Summary
Replication origin assembly is a pivotal step in chromosomal DNA replication. In this process, the ORC complex binds DNA and, together with the CDC6 and CDT1, promotes the loading of the MCM helicase. Chemicals targeting origin assembly might be useful to sensitize highly proliferative cancer cells. However, identifying such compounds is challenging due to the multistage nature of this process. Here, using Xenopus laevis egg extract we set up a high-throughput screening to isolate MCM chromatin loading inhibitors, which led to the identification of NSC-95397 as a powerful inhibitor of replication origin assembly that targets CDC6 protein and promotes its degradation. Using systems developed to test selective drug-induced lethality we show that NSC-95397 triggers cell death both in human cells and Xenopus embryos that have higher proliferative ability. These findings demonstrate the effectiveness of molecules disrupting DNA replication processes in targeting hyperproliferating cells, highlighting their potential as anti-cancer molecules.
Subject areas: Biochemistry, Molecular biology, Cell biology
Graphical abstract

Highlights
-
•
Xl egg extract is a powerful tool to identify replication origin assembly inhibitors
-
•
MCMs chromatin loading is a useful readout to screen for DNA replication inhibitors
-
•
NSC-95397 induces CDC6 protein degradation preventing MCM chromatin loading
-
•
NSC-95397 promotes cell death in highly proliferative human cells and Xenopus embryos
Biochemistry; Molecular biology; Cell biology
Introduction
In eukaryotic cells, DNA replication starts at multiple origins distributed throughout the genome. These origins serve as assembly sites for all multiprotein complexes involved in the cell cycle, promoting the formation of bidirectional replication forks. During this phase, the six-subunit origin recognition complex (ORC; subunit ORC1-6) binds DNA and, together with the cell division cycle 6 (CDC6) and the cell division cycle 10-dependent transcript 1 (CDT1), promotes the loading of minichromosome maintenance 2–7 (MCM2-7) double hexamers, which encircle the double-stranded DNA.1 This reaction, also known as licensing, takes place at the end of mitosis and during the G1 phase when the inhibitor of replication origin assembly, Geminin, is degraded and the activities of CDK1 and CDK2 are low.2 Once in the S-phase, CDK2 activity promotes the binding of CDC45 and the GINS complex to the MCM2-7 double hexamers to form the replicative CMG (Cdc45-MCM-GINS) the activation of which also requires the activity of DBF-dependent kinase (DDK).3
Intriguingly, the number of DNA replication origins assembled onto DNA is higher than the ones used in each cell cycle, with the majority remaining dormant. The excess of DNA replication origins allows cells to efficiently and accurately duplicate DNA even when DNA damage irreversibly halts fork progression.4 This is particularly useful to bypass stalling events blocking the duplication of a defined DNA segment by two stalled converging forks. In this case activation of dormant origins between the convergent forks can promote duplication of the affected segment by promoting the formation of additional forks moving outwards toward the stalled ones.5
Alterations in pathways controlling replication licensing correlate with active cell proliferation, as evidenced by elevated levels of CDC6, CDT1, and MCM proteins observed in many cancer types.6 Therefore, the identification of a compound capable of interfering with the loading of MCM2-7 onto DNA represents a promising therapeutic strategy that specifically targets the dysregulated DNA replication process in cancer cells.
The assembly of the origin of replication has been well characterized in Xenopus egg cell-free extract, a widely used model system to study DNA replication. Following the addition of DNA templates to egg extract, ORC1-6, and MCM2-7 rapidly load onto DNA in a CDC6-dependent fashion and the CMG helicase is formed once the nuclei are assembled.7 The Xenopus egg cell-free system represents a unique tool to identify replication origin assembly inhibitors through robust biochemical assays.8,9,10,11,12,13 Using this system, we recently performed a large chemical screening that led to the identification of 2,3-bis(2-hydroxyethylsulfanyl) naphthalene-1,4-dione, also known as NSC-95397, as a powerful inhibitor of DNA replication. NSC-95397 was previously described as an inhibitor of cell proliferation.14 Here, we report that NSC-95397 prevents the assembly of MCM2-7 onto chromatin by directly binding CDC6 and promoting its destabilization, acting as a molecular glue. Importantly, NSC-95397 triggered cell death in human cells and Xenopus embryos tailored to hyper-proliferate upon SSRP1 overexpression and subsequent increase of replication origin assembly,8,9 demonstrating its effectiveness in vivo.
