Abstract
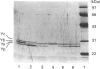
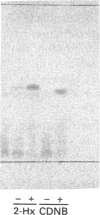
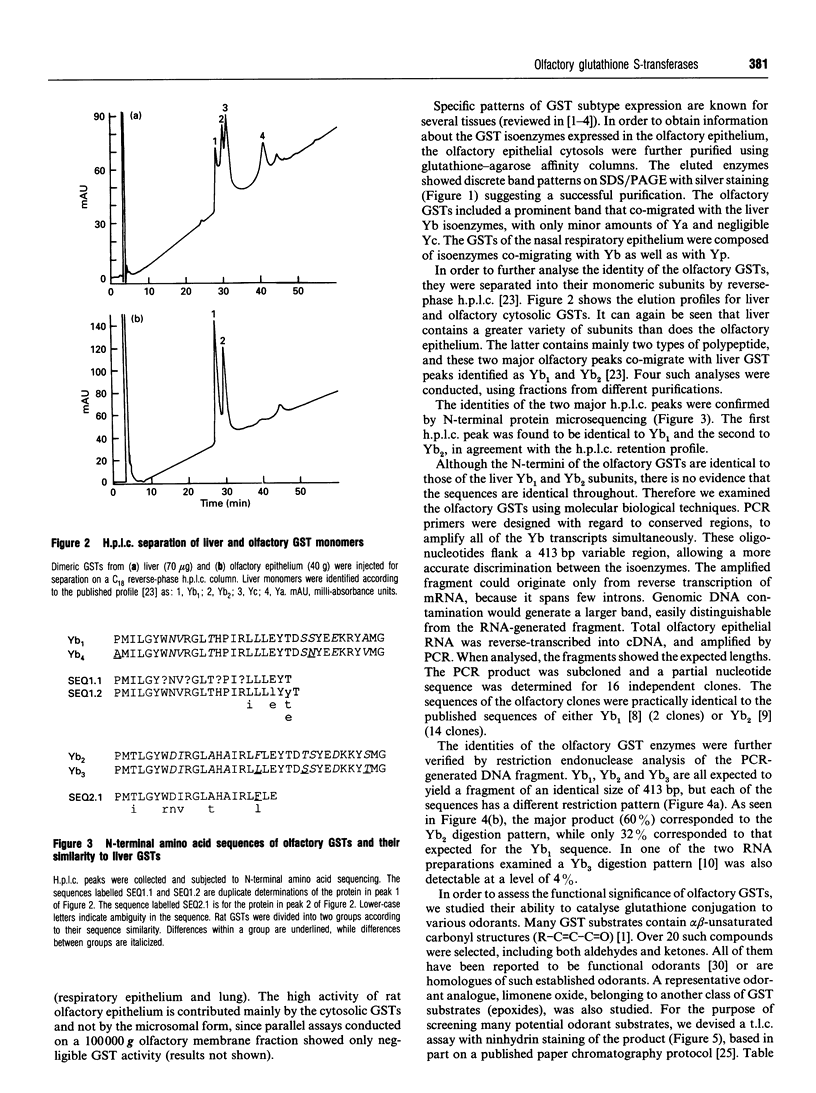
The olfactory epithelium is exposed to a variety of xenobiotic chemicals, including odorants and airborne toxic compounds. Recently, two novel, highly abundant, olfactory-specific biotransformation enzymes have been identified: cytochrome P-450olf1 and olfactory UDP-glucuronosyltransferase (UGT(olf)). The latter is a phase II biotransformation enzyme which catalyses the glucuronidation of alcohols, thiols, amines and carboxylic acids. Such covalent modification, which markedly affects lipid solubility and agonist potency, may be particularly important in the rapid termination of odorant signals. We report here the identification and characterization of a second olfactory phase II biotransformation enzyme, a glutathione S-transferase (GST). The olfactory epithelial cytosol shows the highest GST activity among the extrahepatic tissues examined. Significantly, olfactory epithelium had an activity 4-7 times higher than in other airway tissues, suggesting a role for this enzyme in chemoreception. The olfactory GST has been affinity-purified to homogeneity, and shown by h.p.l.c. and N-terminal amino acid sequencing to constitute mainly the Yb1 and Yb2 subunits, different from most other tissues that have mixtures of more enzyme classes. The identity of the olfactory enzymes was confirmed by PCR cloning and restriction enzyme analysis. Most importantly, the olfactory GSTs were found to catalyse glutathione conjugation of several odorant classes, including many unsaturated aldehydes and ketones, as well as epoxides. Together with UGT(olf), olfactory GST provides the necessary broad coverage of covalent modification capacity, which may be crucial for the acuity of the olfactory process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz M., Ishigaki S., Felix A. M., Listowsky I. Expression of an enzymatically active Yb3 glutathione S-transferase in Escherichia coli and identification of its natural form in rat brain. J Biol Chem. 1988 Nov 25;263(33):17627–17631. [PubMed] [Google Scholar]
- Abramovitz M., Listowsky I. Selective expression of a unique glutathione S-transferase Yb3 gene in rat brain. J Biol Chem. 1987 Jun 5;262(16):7770–7773. [PubMed] [Google Scholar]
- Aceto A., Di Ilio C., Angelucci S., Longo V., Gervasi P. G., Federici G. Glutathione transferases in human nasal mucosa. Arch Toxicol. 1989;63(6):427–431. doi: 10.1007/BF00316443. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Baron J., Voigt J. M., Whitter T. B., Kawabata T. T., Knapp S. A., Guengerich F. P., Jakoby W. B. Identification of intratissue sites for xenobiotic activation and detoxication. Adv Exp Med Biol. 1986;197:119–144. doi: 10.1007/978-1-4684-5134-4_10. [DOI] [PubMed] [Google Scholar]
- Bhargava M. M., Ohmi N., Listowsky I., Arias I. M. Subunit composition, organic anion binding, catalytic and immunological properties of ligandin from rat testis. J Biol Chem. 1980 Jan 25;255(2):724–727. [PubMed] [Google Scholar]
