Abstract
Objective
This expert opinion paper proposes a design for a state-of-the-art magnetic resonance image (MRI) acquisition protocol for knee osteoarthritis clinical trials in early and advanced disease. Semi-quantitative and quantitative imaging endpoints are supported, partly amendable to automated analysis. Several (peri-) articular tissues and pathologies are covered, including synovitis.
Method
A PubMed literature search was conducted, with focus on the past 5 years. Further, osteoarthritis imaging experts provided input. Specific MRI sequences, orientations, spatial resolutions and parameter settings were identified to align with study goals. We strived for implementation on standard clinical scanner hardware, with a net acquisition time ≤30 min.
Results
Short- and long-term longitudinal MRIs should be obtained at ≥1.5T, if possible without hardware changes during the study. We suggest a series of gradient- and spin-echo-sequences, supporting MOAKS, quantitative analysis of cartilage morphology and T2, and non-contrast-enhanced depiction of synovitis. These sequences should be properly aligned and positioned using localizer images. One of the sequences may be repeated in each participant (re-test), optimally at baseline and follow-up, to estimate within-study precision. All images should be checked for quality and protocol-adherence as soon as possible after acquisition. Alternative approaches are suggested that expand on the structural endpoints presented.
Conclusions
We aim to bridge the gap between technical MRI acquisition guides and the wealth of imaging literature, proposing a balance between image acquisition efficiency (time), safety, and technical/methodological diversity. This approach may entertain scientific innovation on tissue structure and composition assessment in clinical trials on disease modification of knee osteoarthritis.
Keywords: Clinical trial, Osteoarthritis (OA), MRI acquisition protocol, Synovitis, Early- and late-stage disease
1. Introduction
Studies investigating osteoarthritis (OA) status, progression, or response to intervention frequently employ liquid or imaging biomarkers [1]. The advantage of imaging is that it visualizes articular tissues directly, providing exquisite soft tissue contrast when magnetic resonance imaging (MRI) is used [2]. Commonly in OA, articular tissue pathology is evaluated by expert radiologists using semi-quantitative MRI scoring systems [[3], [4], [5], [6]], but articular tissue properties may also be measured quantitatively using morphometry [[7], [8], [9]]. Another avenue is relaxometry [10,11] that derives MRI-specific relaxation parameters related to biochemical composition of specific tissues. The detection of specific pathological processes is best supported by different MRI sequences with specific parameters, resolution, orientation, etc.
A dedicated MRI acquisition protocol (i.e. a series of sequential acquisitions within one scanning session) must balance the number of MRI sequences used, enabling specific image assessments that answer study questions within the examination time that the patient is able, or willing, to tolerate. Overly long imaging times can be detrimental to image quality, potentially resulting in motion artifacts due to patient physical discomfort and a negative impact on patient experience that may reduce follow-up visit compliance [12]. Further, clinical trial MRI time often competes with scan time reserved for clinical routine imaging. Shorter protocols that have proven useful in clinical trials are more easily translated into clinical practice, and may help standardizing of general medical diagnostics and generating comparable results across different hospitals and countries.
The image acquisition protocol of the Osteoarthritis Initiative (OAI) is well established [13,14], containing fast (intermediate weighted) spin-echo sequences for semi-quantitative tissue evaluation, and a high resolution coronal spoiled gradient echo and sagittal double echo steady state (DESS) [15] for quantification of cartilage morphology [7,8,16]. The DESS also facilitates morphometry of meniscus morphology and extrusion [[17], [18], [19]], and bone size and shape [8,[20], [21], [22]]. A multi-echo-spin-echo (MESE) sequence was finally acquired to measure transverse (spin-spin) relaxation times (T2) of cartilage. T2 is related to the speed by which protons lose phase coherence after excitation, the rate of decay impacted by free water molecules that prolong transverse magnetization [23]. It thus can be employed to estimate articular cartilage matrix composition, specifically hydration and collagen [24,25]. T2 has been correlated with cartilage histological grading [26], mechanical properties [27], and early OA before matrix loss occurs [10,[28], [29], [30]]. The OAI imaging protocol required 58 min (39 min for the target, and 19 min for the secondary knee), not including patient set-up, coil repositioning, and time for pre-scanning. In 2015, a consensus-driven approach was put forward on how imaging may be best applied to knee OA (KOA) trials [31]. The review provided information on acquisition methods, protocol and hardware recommendations, quality control procedures, performance metrics for assessments, and clinical research recommendations [31]. Given concerns about image quality and time constraints, the OAI protocol is not ideal for clinical trials today. New MRI sequences and modifications have been introduced to replace, supplement, or accelerate traditional sequences [2,11,32,33].
The purpose of this expert opinion design paper is to propose, implement, test, and discuss a state-of-the-art image acquisition protocol for KOA clinical trials, applicable in either early or advanced KOA. A 30-min net acquisition was targeted; yet, the protocol supports analysis of multiple desirable semi-quantitative and quantitative assessments, pertinent to a multitude of articular and peri-articular tissues and pathological processes that include synovitis [34,35]. Quantitative assessment should be amendable to automated analysis [[36], [37], [38], [39]]. The choice of MRI sequences and their specific role in studying KOA relevant structural endpoints will be discussed in context of the scientific literature with the aim of helping to bridge the gap between technical manuals (i.e. protocol acquisition guides) for KOA used in clinical studies and the wealth of imaging literature.
2. Methods
A design for an MRI protocol was developed for use in two clinical trials within the PeRsOnalized Therapies for Osteoarthritis (PROTO) (Advanced PeRsOnalized Therapies for Osteoarthritis) project (No: 101095635, HORIZON-HLTH-2022-STAYHLTH-02-01). (i) PROTO-PTOA (PTOA = posttraumatic OA) studies patients with pre-stage KOA (anterior cruciate ligament (ACL) injury and reconstruction) who will receive a personalized training intervention; (ii) PROTO-PLX (PLX = placental derived mesenchymal like stromal cells) includes patients with moderate to advanced KOA (medial Osteoarthritis Research Society [OARSI] atlas joint space narrowing (JSN) grades 1 or 2) [40,41], in which cell therapy will be tested for safety, symptomatic and structural efficacy. A specific focus was on the assessment of synovitis [34,35], semi-quantitatively and quantitatively, without using contrast enhancement (CE). CE (using intravenous gadolinium administration) is considered the most accurate method to assess synovitis [34,35], as it permits differentiating synovitis as thickened, fluid-rich synovium that allows uptake of Gadolinium contrast molecules, but it is associated with scheduling challenges, higher cost, some patient burden, and potential medical risks [[42], [43], [44], [45], [46], [47]]. Furthermore, CE imaging requires monitoring by a physician, increasing cost and duration of the exam beyond actual scan time, hence advantages of assessing synovitis without its use.
A PubMed literature search was conducted for 2004 to 2024, with a specific focus on the past 5 years, using keywords relevant to MRI of KOA. No hard bibliometric measures were applied, but the subjective evaluation by the authors was used to rate the relevance, importance, and quality of the work with respect to the aims of this project. Relevance was evaluated with a focus on designing and implementing a KOA imaging protocol supporting the image assessments of interest within a short acquisition time, optimally considered less than 30 min. Many high-quality articles on the topic were therefore not included, as this is not a comprehensive literature review, but an expert opinion protocol development, with input from several OA and/or imaging experts (see acknowledgments). Their invitation was not contingent on a formal selection process, or on prescribing a formal structure to the interaction but on their direct or indirect involvement in the PROTO project. The following structural endpoints were deemed desirable for a KOA trial:
2.1. Quantitative
-
1)
Cartilage thickness (ThC), volume (VC), surface areas (tAB, cAB), and denuded areas (dAB) [8,9,48].
-
2)
Cartilage composition based on laminar (deep and superficial) T2;10,30,49
-
3)
Hoffa synovitis (HS); [35,50], or synovitis at the intercondylar notch region [51].
- 4)
-
5)
Joint effusion, and synovial thickness, separately [34,35,52].
-
6)
Meniscus morphology and position/extrusion [[17], [18], [19],50].
-
7)
Bone parameters of size and shape, including osteophyte size [8,[20], [21], [22]].
2.2. Semi-quantitative
The MRI OA Knee Score (MOAKS) [3] or ACL OA Score (ACLOAS) [53]:
-
1)
Cartilage damage/lesions;
-
2)
Meniscus damage and extrusion;
-
3)
Bone marrow lesions;
-
4)
HS, potentially using a scoring system different from MOAKS [54] and different terminology of locations [51].
-
5)
ES;
-
6)
Joint effusion, and synovial thickening, separately;
-
7)
Subchondral bone attrition;
-
8)
Ligament status (cruciate and collateral), and loose bodies;
-
9)
Bone pathology: i.e. subchondral sclerosis, subchondral insufficiency fractures, bone marrow infiltration, bone infection, acute and pathological bone fractures, and bone tumor infiltration.
Based on this selection, a KOA MRI protocol of net <30 min is suggested, supporting the above image assessments, limiting artifacts and imaging inconsistencies.
3. Results
3.1. Study duration, scanner hard- and software
Within PROTO, the MRI protocol will be acquired at baseline for cross-sectional analysis, longitudinal in the short term (6 or 12 months), and in the long term (36 months). If participants must terminate or withdraw from the study before 36 months, an “unscheduled” final MRI visit will be arranged, if possible. For KOA trials, MRI scanners with a field strength of ≥1.5 T (T) are recommended, but 3T is preferred. A multi-channel transmit-receive knee coil should be used, or a different type of coil of similar quality; surface/flex coils should be tested thoroughly before being included. Every attempt should be made to ensure that scanners and coils used in the study are not exchanged during the trial, even if with similar equipment from the same vendor. Although scanner breakdowns and repairs may occur, one should check during an upfront imaging site qualification process that no scheduled replacement takes place during the study. Software updates should also be avoided or kept to a minimum, as vendors do not normally disclose changes in their sequences between software versions. Hence, updates affecting fundamental scanner functions or the MRI sequences (e.g. new reconstruction algorithms) should be initiated before the start of the study, or delayed until the end of it, with the software version “frozen” during the study, to warrant consistent measurement and avoid systematic bias. If alterations in the hard- or software are indispensable, studying healthy volunteers before and after the change can help quantifying a potential bias, or assure that this is smaller than the expected effect of interest.
3.2. Suggested protocol, test-retest, and QC
One knee per participant will be imaged (the “target” knee). The specific sequences facilitating semi-quantitative and quantitative image assessment within ≤30 min net imaging time are shown in Table 1 and Fig. 1, with detailed parameters in Table 2. The order of acquisition will be identical in each participant and visit.
Table 1.
