Abstract
Objective
The European Health Data Space (EHDS) shapes the digital transformation of healthcare in Europe. The EHDS regulation will also accelerate the use of health data for research, innovation, policy-making, and regulatory activities for secondary use of data (known as EHDS2). The Integration of heterogeneous Data and Evidence towards Regulatory and HTA Acceptance (IDERHA) project builds one of the first pan-European health data spaces in alignment with the EHDS2 requirements, addressing lung cancer as a pilot.
Methods
In this study, we conducted a comprehensive review of the EHDS regulation, technical requirements for EHDS2, and related projects. We also explored the results of the Joint Action Towards the European Health Data Space (TEHDAS) to identify the framework of IDERHA’s alignment with EHDS2. We also conducted an internal webinar and an external workshop with EHDS experts to share expertise on the EHDS requirements and challenges.
Results
We identified the lessons learned from the existing projects and the minimum-set of requirements for aligning IDERHA infrastructure with EHDS2, including user journey, concepts, terminologies, and standards. The IDERHA framework (i.e., platform architecture, standardization approaches, documentation, etc.) is being developed accordingly.
Discussion
The IDERHA's alignment plan with EHDS2 necessitates the implementation of three categories of standardization for: data discoverability: Data Catalog Vocabulary (DCAT-AP), enabling semantics interoperability: Observational Medical Outcomes Partnership (OMOP), and health data exchange (DICOM and FHIR). The main challenge is that some standards are still being refined, e.g., the extension of the DCAT-AP (HealthDCAT-AP). Additionally, extensions to the Observational Health Data Sciences and Informatics (OHDSI) OMOP Common Data Model (CDM) to represent the patient-generated health data are still needed. Finally, proper mapping between standards (FHIR/OMOP) is a prerequisite for proper data exchange.
Conclusions
The IDERHA's plan and our collaboration with other EHDS initiatives/projects are critical in advancing the implementation of EHDS2.
Keywords: Artificial Intelligence; European health data space; cancer, digital health; healthcare standards; interoperability; secondary use of data.
Abbreviations
AI, Artificial Intelligence; CDM, Common Data Model; DCAT, Data Catalog Vocabulary; DCAT-AP, DCAT Application profile for data portals in Europe; DICOM, Digital Imaging and Communications in Medicine; EC, European Commission; EFMI, European Federation for Medical Informatics; EHDS, European Health Data Space; EHR, Electronic Health Record; EMA, European Medicines Agency; EOSC, European Open Science Cloud; FAIR, Findability, Accessibility, Interoperability, and Reusability; FHIR, Fast Healthcare Interoperability Resource; FML, Federated Machine Learning; HHR, Holistic Health Records; HL7, Health Level 7; HTA, Health Technology Assessment; EU, European Union; GDPR, General Data Protection Regulation; IDERHA, Integration of heterogenous Data and Evidence towards Regulatory & HTA Acceptance; JA, Joint Action; LC, Lung Cancer; OHDSI, Observational Health Data Sciences and Informatics; OMOP, Observational Medical Outcomes Partnership; PGHD, Patient-Generated Health Data; RWD, Real-World Data; RWE, Real-World Evidence; TEHDAS, Towards the European Health Data Space; WHO, World Health Organization.
The IDERHA project
The Integration of heterogeneous Data and Evidence towards Regulatory & HTA Acceptance (IDERHA) project aims to develop one of the first pan-European health data spaces (URL: https://www.iderha.org), and as such, necessitates an adequate adoption of the European Health Data Space (EHDS) principles 1 . This project has a focus on Lung Cancer (LC), to provide an example of integration and analysis of health data across sectors and along the continuum of care for clinical or medical research questions. There is also an underlying aim to accelerate policy development by building consensus recommendations. These recommendations would further enable the use of heterogeneous health data for product research and development, and are focused on needs of the regulatory and Health Technology Assessment (HTA) community 2 . IDERHA has selected four use cases in LC to demonstrate the value of health data integration through developing Artificial Intelligence (AI) and Machine Learning (ML) tools in a federated data environment 3 , and personalized remote monitoring applications for, 1) risk profiling, 2) diagnosis, 3) prognosis, and 4) well-being and patient engagement using device technology/ application in an at-home environment.
We target both institutional and individual data providers. Among the IDERHA consortium partners and within their wider networks, we identified 26 potential institutional data access providers (e.g., institutions, services, repositories) for LC data.
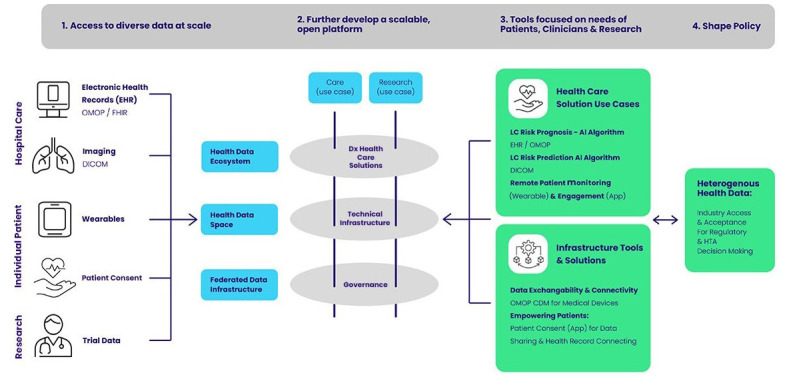
From a technical perspective, the IDERHA data space specifies a federated data infrastructure with participatory governance that keeps decision rights distributed among federated parties 4 . It thus makes health datasets from Data Providers accessible for analysis, including via sophisticated Federated Machine Learning (FML) algorithms 5 , while ensuring both organizationally (e.g. Data Access Committee (DACs)) and technically (e.g., standardized data policies) enforced access controls. Processing operations of the FML framework are executed at the federated endpoints of the network (i.e., directly at data providers’ sites); subsequently, the partial results of the computations are aggregated at a central node. Thus, the IDERHA infrastructure will facilitate centralized discovery and utilization of federated data resources (i.e., stored, managed, and controlled by the data providers at their facilities) for the evaluation of personal data with privacy-preserving and distributed analytics (see Figure 1). To achieve that, IDERHA aims to connect multiple public and private data sources that aggregate health-related data for secondary use and that cover various aspects of: Electronic Health Records (EHR), clinical images, Patient-Generated Health Data (PGHD), Patient Reported Outcome Measures (PROMs), Patient-Reported Experiences Measures (PREMs), environmental and socioeconomic data.
Figure 1. IDERHA Landscape.