- Bignetti E., Cavaggioni A., Pelosi P., Persaud K. C., Sorbi R. T., Tirindelli R. Purification and characterisation of an odorant-binding protein from cow nasal tissue. Eur J Biochem. 1985 Jun 3;149(2):227–231. doi: 10.1111/j.1432-1033.1985.tb08916.x. [DOI] [PubMed] [Google Scholar]
- Bond J. A., Harkema J. R., Russell V. I. Regional distribution of xenobiotic metabolizing enzymes in respiratory airways of dogs. Drug Metab Dispos. 1988 Jan-Feb;16(1):116–124. [PubMed] [Google Scholar]
- Bond J. A. Some biotransformation enzymes responsible for polycyclic aromatic hydrocarbon metabolism in rat nasal turbinates: effects on enzyme activities of in vitro modifiers and intraperitoneal and inhalation exposure of rats to inducing agents. Cancer Res. 1983 Oct;43(10):4805–4811. [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. Enzymes catalysing conjugations of glutathione with alpha-beta-unsaturated carbonyl compounds. Biochem J. 1968 Oct;109(4):651–661. doi: 10.1042/bj1090651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burchell B., Coughtrie M. W. UDP-glucuronosyltransferases. Pharmacol Ther. 1989;43(2):261–289. doi: 10.1016/0163-7258(89)90122-8. [DOI] [PubMed] [Google Scholar]
- Burchell B. Turning on and turning off the sense of smell. Nature. 1991 Mar 7;350(6313):16–17. doi: 10.1038/350016a0. [DOI] [PubMed] [Google Scholar]
- Cammer W., Tansey F., Abramovitz M., Ishigaki S., Listowsky I. Differential localization of glutathione-S-transferase Yp and Yb subunits in oligodendrocytes and astrocytes of rat brain. J Neurochem. 1989 Mar;52(3):876–883. doi: 10.1111/j.1471-4159.1989.tb02536.x. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Coles B., Ketterer B. The role of glutathione and glutathione transferases in chemical carcinogenesis. Crit Rev Biochem Mol Biol. 1990;25(1):47–70. doi: 10.3109/10409239009090605. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Gollan J. L. Hepatocyte cotransport of taurocholate and bilirubin glucuronides: role of microtubules. Am J Physiol. 1988 Jul;255(1 Pt 1):G121–G131. doi: 10.1152/ajpgi.1988.255.1.G121. [DOI] [PubMed] [Google Scholar]
- Ding X. X., Porter T. D., Peng H. M., Coon M. J. cDNA and derived amino acid sequence of rabbit nasal cytochrome P450NMb (P450IIG1), a unique isozyme possibly involved in olfaction. Arch Biochem Biophys. 1991 Feb 15;285(1):120–125. doi: 10.1016/0003-9861(91)90337-i. [DOI] [PubMed] [Google Scholar]
- Foster J. R., Elcombe C. R., Boobis A. R., Davies D. S., Sesardic D., McQuade J., Robson R. T., Hayward C., Lock E. A. Immunocytochemical localization of cytochrome P-450 in hepatic and extra-hepatic tissues of the rat with a monoclonal antibody against cytochrome P-450 c. Biochem Pharmacol. 1986 Dec 15;35(24):4543–4554. doi: 10.1016/0006-2952(86)90777-x. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Lemahieu R. A., Udenfriend S. Studies on glutathione-S-arene oxidase transferase. A sensitive assay and partial purification of the enzyme from sheep liver. Arch Biochem Biophys. 1974 May;162(1):223–230. doi: 10.1016/0003-9861(74)90122-2. [DOI] [PubMed] [Google Scholar]