Summary of the MRI acquisition protocol, detailing the orientations, sequence acquisition durations, capabilities for semi-quantitative (sq) and quantitative (q) image assessments facilitated by the protocol. For sequence #2, two alternatives are provided (in grey).
| Orientation | MRI sequence | Acq. Time (minutes) | Assessments facilitated by the acquisition (sq or q) (assessment capability) | |
|---|---|---|---|---|
| 1A | 3-Plane | Localizer(s) | 0:14 | |
| 1B | Axial | Localizer | 0:30 | |
| 2 | Coronal | 3D qDESS we 1.5/0.31 # | 9:34 | Cartilage thickness & T2 femorotibial joint (q); Meniscus morphology & extrusion (q); Bone size & shape (q) |
| 2 alt. A | Sagittal same resolution | 3D qDESS we 1.5/0.31 # | 9:11 | Cartilage thickness & T2 femorotibial & femoropatellar joint (q); Bone size & shape (q); Effusion synovitis (q); Hoffa synovitis (q) |
| 2 alt. B | Sagittal near-iso-tropic resolution | 3D qDESS we # 0.7/0.31 # | 14:08 | All of the above (2 and 2A) |
| 3 | Sagittal | 2D PD TSE FS | 2:13 | Hoffa synovitis (q)§; Hoffa synovitis (sq) Hagiwara et al. (ref. #54) All tissues & pathologies MOAKS (sq); ACLOAS (sq) |
| 4 | Axial | 2D PD TSE FS | 3:56 | Effusion synovitis (q), All tissues & pathologies MOAKS (sq); ACLOAS (sq) |
| 5 | Axial | 2D FLAIR FS | 6:38 | Effusion & synovial thickness MOAKS (sq) Effusion & synovial thickness (q)§ |
| 6 | Coronal | 2D T1 TSE (no FS) | 2:29 | All tissues & pathologies MOAKS (sq), including medial and lateral collateral ligament status, loose bodies, subchondral sclerosis, subchondral insufficiency fractures, bone marrow & bone tumor infiltration, bone infection, acute and pathological bone fractures |
| Total acquisition time without re-test scan | 25:34 min | |||
Acq. = acquisition; q = quantitative; sq = semi-quantitative; 2D = two-dimensional; 3D = three-dimensional; DESS = double echo steady state; we = water excitation (an alternative form of fat suppression); # = spatial resolution: slice thickness/in-plane resolution (both in mm); T2 = transverse relaxation time = compositional cartilage measure; alt. = alternative to 2 acquisition No 2; ref # = reference No; MOAKS = MRI Osteoarthritis Knee Score; ACLOAS = Anterior Cruciate Ligament Osteoarthritis Score; PD = proton density weighted; TSE = turbo spin echo; FS = fat suppression; § experimental and not yet established; FLAIR = fluid-attenuated inversion-recovery; T1 = T1-weighted.
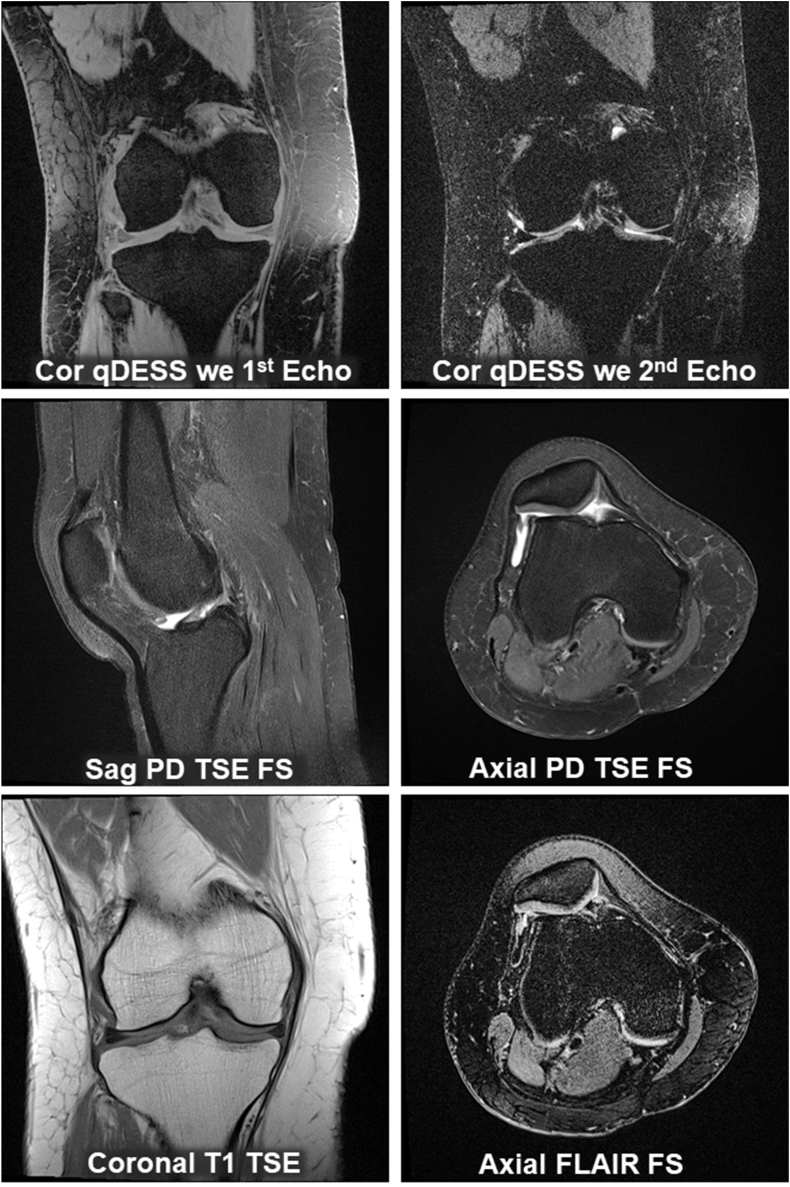
Fig. 1.
Example images of the proposed sample MRI acquisition protocol for clinical trials. These encompass the 1st and 2nd echo of the qDESS MRI sequence with water excitation (we). The 1st echo displays a mixed T1/T2 contrast, whereas the second one exhibits T2 and diffusion weighted imaging contrast. The “clinical” sequences in three orientations include a sagittal (sag) PD weighted TSE sequence with fat-saturation (FS) as well as an axially orientated PD TSE FS; the coronal (Cor) TSE sequence is acquired with T1 weighting (T1) and without FS. Finally, an axial FLAIR is obtained with FS. The MRIs were obtained on a 3T Siemens VIDA scanner (Software Version Numaris VA 50).
Table 2.
Detailed acquisition parameters of the proposed MRI acquisition protocol. For sequence #2, two alternatives are provided (in grey).
| Orientation | Sequence | FS | Acq. T |
TR |
TE |
FA | Sl.Th. |
Slice |
FOV |
Matrix | BandW |
In-pl. reso. |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| minutes | ms | ms | mm | No. | cm | Hz/Pixel | mm x mm | ||||||
| 1A | Localizer | none | 0:14 | 3.3 | 1.3 | 12 | 2.0 | 80 | 45 | 224 × 224 | 540 | 2.01 × 2.01 | |
| 1B | Localizer HR | none | 0:30 | 2630 | 83.0 | 15 | 3.5 | 15 | 16 | 192 × 384 | 246 | 0.21 × 0.21 | |
| 2 | Coronal | 3D qDESS | we | 9:34 | 16.9 | 4.9/29 | 15 | 1.5 | 80 | 16 | 512 × 512 | 296 | 0.31 × 0.31 |
| 2 alt. A | Sagittal | 3D qDESS | we | 9:11 | 16.9 | 4.9/29 | 15 | 1.5 | 80 | 16 | 512 × 512 | 296 | 0.31 × 0.31 |
| 2 alt. B | Sag near iso | 3D qDESS | we | 14:08∗ | 16.9 | 4.9/29 | 15 | 0.7 | 120 | 16 | 512 × 512 | 296 | 0.31 × 0.31 |
| 3 | Sagittal | 2D PD TSE | fs | 2:13 | 3260 | 42 | 180 | 3.0 | 37 | 16 | 328 × 448 | 248 | 0.36 × 0.36 |
| 4 | Axial | 2D PD TSE | fs | 3:56 | 4500 | 33 | 180 | 3.0 | 37 | 16 | 448 × 448 | 248 | 0.36 × 0.36 |
| 5 | Axial | 2D FLAIR | fs | 6:38 | 9000 | 89 | 180 | 3.0 | 37 | 16 | 448 × 448 | 219 | 0.36 × 0.36 |
| 6 | Coronal | 2D T1-w TSE | none | 2:29 | 600 | 9.9 | 180 | 3.0 | 37 | 14 | 307 × 384 | 228 | 0.36 × 0.36 |
FS = fat suppression; Acq. T = acquisition time; TR = repetition time; TE = echo time; FA = flip angle; Sl.Th. = slice thickness; No. = number; FOV = field of view; BandW = Bandwidth; Hz = Herz; In-pl. reso = in-plane resolution; sag near iso = sagittal near isotropic; for other abbreviations please see Table 1; ∗ the acquisition time is given to achieve the same signal-to-noise ratio as for the 1.5 mm sagittal qDESS we; f or sagittal and coronal acquisitions, a superior-inferior readout, and for axial acquisitions an anterior-posterior readout direction may be used to minimize aliasing artifacts.
A second (retest) scan not included in the 30-min net acquisition time) may be obtained during the baseline visit, if tolerated, with repositioning of the knee in the coil. The retest scan should be acquired for one sequence (acquisitions 2, 3, 4, or 5 in Table 1) per patient, assigned in ascending order, so that approximately an equal number of retest acquisitions is obtained for each sequence. A retest scan of the same imaging sequence will then again be acquired at 36 M (or any early termination visit). Acquiring test-retest scans at baseline and follow-up will permit evaluation of the precision (reproducibility) errors of quantitative image assessments and the smallest detectable change (SDC).
To ensure high-quality data, each participant's exam must be quality controlled (QC'd) as soon as possible after the MRI session by a trained person familiar with the imaging acquisition guide and potential sources of errors/artifacts. In case of quality issues or non-adherence to the imaging protocol, the acquisition must be repeated. The QC review should include complete anatomical coverage, proper (double oblique) image orientation (Fig. 2, Fig. 3), proper fat saturation (FS) where applicable, lack of motion, aliasing (anatomical structures appearing at wrong locations) or other artifacts, and adherence to the pre-specified image acquisition parameters. If the parameters were not met and acquisition not repeated before the participant received drug/placebo treatment at baseline, all follow-up scans must be acquired with acquisition parameters identical to those of the baseline visit to ensure consistency of signal, contrast, and resolution, and warrant unbiased longitudinal measurement.
