Abstract
Background
Research on microplastics has largely focused on the environment and marine organisms until recently. A growing body of evidence has detected microplastics in human organs and tissues, with their exact entry routes being unclear and their potential health effects remain unknown. This scoping review aimed to characterise microplastics in human tissues and organs, examine their entry routes and addressing gaps in research analytical techniques.
Methods
Eligibility criteria included English language full text articles, in-vivo human studies only, and searching the databases using pre-defined terms. We based our analysis and reporting on the PRISMA guideline and examined the quality of evidence using the risk of bias assessment tool.
Results
Of 3616 articles screened, 223 evaluated and 26 were eventually included in this review. Nine were high risk for bias, three were unclear risk and the rest low risk for bias. Microplastics were detected in 8/12 human organ systems including cardiovascular, digestive, endocrine, integumentary, lymphatic, respiratory, reproductive and urinary. Microplastics were also observed in other human biological samples such as breastmilk, meconium, semen, stool, sputum and urine. Microplastics can be characterised based on shape, colours, and polymer type. Potential entry routes into human included atmospheric inhalation and ingestion through food and water. The extraction techniques for analysis of microplastics in human tissues vary significantly, each offering distinct advantages and limitations.
Conclusions
Microplastics are commonly detected in human tissues and organs, with distinct characteristics and entry routes, and variable analytical techniques exist.
The global production of plastics in 2020 alone is estimated at 367 million metric tons [1]. Mismanaged plastic wastes may lead to the formation of tiny plastics with the size of less than five mm, called as microplastics, into the environment via wind and water runoff [2]. These plastics can be broken down via weathering processes such as mechanical fragmentation, photo-degradation, thermal degradation, and biodegradation [3,4]. Microplastics are categorised into primary and secondary microplastics [5]. Primary microplastics, such as microbeads in cosmetics and microfibres from synthetic textiles, are intentionally manufactured at small size. Secondary microplastics, in contrast, are the result of the degradation and fragmentation of larger plastic items due to weathering processes. Microplastics are documented widely in aquatic and marine environments [6,7] and can be ingested by marine organisms, including fish [8], mussels [9] and shellfish [10], causing bioaccumulation and biomagnification. While the effects on environment and marine organisms have been extensively studied, similar studies in humans are lacking, with variable characteristics being reported in different studies. The variation in characteristics may be due to gaps and differences in research and analytical techniques [11,12]. Only recently that microplastics are being increasingly detected in various human organs, raising concerns about their health effects. Mechanism for health effects is unclear but microplastics may act as carriers for harmful chemicals and pathogen from the environment into human body. Therefore, this scoping review aimed to characterise microplastics in human tissues and organs, identify their entry routes, and identify gaps in analytical methodology. There may be discussion on potential health effects but these are by no means regarded as definitive due to limitations in current evidence.
METHODS
Study design
This study was formulated following the guidelines provided by the Preferred Reporting Items for Systematic Review and Meta-analysis extension for Scoping Reviews (PRISMA-ScR) (Table S1 in the Online Supplementary Document).
Inclusion and exclusion criteria
Inclusion criteria include English language full text articles focusing on in-vivo human studies published until 2024. Boolean operators such as ‘AND’ and ‘OR’ were employed effectively during the study selection alongside the keywords of ‘microplastics in human’, ‘human organ’, ‘tissues’ and ‘cancer' to prevent data oversaturation and enhance the precision of the retrieved information. Initial screens were conducted using the PubMed and Web of Science databases and detailed search strategy is shown in Appendix S1 in the Online Supplementary Document. Results of searches were exported into Mendeley and duplicates removed through Excel. Exclusion criteria included in vitro human studies or laboratory testing. Commentaries, opinion pieces, reviews, editorials and non-peer-reviewed reports were also excluded.
Data extraction and collection
Three authors (NSR, YSI and LYY) worked on data extraction and initial draft. For each study, information such as type of organ, sample size, abundance, size, shape, colour and polymer composition of microplastics were extracted by the authors. Discrepancies among the authors were resolved through discussion and consensus. If disagreements persisted, a fourth author (STA or LAL) was consulted to make the final decision.
Quality assessment
Risk of bias, methodology quality and reliability were determined using the Risk of Bias (RoB) assessment tool [13]. The tool was based on four domains - study design, sampling, analysis, and reporting (Table S2 in the Online Supplementary Document) [14–16]. The RoB tool also yields three ratings: high risk, low risk, or unclear risk. High risk refers to studies that met the domain criteria but obtained negative result. Low risk indicates studies that thoroughly addressed each domain, while studies that does not elaborate further on any of the domains are categorised as having an unclear risk.
Patient and public involvement
No patient involved.
RESULTS
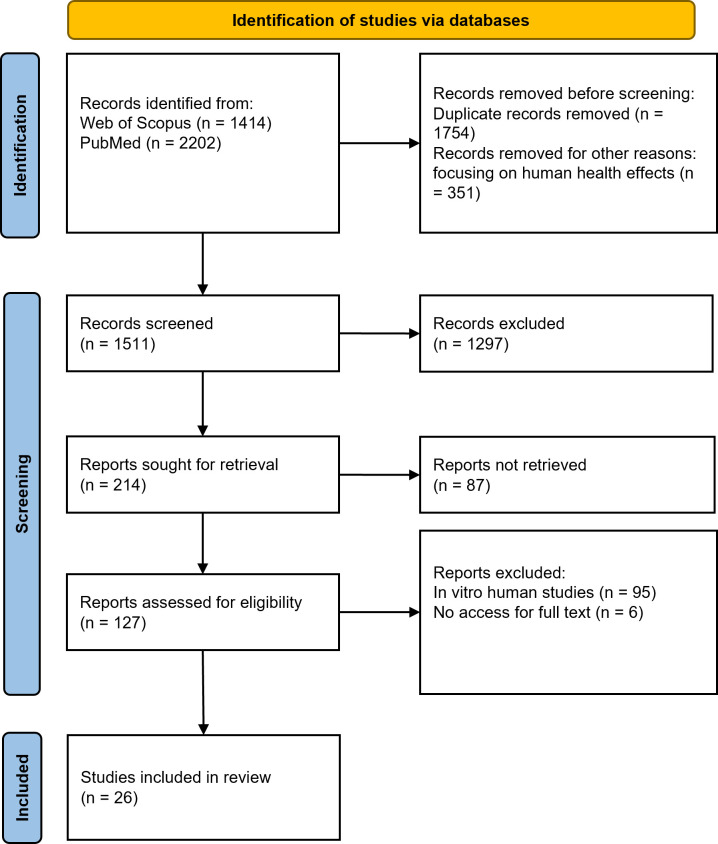
A total of 3616 articles were initially identified. After screening using predetermined criteria, 223 articles were evaluated and 26 were finally included in the study (Figure 1). Our human body is composed of 12 organ systems, and eight of them have evidence of contamination by microplastics (Figure 2). These organ systems are cardiovascular system [17–20], digestive system [21,22], endocrine system [23–26], integumentary system [27], lymphatic system [22], respiratory system [28–30], reproductive system [31], and urinary system [22] (Table 1). In addition to organ systems, microplastics were also reported in other biological human samples such as breastmilk [26,32], meconium [24,26], infant faeces [26], semen [31,33], stool [34–39], sputum [40], and urine [41,42] (Table 2). Microplastics can be further categorised based on origin, morphology, colours, and polymer type (Table 1, Table 2). In addition, we found that atmospheric inhalation and ingestion through food and water were the likely primary routes of entry of microplastics into human body. Furthermore, the extraction methodologies for microplastics in human organs vary significantly, each offering distinct advantages and limitations. These diverse methodologies are comprehensively detailed in Table 3.
Figure 1.
Literature screening flow.
Figure 2.
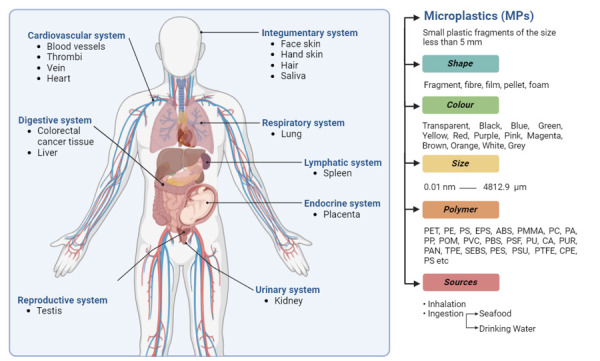
Summary of presence of microplastics in human body systems including their characteristics and possible pathway of microplastics into the body. Schematic representations were generated by BioRender.com. ABS – Acrylonitrile Butadiene Styrene, CA – Cellulose Acetate, CPE – Chlorinated Polyethylene, EPS – Expanded Polystyrene, mm – millimetre, PA – Polyamide, PAN – Polyacrylonitrile, PBS – Phosphate-buffered Saline, PES – Polyethersulfone, PE – Polyethylene, PET – Polyethylene Terephthalate, PC – Polycarbonate, PMMA – Polymethyl Methacrylate, POM – Polyoxymethylene, PP – Polypropylene, PS – Polystyrene, PSF/PSU – Polysulfone, PU/PUR – Polyurethane, PTFE – Polytetrafluoroethylene, PVC – Polyvinyl Chloride, TPE – Thermoplastic Elastomers, SEBS – Styrene-Ethylene-Butylene-Styrene, μm – micrometre.
