing for ligand binding or forming heterodimers with RTKs | Modulation of cell growth, survival, and differentiation pathways |
| G Protein-Coupled Receptors (GPCRs) | Sequestering ligands or forming complexes with GPCRs | Regulation of GPCR-mediated signalling events, impacting neurotransmission, hormone signalling, and immune responses |
| Adhesion Molecules | Interacting with adhesion molecules | Influencing cell adhesion, migration, and tissue organization |
| Immune Checkpoint Proteins | Engaging with immune checkpoint proteins | Modulation of T cell activation, immune surveillance, and immune evasion mechanisms |
| Receptor-associated Signalling Complexes | Interacting with components of signalling complexes | Disruption of signal transduction pathways and alteration of cellular responses |
Table 2:
Examples of DRs discussed in this review (see also main text).
| DR | Pathophysiology and prognosis | Therapeutic potential |
|---|---|---|
| Inflammation DRs | ||
| IL-1R2 | Binds to IL-1 cytokines, regulates inflammation in cardiovascular diseases | Anakinra used for autoimmune diseases, with potential impact in heart failure |
| sTNFRs | Compete with TNF-α receptors, predict CKD progression and cardiovascular events | Etanercept approved for rheumatoid arthritis, biosimilars available |
| TRAIL DRs | Involved in apoptosis, lower levels linked to cardiovascular diseases | Promising in cancer treatment, no studies in cardiovascular or kidney diseases |
| DR for IL-6 | Binds to IL-6, associated with increased cardiovascular risk | Research ongoing for potential treatment in inflammatory diseases |
| DcR1 | Binds to TRAIL ligand, regulates apoptosis pathways | Potential role in cancer therapy |
| DcR2 | Binds to TRAIL ligand, modulates apoptosis signalling pathways | Investigated for cancer treatment |
| DcR3 | Binds to Fas ligand and LIGHT, regulates immune responses and cell death | Implicated in autoimmune diseases, potential therapeutic target |
| DR for IL-17 | Binds to IL-17 cytokines, regulates inflammation and immune responses | Investigated for autoimmune diseases and inflammatory conditions |
| Cardiovascular DRs | ||
| DR for VEGF | Binds to VEGF, modulates angiogenesis and vascular permeability | Potential target in cancer therapy and retinal vascular diseases |
| RAGE (Receptor for Advanced Glycation End Products) | Binds to advanced glycation end products, implicated in inflammation and vascular dysfunction | Potential target for diabetic complications and cardiovascular diseases |
| DR for PDGF | Binds to PDGF, regulates cell growth and proliferation in cardiovascular diseases | Investigated for potential use in vascular remodelling and fibrosis |
As alluded to before, the effect of DRs may also depend on the presence of co-receptors and other interactions [1]. Figure 1C and d schematize the receptor–co-receptor interaction. The soluble nature of DRs allows for easy quantification in biological fluids, such as blood or urine, making them exploitable for diagnostic and prognostic purposes. Their presence and activity can provide insights into specific signalling pathways and aid disease diagnosis and prognosis. In addition, these soluble receptors offer opportunities to develop targeted therapies.
A unique property of some SDRs is that they mimic the effect of an intervention countering the ligand they block. In this respect, a DR of a noxious ligand would be expected to be inversely related to disease outcomes because it limits the exposure of the corresponding tissue receptor to the noxious ligand. However, most DRs affecting inflammation signalling that are measurable in biological fluids are directly rather than inversely associated with organ damage and the risk of adverse health events, as is the case for most DRs of the inflammatory system [1]. This apparently paradoxical relationship depends on their complex interaction with co-receptors and on the fact that some inflammatory DRs have pleiotropic effects and serve to modulate rather than abolish the inflammatory response. Examples of pleiotropic effects of DRs include Decoy Receptor 3 (DcR3) [5], a receptor binding the Fas ligand; a receptor expressed by T lymphocytes, and the ‘Lymphotoxin-like, exhibiting Inducible expression, and competing with Herpes Virus (HSV) Glycoprotein D’ (LIGHT), an entry mediator for HSV regulating both immune responses and cell death. Similarly, the DR for ‘Tumour Necrosis Factor-Related Apoptosis-Inducing Ligand’ (TRAIL) binds to TRAIL, Fas ligand, and ‘TNF-related weak inducer of apoptosis’ (TWEAK) [5], a ligand regulating apoptosis and immune responses.
As remarked, some receptors need co-receptors to function, and soluble co-receptors may either activate or inhibit cell signalling, as depicted in Fig. 1E and F.
Overall, with some notable exceptions, most circulating DRs that interfere with inflammatory mechanisms are direct, rather than inverse, predictors of health outcomes.
Herein, we will discuss a series of inflammatory, cardiovascular (CV), and metabolic DRs, focusing on their relationship with disease status and the risk of clinical events and, in specific cases, underlining their therapeutic potential.