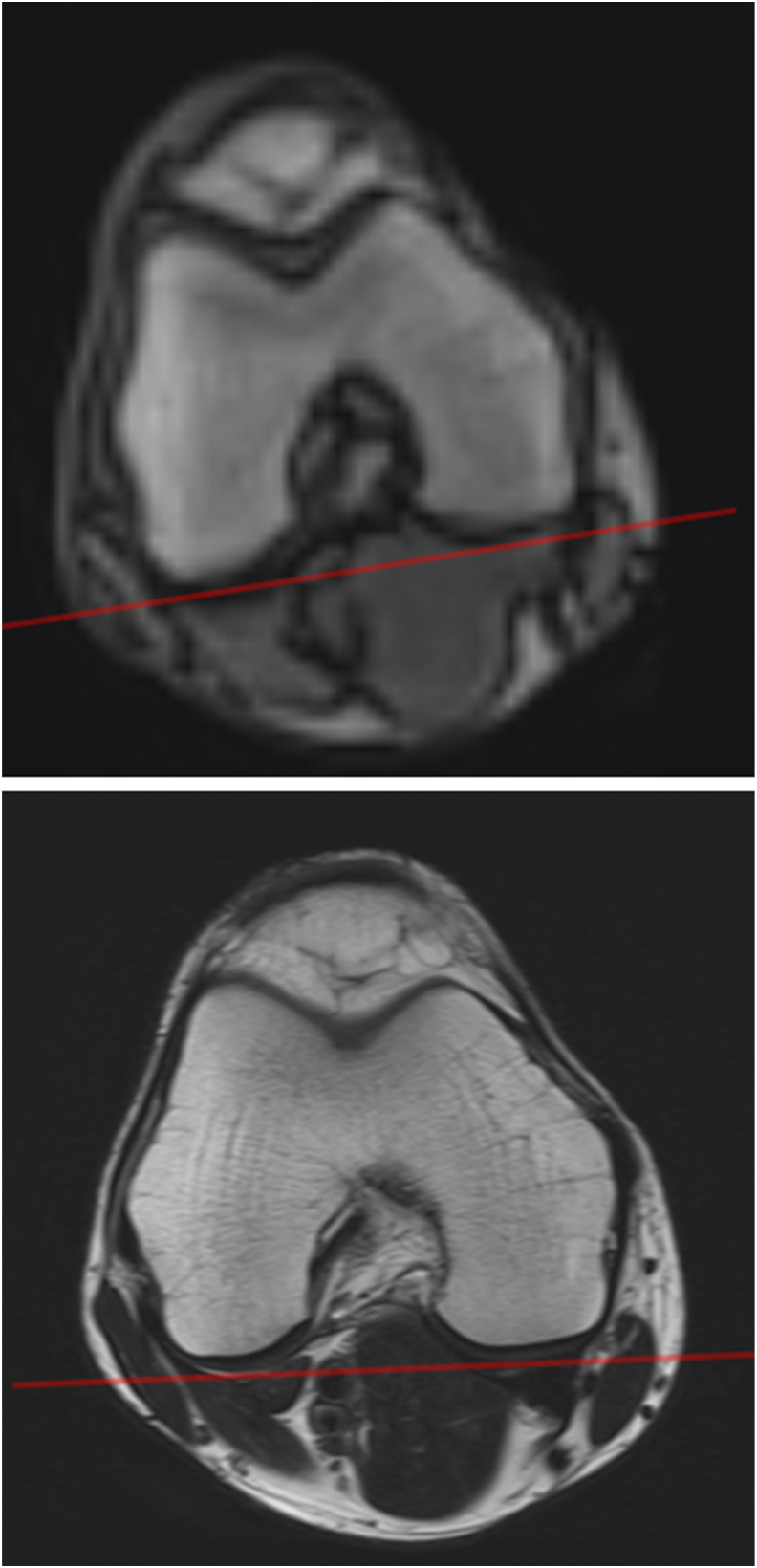
Fig. 2.
Standard (top) and high resolution (HR, bottom) axial localizer image for the double oblique coronal and sagittal acquisitions. Comparison of the localizer images demonstrates that the HR acquisition is superior in obtaining a double oblique coronal orientation (red line) pertinent to the DBEV, and in acquiring sagittal images perpendicular to the DBEV.
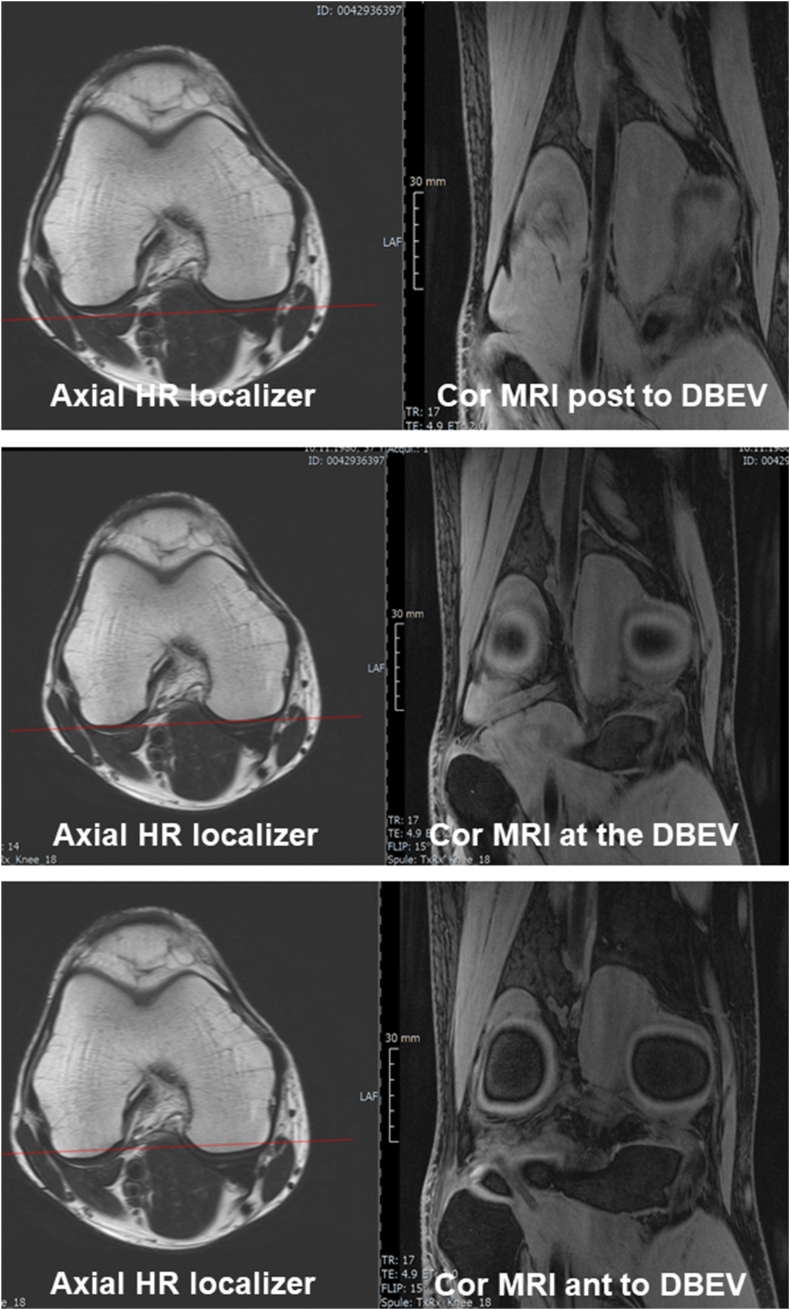
Fig. 3.
Axial HR localizer images shown on the left with a red line displaying the position of the respective coronal MRI. The middle image on the right shows a coronal MRI positioned double oblique to obtain a double bull eyes view (DBEV). The superior image on the right shows a coronal MRI posterior, and the inferior image a coronal MRI anterior to the DBEV. The MRIs were obtained on a 3T Siemens VIDA scanner.
3.3. Patient preparation, positioning, and MRI scan orientations
Participants should avoid severe physical activity that involves knee loading over 12 h prior to imaging and be required to physically rest for 45 min prior to scanning. Participants should be positioned comfortably, supine in the scanner, with an empty bladder; sandbags and padding may be positioned around knee and ankle to fix the leg and minimize motion artifacts. The patella should be aligned with the middle of the coil, with the knee slightly flexed (10–15°). The leg may be placed in defined rotation, using positioning devices, but ensuring that the patient feels comfortable in natural external rotation, avoiding motion artifacts, we consider being more important. The position-sensitive magic angle effect is more pronounced in the femoral trochlea than in the weight-bearing femoro-tibial joint, and less relevant in longitudinal studies of cartilage T2, with “change over time” being the target measure.
The coronal, sagittal and axial acquisitions are planned with reference to the participant's knee position using a 3-plane localizer (scout), taking approximately 15 s (Fig. 2), that permits planning orientation and position of the actual MRI sequences (Table 1). The 3D localizer may be supplemented by a second, high-resolution axial localizer (Fig. 2) that depicts the posterior medial and lateral femoral condyles in better detail for proper orientation of the (double oblique) coronal and sagittal MRIs. For sagittal and coronal acquisitions, a superior-inferior readout, and for axial acquisitions an anterior-posterior readout direction may be used, as these minimize aliasing artifacts, which degrade image quality.
Coronal (acquisitions 2 and 6, Table 1): These sequences should be obtained as a double oblique, “double bull's eye view”. This view delineates the posterior medial and lateral femur in the same coronal image, and the condyle as circles of similar size (Fig. 2, Fig. 3). This approach allows for a consistent region of interest on the weight-bearing femur, ensuring that measurements are not affected by variable positioning, particularly in a longitudinal study. Using the axial localizer (Table 1, acquisition 1A), the slice showing the largest femoral cross-section is identified, and the second (high resolution) axial localizer (acquisition 1B) acquired in this position. A line connecting the posterior edges of both condyles is drawn, the coronal plane then orientated parallel to it (Fig. 2, Fig. 3). Based on the sagittal (localizer) image, the image slice is orientated perpendicular to the tibial plateau. Alternatively to the coronal orientation, a sagittal quantitative dual echo steady state (qDESS) may be acquired (Table 1, Table 2), using the same slice thickness proposed for coronal imaging, or with thin slices and near-isotropic spatial resolution (Table 1, Table 2). The field of view should be positioned to cover the femorotibial joint, to extend beyond the patella superiorly and beyond (approximately 0.5 cm) the margins of the soft tissues in medial-lateral direction to avoid “aliasing”.
Sagittal (acquisition 3, Table 1). This acquisition should be planned in a manner similar to the coronal ones. The axial scout is used to orient sagittal slices perpendicular to the line connecting the posterior femoral condyles, and based on the coronal localizer, perpendicular to the tibial plateau. The sagittal acquisitions should extend beyond the medial and lateral margins of the knee soft tissues to avoid aliasing, and the field of view anteriorly beyond the patella and posteriorly beyond the popliteal soft tissues. To assess synovitis, the position should extend about 5 cm above the patella, whereas the tibia needs to be included only to the level of the proximal tibiofibular joint, i.e. 2 cm distal to the plateau.
Axial (acquisitions 4 and 5, Table 1): These should be planned based on the coronal localizer with the largest diameter of the knee, and be angulated parallel to the tibial plateau. Based on the sagittal localizer, they should be oriented perpendicular to the posterior patellar surface. Because a focus of this protocol is assessment of ES and synovial thickness, the image stack should be placed more proximally than usual (see sagittal acquisition). This is important for comprehensively evaluating SE, but at the same time it is advised that the distal portion of the Hoffa's (or infrapatellar) fat pad (=IPFP) is covered for assessment of HS.
Table 1 summarizes which image assessments can be performed based on which MRI sequence at the expense of which acquisition time. This may be used as a “menu” to omit certain sequences and shorten the protocol, if tailoring to a specific study need is desired.