- Hiratsuka A., Sebata N., Kawashima K., Okuda H., Ogura K., Watabe T., Satoh K., Hatayama I., Tsuchida S., Ishikawa T. A new class of rat glutathione S-transferase Yrs-Yrs inactivating reactive sulfate esters as metabolites of carcinogenic arylmethanols. J Biol Chem. 1990 Jul 15;265(20):11973–11981. [PubMed] [Google Scholar]
- Hsieh J. C., Liu L. F., Chen W. L., Tam M. F. Expression of Yb1 glutathione S-transferase using a baculovirus expression system. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1147–1154. doi: 10.1016/0006-291x(89)90793-6. [DOI] [PubMed] [Google Scholar]
- Ishikawa T. Is the glutathione S-conjugate carrier an mdr1 gene product? Trends Biochem Sci. 1990 Jun;15(6):219–220. doi: 10.1016/0968-0004(90)90032-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Sies H. The isozyme pattern of glutathione S-transferases in rat heart. FEBS Lett. 1984 Apr 24;169(2):156–160. doi: 10.1016/0014-5793(84)80309-9. [DOI] [PubMed] [Google Scholar]
- Kirstein C. L., Coopersmith R., Bridges R. J., Leon M. Glutathione levels in olfactory and non-olfactory neural structures of rats. Brain Res. 1991 Mar 15;543(2):341–346. doi: 10.1016/0006-8993(91)90047-y. [DOI] [PubMed] [Google Scholar]
- Krieger J., Raming K., Breer H. Cloning of genomic and complementary DNA encoding insect pheromone binding proteins: evidence for microdiversity. Biochim Biophys Acta. 1991 Feb 16;1088(2):277–284. doi: 10.1016/0167-4781(91)90064-s. [DOI] [PubMed] [Google Scholar]
- Krishna N. S., Getchell M. L., Tate S. S., Margolis F. L., Getchell T. V. Glutathione and gamma-glutamyl transpeptidase are differentially distributed in the olfactory mucosa of rats. Cell Tissue Res. 1992 Dec;270(3):475–484. doi: 10.1007/BF00645049. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai H. C., Qian B., Grove G., Tu C. P. Gene expression of rat glutathione S-transferases. Evidence for gene conversion in the evolution of the Yb multigene family. J Biol Chem. 1988 Aug 15;263(23):11389–11395. [PubMed] [Google Scholar]
- Lai H. C., Tu C. P. Rat glutathione S-transferases supergene family. Characterization of an anionic Yb subunit cDNA clone. J Biol Chem. 1986 Oct 15;261(29):13793–13799. [PubMed] [Google Scholar]
- Lazard D., Tal N., Rubinstein M., Khen M., Lancet D., Zupko K. Identification and biochemical analysis of novel olfactory-specific cytochrome P-450IIA and UDP-glucuronosyl transferase. Biochemistry. 1990 Aug 14;29(32):7433–7440. doi: 10.1021/bi00484a012. [DOI] [PubMed] [Google Scholar]
- Lazard D., Zupko K., Poria Y., Nef P., Lazarovits J., Horn S., Khen M., Lancet D. Odorant signal termination by olfactory UDP glucuronosyl transferase. Nature. 1991 Feb 28;349(6312):790–793. doi: 10.1038/349790a0. [DOI] [PubMed] [Google Scholar]
- Longo V., Citti L., Gervasi P. G. Biotransformation enzymes in nasal mucosa and liver of Sprague-Dawley rats. Toxicol Lett. 1988 Dec;44(3):289–297. doi: 10.1016/0378-4274(88)90168-3. [DOI] [PubMed] [Google Scholar]