Results
An in vitro assay to isolate inhibitors of MCM2-7 loading onto DNA
To identify inhibitors of replication origin assembly, we used high-speed supernatant (HSS) Xenopus laevis egg extracts arrested in interphase, which can rapidly assemble ORC1-6 and MCM2-7 complexes on chromatin following the addition of DNA templates.15 Streptavidin beads bound to a 2 kilobases (kb) long DNA molecule biotinylated at the 5′ end were incubated in HSS for 30 min and then recovered. After stringent washing MCM2-7 assembly onto DNA could be detected by western blot of the MCM7 subunit. MCM2-7 binding to DNA was effectively inhibited by Geminin (Figure 1), indicating its physiological assembly.
Figure 1.
An in vitro assay to isolate inhibitors of MCM2-7 loading onto DNA
(A) Pull-down. Western blot analysis of interphase Xenopus laevis egg extract incubated for 30 min with or without streptavidin beads bound to a 1.8 kb long DNA molecule biotinylated at the 5′-end (bDNA). MCM2-7 binding to DNA was effectively inhibited by Geminin. No DNA indicates the absence of a DNA template, neither sperm nuclei nor the 1.8 kb long DNA fragment.
(B) Pull-down of biotinylated DNA (bDNA) was performed at 30 min. HSS was diluted with XB buffer two-to 3-fold in the presence or absence of the recombinant protein Geminin. No DNA indicates the absence of nuclei.
(C) Luminescence w/wo geminin. ELISA assay was performed in a 384-well plate using a 2-fold diluted Xenopus extract, in the presence or absence of the recombinant protein Geminin, as well as a crosslinking reagent (formaldehyde). Luminescence was acquired with the Enspire plate reader and it is expressed as Relative Luminescence Unit (RLU). The graph shows a representative experiment.
To optimize this assay for drug screening purposes, we tested the ability of diluted extracts to support the loading of MCM2-7 on DNA. We found that a two-to 3-fold dilution of HSS with egg extract buffer preparation (XB) was still able to support Geminin-sensitive MCM2-7 DNA loading, including all previous reactions leading to chromatin formation and ORC1-6 DNA binding (Figure 1B). This optimization allowed to minimize the egg extract production for the screening of a large number of small molecules.
Setting up a small molecule screening to isolate inhibitors of MCM2-7 loading onto DNA
Once the MCM2-7 DNA loading conditions were validated by WB, we adapted this in vitro binding assay to an enzyme-linked immunosorbent assay (ELISA) suitable for the 384-well plate format. To this end, 1.8 kb long biotinylated DNA (bDNA), working as a scaffold for the ORC-MCM loading, was generated and immobilized on the bottom of a streptavidin-coated 384-well plate. Then, 2-fold diluted HSS was added in each well in the presence or absence of the recombinant protein Geminin. After 30 min of incubation at room temperature, wells were washed twice with a washing buffer, and then the bDNA-protein complexes were cross-linked before being processed with an MCM7-specific ELISA assay. The stable loading of MCM2-7 onto bDNA was detected using a primary antibody specific to MCM7, which, in turn, was recognized by an HRP-labeled secondary antibody. The addition of the chemiluminescence HRP substrate allows a quantitative luminescent readout of antibody binding and, consequently, of the loading of MCM2-7 on bDNA. The ELISA-based assay was extremely sensitive and specific (signal-to-background ratio greater than 10) in revealing the MCM2-7 loading onto bDNA, as well as the ability of Geminin to inhibit it (Figure 1C).
Using this assay, were screened 3828 small molecules for their ability to impact the MCM2-7 loading on DNA (Figure 2A). The molecules selected for the screening represented most of the chemical classes of the in-house collection (ca. 215.000 small molecules) available at the Experimental Therapeutic Program of IFOM. The subset selected for this screening included clinically approved drug molecules, compounds belonging to a kinase-targeting library, and molecules designed to hamper protein-protein interaction to embrace an ample variety of structural chemotypes and biological activities among the targets.
Figure 2.
Setting up a small molecule screening to isolate inhibitors of MCM2-7 loading onto DNA
(A) HTS workflow outline.
(B) Scatterplot of the primary screening data. The plot of Z-scores for each of the pooled compounds subjected to the primary screen for the identification of an inhibitor of MCM2 loading to bDNA. Values represent a single replicate per compound concentration. The screening was performed on 384-well plates where negative controls (DMSO) are reported in red, positive controls are reported in black (RL5a), and hits are in green. Solid lines represent the mean chemiluminescent value for the measurements of the controls and compounds, and dashed lines denote the average ±3 standard deviation of the negative and positive controls, respectively.
(C) Hit confirmation. The pooled compounds were screened in triplicate. Geminin and RL5A were used as positive controls, while DMSO corresponds to the negative control. Data are expressed as the mean percentage of inhibition of MCM7 loading on bDNA compared to vehicle (DMSO). The dashed red line corresponds to the threshold of 50% inhibition.
