Abstract
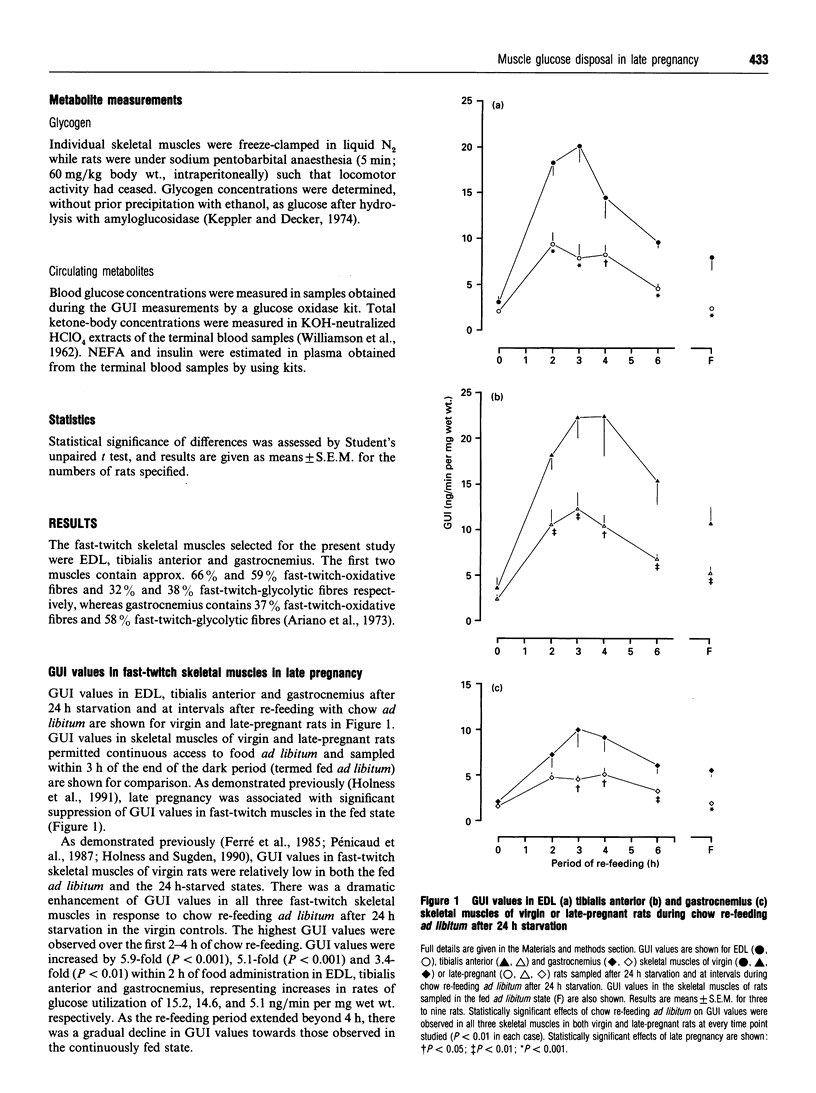
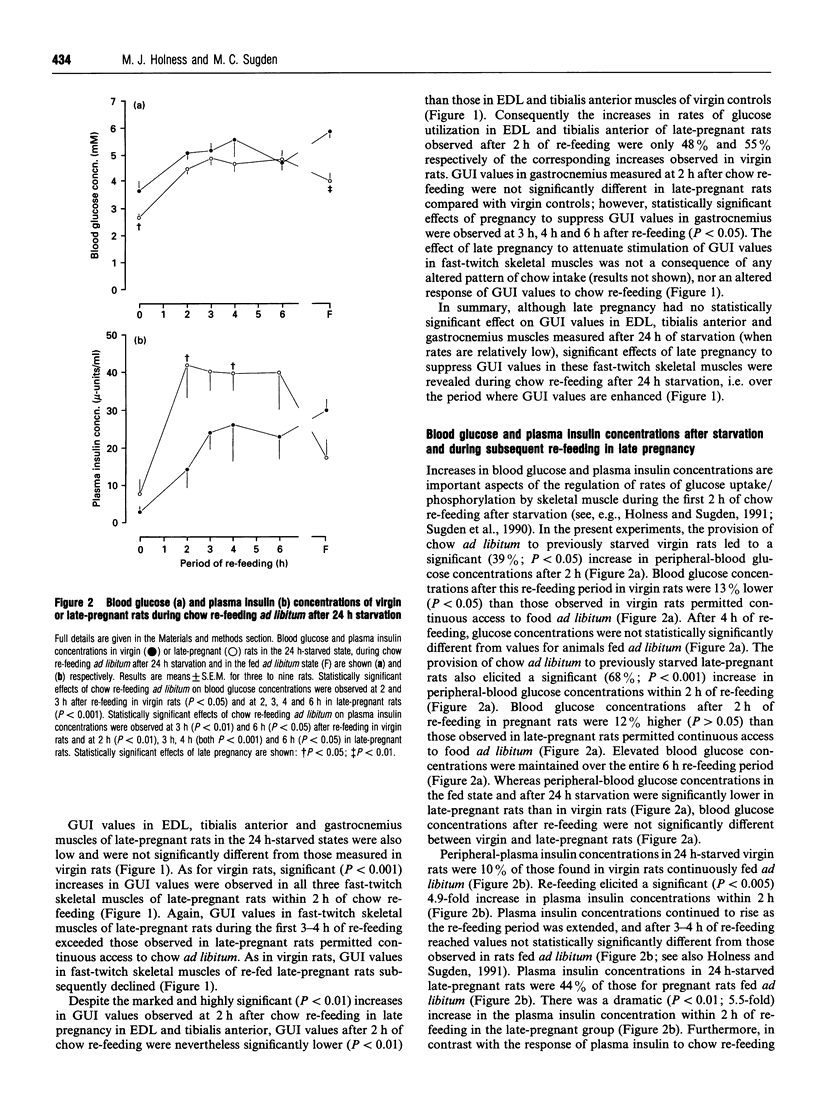
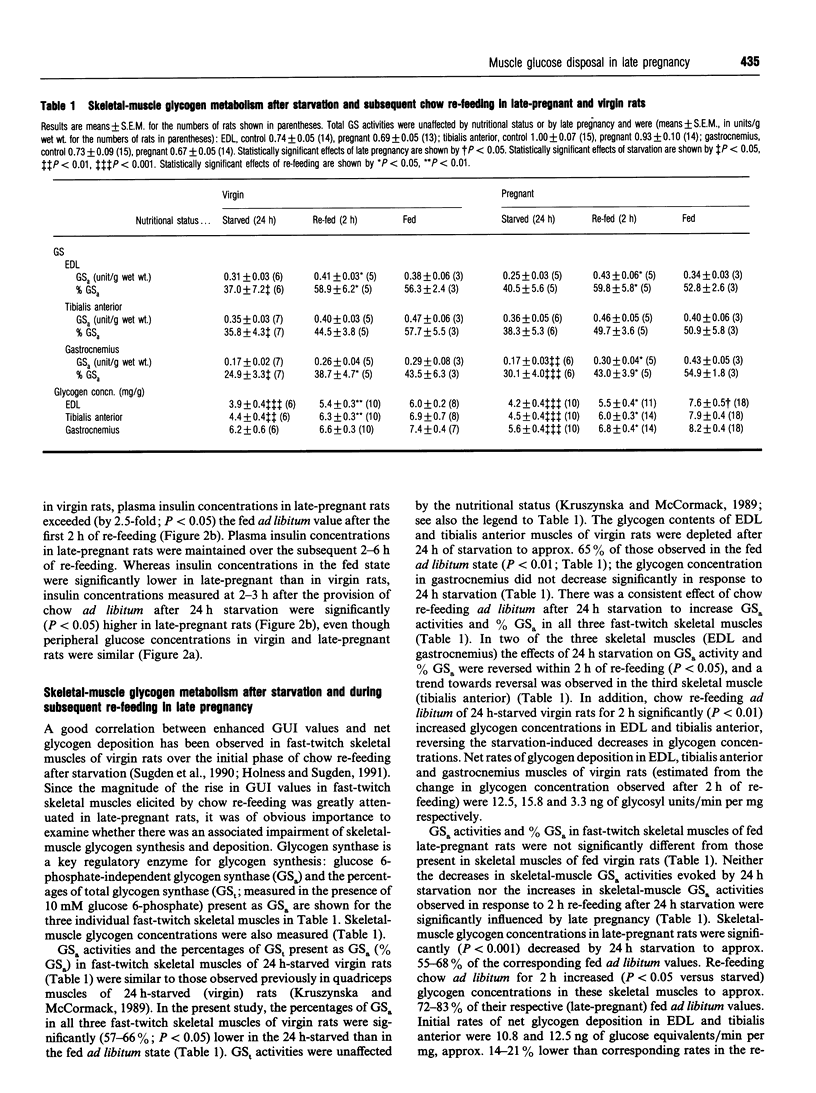
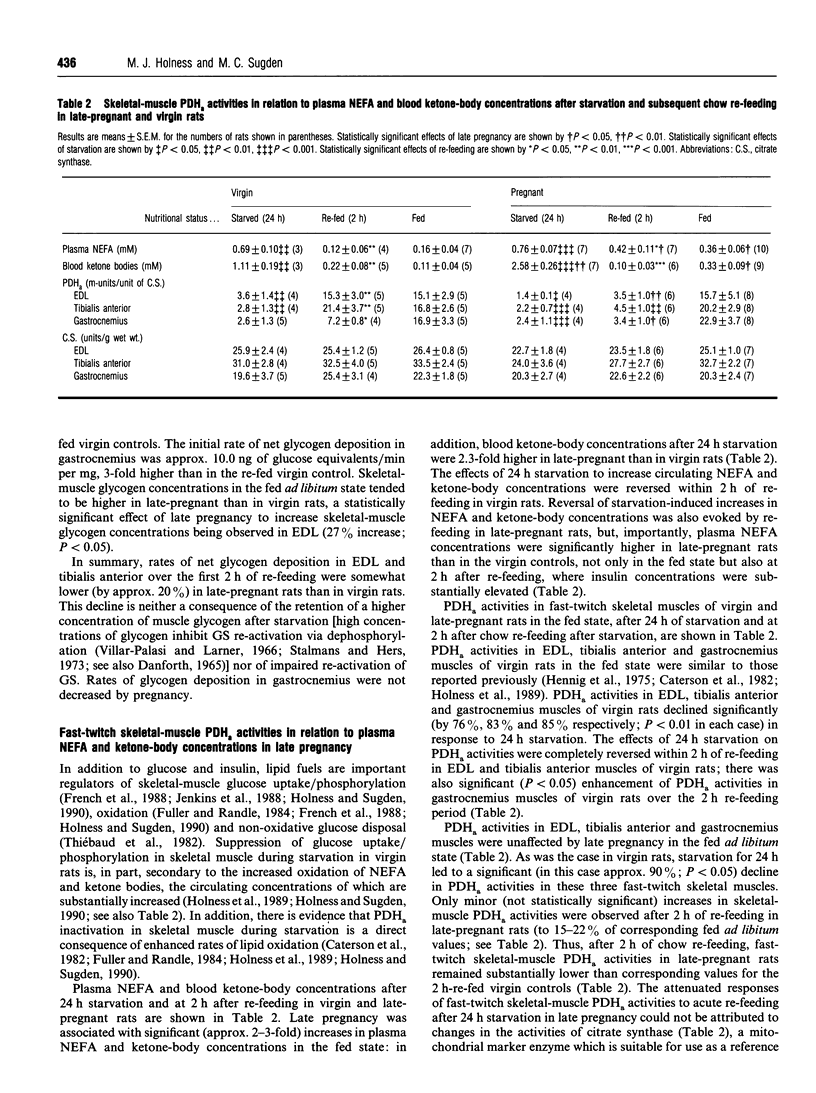
Glucose utilization indices (GUI) were measured in vivo in conjunction with active pyruvate dehydrogenase complex (PDH(a) and glycogen synthase (GS) activities in fast-twitch skeletal muscles [extensor digitorum longus (EDL), tibialis anterior and gastrocnemius] of late-pregnant rats and age-matched virgin control rats in the fed state, after 24 h starvation and at 2 h after re-feeding with standard laboratory chow ad libitum after 24 h starvation. As demonstrated previously [Holness and Sugden (1990) Biochem. J 277, 429-433], GUI values of fast-twitch skeletal muscles of virgin rats were low in the fed ad libitum and the 24 h-starved states, but dramatically increased after subsequent chow re-feeding. GUI values of fast-twitch skeletal muscles of late-pregnant rats were also low in the fed and starved states and were increased by re-feeding, but the increase in GUI values elicited by re-feeding was greatly attenuated. PDHa activities in EDL, tibialis anterior and gastrocnemius in the fed state were unaffected by late pregnancy, and skeletal-muscle PDHa activities were decreased after 24 h of starvation in both groups. Whereas re-feeding of virgin rats with standard diet for 2 h restored PDHa activities in fast-twitch skeletal muscles to values for rats continuously fed ad libitum, PDHa activities in fast-twitch skeletal muscles of late-pregnant rats, although increased in response to re-feeding, remained considerably less than the corresponding fed ad libitum values after 2 h of re-feeding. In contrast, neither skeletal-muscle GS re-activation nor rates of skeletal-muscle glycogen deposition after re-feeding were markedly affected by late pregnancy. The results are discussed in relation to