Abstract
Background and Aims
Paravalvular leak (PVL) is a serious complication of prosthetic valve replacement. Both surgical and percutaneous closure techniques are used for PVL closure, but optimal strategies and comparative outcomes are uncertain. This study aimed to compare the efficacy and safety of percutaneous versus surgical PVL closure by analyzing changes in leak severity, functional status, echocardiographic parameters, and clinical outcomes.
Methods
A total of 72 patients were included in this retrospective cross‐sectional single‐center study comparing percutaneous (n = 25) and surgical (n = 47) PVL closure from 2015 to 2022. Demographics, medical history, echocardiograms, laboratory data, complications, and mortality data were extracted from the records. Changes in leak severity, NYHA class, echocardiographic parameters, and clinical outcomes were compared between the percutaneous and surgical groups.
Results
Both percutaneous and surgical PVL closure significantly reduced leak severity and improved NYHA class (both p < 0.01), with no difference between the quantity of changes in each group. The 30‐day mortality was 4% after percutaneous and 6.4% after surgical closure (p = 0.65). At 90 days, mortality was 24% percutaneous versus 17% surgical (p = 0.48). The length of stay in the hospital and post‐procedural decrease in hemoglobin were considerably lower in the percutaneous group. The rate of complication rates was similar between the groups. Echocardiographic changes were also comparable.
Conclusion
Percutaneous and surgical PVL closure had similar efficacy in reducing leaks and improving symptoms, with no significant difference in early outcomes. Both options should be considered viable for PVL repair after heart team evaluation.
Keywords: paravalvular leak, percutaneous intervention, prosthetic valve, surgical intervention
1. INTRODUCTION
Valvular heart disease is a significant public health concern that is associated with various comorbidities and is estimated to affect over 100 million people globally. 1 , 2 It affects approximately 2.5% of the population of the United States and accounts for 1.9% of all deaths there. 3 The majority of cases involve individuals with aortic or mitral valve dysfunctions that often require valve replacement interventions. Surgical valve replacement carries risks, especially in high‐risk patients, and may result in complications such as paravalvular leaks or endocarditis. 4
Paravalvular leakage (PVL) of prosthetic valves, both mechanical and bioprosthetic, refers to the presence of a leak around the prosthetic valve, specifically in the area between the sewing ring of the valve and the native tissue. 5 The prevalence of paravalvular regurgitation, including both mitral and aortic valves, ranges between 5% and 20% and it is more commonly seen in left‐sided prosthetic valves. 6 The exact cause of PVL is not fully understood, but it is believed to result from suture dehiscence between the sewing ring and the native tissue. 5 This can occur due to factors such as inadequate suturing technique, tissue friability, or annular tissue degeneration. 6
PVL is typically small and benign; It may occur early after the procedure remaining unchangeable until late follow‐up. 7 However, in some cases, it can lead to clinically significant regurgitation, a potentially serious condition. 8 , 9 , 10 The diagnosis of a prosthetic PVL can be challenging. Echocardiography, including both transthoracic echocardiography and transesophageal echocardiography (TEE), which is particularly useful for assessing the severity and location of the leak, is commonly used for initial evaluation. 11 However, these imaging modalities may not always provide a clear visualization of the paravalvular leak. 6 Due to the detection difficulty, PVLs are often associated with increased late mortality. 12 Patients with PVL may remain asymptomatic, or depending on the severity of the leak, they can experience complications such as heart failure, hemodynamically significant hemolysis, and endocarditis. 13
Treatment options for PVL depend on the severity of the leak and the patient's clinical presentation. 4 In some cases, conservative management may be appropriate, especially for small, asymptomatic leaks. 14 , 15 While medication therapy is essential for symptom management in patients with PVL‐induced hemolysis or heart failure, no known medical treatment prevents or reverses PVL or its underlying cause. 16 , 17 Therefore, if the leak is causing significant regurgitation and symptoms, intervention may be necessary. 18 Surgical repair or replacement of the prosthetic valve is the traditional approach for treating paravalvular leaks is still considered the gold standard for treating paravalvular leaks in symptomatic patients. 19 However, repeat open heart surgery carries significant risks of morbidity and mortality. This is especially true for patients with prior sternotomies, multiple comorbidities, or prohibitive surgical risks. 8 Over the past decade, percutaneous PVL closure has emerged as a less invasive alternative, using implanted occlusion devices to repair leaks without surgery. 8 , 20
Percutaneous repair of the PVL involves using catheter‐based techniques and devices to close the paravalvular defect. The Amplatzer Vascular Plug and the Amplatzer Muscular Ventricular Septal Defect Occluder are commonly used devices for the percutaneous closure of PVL. 21 , 22 The procedure is performed under fluoroscopy and guided by multimodality imaging, including TEE and transthoracic imaging. 10
Percutaneous closure of PVL has been successfully performed in all types of prosthetic valves, including mitral, aortic, tricuspid, and pulmonary valves. 5 , 23 , 24 , 25 , 26 It has also been used in patients with complex clinical conditions that are poor surgical candidates. 27 However, there are some challenges associated with the percutaneous closure of PVL. Device dislodgement and late complications have been reported in some cases. 22
Given that choosing the proper method to close PVLs can reduce mortality and complications from re‐surgery, and considering that no technique has been definitively proven superior across all patient populations due to limited direct comparative data, additional comparative data are needed to determine which approach results in the best leak closure, fewest complications, and best outcomes for different patient subgroups. In addition, studies with a sufficient sample size have not been conducted in this area; therefore, the objective of this study was to compare changes in leak severity, functional status, echocardiographic indices, complications, and mortality between percutaneous intervention (PI) and surgical repair for PVL closure, using a sample of consecutive patients treated between 2015 and 2022 at a high‐volume cardiovascular center.