- Longo V., Mazzaccaro A., Naldi F., Gervasi P. G. Drug-metabolizing enzymes in liver, olfactory, and respiratory epithelium of cattle. J Biochem Toxicol. 1991 Summer;6(2):123–128. doi: 10.1002/jbt.2570060206. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Coles B., Pemble S. E., Gilmore K. S., Fraser G. M., Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem J. 1991 Mar 1;274(Pt 2):409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern R., Guthenberg C., Depierre J. W. Microsomal glutathione S-transferase. Purification, initial characterization and demonstration that it is not identical to the cytosolic glutathione S-transferases A, B and C. Eur J Biochem. 1982 Nov;128(1):243–248. [PubMed] [Google Scholar]
- Nef P., Heldman J., Lazard D., Margalit T., Jaye M., Hanukoglu I., Lancet D. Olfactory-specific cytochrome P-450. cDNA cloning of a novel neuroepithelial enzyme possibly involved in chemoreception. J Biol Chem. 1989 Apr 25;264(12):6780–6785. doi: 10.1016/S0021-9258(18)83497-4. [DOI] [PubMed] [Google Scholar]
- Ostlund Farrants A. K., Meyer D. J., Coles B., Southan C., Aitken A., Johnson P. J., Ketterer B. The separation of glutathione transferase subunits by using reverse-phase high-pressure liquid chromatography. Biochem J. 1987 Jul 15;245(2):423–428. doi: 10.1042/bj2450423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner J., Reed R. R., Feinstein P. G., Snyder S. H. Molecular cloning of odorant-binding protein: member of a ligand carrier family. Science. 1988 Jul 15;241(4863):336–339. doi: 10.1126/science.3388043. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Sklar P. B., Snyder S. H. Odorant-binding protein: localization to nasal glands and secretions. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4942–4946. doi: 10.1073/pnas.83.13.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. B., Lu A. Y. Glutathione S-transferases: gene structure, regulation, and biological function. Annu Rev Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases by glutathione-affinity chromatography. Methods Enzymol. 1981;77:235–237. doi: 10.1016/s0076-6879(81)77031-9. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Chang M., Reddy C. C. The major rat heart glutathione S-transferases are anionic isozymes composed of Yb size subunits. Biochem Biophys Res Commun. 1984 Sep 28;123(3):981–988. doi: 10.1016/s0006-291x(84)80230-2. [DOI] [PubMed] [Google Scholar]
- Vogt R. G., Köhne A. C., Dubnau J. T., Prestwich G. D. Expression of pheromone binding proteins during antennal development in the gypsy moth Lymantria dispar. J Neurosci. 1989 Sep;9(9):3332–3346. doi: 10.1523/JNEUROSCI.09-09-03332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt J. M., Guengerich F. P., Baron J. Localization of a cytochrome P-450 isozyme (cytochrome P-450 PB-B) and NADPH-cytochrome P-450 reductase in rat nasal mucosa. Cancer Lett. 1985 Jul;27(3):241–247. doi: 10.1016/0304-3835(85)90180-6. [DOI] [PubMed] [Google Scholar]
- Williams D. E., Ding X. X., Coon M. J. Rabbit nasal cytochrome P-450 NMa has high activity as a nicotine oxidase. Biochem Biophys Res Commun. 1990 Jan 30;166(2):945–952. doi: 10.1016/0006-291x(90)90902-y. [DOI] [PubMed] [Google Scholar]
- Zupko K., Poria Y., Lancet D. Immunolocalization of cytochromes P-450olf1 and P-450olf2 in rat olfactory mucosa. Eur J Biochem. 1991 Feb 26;196(1):51–58. doi: 10.1111/j.1432-1033.1991.tb15784.x. [DOI] [PubMed] [Google Scholar]