See also Figure S1.
To minimize the amount of extract to use, the 3828 compounds were tested as pools of four compounds per well at a fixed concentration of 187 μM each to match the extract’s high protein concentration. In each multiwell plate RL5a, a known licensing inhibitor,16 and DMSO were used as positive and negative controls, respectively. In addition, wells without bDNA were used to define the background level of the measured luminescent signal.
The screening was carried out in three rounds and the hits were identified by applying a robust Z score approach, which was chosen based on the normal distribution of the curve of the experimental data (Figure S1A). After Z score normalization, the majority of the compounds did not inhibit the MCM binding to bDNA and consequently appeared grouped with the DMSO controls (Figure 2B). This “inactive” set was separated from the positive controls and a small number of experimental wells that resulted within the range of the positive controls. Hits were then defined as those molecules (wells) that could reduce the MCM binding to bDNA at least three standard deviations higher than the overall mean of the wells (Z score ≥ - 3). This approach led to the identification of 11 pooled hits with a hit identification rate of 1.1%.
The 11 pooled hits were then tested in triplicate at the dose of 187 μM for confirmation. As reported in the graph (Figure 2C), six of them were confirmed to be able to reduce the MCM chromatin loading on bDNA by at least 50% compared to the DMSO. Finally, the 6 hits pools were deconvoluted by testing the corresponding 24 compounds as single molecules in triplicate, at the dose of 187 μM (Figure S1B). The deconvoluted compounds able to reduce MCM chromatin loading on bDNA by at least 40% compared to the DMSO turned out to be Chelerythrine, Candicidin, Reactive blue 2, NSC-95397, DDP-28574, valsartan, aminacrine, and ethacridine (Figure S1C). After an initial analysis of the structures and activity profiles of these hits, the nonspecific cytotoxic compound Chelerythrine was deprioritized, as well as Candicidin, which is a mixture of antifungal heptaene macrolides and Reactive blue 2, for its non-specific affinity for nucleotide-binding regions present in a variety of proteins.
Validation of the identified compounds
To verify the impact of the remaining five hits, including NSC-95397, DDP-28574, valsartan, aminacrine, and ethacridine (Figure 3A; Table 1) on licensing we used undiluted low-speed supernatant Xenopus egg extracts capable of supporting various processes, such as chromatin formation, origin assembly, nuclear membrane assembly, and origin firing. To evaluate the chromatin loading status of replication proteins in the presence of these compounds, we conducted a chromatin binding assay with a fixed concentration of the drugs (Figure 3B). To avoid any possible interference with sperm chromatin decondensation, the drugs were added 5 min after the beginning of the reaction and before licensing. In this assay, we observed that NSC-95397 was able to potently suppress MCM2-7 loading onto chromatin while aminacrine and ethacridine inhibited MCM loading to a minor extent (Figure 3B). Moreover, compounds such as valsartan and DDP_28574 showed limited effectiveness in preventing MCM loading (Figure 3B).
Figure 3.
Validation of the identified compounds
(A) Chemical structure of the 5 hits identified in the compound screening: NSC-95397, DDP_28574, valsartan, aminacrine, and ethacridine.
(B) Western blot analysis of the indicated proteins bound to chromatin isolated from sperm nuclei incubated with DMSO, used as Control, Geminin, or 200 μM NSC-95397, DDP_28574, valsartan, aminacrine, ethacridine for 30 min.
(C) Autoradiography of a DNA replication assay showing the time course of α32P-dCTP incorporation in sperm nuclei incubated in egg extract treated with DMSO or with increasing concentrations of NSC-95397 (50-100-150-200 μM). Samples were taken at the indicated times after nuclei and α32P-dCTP addition to egg extracts. Optical density (OD) for each lane is indicated.
(D) Representative images of sperm nuclei incubated in interphase extract supplemented with Cy3-dCTP (red) for 30 min (Upper panel) or 90 min (Lower panel) and treated with DMSO, used as control, or 200 μM NSC-95397. Samples were fixed and stained with 4,6-diamidino-2-phenylindole (DAPI) for DNA (blue) and DiOC6 (green) for membranes. Scale bar = 10 μm.
See also Figure S2.
Table 1.
List of hits identified from the compounds screening
| Compound name | IUPAC name |
|---|---|
| NSC-95397 | 2,3-bis(2-hydroxyethylsulfanyl) naphthalene-1,4-dione |
| DDP_28574 | [4-(3,5-dimethylphenyl)tetrahydropyran-4-yl]-[3-(pyrazolo[1,5-a]pyridin-4-ylmethyl)-8-azabicyclo[3.2.1]octan-8-yl]methanone |
| Valsartan | (2S)-3-methyl-2-[pentanoyl-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]amino]butanoic acid |
| Aminacrine | acridin-9-amine |
| Ethacridine | 7-ethoxyacridine-3,9-diamine |
See also Figure 3A.