Table 1.
Abundance of microplastics in human organ systems
| System | Organ | Sample Size | Abundance of microplastics | Size of Microplastics | Shape of Microplastics | Colour of microplastics | Polymer of Microplastics | References |
|---|---|---|---|---|---|---|---|---|
| Cardiovascular system | Blood vessels |
22 |
1.6 ug/mL |
>700 nm |
NA |
NA |
PET, PE, PS, EPS, ABS, PMMA |
[17] |
| Thrombi |
26 |
87 particles |
2.1–26.0 μm |
Block shaped |
Yellow, green, red |
LDPE, Pigment, Chromium Oxide, Phthalocyanine |
[18] |
|
| Vein |
5 |
20 particles or 14.99 ± 17.18 microplastic/g of tissue |
16–1074 μm |
Fragment, fibre |
NA |
Alkyd Resin, Poly(vinyl propionate), Nylon-ethylene-vinyl acetate, nylon-EVA, tie layer |
[19] |
|
|
|
Heart |
15 |
NA |
20–500 μm |
NA |
NA |
PET, PVC, PMMA |
[20] |
| Digestive system | Colorectal cancer tissue |
11 |
331 Microplastics per individual or 28.1–15.4 particles/g tissue |
0.8–1.6 mm |
Fibre |
Transparent, black, red, green, blue, brown, purple, and yellow |
PC, PA, PP |
[21] |
|
|
Liver |
11 |
0–13 particles per sample or 3.2 particles/g tissue |
4–30 μm |
Fragment, microbead |
NA |
PS, PVC, PET, PMMA, POM, PP |
[22] |
| Endocrine system | Placenta |
NA |
12 particles |
>5 μm |
Fragment |
Blue, purple, pink, orange, red |
PP |
[23] |
| Placenta |
|
NA |
>50 μm |
NA |
|
PE, PP, PU |
[24] |
|
| Placenta |
17 |
149 microplastics particles |
20.34–307.24 μm |
Fragment, fibre, film, subspherical particle |
NA |
PVC, PP, PBS, PET, PC, PS, PA, PE, PSF |
[25] |
|
|
|
Placenta |
18 |
NA |
20–500 μm |
NA |
NA |
PU, PA, PE, PET, PC |
[26] |
| Integumentary system | Face skin |
2000 |
4265 microplastics particles |
100–500 μm |
Spheres fragment, film, fibre |
Blue, red, yellow, transparent, black |
PE, PET, PS, PVC |
[27] |
| Hand skin |
2000 |
4051 microplastics particles |
100–500 μm |
Sphere, fragment, film, fibre |
Blue, red, yellow, transparent, black |
PE, PET, PS, PVC |
[27] |
|
| Hair |
2000 |
7462 microplastics particles |
100–500 μm |
Sphere, fragment, film, fibre |
Blue, red, yellow, transparent, black |
PE, PET, PS, PVC |
[27] |
|
|
|
Saliva |
2000 |
645 microplastics particles |
100–500 μm |
Sphere, fragment, film, fibre |
Blue, red, yellow, transparent, black |
PE, PET, PS, PVC |
[27] |
| Lymphatic system |
Spleen |
3 |
4 particles per sample or 1.1 particles/g tissue |
5–25 μm |
Fragment, Microbead |
NA |
PS, PVC, PET, PMMA, POM, PP |
[22] |
| Respiratory system | Lung tissues |
20 |
31 particles |
1.6–16.8 μm |
Fragment, fibre |
Transparent, white, blue, grey, yellow, brown, orange |
PP, PE, Cotton, PVC, CA, PA, PS, PU |
[28] |
| Lung granule nodules |
100 |
65 particles |
>20 μm |
Fibre |
Purple, blue, transparent, yellow, red |
Cotton, PA, Polyester, Denim, Phenoxy resin, |
[29] |
|
|
|
Lung tissue |
13 |
39 particles |
12–2475 μm |
Fibre, fragment, film |
NA |
PP, PET, Resin, PE, PTFE, PS, PAN, PES, PMMA, PUR, SEBS, TPE |
[30] |
| Reproductive system |
Testis |
6 |
31 particles in 4 of 6 testis samples |
20–100 μm |
Fragment, fibre, film, subspherical |
NA |
PS, PVC, PE, PP |
[31] |
| Urinary system | Kidney | 3 | 0 particle per sample | 10–20 μm | NA | NA | NA | [22] |
ABS – Acrylonitrile Butadiene Styrene, CA – Cellulose Acetate, CPE – Chlorinated Polyethylene, EPS – Expanded Polystyrene, EVA – Ethylene-Vinyl Acetate, HDPE – High-Density Polyethylene, LDPE – Low Density Polyethylene, NA – not available, NC – Nitrocellulose, mm – millimetre, PA – Polyamide, PAN – Polyacrylonitrile, PBS – Phosphate-buffered Saline, PBT – Persistent Bioaccumulative Toxic, PES – Polyethersulfone, PET – Polyethylene Terephthalate, PEMA – Phenylethylmalonamide, PC – Polycarbonate, PLA – Polylactic acid, PMMA – Polymethyl methacrylate, POM – Polyoxymethylene, PP – Polypropylene, PS – Polystyrene, PSF/PSU – Polysulfone, PU/PUR – Polyurethane, PTFE – Polytetrafluoroethylene, PVC – Polyvinyl Chloride, PVOH – Polyvinyl Alcohol, TPE – Thermoplastic Elastomere, SEBS – Styrene-Ethylene-Butylene-Styrene, μm – micrometre, μg/mL – microgram per millilitre
Table 2.
