Abstract
Objectives:
With remarkable progress in the field of RSV prophylaxis, it is critical to understand population immunity against RSV. We aim to describe the RSV pre-F immunoglobin G (IgG) antibodies across all age groups in southern China and evaluate the risk factors associated with lower antibody levels.
Methods:
We conducted a community-based cross-sectional sero-epidemiological study in Anhua County, Hunan Province, southern China, from July to November 2021. Serum samples were tested for IgG antibodies against the RSV prefusion F (pre-F) protein using an enzyme-linked immunosorbent assay. We estimated the geometric mean titres (GMTs) and seropositivity rates across all age groups. Generalized linear models (GLMs) were built to identify factors associated with antibody levels.
Results:
A total of 890 participants aged 4 months to >89 years were enrolled. The lowest RSV pre-F IgG GMTs were observed in infants and toddlers aged 4 months to <2 years (3.0, 95% confidence interval [CI]: 2.6–3.5). With increasing age, RSV pre-F IgG GMT increased to 4.3 (95% CI: 4.1–4.4) between the ages of 2 and <5 years and then stabilized at high levels throughout life. All the children had serological evidence of RSV infection by the age of 5 years. Age was associated with RSV pre-F antibody levels in children, with an estimated 1.9-fold (95% CI, 0.8–3.6) increase in titre per year before 5 years of age, while it was not significantly associated with antibody levels in adults aged >60 years.
Conclusions:
Our findings could provide a comprehensive understanding of the gaps in RSV immunity at the population level and inform the prioritization of immunization platforms.
Keywords: respiratory syncytial virus, serology, cross-sectional study, general population, pre-F IgG antibody
Introduction
Respiratory syncytial virus (RSV) is a highly contagious virus that causes acute respiratory infection (ARI) across the age spectrum and has a high burden of disease among infants, older adults, and those with comorbid conditions(1, 2).
Recent breakthroughs in defining the structure of the RSV fusion glycoprotein in its prefusion (pre-F) conformation identified new neutralization-sensitive epitopes(3), yielding remarkable progress in RSV prophylactics. Studies have shown that the neutralizing antibody response is mainly directed against pre-F, which has greater neutralizing activity(4, 5). Moreover, the results of clinical trials showed that prophylaxes directed against pre-F elicited better protection and were more immunogenic. Nirsevimab (Beyfortus, AstraZeneca and Sanofi), a half-life-extended monoclonal antibody (mAb) used to protect infants from RSV-associated acute lower respiratory tract infection (LRTI), has been approved in many regions (e.g., the European Union and the United States) since November 2022 and in China at the beginning of 2024(6–8), with early estimates of 81% effectiveness in the first season against RSV-associated LRTI with hospitalization(9). In May 2023, the world’s first two vaccines for the prevention of RSV-LRTI in adults aged 60 years and older were authorized by US Food and Drug Administration (FDA) (RSVPreF3, Arexvy, GlaxoSmithKline, and RSVpreF, Abrysvo, Pfizer)(10, 11), and RSVpreF was approved for maternal immunization subsequently(12). All recently approved immunization products target the prefusion conformation of the RSV F protein.
Although the RSV prevention landscape is evolving rapidly, there is still an incomplete quantitative understanding of RSV population immunity, especially in low- and middle-income settings, due to a lack of systematic surveillance. This knowledge gap may impede evidence-based policy decisions with respect to the potential introduction of new prophylactic options. Additionally, the COVID-19 pandemic led to a disruption in the typical seasonality of RSV, resulting in an abnormally extended epidemic season in many parts of the world, as well as an increase in disease burden in older pediatric age cohorts after the lifting of pandemic measures. There is an urgent need for more comprehensive data regarding RSV immune profiles among the general population. A community-based serological survey could provide insight into the gaps in RSV immunity at the population level to inform prioritization of immunization platforms.
In this study, we describe the RSV pre-F immunoglobin G (IgG) antibody titres and seropositivity rates across all age groups in rural southern China. Risk factors associated with lower antibody levels were also evaluated in young children and elderly adults. Our findings could be timely and instrumental for the design of RSV immunization strategies for high-risk age groups.