PROMs for symptom monitoring in cancer provide an evidence-based method for recognizing symptoms, providing clinicians with valuable information, and improving clinical management 6 . Furthermore, systematically capturing and evaluating patients' perspectives can enhance both their healthcare experience and outcomes. PROMs record symptoms, health-related quality of life, and functional status and refer to standardized questionnaires that are answered directly by the patients. PREMs, on the other hand, focus on the human aspects of the care process 7 .
The IDERHA project aims to make these heterogeneous health datasets discoverable and utilizable for secondary use in research, innovation, public health, policymaking, regulatory activities, and personalized medicine, by:
Aligning with the EHDS principles of the secondary use of data 8 .
Adopting the principles of Findability, Accessibility, Interoperability, and Reusability (FAIR) 9 for both IDERHA data and infrastructure. A metadata catalogue based on FAIR principles 10 will support effective data discovery and matchmaking, as well as access to algorithms used in IDERHA. The IDERHA platform will implement a core layer of appropriate authentication and authorization services to manage secure data access.
Supporting healthcare data standards and models for interoperability 11 , mainly, Health Level 7 (HL7®) Fast Healthcare Interoperability Resources (FHIR®), Open Health Data Science and Informatics (OHDSI), Observational Medical Outcomes Partnership (OMOP)-Common Data Model (CDM), and Digital Imaging and Communications in Medicine (DICOM®).
Establishing an IDERHA data quality framework, in which specific requirements and assessment methods will be defined from the data users' perspective 12 , especially when using Real-World Data (RWD) for decision-making and Real-World Evidence (RWE) 13 .
The benefits generated through the execution of use cases on the IDERHA platform will be assessed by a Clinical Advisory Board drawn from key stakeholders, including clinicians and RWE/RWD experts.
This article describes our approach for aligning IDERHA with EHDS2 requirements, highlighting the alignment framework, landscape of existing projects and interoperability standards, lessons learned, and next steps.
The European Health Data Space (EHDS) regulation
On the 24th of April 2024, the European Parliament adopted the EHDS regulation to build a health-specific ecosystem comprised of rules, common standards and practices, infrastructures, and a governance framework 8, 14 . The Council will formally adopt the EHDS regulation, which is expected to be published in the Official Journal in autumn. It will then become applicable in different stages according to the use case and data type.
EHDS aims to empower individuals to access and control their health data across the European Union (EU) for 1) the primary use of data (EHDS1) (MyHealth@EU), for healthcare delivery and decision making 15 and 2) secondary use of data (EHDS2) (HealthData@EU) for research, innovation, policy-making and regulatory activities 16 .
In EHDS1, the EU member states will ensure that patient summaries, ePrescriptions, images and image reports, laboratory results, discharge reports among others will be exchanged in a common European format within the cross-border digital infrastructure (MyHealth@EU) 15 . To ensure that citizens' rights are safeguarded, all member states will appoint digital health authorities that will participate in MyHealth@EU.
On the other hand, in EHDS2 (see Figure 2), each member state will set up a health data access body that gives permits to access data by researchers, companies, or institutions using a decentralized EU-infrastructure (HealthData@EU), which will be set up to support cross-border projects 16 . The European principles for the secondary use of health data are provided by the TEHDAS Joint Action (JA) (URL: https://tehdas.eu/) and are being adopted by the HealthData@EU pilot project (URL: https://ehds2pilot.eu/).
Figure 2. EHDS regulation and EHDS2 foreseen architecture, user journey, and requirements.

Because building trust is the main enabler for the success of the EHDS, the EHDS regulation is built further on the General Data Protection Regulation (GDPR), AI Act, the Data Governance Act, the Data Act, and Network and Information Systems (NIS2) Directive 14 . The EHDS legislation aims to facilitate the sharing of data and leverage opportunities for innovation and still acknowledge data protection and security 17 . It also requires implementation approaches like IDERHA to overcome organizational and data silos, especially, the EHDS does not provide technical implementation details. Therefore, aligning the IDERHA data space with the EHDS2 principles and the technical requirements provided by the TEHDAS JA is a cornerstone for IDERHA’s synchronization with EHDS and future sustainability.
The IDERHA’s alignment framework with EHDS2
At first, we conducted a comprehensive review of the EHDS regulation, technical requirements for EHDS2, and related projects that were launched with the EHDS proposal in May 2022. The authors searched PubMed, the European Commission portals, and Google using combinations of terms such as “European Health Data Space,” “secondary use of data” "EHDS", "projects" “infrastructure,” “regulations,” and “standards”. We also used the terms “AI” and “cancer” to search for the main EU-funded projects using AI in cancer since 2020. The first search was conducted in June 2023 and the last search was in January 2024. During the IDERHA internal meetings in November and December 2023, the authors identified 36 projects and categorized them into EHDS1 and EHDS2 projects, personal platforms, cancer projects using AI, and other EHDS supporting projects. We also investigated the deliverables of these projects to identify the lessons learned and the technical approaches that can be adopted during the IDERHA implementation. As the EHDS regulation and related projects are still evolving, we did not apply any exclusion criteria in the planning phase.
The final list of the categorized projects was reviewed by several experts from the IDERHA partners and networks. We also conducted a webinar with EHDS experts in October 2023 to involve their insight and recommendations into the plan. Furthermore, we conducted a workshop with representatives and experts from all IDERHA work-packages in November 2023 to discuss the mapping process of IDERHA architecture with the TEHDAS results and deliverables in terms of concepts, standards, and user journey.
Finally, we adopted the World Health Organization (WHO) process of planning 18 to create the IDERHA's alignment plan with EHDS2. The process comprises seven phases (see Figure 3), as follows:
Figure 3. IDERHA's alignment plan with EHDS2 requirements, adapted from the WHO planning tool 18 .