2. MATERIALS AND METHODS
2.1. Study design and population
This retrospective observational study compared the efficacy and safety of prosthetic PVL closure with PI and surgical intervention (SI). The study population included all consecutive patients who underwent PVL closure at Rajaie Cardiovascular Medical and Research Center, a tertiary referral center in Tehran, Iran, between 2015 and 2022.
Patients were identified retrospectively from the Rajaie Cardiovascular Medical and Research Center hospital database based on ICD‐9/10 codes corresponding to paravalvular leak repair. The sample size was determined by the number of patients with available records undergoing PVL closure in the specified timeframe. Consecutive patients undergoing PVL closure with either percutaneous or SI between March 2015 and December 2022 were screened for eligibility. The decision on choosing the percutaneous or surgical approach were made by a heart team, including interventional cardiologists, cardiac surgeons, and imaging specialists based on a comprehensive assessment of the patient's clinical presentation, anatomical factors, technical feasibility, patient's comorbidities and surgical risks, previous interventions, expected outcomes and efficacy of the procedures, and patient preferences. The team also considered the latest evidence clinical guidelines at the indexed time to ensure the best possible decision aligned with the patient's specific needs and circumstances.
Patients were excluded if they did not have complete medical records or lacked pre‐ and post‐intervention echocardiogram data. Out of 102 potential participants screened, 72 met all eligibility criteria and were included in the final analysis.
This study was a retrospective analysis of medical records and was not registered prospectively in a clinical trial registry. The research was approved by the Ethics Committee of Rajaie Cardiovascular Medical and Research Center, Tehran, Iran (IR.RHC.REC.1401.092). In addition, written informed consent was obtained from all study participants before their enrollment. All participants voluntarily provided consent to have their medical records used in this research.
2.2. Data collection
Demographic information, medical history, comorbidities, type of repair procedure, laboratory, echocardiographic, and clinical data were extracted from electronic medical records. The patient's information has been recorded once before and once after the surgery, at the time of discharge, and at least 3 months after the surgery by calling or visiting the doctor.
Baseline characteristics collected included age, sex, body surface area, body mass index, comorbidities, NYHA class, PVL severity, previous surgeries, and prosthetic valve details. The outcomes collected were a procedural success, post‐procedure NYHA class, echocardiographic parameters, length of stay, complications, and mortality.
2.3. Definitions
Success in PVL closure was defined as post‐intervention reduction by at least 1 degree of severity, regardless of 30‐day clinical outcomes. 28
Left ventricle (LV) function categories were defined as normal (LVEF: 50%–70%), mild dysfunction (LVEF: 40%–49%), moderate dysfunction (LVEF: 30%–39%), severe dysfunction (LVEF less than 30%) according to American College of Cardiology (ACC) classification. 29
2.4. Statistical analysis
Descriptive statistics were used to summarize the demographic and clinical characteristics. Continuous variables are reported as mean ± standard deviation for normally distributed data or median (interquartile range) for skewed data. Categorical variables are reported as numbers (percentages). Comparisons between the PI and SI groups were made using the Chi‐square test for categorical data. For continuous data, normality was assessed using the Shapiro–Wilk test. If the data were normally distributed, an independent‐samples t‐test was used between groups. For non‐normally distributed continuous variables, the Mann–Whitney U‐test was applied.
To compare the efficiency of the percutaneous and surgical approach in improving patients PVL severity, NYHA function class, and LV function we used Chi‐square test to compare the number of patients with at least one‐degree improvement in each of mentioned aspects between groups. The Mann–Whitney U‐test was utilized to compare the periprocedural changes in each echocardiographic index between PI and SI groups.
All statistical tests were two‐sided; a p < 0.05 was considered statistically significant. Analyzes were performed using IBM SPSS Statistics (Version 27). Data visualization was performed using R statistical software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). A Sankey diagram was generated to visualize changes in the NYHA function classification and PVL severity after percutaneous and SI.
3. RESULTS
3.1. Baseline characteristics
A total of 72 patients were included in this study, with 25 in the PI group and 47 in the SI group. The mean age of patients was 53.12 ± 14.13 years, and males comprised 55.56% (n = 40) of the total population. The majority of patients presented with dyspnea (77.78%), followed by symptomatic hemolysis (58.3%), weakness (44.44%), chest pain (44.44%), and anemia (9.72%). Hypertension and coronary artery disease were the most prevalent comorbidities in 34 (47.22%) and 32 (44.44%) patients, respectively.
Prosthetic valve replacement was performed via surgery in all patients, with the mitral valve being the most frequent (72.22%), followed by the aortic (59.72%), tricuspid (16.67%), and pulmonary valves (1.39%). Additionally, 31.94% (n = 23) of patients had their prosthetic valves repaired before, with two cases being due to paravalvular leakage, and the procedure was performed surgically in all of them. Thirty‐two patients (44.44%) had more than one prosthetic valve. Among the valves with regurgitation, 44 mitral valves, 23 aortic valves, 5 tricuspid valves, and one pulmonary valve were observed. Other details on the baseline characteristics of the studied patients are summarized in Table 1. As demonstrated in Table 1, the baseline characteristics of the two groups were compared, and no statistically significant differences were observed in terms of including age, sex, body surface area, body mass index, serum hemoglobin, and creatinine levels, initial presentation, medical history, number of previous sternotomies, replaced prosthetic valves, number of previously repaired valves, NYHA classification, and LV function. However, given the small sample size of our study, particularly in the percutaneous treatment group, the lack of statistically significant differences should be interpreted with caution, as our study may have been underpowered to detect small to moderate differences between the groups.
Table 1.