Methods
Study design and participant enrolment
A community-based cross-sectional sero-epidemiological study was conducted in the rural areas of Anhua County, Hunan Province, southern China, from July 15 to November 5, 2021. A multistage sampling approach was employed (the detailed sampling method is presented in Supplementary method). In brief, 10 villages of three townships (Qingtang, Jiangnan, and Tianzhuang) were randomly selected, with “Anhua County Heicha Senior High School”, a boarding middle school at Dongping Town (hereinafter referred to as the “Heicha School”), chosen as a study site for recruiting of adolescents aged 15–17 years. The geographical location of the study area is shown in Supplementary Figure 1. Participants who met the inclusion criterion of residing at the study site in the preceding 3 months or longer were eligible to participate. Infants younger than 3 months of age were also eligible for inclusion if they were born at the study sites. Individuals whose guardians refused to participate were ineligible.
Participant enrolment was conducted over two time periods due to a local COVID-19 outbreak, which led to a suspension of study activities for more than two months. The first phase (July 15–29, 2021) involved the enrolment of individuals from Tianzhuang town and Jiangnan town. The second phase started on October 22, 2021, during which participants from Qingtang town (October 22–31, 2021) and Heicha School (November 5, 2021) were enrolled.
Data and sample collection
A questionnaire was administered by trained interviewers to obtain detailed information on demographics, health status, and household information from face-to-face interviews with each participant or one of the guardians. We drew venous blood samples from all participants at the time of enrolment for serum isolation (see Supplementary methods for more details).
Laboratory procedures
RSV pre-F IgG antibodies were quantified using an enzyme-linked immunosorbent assay (ELISA) according to previously described methods, yielding endpoint titres(13). Briefly, 96-well plates were coated with stabilized pre-F protein (DS-Cav1) and incubated at 4°C overnight and serially diluted sera were added into the wells and incubated for 2 h at 37°C. HRP conjugated anti-human IgG was added to all wells followed by the substrate, and the reaction was then stopped with sulfuric acid (0.5 M). The optical density (OD) value was read at 450 nm and subtracted using a 650 nm reference. The endpoint titres of the IgG antibodies were calculated as the last dilution for which the absorbance of a sample was closest to OD=0.1 using GraphPad Prism (version 8.0.2) to fit the logistic curve (four parameters). Additionally, to validate the pre-F IgG antibody level, the neutralizing activity against RSV of antibodies was evaluated in a subset of 91 participants using Focus reduction neutralization test (FRNT). The details of laboratory assays are described in Supplementary methods.
Statistical analysis
We calculated that a target sample size of 842 participants would allow the seropositivity rate(14, 15) of RSV infection in each age group to be estimated within ±5%, with a statistical significance level of 5% (Supplementary Table 1).
Demographic information (i.e., age, sex, township, village and education), household information (i.e., household size and number of siblings), health status (i.e, underlying disease), and the RSV pre-F IgG antibody level of each participant, were collected and analysed. Descriptive analyses were conducted to understand the basic characteristics of the study participants. Subsequently, participants were categorized into six age groups (i.e., 4 months to <2 years, 2 to <5 years, 5 to <12 years, 12 to <18 years, 18 to <60 years and ≥60 years). The geometric mean titres (GMTs) with 95% confidence intervals (95% CIs), as well as the seropositivity rates, were estimated across all age groups. Correlations between RSV pre-F IgG antibody and neutralization antibody were calculated using Spearman’s correlation coefficient.
Generalized linear models (GLMs) were used to determine the factors associated with RSV-specific antibody levels. The main analysis was conducted among all participants, and subgroup analyses were performed among high-risk groups (i.e., children aged <5 years and elderly adults aged ≥60 years). Prior to this, a systematic literature review and univariate analyses were conducted to explore potential risk factors associated with lower antibody levels. The variables included in the multivariate model were determined based on previous research findings and the results of the univariate analyses (P<0.05). To handle incomplete data on household size and underlying disease, a multiple imputation method was used, where missing values were imputed by randomly selecting values from the observed distributions of complete cases, stratified by age groups. Additionally, a GLM based on complete cases was built for sensitivity analyses. All analyses were performed in R, version 4.1.2.
Ethics approval and consent to participate
The study was approved by the institutional review board of School of Public Health, Fudan University (IRB#2020-11-0857, #2020-11-0857-S and #2022-02-0948). Consents to participants were described in the Supplementary methods.
Results
A total of 890 participants aged between 4 months and older than 89 years were enrolled. The median age was 23.9 years (interquartile range, IQR: 6.9–52.4). A total of 46.5% (380/817) had a household size of 2–4 persons. 15.4% (124/804) suffered from at least one underlying disease, including cardiovascular disease, diabetes, and chronic respiratory disease (Table 1).