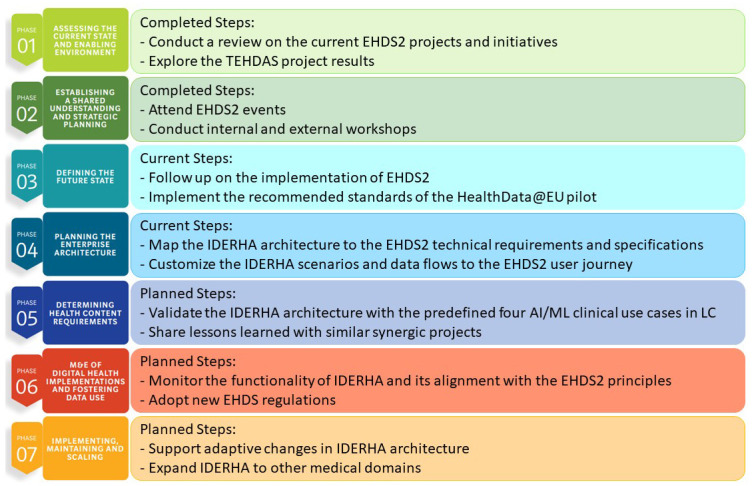
In the first phase, we used the results of the conducted comprehensive review to map the current state and enabling environment for EHDS2 and to explore the current projects and initiatives (see Table 1– Table 6). We adopted the European principles for the secondary use of health data provided by the TEHDAS JA and the HealthData@EU pilot project in identifying the minimum set of alignment requirements as listed in Table 7.
In the second phase, we established a shared understanding and strategic planning with internal and external experts through conducting and attending several EHDS2 events, e.g., meetings, webinars, workshops, etc. In October 2023, we also organized a workshop with EHDS experts and IDERHA consortium members, where we discussed the potential impact of the EHDS on the implementation of IDERHA. We also identified key areas of common interest and priority topics for the upcoming workshops.
In phase 3, we explore the future state of the EHDS2 implementation through networking with other thematically aligned projects, e.g., the HealthData@EU pilot, the European Federation for Cancer Images (EUCAIM) (URL: https://www.eibir.org/projects/eucaim/), and the EHDS2 recent implementation projects in 2024: TEHDAS2 JA and the EHDS Data Quality and Utility Label (Quantum) project.
In phase 4 for planning enterprise architecture, we currently map the EHDS2 specifications and user journey to the IDERHA architecture.
To realize phase 5 for determining health content requirements, we will validate the IDERHA architecture with the predefined four AI clinical use cases in LC. Moreover, we currently participate in the HSbooster.eu (URL: https://hsbooster.eu/) to get consultation services and the OMOP and HL7/FHIR standards for the PGHD and reported health outcomes. Additionally, we created synergies with similar projects for sharing lessons learned and extending expertise in PGHD collection 19 , integration with EHR 20 and establishing need for standardization, for example, the Holistic Health Record approach 21 adopted by the iHelp project (URL: https://ihelp-project.eu/).
After the development of the IDERHA infrastructure, we will start with phase 6 for Monitoring and Evaluation (M&E) and fostering infrastructure use. We will monitor the functionality of the IDERHA data space and its alignment with the EHDS2 principles. We will also aim to ensure the adoption of the EHDS regulation for data access and sharing.
We aim to provide a scalable open architecture of IDERHA to support both the clinicians’ and researchers’ journey in alignment with the EHDS2 data governance principles. We also plan to build synergies with other EHDS2 projects (fulfilling phase 7) to implement, maintain, and scale IDERHA to other medical domains.
Table 1. Main EHDS initiatives and projects: Primary use of data (EHDS1).
| Initiative/
Project |
Scope/Goal | Standards | Lessons Learned |
|---|---|---|---|
| MyHealth@EU | EC cross-border infrastructure for patient’s
data exchange in healthcare delivery |
eHealth Digital Service
Infrastructure (eHDSI) EEHRxF |
ePrescriptions and Patient Summary
(long term, medical images, lab results and hospital discharge reports) 19 |
| X-eHealth | Accelerating the implementation of the
EEHRxF |
EEHRxF | Implementation and deployment of EEHRxF
services 20 |
| XpanDH | Empower individuals and organizations to
create, adapt, and explore interoperable digital health solutions |
EEHRxF | Successful adoption of the EEHRxF 21 |
| Xt-EHR | Joint Action carried out by 25 European
countries for laying the groundwork for the improved primary use of electronic health data for EHDS |
EEHRxF | The project will develop the implementation
guides, technical specifications, and a conformity assessment framework for the adoption of the EEHRxF at the European level |
Table 2. Main EHDS initiatives and projects: Secondary use of data (EHDS2).
| Initiative/Project | Scope/Goal | Standards | Lessons Learned |
|---|---|---|---|
| HealthData@EU pilot | Piloting an infrastructure for the secondary use of health data | Extension of DCAT-AP: HealthDCAT-AP
(Expected in early 2024) |
Proof-of-concept implementation 25 , and exploration of the legal landscape 26 |
| TEHDAS1 | Joint Action carried out by 25 European countries to develop the European principles for the secondary use of health data | Examined the standards for health data discovery, as well as enabling semantic and exchange interoperability | Identification of technical and data governance requirements for EHDS2 alignment 27 |
| Genomic Data Infrastructure (GDI) | Enabling access to genomic and related phenotypic and clinical data across Europe. | DCAT-AP | User journey, federated data access scenarios 28 , connectivity with EHDS and EOSC |
| EUCAIM | A pan-European digital federated infrastructure of FAIR cancer-related, de-identified, real-world images. | OMOP, FHIR, DICOM, DCAT-AP | Development, benchmarking, testing, and piloting of AI-based technologies for cancer diagnosis and treatment 29 and federated data access scenarios 30 |
| DARWIN EU | Delivering real-world evidence from across Europe on diseases, populations and the uses and performance of medicines. | OMOP | Federated data analysis network operational, participation in HealthData@EU Pilot, and incorporating RWE in HTA 31 |
Table 3. Main EHDS initiatives and projects: Personal platforms.
| Initiative/Project | Scope/Goal | Standards | Lessons Learned |
|---|---|---|---|
| Gravitate Health | Foster personal health management and adherence to treatment | FHIR
HL7 Vulcan |
Personal Health Data Space 32 |
| AIDAVA | Automating curation and publishing of Personal Health Data through AI | DCAT-AP, SNOMED, FHIR, LOINC, IPS & EEHRxF | Personal Health Knowledge Graphs (PHKG) 33 |
Table 4. Main EHDS initiatives and projects: AI/ML in Cancer.