Characteristics of patients at baseline.
| Total | PI | SI | p‐value | |
|---|---|---|---|---|
| N = 72 | N = 25 | N = 47 | ||
| Male—n (%) | 40 (55.56%) | 17 (68%) | 23 (48.94) | 0.12 |
| Age— years | 53.12 ± 14.13 | 53.16 ± 14.48 | 53.11 ± 14.1 | 0.95 |
| Body surface area—m2 | 108.2 ± 11.96 | 107.39 ± 10.64 | 108.63 ± 12.70 | 0.66 |
| BMI—kg/m2 | 25.20 ± 3.40 | 24.56 ± 3.14 | 25.54 ± 3.51 | 0.23 |
| Hemoglobin—g/dL | 11.52 ± 2.03 | 11.36 ± 1.80 | 11.60 ± 2.16 | 0.80 |
| Creatinine—mg/dL | 1.27 ± 0.81 | 1.17 ± 0.46 | 1.32 ± 0.95 | 0.74 |
| Initial presentation—n (%) | ||||
| Symptomatic hemolysis | 42 (58.3%) | 18 (72%) | 24 (51.1%) | 0.09 |
| Chest pain | 32 (44.44%) | 13 (52%) | 19 (40.43%) | 0.35 |
| Weakness | 32 (44.44%) | 12 (48%) | 20 (42.55%) | 0.66 |
| Anemia | 7 (9.72%) | 2 (8%) | 5 (10.64%) | 0.72 |
| Dyspnea | 56 (77.78%) | 19 (76%) | 37 (78.72%) | 0.79 |
| Other | 3 (4.17%) | 1 (4%) | 2 (4.26%) | 0.96 |
| Medical history—n (%) | ||||
| Coronary artery disease | 32 (44.44%) | 14 (56%) | 18 (38.30%) | 0.15 |
| Hypertension | 34 (47.22%) | 11 (44%) | 23 (48.94%) | 0.69 |
| Diabetes mellitus | 11 (15.28%) | 3 (12%) | 8 (17.02%) | 0.57 |
| Hyperlipidemia | 13 (18.06%) | 3 (12%) | 10 (21.28%) | 0.33 |
| Chronic kidney disease | 9 (12.50%) | 1 (4%) | 8 (17.02%) | 0.11 |
| Liver disease | 4 (5.56%) | 1 (4%) | 3 (6.38%) | 0.67 |
| Number of previous sternotomies—n (%) | ||||
| 1 | 38 (52.78%) | 14 (56%) | 24 (51.06%) | 0.69 |
| 2 | 28 (38.89%) | 10 (40%) | 18 (38.30%) | 0.89 |
| 3 | 6 (8.33%) | 1 (4%) | 5 (10.64%) | 0.33 |
| Replaced prosthetic valves—n (%) | ||||
| Aortic | 43 (59.72%) | 18 (72%) | 25 (53.19%) | 0.12 |
| Mitral | 52 (72.22%) | 20 (80%) | 32 (68.09%) | 0.28 |
| Pulmonary | 1 (1.39%) | 0 | 1 (2.13%) | 0.46 |
| Tricuspid | 12 (16.67%) | 2 (8%) | 10 (21.28%) | 0.15 |
| Number of patients with ≥1 prosthetic valve—n (%) | 32 (44.44%) | 13 (52%) | 19 (40.43%) | 0.35 |
| Number of previously repaired valves—n (%) | ||||
| 0 | 49 (68.06%) | 18 (72%) | 31 (65.96%) | 0.60 |
| 1 | 21 (29.17%) | 7 (28%) | 14 (29.79%) | 0.87 |
| 2 | 2 (2.78%) | 0 | 2 (4.26%) | 0.30 |
| Valves with PVLa—n (%) | ||||
| Aortic | 23 (31.94%) | 10 (40%) | 13 (27.66%) | 0.28 |
| Mitral | 44 (61.11%) | 14 (56%) | 30 (63.83%) | 0.52 |
| Pulmonary | 1 (1.39%) | 0 | 1 (2.13%) | 0.46 |
| Tricuspid | 5 (6.94%) | 1 (4%) | 4 (8.51%) | 0.47 |
| Previous procedure for PVL repair—n (%) | 2 (2.78%) | 0 | 2 (4.26%) | 0.30 |
| PVL severity—n (%) | <0.01 | |||
| Mild | 0 | 0 | 0 | |
| Mild to moderate | 0 | 0 | 0 | |
| Moderate | 12 (16.67%) | 3 (12%) | 9 (19.15%) | |
| Moderate to severe | 36 (50%) | 6 (24%) | 30 (63.83%) | |
| Severe | 24 (33.33%) | 16 (64%) | 8 (17.02%) | |
| NYHA functional class—n (%) | 0.48 | |||
| I | 4 (5.56%) | 0 | 4 (8.51%) | |
| II | 28 (38.89%) | 11 (44%) | 17 (36.17%) | |
| II | 29 (40.28%) | 14 (56%) | 15 (31.91%) | |
| IV | 11 (15.28%) | 0 | 11 (23.4%) | |
| LV function—n (%) | 0.34 | |||
| Normal | 24 (33.3%) | 9 (36%) | 15 (31.9%) | |
| Mild dysfunction | 34 (47.2%) | 8 (32%) | 26 (55.3%) | |
| Moderate dysfunction | 8 (11.1%) | 5 (20%) | 3 (6.4%) | |
| Severe dysfunction | 6 (8.3%) | 3 (12%) | 3 (6.4%) |
Note: The bold p‐values are the ones with statistical significance.
Abbreviations: BMI, body mass index; LV, left ventricle; PI, percutaneous intervention; PVL, paravalvular leakage; SI, surgical intervention.
One patient undergone valve replacement surgery for mitral and tricuspid valve simultaneously.
