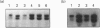Abstract
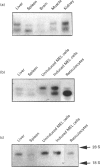
Ferrochelatase, which catalyses the last step in haem biosynthesis, i.e. the insertion of Fe(II) into protophorphyrin IX, is present in all cells, but is particularly abundant in erythroid cells during haemoglobinization. Using mouse ferrochelatase cDNA as a probe two ferrochelatase transcripts, having lengths of 2.9 kb and 2.2 kb, were found in extracts of mouse liver, kidney, brain, muscle and spleen, the 2.9 kb transcript being more abundant in the non-erythroid tissues and the 2.2 kb transcript more predominant in spleen. In mouse erythroleukemia cells the 2.9 kb ferrochelatase transcript is also more abundant; however, following induction of erythroid differentiation by dimethyl sulphoxide there is a preferential increase in the 2.2 kb transcript, which eventually predominates. With mouse reticulocytes, the purest immature erythroid cell population available, over 90% of the total ferrochelatase mRNA is present as the 2.2 kb transcript. Since there is probably only one mouse ferrochelatase gene, the occurrence of two ferrochelatase transcripts could arise from the use of two putative polyadenylation signals in the 3' region of ferrochelatase DNA. This possibility was explored by using a 389 bp DNA fragment produced by PCR with synthetic oligoprimers having sequence similarity with a region between the polyadenylation sites. This fragment hybridized only to the 2.9 kb ferrochelatase transcript, indicating that the two transcripts differ at their 3' ends and suggesting that the 2.2 kb transcript results from the utilization of the upstream polyadenylation signal. The preferential utilization of the upstream polyadenylation signal may be an erythroid-specific characteristic of ferrochelatase gene expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUNS G. P., LONDON I. M. THE EFFECT OF HEMIN ON THE SYNTHESIS OF GLOBIN. Biochem Biophys Res Commun. 1965 Jan 18;18:236–242. doi: 10.1016/0006-291x(65)90746-1. [DOI] [PubMed] [Google Scholar]
- Beaumont C., Deybach J. C., Grandchamp B., da Silva V., de Verneuil H., Nordmann Y. Effects of succinylacetone on dimethylsulfoxide-mediated induction of heme pathway enzymes in mouse friend virus-transformed erythroleukemia cells. Exp Cell Res. 1984 Oct;154(2):474–484. doi: 10.1016/0014-4827(84)90171-x. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Kitchen H., Wood W. A. Evidence for erythroid and nonerythroid forms of delta-aminolevulinate synthetase. Arch Biochem Biophys. 1981 Feb;206(2):380–391. doi: 10.1016/0003-9861(81)90105-3. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Frasier F. Cloning of murine ferrochelatase. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):849–853. doi: 10.1073/pnas.88.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chretien S., Dubart A., Beaupain D., Raich N., Grandchamp B., Rosa J., Goossens M., Romeo P. H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci U S A. 1988 Jan;85(1):6–10. doi: 10.1073/pnas.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conder L. H., Woodard S. I., Dailey H. A. Multiple mechanisms for the regulation of haem synthesis during erythroid cell differentiation. Possible role for coproporphyrinogen oxidase. Biochem J. 1991 Apr 15;275(Pt 2):321–326. doi: 10.1042/bj2750321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. C., Bawden M. J., Martin A., May B. K. Human erythroid 5-aminolevulinate synthase: promoter analysis and identification of an iron-responsive element in the mRNA. EMBO J. 1991 Jul;10(7):1891–1902. doi: 10.1002/j.1460-2075.1991.tb07715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar T., Stripecke R., Gray N. K., Goossen B., Constable A., Johansson H. E., Hentze M. W. Identification of a novel iron-responsive element in murine and human erythroid delta-aminolevulinic acid synthase mRNA. EMBO J. 1991 Jul;10(7):1903–1909. doi: 10.1002/j.1460-2075.1991.tb07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink C. J., Sassa S., May B. K. Regulation of 5-aminolevulinate synthase in mouse erythroleukemic cells is different from that in liver. J Biol Chem. 1988 Sep 15;263(26):13012–13016. [PubMed] [Google Scholar]