DECOY RECEPTORS AFFECTING INFLAMMATION
Interleukin 1 receptor type II (IL-1R2)
Pathophysiology and prognosis
IL-1R2 binds to interleukin-1α, interleukin-1β, and interleukin-1 receptor antagonist (IL-1Ra), preventing their binding to conventional receptors and subsequent signalling. IL-1R2 acts as a DR on the cell surface or in a soluble form (sIL-1R2) in circulation, inhibiting the IL-1β-mediated inflammatory response [6]. IL-1R1 is a receptor that functions as a co-receptor, interacting with the DR IL-1R2, which helps regulate the effects of IL-1β. This interaction between IL-1R1 and IL-1R2 is important for fine-tuning the immune response and maintaining immune balance. Maintaining a balance between agonist and antagonist levels avoids exaggerated inflammatory responses. IL-1β plays a central role as a mediator propagating the inflammatory response and is implicated in atherothrombosis. For this reason, IL-R2 and other receptors functionally linked to IL-1β are expected to be recruited following an inflammatory event such as myocardial infarction. In a study by Orrem et al. [7] sIL-1R2, IL-1Ra, and sIL-1R1 levels were elevated after myocardial infarction. sIL-1R2 levels correlated positively with C-reactive protein, myocardial infarct size, and change in indexed left ventricular end-diastolic and end-systolic volume acutely, and, after 4 months, negatively with LV ejection fraction. Patients with levels of sIL-1R2 above the median value in the acute phase were more likely to develop LV dilatation. Thus, because these receptors are shed as a compensatory response to inflammation, their circulating levels are directly, rather than inversely, associated with heart disease and do not capture their mitigating effect on inflammation in myocardial infarction. Unquestionably, IL-1β, a main ligand of the IL1-R2 receptor, exerts adverse effects on the CV system. Indeed, the Canakinumab Anti-inflammatory Thrombosis Outcomes Study showed that blocking inflammation with the anti-IL-1β monoclonal antibody canakinumab reduced heart attacks, strokes, and new-onset diabetes among patients with prior myocardial infarction [8]. Furthermore, in the same trial, this treatment reduced the risk of incident lung cancer and lung cancer mortality [9]. The situation is different in some renal diseases. Indeed, higher sIL-2R was independently associated with renal recovery in a series of patients with the autoimmune tubule-interstitial disease and, on discrimination analysis, this DR had a good area under the receiver operator curve (80%) for predicting a favourable outcome [10]. In line with a favourable action of high sIL1-R in kidney diseases, high levels of the soluble IL-1 receptor antagonist (sIL-1Ra) predicted progression towards kidney failure in patients with IgA nephropathy [11].
Therapeutic potential
Anakinra (Kineret, Swedish Orphan Biovitrum AB, Stockholm, Sweden) is a recombinant form of the IL-1R antagonist that inhibits the activity of IL-1β and has been primarily used as a treatment for autoimmune diseases that involve dysregulated IL-1 signalling, such as rheumatoid arthritis [12]. In a systematic review that included eight anakinra clinical trials in patients with heart failure, the use of the drug was associated with a reduction in C-reactive protein (CRP) levels, indicating anti-inflammatory effects. However, these trials failed to show meaningful effects of the drug on cardiac function and exercise capacity or adverse events [13]. In the ACTION trial—a pilot, multicentre, randomized, placebo-controlled trial of an IL-1 receptor antagonist, anakinra—the median plasma concentrations of high sensitivity CRP from baseline to week 24 was 41% in the anakinra group and 6% in the placebo group, documenting a relevant anti-inflammatory effect of the drug in these patients [14]. Anakinra's effect needs to be tested in well-powered trials in this population.
Soluble tumour necrosis factor receptors (sTNFRs)
Pathophysiology and prognosis
TNF-α is critical to immune system responses [15]. Inflammatory and stress response pathways are activated when TNF-α binds to TNF receptors (TNFRs). Two main TNF-α receptors exist: type 1 (TNFR1) and type 2 (TNFR2) [15]. CD40 is a co-receptor that interacts with DRs TNFR1 an TNFR2 to modulate the signalling of TNFα family cytokines. Similarly, TNF-related apoptosis-inducing ligands (TRAIL)-R1 and TRAIL-R2 are co-receptors that interact with DRs TRAIL-R3 and TRAIL-R4 to regulate the apoptotic signalling of TNF ligands.
At the tissue level, TNFR1 is ubiquitously expressed and is a death receptor inducing apoptosis, whereas TNFR2 is mainly expressed in T cells, endothelial cells, and fibroblasts and stimulates NFκB signalling [15]. Both contain transmembrane domains but can also be cleaved by ADAM metallopeptidase domain 17 into soluble receptors (sTNFR1 and sTNFR2). sTNFR1 and sTNFR2 compete with the transmembrane forms by binding circulating TNF-α to inhibit/modulate the effects of this cytokine.
In rats treated with streptozocin, TNFα and IL-1β exert damaging effects at the kidney level by mediating the effect of advanced glycosylation end products [16]. However, soluble TNFRs are direct predictors of the progression of CKD in patients with type 1 and 2 diabetes [17]. Similarly, sTNFR1 and sTNFR2 concentration increases after myocardial infarction and correlates directly with infarct size and left ventricular end-diastolic volume and inversely with left ventricular ejection fraction [18] and are direct predictors of a composite endpoint, including death, recurrent myocardial infarction, stroke, or hospitalization for heart failure within 24 months after myocardial infarction [18].
Therapeutic potential
Etanercept (Enbrel, Wyeth Pharmaceuticals) is a fusion protein composed of a soluble TNF-alpha receptor issued by biotechnology. In the United States, etanercept has been approved by the Food and Drug Administration since 1998 to treat rheumatoid arthritis, showing an inadequate response to prior therapy with other disease-modifying antirheumatic drugs. Other marketing authorizations have been obtained in ankylosing spondylitis, polyarticular-course juvenile rheumatoid arthritis, and the treatment of psoriatic arthritis. The etanercept patent expired in the EU in 2015, and some etanercept copies have reached the production phase and are being used. In a recent meta-analysis, etanercept biosimilars were as effective and safe as the originator [19].