Table 1.
Characteristics of the study participants.
| Characteristics | |
|---|---|
|
| |
| Age (N=890) | |
| Range | 4 months to >89 years |
| Median (IQR) | 23.9 (6.9-52.4) years |
| Age group | |
| 4 months to <2 years | 21 (2.4) |
| 2 to <5 years | 106 (11.9) |
| 5 to <12 years | 226 (25.4) |
| 12 to <18 years | 66 (7.4) |
| 18 to <60 years | 336 (37.8) |
| ≥60 years | 135 (15.2) |
| Sex (N=890) | |
| Male | 360 (40.4) |
| Female | 530 (59.6) |
| Location of residence (N=890) | |
| Jiangnan town | 211 (23.7) |
| Tianzhuang town | 234 (26.3) |
| Qingtang town | 397 (44.6) |
| Heicha School | 48 (5.4) |
| Household size* (N=817) | |
| 2-4 | 380 (46.5) |
| >4 | 437 (53.5) |
| Underlying diseases† (N=804) | |
| At least one | 124 (15.4) |
| Cardiovascular diseases | 78 (62.9) |
| Diabetes | 22 (17.7) |
| Chronic respiratory diseases | 13 (10.5) |
| Others | 37 (29.8) |
| Without | 680 (84.6) |
The data are expressed as n (%), unless otherwise specified. The percentages might not total 100% because of rounding. A value <890 indicated missing data; 73 participants declined to provide their household size, and 86 were unwilling to provide information on their underlying disease.
We defined household size as the number of individuals who lived with the participants (those included) for at least 5 days per week, including the colleagues or students living with the participants in the dormitories.
The numbers in parentheses are the constituent ratios; for example, the proportion of participants with cardiovascular diseases was 62.9% (78/124). Other diseases include chronic liver diseases, chronic kidney diseases, tumours, etc.
We found distinct antibody responses in different age groups. The lowest RSV pre-F IgG GMTs were observed in infants and toddlers aged 4 months to <2 years, which was 3.0 (95% CI: 2.6–3.5). As age increased, the GMT increased to 4.3 (95% CI: 4.1–4.4) in young children between the ages of 2 and <5 years and then stabilized at a high level throughout life (Figure 1A). Approximately 76.2% (16/21) and 93.4% (99/106) of the participants were seropositive in the age groups of 4 months to <2 years and 2 to <5 