| Initiative/Project | Scope/Goal | Standards | Lessons Learned |
|---|---|---|---|
| ASCAPE | Creating an open AI infrastructure enabling deployment and execution of AI algorithms locally and results to be shared | EN/ISO 13606, SNOMED CT, LOINC, HL7 CDA & FHIR | ML techniques in cancer 34 , PGHD integration with EHR 35 |
| iHelp | Personalized Health Monitoring and Decision Support Based on AI and Holistic Health Records for early identification and mitigation of risks associated with Pancreatic Cancer | FHIR | Collection, integration, and management of health-related data from various sources in standardized Holistic Health Records (HHR) 36 |
| OPTIMA | Enable shared decision-making using dynamic computer-interpretable guidelines (CIGs), access to broad data sets, AI algorithms and tools | OMOP, FHIR, DICOM | Federated network of data providers on cancer, computer interpretable guidelines 37 |
| UNderstand CANcer (UNCAN.eu) | Creating the UNCAN.eu platform | - | The blueprint for UNCAN.eu proposed to set up a European Federated Cancer 38 |
| INCISIVE | A multimodal AI-based toolbox and an interoperable health imaging repository | FHIR, DICOM, SNOMED, LOINC | Tailoring the legal framework, adopting technological solutions for privacy-preserved data
collection, integration, and harmonization, and how federated data storage and sharing has been achieved 39 |
Table 5. Main EHDS initiatives and projects: EHDS2 supporting and interlinking activities.
| Initiative/Project | Scope/Goal | Standards | Lessons Learned |
|---|---|---|---|
| XShare | Enable personal health data sharing through EHRxF. | EHRxF | Personal Health Data Portability, Standard and Policy development |
|
HSBooster.eu
|
Standardization consultancy service to EU-funded projects | International Organization for Standardization
(ISO) standards |
Synergies between standardization projects |
| FHIR for FAIR - FHIR4FAIR | Guidance on how HL7 FHIR can be used for supporting FAIR health data implementation | FHIR | Leveraging FHIR in health data FAIRfication process 40 |
| Hospitals On FHIR | Establishing Hospitals on FHIR network in Europe | FHIR | Preparing Hospitals for European Health Data Space by introducing an interoperability capabilities maturity model 41 |
| Data Spaces Support Centre (DSSC) | Contribute to the creation of common data spaces | - | Definition of common requirements and best practices on building data spaces 42 |
| GAIA-X | Initiative to develop a digital governance that can be applied to any existing cloud/ edge technology stack to obtain transparency, controllability, portability and interoperability across data and services. | - | Framework specification covering compliance, trust, federation and data exchange 43 |
| International Data Spaces Association | Initiative to enable secure, sovereign data sharing across companies and industries ensuring self-determined control of data use for data providers | - | Framework specification for data exchange and dataspace interoperability 44 |
| FAIRplus | Increase the discovery, accessibility and reusability of data from selected IMI projects, as well as internal data from pharmaceutical industry | Endorsement of domain-specific standards | Assessment of FAIR datasets to ensure community reuse, guidelines and tools on FAIRification, e.g. FAIR cookbook 45 |
| European Health Data Evidence Network – EHDEN | Building a large-scale federated network of data sources standardized to a common data model, following the FAIR approach | OMOP | Metadata on data sources in a database catalogue 46 |
| Health Outcomes Observatory – H2O | Create ‘health outcomes observatories’ that will amplify the patient voice both in their own healthcare and in healthcare systems more broadly, by establishing a data governance and infrastructure system initially in four European countries | OMOP, FHIR | Network of observatories, standardized core outcome sets for diabetes, inflammatory bowel disease and cancer 47 |
| EOSC life | Create an open, digital and collaborative space for biological and medical research with 13 Life Science ‘ESFRI’ research infrastructures | - | Tools and best practices, e.g. Clinical Research Metadata Repository, Ontology Lookup Service 48 |
| HealthyCloud | Generating several guidelines, recommendations and specifications that will enable distributed health research across Europe in the form of a Ready-to-implement Roadmap. | - | Strategic agenda towards the European Health Research and Innovation Cloud (HRIC) - proposal for five core services 49, 50 |
| Personal Health Train | Giving controlled access to data, while ensuring privacy protection and optimal engagement of individual patients and citizens. | - | Implementation reference for decentral data analysis, FML demonstrated 51, 52 |
| GO FAIR | Open ecosystem for collaboration on FAIR data and services organized in implementation networks | Metadata standards per domain | Operational implementation networks, e.g. on personal health train 53 |
| HEALTH-X dataLOFT | Implementing legitimized, open, and federated dataLOFT platform and made accessible to citizens | FHIR, SNOMED-CT, IHE, Gaia-X interfaces | Data Space implementation 54 |
| InteropEHRate | Enabling patient-centered exchange of health records | FHIR | End-to-end data integration methodology and open protocol specifications 55 |
| Sphin-X | Enabling sovereign collaboration with health data from solution provider via healthcare provider to patient | - | End-to-end community and ecosystem building |
Table 6. Main EHDS initiatives and projects: Ongoing and upcoming EHDS implementation projects.
| Initiative/Project | Scope/Goal |
|---|---|
| TEHDAS2 (May 2024- December 2026) | Supporting the successful realization of the EHDS, where data would be available securely on demand across borders for patient care (primary use) and for secondary purposes such as research, innovation, and policymaking. |
| QUANTUM (January 2024 - June 2026) | Performing a mapping of existing data quality and utility principles, initiatives, and frameworks (i.e. EMA/ Heads of Medicines Agencies (HMA), TEHDAS, EOSC-LIFE, Health Data Research UK’s data quality and utility framework, and relevant data principles, resources and tools (FAIR, FAIR Cookbook, etc.) |
| EC and WHO/Europe agreement in December 2023 (Harnessing health data, 4-Year project funded by the EU4HEALTH programme) | The project aims to strengthen health information systems and boost health data governance and interoperability in Europe.
The initiative will be driven by the EHDS framework and principles to facilitate the use and reuse of health data within the EU 56 |
Table 7. IDERHA alignment with EHDS2 requirements based on the TEHDAS results.