DRs for Tumour Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)
Pathophysiology and prognosis
TRAIL is a member of the tumour necrosis factor superfamily [20]. It is expressed as a transmembrane protein on various cell types' cell surfaces or released as a soluble protein. One important characteristic of TRAIL is its ability to induce apoptosis (cell death) in cancer cells [20].
TRAIL binds to DRs TRAIL-R3/DcR1 and R4/DcR2 and Osteoprotegerin, showing a complex system with various biological effects. The anti-cancer activity of this signalling pathway is well-known [21]. Recent studies focused on its role in CV diseases [22]. TRAIL and its receptors are expressed in endothelial cells, vascular smooth muscle cells, and inflammatory cells. Studies suggest that TRAIL is involved in CV diseases, with lower levels linked to acute myocardial infarction and poor prognosis in patients with coronary artery disease or heart failure [23]. Low TRAIL levels are also associated with atheromatosis plaque formation in patients with chronic kidney disease [24]. Lower TRAIL levels increase the risk of death in patients with prevalent CV disease, with higher mortality rates linked to the lowest TRAIL levels [25].
Therapeutic potential
TRAIL-based therapy shows promise in cancer treatment by targeting cancer cells while sparing normal cells [21]. However, resistance to TRAIL in colorectal cancer proved to be a significant issue. No studies with TRAIL have been performed on CV and kidney diseases in humans.
Decoy receptor for IL6
IL-6 is one of the most potent inflammatory cytokines implicated in various pathophysiological responses and diseases, from the response to infectious agents to CV, kidney, rheumatic, and cancer [26].
Pathophysiology and prognosis
One example of a DR for IL-6 is the soluble IL-6 receptor (sIL-6R), which can bind to IL-6 and block its signalling pathway. Elevated levels of sIL-6R are associated with an increased risk of CV diseases such as heart attack and stroke. This is because sIL-6R is involved in the inflammatory processes that contribute to the development of atherosclerosis, a condition where plaque builds up in the arteries and can lead to heart disease. In a large population-based case-control study in Stockholm, individuals in the highest quartile of sIL-6R had 40% higher risk of myocardial infarction [27]. Since the binary IL6: sIL6R complex is inactivated by sgp130 through the formation of the ternary IL6: sIL6R: sgp130 complex, in a subsequent study in the same cohort by the same investigators tested whether the ratio between the binary and ternary complexes (IL6:sIL6R to IL6:sIL6R:sgp130 ratio) associates with incident CV events [28]. In line with the hypothesis that active binary complexes are the main determinant of high CV risk, this study showed that individuals with a high ratio of circulating IL-6/sIL-6R to IL-6/IL-6R/gp130 complexes had an increased risk of disease, including coronary heart disease, sudden cardiac death, and ischaemic stroke. Levels of soluble IL6 receptors may be elevated in patients with kidney disease, potentially serving as a biomarker for disease progression [29] but no prospective cohort studies investigating the link between sILR and the progression of CKD or the risk of kidney failure have been produced so far.
Therapeutic potential
DRs for IL-6 are being researched as potential treatment options for diseases involving inflammation, like rheumatoid arthritis, inflammatory bowel disease, and certain cancers. Scientists are exploring ways to use DRs to regulate the IL-6 pathway and develop new therapies for these conditions. EVs are natural nanoparticles that can be modified to carry therapeutic molecules to specific targets. By engineering EVs to express IL6 signal transducer (IL6ST) DRs, researchers could selectively block the IL6 trans-signalling pathway while leaving the IL6 classical signalling pathway unaffected [30]. In studies using muscle cells and a mouse model of Duchenne muscular dystrophy (DMD), IL6ST DR EVs successfully inhibited the activation of STAT3, a protein involved in inflammation and muscle degeneration [30]. This study highlights the potential of targeting the IL6ST DR EVs as a potential therapy beyond rare muscle diseases.
Decoy receptor 3 (DcR3)
Pathophysiology and prognosis
DR 3, also known as Fas, is a protein encoded by the FAS gene in humans. This receptor is a cell death receptor that causes programmed cell death (apoptosis). Its soluble form can neutralize the biological functions of three members of the tumour necrosis factor superfamily (TNFSF): Fas ligand (FasL), LIGHT, and TL1A, which is a direct predictor of inflammatory disease progression and cancer metastasis [31]. Thus, this soluble DR indicates disease severity and does not capture this molecule's inherent protective effects on inflammatory mechanisms. Studies in CV disease go along with those with cancer, showing that DcR3 levels are raised in myocardial infarction and associated with the severity of inflammation in this disease [32].
Therapeutic potential
DcR3 may have the potential to be a therapeutic target for various diseases, including cancer and autoimmune disorders. Some studies have explored the use of DcR3 as a drug target in preclinical models, with promising results in terms of modulating immune responses and reducing inflammation [5]. However, to date, no studies are testing this DR for the prevention and/or treatment of cardiovascular and renal diseases.
Decoy receptor for Interleukin-17 (IL-17)
The interleukin-17 (IL-17) family, consisting of six members, plays a crucial role in inflammation, immunity, and disease progression. IL-17 involves various biological functions, including inflammatory responses during infections and autoimmune diseases and enhancing protective immunity against pathogens. Despite its importance, the exact mechanisms by which IL-17 operates are still debated [33].