4. Discussion
The aim of this expert opinion design paper was to propose, implement, test, and discuss a state-of-the-art MRI acquisition protocol for KOA trials with <30 min net imaging time, enabling comprehensive and ideally automated [[36], [37], [38], [39]], quantitative image assessments. These included cartilage morphology and T2, synovitis, meniscus morphology and position, and bone size and shape. The protocol also supports semi-quantitative evaluation of structural tissue pathology, including bone disease [[3], [4], [5],34,53].
In clinical trials of KOA it is desirable to obtain short- (6–12 months) and long-term (36–60 months) imaging results. The former may demonstrate early structural effects and permit inclusion of participants who withdraw early, e.g. employing mixed-effects models [55]. Yet, long observation intervals are needed, acknowledging the slow progression rates in OA [56]. Tissue modification by pharmacological or non-pharmacological intervention likely requires a certain time to exceed thresholds above which they translate into symptomatic benefit and become clinically meaningful [57,58]. Particularly in longer trials, imaging centers must be directed to avoid scanner changes, because these may cause substantial deviations in quantitative measurement, even when from the same vendor [59]. Similarly, deviations occur when using different coils [60], so hardware changes are to be avoided. Participants should abstain from strenuous physical activity prior to imaging to avoid involuntary movement, compression of cartilage [[61], [62], [63], [64]], and modification of cartilage T2 [25,65,66].
OA trials of the early disease phase, when cartilage matrix is still intact, may prioritize assessment of cartilage composition [30], because no net cartilage loss occurs over prolonged periods of time [67]. However, subtle alterations in cartilage thickness may be detected in early OA models when specific, location-independent analysis techniques are used [68,69]. Furthermore, a semi-quantitative grading system has been proposed, specifically for ACL-injured patients with early structural changes [53]. Knee joint synovitis occurs at early KOA stages [70,71], including ACL injury [30]. It is associated with various imaging features [72], and considered a distinct “phenotype” of KOA [34,73,74], advocating its inclusion in clinical trials. In advanced disease, with radiographic JSN apparent, structural progression rates become greater and more stable, with cartilage thickness change apparent over relatively short periods of 6–12 months [75,76].
The core sequence of our proposed protocol is qDESS [[77], [78], [79]]. This sequence is not yet commonly available on MRI manufacturer software platforms, but can be installed by application specialists based on research agreements between the MRI manufacturer and the imaging site. This “research version” generates two separate images per repetition time, separated by a spoiler gradient that allows one to compute T2. The first acquisition displays T1/T2 contrast similar to proton density (PD) images, whereas the second acquisition exhibits T2 and diffusion-weighted contrast. Conventional DESS [15], on the other hand, only generates a single image with mixed T1-and T2-contrast [80], as root-sum-of-squares of the two images, permitting measurement of cartilage morphology, but not T2. When stored separately, however, a model-based voxel-by-voxel fit can produce a high-resolution T2 map [81]. Given a relatively long 2nd echo (2∗TR-TE; ∼30 ms) T2 can be estimated in both the deep and superficial cartilage layer [77]. MESE, for comparison, relies on approximate echoes, as it needs to sample non-T2 related effects, such as T1 and the flip angle, whereas with qDESS these can be modelled into the exponential fit so that two echoes suffice [[77], [78], [79]]. QDESS T2 was shown to match MESE T2 [77] and single echo spin echo T2, considered the gold standard [82], and to discriminate between mild vs advanced KOA [83]. With qDESS, T2 and cartilage morphology can be analyzed from the same high-resolution images, obtained with relatively short imaging time, without requiring separate segmentations, and substantially reducing analysis and patient burden [77]. The coronal orientation of the qDESS acquisition (parallel imaging, readout superior-inferior, phase encoding right-left) takes <10min, whereas SPGR (for cartilage thickness) and MESE (for T2) taken together take much longer [80]. Even shorter qDESS imaging times <5 min have been published [77], but the current protocol focusses on high in-plane resolution (0.31 mm) for accurate cartilage thickness analysis. Although such resolution may not be required for measuring bulk T2, but it is advantageous for separating T2 for in the deep and superficial layers, in which compositional properties differ substantially [49,84]. The coronal (and standard sagittal) slice thickness proposed here (1.5 mm) is geared towards a greater signal and contrast-to-noise ratio compared with the near-isotropic OAI protocol [80] (0.7 mm), with sensitivity to longitudinal cartilage thickness changes shown to be similar when analyzing every second vs each 0.7 mm slice [85]. The sequence proposed here is geared towards the greater signal and contrast-to-noise ratio associated with greater slice thickness, whereas isotropic or near-isotropic acquisition can be more easily reformatted to other planes, permitting assessment in all image orientations (see Table 1: 2Alt B). Near-isotropic imaging typically occurs at the expense of a lower in-plane resolution [77], which is disadvantageous when measuring thickness (or laminar T2) of a thin (sometimes denuded) cartilage layer, or comes with the expense of a longer acquisition time. Partial volume effects of femorotibial cartilage are smaller with coronal than with sagittal orientation, with a well-defined and consistent orientation of the double oblique acquisitions warranting consistent and reproducible measurements [86,87]. A coronal acquisition is also beneficial when measuring meniscus extrusion [17,18], with automated methods being developed [50]. Osteophyte measurement at medial or lateral locations also require coronal acquisitions, with a coronal multi-planar reconstruction of a sagittal near-isotropic qDESS as fall back, also applicable for meniscus extrusion [19,88]. If the patellofemoral joint is to be included, a sagittal protocol is mandatory [89] and is advantageous for analysis of bone size and shape [8,[20], [21], [22]] (except for measuring medial and lateral osteophytes). Sagittal qDESS also may be used for evaluating HS and ES [51,90], potentially relying on axial multiplanar reconstruction if acquired with near-isotropic resolution.
With conventional DESS [15,80], fully automated cartilage thickness measurement was achieved using deep learning and convolutional neural networks methods, validated cross-sectionally [91,92] and longitudinally [93]. Fully automated laminar T2 analysis from qDESS hence is straightforward based on validated technology [[91], [92], [93]].
The acquisition time for the three clinical turbo spin echo (TSE) sequences images is < 9min. Recent innovation has reduced acquisition time to approximately 5min, using combined simultaneous multi-slice and parallel imaging acceleration for multi-contrast knee MRI [94], or qDESS with deep learning super-resolution augmentation [95]. Deep learning-based acquisition and image reconstruction may accelerate acquisition of clinical TSE sequences 10-fold, with 2–3 min being sufficient for the exam [96,97], and 5–7 min saved with the present protocol.
PD TSE FS sequences are fluid-sensitive, but they cannot discriminate between the actual (potentially thickened) synovium and joint effusion because both pathologies appear hyper-intense. However, CE imaging, at one time point after gadolinium injection, and particularly “dynamically” at several time points (DCE-MRI) permit differentiation between the (thickened) synovial membrane and effusion [34], synovial thickening representing the most accepted MRI measure of synovitis [35]. In end-stage OA, DCE-MRI was found to be strongly associated with synovitis histology [98]. Whereas static CE-MRI added significant independent information to this association, non-CE-MRI (NCE-MRI) did not [98]. Given the inability of a standard fluid-sensitive sequence to differentiate between effusion and synovial thickening, “ES” has been used as a surrogate for synovitis [3,34]. Likewise, signal heterogeneities in the IPFP or inter-condylar region (HS) [51] have been proposed as surrogates of ES, with evidence that the IPFP plays a role in KOA symptomatology [99]. IPFP signal heterogeneity was shown to be related to synovitis on biopsy, and to synovitis detected on CE T1-weighted TSE MRI [71]. Perfusion variables on DCE MRI of HS were more strongly associated with knee pain severity than MOAKS HS, suggesting inflammation in the IPFP to be associated with knee pain [100]. Yet signal characteristics from the IPFP on NCE-images are non-specific when acquired without CE [34], whereas the latter increases patient burden [[42], [43], [44], [45], [46], [47]]. A specific reading system has been proposed for superior semi-quantitative evaluation of HS [54]. Segmentation and quantification of the IPFP [51] has been shown to be reproducible [101] and responsive to change in weight [102,103]. Furthermore, a correlation of IPFP MRI signal has been demonstrated with KOA status and progression [[104], [105], [106]]. Finally, IPFP signal alterations were shown to be related to synovial fluid cytokine profiles [107] and to higher serum levels of resistin, an adipose-derived hormone and endogenous ligand of Toll-like receptor-4, triggering major inflammatory pathways and mediating inflammatory processes [108]. Although HS has thus far been mainly graded semi-quantitatively [3,34,54,109], efforts are underway to obtain quantitative HS measures [34,50]. Extracting HS measures quantitatively may feed statistical approaches with continuous variables to improve diagnostic accuracy longitudinal sensitivity to change [50]. QDESS also has been explored for semi-quantitative scoring of HS in the intercondylar region, the agreement vs CE-MRI of synovial thickness [51] in this region being similar to other regions around the knee.
Whereas sagittal PD TSE is typically used to assess HS, axial PD TSE is more tailored towards assessing ES. Imaging should reach more proximal for this purpose, aiming at full coverage of effusion volume, with proximal regions being the most common locus [110]. Semi-quantitative evaluation of ES on NCE-MRI was shown to be positively correlated with quantitative measures of synovial thickening and effusion on CE-MRI, this relationship being stronger than that for HS with CE-MRI [35]. Biochemical serum markers of inflammation (i.e. sHA and sMMP-3) were modestly associated with ES; clinical signs of effusion were not highly sensitive but highly specific to presence of ES on NCE-MRI. However, in at least one study, HS (on NCE-MRI) was not associated with ES [111]. Several investigators have taken quantitative approaches to measure ES (mostly maximal area or volume) on NCE or CE TSE [52,[112], [113], [114], [115]]. ES volume was shown to be statistically significantly related to semi-quantitative scores of ES [3,4,34,116]. Although volumetric measurement on NCE PD TSE FS overestimated the amount of ES from T1-weighted CE FS imaging (as a reference) almost two-fold, both measurements were highly correlated [114]. A quantitative approach was applied in a clinical trial reporting volumetric ES to be reproducible in participants with an inflammatory OA phenotype [117]. Studies investigating ES pathophysiology using novel automated methods are underway [50].
Given the inability of classical fluid-sensitive NCE imaging to differentiate between synovial inflammation and effusion, there are efforts in developing novel NCE sequences that measure “synovitis” (with synovial thickening) directly, rather than the surrogate composed of effusion and synovium together. QDESS is one of these sequences, since a weighted subtraction of the two qDESS echoes can null the synovial fluid and create a positive contrast to visualize synovium [90]. Although qDESS was shown to underestimate semi-quantitative synovitis scores compared with CE-MRI, the two methods were highly correlated [90]. Diagnostic confidence was slightly superior for qDESS, although inferior to that of CE-MRI [51]. The attractiveness of the qDESS clearly lies in its multi-functionality of supporting quantitative endpoints in KOA. Another option for evaluating the synovium without CE is double inversion recovery MRI [118,119]; however, this has not been explored much and requires further validation.