3.2. Functional and clinical improvement
A total of 98.61% (n = 71) of the patients (96% [n = 24] in the PI group vs. 100% [n = 47] in the SI group) showed at least one grade improvement in the severity of the paravalvular leak compared to their condition before interventions. Figure 1 illustrates the extent of improvement in the patients. As shown in Table 2, the improvement in PVL severity was similar between the PI and surgery groups, with no statistically significant difference.
Figure 1.
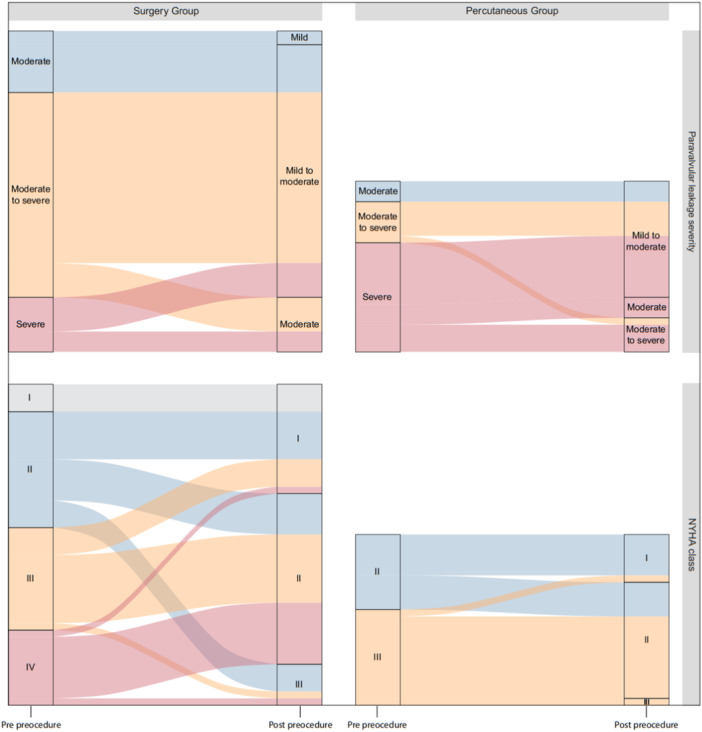
Comparison of periprocedural changes in PVL severity and NYHA functional classification between surgery and percutaneous intervention groups. PVL, paravalvular leakage.
Table 2.
Periprocedural changes in PVL severity, NYHA classification, and LV function.
| PI | SI | |||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | p‐value a | Odds ratio (95% CI) | |
| PVL severity | 0.17 | – | ||||
| Mild | 0 | 0 | 0 | 2 | ||
| Mild to moderate | 0 | 17 | 0 | 37 | ||
| Moderate | 3 | 3 | 9 | 8 | ||
| Moderate to severe | 6 | 5 | 30 | 0 | ||
| Severe | 16 | 0 | 8 | 0 | ||
| NYHA functional class | 0.48 | 1.48 (049–4.48) | ||||
| I | 0 | 7 | 4 | 16 | ||
| II | 11 | 17 | 17 | 25 | ||
| III | 14 | 1 | 15 | 6 | ||
| IV | 0 | 0 | 11 | 0 | ||
| LV function | 0.35 | 0.56 (0.16–1.95) | ||||
| Normal | 9 | 8 | 15 | 21 | ||
| Mild dysfunction | 8 | 11 | 26 | 17 | ||
| Moderate dysfunction | 5 | 4 | 3 | 7 | ||
| Severe dysfunction | 3 | 2 | 3 | 2 | ||
Abbreviations: CI, confidence interval; LV, left ventricle; PI, percutaneous intervention; PVL, paravalvular leakage; SI, surgical intervention.
Total number for reapired valves is 73 and the total number of enrolled patients is 72.
In addition, 70.83% (n = 51) of the patients (76% [n = 19] in the PI group vs. 68.08% [n = 32] in the SI group) experienced at least one class improvement in their functional class compared to their condition before interventions, based on the NYHA classification, which is visualized in Figure 1. The improvement in functional class was similar between the PI and SI groups, with no statistically significant difference (Table 2).
Regarding left ventricular function changes, 22.22% (n = 16) of the patients (25.5% [n = 12] of the SI group and 16% [n = 4] of the PI group) improved by at least one grade and both groups showed similar periprocedural improvement rate in the degree of LV dysfunction with no considerable difference (Table 2).
In the subgroup analysis, there were also no significant differences between the SI and PI groups in terms of the rate of improvement in the periprocedural degrees of PVL severity, NYHA function class, and LV function for the aortic and mitral valves, which are described separately in Tables S1 and S2.
3.3. Laboratory parameters
Serum hemoglobin levels for both groups significantly dropped after the procedure as shown in Table 3. However, the PI group presented considerably lower amounts of drop than the SI group (p < 0.01).
Table 3.
Periprocedural changes in the laboratory data.
| PI | SI | |||||
|---|---|---|---|---|---|---|
| Before | After | p‐value | Before | After | p‐value | |
| Hemoglobin—g/dL | 11.7 (9.75–12.75) | 11.2 (8.8–11.95) | <0.001 | 11.5 (10.1–13.4) | 9 (7.6–10.5) | <0.01 |
| Creatinine—mg/dL | 1.1 (0.9–1.4) | 1.2 (1–1.45) | 0.192 | 1.1 (0.8–1.5) | 1 (8–1.4) | 0.08 |
Abbreviations: PI, percutaneous intervention; SI, surgical intervention.
Note: The bold p‐values are the ones with statistical significance.
Serum creatinine levels elevated after the PIs whereas the SI group appeared to have lower creatinine levels post‐operatively. Nonetheless, neither of the changes is statistically considerable.