Abundance of microplastics in human biological samples
| Type of sample | Sample Size | Abundance of microplastics | Size of microplastics | Shape of microplastics | Colour of microplastics | Polymer of microplastics | References |
|---|---|---|---|---|---|---|---|
| Breastmilk | 7 |
20.2 particles/g |
>20 μm |
NA |
NA |
PA, PU, PE, PET, PP, PVC, POM, EVA, PTFE, CPE, Polybutadiene, PS, PMMA, PLA, Polysulfones |
[26] |
|
|
34 |
58 particles in total |
1–12 μm |
Fragment, sphere |
Orange, blue, black, red, grey, brown, green, transparent, magenta |
PE, PVC, PP, CPE, PVOH, PEVA, PEMA, ABS, PES, PA, PC, PS, NC |
[32] |
| Meconium | 2 |
NA |
>50 μm |
NA |
NA |
PE, PP, PS |
[24] |
|
|
12 |
54.1 particles/g |
>20 μm |
NA |
NA |
PA, PU, PE, PET, PP, PVC, POM, EVA, PTFE, CPE, PS, PMMA, PLA, Polysulfones |
[26] |
| Infant faeces |
12 |
26.6 particles/g |
>20 μm |
NA |
NA |
PA, PU, PE, PET, PP, PVC, POM, EVA, PTFE, CPE, Polybutadiene, PS, PMMA, PLA, Polysulfones |
[26] |
| Semen | 25 semen samples |
24 microplastics in 11 of 25 semen samples (0.23 ± 0.45 particles/mL) |
21.76–286.71 μm |
Fibre, fragment, subspherical, film |
NA |
PVC, PE, PA, PP, PS, PET |
[31] |
|
|
10 healthy young men |
16 microplastics in 6 of 10 semen samples |
2–5 μm |
Fragment, sphere |
Green, black, grey, orange, clear, yellow, blue, magenta |
PP, PS, PET, PVS, PC, POM, Arcylic |
[33] |
| Stool | 8 healthy young men |
9 particles in total |
50–500 μm |
Fragment, film |
NA |
PP, PET, PS, PE, POM, PC, PA, PVC, PU |
[34] |
| 8 participants |
129 particles (20.4–138.9 particles/g) |
40.2–4812.9 μm |
Fragment, fibre |
NA |
PS, PP, PE, PET, PVC |
[35] |
|
| 50 of healthy adult |
3070 particles (28 items/g) |
4.4–333.2 μm |
Sheet, fibre fragment, pellet |
NA |
PET, PA, PP, PE, PC, PVC, POM, PTFE, EVA, PS, PMMA, PBT, AS, PET, TPU |
[36] |
|
| 52 of inflammatory bowel disease patients |
5459 particles (41.8 items/g) |
1.7–393.8 μm |
Sheet, fibre, fragment, pellet |
NA |
PET, PA, PP, PE, PC, PVC, POM, PTFE, EVA, PS, PMMA, PBT, AS, PET, TPU |
[36] |
|
| 11 of coastal fishermen population |
3.33–13.99 μg/g |
<5 mm |
NA |
NA |
HDPE, LDPE, LLDPE, PP, PS, PET |
[37] |
|
| 11 of rural farming community |
6.94–16.55 μg/g |
<5 mm |
NA |
NA |
PET, PS, PP, PE, HDPE, LDPE |
[38] |
|
|
|
26 young male students |
1–36 particles/g |
20–800 μm |
NA |
NA |
PP, PET, PS, PE, PVC, PC, PA, PU |
[39] |
| Sputum |
22 |
18.75 − 91.75 particles/10mL |
20–500 μm |
NA |
NA |
PU, PES, Chlorinated polyethylene, alkyd varnish |
[40] |
| Urine | 6 |
7 particles in total |
4–15 μm |
Fragment, sphere |
Transparent, brown, blue, green, red |
PVA, PVC, PP, PE |
[41] |
| 9 | 98 particles in total | 0.01 nm–871 μm | Fibre, fragment | Black | PP, PA | [42] |
ABS – Acrylonitrile Butadiene Styrene, CA – Cellulose Acetate, CPE – Chlorinated Polyethylene, EPS – Expanded Polystyrene, EVA – Ethylene-Vinyl Acetate, HDPE – High-Density Polyethylene, LDPE – Low Density Polyethylene, NA – not available, NC – Nitrocellulose, mm – millimetre, PA – Polyamide, PAN – Polyacrylonitrile, PBS – Phosphate-buffered Saline, PBT – Persistent Bioaccumulative Toxic, PES – Polyethersulfone, PET – Polyethylene Terephthalate, PEMA – Phenylethylmalonamide, PC – Polycarbonate, PLA – Polylactic acid, PMMA – Polymethyl Methacrylate, POM – Polyoxymethylene, PP – Polypropylene, PS – Polystyrene, PSF/PSU – Polysulfone, PU/PUR – Polyurethane, PTFE – Polytetrafluoroethylene, PVC – Polyvinyl Chloride, PVOH – Polyvinyl Alcohol, TPE – Thermoplastic Elastomers, SEBS – Styrene-Ethylene-Butylene-Styrene, μm – micrometre, μg/g – microgram per gram
Table 3.
Summary of advantages and limitations of methodology applied in the sample matrices
| Laboratory equipment and instrumentation | Type of sample | Advantages | Limitations |
|---|---|---|---|
|
Sample pre-treatment
|
|
|
|
| 10–30% KOH |
Blood thrombi [18], heart [20], colectomy tissues [21], placenta [22], breastmilk [32], semen [33], stool [34,35], urine [41] |
Cheap and effective that allows the isolation of microplastics from the sample. Efficiency of KOH may increase when incorporated with higher temperature at 60–70°C |
Higher percentage may influence the degradation of microplastics. Time-consuming |
| 10% KOH + CHKO2 |
Placenta [25] |
CHKO2 increased the efficiency of the digestion process |
Newly developed method is considered risky to use due to a lack of substantial supporting studies |
| 10M KOH + sodium hypochlorite |
Liver, kidney, spleen [22] |
Sodium hypochlorite acts as a catalyst in increasing the efficiency of the digestion process |
Sodium hypochlorite is expensive |
| 30% H2O2 |
Vein [19], lung ground nodules [29], lung tissue [30], stool [39] |
Readily available and relatively inexpensive. Effectively digest organic matter |
Requires PPE as H2O2 is a strong oxidising agent. H2O2 may lead to formation of by-products that can interfere with the analysis of microplastics |
| 30% H2O2 + 0.05M NaOH |
Placenta [24], meconium [24] |
Readily available and relatively inexpensive. Effectively digest organic matter. NaOH is cheap and can enhance the efficiency of the digestion process. |
Higher percentage may influence the degradation of microplastics |
| 30% H2O2 + 0.05M Fenton reagent |
Urine [42] |
Effectively digest organic matter. Fenton reagent acts as a catalyst |
Expensive reagent |
| 35% H2O2 + ZnCl2 |
Hand, hair, faces [27] |
Effectively digest organic matter |
ZnCl2 is highly toxic to the environment |
| HNO3 |
Placenta, infant faeces, meconium [26], stool [37,38] |
Effectively digest organic matter |
Highly corrosive. Can be hazardous to handle. |
| ZnCl2 |
Sputum [40] |
Efficiency of ZnCl2 remains above 95% after five filtrations. Can be reused |
Highly corrosive. Can be hazardous to handle. Highly toxic to the environment. |
| TRIS HCl buffer |
Blood [17] |
Works in denaturing proteins for blood sample |
Newly developed method is considered risky to use due to a lack of substantial supporting studies |
| 0.05% SDS solution +5 mM CaCl2 + 1 M TRIS HCl |
Testis [31], semen [31] |
SDS (sodium dodecyl sulfate) is a surfactant that can solubilise proteins and lipids, and it can also solubilise microplastics. The addition of CaCl2 can enhance the efficiency of the digestion process. |
Newly developed method is considered risky to use due to a lack of substantial supporting studies. |
| NaOH + HNO3 + Protease |
Lung tissues [28] |
The addition of HNO3 and protease can enhance the efficiency of the digestion process |
Newly developed method is considered risky to use due to a lack of substantial supporting studies |
|
Physical characterisation
| |||
| Microscopic observation |
Blood [17], thrombi [18], vein [19], colectomy [21], placenta [23,25,26], meconium [24], infant faeces [26], breastmilk [26,32], hand, hair, faces [27], lung ground nodules [29], lung tissue [30], testis [31], semen [31,33], stool [35,39], urine [41,42] |
Obtain clear view of microplastic particles including their shape, size and colour. Easy to use. Non-destructive |
Unable to detect the polymer type of microplastic. Prone to significant human error. Labour intensive. |
| Nile Red fluorescence microscopy |
Liver, kidney, spleen [22] |
Rapidly estimate microplastic count under the microscope. Easy to use. |
Does not specify polymer composition of microplastics. Staining can conceal the original colour and surface morphology of microplastics. |
| SEM-EDX |
Colectomy [21], lung ground nodules [29] |
Able to observe any adherence of foreign particles on the microplastic sample. High resolution imaging machine that can provide detailed images of microplastics |
Destructive to the sample. Time consuming and expensive. |
|
Chemical characterisation
| |||
| Raman/μRaman |
Thrombi [8], liver, kidney, spleen [22], placenta [23], hand, hair, faces [27], lung tissues [28], lung ground nodules [29], breastmilk [32], semen [33], stool [35,37,38], urine [41,42] |
Offer precise and reliable results. Non-destructive to the microplastic particles. |
Requires meticulous sample preparation. Prolonged processing time. |
| FTIR/μFTIR |
Vein [19], colectomy tissues [21], placenta [24], meconium [24], lung ground, nodules [29], lung tissue [30], stool [34,39], sputum [40], urine [42] |
Common method for analysing microplastic polymers. Offer precise and reliable results. Can detect up to 10 μm in size (for μFTIR). |
Can be affected by the presence of other materials adhered on the microplastic particles. ATR-FTIR may be destructive to the surface morphology of the sample |
| Py-GC/MS |
Blood [17], testis [31], semen [31] |
Utilises various types of microplastic polymers. Offer both accuracy and high sensitivity in obtaining results. Efficient and effective approach for analysis. |
Prolonged processing times. Requires high count of microplastics particles especially fibre shaped due to their low weight. |
| LDIR | Placenta [25,26], infant faeces [26], breastmilk [26] | Can detect up to 10 μm in size. High automation and integration | Extensive sample pre-treatment. |
ATR-FTIR – Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy, CaCl2 – Calcium Chloride, CHKO2 – Potassium Formate, HNO3 – Nitric Acid, H2O2 – Hydrogen peroxide, KOH – Potassium Hydroxide, LD-IR – Laser Direct Infrared Spectrometry, NaOH – Sodium Hydroxide, M – molar, TRIS HCl – Tris (Hydroxymethyl) Aminomethane, mM – millimolar, PPE – Personal Protective Equipment, PY-GC/MS – Pyrolysis–Gas Chromatography Tandem Mass Spectrometry, SDS – Solution Sodium Dodecyl Sulfate Solution, SEM-EDX – Scanning Electron Microscopy/Energy Dispersive Spectroscopy, ZnCl2 – Zinc Chloride, μFTIR – microFourier Transform Infrared Spectrometry, μm – micrometre, μRaman – microRaman
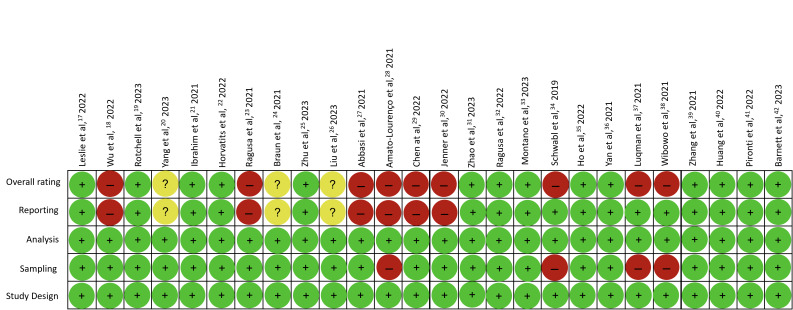
The Quality of studies
RoB assessment is presented in Figure 3. Nine studies were deemed to have high risk [18,23,27-30,34,37,38], while three were of unclear risk [20,24,26], with the remaining being low risk [17,19,21,22,25,31-33,35,36,39-42]. Studies with low risk of bias in the study design reported clear and comprehensive methodologies to identify and quantify microplastics. Four studies [28,34,37,38] have high RoB in the sampling domain due to absence of quality control measures when handling microplastics which may cause contamination from atmospheric microplastics. Additionally, six studies [18,23,27-30] have high RoB and three studies [20,24,26] with unclear risk in the reporting domain, as these studies did not report specific concentration of microplastics particles per g of tissue or ml of solution. Additionally, sample size was often mentioned as limitation in all studies.
