Abstract
The H+ hypothesis of lateral feedback inhibition in the outer retina predicts that depolarizing agents should increase H+ release from horizontal cells. To test this hypothesis, self-referencing H+-selective microelectrodes were used to measure extracellular H+ fluxes from isolated goldfish horizontal cells. We found a more complex pattern of cellular responses than previously observed from horizontal cells of other species examined using this technique. One class of cells had an initial standing signal indicative of high extracellular H+ adjacent to the cell membrane; challenge with glutamate, kainate or high extracellular potassium induced an extracellular alkalinization. This alkalinization was reduced by the calcium channel blockers nifedipine and cobalt. A second class of cells displayed spontaneous oscillations in extracellular H+ that were abolished by cobalt, nifedipine and low extracellular calcium. A strong correlation between changes in intracellular calcium and extracellular proton flux was detected in experiments simultaneously monitoring intracellular calcium and extracellular H+. A third set of cells was characterized by a standing extracellular alkalinization which was turned into an acidic signal by cobalt. In this last set of cells, addition of glutamate or high extracellular potassium did not significantly alter the proton signal. Taken together, the response characteristics of all three sets of neurons are most parsimoniously explained by activation of a plasma membrane Ca2+ ATPase (PMCA) pump, with an extracellular alkalinization resulting from exchange of intracellular calcium for extracellular H+. These findings argue strongly against the hypothesis that H+ release from horizontal cells mediates lateral inhibition in the outer retina.
Keywords: retina, feedback, horizontal cell, pH
INTRODUCTION
Lateral inhibition is fundamental in shaping sensory signals throughout the nervous system and can be mediated by both feedforward and feedback mechanisms. A number of substances have been hypothesized to mediate lateral feedback inhibition by horizontal cells in the outer retina, but the exact molecular mechanisms remain unclear (cf. Kamermans & Spekreijse, 1999). The high sensitivity of retinal signals to changes in extracellular pH has made protons (H+) an attractive candidate as the lateral inhibitory agent (Kleinschmidt,1991; Barnes et al., 1991, 1993; Harsanyi & Mangel, 1993). It has been demonstrated previously that increases in extracellular H+ inhibit influx of calcium into photoreceptor terminals through voltage-gated calcium channels and in turn reduces glutamate release (Barnes et al., 1993). This is thought to be mediated by a decrease in the overall conductance of voltage-gated calcium channels and a shift in their voltage activation curve to more depolarized potentials. Increasing the pH buffering capacity reduced feedback inhibition mediated by surround light stimuli (Kaneko & Hirasawa, 2003) and reduced the voltage-dependent activation shift resulting from direct depolarization of horizontal cells (Cadetti & Thoreson, 2006; Thoreson et al., 2008).
A key expectation of the H+ hypothesis of lateral feedback inhibition is that H+ is released from horizontal cells upon depolarization. It has proven difficult to test this in the intact retina due to technical limitations in measuring changes in pH within the small volume of extracellular milieu where such inhibition is mediated. Two alternative approaches have been used to test this hypothesis by measuring H+ concentrations adjacent to isolated horizontal cells. Fluorescence changes of the pH-sensitive dye 5-hexadecanoylaminofluorescein (HAF) applied to isolated horizontal cells of carp (Jouhou et al., 2007) and goldfish (Trenholm & Baldridge 2010) indicated an acidification upon challenge with depolarizing agents. However, self-referencing H+ selective electrodes adjacent to isolated horizontal cells of skate (Molina et al., 2004) and catfish (Kreitzer et al., 2007; Jacoby et al., 2012) reported an extracellular alkalinization upon application of depolarizing agents.
In the present study, we used self-referencing methodology with H+ selective microelectrodes to examine glutamate- and high extracellular potassium-induced changes in extracellular H+ from isolated goldfish retinal horizontal cells. We found a more complex pattern of cellular responses than observed in skate and catfish, with some cells displaying acidic extracellular proton fluxes at rest, some showing oscillatory changes in extracellular pH even without stimulation, and others showing initial alkaline fluxes that are not altered by glutamate or high extracellular potassium. These responses are most parsimoniously explained by the hypothesis that calcium entry into horizontal cells leads to activation of a plasmalemma Ca2+/H+ ATPase (PMCA) that extrudes intracellular calcium from the cells in exchange for extracellular protons. We also examined the distribution of the dye HAF in isolated goldfish horizontal cells and found the dye widespread in the interior of the cells. The findings reported here strengthen the argument against the hypothesis that H+ release from horizontal cells mediates lateral inhibition in the outer retina.
MATERIALS AND METHODS
Cell Dissociation
Goldfish (Carassius auratus) were maintained on a 12 hour light/dark cycle at room temperature for up to 2 months. Fish were anaesthetized for approximately 5-10 minutes with 1 g/gal of MS222 (tricaine, Sigma-Aldrich) pH-buffered with 2.5 g/gal NaHCO3 and then cervically transected and double-pithed. Eyes were enucleated and hemi-sected with the posterior portion being placed in a Leibovitz media (L-15; Sigma-Aldrich, L4386) modified with 10 mM HEPES and adjusted to pH 7.40, containing 1mg/4.4mL hyaluronidase for 20 minutes to decrease the viscosity of the vitreous humor in the posterior eye cup. Retinae was removed and placed in modified L-15 media containing 1 mg/mL papain and 0.5 mg/mL L-cysteine and incubated for 30 minutes. Retinae were then rinsed eight times with enzyme-free modified L-15 and mechanically triturated in 2 mL of modified L-15 media. 1-2 drops of cell-suspension were plated in 2 mL of modified L-15 in Falcon 3001 35mm Petri dishes. Plated cells were maintained for up to 3 days at 14°C. Recordings were made at room temperature from isolated retinal horizontal cells separated from cellular neighbors by at least 1mm.
Preparation of H+-selective Electrodes
H+-selective microelectrodes were prepared as described in Molina et al. (2004) (see Smith et al., 1999 for a more complete description). Silanized microelectrodes with 2-4 μm tip diameters were backfilled with a solution containing 100 mM KCl and 10 mM HEPES, adjusted to a pH of 7.0. The microelectrodes were front-filled to a length of 30 μm with a highly H+-selective ionophore (hydrogen ionophore 1-cocktail B; Fluka). This cocktail is reported to be 109 more sensitive to H+ than Na+ or K+ (Fluka, 1996).
Self-referencing Recordings
H+-selective microelectrodes were used in a self-referencing manner (Smith et al., 2007). The microelectrode was first positioned about 1-2 μm away from an isolated horizontal cell, a reading taken, and the electrode then moved to a distant position 30 μm away and a second reading was taken. An H+-dependent differential voltage signal was obtained by subtracting the voltage signal at the distant position from the near position. This differential measurement removes the inherent electrical drift that is present in all ion-selective electrodes. An important assumption of the technique is that the frequency of movement of the microelectrode between the two points (0.3 Hz) is fast enough so that the electrical drift is virtually identical at the two points, but not so fast as to stir the solution and disturb the diffusional H+ gradient being measured from the cell. This differential measurement combined with signal averaging is estimated to increase the sensitivity of the ion-selective microelectrodes by 1000 times (Somieski and Nagel, 2001). The electronics, software, and mechanical control of electrode movement were the same as described in Molina et al. (2004), and are products of the BioCurrents Research Center. H+-selective microelectrodes were calibrated with standard pH 6.0, 7.0, and 8.0 solutions (Fisher, SB104, SB108, SB112 respectively). Only microelectrodes with Nernstian voltage slopes of 45-60mV/per pH unit were used in experiments. The 1-2 μm distance between the H+-selective microelectrode and the cell membrane makes it highly unlikely that extracellular voltage fields and membrane surface charges associated with natural cellular currents contribute to the signal reported here with self-referencing.
The self-referencing system is normally configured to produce results using a running average of 10 data points. The running average was adjusted to produce finer temporal resolution of the signal in experiments combining self-referencing with calcium imaging. This increases the baseline noise in the signal but increases temporal resolution to approximately 3.33 seconds for each point acquired. Thus, even with the reduction in averaging of the signal, changes in H+ on the millisecond time scale will not be resolved by this technique.