years, respectively. The seropositivity rates reached and remained at 100% after age five (Figure 1B). We also observed spatial heterogeneities in RSV population immunity levels, with participants from Qingtang town exhibiting lower antibody levels than those from the other two towns. However, it should be noted that the enrolment and sample collection from Qingtang town was nearly three months after the participants’ enrolment in Jiangnan and Tianzhuang towns (Supplementary Figure 2).
Figure 1. Dynamic patterns of RSV pre-F IgG antibody titres (A) and seropositivity rates (B) across all age groups.
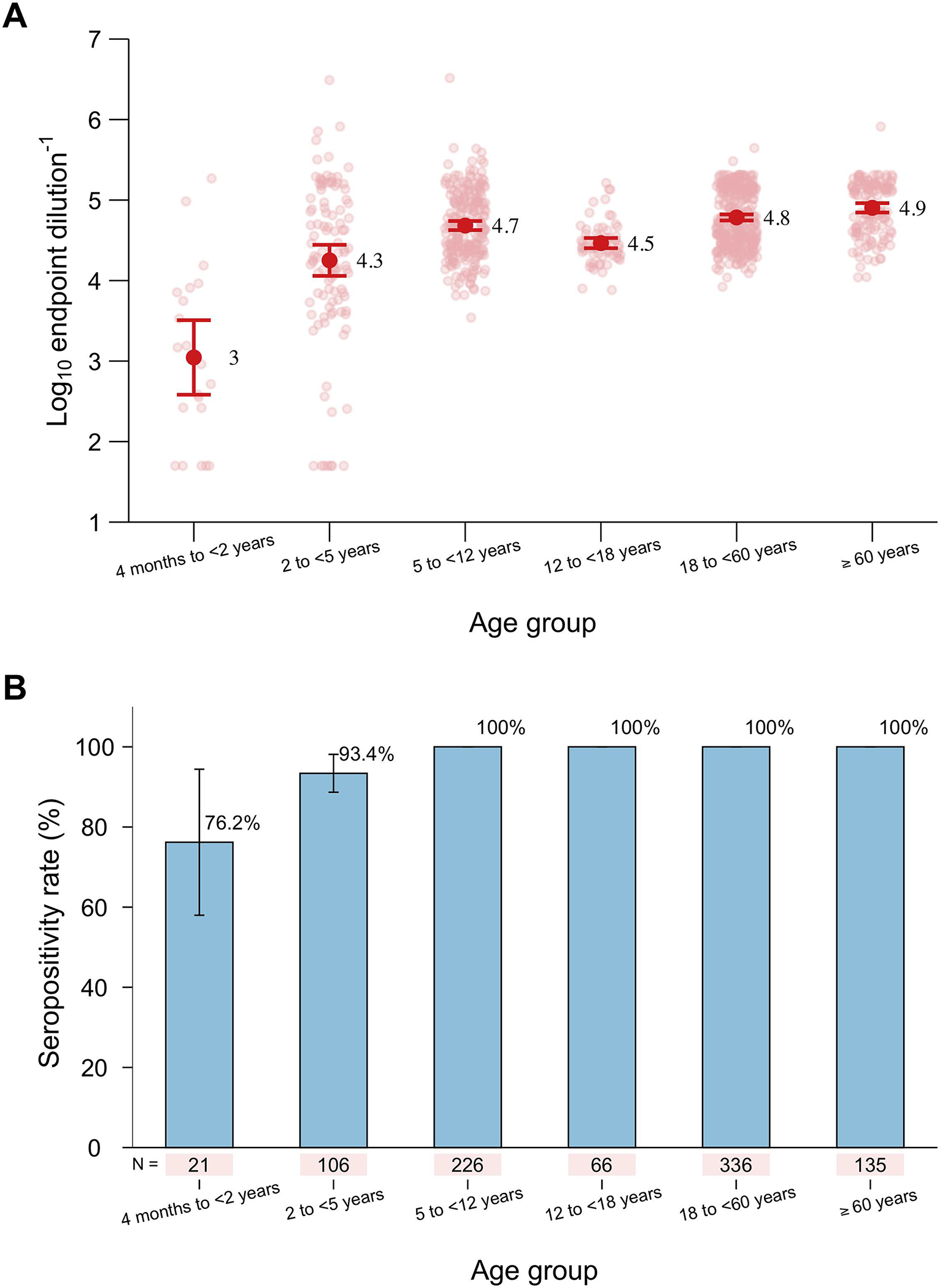
The red points and error bars in (A) refer to the GMTs and 95% CIs, respectively. The pink points in (A) refer to the individual data of each participant. The blue bars and error bars in (B) refer to the seropositivity rates and 95% CIs, respectively.
Univariate generalized linear models showed that individual’s age, location of residence, household size and underlying health conditions are potential factors affecting their RSV pre-F antibody levels (Supplementary Table 2), and the daycare attendance may have an impact on pediatric antibody titres (Supplementary Table 3). A multivariate model accounting for the influence of individuals’ household size and potential spatial heterogeneities showed that infancy and toddler status (4 months to <2 years) and young age (2 to <5 years) were significantly associated with lower RSV pre-F antibody levels (P<0.001, Table 2). Subgroup analyses demonstrated increasing antibody levels with age in children <5 years, with an annually estimated increase in GMT of 0.5 (95% CI: 0.3–0.7), corresponding to a 1.9-fold increase (95% CI: 0.8–3.6) in titre per year on average. Daycare attendance was no longer to be a significant risk factor for lower antibody levels among children after controlling for age. Neither age nor underlying conditions were significantly associated with RSV pre-F antibody levels in adults aged 60 years or older (Table 3). Similar results were observed in the sensitivity analyses based on complete data (Supplementary Table 4).
Table 2.