Figure 3.
Risk of bias (RoB) adopted to this study. The RoB displays the evaluation scores for each of the four domains, as well as the overall rating for each study. A red (−) rating signifies a high risk of bias, a green (+) rating indicates a low risk of bias and a yellow (?) rating indicates an unclear risk of bias.
DISCUSSION
Microplastics in human organ systems
Environmental plastic particles can be ingested, absorbed, digested, and removed (or remained) by the human intestines, as is similarly observed in fishes or other organisms [9,10]. The intrusion of microplastics in the digestive tract could potentially modify the gut microbiota as evidenced from various studies of human organoids [43]. Furthermore, microplastics might cause abrasions, perforations, malnutrition, mechanical injuries and even blockages of the digestive system [44]. Not only that, translocation of microplastics to other digestive organs such as the liver could occur, e.g. 11 particles from 2 cm3 tissue samples were reported in normal and cirrhotic liver, and interestingly more microplastics were reported in liver cirrhosis than in normal liver [22].
While adverse effects are known in marine organisms, but health effects in human are less well-studied. Hence the following discussion on health and diseases associated with microplastic is largely based on in-vitro studies, small pilot human studies and some speculation based on changes found in marine organisms. For example, notable adverse effects of microplastics to the blood vessels have included genotoxicity and cytotoxicity. In an in-vitro study, isolated human peripheral blood lymphocytes were incubated with 10–45 µm (μm) of polyethylene microplastics [45], and it was found that microplastics increased the frequency of micronucleation, nucleoplasm bridge formation and nuclear bud formation in the bloodstream. These effects have been linked to disorders such as infertility, diabetes, obesity, cardiovascular disease (coronary artery disease), chronic renal disease, cancer and neurological diseases (including Alzheimer disease and Parkinson disease) [46]. Microplastics may likely reach placenta via translocation [17], becoming vectors for transporting substances such as metals and chemicals that are endocrine disruptors [47]. They may interrupt the immune mechanisms, maternal-foetal communication, signalling between the embryo and the uterus, and trafficking of uterine dendritic cells, natural killer cells, T cells, and macrophages during a typical pregnancy [48].
Microplastic retrieved from filtered washes of hands and faces, head hairs and saliva [27] might have been attributed to the ubiquity of atmospheric microplastics but also headgears such as veils or caps, other than coming from contaminated saliva [49]. Climatic conditions may also play a role, for example, a greater quantity of microplastics was recorded in the Bushehr area of Iran due to a higher humid climate that promoted adherence of microplastics to hairs and skins [27]. Interestingly, hand skin samples have reportedly lower abundance of microplastics despite being in greater contacts with numerous sources of microplastics, and this was likely because of hand transfer and hand washing.
Microplastics have been reported in human spleen where five particles per sample of three individuals have been found [22], and despite the many vital functions of spleen, it is unclear at the moment if microplastics can cause spleen dysfunction. Alarmingly, studies have shown that microplastics could absorb and accumulate environmental contaminants, and act as vectors of bodily contaminants [49]. As microplastics can circulate in the bloodstream and potentially accumulate in various organs, including the spleen, it is possible that other potential contaminants attached to the microplastics could also be transported to the spleen. Therefore, while further research is needed to better understand the potential for microplastics to transport other pollutants to the spleen, it is possible that microplastics could play a role in the bodily accumulation of toxic chemicals.
Inhalation is the major route of translocation of environmental microplastics into the respiratory tissues [30]. Microfibre are believed to gradually accumulated with age, and the embedded microfibre in lung tissues may account for the formation of ground glass nodules; a lesion associated with chronic lung diseases. Inhaled microplastics could have negative clinical effects on the respiratory system and other organs as well [28]. For example, microfibres may accumulate in terminal bronchioles, alveolar ducts and alveoli which may eventually lead to formation of granulomas, fibrosis and chronic inflammation [50].
A recent pilot study has also reported the pollution of microplastics in human male reproductive system [31]. The exposure to microplastics may possibly cause male reproductive dysfunction as seen in mice experimented to continual contact with polystyrene which leads to a decrease in serum testosterone levels and a deterioration in sperm quality [51,52]. Even worse, a related study has unveiled nanoplastics possess more pronounced adverse effect compared to microplastics, and this includes their capability in contributing to male infertility [53].
Kidneys are particularly susceptible to water pollutants and considering that drinking water being a major source of microplastics. Advantageously, no contamination of microplastics were observed in the three samples of kidneys obtained from three healthy patients. Nonetheless, there are likely detrimental health effects of microplastics on the human kidneys, and further research is needed. Based on a study by Wang et al, exposure of human kidney proximal tubular epithelial cells (HK-2 cells) and male inbred strain mice (C57BL/6) to polystyrene microplastics resulted in mitochondrial dysfunction, endoplasmic reticulum stress, inflammation, and autophagy [54].
Microplastics in human biological samples
Besides human organs, microplastics have been found in other human biological samples such as breastmilk, stool, sputum, or urine (Table 2). The presence of microplastics in these samples is due to passage storage or excretion pathways. Polypropylene, common plastic polymer utilised in various household and personal care products, was identified as the predominant form of microplastics detected in breast milk [32]. The exact mechanism by which the microplastics get into breast milk is not yet fully understood, however, it is possible that individuals, including lactating mothers, may ingest microplastics through foods or water, which are then transported to the mammary gland.
Studies have shown that microplastics is prevalent in breast milk, but, notably, the presence of microplastics in meconium and infant faeces adds another layer of concern, suggesting that exposure to these particles may continue beyond breastfeeding. Meconium, the first stool of newborns is composed of materials ingested by the foetus in the womb such as amniotic fluid and mucus. It is believed that microplastics can penetrate the foetal gut via the placenta, which is the organ that connects the foetus to the mother's womb. Since microplastics have been clearly found in the placenta [23,25] it is hypothesised that they may cross the placenta barrier and enter the foetal bloodstream, ultimately reaching the foetal gut and being excreted in the meconium [24,26]. As newborn’s digestive system matures, their stool transitions from meconium to more typical infant faeces.
For studies of microplastics in stools, dietary consumption including drinking water should be documented. Most studies recorded diet of participants for about a week before collecting their stool samples. Some studies have attempted to correlate polymers found in stools with types of diet, but despite the abundance of polymers found, there was only a moderate correlation. It is unknown if polymers found in colectomy specimens correlated with polymers found in stools. Indirect correlation seems to suggest so, with polypropylene being found in colectomy specimens in our study [24] and also stools from Schwabl et al. [34]. There are likely variations in polymer types found between geographical areas, and besides diet, other consumables may be important in explaining the difference. For example, high-density polyethylene was most common in 11 participants living in the coastal region of Surabaya, Indonesia [37] but polypropylene was commonest found in community living in rural highland village in Indonesia [38]. Besides local staple foods e.g. tempeh in Indonesia, other consumables for e.g. toothpaste and table salts have been linked to microplastics in stools.