the specific targeting of individual pathways of glucose disposal in fast-twitch skeletal muscles during re-feeding in late pregnancy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Caterson I. D., Fuller S. J., Randle P. J. Effect of the fatty acid oxidation inhibitor 2-tetradecylglycidic acid on pyruvate dehydrogenase complex activity in starved and alloxan-diabetic rats. Biochem J. 1982 Oct 15;208(1):53–60. doi: 10.1042/bj2080053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANFORTH W. H. GLYCOGEN SYNTHETASE ACTIVITY IN SKELETAL MUSCLE. INTERCONVERSION OF TWO FORMS AND CONTROL OF GLYCOGEN SYNTHESIS. J Biol Chem. 1965 Feb;240:588–593. [PubMed] [Google Scholar]
- Denyer G. S., Lam D., Cooney G. J., Caterson I. D. Effect of starvation and insulin in vivo on the activity of the pyruvate dehydrogenase complex in rat skeletal muscles. FEBS Lett. 1989 Jul 3;250(2):464–468. doi: 10.1016/0014-5793(89)80777-x. [DOI] [PubMed] [Google Scholar]
- Ferré P., Leturque A., Burnol A. F., Penicaud L., Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985 May 15;228(1):103–110. doi: 10.1042/bj2280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French T. J., Goode A. W., Holness M. J., MacLennan P. A., Sugden M. C. The relationship between changes in lipid fuel availability and tissue fructose 2,6-bisphosphate concentrations and pyruvate dehydrogenase complex activities in the fed state. Biochem J. 1988 Dec 15;256(3):935–939. doi: 10.1042/bj2560935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. J., Randle P. J. Reversible phosphorylation of pyruvate dehydrogenase in rat skeletal-muscle mitochondria. Effects of starvation and diabetes. Biochem J. 1984 Apr 15;219(2):635–646. doi: 10.1042/bj2190635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M., Pere M. C., Baudelin A., Battaglia F. C. Role of free fatty acids in hepatic insulin resistance during late pregnancy in conscious rabbits. Am J Physiol. 1991 Jun;260(6 Pt 1):E938–E945. doi: 10.1152/ajpendo.1991.260.6.E938. [DOI] [PubMed] [Google Scholar]