Differential measurements made during self-referencing rely on a stable diffusional H+ gradient produced from isolated horizontal cells. Application of test agents through superfusion systems disrupt this gradient, hindering H+ flux measurements. The present studies relied on bolus applications of 1mL of Ringer’s solution containing test agents designed to challenge the cell. Experimental protocols began typically with 2 mL of normal Ringer’s solution in the dish. After a stable differential voltage measurement was obtained, 1 mL of plain Ringer’s solution was applied as a control to examine whether bath application of solution by itself altered H+-dependent voltage measurement. After the signal had again stabilized, an additional 1 mL Ringer’s solution containing the test agent was applied. As an additional control, during the course of every experiment the microelectrode was moved 200 μm vertically away from the cell. At this background position there should be no difference in the H+ concentration at the two electrode positions. The resulting differential measurement should be near zero. During these experiments all drugs added during the experiment persisted in the bath until the end of the trial.
In experiments in which ions were removed from the extracellular environment or concentrations of ions in the bath were substantially changed, the bath solution was completely exchanged by manually removing the initial Ringer’s solution and replacing it with the modified Ringer’s solution. All Ringer’s solutions contained 1 mM HEPES, and the normal Ringer’s solution additionally contained 120 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, and 10 mM glucose. All solutions were adjusted pH to 7.4 ± 0.01 with NaOH or HCl. It should be noted that although the present studies use HEPES as a pH buffer, previous studies (Kreitzer et al., 2007) demonstrated glutamate-induced extracellular alkalinizations persist while buffering pH with HCO3−; or phosphate.
Drugs used in these experiments were tested to make sure they did not alter the ability of the electrodes to respond to changes in H+. H+-selective electrodes were placed in buffered pH standards 6.0, 7.0, and 8.0 in the presence and absence of the test agent to see if the drug changed the Nernstian slope. No effects were seen except as noted for nifedipine by Kreitzer et al. (2007).
Calcium Imaging
Two methods were used to measure intracellular calcium dynamics from isolated goldfish horizontal cells. The calcium indicator dye Oregon Green 488 BAPTA-1, AM (Invitrogen Molecular Probes, Eugene, OR), was employed in initial studies examining intracellular calcium changes in populations of cells. A stock solution was created by dissolving 50 μg Oregon Green in 20 μl dimethyl sulfoxide (DMSO) with 20% pluronic F-127 detergent. Oregon Green stock solutions were diluted in goldfish Ringer’s solution to a final concentration of 5 μM. Isolated retinal cells were incubated in Oregon-Green-containing Ringer’s solution in the dark for 30 minutes at 14°C. Cells were imaged immediately following full wash-off with dye-free Ringer’s solution. Epifluorescent images were collected from a CCD camera (Hamamatsu ORCA) mounted onto a compound microscope (Olympus BX50WI) equipped with an Olympus LUMPlanFL 40x long working distance water immersion objective lens. A rapidly switchable Xenon source (Sutter Lambda DG4) excited the dye at 488 nm. Images were filtered by a dichroic mirror at a wavelength of 530 nm at a resolution of 640 x 512 pixels (binning 2 x 2). Images were acquired every 5 seconds using MicroManager software and quantified with ImageJ.
The calcium indicator dye Fluo-4 (Invitrogen, cat # F-14201) was used in experiments in which intracellular calcium measurements were simultaneously recorded with changes in extracellular H+ with self-referencing microelectrodes. A stock solution was prepared by dissolving 50 μg Fluo-4 in 20 μl DMSO without pluronic detergent. Fluo-4 stock solutions were diluted in goldfish Ringer’s solution to a final concentration of 5 μM, and cells loaded in the dark at 10°C for 1 hour. Cells were imaged immediately following full wash-off with dye-free Ringer’s solution on a Zeiss Axiovert 25 microscope equipped with a 40X LD Acroplan 0.60 NA objective. The dye was continuously excited using an Exfo X-cite 120 lamp equipped with a liquid light. The excitation filter had maximal transmittance at 490 nm with a 20 nm bandpass cutoff. Emitted light passed through a 535 nm filter and was centered upon an American Scientific photomultiplier tube (#10-222) set for maximal sensitivity with damping on and adjusted to a common baseline reading for each experiment. The output of the photomultiplier tube was connected to a Digidata 1322A A/D converter controlled by Axoscope software (Axon Instruments, Burlingame, CA).
Confocal microscopic localization of HAF fluorescence
The HAF (5-hexadecanoylaminofluorescein) staining protocol used in these experiments was identical to the protocol described in Jouhou et al. (2007). A stock solution was created by dissolving 1 mg HAF in 500 μl DMSO and aliquots stored in the freezer. HAF stock solutions were diluted in goldfish Ringer’s solution to 5 μM and isolated horizontal cells were incubated in HAF-containing Ringer solution in the dark for 20 minutes at 4°C. In control experiments, cells were loaded with the dye FM 1-43. A 5 mM stock solution was prepared in distilled water and stock solution (2 μl) was added directly to the cell culture dish containing 2 ml of Ringer solution, leading to a final working concentration of 5 μM. Cells were imaged immediately following full wash-off with dye-free Ringer’s solution.
Experiments to determine the location of HAF and FM 1-43 were performed using confocal microscopy by utilizing either a Zeiss LSM-510 NLO META, equipped with a 20x water immersion objective (Apochromat 20x / 1.0 n.a.), or a Zeiss LSM-710 NLO equipped with an inverted 40x water immersion objective (C-Apochromat 40x/1.1 n.a.), present at the Marine Biological Laboratory in Woods Hole, Massachusetts and the Confocal Microscopy Facility at the University of Illinois at Chicago, respectively. In experiments using the Zeiss LSM-510, cells were plated on Falcon 3001 plastic culture dishes and illuminated from above. In experiments using the Zeiss LSM-710 microscope, cells were plated on Ibidi 81156 culture dishes having thin plastic bottoms 180 μm thick and illuminated from below. Images were acquired using Zeiss Zen LE software (2009 and 2010) and analyzed further using ImageJ.
Data Treatment and Statistical Analysis
Student’s paired t-tests were used throughout to determine statistical significance, with a criterion of P < 0.01 selected as indicating significantly different distributions. Data are presented throughout as the mean ± SEM. For most experiments, values reported reflect the average of the 10 points before application of a drug and the 10 points after application. All data was statistically analyzed and graphically displayed using Prism 5 (GraphPad) Software. A random examination of 113 cells from a total of 23 dissociations was done to estimate the percentage of cells in each of the three response categories cited below (cells with a standing acidic flux, cells displaying oscillations in extracellular H+, and cells with a standing alkaline flux). Studies examining modulation of H+ fluxes by pharmacological agents and correlations between intracellular calcium and extracellular H+ included an additional 107 cells. In sum, the present study reports data collected from 220 self-referencing trials from a total of 41 goldfish.
RESULTS
The goldfish is known to possess four distinct types of horizontal cells, three cone-driven types (H1, H2, & H3) as well as a rod-driven horizontal cell (H4) (Stell & Lightfoot, 1975). As noted by Tachibana (1981, 1983), while it is straightforward to distinguish horizontal cells from other neurons and glia produced by the dissociation process by their highly characteristic morphology, it is difficult to unambiguously assign isolated horizontal cells to a given class based solely on morphological grounds. We therefore chose to group cells based on the nature of their initial H+ signals prior to stimulation with depolarizing agents. In an examination of 113 cells isolated from a total of 23 goldfish retinae most dissociations resulted in a distribution of three groups of horizontal cells distinguished by their initial H+ signals. From this population of trials 50 cells (44%) displayed consistent standing positive signals (54.1 + 5.6 μV) indicative of a higher level of protons adjacent to the cell membrane compared to a point 30 μm distant. In the second group (44 cells, 39%), spontaneous oscillations in extracellular pH were observed in unstimulated cells (peak 37.5 ± 2.6 μV; trough −45.9 ± 5.1 μV). Finally, in 19 cells (17%), a standing negative (alkaline) signal was detected, with average signal of −27.3 ± 4.9 μV). In the following sections, we will examine changes in proton flux in response to external stimuli for these three classes of goldfish horizontal cells.