In addition to being eliminated through stools, microplastics in the body can be excreted into the urine [41]. The sources of microplastics in urine are not entirely clear, but it is likely that they come from a variety of sources, including food packaging, personal care products and environmental contamination. Other contaminants such as Bisphenol-A (BPA) were also isolated in the human urine through gas chromatography technique coupled with mass spectrometry (GC-MS) processes [55]. The presence of BPA may explain the environmental paths of microplastics into the human urine. BPA is a chemical compound commonly used in production of certain types of plastics, including polycarbonate plastics and epoxy resins [56].
There are growing interests of finding microplastics in other bodily fluids including sputum, which is a mixture of saliva and mucus that is coughed up from the respiratory tract [40]. Interestingly, the levels of microplastics in the sputum correlated with microplastics found in dust, and dust is known to be affected by occupational background. At this moment, it is unclear the potential effects of microplastics in causing damage or inflammation within the respiratory tract.
Physical characteristics of microplastics in human samples
Shape of microplastics in human samples
Microplastics can be found in different shapes depending on their sources and how they are broken down in the environment. For example, fibres or microfibres can be shed from clothing and other textiles during washing, while fragments can result from breakdown of larger plastic items, such as bottles or bags [57]. Microfibres appeared to be more durable than other types of microplastics such as fragments, films, pellets and foams. Additionally, microplastics can take on different shapes and textures as they are exposed to different environmental conditions, such as sunlight, heat, and water [36]. Findings across multiple studies indicate that microfibres could be accumulated at high levels in various human organs [21–23]. They can be as small as a few micrometres in diameter and also lightweight, which allow them to be easily inhaled and ingested by humans. Recent studies have reported excretion of two shapes, i.e. microfibres and sheets (also called, films) in stools of healthy participants and in patients with inflammatory bowel disease [36].
Colour of microplastics in human samples
The colour of microplastics discovered in human tissues can vary depending on a range of factors, such as polymer type and degree of degradation from human biological activities. Examples of human activities may include external actions like washing hair and hands [27] but also internal actions from digestive juices of acid and bile [21]. In general, microplastics found in human tissues tend to be transparent or translucent rather than brightly coloured. Smaller particles are more likely to be transparent or translucent [21], whereas larger particles are more opaque and coloured [27]. Furthermore, many consumer products, such as packaging and personal care items, are often made from transparent or translucent polymers [58]. Nonetheless, some studies have reported finding microplastics with various colours in human tissues, including yellow, blue, green, and red [28,32]. These colours could be attributed to additives or pigments used during the production of plastic products or from environmental factors, such as exposure to UV radiation, which can cause plastics to degrade and to discolour [5]. Overall, while the dominant colours of microplastics found in humans may vary, there is currently no evidence to suggest that the colours of microplastics have any direct effects on human health.
Size of microplastics in human samples
The size of microplastics is a critical factor in their ability to penetrate human tissues, with smaller particles being more likely to do so and potentially causing more harm, due to larger surface area relative to their volume, and thus increasing the potential for interactions with biological molecules. Research has shown that particles smaller than 100 μm can penetrate biological barriers and accumulate in various tissues, including the placenta [23–25]. This finding is consistent with previous observations made in blood clots, where microplastics of 2.1–26.0 μm in length were extracted [18]. Shockingly, these particles are also available in nanoscale level in urine samples, with the size of 0.01 nanometre (nm) to 0.60 μm [42], raising additional concerns about the potential health effects that these minuscule particles may bring along the human organ systems before being eliminated through biological processes. At this tiny size, their behaviour tends to exhibit complex interactions with cellular membranes of which their transport mechanisms are still being studied [59]. Several factors, such as the tissue type and location of exposure, also determine the precise size of microplastics that can penetrate human tissue. For instance, microplastics were seen more abundantly in soft tissues than hard tissues [21,22,28]. Soft tissues are composed of cells and extracellular matrix, and include connective tissue, muscle tissue, nervous tissue, and epithelial tissue. Notably, larger microplastics exceeding 4000 μm have been observed in human stool samples [36].
Polymer of microplastics in human samples
Microplastics have been identified as a potential vector for pollutants and chemicals, facilitating their entry into human tissues. Chemicals could leach either from plastics themselves or from chemicals absorbed from the environment. Studies have shown that microplastics contained a range of toxic chemicals, such as phthalates and BPA [55,60]. These have been linked to various health problems, including cancer, developmental disorders, and reproductive problems [55]. Other studies have shown that polypropylene and polyethylene microplastics can accumulate in various human tissues but are more abundant in digestive tract, placenta and lungs [21,24,29]. The reason for greater abundance of the polymers polyethylene in these human organs compared to others is unknown but these polymers are commonly found in consumer goods, such as food packaging, cosmetics, and textiles [57].
Potential pathway of microplastics into human
Microplastics can enter the body through inhalation and ingestion. Contaminated food, water, and polluted air are common sources of microplastics [61]. Studies have shown that inhalable microplastics particles with a size of less than 10 μm [28] can enter the respiratory system and intrathoracic cavity of humans, with an estimated annual inhalation exposure of 53 700 particles per person [61], assuming an inhalation rate of 15 m3/d [62]. Even though the mucociliary function in the respiratory system can be effective barrier against intruding particles such as microplastics, a small number of microplastics can still persist in the lungs and cause certain bodily reactions [52]. For instance, inflammation could happen due to suspension of pollutants such as polycyclic aromatic hydrocarbons and metals on the hydrophobic surfaces of atmospheric microplastics [63]. Microplastics are ubiquitous in the atmosphere due to their small size and various meteorological factors [64], and their abundance varies across different countries, with megacities in China [65] reporting a higher abundance of microplastics than urban and suburban areas in Indonesia [66]. Exact reasons behind the differences between the two countries are unclear but lack of a standardised sampling method may be a reason.
Being rich in vital nutrients, seafood is essential for human nutrition and global food security [67]. However, microplastics are abundant in seafood. In commercial fish (Atule mate, Crenimugil seheli, Sardinella fimbriata, and Rastrelliger brachysoma) from Malaysia Northwest Peninsular seawater, microplastics were present in 100% of the samples with S. fimbriata has the highest average microplastic abundance at 6.5 ± 4.3 microplastics per organism [9]. Microplastics are also abundant in marine dried fish products that are widely consumed in Asian countries including Taiwan, Japan, Thailand, South Korea and Sri Lanka [68,69].
Microplastics can be present in abundance in drinking water, especially bottled ones. The concentration of microplastics in bottled water varies depending on the country and the brand, with some brands containing high levels of microplastics (>25 μm) [70]. The morphology of microplastics found in bottled water may include fragments and fibres of different lengths [71,72] and the predominant polymers found in bottled water were polyethylene, polystyrene and polyethylene terephthalate [73]. An average adult, with a body weight of 61.57 kg, may consume around 0.09–0.19 million microplastics particles per day from bottled water [74]. This estimation is based on the average daily intake (EDI) of microplastics in bottled water. The amount of microplastics consumed may vary based on the type and quality of bottled water and the individual's drinking habits. Therefore, it may be essential to monitor the levels of microplastics in bottled water and to take measures to reduce their presence to safeguard the public health.
Potential health effects of microplastics
At the cellular and molecular level, microplastics can induce oxidative stress in skeletal muscle by generating reactive oxygen (ROS) as seen when PS subjected to satellite cell [75]. Microplastics of the size 0.5 μm could be phagocytosed by macrophages, leading to the increase levels of ROS [76], disrupting mitochondrial kinetic homeostasis [77]. Additionally, with the increasing ROS, it may also lead to lipid peroxidation, damaging other lipid-containing structures such as cell membranes [78]. In another study, a cytotoxic effect was observed after introducing PVC microplastics into a simulated digestive tract model. The gene expression levels of DDIT3 and OXR1 significantly increased, indicating that the Caco-2 cell membrane was under oxidative stress, contributing to the observed cytotoxicity [79].
Limitations and advantages of each methodology employed
Limitations and advantages of existing methodologies to detect microplastics is presented in Table 3. Chemicals used for sample digestion in most studies include 10–30% potassium hydroxide (KOH), 30% hydrogen peroxide (H2O2), nitric acid (HNO3), and zinc chloride (ZnCI2). Catalysts such as potassium formate (CHKO2), Fenton reagent and sodium hydroxide (NaOH) were also employed to improve efficiency in addition of chemical digestion of HNO3, ZnCI2 and H2O2. Commonly observed techniques for processing diverse human samples such as blood, tissues, stools, semen and urine, involve the utilisation of a 10% KOH for digestion. This readily available solution is applied to facilitate the breakdown and preparation of these samples for further analysis or testing [80]. Their efficiency may also increase when incorporating with higher temperatures at 60–70°C [81]. Additionally, KOH is a common choice for digesting various other types of samples and matrices including fish [9], shellfish [10] and even sediments [82]. Meanwhile, a less documented method involving the use of TRIS HCl buffer along with a combination of sodium dodecyl sulphate solution and calcium chloride (CaCl2), as well as additional HNO3 and protease as catalysts has been shown to yield microplastics [17,28,31]. However, this method lacks significant supporting studies, as it is not widely employed yet.