3.4. Echocardiogram indices
Changes in echocardiographic parameters after PI and surgery are outlined in Table 4. No significant differences were found between PI and surgery concerning changes in left ventricular end‐diastolic diameter (LVEDD), left ventricular end‐systolic diameter (LVESD), left ventricular end‐diastolic volume (LVEDV), right ventricular (RV) size, tricuspid annular plane systolic excursion (TAPSE), pulmonary artery pressure (PAP), left atrial (LA) size, LA volume, right atrial (RA) size, aortic annulus (AA) size, right atrial pressure (RAP), and Right Ventricular Ejection (RVE).
Table 4.
Periprocedural echocardiogram indexes. a
| Index | PI | SI | p‐valueb | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| LVEDD | 5.5 (5.05–6.2) | 5.6 (4.8–5.8) | 5 (4.5–5.6) | 5.2 (4.6–5.7) | 0.35 |
| LVESD | 3.9 (3.25–4.3) | 3.8 (3.3–4) | 3.5 (3–4.03) | 3.6 (3.2–4.1) | 0.58 |
| LVEDV | 136 (115–169) | 150 (115–169) | 113 (86–135.2) | 116 (93–145) | 0.46 |
| RV size | 3.3 (3–3.85) | 3.4 (3.05–3.9) | 3.3 (3.06–3.7) | 3.4 (3.2–3.6) | 0.39 |
| TAPSE | 15 (14–17) | 15 (13–16) | 16 (13–18) | 16 (13–18) | 0.70 |
| PAP | 44 (35–61) | 45 (35–57) | 42 (34–45) | 40 (30–48) | 0.11 |
| LA size | 4.1 (3.55–4.6) | 4.2 (3.45–5.35) | 4.3 (4–5.6) | 4 (3.4–5) | 0.06 |
| LA volume | 78 (70–88) | 85 (74–87) | 75 (64–88) | 75 (63–88) | 0.09 |
| RA size | 2 (2–3) | 2 (2–4) | 2 (1–3) | 2 (1–4) | 0.51 |
| AA size | 3.4 (3.3–3.5) | 3.4 (3.3–3.5) | 3.3 (3–3.5) | 3.3 (3.1–3.6) | 0.77 |
| RAP | 5 (5–13.75) | 5 (5–10) | 5 (5–10) | 5 (5–8) | 0.44 |
| RVE | 2 (1–3) | 2 (2–3) | 2 (1–3) | 2 (1–3) | 0.09 |
Abbreviations: AA size, aortic annulus size; LA size, left atrial size; LA volume, left atrial volume; LVEDD, left ventricular end diastolic diameter; LVEDV, left ventricular end diastolic volume; LVESD, left ventricular end systolic diameter; PAP, pulmonary artery pressure; PI, percutaneous intervention; RA size, right atrial size; RAP, right atrial pressure; RVE, right ventricular ejection; RV size, right ventricular size; SI, surgical intervention; TAPSE, tricuspid annular plane systolic excursion.
The numbers are reported as median and interquartile (IQR) range; median (25th percentile to 75th percentile).
The reported p‐values refer to the differences between changes in each parameter between the studied groups.
3.5. Complications and mortality
In‐hospital (30‐day) mortality was low in both groups, with 1 (4%) death in the PI group and 3 (6.4%) deaths in the SI group (p = 0.65). A 90‐day all‐cause mortality (p = 0.48) and complications like pericardial effusion (p = 0.64) and thromboembolism (p = 0.30) were also comparable between the two groups. The length of hospital stay after the procedure was significantly different between the PI (7.36 ± 8.36 days) and SI (8.81 ± 8.67 days) groups with lower numbers belonging to PI (p = 0.04) (Table 5).
Table 5.
Short–term and mid–term outcomes.
| Total | PI | SI | |||
|---|---|---|---|---|---|
| N = 72 | N = 25 | N = 47 | p‐value | Odds ratio (95% CI) | |
| In‐hospital (30 days) outcomes | |||||
| All‐cause mortality | 4 | 1 (4%) | 3 (6.38%) | 0.65 | 0.61 (0.06–6.2) |
| Length of stay in the hospital after procedure—days |
8.31 ± 8.53a 6 (3–7)b |
7.36 ± 8.36 5 (3–7) | 8.81 ± 8.67 7 (5–10) | 0.04 | – |
| 90‐Day outcomes | |||||
| All‐cause mortality | 14 | 6 | 8 | 0.48 | 1.53 (0.47–5.07) |
| Pericardial Effusion | 2 | 1 | 1 | 0.64 | 1.91 (0.11–32) |
| Thromboembolism | 2 | 0 | 2 | 0.30 | – |
Note: The bold p‐values are the ones with statistical significance.
Abbreviations: CI, confidence interval; PI, percutaneous intervention; SI, surgical intervention.
Mean ± standard deviation.
Median (25th percentile to 75th percentile).
4. DISCUSSION
In recent years, there has been increasing interest in PIs as an alternative to surgery, and comparing the percutaneous approach to surgical repair of prosthetic PVL is a topic of interest in the field of cardiology. 30 , 31 Traditionally, repeat surgery has been the standard treatment for symptomatic patients with PVL, but it is associated with high operative risk and variable results. 32 However, percutaneous treatment of PVL has emerged as a less invasive alternative, with successful outcomes and the potential to eliminate the need for open surgical correction. 33 Our results suggest that both percutaneous treatment and surgery are effective options for reducing the severity of prosthetic paravalvular leaks, with similar improvements in patient function class, leak severity, and echocardiographic parameters. In‐hospital mortality and mid‐term clinical outcomes appeared to be comparable between the PI and surgery group.