Response properties of cells with a standing steady acidic flux
Figure 1 shows a representative self-referencing trace from one cell in the first class (i.e. with a standing acidic flux). As in all our self-referencing experiments, a microelectrode was moved repeatedly first from a position 1-2 μm from the cell, then to a distant position 30 μm away, and a differential signal obtained. The positive voltage signal at the beginning of the trace in figure 1A reflects a greater concentration of H+ adjacent to the cell than at the distal position. Prior studies in skate and catfish horizontal cells indicated that the standing acidic fluxes characteristic of horizontal cells in those species were mediated in large part by Na+/H+ exchange and could be abolished with the Na+/H+ exchange antagonist, EIPA, or by removal of extracellular Na+ (Molina et al., 2004; Kreitzer et al., 2007). The Na+ dependence of the standing acidic flux in this class of goldfish horizontal cells was confirmed by removing extracellular Na+ and replacing it with an equimolar concentration of NMDG. This exchange in 8 cells reduced the standing H+ flux from 60.6 ± 9.6 μV to −0.5 ± 0.8 μV. The asterisk in figure 1A (and all representative self-referencing traces) denotes a background control during which the microelectrode was moved 200 μm away from the cell. A differential voltage measurement near 0 at this location would be expected given that the pH at the two locations should be virtually identical. Following this, the electrode was repositioned to its original location, and the standing acidic differential signal was again observed. At approximately 350 sec into the recording, a 1mL addition of 300 μM glutamate was added to normal goldfish Ringer’s solution making a final concentration of 100 μM glutamate around the cell. The break in the trace (and all subsequent traces) represents points removed due to voltage artifacts resulting from solution application. Challenging the cell with 100 μM glutamate resulted in a negative differential voltage measurement indicating that the H+ concentration next to the isolated horizontal cell was now less than the H+ concentration 30 μm away. The glutamate-induced alkalinization had a time-dependent decay after which the differential voltage measurement came to a new steady-state level despite the continued presence of glutamate. The average standing H+ signal before glutamate application was 75.1 ± 15.6 μV for 7 cells (Fig. 1B). Following glutamate application the H+ signal was initially −52.2 ± 16.9 μV and after 100 seconds was −28.3 ± 12.6 μV (Fig. 1B). Figure 1A also shows that addition of 75 μM CNQX (a kainate/AMPA ionotropic glutamate receptor antagonist) while maintaining the glutamate concentration at 100 μM reversed the glutamate-induced alkalinization. In 7 cells the average H+ flux in the presence of CNQX was 78.4 ± 8.9 μV (Fig. 1B).
Figure 1.
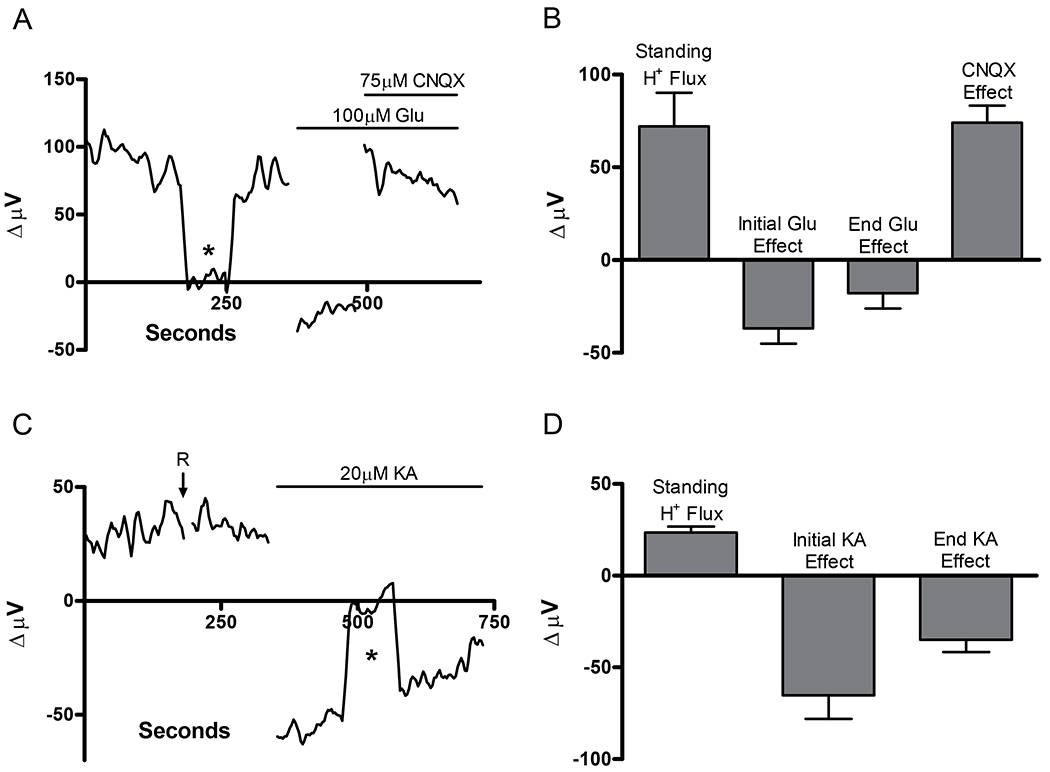
Responses of cells with standing initial positive (acidic) H+ signals in response to application of glutamate and kainate measured using self-referencing H+ selective microelectrodes. (A) Representative trace showing the alkalinization response to application of 1mL bolus of 100 μM glutamate from one horizontal cell. The alkalization was reversed with additional application of 1mL of a solution containing glutamate (100 μM) and the ionotropic glutamate receptor blocker CNQX (75 μM). Asterisk in this and other figures shows control recording 200 μm away from the cell. (B) Average result from 6 cells before glutamate, initially after glutamate, 100 sec after glutamate, and following co-application of CNQX. (C) Representative trace from a different horizontal cell. 1mL bath application of additional Ringer’s solution (R) by itself did not change the standing differential recording. Addition of 1mL of kainate induced an extracellular alkalization. (D) Average result from 8 cells before kainate, initially after kainate, and 100s after kainate.
Figure 1C is a representative trace showing that addition of 20 μM of the ionotropic glutamate receptor activator kainate also resulted in an extracellular alkalinization. The “R” label with the arrow represents a control application of 1mL of Ringer’s solution by itself to emphasize that the alteration in the differential voltage measurements did not result simply from the addition of solution to the dish. The average initial voltage differential (standing H+ signal) from 8 cells was 23.5 ± 3.3 μV. After challenging these cells with kainate the average voltage differential was −65.2 ± 12.9 μV and −34.9 ± 6.7 μV after 100 seconds. Taken together the data from figure 1 suggests that activation of kainate/AMPA receptors induces an extracellular alkalinization around this class of isolated goldfish horizontal cells.
Figure 2 shows self-referencing recordings measuring the response of isolated goldfish horizontal cells with standing acidic fluxes to challenges with a Ringer’s solution high in extracellular potassium. As demonstrated previously (Kaneko & Shimazaki, 1975; Tachibana, 1981; Kaneko & Tachibana, 1985, Jouhou et al. 2007), the membrane potential of goldfish and carp horizontal cells are depolarized by high extracellular potassium and follow the Nernst equation for potassium. Figure 2A shows an example of the response in one cell in which 43 mM of the standard NaCl in the solution had been replaced by 43 mM KCl. A typical standing positive signal was initially observed, and upon addition of the high external potassium solution, a significant alkalinization was detected. This extracellular alkalinization could be abolished by co-application of 10 μM of the voltage-gated L-type calcium channel antagonist, nifedipine (figure 2A). In 6 cells the average standing H+ signal was 59.4 ± 14.3 μV. After introducing high extracellular potassium, the differential voltage measurement was reduced to −44.8 ± 16.6 μV just after application and −33.2 ± 8.5 μV after 100 seconds. While in high external potassium Ringer’s solution, co-application of 10 μM nifedipine restored the differential voltage measurement to 28.7 ± 2.6 μV in these cells (Figure 2B). Figure 2C shows an alkalinization induced in a second cell by 43 mM potassium. Application of 4 mM cobalt, another blocker of voltage-gated calcium channels, greatly diminished the alkalinization. In 9 cells in which normal Ringer’s solution was exchanged for one containing 43 mM potassium Ringer’s solution, a significant decrease in the differential voltage indicative of an alkalinization was observed. In these cells, the average initial signal was 44.7 ± 8.5 μV; upon addition of 43 mM potassium, the signal changed to a value of −17.2 ± 12.7 μV (initial) and −11.1 ± 7.5 μV after 100 seconds. Subsequent addition of 4 mM cobalt in the high potassium environment restored the differential voltage measurement to 21.3 ± 2.9 μV in these same cells (figure 2D). Taken together these results suggest that an extracellular alkalinization can be induced by depolarizing horizontal cells with high extracellular potassium and that the alkalinization is dependent upon the activation of voltage-gated calcium channels.
Figure 2.