Factors associated with RSV pre-F IgG antibodies according to a generalized linear model.
| Characteristics | β (95% CI) | Fold changes (10β-1, 95% CI) | P value |
|---|---|---|---|
|
| |||
| Age group | |||
| 4 months to <2 years | −1.574 (−1.804, −1.344) | −0.973 (−0.984, −0.955) | <0.001 |
| 2 to <5 years | −0.410 (−0.529, −0.292) | −0.611 (−0.704, −0.489) | <0.001 |
| 5 to <12 years | 0.037 (−0.059, 0.134) | 0.090 (−0.127, 0.360) | 0.447 |
| 12 to <18 years | −0.192 (−0.434, 0.050) | −0.357 (−0.632, 0.122) | 0.120 |
| 18 to <60 years | Reference | Reference | - |
| ≥60 years | 0.032 (−0.077, 0.140) | 0.076 (−0.162, 0.382) | 0.564 |
| Location of residence | |||
| Jiangnan town | Reference | Reference | - |
| Tianzhuang town | −0.082 (−0.177, 0.013) | −0.172 (−0.334, 0.030) | 0.090 |
| Qingtang town | −0.296 (−0.394, −0.199) | −0.494 (−0.596, −0.367) | <0.001 |
| Heicha School | −0.254 (−0.537, 0.029) | −0.443 (−0.710, 0.069) | 0.079 |
| Household size | |||
| 2–4 | Reference | Reference | - |
| >4 | 0.009 (−0.064, 0.081) | 0.020 (−0.137, 0.205) | 0.815 |
| Underlying diseases | |||
| No | Reference | Reference | - |
| Yes | 0.096 (−0.013, 0.205) | 0.247 (−0.029, 0.602) | 0.085 |
We quantified RSV pre-F IgG antibodies using log10 endpoint titres. β > 0 indicates that the factor was associated with an increase in RSV pre-F IgG antibodies by 10β-1 fold; β < 0 indicates that the factor was associated with a decrease in RSV pre-F IgG antibodies by 1-10β fold.
Table 3.
Factors associated with RSV pre-F IgG antibodies among children aged <5 years and elderly adults aged ≥60 years.
| Characteristics | β (95% CI) | Fold changes (10β-1, 95% CI) | P value |
|---|---|---|---|
|
| |||
| Children aged <5 years | |||
| Age, years | |||
| Contiguous age | 0.459 (0.253, 0.665) | 1.877 (0.790, 3.625) | <0.001 |
| Location of residence | |||
| Jiangnan town | Reference | Reference | - |
| Tianzhuang town | −0.166 (−0.856, 0.523) | −0.318 (−0.861, 2.336) | 0.637 |
| Qingtang town | −0.488 (−1.009, 0.032) | −0.675 (−0.902, 0.077) | 0.068 |
| Household size | |||
| 2-4 | Reference | Reference | - |
| >4 | 0.265 (−0.113, 0.644) | 0.841 (−0.230, 3.401) | 0.172 |
| Use of daycare | |||
| Yes | −0.020 (−0.531, 0.492) | −0.045 (−0.706, 2.101) | 0.940 |
| No | Reference | Reference | - |
| Elderly adults aged ≥60 years | |||
| Age, years | |||
| Contiguous age | −0.003 (−0.013, 0.006) | −0.008 (−0.030, 0.015) | 0.493 |
| Location of residence | |||
| Jiangnan town | Reference | Reference | - |
| Tianzhuang town | −0.092 (−0.219, 0.034) | −0.192 (−0.396, 0.082) | 0.154 |
| Qingtang town | −0.052 (−0.272, 0.168) | −0.113 (−0.466, 0.474) | 0.645 |
| Household size | |||
| 2-4 | Reference | Reference | - |
| >4 | 0.081 (−0.053, 0.215) | 0.205 (−0.114, 0.639) | 0.237 |
| Underlying diseases | |||
| No | Reference | Reference | - |
| Yes | 0.011 (−0.107, 0.129) | 0.026 (−0.218, 0.347) | 0.853 |
We quantified RSV pre-F IgG antibodies using log10 endpoint titres. β > 0 indicates that the factor was associated with an increase in RSV pre-F IgG antibodies by 10β-1 fold; β < 0 indicates that the factor was associated with a decrease in RSV pre-F IgG antibodies by 1-10β fold.
In addition, we demonstrated that pre-F IgG titres correlate well with the “gold-standard” serology assay FRNT (spearman ρ=0.69, P< 0.001, Supplementary Figure 4), indicating high levels of measurement reliability.
Discussion
This serological study spans age groups of the general population, allowing us to analyse the antibody profiles and their associated factors at the population level. The strengths of our study include the use of representative samples and testing of RSV pre-F IgG antibodies. We demonstrated that infants and toddlers (4 months to <2 years), as well as young children (2 to <5 years), had lower antibody levels, with GMTs gradually increasing by 5 years of age; however, in older adults, the antibody titres remained stable.