We further selected an axial fluid attenuated inversion-recovery (FLAIR) sequence with FS, with acquisition parameters at 3T having been explored [33]. Compared with PD TSE, the acquisition time is relatively long due to the need for nulling the fluid signal [120] and the inversion time is longer (2200 ms) than that commonly used in brain imaging [33]. Before a 90° excitation radiofrequency pulse is applied with FLAIR, a 180° inversion pulse is administered and a saturation pulse is utilized for chemical shift-selective FS. The FLAIR FS improves delineation of the synovium in the intra-articular space by suppressing both the fluid and fat signal. The absence of the fluid signal increases the dynamic range and improves delineation of other tissues such as synovium [120]. The FLAIR sequence is acquired in identical location, orientation, and resolution as the axial PD TSE FS. Given improved conspicuity of the synovium and the superior separation between thickened synovial membrane and synovial fluid (without CE), FLAIR has shown superior diagnostic performance to standard clinical PD TSE sequences in relation to CE T1-weighted SE FS as a reference standard [33]. If effusion volume is to be measured, axial acquisitions should extend 5 cm above the patella, particularly when evaluating longitudinal change over time. An “accelerated” FLAIR [120] (at 3T) has been introduced with acquisition times of approximately 50% less, similar to that of axial PD TSE FS. The accelerated FLAIR has shown equivalence to CE T1-weighted SE FS in terms of intra-reader and inter-reader reproducibility, and the diagnosis of inflammatory knee synovitis [120]. Neither qDESS or FLAIR have yet been explored in terms of quantitative assessment of HS, ES, or synovial thickness.
Contrary to other clinical protocols, the proposed coronal TSE is acquired T1-weighted and without FS. This better suits for evaluation of the collateral ligament complexes and bone pathology, e.g. subchondral bone sclerosis, subchondral insufficiency fractures, bone marrow infiltration, bone infection, acute and pathological fractures, bone tumor infiltration, and other pathologies, important to monitor or exclude from KOA clinical trials.
Repeat (test-retest) scanning for each sequence is useful from a clinical research perspective, permitting quantification of the test-retest precision (root mean square standard deviation and coefficient of variation) ≤and specific to the study [121]. Furthermore, if performed at two longitudinal time points, repeat scanning allows for determining the smallest detectable change (SDC), relating test-retest precision to the actual longitudinal change within the study [[122], [123], [124]]. This provides thresholds of progression/non-progression and enables transformation of continuous data into number of progressors per treatment and placebo arm, a metric often more straightforward to communicate to regulators or the public. Ideally, retest scanning should be performed at another day, to include variability originating from scanner drift, imaging conditions, and patient conditions. However, longer term (8–9 month) measurement variability in cartilage morphology only is marginally greater than that within the same imaging session (with repositioning of the joint), the relative position of the images vs the joint anatomy apparently being the major driver of test-retest error [125]. It is of utmost importance that all MRIs are thoroughly checked as soon as possible after the session. In case of quality issues, any unacceptable acquisitions should be repeated, ideally while the patient is still on site. This must occur before the patient is receiving drug or placebo treatment.
Limitations of the current protocol include the lack of inclusion of deep learning-assisted clinical sequences that may save 5–7 min over the “clinical” TSE [96,97], but these were not available on the VIDA scanner used for the PROTO studies. The qDESS currently has “research” status, and inclusion in a clinical trial requires administrative and technical work between the MRI site, application specialists, and the MRI manufacturer. While T2 may be sensitive to proteoglycan content [23,126], other MRI techniques such as T1rho, sodium imaging, or gagCEST, are more specific to these or other matrix components [10,23,32,127]. Such sequence hence should to be added if these measures are of particular interest. In the current protocol, no gold standard CE-MRI is obtained for assessing synovitis. For logistical and safety reasons [[42], [43], [44], [45], [46], [47]], use of CE appears feasible in dedicated centers for early phase trials, but measurement from NCE-MRI (even if only surrogates) is often preferred from a logistic perspective. We decided to include the NCE FLAIR [33], with the accelerated version [120] not available on the scanner. To reduce whole study imaging time, FLAIR of course may be omitted if acquisition time is limited and synovitis is not a focus of the study.
In conclusion, this work is intended to bridge the gap between common technical manuals for image acqusitions in clinical studies and the overwhelming amount of imaging literature. In this expert opinion clinical study protocol design paper, we propose an MRI acquision protocol for KOA trials suitable for early and advanced KOA. The protocol aims to balance imaging efficiency (<30min), safety (no intravenous CE), and academic interest, with a comprehensive number of relevant semi-quantitative and quantitative imaging endpoints supported. These are pertinent to the multitude of articular tissues and pathological processes, including synovitis. The availability of a relatively short and efficient image acquisition protocol, supporting relevant semi-quantitative and quantitative imaging endpoints with the potential for automated analysis, is instrumental for conduct of clinical research. The protocol should provide sufficient opportunity for technical innovation and for setting up innovative clinical trials on pharmacological, non-pharmacological, “advanced therapy medicinal product”, surgical, or other interventions, either for improving knee tissue status or for ameliorating articular and peri-articular structural pathology. Once an effective disease-modifying therapy is established, abbreviated protocols may be used to monitor the structural efficacy of such interventions in the long term [58].
Consent for publication
All authors have consented to publication.
Authors’ contributions
Study conception and design: FE, TCWR, WW, TW.
Acquisition of data: TCWR.
Analysis & interpretation of data: all authors.
Writing of the first manuscript draft: FE.
Critical manuscript revision and approval of final manuscript: all authors.
Role of the funding source
This work was funded by the European Union under Grant Agreement Nr. 101095635 (PROTO – Horizon Europe). Views and opinions expressed are however those of the authors only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency. Neither the European Union nor the granting authority can be held responsible for them. The design of the image acquisition protocol and the content of this article has not been contingent upon approval from the study sponsor.
Ethics approval
Ethics approval of the two PROTO clinical studies is pending.
Declaration of competing interest
FE is CEO/CMO and co-owner of Chondrometrics GmbH; has provided consulting services to Merck KGaA, Kolon-Tissuegene, Servier, Galapagos, Novartis, 4P Pharma/4Moving and Trialspark/Formation Bio; and has – related to this paper – received funding through PROTO from the EU.
TCWR has no conflict of interest to declare
AC has received research support from the National Institutes of Health, GE Healthcare and Philips and has provided consulting services to Patient Square Capital and Elucid Bioimaging Inc – unrelated to this paper.
NMB has – related to this paper – received funding through PROTO from the EU
TM has -– related to this paper – received funding through PROTO from the EU
GND has – related to this paper – received funding through PROTO from the EU
AW is a part-time employee of Chondrometrics GmbH and has – related to this paper – received funding through PROTO from the EU
WW is part-time employee and share-holder of Chondrometrics GmbH and has – related to this paper – received funding through PROTO from the EU
TW is part of the Executive Board of the Advanced Therapies in Orthopaedics Foundation and has – related to this paper– received funding through PROTO from the EU.
Acknowledgment
This work was funded by the European Union (Horizon Europe) under Grant Agreement Nr. 101095635 (PROTO). Views and opinions expressed are however those of the authors only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them.
We would like to acknowledge the technical personnel at the Radiology Department of Charité – Universitätsmedizin Berlin for their invaluable support, in particular Sabine Wagner and David Kohnert for their help with implementing the sample image acquisition protocol on the 3T Siemens Vida scanners. We thank David Kohnert for proposing and implementing acquisition of an additional axial localizer image with higher in-plane resolution, for appropriate orientation of the double oblique coronal and sagittal images. We would further like to express our appreciation to Pete Lally and Neal Bangerter from Imperial College London for providing and adapting the qDESS to the MRI scanners at the Radiology Department at Charité - Universitätsmedizin Berlin under a C2P research agreement with SiemensHealthineers. We thank Mikael Boesen (Professor, MD PhD, Consultant Radiologist.
Head of Imaging Research, Department of Radiology, Copenhagen University Hospital, Bispebjerg and Frederiksberg, Denmark), Florian N. Fleckenstein (Board-certified Radiologist, Department of Diagnostic and Interventional Radiology & Clinician Scientist, BIH Biomedical Innovation Academy and BIH Charité Clinician Scientist Program, Berlin Institute of Health at Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität, Berlin, Germany, Chris Ladel (Consultant, CHL4special consulting, Darmstadt, Germany), Pete Lally (Fellow, Department of Bioengineering - Faculty of Engineering, Imperial College, London, UK), Ali Mobasheri (Professor, PhD, Research Unit of Health Sciences and Technology, Faculty of Medicine, University of Oulu, Finland & Department of Regenerative Medicine, State Research Institute Centre for Innovative Medicine, Vilnius, Lithuania), Frank W. Roemer (Professor of Radiology, MD, Chief Musculoskeletal Imaging, Attending Radiologist, Department of Radiology, Universitätsklinikum Erlangen and Friedrich-Alexander Universität Erlangen-Nürnberg (FAU), Germany & Adjunct Professor of Radiology, Co-Director, Quantitative Imaging Center (QIC), Department of Radiology, Chobanian & Avedisian School of Medicine, Boston University, MA, USA and Tom Turmezei (Honorary Professor, MD PhD, Norfolk and Norwich University Hospital & University of East Anglia, Norfolk, UK) for advice and critical discussion of the manuscript.