| TEHDAS deliverable | Scope | Mapping to IDERHA |
|---|---|---|
| Deliverable 5.4: Options for governance models for the European Health Data Space 58 | Data governance | Understanding the EHDS2 legal interoperability and how it might be used, customized or reflected to the IDERHA data lifecycle and associated data governance models. |
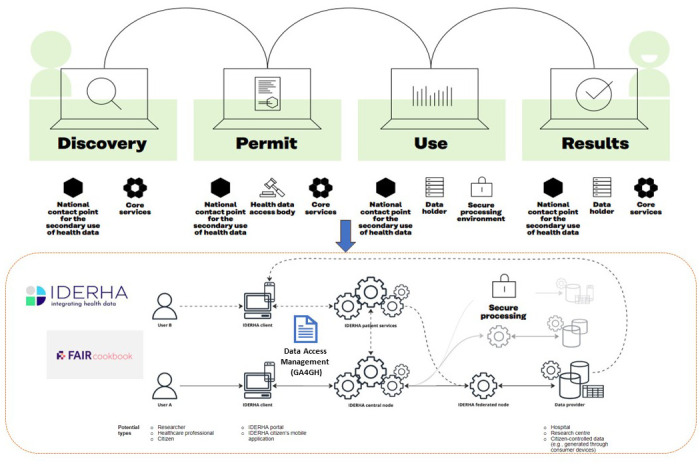
| Deliverable 7.2: Options for the services and services architecture and infrastructure for secondary use of data in the EHDS 59 | User journey | Adopting the proposed EHDS2 user journey, concepts, and terminologies into the IDERHA patient/researcher scenarios and overall architecture (see Figure 4). |
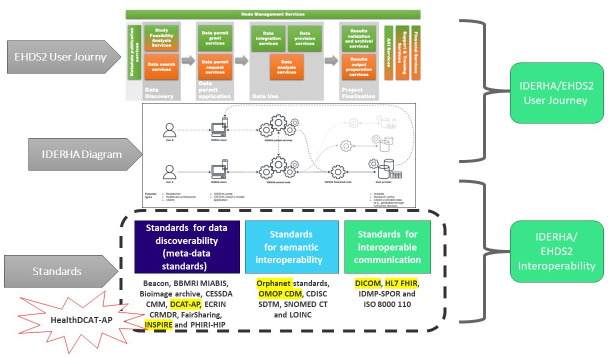
| Deliverable 6.2: Recommendations to enhance interoperability within HealthData@EU 60 | Standards and interoperability | Considering the recommended standards for data discovery (DCAT-AP), enabling semantic interoperability (OMOP) and data exchange (DICOM and FHIR). |
| Deliverable 6.3: Recommendations on a Data Quality Framework for the European Health Data Space for secondary use 61 | Data quality | Following the provided Data Quality Framework in developing IDERHA's data quality approach. |
| Deliverable 5.3: Guidelines document for multicountry data access applications, including mutual recognition and cross-border applications 62 | Data access applications | Guiding in developing the IDERHA's data access approaches with the EHDS2 perspective on data access and data permit processes in different national settings. |
Figure 4. IDERHA infrastructure in alignment with EHDS2 user journey, adapted from 57.
The landscape of existing projects and interoperability standards
The conducted comprehensive review explored the current state and enabling environment for EHDS2. The following tables summarize the main projects and initiatives shaping the EHDS, the recommended standards for health data interoperability in these projects, and the lessons learned that are relevant to IDERHA. Table 1 addresses the main existing projects shaping the MyHealth@EU using the European Electronic Health Record Exchange Format (EEHRxF)
Table 2 mainly addresses the HealthData@EU pilot and TEHDAS1 JA. It also introduces the other projects that support the EHDS2 implementation. The current key standards for EHDS2 are OMOP, FHIR, DICOM, and DCAT-AP.
Table 3 explores the projects that build personal platforms that empower patients to take an active role in managing and sharing his/her health data in alignment with the EHDS2. The main standards used in the personal health data space are FHIR, HL7 Vulcan, DCAT-AP, and others.
Table 4 highlights similar EU-funded projects that utilize AI and ML in the cancer domain. Besides using the OMOP, FHIR, and DICOM standards, the iHelp and ESCAPE projects adopt innovative approaches for personalized healthcare through the integration of personal data with EHR.
Table 5 lists the major projects that support the implementation and standardization of EHDS, in terms of”
-
-
EHDS architecture and main principles, such as GAIA-X, Data Spaces Support Centre – DSSC, etc.
-
-
European research infrastructure, such as, EOSC life, HealthyCloud
-
-
Standardization, such as XShare, HSBooster.eu, EHDEN, Hospitals On FHIR projects, etc.
-
-
Data FAIRfication, such as FAIRplus, GO FAIR, etc.
Finally, Table 6 highlights the recently kicked-off projects concerning the real-world implementation of the EHDS and related technical needs.
IDERHA’s minimum set of requirements for EHDS2 alignment
The TEHDAS JA developed the European principles for the secondary use of health data with the involvement of 25 countries. The results of TEHDAS are currently adopted to shape the HealthData@EU pilot project, mainly the user journey 57 . Similarly, we selected the relevant TEHDAS guidelines and recommendations that would be considered in IDERHA (as listed in Table 7).
Figure 4 provides an overview of the IDERHA platform, including its potential actors/components and their roles. Two exemplary scenarios/data flows are presented for two types of users: Researcher (federated data analysis use case) and Citizen (own personal health data access). These processes are aligned with the EHDS2 user journey, covering discovery, permit, use, and results processes.
Figure 5– Figure 9 and Table 8, Table 9 provide the detailed results of mapping the EHDS2 requirements to IDERHA using the TEHDAS deliverables.
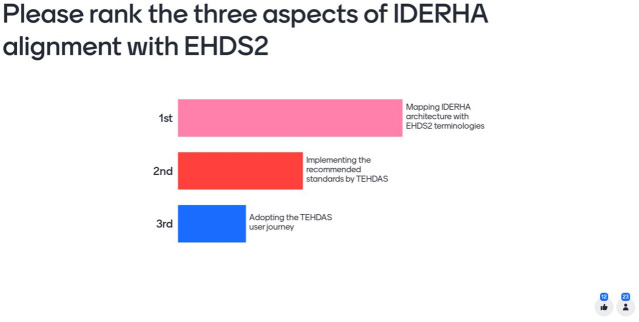
Figure 5. Mentimeter results ranking the main aspects for aligning IDERHA with EHDS2.
Figure 9. Mapping the TEHDAS user journey and recommended standards to IDERHA 59 .
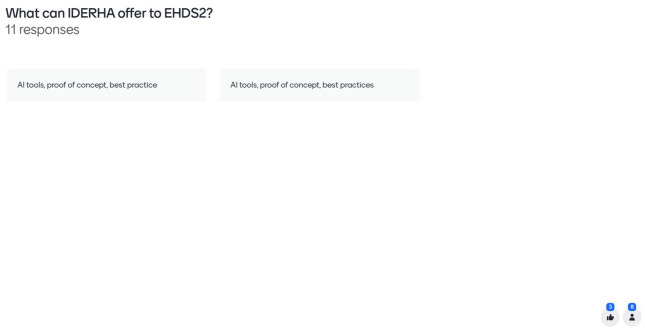
Figure 6. Mentimeter results – how can IDERHA shape the EHDS2 implementation (1/2).
Figure 7. Mentimeter results – how can IDERHA shape the EHDS2 implementation (2/2).
Figure 8. Mapping the EHDS2 terminologies and concepts to IDERHA 57 .