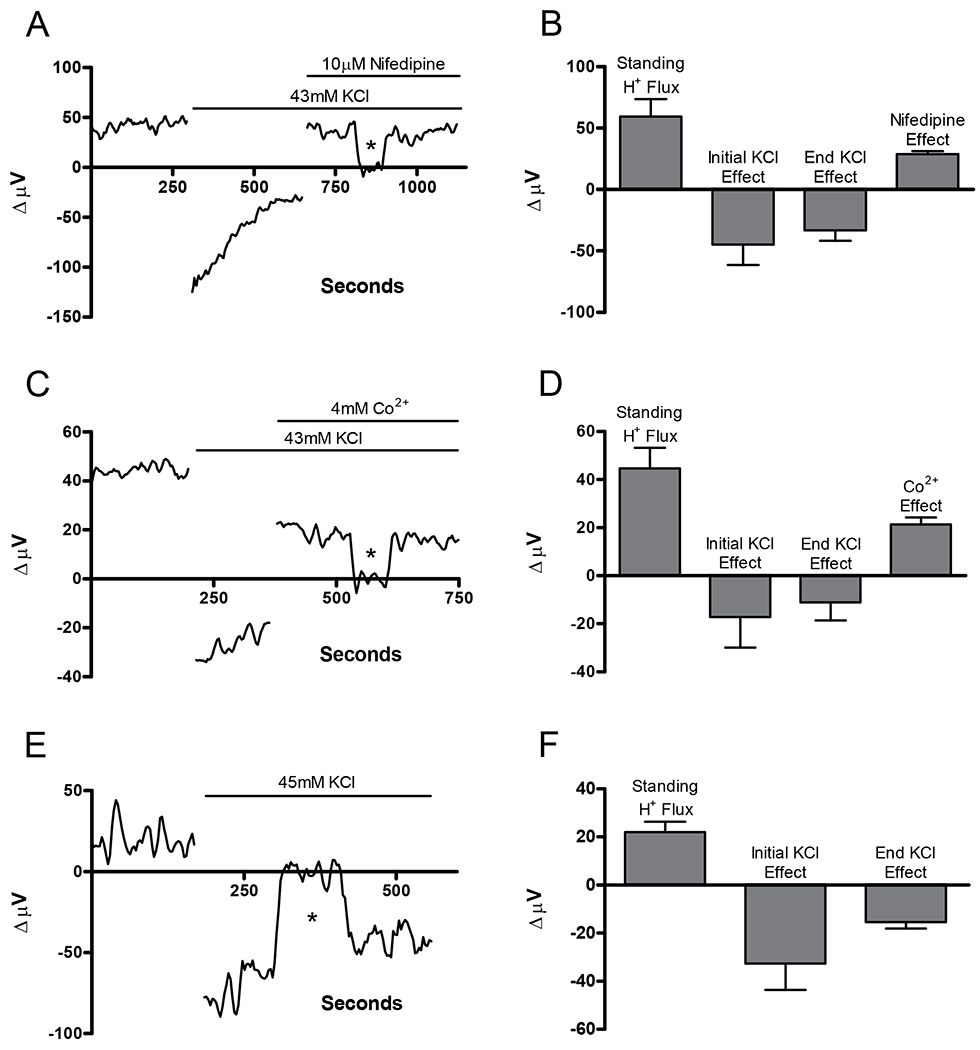
The alkalinization induced by high external potassium in cells with a standing acidic initial flux is markedly reduced by nifedipine and cobalt (Co2+). (A) Representative trace from one horizontal cell showing the alkalinizing effect of high extracellular potassium, and its subsequent abolishment with co-application of nifedipine. (B) Average results from 6 cells prior to stimulation, initially after the addition of 43 mM extracellular potassium, 100 sec after high potassium addition, and following co-application of 10 μM nifedipine. (C) Representative trace from a second horizontal cell showing the extracellular alkalization induced by high extracellular potassium and its elimination upon co-application of Co2+. (D) Average results from 9 cells showing signals prior to stimulation, initially after the addition of 43 mM extracellular potassium, 100 sec after KCl, and following co-application of 4 mM Co2+. (E) Representative trace from a third horizontal cell showing the extracellular alkalinization induced by high extracellular potassium. In this experiment cells were bathed in a solution in which 45 mM NMDG replaced 45mM Na+. High potassium was then added by an equimolar exchange of NMDG rather than reducing the extracellular sodium concentration. (F) Average signals from 6 cells prior to stimulation, initially after the addition of high extracellular potassium, and 100 sec after KCl.
In the experiments described above, 43 mM KCl was introduced by making an equimolar exchange of extracellular NaCl. It is possible that changes in extracellular pH could result from changes in the sodium driving force, perhaps affecting Na+/H+ exchange. We therefore also conducted a set of experiments in 6 cells in which 45 mM of the sodium concentration in the initial Ringer’s solution had been replaced by 45 mM n-methyl-d-glucamine (NMDG). In challenging the cell with high KCl, we now replaced the 45 mM NMDG in the Ringer’s solution with 45 mM potassium. This insured that subsequent addition of 45 mM potassium resulted in no change in the Na+ driving force. The KCl-induced alkalinization persisted in these experiments with the differential voltage measurement decreasing from a standing value of 22.1 ± 4.3 μV to −32.6 ± 11.0 μV (initial) and −15.5 ± 2.7 μV after 100 seconds (figure 2E & F).
Extracellular H+ oscillations from isolated goldfish horizontal cells
In the second class of goldfish horizontal cells, oscillations in extracellular H+ were observed in unstimulated cells. Figure 3 shows examples of these oscillations from four individual cells. These oscillations varied in timing and duration with no particular pattern being obvious. Figure 3A shows alkaline oscillations with an irregular time course starting from what appears to be an acidic “baseline” value of about +20 μV. The application of the L-type calcium-channel blocker nifedipine abolished the oscillations, and the cell maintained a positive standing proton signal. In 10 cells exhibiting spontaneous extracellular pH oscillations and treated in the same fashion, the positive standing H+ flux in between oscillations was 25.2 ± 5.8 μV. Following application of 10 μM nifedipine, 7 of the cells exhibited a stable positive acidic H+ flux of 23.8 ± 4.6 μV. In the remaining three cells oscillations were still present but significantly reduced in frequency and duration. The spontaneous oscillations in extracellular pH were also abolished by the application of the L-type calcium channel blocker cobalt (representative trace; figure 3B). In 6 cells examined, addition of 4 mM cobalt completely abolished the H+ oscillations from 5 of the cells and reduced the number and size of oscillations in the remaining cell. The average standing positive flux between oscillations in these cells was 34.0 ± 12.8 μV and 40.9 ± 10.6 μV after introducing 4 mM cobalt.
Figure 3.
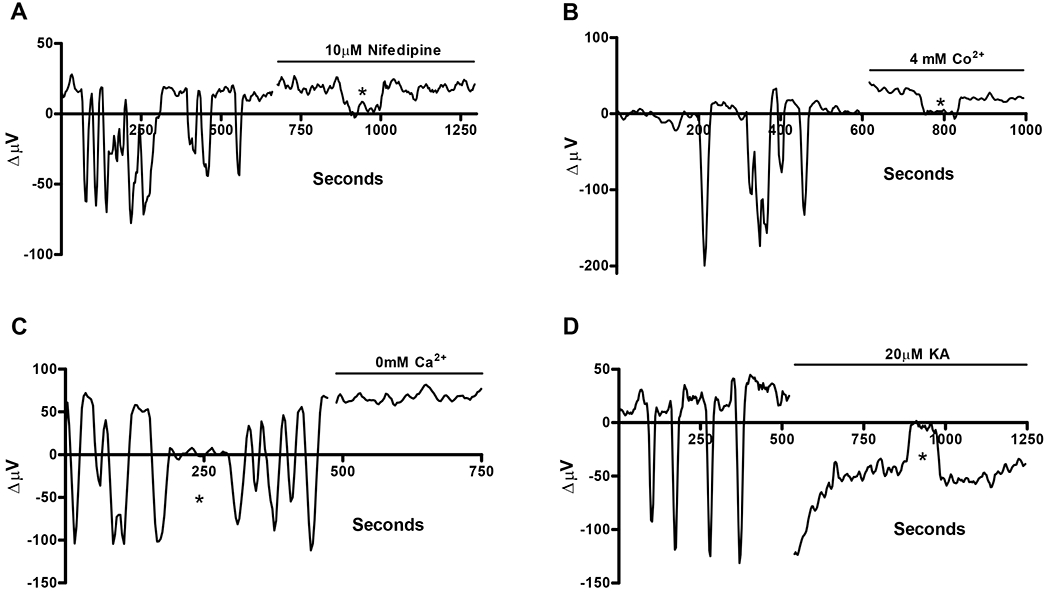
Spontaneous extracellular H+ oscillations and their abolishment by nifedipine, cobalt, nominally 0mM extracellular Ca2+, and kainate. (A) Representative trace from one cell showing spontaneous extracellular H+ oscillations; addition of 10 μM nifedipine abolished the oscillations and resulted in a standing acidic H+ signal. (B) Representative trace showing oscillations from a second cell that were abolished reversed upon addition of 4 mM Co2+; a standing extracellular acidification was observed following cobalt application. (C) H+ oscillations observed in a third cell were eliminated by applying a solution containing nominally 0 mM external Ca2+. A continuous extracellular acidification was observed following the addition of the nominally 0 mM external calcium solution. (D) H+ oscillations in the final cell were quieted in an extracellular alkalinized state following the addition of 20 μM kainate.