Handling Editor: Professor H Madry
References
- 1.Hunter D.J., Nevitt M., Losina E., Kraus V. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract. Res. Clin. Rheumatol. 2014;28(1):61–71. doi: 10.1016/j.berh.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roemer F.W., Wirth W., Demehri S., et al. Imaging biomarkers of osteoarthritis. Semin. Muscoskel. Radiol. 2024;28(1):14–25. doi: 10.1055/s-0043-1776432. [DOI] [PubMed] [Google Scholar]
- 3.Hunter D.J., Guermazi A., Lo G.H., et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19(8):990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roemer F.W., Jarraya M., Hayashi D., et al. A perspective on the evolution of semi-quantitative MRI assessment of osteoarthritis: past, present and future. Osteoarthritis Cartilage. January 2024 doi: 10.1016/j.joca.2024.01.001. Published online. [DOI] [PubMed] [Google Scholar]
- 5.Roemer F.W., Hunter D.J., Crema M.D., Kwoh C.K., Ochoa-Albiztegui E., Guermazi A. An illustrative overview of semi-quantitative MRI scoring of knee osteoarthritis: lessons learned from longitudinal observational studies. Osteoarthritis Cartilage. 2016;24(2):274–289. doi: 10.1016/j.joca.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guermazi A., Roemer F.W., Haugen I.K., Crema M.D., Hayashi D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat. Rev. Rheumatol. 2013;9:1759–4804. doi: 10.1038/nrrheum.2012.223. (Electronic)):236-251. [DOI] [PubMed] [Google Scholar]
- 7.Wirth W., Ladel C., Maschek S., Wisser A., Eckstein F., Roemer F. Quantitative measurement of cartilage morphology in osteoarthritis: current knowledge and future directions. Skeletal Radiol. 2023;52(11):2107–2122. doi: 10.1007/s00256-022-04228-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckstein F., Wluka A.E., Wirth W., Cicuttini F. 30 Years of MRI-based cartilage & bone morphometry in knee osteoarthritis – from correlation to clinical trials. Osteoarthritis Cartilage. February 2024 doi: 10.1016/j.joca.2024.02.002. Published online. [DOI] [PubMed] [Google Scholar]
- 9.Buck R.J., Wyman B.T., Le Graverand M.P., Wirth W., Eckstein F. An efficient subset of morphological measures for articular cartilage in the healthy and diseased human knee. Magnes. Res. 2010;63(3):680–690. doi: 10.1002/mrm.22207. [DOI] [PubMed] [Google Scholar]
- 10.Link T.M., Joseph G.B., Li X. MRI-based T1rho and T2 cartilage compositional imaging in osteoarthritis: what have we learned and what is needed to apply it clinically and in a trial setting? Skeletal Radiol. 2023;52(11):2137–2147. doi: 10.1007/s00256-023-04310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhari A.S., Kogan F., Pedoia V., Majumdar S., Gold G.E., Hargreaves B.A. Rapid knee MRI acquisition and analysis techniques for imaging osteoarthritis. J. Magn. Reson. Imag. 2020;52(5):1321–1339. doi: 10.1002/jmri.26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacKenzie R., Sims C., Owens R.G., Dixon A.K. Patients' perceptions of magnetic resonance imaging. Clin. Radiol. 1995;50(3):137–143. doi: 10.1016/s0009-9260(05)83042-9. [DOI] [PubMed] [Google Scholar]
- 13.Eckstein F., Kwoh C.K., Link T.M. Imaging research results from the Osteoarthritis Initiative (OAI): a review and lessons learned 10 years after start of enrolment. Ann. Rheum. Dis. 2014;73(7):1289–1300. doi: 10.1136/annrheumdis-2014-205310. [DOI] [PubMed] [Google Scholar]
- 14.Peterfy C.G., Schneider E., Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy P.A., Recht M.P., Piraino D., Thomasson D. Optimization of a dual echo in the steady state (DESS) free-precession sequence for imaging cartilage. JMagn Reson Imaging. 1996;6(2):329–335. doi: 10.1002/jmri.1880060212. [DOI] [PubMed] [Google Scholar]
- 16.Schneider E., Nevitt M., McCulloch C., et al. Equivalence and precision of knee cartilage morphometry between different segmentation teams, cartilage regions, and MR acquisitions. Osteoarthritis Cartilage. 2012;20(8):869–879. doi: 10.1016/j.joca.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirth W., Frobell R.B., Souza R.B., et al. A three-dimensional quantitative method to measure meniscus shape, position, and signal intensity using MR images: a pilot study and preliminary results in knee osteoarthritis. Magnes. Res. 2010;63(5):1162–1171. doi: 10.1002/mrm.22380. [DOI] [PubMed] [Google Scholar]
- 18.Siorpaes K., Wenger A., Bloecker K., Wirth W., Hudelmaier M., Eckstein F. Interobserver reproducibility of quantitative meniscus analysis using coronal multiplanar DESS and IWTSE MR imaging. Magnes. Res. 2012;67(5):1419–1426. doi: 10.1002/mrm.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma K., Eckstein F., Maschek S., Roth M., Hunter D.J., Wirth W. Association of quantitative measures of medial meniscal extrusion with structural and symptomatic knee osteoarthritis progression - data from the OAI FNIH biomarker study. Osteoarthritis Cartilage. 2023;31(10):1396–1404. doi: 10.1016/j.joca.2023.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Bowes M.A., Kacena K., Alabas O.A., et al. Machine-learning, MRI bone shape and important clinical outcomes in osteoarthritis: data from the Osteoarthritis Initiative. Ann. Rheum. Dis. 2021;80(4):502–508. doi: 10.1136/annrheumdis-2020-217160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brett A., Bowes M.A., Conaghan P.G. Comparison of 3D quantitative osteoarthritis imaging biomarkers from paired CT and MR images: data from the IMI-APPROACH study. BMC Muscoskel. Disord. 2023;24(1):76. doi: 10.1186/s12891-023-06187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowes M.A., Guillard G.A., Vincent G.R., Brett A.D., Wolstenholme C.B.H., Conaghan P.G. Precision, reliability, and responsiveness of a novel automated quantification tool for cartilage thickness: data from the osteoarthritis initiative. J. Rheumatol. 2020;47(2):282–289. doi: 10.3899/jrheum.180541. [DOI] [PubMed] [Google Scholar]
- 23.Matzat S.J., van Tiel J., Gold G.E., Oei E.H.G. Quantitative MRI techniques of cartilage composition. Quant. Imag. Med. Surg. 2013;3(3):162–174. doi: 10.3978/j.issn.2223-4292.2013.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosher T.J., Dardzinski B.J. Cartilage MRI T2 relaxation time mapping: overview and applications. SeminMusculoskeletRadiol. 2004;8(4):355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 25.Liess C., Luesse S., Karger N., Heller M., Glueer C.C. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10:907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 26.Kim T., Min B.H., Yoon S.H., et al. An in vitro comparative study of T2 and T2∗ mappings of human articular cartilage at 3-Tesla MRI using histology as the standard of reference. Skeletal Radiol. 2014;43(7):947–954. doi: 10.1007/s00256-014-1872-z. [DOI] [PubMed] [Google Scholar]
- 27.Lammentausta E., Kiviranta P., Nissi M.J., et al. T2 relaxation time and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of human patellar cartilage at 1.5 T and 9.4 T: Relationships with tissue mechanical properties. J. Orthop. Res. 2006;24(3):366–374. doi: 10.1002/jor.20041. [DOI] [PubMed] [Google Scholar]
- 28.Baum T., Joseph G.B., Karampinos D.C., Jungmann P.M., Link T.M., Bauer J.S. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthritis Cartilage. 2013;21(10):1474–1484. doi: 10.1016/j.joca.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liebl H., Joseph G., Nevitt M.C., et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann. Rheum. Dis. 2015;74(7):1353–1359. doi: 10.1136/annrheumdis-2013-204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Sullivan O., Ladlow P., Steiner K., et al. Knee MRI biomarkers associated with structural, functional and symptomatic changes at least a year from ACL injury - a systematic review. Osteoarthr Cartil open. 2023;5(3) doi: 10.1016/j.ocarto.2023.100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunter D.J., Altman R.D., Cicuttini F., et al. OARSI Clinical Trials Recommendations: knee imaging in clinical trials in osteoarthritis. Osteoarthritis Cartilage. 2015;23(5):698–715. doi: 10.1016/j.joca.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Piccolo C.L., Mallio C.A., Vaccarino F., Grasso R.F., Zobel B.B. Imaging of knee osteoarthritis: a review of multimodal diagnostic approach. Quant. Imag. Med. Surg. 2023;13(11):7582–7595. doi: 10.21037/qims-22-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo H.J., Hong S.H., Oh H.Y., et al. Diagnostic accuracy of a fluid-attenuated inversion-recovery sequence with fat suppression for assessment of peripatellar synovitis: preliminary results and comparison with contrast-enhanced MR imaging. Radiology. 2017;283(3):769–778. doi: 10.1148/radiol.2016160155. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi D., Roemer F.W., Jarraya M., Guermazi A. Update on recent developments in imaging of inflammation in osteoarthritis: a narrative review. Skeletal Radiol. 2023;52(11):2057–2067. doi: 10.1007/s00256-022-04267-3. [DOI] [PubMed] [Google Scholar]
- 35.Crema M.D., Roemer F.W., Li L., et al. Comparison between semiquantitative and quantitative methods for the assessment of knee synovitis in osteoarthritis using non-enhanced and gadolinium-enhanced MRI. Osteoarthritis Cartilage. 2017;25(2):267–271. doi: 10.1016/j.joca.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Lenchik L., Heacock L., Weaver A.A., et al. Automated segmentation of tissues using CT and MRI: a systematic review HHS public access. Acad. Radiol. 2019;26(12):1695–1706. doi: 10.1016/j.acra.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S., Roemer F., Ge Y., et al. Comparison of evaluation metrics of deep learning for imbalanced imaging data in osteoarthritis studies. Osteoarthritis Cartilage. 2023;31(9):1242–1248. doi: 10.1016/j.joca.2023.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cigdem O., Deniz C.M. Osteoarthritis Imaging Artificial intelligence in knee osteoarthritis : a comprehensive review for 2022. Osteoarthr Imaging. 2023;3(3) doi: 10.1016/j.ostima.2023.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kijowski R., Fritz J., Deniz C.M. Deep learning applications in osteoarthritis imaging. Skeletal Radiol. 2023;52(11):2225–2238. doi: 10.1007/s00256-023-04296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altman R.D., Hochberg M., Murphy Jr.WA., Wolfe F., Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarhritis Cartil. 1995;3(Suppl A):3–70. [PubMed] [Google Scholar]
- 41.Altman R.D., Gold G.E. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A) doi: 10.1016/j.joca.2006.11.009. A1--56. [DOI] [PubMed] [Google Scholar]
- 42.McDonald R.J., McDonald J.S., Kallmes D.F., et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275(3):772–782. doi: 10.1148/radiol.15150025. [DOI] [PubMed] [Google Scholar]
- 43.Gulani V., Calamante F., Shellock F.G., Kanal E., Reeder S.B. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16(7):564–570. doi: 10.1016/S1474-4422(17)30158-8. [DOI] [PubMed] [Google Scholar]
- 44.Maximova N., Gregori M., Zennaro F., Sonzogni A., Simeone R., Zanon D. Hepatic gadolinium deposition and reversibility after contrast agent-enhanced MR imaging of pediatric hematopoietic stem cell transplant recipients. Radiology. 2016;281(2):418–426. doi: 10.1148/radiol.2016152846. [DOI] [PubMed] [Google Scholar]