Significant increases in RSV pre-F IgG antibodies were observed in young children aged 2 to <5 years compared with infants and toddlers, which was in line with the findings of serological studies in the Netherlands(16, 17). We also found increasing seropositivity rates, from 76.2% in children between 4 months and <2 years to 100% by the age of 5 years, indicating a high rate of natural infection in early childhood, which was congruent with the findings of prior studies(18). Only one 4-month-old boy was enrolled in this age group, with a titre of 4.2, which was higher than that of most of the participants aged 6 to <24 months (Supplementary Figure 3).
The observed lower antibody levels in participants from Qingtang town, as well as in those from Heicha School (although not significantly different, Supplementary Figure 2), should be interpreted with caution. Despite the longer spatial distance from Qingtang to the other two towns (Supplementary Figure 1), public health interventions (e.g., social distancing) for COVID-19 during the local outbreak could alter RSV seasonality in the autumn of 2021. The delay in sample collection in Qingtang town and Heicha School could have contributed to the differences in RSV antibody levels. A recent study of pediatric inpatients in Hunan province report that both the number and positive rate of RSV infections in August and September, 2021 were at a low level(19). While the data from children in singe hospital with median age 16 months, potentially not accurately reflect the RSV epidemic characteristics in community, it does imply that disruptions in RSV circulation could impact population-level immunity during the conduct of this trial.
Although RSV infections can occur throughout life, young infants and children < 5 are at a greater risk of hospitalization due to LRTI than individuals over the age of 5 years (16, 17), as lower pre-F antibody levels are associated with increased disease burden in this age group(4). We found that young age was a risk factor for decreased RSV pre-F antibodies, with an estimated 1.9-fold increase in titre per year in children between 4 months and 5 years. This highlights the importance of preventive efforts focused on infants and maternal immunization for RSV protection in early life. We found that having at least one sibling in the household had no significant impact on RSV antibody among children (Supplementary Table 3). However, it’s important to interpret this result cautiously, considering a previous study has demonstrated that living with a child aged 0–4 years is associated with an increased probability of RSV infection, while living with older children (≥5 years) is not a risk factor(17). In our study, the majority (85.2%, 46/54) of siblings of the participating children were aged >5 years, which might explain the non-significant result. The use of daycare is significantly associated with RSV pre-F IgG antibodies in univariate analysis (Supplementary Table 3). Its highly correlation with participant age is likely due to few preschools in the study site providing daycare service for children younger than 3 years old. Therefore, the use of daycare was no longer a significant risk factor after controlling for participants’ age in multiple regression (Table 3).
Moreover, for the adults in our study, we estimated that the antibody levels in adults aged between 18 and <60 years were comparable to those in the vaccine DS-Cav-1 clinical trial using an ELISA based on the pre-F conformation antigen(20). The magnitudes of the antibody response were comparable among healthy adults and elderly individuals, similar to the findings of Cherukuri et al.(21) and Falsey et al.(22). Underlying cardiopulmonary disease is a risk factor for severe RSV infection in elderly individuals(2, 23–26). According to our analysis, neither older age (≥60 years) nor the presence of underlying disease was significantly associated with RSV pre-F antibody titres. Aging-related immunosenescence can contribute to reinfection in older adults with high antibody levels. In this population, antibody titres may not be as informative in defining at-risk populations (27, 28).
Although our neutralization assay has demonstrated correlation between the two serological indicators, using RSV pre-F IgG-binding antibodies still have limitation, as neutralizing antibodies are typically used as proxy indicators for correlate of protection (CoP) in clinical trials. However, use of pre-F ELISA assays is substantially less time and cost-intensive. An additional limitation is that this study was conducted during COVID-19 pandemic. A temporary decline of RSV epidemic in the spring of 2020, followed by a rapid recovery in the summer and lasted for 87 weeks before concluding in March 2022 in Hunan(19). There is no agreement on the impact of the COVID-19 pandemic on the trend of RSV antibody dynamics. The concept of “immunity debt” was proposed to elucidate the atypical resurgence of RSV in recent years following the relaxation of strict non-pharmaceutical interventions implemented during early stage of COVID-19 pandemic. Waning of RSV immunity at the population level is a relatively consistent view. However, evidence supporting this remain insufficient. More studies are needed to understand and substantiate the changes in population immunity and the mechanism after COVID-19 pandemic(19, 29, 30). Finally, the relatively small sample of enrolled infants limits our ability to understand the overall antibody profiles in the initial months of life.