To exclude that the observed effect on MCM loading was due to the direct binding to bDNA, we conducted a fluorescent intercalator displacement assay with NSC-95397, aminacrine, and ethacridine (Figure S1D). In this assay, SYBR green binding to bDNA was used as a fluorescent indicator, while bDNA was used as DNA template. Doxorubicin, a well-known DNA intercalator,17 was used as a positive control. The displacement of the SYBR green determined a strong decrease in the fluorescent signal readout compared to DMSO. Similarly, aminacrine and ethacridine binding to bDNA displaced SYBR green determining a clear decrease of the fluorescent signal at the dose of 100 μM (Figure S1D). We concluded that aminacrine and ethacridine disrupt MCM loading because of their DNA intercalator properties. Instead, NSC-95397 did not cause a major displacement of SYBR. These results indicate that the effects observed with NSC-95397 on MCM loading are not a consequence of its direct binding to bDNA.
In agreement with the inhibition of MCM2-7 chromatin loading, NSC-95397 affected the binding of DNA polymerases α alpha and δ delta, and the Psf3 component of the GINS complex onto DNA. This impairment of replication machinery assembly is likely a consequence of the absence of MCM2-7 assembly (Figure 3B). Importantly, we noticed that NSC-95397 also prevented the binding of CDC6, a critical component of the licensing reaction, while it did not affect ORC1-6 binding to DNA (Figure 3B). These results collectively indicate that NSC-95397 disrupt the chromatin loading of essential replication proteins such as MCM2-7 and CDC6 while sparing the binding of ORC1-6 to DNA.
In line with these results, α32P-dCTP incorporation in nascent DNA measured through standard DNA replication time course was reduced by NSC-95397 in a dose-dependent manner (Figure 3C). DNA replication inhibitory concentrations of NSC-95397 were also able to inhibit MCM2-7 chromatin loading (Figure S2A).
We then monitored fluorescently labeled dNTP incorporation in intact nuclei and found that in NSC-95397-treated extracts, the incorporation of Cy3-dCTP into nascent DNA showed a significant reduction compared to the DMSO-treated control extracts (Figures 3D and S2B), further confirming that NSC-95397 treatment inhibits chromosomal DNA replication in a dose-dependent manner.
These results collectively provided the first insights into the mechanism of action of NSC-95397, demonstrating its early interference with CDC6 binding and its inhibitory effect on DNA replication in Xenopus egg extracts.
NSC-95397 targeting of CDC6
Having excluded a direct DNA binding effect we performed a time course experiment to elucidate the impact of NSC-95397 on the licensing reaction dynamics and DNA replication (Figure 4A). We found that NSC-95397 prevents CDC6 chromatin loading after only 1 min from the addition of sperm nuclei to egg extract (Figure 4A). We also conducted a comparative analysis between NSC-95397 and the already established RL5a, which is known to prevent origin assembly.16 We found that, in contrast to NSC-95397, RL5a suppressed MCM loading without impacting CDC6, in agreement with previous findings16 (Figure 4A).
Figure 4.
A direct effect of NSC-95397 on CDC6
(A) Western blot analysis of the indicated proteins bound to chromatin isolated at the indicated time (1, 5, 15, 30 min) after sperm nuclei addition in egg extract incubated with DMSO, 200 μM NSC-95397, and 200 μM RL5a. No DNA indicates the absence of nuclei.
(B) Western blot analysis of the indicated proteins incubated for 5 min at 23°C with 300 μM H2O2, 500 μM roscovitine, 200 μM NSC-95397, or 200 μM MG-132 and then kept on ice.
(C) Western blot analysis of the indicated proteins bound to chromatin isolated from sperm nuclei incubated for 30 min with DMSO, used as Control, or 200 μM NSC-95397 analogs (IFM_47059, IFM_47060, IFM_47061, IFM_47062, IFM_47063, IFM_47064, IFM_47065).
(D) ITC analysis of NSC-95397 binding to recombinant Xenopus laevis CDC6.
See also Figure S3.
(E) Representative images of sperm nuclei incubated in interphase extract supplemented with Cy3-dCTP (red) for 90 min with DMSO, used as control, or 100 μM NSC-95397, in the presence of increasing concentrations of recombinant CDC6 protein (0, 80, 160, 320 nM). Samples were fixed and stained with 4,6-diamidino-2-phenylindole (DAPI) for DNA (blue) and DiOC6 (green) for membranes. Scale bar = 10 μm.