- 45.Roberts D.R., Lindhorst S.M., Welsh C.T., et al. High levels of gadolinium deposition in the skin of a patient with normal renal function. Invest. Radiol. 2016;51(5):280–289. doi: 10.1097/RLI.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 46.Shamam Y.M., De Jesus O. 2023. Nephrogenic Systemic Fibrosis - StatPearls - NCBI Bookshelf. [PubMed] [Google Scholar]
- 47.Ramalho J., Semelka R.C., Ramalho M., Nunes R.H., AlObaidy M., Castillo M. Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol. 2016;37(7):1192–1198. doi: 10.3174/AJNR.A4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckstein F., Ateshian G., Burgkart R., et al. Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthritis Cartilage. 2006;14(10):974–983. doi: 10.1016/j.joca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Wirth W., Maschek S., Eckstein F. Sex- and age-dependence of region- and layer-specific knee cartilage composition (spin–spin–relaxation time) in healthy reference subjects. Ann Anat - Anat Anzeiger. 2017;210(March):1–8. doi: 10.1016/j.aanat.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith S.E., Bahouth S.M., Duryea J. Quantitative bone marrow lesion, meniscus, and synovitis measurement: current status. Skeletal Radiol. 2023;52(11):2123–2135. doi: 10.1007/s00256-023-04311-w. [DOI] [PubMed] [Google Scholar]
- 51.Thoenen J., Stevens K.J., Turmezei T.D., et al. Non-contrast MRI of synovitis in the knee using quantitative DESS. Eur. Radiol. 2021;31(12):9369–9379. doi: 10.1007/s00330-021-08025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fotinos-Hoyer A.K., Guermazi A., Jara H., et al. Assessment of synovitis in the osteoarthritic knee: comparison between manual segmentation, semiautomated segmentation, and semiquantitative assessment using contrast-enhanced fat-suppressed T1-weighted MRI. Magnes. Res. 2010;64(2):604–609. doi: 10.1002/mrm.22401. [DOI] [PubMed] [Google Scholar]
- 53.Roemer F.W., Frobell R., Lohmander L.S., Niu J., Guermazi A. Anterior cruciate ligament osteoarthritis score (ACLOAS): longitudinal MRI-based whole joint assessment of anterior cruciate ligament injury. Osteoarthritis Cartilage. 2014;22(5):668–682. doi: 10.1016/j.joca.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Hagiwara S., Yang A., Takao S., Kaneko Y., Nozaki T., Yoshioka H. New scoring system in assessment of Hoffa's fat pad synovitis: a comparative study with established scoring systems. World J. Radiol. 2018;10(11):162–171. doi: 10.4329/wjr.v10.i11.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hochberg M., Guermazi A., Guehring H., et al. Efficacy and safety of intra-articular Sprifermin in symptomatic radiographic knee osteoarthritis: pre-specified analysis of 3-year data from a 5-year randomized, placebo-controlled, phase II study. Osteoarthritis Cartilage. 2018;26:S26–S27. doi: 10.1016/J.JOCA.2018.02.069. [DOI] [Google Scholar]
- 56.Eckstein F., Wirth W., Nevitt M.C. Recent advances in osteoarthritis imaging-the Osteoarthritis Initiative. Nat. Rev. Rheumatol. 2012;8(10):622–630. doi: 10.1038/nrrheum.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guehring H., Moreau F., Daelken B., et al. The effects of sprifermin on symptoms and structure in a subgroup at risk of progression in the FORWARD knee osteoarthritis trial. Semin. Arthritis Rheum. 2021;51(2):450–456. doi: 10.1016/j.semarthrit.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Eckstein F., Hochberg M.C., Guehring H., et al. Long-term structural and symptomatic effects of intra-articular sprifermin in patients with knee osteoarthritis: 5-year results from the FORWARD study. Ann. Rheum. Dis. 2021;80(8):1062–1069. doi: 10.1136/annrheumdis-2020-219181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hudelmaier M., Glaser C., Pfau C., Eckstein F. Comparison between different implementations of the 3D FLASH sequence for knee cartilage quantification. Magma. 2012;25(4):305–312. doi: 10.1007/s10334-011-0296-1. [DOI] [PubMed] [Google Scholar]
- 60.Eckstein F., Kunz M., Hudelmaier M., et al. Impact of coil design on the contrast-to-noise ratio, precision, and consistency of quantitative cartilage morphometry at 3 Tesla: a pilot study for the osteoarthritis initiative. Magn. Reson. Med. 2007;57(2):448–454. doi: 10.1002/mrm.21146. [DOI] [PubMed] [Google Scholar]
- 61.Eckstein F., Tieschky M., Faber S., Englmeier K.H., Reiser M. Functional analysis of articular cartilage deformation, recovery, and fluid flow following dynamic exercise in vivo. Anat. Embryol. 1999;200:340–2061. doi: 10.1007/s004290050291. 419-424. [DOI] [PubMed] [Google Scholar]
- 62.Eckstein F., Lemberger B., Stammberger T., Englmeier K.H., Reiser M. Patellar cartilage deformation in vivo after static versus dynamic loading. J. Biomech. 2000;33(0021–9290):819–825. doi: 10.1016/s0021-9290(00)00034-8. [DOI] [PubMed] [Google Scholar]
- 63.Sitoci K.H., Hudelmaier M., Eckstein F. Nocturnal changes in knee cartilage thickness in young healthy adults. Cells Tissues Organs. 2012;196:1422–6421. doi: 10.1159/000333456. (Electronic)):189-194. [DOI] [PubMed] [Google Scholar]
- 64.Herberhold C., Faber S., Stammberger T., et al. In situ measurement of articular cartilage deformation in intact femoropatellar joints under static loading. J. Biomech. 1999;32(0021–9290):1287–1295. doi: 10.1016/s0021-9290(99)00130-x. [DOI] [PubMed] [Google Scholar]
- 65.Crowder H.A., Mazzoli V., Black M.S., et al. Characterizing the transient response of knee cartilage to running: decreases in cartilage T(2) of female recreational runners. J Orthop Res Off Publ Orthop Res Soc. 2021;39(11):2340–2352. doi: 10.1002/jor.24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mosher T.J., Smith H.E., Collins C., et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005;234(1):245–249. doi: 10.1148/radiol.2341040041. [DOI] [PubMed] [Google Scholar]
- 67.Eckstein F., Wirth W., Lohmander L.S., Hudelmaier M.I., Frobell R.B. Five-year followup of knee joint cartilage thickness changes after acute rupture of the anterior cruciate ligament. Arthritis Rheumatol. 2015;67(1):152–161. doi: 10.1002/art.38881. [DOI] [PubMed] [Google Scholar]
- 68.Eckstein F., Maschek S., Roemer F.W., Duda G.N., Sharma L., Wirth W. Cartilage loss in radiographically normal knees depends on radiographic status of the contralateral knee – data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2019;27(2):273–277. doi: 10.1016/j.joca.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eckstein F., Buck R., Wirth W. Location-independent analysis of structural progression of osteoarthritis - taking it all apart, and putting the puzzle back together makes the difference. Semin. Arthritis Rheum. 2017;46(4):404–410. doi: 10.1016/j.semarthrit.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 70.Loeuille D., Chary-Valckenaere I., Champigneulle J., et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52(11):3492–3501. doi: 10.1002/art.21373. [DOI] [PubMed] [Google Scholar]
- 71.Fernandez-Madrid F., Karvonen R.L., Teitge R.A., Miller P.R., An T., Negendank W.G. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn. Reson. Imaging. 1995;13(2):177–183. doi: 10.1016/0730-725X(94)00119-N. [DOI] [PubMed] [Google Scholar]
- 72.Hart H.F., Culvenor A.G., Patterson B.E., et al. Infrapatellar fat pad volume and Hoffa-synovitis after ACL reconstruction: association with early osteoarthritis features and pain over 5 years. J Orthop Res Off Publ Orthop Res Soc. 2022;40(1):260–267. doi: 10.1002/jor.24987. [DOI] [PubMed] [Google Scholar]
- 73.Roemer F.W., Jarraya M., Collins J.E., et al. Structural phenotypes of knee osteoarthritis: potential clinical and research relevance. Skeletal Radiol. 2023;52(11):2021–2030. doi: 10.1007/s00256-022-04191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roemer F.W., Collins J., Kwoh C.K., et al. MRI-based screening for structural definition of eligibility in clinical DMOAD trials: rapid OsteoArthritis MRI Eligibility Score (ROAMES) Osteoarthritis Cartilage. 2020;28(1):71–81. doi: 10.1016/J.JOCA.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schnitzer T.J., Pueyo M., Deckx H., et al. Efficacy and safety of s201086/GLPG1972, an ADAMTS-5 inhibitor, in patients with knee osteoarthritis: roccella, a 52-week, randomized, double-blind, dose-ranging phase 2 study. Osteoarthritis Cartilage. 2021;29:S264. doi: 10.1016/j.joca.2021.02.348. [DOI] [Google Scholar]
- 76.Imbert O., Deckx H., Bernard K., et al. The design of a randomized, placebo-controlled, dose-ranging trial to investigate the efficacy and safety of the ADAMTS-5 inhibitor S201086/GLPG1972 in knee osteoarthritis. Osteoarthr Cartil Open. 2021;3(4) doi: 10.1016/j.ocarto.2021.100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaudhari A.S., Black M.S., Eijgenraam S., et al. Five-minute knee MRI for simultaneous morphometry and T2 relaxometry of cartilage and meniscus and for semiquantitative radiological assessment using double-echo in steady-state at 3T. J. Magn. Reson. Imag. 2018;47(5):1328–1341. doi: 10.1002/jmri.25883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heule R., Ganter C., Bieri O. Rapid estimation of cartilage T2 with reduced T1 sensitivity using double echo steady state imaging. Magn. Reson. Med. 2014;71(3):1137–1143. doi: 10.1002/mrm.24748. [DOI] [PubMed] [Google Scholar]
- 79.Welsch G.H., Scheffler K., Mamisch T.C., et al. Rapid estimation of cartilage T2 based on double echo at steady state (DESS) with 3 Tesla. Magn. Reson. Med. 2009;62(2):544–549. doi: 10.1002/mrm.22036. [DOI] [PubMed] [Google Scholar]
- 80.Peterfy C.G., Schneider E., Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sveinsson B., Chaudhari A.S., Gold G.E., Hargreaves B.A. A simple analytic method for estimating T2 in the knee from DESS. Magn. Reson. Imaging. 2017;38:63–70. doi: 10.1016/j.mri.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matzat S.J., McWalter E.J., Kogan F., Chen W., Gold G.E. T2 Relaxation time quantitation differs between pulse sequences in articular cartilage. J. Magn. Reson. Imag. 2015;42(1):105–113. doi: 10.1002/jmri.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eijgenraam S.M., Chaudhari A.S., Reijman M., et al. Time-saving opportunities in knee osteoarthritis: T(2) mapping and structural imaging of the knee using a single 5-min MRI scan. Eur. Radiol. 2020;30(4):2231–2240. doi: 10.1007/s00330-019-06542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dardzinski B.J., Schneider E. Radiofrequency (RF) coil impacts the value and reproducibility of cartilage spin-spin (T2) relaxation time measurements. Osteoarthritis Cartilage. 2013;21(5):710–720. doi: 10.1016/j.joca.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wirth W., Nevitt M., Hellio Le Graverand M.P., et al. Sensitivity to change of cartilage morphometry using coronal FLASH, sagittal DESS, and coronal MPR DESS protocols--comparative data from the Osteoarthritis Initiative (OAI) Osteoarthritis Cartilage. 2010;18(4):547–554. doi: 10.1016/j.joca.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hudelmaier M., Wirth W., Wehr B., et al. Femorotibial cartilage morphology: reproducibility of different metrics and femoral regions, and sensitivity to change in disease. Cells Tissues Organs. 2010;192(5):340–350. doi: 10.1159/000318178. [DOI] [PubMed] [Google Scholar]
- 87.Hyhlik-Duerr A., Faber S., Burgkart R., et al. Precision of tibial cartilage morphometry with a coronal water-excitation MR sequence. Eur. Radiol. 2000;10(2):297–303. doi: 10.1007/s003300050047. [DOI] [PubMed] [Google Scholar]