Pathophysiology and prediction
DRs for IL17 can help regulate the immune response and reduce inflammation in conditions such as autoimmune diseases (e.g. rheumatoid arthritis, psoriasis), inflammatory bowel disease, and certain types of cancer. By binding to IL17 and preventing it from signalling through its regular receptors, DRs can help dampen the inflammatory response and alleviate symptoms associated with these conditions. There are still no studies about the prognostic value of these DRs for cardiovascular and renal diseases.
Therapeutic potential
DRs are a therapeutic agent that can bind to specific cytokines and prevent them from interacting with their natural receptors. This can reduce the cytokine's signalling activity, which can be beneficial in diseases where the cytokine is overactive or contributes to pathology. IL-17 (interleukin-17) is a pro-inflammatory cytokine that plays a key role in the immune response, particularly in the defence against fungal and bacterial infections. However, dysregulation of IL-17 signalling is implicated in various inflammatory and autoimmune diseases, such as psoriasis, ankylosing spondylitis, and rheumatoid arthritis.
In theory, DRs for IL-17, such as IL-17RA and IL-17RC, can treat these diseases by binding to IL-17 and preventing it from interacting with its natural receptors, thus inhibiting the IL-17-mediated inflammatory response. Two randomized clinical trials showed that secukinumab, a monoclonal antibody that targets IL-17A, mitigates psoriasis severity [34]. Another IL-17A inhibitor, ixekizumab, improved symptoms of ankylosing spondylitis in patients with this disease in two double-blind randomized trials [35]. While these studies focus on monoclonal antibodies that target IL-17A directly rather than DRs, they illustrate the therapeutic potential of modulating IL-17 activity in treating inflammatory diseases.
G protein-coupled decoy chemokine receptors
These receptors, formerly known as DARC (Duffy antigen receptor for chemokines) and D6, act as ‘sinks’ for circulating chemokines in the body. They bind to chemokines in the bloodstream, preventing them from interacting with their target receptors on immune cells and thus regulating the levels of chemokines in the body [36]. This process helps to control the immune response and inflammation. The role of these DRs in various diseases and conditions, including inflammation, cancer, and infectious diseases, is being actively investigated [37].
CARDIOVASCULAR AND METABOLIC DECOY RECEPTORS
Vascular Endothelial Growth Factor Receptor-1 (VEGFR-1)
Pathophysiology and prognosis
EGFR-1 is a kinase-impaired tyrosine kinase receptor that negatively modulates angiogenesis by acting as a DR of VEGF, a fundamental regulator of both physiological and pathological angiogenesis [38]. The decoy characteristic of VEGFR-1 (also named fms‐like tyrosine kinase 1 [Flt‐1]) is required for normal development and angiogenesis [38]. VEGFR-2 is a co-receptor that interacts with DR VEGFR-1 to modulate the signalling of VEGF and regulate angiogenesis and tumour growth and another crucial factor coordinating angiogenesis with cardiomyocyte growth [39].
The relationship of these DRs with CV events is complex and incompletely understood. Low levels of VEGFR2 are independently associated with CV death and all-cause death in patients with chronic HF [40]. Since VEGF per se probably plays a favourable role in heart failure [41], such an inverse association suggests that low circulating levels of this receptor reflect a reduced representation of the same receptors at the myocardial level [41] rather than antagonism/capture of circulating VEGF. By contrast, high rather than low VEGFR1 and VEGFR2 predict a high risk of death in patients with acute coronary syndromes [42]. This effect, opposite to that registered in heart failure, is attributed to the fact that these DR are synthesized following cardiac ischaemia and mainly reflect an ongoing inflammatory process.
The kidney is a highly vascularized organ, and damage to the microvascular structure in the kidney is implicated in CKD progression. VEGF expression and signalling in the kidney are reduced in some experimental models, like in a model of ADPKD [43], and the administration of VEGF is protective in the same model [44]. However, antagonism of VEGF by its endogenous inhibitor (soluble VEGFR-1/sFlt-1) in preeclampsia and monoclonal antibody against VEGF in cancer patients causes proteinuria and renal dysfunction [44]. VEGF and its receptors are upregulated in other experimental animals and human type 1 and 2 diabetes, and inhibition of VEGF limits functional and structural alterations of the kidney in models of diabetic nephropathy [45], suggesting a deleterious role for VEGF in the pathophysiology of diabetic nephropathy [46]. Furthermore, deleterious effects of VEGF administration have been demonstrated in experimental sepsis and, conversely, a beneficial effect of VEGF-R1 in the same model [47]. Overall, the effects of VEGF and its receptors, both tissue-level receptors and soluble receptors, are model and disease-dependent, and the soluble DR of VEGF have no real potential for application in clinical and epidemiologic studies looking at CV [48] or kidney [49] outcomes in CKD.
Therapeutical potential
Aflibercept (Eylea), a DR for VEGF, is used to treat eye conditions like macular degeneration and diabetic retinopathy [50]. However, no specific intervention studies on CV and kidney diseases have been performed with this or similar drugs. In diabetic patients with macular disease receiving intravitreal anti-VEGF treatment, the eGFR declined during treatment, and the decline was maximal at the high and low extremes of the eGFR distribution in the study population. Patients with low baseline eGFR tended to require dialysis after P anti-VEGF treatment [51]. These findings clearly indicate that antagonizing VEGF with its DR exerts noxious kidney effects.