- Girard J. R., Ferré P., Gilbert M., Kervran A., Assan R., Marliss E. B. Fetal metabolic response to maternal fasting in the rat. Am J Physiol. 1977 May;232(5):E456–E463. doi: 10.1152/ajpendo.1977.232.5.E456. [DOI] [PubMed] [Google Scholar]
- Groop L. C., Saloranta C., Shank M., Bonadonna R. C., Ferrannini E., DeFronzo R. A. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991 Jan;72(1):96–107. doi: 10.1210/jcem-72-1-96. [DOI] [PubMed] [Google Scholar]
- Hauguel S., Gilbert M., Girard J. Pregnancy-induced insulin resistance in liver and skeletal muscles of the conscious rabbit. Am J Physiol. 1987 Feb;252(2 Pt 1):E165–E169. doi: 10.1152/ajpendo.1987.252.2.E165. [DOI] [PubMed] [Google Scholar]
- Hennig G., Löffler G., Wieland O. H. Active and inactive forms of pyruvatedehydrogenase in skeletal muscle as related to the metabolic and functional state of the muscle cell. FEBS Lett. 1975 Nov 15;59(2):142–145. doi: 10.1016/0014-5793(75)80361-9. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O. Adaptation of skeletal muscle to endurance exercise. Med Sci Sports. 1975 Fall;7(3):155–164. [PubMed] [Google Scholar]
- Holloszy J. O. Biochemical adaptations to exercise: aerobic metabolism. Exerc Sport Sci Rev. 1973;1:45–71. [PubMed] [Google Scholar]
- Holness M. J., Changani K. K., Sugden M. C. Progressive suppression of muscle glucose utilization during pregnancy. Biochem J. 1991 Dec 1;280(Pt 2):549–552. doi: 10.1042/bj2800549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., Liu Y. L., Sugden M. C. Time courses of the responses of pyruvate dehydrogenase activities to short-term starvation in diaphragm and selected skeletal muscles of the rat. Biochem J. 1989 Dec 15;264(3):771–776. doi: 