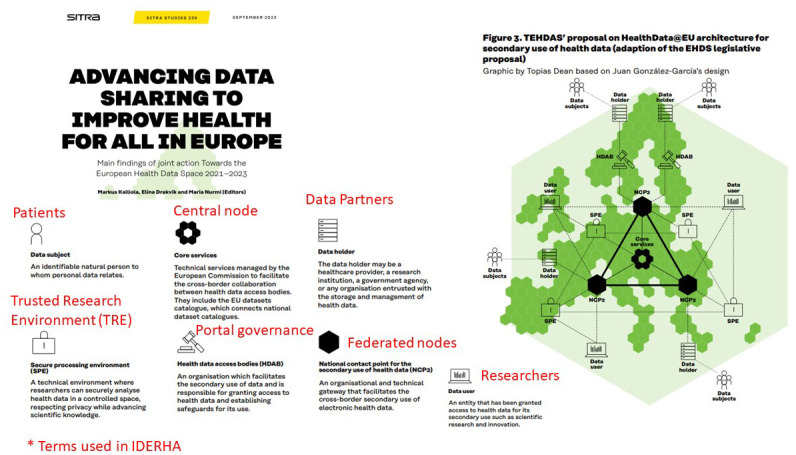
Table 8. Mapping the EHDS2 terms to IDERHA.
| EHDS2 terms | Mapping to
IDERHA |
|---|---|
| Data User | Yes |
| Data subject | Yes |
|
Secure processing environment
(SPE) |
Planned |
| Core services | Yes |
| Health data access bodies (HDAB) | TBD |
| Data holder | Yes |
|
National contact point for the
secondary use of health data (NCP2) |
TBD |
Table 9. Mapping the EHDS2 recommended standards to IDERHA.
| EHDS2 Standards | Used
Standards in IDERHA |
|---|---|
| Data Discoverability | TBD |
| Enabling semantic Interoperability for the
secondary use of health data |
OMOP CDM |
The IDERHA data space
IDERHA adopts the principles of the EHDS2, and it is oriented towards the Gaia-X principles (i.e., decentralization, data sovereignty, federation) and developments of important European initiatives (see Table 5). Thus, the IDERHA architecture essentially relies on two core processes: (1) Data Access Request and (2) FML Execution. The federated architecture of IDERHA along with the associated Secure Processing Environments (SPEs), alongside data FAIRification meets the currently proposed EHDS2 user journey and service requirements. In addition, the data governance model of IDERHA is realized through synchronization and linking between the Data Management Plan (DMP), the Data Protection Impact Assessment (DPIA), and the Data Sharing Agreements (DSA) with data partners.
We plan to implement the recommended standards for data discovery (DCAT-AP), enabling semantic interoperability (OMOP) and data exchange (DICOM and FHIR). We will also investigate the possibility of implementing the extension of the DCAT-AP for health (HealthDCAT-AP) that is being developed by the HealthData@EU pilot project. Furthermore, we build synergy with similar projects through the HSbooster.eu activities and the European Health Data Evidence Network (EHDEN) to share expertise in healthcare standards and interoperability, especially proposing extensions to standards development organizations for PGHD and the need for addressing the mapping challenges between the different standards, for example, mapping OMOP and HL7 FHIR 46 .
As the EHDS infrastructure and requirements are still evolving 63 , we will continue to share the lessons learned among the related projects, e.g., EUCAIM, GDI, HealthData@EU pilot, TEHDAS2, the European Federated Cancer Research Data Hub, and others (see Table 2).
Recommendations to International and European Organizations
For efficient implementation of the EHDS2 ecosystem, the authors recommend establishing communication channels and foster networking between all stakeholders, for instance:
-
-
The EC can promote more collaboration and build synergy among EHDS projects, for example the HSbooster.eu pilot provides a framework for gathering forces for standardization. This model can be also extended to build the EHDS2 community in addition to the EC planned activities for capacity building.
-
-
The WHO/Europe can provide designated EHDS alignment toolkits, M&E framework, and an EHDS atlas for locating the national health data access bodies, data registries, projects, etc. matching with the WHO/Europe digital health roadmap action plan for the WHO European Region 2023–2030 64 . This is in addition to the EU and WHO/Europe and the EC new partnership to strengthen health information systems and boost health data governance and interoperability in the WHO European Region.
-
-
The European Federation for Medical Informatics (EFMI) can provide expertise for modeling and building an interoperability as a service layer to facilitate the connectivity of data holders to the EHDS infrastructure.
The way ahead
The IDERHA project aims to provide a disease and use case-agnostic framework for federated access and processing of anonymized and pseudonymized health data, ensuring data protection and sovereignty through state-of-the-art privacy-preserving technologies. This work describes our plan to align IDERHA with the EHDS2 requirements, including, user journey, services and architecture, and standards. This described framework for aligning IDERHA with EHDS2 requirements can be used as a template for similar and upcoming projects.
The next step is to implement this plan and monitor the outcomes. Concurrently, we will follow up the development of the HealthData@EU to consider the new recommendations for proper implementation of the regulation and better health data interoperability. In addition, we establish a dialogue with similar projects and related organizations to share expertise in implementing the EHDS infrastructure. In this way, IDERHA will actively participate in shaping EHDS2 as one of the first pan-European initiatives.
Ethics and consent
Ethics and consent were not required.
Funding Statement
This project has received funding from the European Union’s Horizon Europe research and innovation programme under grant agreement No [101112135] (Integration of heterogeneous Data and Evidence towards Regulatory and HTA Acceptance [IDERHA]) through the Innovative Health Initiative (IHI) Joint Undertaking (JU). Support is also received from life science industries represented by COCIR, EFPIA / Vaccines Europe, EuropaBio and MedTech Europe. Support is also received from our Swiss and UK partners.
[version 1; peer review: 3 approved, 1 approved with reservations]
Data availability
Underlying data
There are no new data associated with this article.