Physical characterisation involves the optical microscopy observation which is widely applied in across studies as it is easy to use and to classify microplastics particles according to their colour, shape and size. Following this, two reported studies [21,29] has further investigated their samples with Scanning Electron Microscopy/Energy Dispersive Spectroscopy (SEM-EDX) to visualise microplastics at high magnifications [83]. Furthermore, there are multiple methods available for detecting polymer composition of microplastics including Raman/(μRaman) spectroscopy, fourier transform infrared spectroscopy (FTIR)/micro-FTIR (μFTIR), pyrolysis-gas chromatography mass spectrometry (Py-GC/MS), and laser-induced laser direct infrared spectroscopy (LDIR). Each of this approach has its own advantages and limitations mentioned in Table 3.
Potential sources of contamination
Despite the variety of approaches for detecting microplastics in human sample, there could be potential sources of contamination in each methodology if not handled thoroughly. Several studies do not report on the preparation and use of blanks in their laboratory processes [20,24,26]. Blanks are being performed by mimicking the same process of laboratory experiment to identify potential contaminants [84]. For instance, a blank chemical solution without any matrix should be processed alongside the digestion of samples [21]. During physical characterisation using stereomicroscope, another set of blank either dry or wet filter paper should be placed near the working environment until the step is over [85,86]. These blanks are then observed for atmospheric microplastics that may contaminate the samples during processing. Conducting these steps in future studies would generate more reliable and accurate results as the presence of microplastics in the atmosphere is undeniable and could contaminate the samples.
CONCLUSIONS
Microplastics have been detected in more than half of human organ systems. These microplastics may be classified according to their morphology, colours, size, and type of polymers. The exposure routes to microplastics are likely from inhalation and ingestion with subsequent translocation into the organ systems. We have also systematically evaluated the risk of bias associated with each research methodology employed to extract microplastics in human samples, providing a comprehensive list of the strengths and limitations inherent to each approach. However, correlation between microplastics with their sources remains poorly studied, and likewise correlations with adverse health effects. More research is needed to better understand the long-term effects of microplastics on human health and ways to mitigate exposure to microplastics. Future studies should employ strict control contamination procedures during handling and processing of the samples to avoid atmospheric microplastics.
Additional material
Acknowledgements
The authors thank FSSM, CRAFTS, RMO, UMT, HUSM and all members of MRIG for the research facilities, technical support and moral support to the team.
Data availability: The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Footnotes
Funding: This work was funded by a grant (FRGS59655: FRGS/1/2021/WAB02/UMT/02/1) under the Ministry of Higher Education, Malaysia to YSI. The authors also acknowledge support from Research Management Office of UMT.
Authorship contributions: YSI, LYY and TB designed the conceptualisation of the study. NSR, YSI and LYY wrote the original draft. NSR, YSI, STA and LAL worked on methodology, editing and format analysis. YSI, LYY and KMKKY validated the format analysis and data accuracy. TB, LAL and YSI funded the acquisition. All authors have read, reviewed and approved the final manuscript.
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest (available upon request from the corresponding author) and disclose no relevant interests.
REFERENCES
- 1.Rajput H, Maraqa MA, Zryadi F, Al Khatib LA, Ameen N, Ben ElKaid R, et al. A survey on the use of plastic versus biodegradable bottles for drinking water packaging in the United Arab Emirates. Sustainability. 2022;14:2664. 10.3390/su14052664 [DOI] [Google Scholar]
- 2.Anuar ST, Abdullah NS, Yahya NK, Chin TT, Yusof KM, Mohamad Y, et al. A multidimensional approach for microplastics monitoring in two major tropical river basins, Malaysia. Environ Res. 2023;227:115717. 10.1016/j.envres.2023.115717 [DOI] [PubMed] [Google Scholar]
- 3.Duan J, Bolan N, Li Y, Ding S, Atugoda T, Vithanage M, et al. Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Res. 2021;196:117011. 10.1016/j.watres.2021.117011 [DOI] [PubMed] [Google Scholar]
- 4.Liu P, Zhan X, Wu X, Li J, Wang H, Gao S.Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere. 2020;242:125193. 10.1016/j.chemosphere.2019.125193 [DOI] [PubMed] [Google Scholar]
- 5.Lamichhane G, Acharya A, Marahatha R, Modi B, Paudel R, Adhikari A, et al. Microplastics in environment: global concern, challenges, and controlling measures. Int J Environ Sci Technol (Tehran). 2023;20:4673–94. 10.1007/s13762-022-04261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusof KM, Anuar ST, Mohamad Y, Jaafar M, Mohamad N, Bachok Z, et al. First evidence of microplastic pollution in the surface water of Malaysian Marine Park islands, South China Sea during COVID-19. Mar Pollut Bull. 2023;194:115268. 10.1016/j.marpolbul.2023.115268 [DOI] [PubMed] [Google Scholar]
- 7.Amelia TS, Khalik WM, Ong MC, Shao YT, Pan HJ, Bhubalan K.Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog Earth Planet Sci. 2021;8:1–26. 10.1186/s40645-020-00405-4 [DOI] [Google Scholar]
- 8.Zarawi NA, Yatim SR, Dasiman R, Rasdi NW, Abdullah S, Zaki MA, et al. Determination of microplastic in selected freshwater fish species from agriculture fishpond in Tanjong Karang, Selangor, Malaysia. Journal of Health and Translational Medicine. 2023;2:326–33. [Google Scholar]
- 9.Foo YH, Ratnam S, Lim EV, Abdullah M, Molenaar VJ, Hwai AT, et al. Microplastic ingestion by commercial marine fish from the seawater of Northwest Peninsular Malaysia. PeerJ. 2022;10:e13181. 10.7717/peerj.13181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abd Rahim NH, Cannicci S, Ibrahim YS, Not C, Idris I, Jani JM, et al. Commercially important mangrove crabs are more susceptible to microplastic contamination than other brachyuran species. Sci Total Environ. 2023;903:166271. 10.1016/j.scitotenv.2023.166271 [DOI] [PubMed] [Google Scholar]
- 11.Kutralam-Muniasamy G, Shruti VC, Pérez-Guevara F, Roy PD.Microplastic diagnostics in humans: “The 3Ps” Progress, problems, and prospects. Sci Total Environ. 2023;856:159164. 10.1016/j.scitotenv.2022.159164 [DOI] [PubMed] [Google Scholar]
- 12.Daud A, Astuti RD, Basri K.Detection of Exposure to Microplastics in Humans: A Systematic Review. Open Access Macedonian Journal of Medical Sciences. 2021;9:275–280. 10.3889/oamjms.2021.6494 [DOI] [Google Scholar]
- 13.Danopoulos E, Twiddy M, Rotchell JM.Microplastic contamination of drinking water: A systematic review. PLoS One. 2020;15:e0236838. 10.1371/journal.pone.0236838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP.The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 15.West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, et al. Systems to rate the strength of scientific evidence: summary. Evid Rep Technol Assess (Summ). 2002;47:1–11. [PMC free article] [PubMed] [Google Scholar]
- 16.Bilotta GS, Milner AM, Boyd IL.Quality assessment tools for evidence from environmental science. Environ Evid. 2014;3:1–4. 10.1186/2047-2382-3-14 [DOI] [Google Scholar]
- 17.Leslie HA, Van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH.Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. 10.1016/j.envint.2022.107199 [DOI] [PubMed] [Google Scholar]