10.1042/bj2640771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., Sugden M. C. Glucose disposal by skeletal muscle in response to re-feeding after progressive starvation. Biochem J. 1991 Jul 15;277(Pt 2):429–433. doi: 10.1042/bj2770429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., Sugden M. C. Glucose utilization in heart, diaphragm and skeletal muscle during the fed-to-starved transition. Biochem J. 1990 Aug 15;270(1):245–249. doi: 10.1042/bj2700245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issad T., Pénicaud L., Ferré P., Kandé J., Baudon M. A., Girard J. Effects of fasting on tissue glucose utilization in conscious resting rats. Major glucose-sparing effect in working muscles. Biochem J. 1987 Aug 15;246(1):241–244. doi: 10.1042/bj2460241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Kraegen E. W., Chisholm D. J. Muscle glucose metabolism in exercising rats: comparison with insulin stimulation. Am J Physiol. 1985 May;248(5 Pt 1):E575–E580. doi: 10.1152/ajpendo.1985.248.5.E575. [DOI] [PubMed] [Google Scholar]
- Jenkins A. B., Storlien L. H., Chisholm D. J., Kraegen E. W. Effects of nonesterified fatty acid availability on tissue-specific glucose utilization in rats in vivo. J Clin Invest. 1988 Jul;82(1):293–299. doi: 10.1172/JCI113586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp R. H., Ruder H. J., Herrera E., Freinkel N. Carbohydrate metabolism in pregnancy. VII. Insulin tolerance during late pregnancy in the fed and fasted rat. Acta Endocrinol (Copenh) 1970 Oct;65(2):352–360. [PubMed] [Google Scholar]
- Kruszynska Y. T., McCormack J. G. Effect of nutritional status on insulin sensitivity in vivo and tissue enzyme activities in the rat. Biochem J. 1989 Mar 15;258(3):699–707. doi: 10.1042/bj2580699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman S. A., Rosso P. Effects of fasting during pregnancy on maternal and fetal weight and body composition in well-nourished and undernourished rats. J Nutr. 1981 Oct;111(10):1823–1832. doi: 10.1093/jn/111.10.1823. [DOI] [PubMed] [Google Scholar]