- Elferink C. J., Srivastava G., Maguire D. J., Borthwick I. A., May B. K., Elliott W. H. A unique gene for 5-aminolevulinate synthase in chickens. Evidence for expression of an identical messenger RNA in hepatic and erythroid tissues. J Biol Chem. 1987 Mar 25;262(9):3988–3992. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs O., Ponka P., Borova J., Neuwirt J., Travnicek M. Effect of heme on globin messenger RNA synthesis in spleen erythroid cells. J Supramol Struct Cell Biochem. 1981;15(1):73–81. doi: 10.1002/jsscb.1981.380150108. [DOI] [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Grandchamp B., Beaumont C., de Verneuil H., Nordmann Y. Accumulation of porphobilinogen deaminase, uroporphyrinogen decarboxylase, and alpha- and beta-globin mRNAs during differentiation of mouse erythroleukemic cells. Effects of succinylacetone. J Biol Chem. 1985 Aug 15;260(17):9630–9635. [PubMed] [Google Scholar]
- Grandchamp B., De Verneuil H., Beaumont C., Chretien S., Walter O., Nordmann Y. Tissue-specific expression of porphobilinogen deaminase. Two isoenzymes from a single gene. Eur J Biochem. 1987 Jan 2;162(1):105–110. doi: 10.1111/j.1432-1033.1987.tb10548.x. [DOI] [PubMed] [Google Scholar]
- Granick J. L., Sassa S. Hemin control of heme biosynthesis in mouse Friend virus-transformed erythroleukemia cells in culture. J Biol Chem. 1978 Aug 10;253(15):5402–5406. [PubMed] [Google Scholar]
- Hu H. Y., Gardner J., Aisen P. Inducibility of transferrin receptors on friend erythroleukemic cells. Science. 1977 Aug 5;197(4303):559–561. doi: 10.1126/science.267327. [DOI] [PubMed] [Google Scholar]
- KARIBIAN D., LONDON I. M. CONTROL OF HEME SYNTHESIS BY FEEDBACK INHIBITION. Biochem Biophys Res Commun. 1965 Jan 18;18:243–249. doi: 10.1016/0006-291x(65)90747-3. [DOI] [PubMed] [Google Scholar]
- Krowczynska A., Yenofsky R., Brawerman G. Regulation of messenger RNA stability in mouse erythroleukemia cells. J Mol Biol. 1985 Jan 20;181(2):231–239. doi: 10.1016/0022-2836(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Laskey J. D., Ponka P., Schulman H. M. Control of heme synthesis during Friend cell differentiation: role of iron and transferrin. J Cell Physiol. 1986 Nov;129(2):185–192. doi: 10.1002/jcp.1041290209. [DOI] [PubMed] [Google Scholar]
- Malik Z., Bessler H., Djaldetti M. The role of hemin in the regulation of heme synthesis by fetal mouse liver erythroblasts in culture. Exp Hematol. 1979 Apr;7(4):183–188. [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- McCoubrey W. K., Jr, Ewing J. F., Maines M. D. Human heme oxygenase-2: characterization and expression of a full-length cDNA and evidence suggesting that the two HO-2 transcripts may differ by choice of polyadenylation signal. Arch Biochem Biophys. 1992 May 15;295(1):13–20. doi: 10.1016/0003-9861(92)90481-b. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mignotte V., Wall L., deBoer E., Grosveld F., Romeo P. H. Two tissue-specific factors bind the erythroid promoter of the human porphobilinogen deaminase gene. Nucleic Acids Res. 1989 Jan 11;17(1):37–54. doi: 10.1093/nar/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahashi Y., Taketani S., Okuda M., Inoue K., Tokunaga R. Molecular cloning and sequence analysis of cDNA encoding human ferrochelatase. Biochem Biophys Res Commun. 1990 Dec 14;173(2):748–755. doi: 10.1016/s0006-291x(05)80099-3. [DOI] [PubMed] [Google Scholar]