The H+ oscillations could also be eliminated when the Ringer’s solution surrounding an oscillating horizontal cell was exchanged with a nominally 0 mM calcium Ringer’s solution, in which the normal 2.5 mM calcium had been replaced by an equimolar amount of magnesium. An example of this effect can be seen in figure 3C. Following removal of extracellular calcium the extracellular H+ oscillations were abolished and the cells displayed a positive stable standing H+ flux. In 4 cells examined, there was striking similarity between the average positive standing flux between oscillations (45.3 ± 22.1 μV) and the average standing flux after removal of calcium from the bath (45.7 ± 14.2 μV). Taken together, the ability of nifedipine, cobalt, and nominally 0 extracellular calcium to reduce or completely block the oscillations suggests that the extracellular pH oscillations are dependent on calcium influx through voltage-gated calcium channels.
The spontaneous oscillations in H+ signal were also abolished when horizontal cells were challenged with 20 μM kainate (representative trace, figure 3D). Kainate application resulted in a standing extracellular alkalinization, similar to that seen in cells with an initial positive standing H+ flux (cf. figure 1C). The average alkaline voltage differential from 8 oscillating cells after kainate application was −59.3 ± 14.7 μV and −25.2 ± 6.7 μV after 100 seconds. The spontaneous oscillations were removed completely in 6 of 8 cells upon addition of 20 μM KA; in two cells, oscillations persisted but were significantly less frequent. It is worth noting the similarity of the average trough values of spontaneous alkalinizations (−54.4 ± 11.1 μV) compared to the initial values following application of kainate (−59.3 ± 14.7 μV).
Oscillations of extracellular H+ correlated with oscillations in intracellular calcium
The block of the oscillations in extracellular H+ by calcium channel antagonists, as well as nominally 0 mM extracellular calcium solutions, suggests that the oscillations might result from periodic influxes of calcium into the cells. Oscillations in the membrane potential of isolated horizontal cells have previously been reported, along with observations of prolonged “action potentials” on the order of many seconds (Tachibana, 1983; Lasater et al., 1984). Such prolonged depolarizations would be expected to lead to a significant influx of calcium through voltage-gated L-type calcium channels. This in turn would activate the plasma membrane Ca2+ ATPase (PMCA) pump, resulting in the exchange of intracellular calcium for extracellular protons and a consequent extracellular alkalinization. To test this hypothesis, we first sought to determine whether spontaneous calcium changes could be detected in unstimulated isolated horizontal cells. Cells were loaded with the calcium indicator dye Oregon Green, stimulated periodically with 488 nm light, and changes in fluorescence as a function of time examined. Figure 4 shows examples of the responses from several cells. The three traces in fig 4A show changes in normalized fluorescence from three individual cells; the traces have been separated for clarity by a constant amount. The bottom two traces show the results from two cells in the same field that displayed calcium oscillations. It is notable that while both are oscillating, there does not appear to be any obvious correlation between the oscillations of the cells; this was a common feature of the oscillations we encountered. As the top-most trace indicates, we also encountered cells in which no calcium oscillations were evident.
Figure 4.
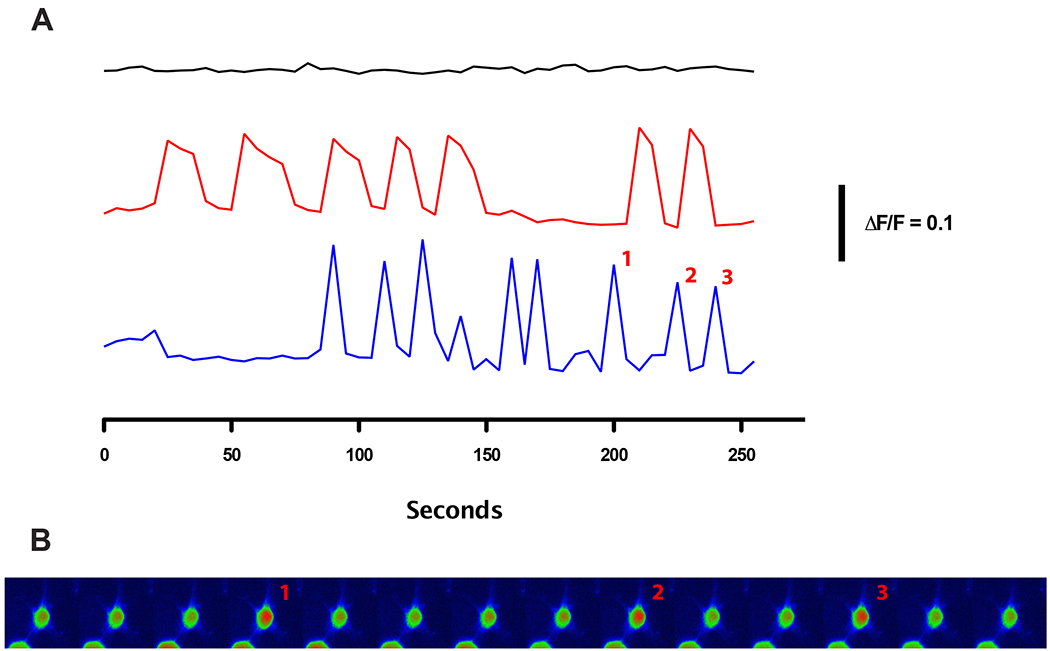
Spontaneous oscillations in intracellular calcium in isolated goldfish horizontal cells as revealed by the calcium-sensitive dye Oregon Green. (A) Traces show changes in Oregon Green fluorescence in three different cells as a function of time. Two of the cells in the same field of view displayed oscillations in fluorescence indicative of calcium oscillations, but the timing and length of oscillations differed between the two cells (lower two traces). The top trace shows the fluorescence of a third horizontal cell that did not display any oscillations. Traces have been offset by a constant amount for clarity. (B) A montage of oscillations in Oregon Green fluorescence from the cell whose data was plotted as the lowest trace in (A). Numbers on the image correspond with the numbers on the oscillations in the lowest trace.
We next looked to see if we could correlate changes in calcium-induced fluorescence in oscillating cells with changes in H+ signals measured with self-referencing microelectrodes. To accomplish this, cells were loaded with the calcium-sensitive dye Fluo-4, stimulated with 488 nm light, and fluorescence measured using a photomultiplier tube while simultaneously examining differential H+ signals using an H+-selective microelectrode. We also took steps to attempt to improve our temporal resolution of the changes in extracellular H+ associated with these oscillations. In the data reported in figures 1–4 and in our previously published measurements of extracellular H+ changes from isolated retinal horizontal cells of skate (Molina et al., 2004) and catfish (Kreitzer et al., 2007; Jacoby et al., 2012), data were collected with the electrode moving between the two positions at 0.3 Hz and with a running average of ten points. In the present experiments, we kept the frequency of movement the same but eliminated the running average. This increases the baseline noise observed, but allows for a temporal resolution of approximately 3 seconds. Figure 5 shows a recording from one cell monitored using this paradigm. The top trace shows the changes in fluorescence of Fluo-4 occurring spontaneously in one cell, while the bottom trace shows the corresponding measurement of extracellular pH with improved temporal resolution at the same time. It is noteworthy that changes in intracellular calcium are locked one-for-one with changes in pH, with an increase in intracellular calcium always associated with an extracellular alkalinization. Similar responses were obtained in 9 additional cells, where increases in intracellular calcium were also locked each and every time with an extracellular alkalinization. Despite the improved resolution by removing the running averaging of the self-referencing signal, the relatively slow degree of data acquisition at 0.3 Hz with the self-referencing electrode makes a precise match with changes in calcium difficult to discern – we cannot tell with precision, for example, which of the two signals is changing first; however, what is clear is the one-to-one change of calcium and extracellular H+ in every oscillating cell encountered. Moreover, in 15 additional cells in which no oscillations in intracellular calcium were observed, there were also no oscillatory changes in extracellular H+.