See also Figure S4.
(F) The Cy3-dCTP incorporation of 45 nuclei was quantified as fluorescence-integrated intensity per nucleus using ImageJ. Data are represented as scatter dot plots. Horizontal bars indicate mean ± SEM. A two-way ANOVA test was used to perform multiple comparisons between DMSO and NSC-treated samples with 0 nM of rCDC6 and between NSC-treated samples with 0 nM of rCDC6 and increasing concentrations of recombinant protein added. ∗∗∗∗ = p < 0.0001, ns, not significant.
To dissect the molecular mechanism underlying the inhibitory activity of NSC-95397, we initially assessed whether it directly impacted replication protein levels in egg extract under the same conditions employed in our screening. To this end, we incubated interphase egg extract with NSC-95397 for 5 min at room temperature and performed a western blot analysis of the major replication proteins. Surprisingly, we observed a rapid decrease in the total levels of CDC6 in egg extracts treated with NSC-95397. In contrast, the total levels of other DNA binding proteins such as ORC1, MCM7, polymerase α and δ, and the DNA repair protein RAD51 were not affected by the treatment (Figure 4B).
As NSC-95397 is a quinone compound potentially able to induce toxicity by inducing oxidative stress, we tested whether the levels in our experimental settings could be affected by the action of a standard reactive oxygen species (ROS) inducer. To explore this, we exposed the egg extract to 300 μM H2O2 for 5 min. Notably, this treatment did not affect the CDC6 protein levels, suggesting that the NSC-95397 activity on CDC6 is not mediated by ROS (Figure 4B).
To verify whether CDC6 degradation was dependent on proteasomal-mediated processing, we also inhibited proteasome activity using 200 μM MG-132. As shown in Figure 4B, the treatment of extract with proteasome inhibitor MG-132, either alone or in combination with NSC-95397, failed to increase CDC6 levels. This experiment revealed that NSC-95397 induces CDC6 degradation despite the treatment with proteasome inhibitor MG-132. Thus, the decline in levels of CDC6 induced by NSC-95397 is proteasome-independent and might rely on other proteases degrading unfolded intracellular proteins.18
We further tested whether CDC6 degradation upon NSC-95397 is mediated by Cyclin-dependent kinases (CDKs). However, the addition of a CDK inhibitor such as roscovitine did not result in any observable effects on the rate of CDC6 degradation (Figure 4B). This suggests that the degradation of CDC6 triggered by NSC-95397 treatment occurs through a CDK-independent pathway.
To test whether the other quinone compounds were able to destabilize CDC6, we examined the effects of several commercial analogs (Figure S4A; Table 2) on the CDC6 protein levels in interphase egg extract (Figure 4C). We found that only a close structural analog of NSC-95397 IFM_47060 showed some CDC6 protein destabilization (Figure 4C).
Table 2.
List of the analog compounds of NSC-95397
| ID identification | IUPAC name |
|---|---|
| NSC-95397 | 2,3-bis(2-hydroxyethylsulfanyl) naphthalene-1,4-dione |
| IFM_47059 | 2,3-bis(butylsulfanyl)naphthoquinone |
| IFM_47060 | 2,3-bis(methylsulfanyl)naphthoquinone |
| IFM_47061 | 3-(3,4-dimethoxyphenyl)-1-ethyl-6-methylpyrimido[5,4-e][1,2,4]triazine-5,7(1H,6H)-dione |
| IFM_47062 | 2-((2-methoxyethyl)thio)-3-methylnaphthalene-1,4-dione |
| IFM_47063 | 2-methyl-3-(methylsulfanyl)naphthoquinone |
| IFM_47064 | 3-(4-fluorophenyi)-1,6-dimethylpyrimido[5,4-e][1,2,4]triazine-5,7(1H,6H)-dione |
| IFM_47065 | 2-(2-Hydroxyethylthio)-3-methylnaphthalene-1,4-dione |
To assess whether CDC6 destabilization was due to the direct interaction of NSC-95397 with CDC6, we expressed Xenopus laevis recombinant CDC6 protein (rCDC6) (Figures S3A and S3B) and performed isothermal titration calorimetry (ITC) experiments.19 The results of ITC shown in the graph on the left of Figure 4D display the differential power (DP) in microcalories per second plotted against time in minutes, with characteristic exothermic peaks indicating binding events.19 The panel on the right instead shows the integrated heat data (ΔH in kilocalories per mole) plotted against the molar ratio of NSC-95397 to CDC6, with the data points fitting a sigmoidal curve typical of a binding isotherm. The experiment was conducted at a fixed CDC6 concentration (74 μM) with titrations of NSC-95397 up to 1 mM. The fit parameters suggest a single set of binding sites with a dissociation constant (KD) in the nanomolar range (323 ± 269 nM), indicating a strong interaction, and a negative enthalpy (ΔH) change (−46 ± 3.78 kcal/mol) consistent with an energetically favorable binding process. These results indicate that NSC-95397 directly interacts with CDC6 protein in vitro possibly causing its destabilization and degradation in vivo through non-proteasome-dependent chaperone-controlled pathways.18
To confirm that CDC6 is a major target of NSC-95397, we performed a rescue experiment by adding increasing amounts of the rCDC6 protein to overcome the effect of the NSC-95397 and examined the total DNA replication after 90 min of incubation of sperm nuclei with DMSO or with the drug. We observed an NSC-95397-dependent impairment of fluorescent Cy3-dCTP incorporation in intact nuclei that was significantly prevented by increasing concentrations of rCDC6 (Figures 4E and 4F). Similar results were obtained by monitoring the α32P-dCTP incorporation in replicating DNA (Figure S4B). The partial rescue observed in both assays may be possibly attributed to NSC-95397 potentially affecting additional targets involved in DNA replication besides CDC6.