- 18.Wu D, Feng Y, Wang R, Jiang J, Guan Q, Yang X, et al. Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence. J Adv Res. 2023;49:141–50. 10.1016/j.jare.2022.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rotchell JM, Jenner LC, Chapman E, Bennet RT, Bolanie IO, Loubani M, et al. Detection of microplastics in human saphenous vein tissue using μFTIR: A pilot study. PLoS One. 2023;18:e0280594. 10.1371/journal.pone.0280594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Xie E, Du Z, Peng Z, Han Z, Li L, et al. Detection of Various Microplastics in Patients Undergoing Cardiac Surgery. Environ Sci Technol. 2023;57:10911–8. 10.1021/acs.est.2c07179 [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim YS, Tuan Anuar S, Azmi AA, Wan Mohd Khalik WM, Lehata S, Hamzah SR, et al. Detection of Microplastics in Human Colectomy Specimens. JGH Open. 2020;5:116–21. 10.1002/jgh3.12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horvatits T, Tamminga M, Liu B, Sebode M, Carambia A, Fischer L, et al. Microplastics detected in cirrhotic liver tissue. EBioMedicine. 2022;82:104147. 10.1016/j.ebiom.2022.104147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragusa A, Slevato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, et al. Plasticenta: First evidence of microplastics in human placenta. Environ Int. 2021;146:106274. 10.1016/j.envint.2020.106274 [DOI] [PubMed] [Google Scholar]
- 24.Braun T, Ehrlich L, Henrich W, Koppel S, Lomako I, Schwabl P, et al. Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics. 2021;13:921. 10.3390/pharmaceutics13070921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, Zhu J, Zuo R, Xu Q, Qian Y, Lihui AN.Identification of microplastics in human placenta using laser direct infrared spectroscopy. Sci Total Environ. 2023;856:159060. 10.1016/j.scitotenv.2022.159060 [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Guo J, Liu X, Yang R, Wang H, Sun Y, et al. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: a pilot prospective study. Sci Total Environ. 2023;854:158699. 10.1016/j.scitotenv.2022.158699 [DOI] [PubMed] [Google Scholar]
- 27.Abbasi S, Turner A.Human exposure to microplastics: A study in Iran. J Hazard Mater. 2021;403:123799. 10.1016/j.jhazmat.2020.123799 [DOI] [PubMed] [Google Scholar]
- 28.Amato-Lourenço LF, Carvalho-Oliveira R, Júnior GR, dos Santos Galvão L, Ando RA, Mauad T.Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126124. 10.1016/j.jhazmat.2021.126124 [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Gao J, Yu H.An emerging role of microplastics in the etiology of lung ground glass nodules. Environ Sci Eur. 2022;34:25. 10.1186/s12302-022-00605-3 [DOI] [Google Scholar]
- 30.Jenner LC, Rotchell JM, Bennett RT, Cowen M, Tentzeris V, Sadofsky LR.Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci Total Environ. 2022;831:154907. 10.1016/j.scitotenv.2022.154907 [DOI] [PubMed] [Google Scholar]
- 31.Zhao Q, Zhu L, Weng J, Jin Z, Cao Y, Jiang H, et al. Detection and characterization of microplastics in the human testis and semen. Sci Total Environ. 2023;877:162713. 10.1016/j.scitotenv.2023.162713 [DOI] [PubMed] [Google Scholar]
- 32.Ragusa A, Notarstefano V, Svelato A, Belloni A, Gioacchini G, Blondeel C, et al. Raman Microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers (Basel). 2022;14:2700. 10.3390/polym14132700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montano L, Giorgini E, Notarstefano V, Notari T, Ricciardi M, Piscopo M, et al. Raman Microspectroscopy evidence of microplastics in human semen. Sci Total Environ. 2023;901:165922. 10.1016/j.scitotenv.2023.165922 [DOI] [PubMed] [Google Scholar]
- 34.Schwabl P, Koppel S, Konigshofer P, Bucsics T, Trauner M, Reiberger T, et al. Detection of various microplastics in human stool: a prospective case series. Ann Intern Med. 2019;171:453–7. 10.7326/M19-0618 [DOI] [PubMed] [Google Scholar]
- 35.Ho YW, Lim JY, Yeoh YK, Chiou JC, Zhu Y, Lai KP, et al. Preliminary findings of the high quantity of microplastics in faeces of Hong Kong residents. Toxics. 2022;10:414. 10.3390/toxics10080414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan Z, Liu Y, Zhang T, Zhang F, Ren H, Zhang Y.Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ Sci Technol. 2022;56:414–21. 10.1021/acs.est.1c03924 [DOI] [PubMed] [Google Scholar]
- 37.Luqman A, Nugrahapraja H, Wahyuono RA, Islami I, Haekal MH, Fardiansyah Y, et al. Microplastic contamination in human stools, foods, and drinking water associated with Indonesian coastal population. Environments. 2021;8:138. 10.3390/environments8120138 [DOI] [Google Scholar]
- 38.Wibowo AT, Nugrahapraja H, Wahyuono RA, Islami K, Haekal MH, Fardiansyah Y, et al. Microplastic contamination in the human gastrointestinal tract and daily consumables associated with an Indonesian farming community. Sustainability. 2021;13:12840. 10.3390/su132212840 [DOI] [Google Scholar]
- 39.Zhang N, Li YB, He HR, Zhang JF, Ma GS.You are what you eat: microplastics in the feces of young men living in Beijing. Sci Total Environ. 2021;767:144345. 10.1016/j.scitotenv.2020.144345 [DOI] [PubMed] [Google Scholar]
- 40.Huang S, Huang X, Bi R, Guo Q, Yu X, Zeng Q, et al. Detection and analysis of microplastics in human sputum. Environ Sci Technol. 2022;56:2476–86. 10.1021/acs.est.1c03859 [DOI] [PubMed] [Google Scholar]
- 41.Pironti C, Notarstefano V, Ricciardi M, Motta O, Giorgini E, Montano L.First evidence of microplastics in human urine, a preliminary study of intake in the human body. Toxics. 2022;11:40. 10.3390/toxics11010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnett AN, Arshad M, Nabi D.A Snapshot into the Invasion of Plastics in Human Urine. ChemRxiv. 2023. 10.26434/chemrxiv-2023-rp3vd [DOI]
- 43.Fournier E, Etienne-Mesmin L, Grootaert C, Jelsbak L, Syberg K, Blanquet-Diot S, et al. Microplastics in the human digestive environment: a focus on the potential and challenges facing in vitro gut model development. J Hazard Mater. 2021;415:125632. 10.1016/j.jhazmat.2021.125632 [DOI] [PubMed] [Google Scholar]
- 44.Yin K, Wang Y, Zhao H, Wang D, Guo M, Mu M, et al. A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci Total Environ. 2021;774:145758. 10.1016/j.scitotenv.2021.145758 [DOI] [Google Scholar]
- 45.Çobanoğlu H, Belivermiş M, Sıkdokur E, Kiliç Ö.C Çayır A. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere. 2021;272:129805. 10.1016/j.chemosphere.2021.129805 [DOI] [PubMed] [Google Scholar]
- 46.Fenech M, Kirsch-Volders M, Natarajan AT, Surralles J, Crott JW, Parry J, et al. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis. 2011;26:125–32. 10.1093/mutage/geq052 [DOI] [PubMed] [Google Scholar]
- 47.Mortensen NP, Johnson LM, Grieger KD, Ambroso JL, Fennell TR.Biological interactions between nanomaterials and placental development and function following oral exposure. Reprod Toxicol. 2019;90:150–65. 10.1016/j.reprotox.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 48.Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215:S1–46. 10.1016/j.ajog.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mortensen NP, Fennell TR, Johnson LM.Unintended human ingestion of nanoplastics and small microplastics through drinking water, beverages, and food sources. NanoImpact. 2021;21:100302. 10.1016/j.impact.2021.100302 [DOI] [PubMed] [Google Scholar]
- 50.Gasperi J, Wright SL, Dris R, Collard F, Mandin C, Guerrouache M, et al. Microplastics in air: are we breathing it in? Curr Opin Environ Sci Health. 2018;1:1–5. 10.1016/j.coesh.2017.10.002 [DOI] [Google Scholar]
- 51.Wu D, Zhang M, Bao TT, Lan H.Long-term exposure to polystyrene microplastics triggers premature testicular aging. Part Fibre Toxicol. 2023;20:35. 10.1186/s12989-023-00546-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin H, Yan M, Pan C, Liu Z, Sha X, Jiang C, et al. Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Part Fibre Toxicol. 2022;19:13. 10.1186/s12989-022-00453-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu W, Yuan Y, Tian Y, Cheng C, Chen Y, Zeng L, et al. Oral exposure to polystyrene nanoplastics reduced male fertility and even caused male infertility by inducing testicular and sperm toxicities in mice. J Hazard Mater. 2023;454:131470. 10.1016/j.jhazmat.2023.131470 [DOI] [PubMed] [Google Scholar]