Figure 5.
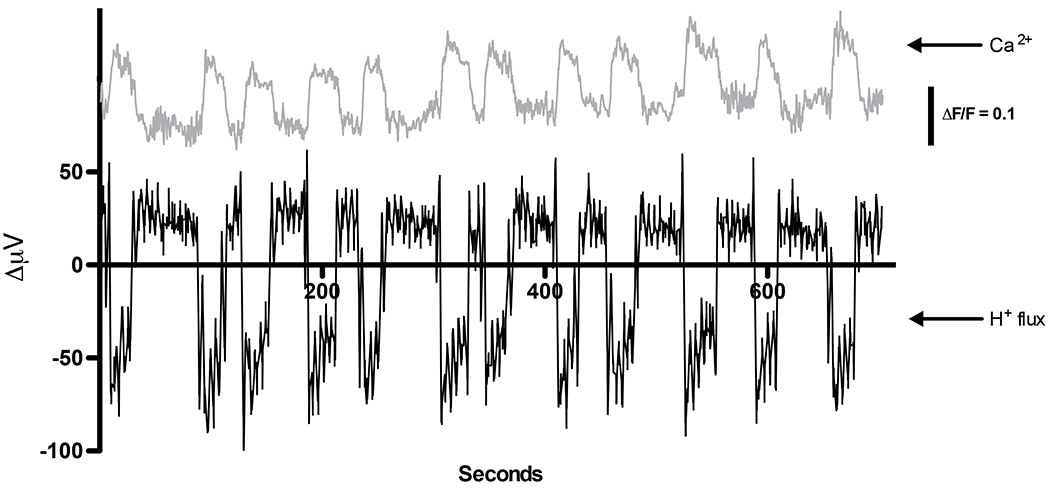
Simultaneous measurements of changes in the fluorescence of the calcium indicator dye (top trace) and alterations in extracellular H+ from a single goldfish horizontal cell. Dye fluorescence is presented as intensity normalized to the beginning of the trace prior to the initial oscillation; the trace has been shifted vertically for clarity. The lower trace represents the differential output of the self-referencing H+ electrode with the 10 point running averaging removed.
Cells displaying a standing negative, alkaline signal
17% of goldfish horizontal cells examined displayed stable initial negative voltage differentials suggesting a standing extracellular alkalinized state adjacent to these cells. This alkaline state was similar in magnitude to what was observed after challenging cells that possessed a stable positive H+ flux with glutamate, kainate or high extracellular potassium. The standing positive H+ flux could be restored in these ”locked-down” cells if they were challenged with 4 mM cobalt (Fig 6A). Application of cobalt flipped the voltage differential from −21.3 ± 4.1 μV to 27.1 ± 6.4 μV in six cells where a standing negative (alkaline) signal was observed. This value is similar in magnitude to the value obtained in 4 mM cobalt after cells had been challenged with 43 mM extracellular potassium. Since the standing alkalinization of these cells was abolished when the calcium channel blocker was added to the bath, these results suggest that the “locked-down” cells displayed an extracellular alkaline level adjacent to the cell membrane because of the continuous influx of extracellular calcium through voltage-gated calcium channels.
Figure 6.
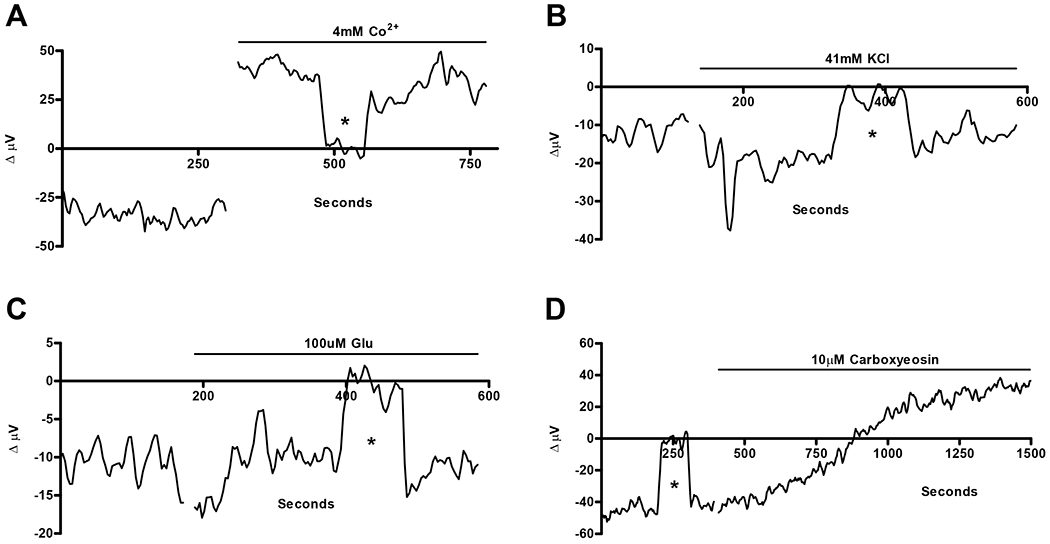
Responses of cells with standing initial negative (alkaline) H+ signals in response to (A) cobalt, (B) high extracellular potassium, (C) glutamate, and (D) carboxyeosin. The calcium channel blocker cobalt reduced the standing alkalinization and turned it into a standing acidic flux, while glutamate and high extracellular potassium were without effect on the standing alkaline flux. Similar to calcium channel blockers, the plasma membrane calcium ATPase blocker, carboxyeosin, also flipped the standing alkalinization to a standing acidic flux.
If the hypothesis above is correct and calcium levels are already high in these cells, one might expect that addition of glutamate or high extracellular potassium to “locked-down” cells would no longer have the alkalinizing effect on H+ flux seen in other cells. Figure 6 B & C shows that this was indeed the case. Figure 6B shows no significant alteration in the standing H+ signal when one “locked-down” cell was challenged with 43 mM external potassium. In 6 cells with standing alkaline fluxes, the average initial signal was −24.4 ± 6.3 μV and in the presence of high external potassium −26.4 ± 5.4 μV. We also examined the responses of “locked-down” cells when challenged with glutamate and found a similar inability of glutamate to induce an alteration in the H+ signal (figure 6C). In 6 cells with standing alkaline signals, the average initial signal was −33.6 ± 9.9 μV and in the presence of 100 μM glutamate −43.9 ± 15.6 μV. Finally, we found that the addition of the PMCA antagonist carboxyeosin led to a marked reduction in the size of the alkalinization (Fig 6D). In 5 cells with standing alkaline fluxes, average initial signal was −38.2 ± 2.8 μV, and following application of carboxyeosin, the average signal was now 33.0± 8.2 μV. These results are consistent with the hypothesis that the “locked-down” cells already have a high internal concentration of calcium with consequent activation of the PMCA pump leading to an extracellular alkalinization.
Distribution of the pH-sensitive dye HAF in isolated retinal goldfish neurons
In two previous studies, the pH-sensitive dye HAF had been used in an attempt to monitor changes in extracellular pH around isolated goldfish and carp horizontal cells (Jouhou et al., 2007; Trenholm & Baldridge 2010). In both sets of experiments, it was reported that challenge of the cells with glutamate or potassium led to an extracellular acidification, precisely the opposite of what we report here using H+-selective microelectrodes. The interpretation that the changes in HAF fluorescence observed in these experiments reflect alterations in extracellular pH depends critically on the assumption that HAF is localized exclusively to the external face of the plasma membrane. To address the question, we loaded cells with HAF using the protocol employed by Jouhou et al. (2007) and examined the distribution of the dye using confocal microscopy. Figure 7 shows confocal slices and corresponding Z-stacks of the distribution of the dye in a horizontal cell stained with HAF (7A) and a horizontal cell stained with FM 1-43 (7B). If the dye were localized to the external surface of the cells, a fluorescent ring on the edge of the cell should be detected in an optical slice with little or not staining in the interior of the cell. This is apparent for the cell stained with FM 1-43. However, it is clear from the optical sections and z-stacks of the cells that the cell stained with HAF instead shows high fluorescent labeling throughout the interior of the cell, and is thus positioned to be able to detect changes in intracellular pH.
Figure 7.