Importantly, consistent with NSC-95397 mediated targeting of CDC6, which acts early in the replication origin assembly reaction, NSC-95397 added to interphase egg extracts 20 min after sperm nuclei, when the replication origins had already formed, was unable to inhibit DNA replication (Figures S4C and S4D). These results confirmed that NSC-95397 inhibits the early step of the assembly of DNA replication origins mediated by CDC6.
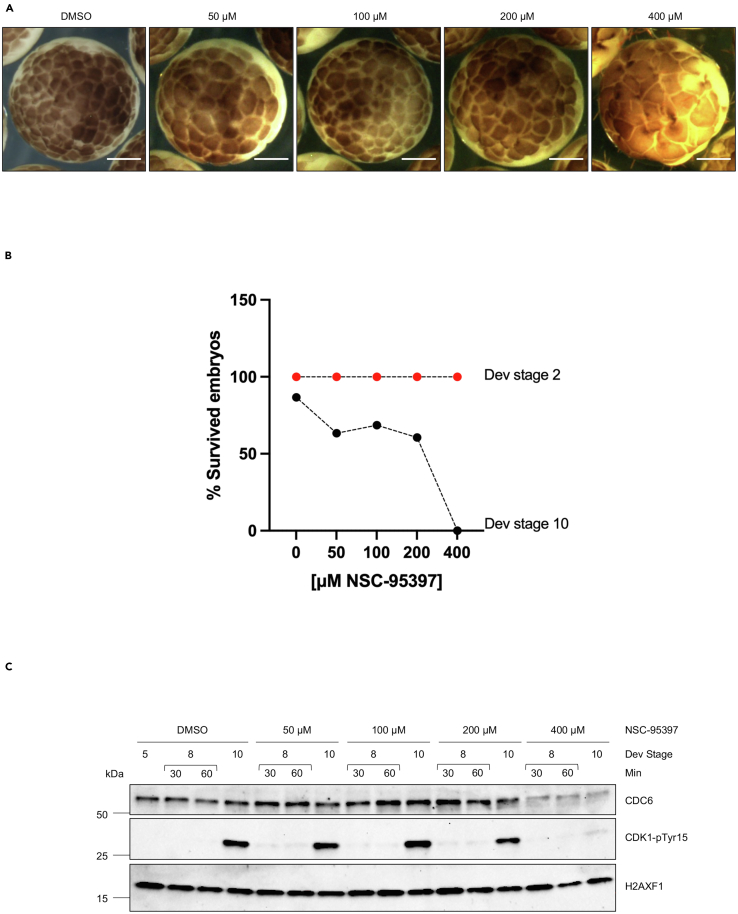
Effects of NSC-95397 on Xenopus development
To validate the effects of NSC-95397 on the cell cycle in vivo we investigated its effects on developing embryos following Xenopus laevis eggs fertilization. Embryos reaching the two-cell stage were arrayed into 6-well plates (100 eggs per well) and exposed to increasing concentrations of NSC-95397 (50–400 μM) and the appropriate control (DMSO) (Figure 5A) placed in a developing buffer. The embryos were left to develop until stage 10, which is reached after few cell divisions.9 We then analyzed the survival rate and the expression levels of CDC6 protein of the developing embryos (Figure 5). NSC-95397 did not show apparent toxicity below 200 μM (Figure 5A). Above this concentration, the compound altered the cleavage of the eggs and the survival ratio. The embryos grown in the presence of NSC-95397 showed a significant dose-dependent increase in mortality (Figure 5B). As shown in Figure 5C, WB analysis indicated that NSC-95397 at high doses (400 μM) induced a significant downregulation of CDC6 correlating with a decrease in the Cdk1 Tyr15 phosphorylation (CDK1-pTyr15) normally associated to active S-phase.20 These findings underscore the dose-dependent impact of NSC-95397 on both embryonic survival and CDC6 protein levels, suggesting its potential as a regulator of DNA replication and cell cycle progression processes during Xenopus laevis development. NSC-95397 has been reported to inhibit Cdk1 Tyr-15 phosphatase CDC25.14 Although we cannot completely exclude an effect on CDC25, we did not observe increased Cdk1 Tyr-15 phosphorylation induced at the midblastula transition9 as would be expected by the inhibition of CDC25, which normally removes Tyr-15 phosphorylation. These results suggest that the action of NSC-95397 on developing Xenopus embryos is mostly independent of its known effect on CDC25 (Figure 5C).