In conclusion, we provided quantitative estimates of the prevalence of RSV infection across all age groups and showed that age was an important factor associated with RSV pre-F antibody levels. The evidence of low antibody titres and high seropositivity rates in young age groups underlines the necessity of protecting infants from RSV infection. With the rapid development and approval of new preventive measures in recent years and the need for reliable data on the antibody response against RSV, our findings could provide timely and instrumental information for the design of strategies for active and passive RSV immunization.
Supplementary Material
Acknowledgements
We thank all study participants for their cooperatively participating in the study. We acknowledge Anhua County CDC for helping with project administration and data collection; and thank staff members at the Anhua County CDC and township health center in the study sites for helping with field investigation, administration, and data collection. We also acknowledge the valuable contributions of Dr. Barney Graham’s team at NIAID for assay setup and support.
Funding
This work was supported by grants from the Key Program of the National Natural Science Foundation of China (82130093) and Shanghai Municipal Science and Technology Major Project (ZD2021CY001). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Transparency declaration
H.Y. has received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang and Shanghai Roche Pharmaceutical Company. None of the research funding is related to this work.
H.Y.C. reports consulting with Ellume, Pfizer, and the Bill and Melinda Gates Foundation. She has served on advisory boards for Vir, Merck and Abbvie. She has conducted CME teaching with Medscape, Vindico, and Clinical Care Options. She has received research funding from Gates Ventures, and support and reagents from Ellume and Cepheid, all outside of the submitted work.
All other authors report no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
All data and materials are available from the corresponding author upon reasonable request.
References
- 1.Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64. 10.1016/S0140-6736(22)00478-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi T, Denouel A, Tietjen AK, Campbell I, Moran E, Li X, et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J Infect Dis. 2020;222(Suppl 7):S577–s83. 10.1093/infdis/jiz059 [DOI] [PubMed] [Google Scholar]
- 3.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340(6136):1113–7. 10.1126/science.1234914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capella C, Chaiwatpongsakorn S, Gorrell E, Risch ZA, Ye F, Mertz SE, et al. Prefusion F, Postfusion F, G Antibodies, and Disease Severity in Infants and Young Children With Acute Respiratory Syncytial Virus Infection. J Infect Dis. 2017;216(11):1398–406. 10.1093/infdis/jix489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med. 2015;7(309):309ra162. 10.1126/scitranslmed.aac4241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration. FDA Approves New Drug to Prevent RSV in Babies and Toddlers 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers.[Accessed 23 May, 2024]
- 7.European Medicines Agency. Beyfortus. https://www.ema.europa.eu/en/medicines/human/EPAR/beyfortus. [Accessed 10 May, 2024]
- 8.AstraZeneca. Beyfortus approved in China for the prevention of RSV disease in infants, https://www.astrazeneca.com/media-centre/press-releases/2024/beyfortus-approved-in-china-for-the-prevention-of-rsv-disease-in-infants.html. [Accessed 5 May, 2024]
- 9.Moline HL, Tannis A, Toepfer AP, Williams JV, Boom JA, Englund JA, et al. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus-Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season - New Vaccine Surveillance Network, October 2023-February 2024. MMWR Morb Mortal Wkly Rep. 2024;73(9):209–14. 10.15585/mmwr.mm7309a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. FDA Approves First Respiratory Syncytial Virus (RSV) Vaccine 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine. [Accessed 2 May, 2024]
- 11.Pfizer Inc. U.S. FDA Approves ABRYSVO™, Pfizer’s Vaccine for the Prevention of Respiratory Syncytial Virus (RSV) in Older Adults 2023. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-abrysvotm-pfizers-vaccine-prevention. [Accessed 2 May, 2024]
- 12.U.S. Food and Drug Administration. FDA Approves First Vaccine for Pregnant Individuals to Prevent RSV in Infants 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants. [Accessed 2 May, 2024]