- 54.Wang YL, Lee YH, Hsu YH, Chiu IJ, Huang CC, Huang CC, et al. The kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells HK-2 and male C57BL/6 mice. Environ Health Perspect. 2021;129:57003. 10.1289/EHP7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.González N, Cunha SC, Monteiro C, Fernandes JO, Marques M, Domingo JL, et al. Quantification of eight bisphenol analogues in blood and urine samples of workers in a hazardous waste incinerator. Environ Res. 2019;176:108576. 10.1016/j.envres.2019.108576 [DOI] [PubMed] [Google Scholar]
- 56.Vilarinho F, Sendón R, Van der Kellen A, Vaz MF, Silva AS.Bisphenol a in food as a result of its migration from food packaging. Trends Food Sci Technol. 2019;91:33–65. 10.1016/j.tifs.2019.06.012 [DOI] [Google Scholar]
- 57.Akhbarizadeh R, Dobaradaran S, Nabipour I, Tangestani M, Abedi D, Javanfekr F, et al. Abandoned Covid-19 personal protective equipment along the Bushehr shores, the Persian Gulf: An emerging source of secondary microplastics in coastlines. Mar Pollut Bull. 2021;168:112386. 10.1016/j.marpolbul.2021.112386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madhumitha CT, Karmegam N, Biruntha M, Arun A, Al Kheraif AA, Kim W, et al. Extraction, identification, and environmental risk assessment of microplastics in commercial toothpaste. Chemosphere. 2022;296:133976. 10.1016/j.chemosphere.2022.133976 [DOI] [PubMed] [Google Scholar]
- 59.Smith DJ, Leal LG, Mitragotri S, Shell MS.Nanoparticle transport across model cellular membranes: when do solubility-diffusion models break down? J Phys D Appl Phys. 2018;51:294004. 10.1088/1361-6463/aacac9 [DOI] [Google Scholar]
- 60.Cao Y, Lin H, Zhang K, Xu S, Leung KM, Lam PK.Microplastics: A major source of phthalate esters in aquatic environments. J Hazard Mater. 2022;432:128731. 10.1016/j.jhazmat.2022.128731 [DOI] [PubMed] [Google Scholar]
- 61.Kannan K, Vimalkumar K.A review of human exposure to microplastics and insights into microplastics as obesogens. Front Endocrinol. 2021;12:724989. 10.3389/fendo.2021.724989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE.Human consumption of microplastics. Environ Sci Technol. 2019;53:7068–74. 10.1021/acs.est.9b01517 [DOI] [PubMed] [Google Scholar]
- 63.Vianello A, Jensen RL, Liu L, Vollertsen J.Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci Rep. 2019;9:8670. 10.1038/s41598-019-45054-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehmood T, Peng L.Polyethylene scaffold net and synthetic grass fragmentation: a source of microplastics in the atmosphere? J Hazard Mater. 2022;429:128391. 10.1016/j.jhazmat.2022.128391 [DOI] [PubMed] [Google Scholar]
- 65.Luo D, Chu X, Wu Y, Wang Z, Liao Z, Ji X, et al. Micro-and Nano-plastics in the atmosphere: a review of occurrence, properties and human health risks. Journal Hazard Mater. 2024;465:133412. 10.1016/j.jhazmat.2023.133412 [DOI] [PubMed] [Google Scholar]
- 66.Zhu X, Huang W, Fang M, Liao Z, Wang Y, Xu L, et al. Airborne microplastic concentrations in five megacities of Northern and Southeast China. Environ Sci Technol. 2021;55:12871–81. 10.1021/acs.est.1c03618 [DOI] [PubMed] [Google Scholar]
- 67.Syafina PR, Yudison AP, Sembiring E, Irsyad M, Tomo HS.Identification of fibrous suspended atmospheric microplastics in Bandung Metropolitan Area, Indonesia. Chemosphere. 2022;308:136194. 10.1016/j.chemosphere.2022.136194 [DOI] [PubMed] [Google Scholar]
- 68.FAO. The State of World Fisheries and Aquaculture 2018-Meeting the sustainable development goals. Rome: Food and Agriculture Organization of the United Nations; 2018. Available: https://openknowledge.fao.org/server/api/core/bitstreams/6fb91ab9-6cb2-4d43-8a34-a680f65e82bd/content. Accessed: 14 August 2024. [Google Scholar]
- 69.Hasan J, Islam SM, Alam MS, Johnson D, Belton B, Hossain MA, et al. Presence of microplastics in two common dried marine fish species from Bangladesh. Mar Pollut Bull. 2022;176:113430. 10.1016/j.marpolbul.2022.113430 [DOI] [PubMed] [Google Scholar]
- 70.Piyawardhana N, Weerathunga V, Chen HS, Guo L, Huang PJ, Ranatunga RR, et al. Occurrence of microplastics in commercial marine dried fish in Asian countries. J Hazard Mater. 2022;423:127093. 10.1016/j.jhazmat.2021.127093 [DOI] [PubMed] [Google Scholar]
- 71.Zhou XJ, Wang J, Li HY, Zhang HM, Zhang DL.Microplastic pollution of bottled water in China. J Water Process Eng. 2021;40:101884. 10.1016/j.jwpe.2020.101884 [DOI] [Google Scholar]
- 72.Makhdoumi P, Amin AA, Karimi H, Pirsaheb M, Kim H, Hossini H.Occurrence of microplastic particles in the most popular Iranian bottled mineral water brands and an assessment of human exposure. J Water Process Eng. 2021;39:101708. 10.1016/j.jwpe.2020.101708 [DOI] [Google Scholar]
- 73.Schymanski D, Goldbeck C, Humpf HU, Fürst P.Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018;129:154–62. 10.1016/j.watres.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 74.Almaiman L, Aljomah A, Bineid M, Aljeldah FM, Aldawsari F, Liebmann B, et al. The occurrence and dietary intake related to the presence of microplastics in drinking water in Saudi Arabia. Environ Monit Assess. 2021;193:390. 10.1007/s10661-021-09132-9 [DOI] [PubMed] [Google Scholar]
- 75.Praveena SM, Ariffin NI, Nafisyah AL.Microplastics in Malaysian bottled water brands: Occurrence and potential human exposure. Environ Pollut. 2022;315:120494. 10.1016/j.envpol.2022.120494 [DOI] [PubMed] [Google Scholar]
- 76.Shengchen W, Jing L, Yujie Y, Yue W, Shiwen X.Polystyrene microplastics-induced ROS overproduction disrupts the skeletal muscle regeneration by converting myoblasts into adipocytes. J Hazard Mater. 2021;417:125962. 10.1016/j.jhazmat.2021.125962 [DOI] [PubMed] [Google Scholar]
- 77.Wang L, Pei W, Li J, Feng Y, Gao X, Jiang P, et al. Microplastics induced apoptosis in macrophages by promoting ROS generation and altering metabolic profiles. Ecotoxicol Environ Saf. 2024;271:115970. 10.1016/j.ecoenv.2024.115970 [DOI] [PubMed] [Google Scholar]
- 78.Yin K, Wang D, Zhang Y, Lu H, Hou L, Guo T, et al. Polystyrene microplastics promote liver inflammation by inducing the formation of macrophages extracellular traps. J Hazard Mater. 2023;452:131236. 10.1016/j.jhazmat.2023.131236 [DOI] [PubMed] [Google Scholar]
- 79.Kadac-Czapska K, Ośko J, Knez E, Grembecka M.Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants. 2024;13:579. 10.3390/antiox13050579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu G, Jiang Q, Qin L, Zeng Z, Zhang P, Feng B, et al. The influence of digestive tract protein on cytotoxicity of polyvinyl chloride microplastics. Sci Total Environ. 2024;945:174023. 10.1016/j.scitotenv.2024.174023 [DOI] [PubMed] [Google Scholar]
- 81.Lavers JL, Stivaktakis G, Hutton I, Bond AL.Detection of ultrafine plastics ingested by seabirds using tissue digestion. Mar Pollut Bull. 2019;142:470–4. 10.1016/j.marpolbul.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 82.Hurley RR, Lusher AL, Olsen M, Nizzetto L.Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ Sci Technol. 2018;52:7409–17. 10.1021/acs.est.8b01517 [DOI] [PubMed] [Google Scholar]
- 83.Razak NI, Khalid AA, Anuar ST, Ibrahim YS, Mohamad N, Jaafar M.Microplastics in ASEAN freshwater sediments: a review of methodologies, occurrence levels and effects on aquatic organisms. Malays J Chem. 2023;25:120–38. [Google Scholar]
- 84.Shi B, Patel M, Yu D, Yan J, Li Z, Petriw D, et al. Automatic quantification and classification of microplastics in scanning electron micrographs via deep learning. Sci Total Environ. 2022;825:153903. 10.1016/j.scitotenv.2022.153903 [DOI] [PubMed] [Google Scholar]
- 85.Rotchell JM, Austin C, Chapman E, Atherall CA, Liddle CR, Dunstan TS, et al. Microplastics in human urine: Characterisation using μFTIR and sampling challenges using healthy donors and endometriosis participants. Ecotoxicol Environ Saf. 2024;274:116208. 10.1016/j.ecoenv.2024.116208 [DOI] [PubMed] [Google Scholar]
- 86.Shruti VC, Kutralam-Muniasamy G.Blanks and bias in microplastic research: Implications for future quality assurance. Trends in Environmental Analytical Chemistry. 2023;38:e00203. 10.1016/j.teac.2023.e00203 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.