Soluble receptors of advanced glycosylation end products (sRAGE)
Pathophysiology and prognosis
sRAGE inhibit the binding of AGEs to their receptors, deterring the inflammatory response that generally follows the activation of this receptor. sRAGEs are of utmost interest because circulating levels of this biomarker are inversely associated with coronary heart disease in nondiabetic men [52] as well as with atherosclerosis [53] and left ventricular hypertrophy [54] in CKD patients, thereby providing circumstantial evidence that countering AGEs may translate into beneficial CV effects in patients with coronary heart disease and CKD patients with CV disease. Indeed, these studies indicate that AGEs are noxious to the CV system [55] and, in a way, mimic clinical trials testing compounds that block the noxious effects of AGEs.
Therapeutic potential
A study conducted at two separate research centres found that the short-term administration of an antagonist to sRAGE protected against diabetes in mice [56]. This treatment with sRAGE led to an increase in regulatory T cells (Tregs) within various key areas such as the islets, pancreatic lymph nodes, and spleen, and Tregs ultimately resulted in enhanced insulin expression and function within the islets, thereby contributing to the overall protection against diabetes [56]. These findings suggest that targeting the RAGE receptor with sRAGE could be a potential therapeutic strategy for managing diabetes. Furthermore, sRAGE exerted an anti-atherogenic effect by blocking the activation of the RAGE signalling pathway induced by disturbed blood flow in a mouse model of partial carotid artery ligation [57]. AGEs have various effects on cell damage that can lead to neurological disorders. They interact with RAGE receptors, which activate intracellular signalling and lead to the expression of pro-inflammatory factors and cytokines [58]. This inflammatory cascade is linked to diseases such as Alzheimer's, traumatic brain injury, ALS, diabetic neuropathy, diabetes, and atherosclerosis. Imbalances in gut microbiota and intestinal inflammation are also connected to endothelial dysfunction, disrupted BBB, and the development of neurological diseases [58].
AGE antagonists manifested favourable effects in various experimental models [50]. In mice, sRAGE acts as a scavenger, blocking the corresponding membrane-bound receptor activation and inhibiting cardiac fibroblast differentiation and age-dependent cardiac fibrosis [59].
Inhibiting the interactions between AGE and RAGE with small molecule-based therapeutics can prevent the inflammatory events linked to these interactions, slowing down disease progression. RAGE antagonists such as Azeliragon [60] are being developed for treating neurological diseases like AD, but as of now, there are no FDA-approved therapeutics based on sRAGE or RAGE antagonists.
DRs for Platelet-Derived Growth Factor (PDGF)
Pathophysiology and prognosis
PDGF receptors are cell surface tyrosine kinase receptors that bind to PDGF proteins. There are two main types of PDGF receptor, PDGFR-α and PDGFR-β, which play important roles in cell growth, development, and wound healing [61]. These receptors are often targeted in cancer therapy due to their involvement in cell proliferation and survival [61].
DRs of PDGF play a crucial role in CV disease by modulating the signalling pathways of PDGF and its receptors [61]. In CV diseases such as atherosclerosis and restenosis, the overactivation of PDGF signalling can lead to excessive smooth muscle cell proliferation and migration, contributing to the progression of the disease.
Therapeutic potential
DRs for PDGF, such as soluble forms of PDGFR-α and PDGFR-β, can potentially be used as therapeutic agents in CV disease. By blocking PDGF signalling, DRs may help to inhibit smooth muscle cell proliferation, reduce inflammation, and ultimately improve outcomes in patients with CV conditions. Specific anti-PDGF and anti-PDGFR molecules can also be designed, such as soluble receptors, aptamers, or oligonucleotides, but these potential interventions for the prevention and treatment of CV and renal disease remain to be explored [62].
AN OVERVIEW
Overall, soluble DR are interesting biomarkers because, in some cases, they may help to dissect the causal implication of their ligand in human diseases. However, perhaps except sRAGE, they often show relationships with clinical outcomes difficult to interpret, a phenomenon depending on the counter-regulatory increase of their synthesis in high-risk conditions and, at least for some of these, on their pleiotropic effect that goes beyond their ‘decoy’ action.
Historically, observational epidemiology has only been able to infer causality. However, biological markers such as some DRs may allow a more refined view of the exposure-disease continuum and help the design of new drugs.
PERSPECTIVE
Exploring inflammatory DRs, including IL-1R2 and sTNFRs, highlights their significance as biomarkers and modulators in disease progression. Their expression in myocardial infarction and renal diseases underscores the complexity of their role in inflammation and tissue injury. These receptors exhibit a nuanced relationship with clinical outcomes, suggesting their potential as targets for therapeutic intervention.
In CV and metabolic disorders, DRs such as VEGFR-1 and sRAGE command attention due to their impact on disease pathogenesis. The dichotomy of their effects in heart failure, acute coronary syndromes, and CKD emphasizes the context-dependent nature of their function. These findings indicate the necessity for precise interpretation of DR levels in various disease settings.
In conclusion, DR represent a unique class of biomarkers that can neutralize ligands and modulate signalling pathways, offering valuable insights into disease processes and potential therapeutic targets. While the relationships between DR and clinical outcomes can be complex, further research and understanding of their intricate roles will contribute to improved disease prognostication and the development of targeted interventions. Harnessing the full potential of DR as biomarkers holds promise for advancing precision medicine and improving patient outcomes.