- 13.Crank MC, Ruckwardt TJ, Chen M, Morabito KM, Phung E, Costner PJ, et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science. 2019;365(6452):505–9. 10.1126/science.aav9033 [DOI] [PubMed] [Google Scholar]
- 14.Arankalle VA, Kulkarni R, Malshe N, Palkar S, Lalwani S, Mishra AC. Seroepidemiology of respiratory syncytial virus in western India with special reference to appropriate age for infant vaccination. J Med Virol. 2019;91(8):1566–70. 10.1002/jmv.25489 [DOI] [PubMed] [Google Scholar]
- 15.Sastre P, Ruiz T, Schildgen O, Schildgen V, Vela C, Rueda P. Seroprevalence of human respiratory syncytial virus and human metapneumovirus in healthy population analyzed by recombinant fusion protein-based enzyme linked immunosorbent assay. Virol J. 2012;9:130. 10.1186/1743-422X-9-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berbers G, Mollema L, van der Klis F, den Hartog G, Schepp R. Antibody Responses to Respiratory Syncytial Virus: A Cross-Sectional Serosurveillance Study in the Dutch Population Focusing on Infants Younger Than 2 Years. J Infect Dis. 2021;224(2):269–78. 10.1093/infdis/jiaa483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andeweg SP, Schepp RM, van de Kassteele J, Mollema L, Berbers GAM, van Boven M. Population-based serology reveals risk factors for RSV infection in children younger than 5 years. Sci Rep. 2021;11(1):8953. 10.1038/s41598-021-88524-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox MJ, Azevedo RS, Cane PA, Massad E, Medley GF. Seroepidemiological study of respiratory syncytial virus in Sa o Paulo state, Brazil. J Med Virol. 1998;55(3):234–9. [DOI] [PubMed] [Google Scholar]
- 19.Xie LY, Wang T, Yu T, Hu X, Yang L, Zhong LL, et al. Seasonality of respiratory syncytial virus infection in children hospitalized with acute lower respiratory tract infections in Hunan, China, 2013–2022. Virol J. 2024;21(1):62. 10.1186/s12985-024-02336-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruckwardt TJ, Morabito KM, Phung E, Crank MC, Costner PJ, Holman LA, et al. Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Respir Med. 2021;9(10):1111–20. 10.1016/S2213-2600(21)00098-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherukuri A, Patton K, Gasser RA Jr., Zuo F, Woo J, Esser MT, et al. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol. 2013;20(2):239–47. 10.1128/CVI.00580-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falsey AR, Walsh EE, Scott DA, Gurtman A, Zareba A, Jansen KU, et al. Phase 1/2 Randomized Study of the Immunogenicity, Safety, and Tolerability of a Respiratory Syncytial Virus Prefusion F Vaccine in Adults With Concomitant Inactivated Influenza Vaccine. J Infect Dis. 2022;225(12):2056–66. 10.1093/infdis/jiab611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackerson B, Tseng HF, Sy LS, Solano Z, Slezak J, Luo Y, et al. Severe Morbidity and Mortality Associated With Respiratory Syncytial Virus Versus Influenza Infection in Hospitalized Older Adults. Clin Infect Dis. 2019;69(2):197–203. 10.1093/cid/ciy991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyffels V, Kariburyo F, Gavart S, Fleischhackl R, Yuce H. A Real-World Analysis of Patient Characteristics and Predictors of Hospitalization Among US Medicare Beneficiaries with Respiratory Syncytial Virus Infection. Adv Ther. 2020;37(3):1203–17. 10.1007/s12325-020-01230-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis. 2004;189(2):233–8. 10.1086/380907 [DOI] [PubMed] [Google Scholar]
- 26.Walsh EE, Peterson DR, Kalkanoglu AE, Lee FE, Falsey AR. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis. 2013;207(9):1424–32. 10.1093/infdis/jit038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cusi MG, Martorelli B, Di Genova G, Terrosi C, Campoccia G, Correale P. Age related changes in T cell mediated immune response and effector memory to Respiratory Syncytial Virus (RSV) in healthy subjects. Immun Ageing. 2010;7:14. 10.1186/1742-4933-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190(2):373–8. 10.1086/421524 [DOI] [PubMed] [Google Scholar]
- 29.Abu-Raya B, Vineta Paramo M, Reicherz F, Lavoie PM. Why has the epidemiology of RSV changed during the COVID-19 pandemic? EClinicalMedicine. 2023;61:102089. 10.1016/j.eclinm.2023.102089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.den Hartog G, van Kasteren PB, Schepp RM, Teirlinck AC, van der Klis FRM, van Binnendijk RS. Decline of RSV-specific antibodies during the COVID-19 pandemic. Lancet Infect Dis. 2023;23(1):23–5. 10.1016/S1473-3099(22)00763-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials are available from the corresponding author upon reasonable request.