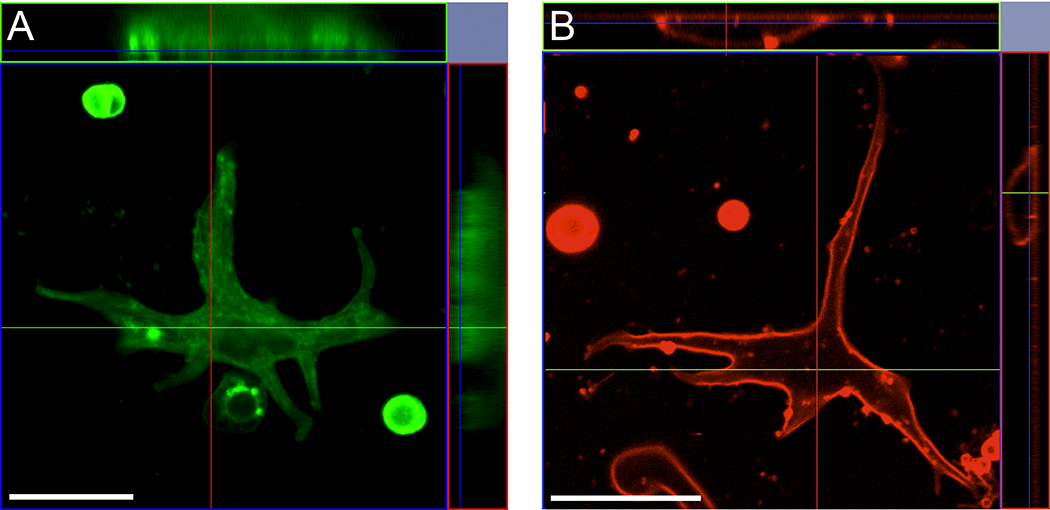
Confocal microscopy reveals HAF staining within the intracellular compartment of isolated retinal neurons. Image taken from an optical section approximately in the center of the Z axis of (A) a horizontal cell stained with HAF showing high fluorescent labeling through the interior of the cell and (B) a horizontal cell stained with FM 1-43. The center slice through the horizontal cell stained with HAF shows extensive intracellular staining in the cell; flanking the center optical section, above and to the right are orthographic projections showing extensive intracellular staining throughout the cells’ interior. The optical slice and orthographic projections obtained from the horizontal cell stained with FM 1-43 displays staining located primarily to the plasmalemma Scale bar, 20 μm.
DISCUSSION
Our examination of extracellular H+ responses from goldfish horizontal cells using self-referencing H+-selective electrodes reveals a level of complexity not seen from horizontal cells of other species previously examined. One class of cells had an initial standing signal indicating higher H+ near the membrane than a point 30 μm distant, and challenge with glutamate, kainate or high extracellular potassium induced an extracellular alkalinization. The extracellular alkalinization was reduced by the calcium channel blockers nifedipine and cobalt. The results from this set of goldfish horizontal cells are very similar to the measurements previously reported for horizontal cells of skate (Molina et al., 2004) and catfish (Kreitzer et al., 2007; Jacoby et al., 2012). We interpret the data from this class of goldfish horizontal cells to indicate that challenge with depolarizing agents leads to increased intracellular calcium, which activates a plasma membrane Ca2+ ATPase with extrusion of intracellular calcium in exchange for H+ from the extracellular fluid, resulting in the extracellular alkalinization observed.
A significant fraction of unstimulated cells displayed spontaneous oscillations in extracellular H+. In this “oscillatory” set of cells, extracellular H+ oscillations were abolished by cobalt and nifedipine, blockers of voltage-gated calcium channels, and by the removal of extracellular calcium. This suggests that calcium influx was a key driver of the oscillations in extracellular H+. A strong correlation between changes in intracellular calcium and extracellular H+ was also detected in experiments monitoring intracellular calcium levels using a calcium-indicator dye while simultaneously measuring extracellular H+ with self-referencing microelectrodes. While the time resolution of our measurements was not sufficient to be able to state clearly whether calcium increases preceded changes in extracellular H+, it was clear that increases in calcium were always paired with alkalinization of the extracellular fluid. Significantly, no oscillatory changes in extracellular H+ were observed in cells that did not display oscillatory changes in intracellular calcium. Collectively, these data argue that oscillations result from spontaneous increases in intracellular calcium which in turn lead to activation of plasmalemma calcium pumps and consequent alkalinization of the extracellular fluid.
A third set of cells had standing negative signals from the self-referencing pH-selective microelectrodes, indicating that extracellular H+ next to the membrane was lower (more alkaline) than the point 30 μm away. The standing alkalinization was reduced or turned into an acidic positive signal by the calcium channel blocker cobalt. Further, glutamate or high extracellular potassium did not induce further significant alterations in the H+ signal from these cells, but the PMCA blocker carboxyeosin reduced the standing alkalinization, flipping it entirely to an acidic proton signal. We take these findings to indicate that this class of cells were already depolarized and possessed high levels of calcium, leading to the standing alkalinization. Addition of cobalt would block entry through voltage-gated calcium channels, leading to a decrease in PMCA activity and a reduction of the extracellular alkalinization, which is what was observed. In such cells, addition of glutamate or high potassium would be expected to have little or no effect on H+ signals, since the PMCA pump would already be operating to remove high levels of intracellular calcium in exchange for extracellular H+, and again, this is what was observed. Finally, the PMCA blocker carboxyeosin would be expected to reduce the standing alkalinization of such cells, and this was also observed.
Taken together, the response characteristics of all three sets of neurons can be most parsimoniously explained by activation of the PMCA pump by high levels of intracellular calcium, with an extracellular alkalinization resulting from the exchange of intracellular calcium for extracellular H+. Moreover, it is striking that in all of the cells examined, regardless of category, we never observed an extracellular acidification upon addition of glutamate, kainate or high extracellular potassium. This is a notable observation in light of the current prominence of the proton hypothesis of lateral inhibition in the outer retina. According to this hypothesis, upon depolarization, horizontal cells release protons into the extracellular space, which bind to calcium channels of photoreceptors and reduce the influx of calcium into these cells, leading to a decrease in the release of glutamate from the photoreceptors. A considerable amount of evidence in favor of this hypothesis has come from studies done in goldfish and the closely related cyprinid fish, carp. For example, Vessey et al. (2005) reported that high levels of the pH buffer HEPES reduced electrical responses (“voltage rollback”) associated with lateral inhibitory feedback from goldfish horizontal cells onto photoreceptors. This effect of HEPES and other aminosulfonate buffers was also observed by Trenholm & Baldridge (2010). Moreover, both Jouhou et al. (2007) and Trenholm & Baldridge (2010) claim evidence for a direct detection of the release of protons from goldfish and carp horizontal cells upon the addition of depolarizing agents. Jouhou et al. (2007) stained isolated carp horizontal cells with the pH-sensitive dye HAF, and reported that when cells were challenged by bath-application of glutamate, kainate, or high extracellular potassium, the dye reported an acidification. A critical assumption in the interpretation of the results of these studies, however, is that the pH-sensitive HAF is located exclusively on the external side of the cellular membrane and is measuring strictly extracellular H+ changes. Our confocal microscopy studies examining the distribution of HAF in isolated goldfish horizontal cells demonstrate, however, that the dye is not exclusively located to the plasma membrane of isolated goldfish neurons, but rather is to be found widely distributed on the inside of the cells. As recently shown (Jacoby et al., 2012), HAF within the interior of catfish cone-driven horizontal cells reports the intracellular acidification known to occur upon challenge with depolarizing agents (Dixon et. al. 1993). Thus, data obtained using HAF as a pH indicator cannot be considered as necessarily indicating extracellular pH changes exclusively, and we hypothesize that the previous data by Jouhou et al. (2007) and Trenholm & Baldridge (2010) reflect intracellular acidifications rather than the extracellular changes as assumed.