References
- 1. European Commission: European Health Data Space. Reference Source
- 2. Hogervorst MA, Møllebæk M, Vreman RA, et al. : Perspectives on how to build bridges between regulation, health technology assessment and clinical guideline development: a qualitative focus group study with European experts. BMJ Open. 2023;13(8): e072309. 10.1136/bmjopen-2023-072309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hallock H, Marshall SE, ’t Hoen PAC, et al. : Federated networks for distributed analysis of health data. Front Public Health. 2021;9: 712569. 10.3389/fpubh.2021.712569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Otto B, Jarke M: Designing a multi-sided data platform: findings from the international data spaces case. Electron Markets. 2019;29(4):561–80. 10.1007/s12525-019-00362-x [DOI] [Google Scholar]
- 5. Dasaradharami Reddy K, Gadekallu TR: A comprehensive survey on federated learning techniques for healthcare informatics.Doulamis AD, editor. Comput Intell Neurosci. 2023;2023: 8393990. 10.1155/2023/8393990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Maio M, Basch E, Denis F, et al. : The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO clinical practice guideline. Ann Oncol. 2022;33(9):878–92. 10.1016/j.annonc.2022.04.007 [DOI] [PubMed] [Google Scholar]
- 7. Churruca K, Pomare C, Ellis LA, et al. : Patient-Reported Outcome Measures (PROMs): a review of generic and condition-specific measures and a discussion of trends and issues. Health Expect. 2021;24(4):1015–24. 10.1111/hex.13254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Commission: Regulation of the European Parliament and of the Council on the European Health Data Space.[cited 2024 Feb 8]. Reference Source
- 9. Martínez-García A, Alvarez-Romero C, Román-Villarán E, et al. : FAIR principles to improve the impact on health research management outcomes. Heliyon. 2023;9(5): e15733. 10.1016/j.heliyon.2023.e15733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rocca-Serra P, Gu W, Ioannidis V, et al. : The FAIR cookbook - the essential resource for and by FAIR doers. Sci Data. 2023;10(1): 292. 10.1038/s41597-023-02166-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Torab-Miandoab A, Samad-Soltani T, Jodati A, et al. : Interoperability of heterogeneous health information systems: a systematic literature review. BMC Med Inform Decis Mak. 2023;23(1): 18. 10.1186/s12911-023-02115-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Declerck J, Kalra D, Vander Stichele R, et al. : Frameworks, dimensions, definitions of aspects and assessment methods for the appraisal of quality of health data in secondary use: a review of reviews. JMIR Med Inform. 2024;12: e51560. 10.2196/51560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christopoulos P, Schlenk R, Kazdal D, et al. : Real-world data for precision cancer medicine—A European perspective. Genes Chromosomes Cancer. 2023;62(9):557–63. 10.1002/gcc.23135 [DOI] [PubMed] [Google Scholar]
- 14. Marcus JS, Martens B, Carugati C, et al. : The European Health Data Space. Rochester, NY;2022; [cited 2024 Feb 8]. Reference Source
- 15. European Commission: Electronic cross-border health services. European Commission,2024; [cited 2024 Feb 8]. Reference Source
- 16. European Commission: Data sharing through eDelivery in the HealthData@EU. [cited 2024 Feb 8]. Reference Source
- 17. Raab R, Küderle A, Zakreuskaya A, et al. : Federated electronic health records for the European Health Data Space. Lancet Digit Health. 2023;5(11):e840–e847. 10.1016/S2589-7500(23)00156-5 [DOI] [PubMed] [Google Scholar]
- 18. Digital Implementation Investment Guide (DIIG): integrating digital interventions into health programmes. Geneva: World Health Organization;2020; [cited 2024 Feb 8]. Reference Source
- 19. Pyper E, McKeown S, Hartmann-Boyce J, et al. : Digital health technology for real-world clinical outcome measurement using patient-generated data: systematic scoping review. J Med Internet Res. 2023;25: e46992. 10.2196/46992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dinh-Le C, Chuang R, Chokshi S, et al. : Wearable health technology and electronic health record integration: scoping review and future directions. JMIR Mhealth Uhealth. 2019;7(9): e12861. 10.2196/12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gallos P, Aso S, Autexier S, et al. : CrowdHEALTH: big data analytics and holistic health records. Stud Health Technol Inform. 2019;258:255–256. [PubMed] [Google Scholar]
- 22. Stellmach C, Muzoora MR, Thun S: Digitalization of health data: interoperability of the proposed European Health Data Space. In: Scott P, Mantas J, Benis A, Ognjanovic I, Saranto K, Ware A., et al., editors. Stud Health Technol Inform. IOS Press;2022;298:132–136. 10.3233/SHTI220922 [DOI] [PubMed] [Google Scholar]
- 23. X-eHealth Project: D7.1- X-eHealth architecture definition to implement and deploy EEHRxF services. [cited 2024 Feb 8]. Reference Source
- 24. Martins H, Carmo A, Asamoah L: Towards the European electronic health record exchange format: XpanDH project support and risks of a delayed regulation on the EHDS.2023; [cited 2024 Feb 8]. Reference Source
- 25. HealthData@EU Pilot: Launch of the proof Of concept.2023; [cited 2024 Feb 8]. Reference Source
- 26. HealthData@EU Pilot identifies common elements for health data access and data use within the legal frameworks of the participating nodes.2023; [cited 2024 Feb 8]. Reference Source
- 27. Abboud L, Cosgrove S, Kessissoglou I: Country factsheets. Zenodo. 2023; [cited 2024 Feb 8]. 10.5281/zenodo.8329552 [DOI] [Google Scholar]
- 28. Spalding D, Marquez JA, Capella-Gutierez S, et al. : Data standards and the European genomic data infrastructure.2023; [cited 2024 Feb 8]. Reference Source
- 29. EUCAIM Project: D5.1. early release of the data federation framework.2023; [cited 2024 Feb 8]. Reference Source
- 30. Rambla J, Beltran S, D’Altri T: European Genomic Data Infrastructure project (GDI) D8.4 report on federated data access scenarios.2023; [cited 2024 Feb 8]. Reference Source
- 31. Claire R, Elvidge J, Hanif S, et al. : Advancing the use of real world evidence in health technology assessment: insights from a multi-stakeholder workshop. Front Pharmacol. 2024;14: 1289365. 10.3389/fphar.2023.1289365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gravitate health FHIR implementation guide v0.1.0. [cited 2024 Feb 9]. Reference Source
- 33. Bihari B, Dallos D, Ferencz L, et al. : D3.1 VA architecture (application and technical).2024; [cited 2024 Feb 9]. 10.5281/zenodo.10593465 [DOI] [Google Scholar]