- Neuwirt J., Ponka P., Borová J. The role of heme in the regulation of delta-aminolevulinic acid and heme synthesis in rabbit reticulocytes. Eur J Biochem. 1969 May 1;9(1):36–41. doi: 10.1111/j.1432-1033.1969.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Ponka P., Neuwirt J. Regulation of iron entry into reticulocytes. I. Feedback inhibitory effect of heme on iron entry into reticulocytes and on heme synthesis. Blood. 1969 May;33(5):690–707. [PubMed] [Google Scholar]
- Ponka P., Schulman H. M. Acquisition of iron from transferrin regulates reticulocyte heme synthesis. J Biol Chem. 1985 Nov 25;260(27):14717–14721. [PubMed] [Google Scholar]
- Ponka P., Schulman H. M. Regulation of heme synthesis in erythroid cells: hemin inhibits transferrin iron utilization but not protoporphyrin synthesis. Blood. 1985 Apr;65(4):850–857. [PubMed] [Google Scholar]
- Riddle R. D., Yamamoto M., Engel J. D. Expression of delta-aminolevulinate synthase in avian cells: separate genes encode erythroid-specific and nonspecific isozymes. Proc Natl Acad Sci U S A. 1989 Feb;86(3):792–796. doi: 10.1073/pnas.86.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roméo P. H., Raich N., Dubart A., Beaupain D., Pryor M., Kushner J., Cohen-Solal M., Goossens M. Molecular cloning and nucleotide sequence of a complete human uroporphyrinogen decarboxylase cDNA. J Biol Chem. 1986 Jul 25;261(21):9825–9831. [PubMed] [Google Scholar]
- Ross J., Sautner D. Induction of globin mRNA accumulation by hemin in cultured erythroleukemic cells. Cell. 1976 Aug;8(4):513–520. doi: 10.1016/0092-8674(76)90219-1. [DOI] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenhaut D. S., Curtis P. J. Structure of a mouse erythroid 5-aminolevulinate synthase gene and mapping of erythroid-specific DNAse I hypersensitive sites. Nucleic Acids Res. 1989 Sep 12;17(17):7013–7028. doi: 10.1093/nar/17.17.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H. M., Martinez-Medellin J., Sidloi R. The reticulocyte-mediated release of iron and bicarbonate from transferrin: effect of metabolic inhibitors. Biochim Biophys Acta. 1974 May 24;343(3):529–534. doi: 10.1016/0304-4165(74)90270-0. [DOI] [PubMed] [Google Scholar]
- Schulman H. M., Wilczynska A., Ponka P. Transferrin and iron uptake by human lymphoblastoid and K-562 cells. Biochem Biophys Res Commun. 1981 Jun;100(4):1523–1530. doi: 10.1016/0006-291x(81)90691-4. [DOI] [PubMed] [Google Scholar]
- Taketani S., Inazawa J., Nakahashi Y., Abe T., Tokunaga R. Structure of the human ferrochelatase gene. Exon/intron gene organization and location of the gene to chromosome 18. Eur J Biochem. 1992 Apr 1;205(1):217–222. doi: 10.1111/j.1432-1033.1992.tb16771.x. [DOI] [PubMed] [Google Scholar]
- Taketani S., Nakahashi Y., Osumi T., Tokunaga R. Molecular cloning, sequencing, and expression of mouse ferrochelatase. J Biol Chem. 1990 Nov 15;265(32):19377–19380. [PubMed] [Google Scholar]
- Tsai S. F., Bishop D. F., Desnick R. J. Human uroporphyrinogen III synthase: molecular cloning, nucleotide sequence, and expression of a full-length cDNA. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7049–7053. doi: 10.1073/pnas.85.19.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynska A., Ponka P., Schulman H. M. Transferrin receptors and iron utilization in DMSO-inducible and -uninducible Friend erythroleukemia cells. Exp Cell Res. 1984 Oct;154(2):561–566. doi: 10.1016/0014-4827(84)90180-0. [DOI] [PubMed] [Google Scholar]
- Woods J. S. Studies on the role of heme in the regulation of delta-aminolevulinic acid synthetase during fetal hepatic development. Mol Pharmacol. 1974 May;10(3):389–397. [PubMed] [Google Scholar]
- Yamamoto M., Fujita H., Watanabe N., Hayashi N., Kikuchi G. An immunochemical study of delta-aminolevulinate synthase and delta-aminolevulinate dehydratase in liver and erythroid cells of rat. Arch Biochem Biophys. 1986 Feb 15;245(1):76–83. doi: 10.1016/0003-9861(86)90191-8. [DOI] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]