Collectively, this body of findings argues strongly against the H+ hypothesis of lateral inhibition in the outer retina. Given the inability of HAF to distinguish clearly between intracellular and extracellular changes in H+, measurements using self-referencing electrodes are the only reliable indicators of changes in extracellular H+ adjacent to isolated horizontal cells. In every case and every species examined so far, including skate (Molina et al., 2005), catfish (Kreitzer et al., 2007; Jacoby et al., 2012) and goldfish (the present study), addition of depolarizing agents such as glutamate or high extracellular potassium has never led to a detectable extracellular acidification. In most instances, a clear alkalinization upon addition of glutamate and high extracellular potassium has been observed, and in those cases in which no effect has been seen, the lack of a response has been associated with cells already showing an extracellular alkalinization, which we argue reflects cells already depolarized and possessing high levels of calcium and an active PMCA pump. Thus, one of the key criteria normally accepted as indicating that a substance is acting as a neurotransmitter mediating a given function in the nervous system, is lacking – there is no convincing evidence for an increase in extracellular H+ upon depolarization of horizontal cells. However, the extrapolation of our results obtained using isolated cells to the regulation of pHo in the intact retina requires some caution. It is conceivable that microdomains of extracellular pH regulation might exist and that such microdomains might be present within the synaptic pedicles and spherules of individual photoreceptors. One could imagine the existence of a set of proteins at the dendritic tips of horizontal cells promoting efflux of protons, and another set of proteins elsewhere on the cell that might act to take in protons. The self-referencing electrodes used in this study cannot be used to examine such potential microdomains due to the relatively large size (2-4 μm) of the electrode tips and the inability to place the electrodes within these selective microdomains.
Despite the caveats above, our data are most consistent with the hypothesis that challenge of horizontal cells with depolarizing agents such as glutamate and high extracellular potassium induces a calcium-dependent extacellular alkalinization dependent upon activation of a plasmalemma calcium pump that exchanges intracellular calcium for extracellular H+. These results are in agreement with prior studies on the horizontal cells of skate and catfish and argue strongly against the hypothesis that H+ release from horizontal cells mediates lateral inhibition in the outer retina. Our findings also demonstrate that many isolated goldfish cells display non-evoked oscillations in intracellular calcium and extracellular pH and may suggest an additional complex physiological role for pH dynamics in the outer retina.
ACKNOWLEDGEMENTS
We thank Richard Sanger and Mark Messerli of the MBL for assistance with self-referencing and calcium imaging at the Marine Biological Laboratory and without whose help this work could not have been done. We thank Blair Rossetti and Louis Kerr at the MBL and Jim McIlvain and Chris Rieken of Zeiss Corp. for assistance with confocal microscopy, and undergraduate students Blair Skinner, Tom Cully, Luke Montgomery, Karisa Burkholder, Paul Garverick, Matthew Decker, Sonja Vogel, Kyle Mazellan and Meredith Osborn at Indiana Wesleyan University for their contribution to the self-referencing measurements reported. This work was supported by National Science Foundation Grants 0924372, 0924383, a Laura and Arthur Colwin Summer Fellowship from the Marine Biological Laboratory, and the Midwest Eye-Banks.
ABBREVIATIONS
- DMSO
dimethyl sufoxide
- HAF
5-hexadecanoylaminofluorescein
- NMDG
n-methyl-d-glucamine
- PMCA
plasmalemma Ca2+/H+ ATPase
REFERENCES
- Akopian A, Krizaj D & Witkovsky P (1997) Both high- and low voltage-activated calcium currents contribute to the light-evoked responses of luminosity horizontal cells in the Xenopus retina. Brain Res., 762, 121–130. [DOI] [PubMed] [Google Scholar]
- Barnes S & Bui Q (1991) Modulation of calcium-activated chloride current via pH-induced changes of calcium channel properties in cone photoreceptors. J. Neurosci, 11, 4015–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Merchant V & Mahmud F (1993) Modulation of transmission gain by protons at the photoreceptor output synapse. Proc. Natl. Acad. Sci. U.S.A, 90, 10081–10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Fuortes MG & O’Bryan PM (1971) Receptive fields of cones in the retina of the turtle. J. Physiol. (Lond.), 214, 265–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J & Lagnado L (1997) Electrical resonance and Ca2+ influx in the synaptic terminal of depolarizing bipolar cells from the goldfish retina. J. Physiol. (Lond.), 505, 571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadetti L & Thoreson W (2006) Feedback effects of horizontal cell membrane potential on cone calcium currents studied with simultaneous recordings. J. Neurophysiol, 95, 1992–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, Tsunenari T & Yau K-W (2009) Intrinsic light response of retinal horizontal cells of teleosts. Nature, 460, 899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DB, Takahashi K & Copenhagen DR (1993) L-glutamate suppresses HVA calcium current in catfish horizontal cells by raising intracellular proton concentration. Neuron, 11, 267–277. [DOI] [PubMed] [Google Scholar]
- Harsanyi K & Mangel SC (1993) Modulation of cone to horizontal cell transmission by calcium and pH in the fish retina. Vis. Neurosci, 10, 81–91. [DOI] [PubMed] [Google Scholar]
- Hirasawa H & Kaneko A (2003) pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J. Gen. Physiol, 122, 657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa Y & Naka K (1985) Spontaneous membrane fluctuation in catfish type-N cells. Vision Res, 25, 539–542. [DOI] [PubMed] [Google Scholar]
- Jacoby J, Kreitzer MA, Alford S, Qian H, Tchernookova BK, Naylor ER, & Malchow RP (2012) Extracellular pH dynamics of retinal horizontal cells examined using electrochemical and fluorometric methods. J. Neurophysiol, 107, 868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouhou H, Yamamoto K, Homma A, Hara M, Kaneko A & Yamada M (2007) Depolarization of isolated horizontal cells of fish acidifies their immediate surrounding by activating V-ATPase. J. Physiol. (Lond.), 585, 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M & Spekreijse H (1999) The feedback pathway from horizontal cells to cones. A mini review with a look ahead. Vision Res, 39, 2449–2468. [DOI] [PubMed] [Google Scholar]
- Kaneko A & Shimazaki H (1975) Effects of external ions on the synaptic transmission from photorecptors to horizontal cells in the carp retina. J. Physiol. (Lond.), 252, 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J. (1991) Signal transmission at the photoreceptor synapse. Role of calcium ions and protons. Ann. N. Y. Acad. Sci, 635, 468–470. [DOI] [PubMed] [Google Scholar]
- Kolb H, Nelson R, Ahnelt P & Cuenca N (2001) Cellular organization of the vertebrate retina. Prog. Brain Res, 131, 3–26. [DOI] [PubMed] [Google Scholar]
- Kreitzer MA, Collis LP, Molina AJA, Smith PJS & Malchow RP (2007) Modulation of extracellular proton fluxes from retinal horizontal cells of the catfish by depolarization and glutamate. J. Gen. Physiol, 130, 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater EM, Dowling JE & Ripps H (1984) Pharmacological properties of isolated horizontal and bipolar cells from the skate retina. J. Neurosci, 4, 1966–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina AJA, Verzi MP, Birnbaum AD, Yamoah EN, Hammar K, Smith PJS & Malchow RP (2004) Neurotransmitter modulation of extracellular H+ fluxes from isolated retinal horizontal cells of the skate. J. Physiol. (Lond.), 560, 639–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann RA & Pochobradský J (1976) Oscillations in rod and horizontal cell membrane potential: evidence for feed-back to rods in the vertebrate retina J. Physiol. (Lond.), 261, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ & Trimarchi J (2001) Noninvasive measurement of hydrogen and potassium ion flux from single cells and epithelial structures. Am. J. Physiol., Cell Physiol, 280, C1–11. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Hammar K, Porterfield DM, Sanger RH & Trimarchi JR (1999) Self-referencing, non-invasive, ion selective electrode for single cell detection of transplasma membrane calcium flux. Microsc. Res. Tech, 46, 398–417. [DOI] [PubMed] [Google Scholar]
- Somieski P & Nagel W (2001) Measurement of pH gradients using an ion-sensitive vibrating probe technique (IP). Pflugers Arch, 442, 142–149. [DOI] [PubMed] [Google Scholar]
- Stell WK & Lightfoot DO (1975) Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J. Comp. Neurol, 159, 473–502. [DOI] [PubMed] [Google Scholar]
- Tachibana M. (1981) Membrane properties of solitary horizontal cells isolated from goldfish retina. J. Physiol. (Lond.), 321, 141–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. (1983) Solitary Horizontal Cells in Culture - 1. Their electrical properties. Vision Res, 23, 1209–216. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Babai N & Bartoletti TM (2008) Feedback from horizontal cells to rod photoreceptors in vertebrate retina. J. Neurosci, 28, 5691–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S & Baldridge WH (2010) The effect of aminosulfonate buffers on the light responses and intracellular pH of goldfish retinal horizontal cells. J. Neurochem, 115, 102–111. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH & Barnes S (2005) Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J. Neurosci, 25, 4108–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS & Dowling JE (1969) Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J. Neurophysiol, 32, 339–355. [DOI] [PubMed] [Google Scholar]