The most important finding of our study was the absence of any statistically significant difference in rate of patients with at least one degree improvement in PVL severity, NYHA functional class, and LV function between PI and SI groups. Both interventions resulted in significant improvement of PVL severity and NYHA class without dramatic changes in LV function. The lack of significant improvement in LV ejection fraction after percutaneous or surgical PVL repair in our study can be explained in multiple ways. For instance, the baseline LV dysfunction in our cohort was likely chronic and partially irreversible due to longstanding volume overload from the PVL. Besides, the follow‐up duration has been too short to allow reverse remodeling of the left ventricle, which occurs gradually over time. 34 , 35 Based on these results, we can infer that percutaneous closure can be considered an effective alternative to repeat surgery in suitable patients with paravalvular prosthetic leaks. This study supports evidence from previous observations by Taramasso et al., 36 Zhang et al., 37 and Zorinas et al. 30 which have also shown comparable outcomes between percutaneous and surgical treatments for PVLs.
The PI group showed a significantly lower amount of decrease in serum hemoglobin levels than the SI group which is not unexpected due to the minimally invasive nature of the percutaneous approach. Our results align with other studies showing significantly higher blood loss and transfusion requirements with redo valve surgery compared to percutaneous approaches. An investigation by Panaich et al. revealed that PI leads to small but significant improvements in markers of hemolysis such as lactate dehydrogenase and haptoglobin levels. 38 Additionally, patients undergoing percutaneous repair experience lower requirements for blood transfusion after the procedure compared to baseline. 38
Comparative analysis of changes in echocardiographic parameters after PI and surgery did not show any considerable difference in all indices. To the best of our knowledge, a comparison of changes in echocardiographic parameters between percutaneous and surgical approaches has not been performed before. Thus, these results need to be interpreted with caution.
Regarding outcomes, both procedures resulted in low rates of 30‐ and 90‐day mortality, and clinical complications with no considerable difference between them. The reports in this regard are somehow heterogeneous. In line with our study, Taramasso et al. 36 reported no in‐hospital deaths in the percutaneous group compared with 9.3% mortality in the surgical group. Similarly, Wells et al. 39 reported 7.1% versus 6.9% 30‐day mortality in the transcatheter and surgical groups, respectively. However, Pinheiro et al. 8 reported that complication rates were high in both groups during hospitalization, with no statistically significant differences. In another investigation by Angulo‐Llanos et al., in‐hospital mortality was significantly lower with percutaneous treatment (2.2% vs. 14.6%). In‐hospital mortality was also noticeably lower with percutaneous repair (0% vs. 10%) in the study by Yang et al. 40
Remarkably, the average length of in‐hospital stay was significantly lower in PI than in SI groups. Angulo‐Llanos et al. 41 and Yang et al. 40 also reported a significantly lower length of hospital stay in the PI group. This can be attributed to the less invasive nature of the percutaneous approach, which avoids repeat sternotomy and cardiopulmonary bypass used for surgical closure. The shorter length of stay is an important advantage of the percutaneous technique, resulting in lower cost and resource utilization for these repairs when suitable.
Overall, our study supports both percutaneous approaches and surgery as suitable options for PVL repair, with the choice between them being dependent on multiple patient factors. Surgery remains the gold standard for patients who require concomitant procedures such as valve replacement or coronary bypass grafting. However, PI offers a less invasive approach that may be preferable for high‐risk surgical patients. Echo findings, PVL location, comorbidities, and heart team recommendations must be considered on a case‐by‐case basis. Although percutaneous closure of PVLs has shown promising results, it is important to consider the limitations and potential complications of the procedure. Percutaneous closure can be time‐consuming and may require a second procedure in some cases. 42 Complications, such as device dislodgement and mechanical valve dysfunction, have been reported. 22 , 43 Percutaneous closure of PVL is a recently developed operation and is still considered to be technically challenging and not devoid of potential dangers. 10 There are potential complications associated with the technique that interventional cardiologists still encounter on occasion. More clinical experience will be required for percutaneous PVL closure to become an established, mainstream therapy option. Therefore, larger comparative trials are warranted to further define best practices for PVL management.
Our study has several limitations. First, the exclusion of 29% of the initial patients due to missing data may introduce selection bias and reduce generalizability. Second, the small sample size from a single center and the lack of randomization between the treatment groups, which may have introduced selection bias, as patients with higher surgical risk, advanced age, or multiple comorbidities were more likely to be referred for percutaneous treatment, limit the statistical power and may not fully account for potential confounding factors. Third, our subgroup analysis was limited to the mitral and aortic valves due to the small sample sizes for the tricuspid (n = 5) and pulmonary (n = 1) valves, which were insufficient to perform meaningful statistical analyzes and draw reliable conclusions about the comparative effectiveness of percutaneous and surgical treatments for paravalvular leaks in these valve sites. Fourth, while our study provides a valuable insight into the short‐term outcomes of percutaneous and surgical treatments for paravalvular leak closure, longer‐term follow‐up is necessary to better assess late clinical outcomes and treatment durability. Future prospective, randomized studies with larger sample sizes and longer follow‐up periods are needed to validate our findings and provide more definitive evidence regarding the comparative effectiveness and safety of percutaneous and surgical treatments for paravalvular leak closure.
5. CONCLUSIONS
This study suggests that percutaneous PVL closure may be a viable alternative to surgical repair, with comparable improvements in clinical and echocardiographic outcomes. These findings should be interpreted as hypothesis‐generating rather than conclusive due to several limitations, including the small sample size, non‐randomized design, and potential for selection bias. While both options may be considered for PVL repair after a multidisciplinary heart team assessment, larger, prospective, and randomized studies are needed to confirm these results and provide more definitive evidence regarding the comparative effectiveness and safety of percutaneous and surgical treatments for paravalvular leak closure.