- Leturque A., Burnol A. F., Ferré P., Girard J. Pregnancy-induced insulin resistance in the rat: assessment by glucose clamp technique. Am J Physiol. 1984 Jan;246(1 Pt 1):E25–E31. doi: 10.1152/ajpendo.1984.246.1.E25. [DOI] [PubMed] [Google Scholar]
- Leturque A., Ferre P., Burnol A. F., Kande J., Maulard P., Girard J. Glucose utilization rates and insulin sensitivity in vivo in tissues of virgin and pregnant rats. Diabetes. 1986 Feb;35(2):172–177. doi: 10.2337/diab.35.2.172. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Proud C. G., Cohen P. The phosphorylation of rabbit skeletal muscle glycogen synthase by glycogen synthase kinase-2 and adenosine-3':5'-monophosphate-dependent protein kinase. Eur J Biochem. 1976 Sep;68(1):31–44. doi: 10.1111/j.1432-1033.1976.tb10762.x. [DOI] [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The significance of changes in tissue clearing-factor lipase activity in relation to the lipaemia of pregnancy. Biochem J. 1968 Feb;106(3):677–682. doi: 10.1042/bj1060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pénicaud L., Ferré P., Kande J., Leturque A., Issad T., Girard J. Effect of anesthesia on glucose production and utilization in rats. Am J Physiol. 1987 Mar;252(3 Pt 1):E365–E369. doi: 10.1152/ajpendo.1987.252.3.E365. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie M. J., Winder W. W., Holloszy J. O. A sparing effect of increased plasma fatty acids on muscle and liver glycogen content in the exercising rat. Biochem J. 1976 Jun 15;156(3):647–655. doi: 10.1042/bj1560647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOW R. O., CHERNICK S. S., BRINLEY M. S. HYPERLIPEMIA AND KETOSIS IN THE PREGNANT RAT. Am J Physiol. 1964 Apr;206:796–804. doi: 10.1152/ajplegacy.1964.206.4.796. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Stace P. B., Fatania H. R., Jackson A., Kerbey A. L., Randle P. J. Cyclic AMP and free fatty acids in the longer-term regulation of pyruvate dehydrogenase kinase in rat soleus muscle. Biochim Biophys Acta. 1992 Jun 10;1135(2):201–206. doi: 10.1016/0167-4889(92)90137-z. [DOI] [PubMed] [Google Scholar]
- Stace P. B., Marchington D. R., Kerbey A. L., Randle P. J. Long term culture of rat soleus muscle in vitro. Its effects on glucose utilization and insulin sensitivity. FEBS Lett. 1990 Oct 29;273(1-2):91–94. doi: 10.1016/0014-5793(90)81058-v. [DOI] [PubMed] [Google Scholar]
- Storlien L. H., Jenkins A. B., Chisholm D. J., Pascoe W. S., Khouri S., Kraegen E. W. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991 Feb;40(2):280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Holness M. J. Substrate interactions in the development of insulin resistance in type II diabetes and obesity. J Endocrinol. 1990 Nov;127(2):187–190. doi: 10.1677/joe.0.1270187. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Liu Y. L., Holness M. J. Glucose utilization and disposal in cardiothoracic and skeletal muscles during the starved-to-fed transition in the rat. Biochem J. 1990 Nov 15;272(1):133–137. doi: 10.1042/bj2720133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiébaud D., DeFronzo R. A., Jacot E., Golay A., Acheson K., Maeder E., Jéquier E., Felber J. P. Effect of long chain triglyceride infusion on glucose metabolism in man. Metabolism. 1982 Nov;31(11):1128–1136. doi: 10.1016/0026-0495(82)90163-9. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder W. W., Baldwin K. M., Holloszy J. O. Enzymes involved in ketone utilization in different types of muscle: adaptation to exercise. Eur J Biochem. 1974 Sep 16;47(3):461–467. doi: 10.1111/j.1432-1033.1974.tb03713.x. [DOI] [PubMed] [Google Scholar]