- 88.Sharma K., Eckstein F., Wirth W., Emmanuel K. Meniscus position and size in knees with versus without structural knee osteoarthritis progression: data from the osteoarthritis initiative. Skeletal Radiol. 2021 doi: 10.1007/S00256-021-03911-8. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eckstein F., Hudelmaier M., Wirth W., et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann. Rheum. Dis. 2006;65(4):433–441. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Vries B.A., Breda S.J., Sveinsson B., et al. Detection of knee synovitis using non-contrast-enhanced qDESS compared with contrast-enhanced MRI. Arthritis Res. Ther. 2021;23(1):55. doi: 10.1186/s13075-021-02436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wirth W., Eckstein F., Kemnitz J., et al. Accuracy and longitudinal reproducibility of quantitative femorotibial cartilage measures derived from automated U-Net-based segmentation of two different MRI contrasts: data from the osteoarthritis initiative healthy reference cohort. Magma. 2021;34(3):337–354. doi: 10.1007/s10334-020-00889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eckstein F., Chaudhari A.S., Kemnitz J., Baumgartner C.F., Wirth W. Agreement and accuracy of fully automated morphometric femorotibial cartilage analysis in radiographic knee osteoarthritis. Osteoarthr Imaging. 2023;3(2) doi: 10.1016/j.ostima.2023.100156. [DOI] [Google Scholar]
- 93.Eckstein F., Chaudhari A.S., Fuerst D., et al. Detection of differences in longitudinal cartilage thickness loss using a deep-learning automated segmentation algorithm: data from the foundation for the national Institutes of Health biomarkers study of the osteoarthritis initiative. Arthritis Care Res. 2022;74(6):929–936. doi: 10.1002/acr.24539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Del Grande F., Rashidi A., Luna R., et al. Five-minute five-sequence knee MRI using combined simultaneous multislice and parallel imaging acceleration: comparison with 10-minute parallel imaging knee MRI. Radiology. 2021;299(3):635–646. doi: 10.1148/radiol.2021203655. [DOI] [PubMed] [Google Scholar]
- 95.Chaudhari A.S., Grissom M.J., Fang Z., et al. Diagnostic accuracy of quantitative multicontrast 5-minute knee MRI using prospective artificial intelligence image quality enhancement. AJR Am. J. Roentgenol. 2021;216(6):1614–1625. doi: 10.2214/AJR.20.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin D.J., Walter S.S., Fritz J. Artificial intelligence-driven ultra-fast superresolution MRI: 10-fold accelerated musculoskeletal turbo spin echo MRI within reach. Invest. Radiol. 2023;58(1):28–42. doi: 10.1097/RLI.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 97.Recht M.P., Zbontar J., Sodickson D.K., et al. Using deep learning to accelerate knee MRI at 3 T: results of an interchangeability study. AJR Am. J. Roentgenol. 2020;215(6):1421–1429. doi: 10.2214/AJR.20.23313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Riis R.G.C., Gudbergsen H., Simonsen O., et al. The association between histological, macroscopic and magnetic resonance imaging assessed synovitis in end-stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage. 2017;25(2):272–280. doi: 10.1016/j.joca.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 99.Zhou S., Maleitzke T., Geissler S., et al. Source and hub of inflammation: the infrapatellar fat pad and its interactions with articular tissues during knee osteoarthritis. J. Orthop. Res. 2022;40(7):1492–1504. doi: 10.1002/jor.25347. [DOI] [PubMed] [Google Scholar]
- 100.Ballegaard C., Riis R.G.C., Bliddal H., et al. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage. 2014;22(7):933–940. doi: 10.1016/j.joca.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 101.Steidle-Kloc E., Wirth W., Ruhdorfer A., Dannhauer T., Eckstein F. Intra- and inter-observer reliability of quantitative analysis of the infra-patellar fat pad and comparison between fat- and non-fat-suppressed imaging-data from the Osteoarthritis Initiative. Ann. Anat. 2016;204:29–35. doi: 10.1016/j.aanat.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steidle-Kloc E., Dannhauer T., Wirth W., Eckstein F. Responsiveness of infrapatellar fat pad volume change to body weight loss or gain: data from the osteoarthritis initiative. Cells Tissues Organs. 2018;205(1):53–62. doi: 10.1159/000485833. [DOI] [PubMed] [Google Scholar]
- 103.Pogacnik Murillo A.L., Eckstein F., Wirth W., et al. Impact of diet and/or exercise intervention on infrapatellar fat pad morphology: secondary analysis from the intensive diet and exercise for arthritis (IDEA) trial. Cells Tissues Organs. 2017;203(4):258–266. doi: 10.1159/000449407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruhdorfer A., Haniel F., Petersohn T., et al. Between-group differences in infra-patellar fat pad size and signal in symptomatic and radiographic progression of knee osteoarthritis vs non-progressive controls and healthy knees – data from the FNIH Biomarkers Consortium Study and the Osteoarthritis in. Osteoarthritis Cartilage. 2017;25(7):1114–1121. doi: 10.1016/j.joca.2017.02.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu M., Chen Z., Han W., et al. A novel method for assessing signal intensity within infrapatellar fat pad on MR images in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2016;24(11):1883–1889. doi: 10.1016/j.joca.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 106.Cen H., Yan Q., Han W., et al. Longitudinal association of infrapatellar fat pad signal intensity alteration with biochemical biomarkers in knee osteoarthritis. Rheumatology. 2022;62(1):439–449. doi: 10.1093/rheumatology/keac214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heilmeier U., Mamoto K., Amano K., et al. Infrapatellar fat pad abnormalities are associated with a higher inflammatory synovial fluid cytokine profile in young adults following ACL tear. Osteoarthritis Cartilage. 2020;28(1):82–91. doi: 10.1016/j.joca.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han W., Aitken D., Zheng S., et al. Higher serum levels of resistin are associated with knee synovitis and structural abnormalities in patients with symptomatic knee osteoarthritis. J. Am. Med. Dir. Assoc. 2019;20(10):1242–1246. doi: 10.1016/j.jamda.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 109.Guermazi A., Kwoh C.K., Hannon M.J., et al. Hoffa synovitis and effusion synovitis are associated with knees undergoing total knee replacement: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20(Suppl):S235. ([abstract]) [Google Scholar]
- 110.Roemer F.W., Kassim Javaid M., Guermazi A., et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. 2010;18(10):1269–1274. doi: 10.1016/j.joca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 111.Deveza L.A., Kraus V.B., Collins J.E., et al. Is synovitis detected on non-contrast-enhanced magnetic resonance imaging associated with serum biomarkers and clinical signs of effusion? Data from the Osteoarthritis Initiative. Scand. J. Rheumatol. 2018;47(3):235–242. doi: 10.1080/03009742.2017.1340511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harkey M.S., Davis J.E., Price L.L., et al. Composite quantitative knee structure metrics predict the development of accelerated knee osteoarthritis: data from the osteoarthritis initiative. BMC Muscoskel. Disord. 2020;21(1):299. doi: 10.1186/s12891-020-03338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang X., Blizzard L., Jin X., et al. Quantitative assessment of knee effusion-synovitis in older adults: association with knee structural abnormalities. Arthritis Rheumatol. 2016;68(4):837–844. doi: 10.1002/art.39526. [DOI] [PubMed] [Google Scholar]
- 114.Habib S., Guermazi A., Ozonoff A., Hayashi D., Crema M.D., Roemer F.W. MRI-based volumetric assessment of joint effusion in knee osteoarthritis using proton density-weighted fat-suppressed and T1-weighted contrast-enhanced fat-suppressed sequences. Skeletal Radiol. 2011;40(12):1581–1585. doi: 10.1007/s00256-011-1200-9. [DOI] [PubMed] [Google Scholar]
- 115.Driban J.B., Price L.L., LaValley M.P., et al. Novel framework for measuring whole knee osteoarthritis progression using magnetic resonance imaging. Arthritis Care Res. 2022;74(5):799–808. doi: 10.1002/acr.24512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roemer F.W., Guermazi A., Collins J.E., et al. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort − Methodologic aspects and definition of change. BMC Muscoskel. Disord. 2016;17(1):466. doi: 10.1186/s12891-016-1310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang X., Cicuttini F., Jin X., et al. Knee effusion-synovitis volume measurement and effects of vitamin D supplementation in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2017;25(8):1304–1312. doi: 10.1016/j.joca.2017.02.804. [DOI] [PubMed] [Google Scholar]
- 118.Jahng G.H., Jin W., Yang D.M., Ryu K.N. Optimization of a double inversion recovery sequence for noninvasive synovium imaging of joint effusion in the knee. Med. Phys. 2011;38(5):2579–2585. doi: 10.1118/1.3581060. [DOI] [PubMed] [Google Scholar]
- 119.Son Y.N., Jin W., Jahng G.H., et al. Efficacy of double inversion recovery magnetic resonance imaging for the evaluation of the synovium in the femoro-patellar joint without contrast enhancement. Eur. Radiol. 2018;28(2):459–467. doi: 10.1007/s00330-017-5017-3. [DOI] [PubMed] [Google Scholar]
- 120.Feuerriegel G.C., Goller S.S., von Deuster C., Sutter R. Inflammatory knee synovitis: evaluation of an accelerated FLAIR sequence compared with standard contrast-enhanced imaging. Invest. Radiol. 2024 doi: 10.1097/RLI.0000000000001065. Published online February 8. [DOI] [PubMed] [Google Scholar]
- 121.Gluer C.C., Blake G., Lu Y., et al. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos. Int. 1995;5(0937–941X):262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 122.Wirth W., Maschek S., Marijnissen A.C.A., et al. Test-retest precision and longitudinal cartilage thickness loss in the IMI-APPROACH cohort. Osteoarthritis Cartilage. 2023;31(2):238–248. doi: 10.1016/j.joca.2022.10.015. [DOI] [PubMed] [Google Scholar]
- 123.Eckstein F., Bernard K., Deckx H., et al. Test-retest reliability and smallest detectable change (SDC) of MRI-based cartilage thickness analysis in a large multicenter randomized controlled clinical trial of knee osteoarthritis. Osteoarthritis Cartilage. 2021;29 doi: 10.1016/j.joca.2021.02.428. S327--S328. [DOI] [Google Scholar]
- 124.Bruynesteyn K., Boers M., Kostense P., et al. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Ann. Rheum. Dis. 2005;64(2):179–182. doi: 10.1136/ard.2003.018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eckstein F., Heudorfer L., Faber S.C., et al. Long-term and resegmentation precision of quantitative cartilage MR imaging (qMRI) Osteoarthritis Cartilage. 2002;10(1063–4584):922–928. doi: 10.1053/joca.2002.0844. [DOI] [PubMed] [Google Scholar]
- 126.Keenan K.E., Besier T.F., Pauly J.M., et al. Prediction of glycosaminoglycan content in human cartilage by age, T1ρ and T2 MRI. Osteoarthritis Cartilage. 2011;19(2):171–179. doi: 10.1016/j.joca.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheng K.Y., Moazamian D., Ma Y., et al. Clinical application of ultrashort echo time (UTE) and zero echo time (ZTE) magnetic resonance (MR) imaging in the evaluation of osteoarthritis. Skeletal Radiol. 2023;52(11):2149–2157. doi: 10.1007/s00256-022-04269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]