Contributor Information
Carmine Zoccali, Renal Research Institute, NY, USA; Institute of Molecular Biology and Genetics (Biogem), Ariano Irpino, Italy; Associazione Ipertensione Nefrologia Trapianto Renale (IPNET), c/o Nefrologia, Grande Ospedale Metropolitano, Reggio Calabria, Italy.
Giovanni Tripepi, CNR-IFC, Institute of Clinical Physiology, Research Unit of Clinical Epidemiology, Reggio Calabria, Italy.
Vianda Stel, ERA Registry, Amsterdam UMC location and the University of Amsterdam, Department of Medical Informatics, Amsterdam, the Netherlands; Amsterdam Public Health, Quality of Care, Amsterdam, the Netherlands.
Edouard L Fu, Fresenius Medical Care, Global Medical Office, Crema, Italy.
Francesca Mallamaci, CNR-IFC, Institute of Clinical Physiology, Research Unit of Clinical Epidemiology, Reggio Calabria, Italy; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, the Netherlands.
Friedo Dekker, Fresenius Medical Care, Global Medical Office, Crema, Italy.
Kitty J Jager, Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, the Netherlands; Nephrology, Dialysis and Transplantation Unit, Azienda Ospedaliera “Bianchi-Melacrino-Morelli” Grande Ospedale Metropolitano of Reggio Calabria, Italy.
CONFLICT OF INTEREST STATEMENT
C.Z. and F.M. are members of the Clinical Kidney Journal Editorial Board.
REFERENCES
- 1. Mantovani A, Locati M, Vecchi Aet al. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol 2001;22:328–36. https://linkinghub.elsevier.com/retrieve/pii/S147149060101941X [DOI] [PubMed] [Google Scholar]
- 2. Kefaloyianni E. Soluble forms of cytokine and growth factor receptors: mechanisms of generation and modes of action in the regulation of local and systemic inflammation. FEBS Lett 2022;596:589–606. https://febs.onlinelibrary.wiley.com/doi/10.1002/1873-3468.14305 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 3. Julier Z, Park AJ, Briquez PSet al. Promoting tissue regeneration by modulating the immune system. Acta Biomater 2017;53:13–28. https://linkinghub.elsevier.com/retrieve/pii/S1742706117300661 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 4. Gershoni JM. Molecular decoys: antidotes, therapeutics and immunomodulators. Curr Opin Biotechnol 2008;19:644–51. https://linkinghub.elsevier.com/retrieve/pii/S0958166908001304 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsieh S-L, Lin W-W. Decoy receptor 3: an endogenous immunomodulator in cancer growth and inflammatory reactions. J Biomed Sci 2017;24:39. http://www.ncbi.nlm.nih.gov/pubmed/28629361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters VA, Joesting JJ, Freund GG. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav Immun 2013;32:1–8. https://linkinghub.elsevier.com/retrieve/pii/S0889159112004928 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Orrem HL, Shetelig C, Ueland Tet al. Soluble IL-1 receptor 2 is associated with left ventricular remodelling in patients with ST-elevation myocardial infarction. Int J Cardiol 2018;268:187–92. https://linkinghub.elsevier.com/retrieve/pii/S0167527318310660 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 8. Ridker PM, Everett BM, Thuren Tet al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. New Engl J Med 2017;377:1119–31. http://www.nejm.org/doi/10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM, MacFadyen JG, Thuren Tet al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390:1833–42. https://pubmed.ncbi.nlm.nih.gov/28855077/ [DOI] [PubMed] [Google Scholar]
- 10. Shiratori-Aso S, Nakazawa D, Nishio Set al. Soluble interleukin-2 receptor predicts treatment outcome in patients with autoimmune Tubulointerstitial nephritis. A preliminary study. Front Med 2022;9:827388. https://www.frontiersin.org/articles/10.3389/fmed.2022.827388/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lundberg S, Lundahl J, Gunnarsson Iet al. Soluble interleukin-2 receptor alfa predicts renal outcome in IgA nephropathy. Nephrol Dialysis Transplant 2012; 27:1916–23. http://www.ncbi.nlm.nih.gov/pubmed/21940483 [DOI] [PubMed] [Google Scholar]
- 12. Delpech C, Laborne FX, Hilliquin P.. Comparison of biological agent monotherapy and associations including disease-modifying antirheumatic drugs for rheumatoid arthritis: literature review and meta-analysis of randomized trials. J Clin Med 2022;12. https://pubmed.ncbi.nlm.nih.gov/36615086/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahfooz K, Rana A, Palagati Ket al. Anakinra in heart failure: a systematic review and meta-analysis of randomized controlled trials. Med Sci 2022;11:4. https://www.mdpi.com/2076-3271/11/1/4 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dember LM, Hung A, Mehrotra Ret al. A randomized controlled pilot trial of anakinra for hemodialysis inflammation. Kidney Int 2022; 102:1178–87. http://www.ncbi.nlm.nih.gov/pubmed/35863559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gough P, Myles IA.. Tumor necrosis factor receptors: pleiotropic signaling complexes and their differential effects. Front Immunol 2020;11:585880. https://www.frontiersin.org/articles/10.3389/fimmu.2020.585880/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hasegawa G, Nakano J, Sawada Met al. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int 1991;40:1007–12. 10.1038/ki.1991.308 [DOI] [PubMed] [Google Scholar]