AUTHOR CONTRIBUTIONS
Mohammadsaleh Baghi: Investigation; Conceptualization; Methodology; Writing—original draft. Erfan Kohansal: Conceptualization; Methodology; Writing—original draft; Writing—review and editing. Mahsa Akbarian: Investigation; Writing—original draft; Methodology. Sara Adimi: Investigation; Methodology; Writing—review and editing. Hooman Bakhshandeh: Visualization; Formal analysis; Data curation. Ata Firoozi: Conceptualization; Supervision. Pegah Salehi: Investigation; Methodology. Kasra Mehdizadeh: Visualization; Writing—original draft; Writing—review and editing. Hamed Hesami: Investigation; Methodology. Mina Yousefi: Writing—original draft. Sajjad Erami: Investigation; Methodology. Yeganeh Dehghani: Investigation; Methodology. Zahra Hosseini: Investigation; Methodology. Maryam Shojaeifard: Methodology; Supervision; Conceptualization.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
TRANSPARENCY STATEMENT
The lead author Maryam Shojaeifard affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
Baghi M, Kohansal E, Akbarian M, et al. Percutaneous Versus Surgical Closure of Paravalvular Leaks in Prosthetic Valves: a cross‐sectional Comparison of Clinical Outcomes. Health Sci Rep. 2024;7:e70001. 10.1002/hsr2.70001
During the preparation of this work, the authors used Claude 2 and Grammarly to assist with drafting sections of the manuscript and revising the content. After using these tools/services, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8(3):162‐172. [DOI] [PubMed] [Google Scholar]
- 2. Ogah OS, Davison BA, Sliwa K, et al. Gender differences in clinical characteristics and outcome of acute heart failure in sub‐saharan Africa: results of the THESUS‐HF study. Clin Res Cardiol. 2015;104(6):481‐490. [DOI] [PubMed] [Google Scholar]
- 3. Matiasz R, Rigolin VH. 2017 focused update for management of patients with valvular heart disease: summary of new recommendations. J Am Heart Assoc. 2018;7(1):e007596. [Google Scholar]
- 4. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Rev Esp Cardiol (Engl Ed). 2022;75(6):524. [DOI] [PubMed] [Google Scholar]
- 5. Seery TJ, Slack MC. Percutaneous closure of a prosthetic pulmonary paravalvular leak. Congenit Heart Dis. 2014;9(1):E19‐22. [DOI] [PubMed] [Google Scholar]
- 6. Lampropoulos K, Aggeli C, Megalou A, Barbetseas J, Budts W. Diagnosis and treatment of left‐sided prosthetic paravalvular regurgitation. Cardiology. 2016;133(1):27‐34. [DOI] [PubMed] [Google Scholar]
- 7. Garg A, Azad S, Radhakrishnan S. Percutaneous paravalvular leak closure with their outcomes: a single center experience. Ann Card Anaesth. 2021;24:302‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pinheiro CP, Rezek D, Costa EP, et al. Paravalvular regurgitation: clinical outcomes in surgical and percutaneous treatments. Arq Bras Cardiol. 2016;107(1):55‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Rourke DJ, Palac RT, Malenka DJ, Marrin CAS, Arbuckle BE, Plehn JF. Outcome of mild periprosthetic regurgitation detected by intraoperative transesophageal echocardiography. J Am Coll Cardiol. 2001;38(1):163‐166. [DOI] [PubMed] [Google Scholar]
- 10. Cappelli F, del Bene MR, Santoro G, Meucci F, Attanà P, Barletta G. The challenge of integrated echocardiographic approach in percutaneous closure of paravalvular leak. Echocardiography. 2011;28:E168‐E171. [DOI] [PubMed] [Google Scholar]
- 11. Singh P, Manda J, Hsiung MC, et al. Live/real time three‐dimensional transesophageal echocardiographic evaluation of mitral and aortic valve prosthetic paravalvular regurgitation. Echocardiography. 2009;26:980‐987. [DOI] [PubMed] [Google Scholar]
- 12. Genereux P, Head SJ, Hahn R, et al. Paravalvular leak after transcatheter aortic valve replacement: the new Achilles’ heel? A comprehensive review of the literature. J Am Coll Cardiol. 2013;61(11):1125‐1136. [DOI] [PubMed] [Google Scholar]
- 13. Helmy T, Kumar S, Khan AA, Raza A, Smart S, Bailey SR. Review of prosthetic paravalvular leaks: diagnosis and management. Curr Cardiol Rep. 2022;24(10):1287‐1297. [DOI] [PubMed] [Google Scholar]
- 14. Qamar F, Attar RH, Nabi F. Paravalvular leak. Methodist Debakey Cardiovasc J. 2022;18(1):7‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Randall M, Betageri O, Hanayneh S, Anderson RD. Paravalvular leak: a systemic review. Curr Cardiol Rev. 2022;18(6):e110522204571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129(23):e521‐e643. [DOI] [PubMed] [Google Scholar]
- 17. Baumgartner H. The 2017 ESC/EACTS guidelines on the management of valvular heart disease: what is new and what has changed compared to the 2012 guidelines? Wien Klin Wochenschr. 2018;130:168‐171. [DOI] [PubMed] [Google Scholar]
- 18. Sato H, Sorajja P. Paravalvular regurgitation: an overview of indications for closure and management strategies. Curr Cardiol Rep. 2022;24(5):577‐586. [DOI] [PubMed] [Google Scholar]
- 19. Altarabsheh SE, Deo SV, Rihal CS, Park SJ. Mitral paravalvular leak: caution in percutaneous occluder device deployment. Heart Surg Forum. 2013;16(1):21. [DOI] [PubMed] [Google Scholar]
- 20. Eng MH, Tandon V, Greenbaum AB, Fang K. Percutaneous paravalvular leak repair. Interv Cardiol Clin. 2022;11(3):233‐243. [DOI] [PubMed] [Google Scholar]