- 34. Savic M, Kurbalija V, Ilic M, et al. : The application of machine learning techniques in prediction of quality of life features for cancer patients. Comput Sci Inf Syst. 2023;20(1):381–404. 10.2298/CSIS220227061S [DOI] [Google Scholar]
- 35. Frid S, Fuentes Expósito MA, Grau-Corral I, et al. : Successful integration of EN/ISO 13606-standardized extracts from a patient mobile app Into an electronic health record: description of a methodology. JMIR Med Inform. 2022;10(10): e40344. 10.2196/40344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manias G, Op Den Akker H, Azqueta A, et al. : iHELP: personalised health Monitoring and decision support based on artificial intelligence and holistic health records. In: 2021 IEEE Symposium on Computers and Communications (ISCC). Athens, Greece: IEEE,2021;1–8. 10.1109/ISCC53001.2021.9631475 [DOI] [Google Scholar]
- 37. Oyen W, Catalano C: The European Health Data Space and cancer. applying lessons learnt for successful implementation. European Cancer Organisation,2022; [cited 2024 Feb 9]. Reference Source
- 38. Boutros M, Baumann M, Bigas A: UNCAN.eu: toward a European federated cancer research data hub. Cancer Discov. 2024;14(1):30–35. 10.1158/2159-8290.CD-23-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lazic I, Agullo F, Ausso S, et al. : The Holistic perspective of the INCISIVE project—artificial intelligence in screening mammography. Appl Sci. 2022;12(17):8755. 10.3390/app12178755 [DOI] [Google Scholar]
- 40. Martínez-García A, Cangioli G, Chronaki C, et al. : FAIRness for FHIR: towards making health datasets FAIR using HL7 FHIR. In: Otero P, Scott P, Martin SZ, Huesing E, editors. Studies in Health Technology and Informatics. IOS Press,2022;290:22–26. 10.3233/SHTI220024 [DOI] [PubMed] [Google Scholar]
- 41. Martins H, Cangioli G, Chronaki C: Hospitals-on-FHIR: preparing hospitals for European Health Data Space. Health Management. 2022;22(3). Reference Source [Google Scholar]
- 42. Data Spaces Support Centre: Starter kit for data space designers, Version 1.0.2023; [cited 2024 Feb 9]. Reference Source
- 43. GAIA-X European Association for Data and Cloud: Gaia-X architecture document - 23.1O release. [cited 2024 Feb 9]. Reference Source
- 44. Nagel L, Lycklama D: Design principles for data spaces - position paper. Zenodo. 2021; [cited 2024 Feb 9]. 10.5281/zenodo.5105744 [DOI] [Google Scholar]
- 45. Lynch N, Williams-Jones B: D3.8 FAIRplus sustainability white paper.2022; [cited 2024 Feb 9]. Reference Source
- 46. Bochove KV, Vos E, Moinat M, et al. : EHDEN - D4.5 - roadmap for interoperability solutions.2020; [cited 2024 Feb 9]. Reference Source
- 47. De Ligt KM, De Rooij BH, Hedayati E, et al. : International development of a patient-centered core outcome set for assessing health-related quality of life in metastatic breast cancer patients. Breast Cancer Res Treat. 2023;198(2):265–81. 10.1007/s10549-022-06827-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. David R, Rybina A, Burel JM, et al. : “Be sustainable”: EOSC‐Life recommendations for implementation of FAIR principles in life science data handling. EMBO J. 2023;42(23): e115008. 10.15252/embj.2023115008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Canham S, Ohman C, Demotes-Mainard J, et al. : Final healthycloud strategic agenda for the health research innovation cloud.2023; [cited 2024 Feb 9]. Reference Source
- 50. Alvarez-Romero C, Rodríguez-Mejias S, Parra-Calderón CL: Desiderata for the data governance and FAIR principles adoption in health data hubs. In: Mantas J, Gallos P, Zoulias E, Hasman A, Househ MS, Charalampidou M, et al., editors. Studies in Health Technology and Informatics. IOS Press,2023. 10.3233/SHTI230452 [DOI] [PubMed] [Google Scholar]
- 51. Beyan O, Choudhury A, Van Soest J, et al. : Distributed analytics on sensitive medical data: the personal health train. Data Intell. 2020;2(1–2):96–107. 10.1162/dint_a_00032 [DOI] [Google Scholar]
- 52. da Silva Santos LOB, Ferreira Pires L, Graciano Martinez V, et al. : Personal health train architecture with dynamic cloud staging. SN Comput Sci. 2022;4(1): 14. 10.1007/s42979-022-01422-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schultes E, Magagna B, Hettne KM, et al. : Reusable FAIR implementation profiles as accelerators of FAIR convergence. In: Grossmann G, Ram S. editors. Advances in Conceptual Modeling. Cham: Springer International Publishing,2020;138–47. 10.1007/978-3-030-65847-2_13 [DOI] [Google Scholar]
- 54. HEALTH-X dataLOFT - legitimate, open and federated health data space in GAIA-X. Fraunhofer Institute for Software and Systems Engineering, [cited 2023 Sep 5]. Reference Source
- 55. Kiourtis A, Mavrogiorgou A, Mavrogiorgos K, et al. : Electronic health records at people’s hands across Europe: the interopEHRate protocols. In: Blobel B, Yang B, Giacomini M, editors. Stud Health Technol Inform. IOS Press;2022;299:145–150. 10.3233/SHTI220973 [DOI] [PubMed] [Google Scholar]
- 56. European commission and WHO/Europe sign €12 million agreement to strengthen health information systems and boost health data governance and interoperability in Europe.2023; [cited 2024 Feb 9]. Reference Source
- 57. TEHDAS project: Advancing data sharing to improve health for all in Europe.2023; [cited 2024 Feb 9]. Reference Source
- 58. TEHDAS project: Deliverable 5.4: options for governance models for the European Health Data Space.2023; [cited 2024 Feb 9]. Reference Source
- 59. TEHDAS project: Deliverable 7.2: options for the services and services architecture and infrastructure for secondary use of data in the EHDS.2023; [cited 2024 Feb 9]. Reference Source
- 60. TEHDAS project: Deliverable 6.2: EHDS semantic interoperability framework- recommendations to enhance interoperability within HealthData@EU- a framework for semantic, technical and organisational interoperability.2022; [cited 2024 Feb 9]. Reference Source
- 61. TEHDAS project: Deliverable 6.3: recommendations on a data quality framework for the European Health Data Space for secondary use.2023; [cited 2024 Feb 9]. Reference Source
- 62. TEHDAS project: Deliverable 5.3: guidelines document for multicounty data access applications, including mutual recognition and cross-border applications.2023; [cited 2024 Feb 9]. Reference Source
- 63. EIT Health’s Think Tank: Implementing the European Health Data Space across Europe.2024; [cited 2024 May 10]. Reference Source
- 64. The ongoing journey to commitment and transformation: digital health in the WHO European region. Copenhagen: WHO Regional Office for Europe,2023; [cited 2024 Feb 9]. Reference Source