- 17. Gohda T, Niewczas MA, Ficociello LHet al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 2012; 23:516–24. https://journals.lww.com/jasn/Fulltext/2012/03000/Circulating_TNF_Receptors_1_and_2_Predict_Stage_3.20.aspx (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paccalet A, Crola Da Silva C, Mechtouff Let al. Serum soluble tumor necrosis factor receptors 1 and 2 are early prognosis markers after ST-segment elevation myocardial infarction. Front Pharmacol 2021; 12:656928. https://www.frontiersin.org/articles/10.3389/fphar.2021.656928/full (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu R, Yuan T, Wang Het al. Efficacy, safety and immunogenicity of etanercept biosimilars versus reference biologics in patients with rheumatoid arthritis: a meta-analysis. Front Pharmacol 2023;14. https://www.frontiersin.org/articles/10.3389/fphar.2023.1089272/full (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiley SR, Schooley K, Smolak PJet al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995; 3:673–82. http://www.ncbi.nlm.nih.gov/pubmed/8777713 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 21. Jong KXJ, Mohamed EHM, Ibrahim ZA.. Escaping cell death via TRAIL decoy receptors: a systematic review of their roles and expressions in colorectal cancer. Apoptosis 2022;27:787–99. https://link.springer.com/10.1007/s10495-022-01774-5 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 22. Grisanti LA. TRAIL and its receptors in cardiac diseases. Front Physiol 2023; 14. /pmc/articles/PMC10445540 / (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kakareko K, Rydzewska-Rosołowska A, Zbroch Eet al. TRAIL and cardiovascular disease—a risk factor or risk marker: a systematic review. J Clin Med 2021;10:1252. https://www.mdpi.com/2077-0383/10/6/1252 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arcidiacono MV, Rimondi E, Maietti Eet al. Relationship between low levels of circulating TRAIL and atheromatosis progression in patients with chronic kidney disease. PLoS ONE 2018;13:e0203716. https://dx.plos.org/10.1371/journal.pone.0203716 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Volpato S, Ferrucci L, Secchiero Pet al. Association of tumor necrosis factor-related apoptosis-inducing ligand with total and cardiovascular mortality in older adults. Atherosclerosis 2011; 215:452–8. https://pubmed.ncbi.nlm.nih.gov/21122855/ (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanaka T, Narazaki M, Kishimoto T.. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect Biol 2014; 6:a016295. http://www.ncbi.nlm.nih.gov/pubmed/25190079 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moreno Velásquez I, Golabkesh Z, Källberg Het al. Circulating levels of interleukin 6 soluble receptor and its natural antagonist, sgp130, and the risk of myocardial infarction. Atherosclerosis 2015;240:477–81. 10.1016/j.atherosclerosis.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 28. Ziegler L, Gajulapuri A, Frumento Pet al. Interleukin 6 trans-signalling and risk of future cardiovascular events. Cardiovasc Res 2019; 115:213–21. https://academic.oup.com/cardiovascres/article/115/1/213/5057037 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 29. Su H, Lei CT, Zhang C.. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front Immunol 2017; 8. /pmc/articles/PMC5399081 / (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuan Q, Tang B, Zhang C.. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transd Targeted Therapy 2022 7:1 2022; 7:1–27. https://www.nature.com/articles/s41392-022-01036-5 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bien E, Balcerska A.. Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: a review. Biomarkers 2008; 13:1–26. http://www.ncbi.nlm.nih.gov/pubmed/17906988 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 32. Doustkami H, Avesta L, Babapour Bet al. Correlation of serum decoy receptor 3 and interleukin-6 with severity of coronary artery diseases in male acute myocardial infarction patients. Acta Biomed 2021;92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mills KHG. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol 2023;23:38–54. https://www.nature.com/articles/s41577-022-00746-9 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Langley RG, Elewski BE, Lebwohl Met al. Secukinumab in plaque psoriasis—results of two phase 3 trials. New Engl J Med 2014;371:326–38. http://www.nejm.org/doi/10.1056/NEJMoa1314258 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 35. Baeten D, Sieper J, Braun Jet al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. New Engl J Medicine 2015;373:2534–48. http://www.nejm.org/doi/10.1056/NEJMoa1505066 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 36. Bonecchi R, Savino B, Borroni EMet al. Chemokine decoy receptors: structure-function and biological properties. Curr Top Microbiol Immunol 2010;341. [DOI] [PubMed] [Google Scholar]
- 37. Jang W, Lu S, Xu Xet al. The role of G protein conformation in receptor–G protein selectivity. Nat Chem Biol 2023;19:687–94. https://www.nature.com/articles/s41589-022-01231-z (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferrara N, Davis-Smyth T.. The biology of vascular endothelial growth factor. Endocrine Rev 1997; 18:4–25. http://www.ncbi.nlm.nih.gov/pubmed/9034784 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 39. Stevens M, Oltean S.. Modulation of receptor tyrosine kinase activity through alternative splicing of ligands and receptors in the VEGF-A/VEGFR axis. Cells 2019;8:288. https://www.mdpi.com/2073-4409/8/4/288 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iguchi M, Wada H, Shinozaki Tet al. Soluble vascular endothelial growth factor receptor 2 and prognosis in patients with chronic heart failure. ESC Heart Failure. 