- 21. Alderweireldt AS, De Wolf D. Percutaneous closure of paravalvular leak after cone repair for ebstein's anomaly. Catheter Cardiovasc Interv. 2019;93(1):E46‐E48. [DOI] [PubMed] [Google Scholar]
- 22. Ussia GP, Scandura S, Calafiore AM, et al. Late device dislodgement after percutaneous closure of mitral prosthesis paravalvular leak with amplatzer muscular ventricular septal defect occluder. Circulation. 2007;115(8):e208‐e210. [DOI] [PubMed] [Google Scholar]
- 23. Baztarrica GEP, Heredia G, Arellano J, Fernández J, Porcile R. Symptomatic aortic paravalvular leak: percutaneous treatment with amplatzer vascular plug iii device as an alternative to surgery. Braz J Cardiovasc Surg. 2018;33:107‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turner ME, Lai WW, Vincent JA. Percutaneous closure of tricuspid paravalvular leak. Catheter Cardiovasc Interv. 2013;82(4):E511‐E515. [DOI] [PubMed] [Google Scholar]
- 25. Webb JG, Pate GE, Munt BI. Percutaneous closure of an aortic prosthetic paravalvular leak with an Amplatzer duct occluder. Catheter Cardiovasc Interv. 2005;65(1):69‐72. [DOI] [PubMed] [Google Scholar]
- 26. Nietlispach F, Maisano F, Sorajja P, Leon MB, Rihal C, Feldman T. Percutaneous paravalvular leak closure: chasing the chameleon. Eur Heart J. 2016;37(47):3495‐3502. [DOI] [PubMed] [Google Scholar]
- 27. Kort HW, Sharkey AM, Balzer DT. Novel use of the Amplatzer duct occluder to close perivalvar leak involving a prosthetic mitral valve. Catheter Cardiovasc Interv. 2004;61(4):548‐551. [DOI] [PubMed] [Google Scholar]
- 28. García E, Arzamendi D, Jimenez‐Quevedo P, et al. Outcomes and predictors of success and complications for paravalvular leak closure: an analysis of the SpanisH real‐world paravalvular LEaks closure (HOLE) registry. EuroIntervention. 2017;12(16):1962‐1968. [DOI] [PubMed] [Google Scholar]
- 29. Kosaraju A, Goyal A, Grigorova Y, Makaryus AN Left ventricular ejection fraction. 2017.
- 30. Zorinas A, Janušauskas V, Austys D, et al. A comparison of the catheter‐based transapical and surgical treatment modalities for mitral paravalvular leak. J Clin Med. 2022;11(17):4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calvert PA, Northridge DB, Malik IS, et al. Percutaneous device closure of paravalvular leak: combined experience from the United Kingdom and Ireland. Circulation. 2016;134(13):934‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sorajja P, Cabalka AK, Hagler DJ, et al. Successful percutaneous repair of perivalvular prosthetic regurgitation. Catheter Cardiovasc Interv. 2007;70(6):815‐823. [DOI] [PubMed] [Google Scholar]
- 33. Sorajja P, Bae R, Lesser JA, Pedersen WA. Percutaneous repair of paravalvular prosthetic regurgitation: patient selection, techniques and outcomes. Heart. 2015;101(9):665‐673. [DOI] [PubMed] [Google Scholar]
- 34. Nam JS, Chin J‐H, Kang HU, Kim J, Joung KW, Choi IC. Prognostic value of left ventricular apical four‐chamber longitudinal strain after heart valve surgery in real‐world practice. Korean J Anesthesiol. 2022;75(5):416‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen M, Yao X, Wang D, et al. Long‐term cardiac remodeling associated with heart failure following left‐ventricular valve replacement surgery: a retrospective study. Medicine. 2021;100(30):e26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taramasso M, Maisano F, Latib A, et al. Conventional surgery and transcatheter closure via surgical transapical approach for paravalvular leak repair in high‐risk patients: results from a single‐centre experience. Eur Heart J Cardiovasc Imaging. 2014;15(10):1161‐1167. [DOI] [PubMed] [Google Scholar]
- 37. Zhang Y, Pan X, Qu X, et al. Comparison of transcatheter and surgical treatment of paravalvular leak: results from a 5‐year follow‐up study. Catheter Cardiovasc Interv. 2019;94(2):E88‐E95. [DOI] [PubMed] [Google Scholar]
- 38. Panaich SS, Maor E, Reddy G, et al. Effect of percutaneous paravalvular leak closure on hemolysis. Catheter Cardiovasc Interv. 2019;93(4):713‐719. [DOI] [PubMed] [Google Scholar]
- 39. Wells JA, Condado JF, Kamioka N, et al. Outcomes after paravalvular leak closure. JACC: Cardiovascular Interventions. 2017;10(5):500‐507. [DOI] [PubMed] [Google Scholar]
- 40. Yang C, Liu Y, Tang J, et al. Prognosis of transcatheter closure compared with surgical repair of paravalvular leak after prosthetic valve replacement: a retrospective comparison. Thorac Cardiovasc Surg. 2020;68(2):148‐157. [DOI] [PubMed] [Google Scholar]
- 41. Angulo‐Llanos R, Sarnago‐Cebada F, Rivera AR, et al. Two‐year follow up after surgical versus percutaneous paravalvular leak closure: a non‐randomized analysis. Catheter Cardiovasc Interv. 2016;88(4):626‐634. [DOI] [PubMed] [Google Scholar]
- 42. Pate GE, Al Zubaidi A, Chandavimol M, Thompson CR, Munt BI, Webb JG. Percutaneous closure of prosthetic paravalvular leaks: case series and review. Catheter Cardiovasc Interv. 2006;68(4):528‐533. [DOI] [PubMed] [Google Scholar]
- 43. Brilakis ES, Collins LJ, Obel O, Banerjee S. Mechanical valve dysfunction after percutaneous perimitral leak closure: salvage by percutaneous occluder retrieval. Catheter Cardiovasc Interv. 2010;75(6):876‐881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