Figure 5.
Effect of NSC-95397 during early development
(A) Xenopus eggs were subjected to in vitro fertilization (IVF) and then incubated in the absence (DMSO) or in the presence of NSC-95397 (50 μM, 100 μM, 200 μM or 400 μM), and the survival rate was assessed at 8 h after IVF. Scale bar = 500 μm.
(B) The percentage of survived embryos at developmental stage 10 was normalized to the total number of eggs that underwent IVF.
(C) Western blot of whole embryos incubated in the absence (DMSO) or in the presence of NSC-95397 (50 μM, 100 μM, 200 μM or 400 μM) and taken at the indicated developmental stages. Stage 8 was sampled every 30 min.
Effects of NSC-95397 on cells and embryos with increased DNA replication origin assembly
To be useful a drug interfering with DNA replication should be able to preferentially target cancer cells, which tend to have higher levels of replication origins assembled onto chromatin.21 We hypothesized that higher levels of replication origins confer intrinsic vulnerability to drugs interfering with DNA replication. To reproduce this condition in a controlled fashion we set up assays based on Xenopus laevis embryos and human cells overexpressing the chromatin factor SSRP1. SSRP1 is highly expressed in cancer and potently enhances replication origin assembly as we have previously shown.9 Using these systems we tested whether low doses of NSC-95397 confer an intrinsic vulnerability to cells addicted to higher levels of replication origin assembly. Consistent with this hypothesis, we observed that the low doses of NSC-95397 tolerated in control embryos were severely cytotoxic in SSRP1-injected embryos, resulting in early embryonic lethality within 8 h of treatment (Figures 6A and 6B). The phenotypes observed were highly reproducible as all SSRP1-injected embryos treated with the NSC-95397 showed the same phenotype involving early embryonic death.
Figure 6.
Effects of NSC-95397 in SSRP1 overexpression conditions
(A) Images of embryos injected with water (control, first row) or SSRP1 mRNA (second row) and then incubated in the absence (DMSO) or in the presence of 200 μM NSC-95397. Scale bar = 500 μm.
See also Figure S5.
(B) The percentage of survived embryos at developmental stage 10 was normalized to the total number of eggs that underwent IVF. The results from independent experiments are expressed as the raw percentage of embryos at each developmental stage. Horizontal bars indicate the median with range. One-way ANOVA test was used to analyze differences among multiple groups compared to the control (DMSO). ∗∗ = p < 0.0097, ns = not significant.
(C) Western blot of whole embryos injected with water, or Myc-SSRP1 mRNA, treated without (DMSO) or with 200 μM NSC-95397 and taken at the indicated developmental stages.
WB analysis indicated that low doses of NSC-95397, which were tolerated by normal embryos (Figures 5A–5C), induced a significant downregulation of CDC6 protein levels correlating with a decrease in the Cdk1 Tyr-15 phosphorylation normally associated with the onset of midblastula S-phase in SSRP1 injected embryos (Figure 6C). These findings confirmed selective cytotoxic effects of NSC-95397 in cells with high levels of replication origins.
We next asked whether the in vivo activities of the compound NSC-95397 extended also to human cells. To investigate this aspect, we used immortalized, non-tumorigenic MCF10A and hTERT-RPE1 cells to construct SSRP1 inducible expression model systems (Figures S5A and S5B). We conducted cell proliferation assays on MCF10A-SSRP1 and hTERT-RPE1-SSRP1 human cells following exposure to different concentrations of NSC-95397 for 48 h (Figures S5C and S5D). Consistent with the embryo results, we observed a significant level of cytotoxicity induced by NSC-95397 (Figures S5C and S5D), enhanced in cells overexpressing SSRP1 as shown by the evaluation of the relative IC50 (Figures S5E and S5F). Similar findings were obtained in HCT116, SW620, and MCF7 cancer cells selected among lines naturally expressing high levels of SSRP1 (Figures S5G and S5H). These results suggest that NSC-95397 induces increased lethality in cells with increased levels of SSRP1 and, possibly, high levels of DNA replication origins assembled onto chromatin. These observations highlight the importance of targeting DNA replication origins assembly processes as an anti-proliferative strategy.