2021; 8:4187–98. https://onlinelibrary.wiley.com/doi/10.1002/ehf2.13555 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taimeh Z, Loughran J, Birks EJet al. Vascular endothelial growth factor in heart failure. Nat Rev Cardiol 2013; 10:519–30. http://www.ncbi.nlm.nih.gov/pubmed/23856679 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 42. Marks ECA, Wilkinson TM, Frampton CMet al. Plasma levels of soluble VEGF receptor isoforms, circulating pterins and VEGF system SNPs as prognostic biomarkers in patients with acute coronary syndromes. BMC Cardiovasc Disorders 2018;18:169. https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-018-0894-1 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang JL, Woolf AS, Kolatsi-Joannou Met al. Vascular endothelial growth factor C for polycystic kidney diseases. J Am Soc Nephrol 2016;27:69–77. https://journals.lww.com/00001751-201601000-00013 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doi K, Noiri E, Fujita T.. Role of vascular endothelial growth factor in kidney disease. Current Vasc Pharmac 2010; 8:122–8. http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1570-1611&volume=8&issue=1&spage=122 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 45. Falkevall A, Mehlem A, Palombo Iet al. Reducing VEGF-B signaling ameliorates renal lipotoxicity and protects against diabetic kidney disease. Cell Metab 2017; 25:713–26. http://www.ncbi.nlm.nih.gov/pubmed/28190774 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 46. Schrijvers BF, Flyvbjerg A, De Vriese AS.. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int 2004;65:2003–17. https://linkinghub.elsevier.com/retrieve/pii/S0085253815499466 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 47. Yano K, Liaw PC, Mullington JMet al. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med 2006;203:1447–58. https://rupress.org/jem/article/203/6/1447/54107/Vascular-endothelial-growth-factor-is-an-important (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wada H, Shinozaki T, Suzuki Met al. Impact of chronic kidney disease on the associations of cardiovascular biomarkers with adverse outcomes in patients with suspected or known coronary artery disease: the EXCEED-J study. J Am Heart Assoc 2022;11. https://www.ahajournals.org/doi/10.1161/JAHA.121.023464 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anderson CE, Hamm LL, Batuman Get al. The association of angiogenic factors and chronic kidney disease. BMC Nephrol 2018; 19:117. https://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-018-0909-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anguita R, Tasiopoulou A, Shahid Set al. A review of Aflibercept treatment for macular disease. Ophthalmol Ther 2021; 10:413. /pmc/articles/PMC8319283/. https://doi.org/10.1007/s40123-021-00354-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fang Y-C, Lai IP, Lai T-Tet al. Long-term change in renal function after intravitreal anti-VEGF treatment for diabetic macular edema: a 2-year retrospective cohort study. Ophthalmol Ther 2023;12:2977–88. https://link.springer.com/10.1007/s40123-023-00771-4 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Falcone C, Emanuele E, D'Angelo Aet al. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arteriosclerosis Thrombosis Vasc Biol 2005; 25:1032–7. http://www.ncbi.nlm.nih.gov/pubmed/15731496 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 53. Basta G, Leonardis D, Mallamaci Fet al. Circulating soluble receptor of advanced glycation end product inversely correlates with atherosclerosis in patients with chronic kidney disease. Kidney Int 2010; 77:225–31. https://linkinghub.elsevier.com/retrieve/pii/S008525381554221X (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 54. Leonardis D, Basta G, Mallamaci Fet al. Circulating soluble receptor for advanced glycation end product (sRAGE) and left ventricular hypertrophy in patients with chronic kidney disease (CKD). Nutr Metab Cardiovasc Dis 2012;22. 10.1016/j.numecd.2010.11.008 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 55. Yoshida N, Okumura K, Aso Y.. High serum pentosidine concentrations are associated with increased arterial stiffness and thickness in patients with type 2 diabetes. Metabolism Clin Exp 2005; 54:345–50. http://www.ncbi.nlm.nih.gov/pubmed/15736112 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 56. Leung SS, Borg DJ, McCarthy DAet al. Soluble RAGE prevents type 1 diabetes expanding functional regulatory T cells. Diabetes 2022;71:1994–2008. https://diabetesjournals.org/diabetes/article/71/9/1994/147090/Soluble-RAGE-Prevents-Type-1-Diabetes-Expanding (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ha CH, Kim S, Chung Jet al. Inhibitory effect of soluble RAGE in disturbed flow-induced atherogenesis. Int J Mol Med 2013;32:373–80. https://www.spandidos-publications.com/10.3892/ijmm.2013.1393 (22 May 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 58. Reddy VP, Aryal P, Darkwah EK.. Advanced glycation end products in health and disease. Microorganisms 2022; 10:1848. https://www.mdpi.com/2076-2607/10/9/1848 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Scavello F, Zeni F, Milano Get al. Soluble receptor for advanced glycation end-products regulates age-associated cardiac fibrosis. Int J Biol Sci 2021; 17:2399–416.https://www.ijbs.com/v17p2399.htm (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Burstein AH, Sabbagh M, Andrews Ret al. Development of Azeliragon, an oral small molecule antagonist of the receptor for advanced glycation endproducts, for the potential slowing of loss of cognition in mild Alzheimer's disease. J Prev Alzheimers Dis 2018; :1–6.https://link.springer.com/article/10.14283/jpad.2018.18 (22 May 2024, date last accessed). [DOI] [PubMed]
- 61. Kazlauskas A. PDGFs and their receptors. Gene 2017; 614:1–7.https://linkinghub.elsevier.com/retrieve/pii/S0378111917301385 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Solinc J, Ribot J, Soubrier Fet al. The platelet-derived growth factor pathway in pulmonary arterial hypertension: still an interesting target? Life 2022; 12:658. http://www.ncbi.nlm.nih.gov/pubmed/35629326 (22 May 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]