Discussion
DNA replication is tightly controlled by a process known as origin licensing which ensures the proper duplication of the genome during cell cycle progression. Aberrant expression of proteins involved in the licensing process is associated with replication stress, leading to under- or re-replicated DNA, thus resulting in the occurrence of genomic instability typical of cancer cells.22,23,24,25,26 Indeed, some studies have shown that neoplastic tumor growths often exhibit an increased expression of replication licensing factor CDC6, responsible for the loading of MCM proteins onto replication origins.27,28,29,30 In addition, CDC6 overexpression has been shown to promote aggressiveness in cervical,28 bladder,27 and brain tumors.31
Deregulated levels of MCM2-7 complex subunits are also closely associated with cancer.32,33,34 In cancer cells, the excess of MCMs on chromatin serves as a crucial survival mechanism by activating dormant origins as backups during DNA replication, particularly in the presence of replicative stress.35,36 Conversely, a reduction or deficiency in MCMs to a limited extent, does not impair DNA replication.24,37,38 However, in the presence of replicative stress lack of sufficient amount of replication origins assembled onto chromatin challenges the completion of DNA replication, giving rise to genomic instability.35,36 Therefore, cancer cells become addicted to high levels of MCMs and other licensing factors. Such addiction generates a vulnerability that could be selectively exploited to kill cells with high levels of replication origins typically associated with high levels of MCM and CDC6 protein expression.
Here, we identified small molecules with the ability to interfere with the binding of the MCM2-7 complex to DNA, a crucial step in DNA replication origin assembly. In particular, we discovered that a compound, known as NSC-95397, a quinone-based small molecule, directly targets CDC6 protein inducing its destabilization, and preventing MCM complex binding to DNA.
NSC-95397-mediated decreased levels of CDC6 in egg extracts are independent of proteasomal degradation and are not affected by the activity of CDKs. This suggests a unique mechanism of CDC6 degradation induced by NSC-95397.
NSC-95397 was first shown to have anti-proliferative potential in several cancers39,40 by promoting inhibition of CDC25 dual-specificity phosphatases.14 Here we show that NSC-95397 also inhibits replication origin assembly, triggering cytotoxicity without increasing the levels of CDK1 phosphorylation as expected by an inhibitory effect on CDC25 activity. Any possible relation between CDC6 destabilization and CDC25 activity regulation by NSC-95397 requires further work to be better understood.
To validate the effects of NSC-95397 in vivo we set up an assay based on the overexpression of SSRP1, a subunit of the FACT complex, which we have previously shown to remove histone H1 from chromatin promoting increased ORC- and MCM-dependent replication origin assembly.9 SSRP1 is often overexpressed in several tumors and this correlates with increased replication origin activity. Accordingly, SSRP1 accelerates DNA replication, cell cycle, and embryo growth, rendering the cells addicted to elevated levels of MCMs assembled onto the chromatin.9,41 Consistent with this, NSC-95397 specifically killed embryos expressing high levels of SSRP1 possibly by targeting cells addicted to high levels of the MCMs loaded on chromatin. This lethality suggests the potential of NSC-95397 in SSRP1 overexpressing tumors and in general in cells that have increased replication origins.
In agreement with the embryo results, we found reduced viability of immortalized and transformed cancer cells overexpressing SSRP1 after the treatment with NSC-95397. Collectively, these results suggest that NSC-95397-mediated targeting of replication origin assembly through the destabilization of CDC6 could be exploited as a powerful strategy to selectively kill highly proliferative cancer cells. The discovery that inhibitors of DNA replication origins assembly induce growth arrest and eventually death of hyperproliferating cells offers promising opportunities for targeted therapies.
Limitations of the study
The mechanism that leads to proteasome-independent Cdc6 degradation induced by NSC-95397 remains to be identified.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-CDC45 (Rabbit polyclonal) | Aze et al.42 | N/A |
| Anti-ORC1 (Mouse monoclonal) | Aze et al.42 | N/A |
| Anti-CDK1 (Mouse monoclonal) [A17] | Falbo et al.9 | N/A |
| Anti-phospho-Cdk1 (Thr14, Tyr15) (Mouse monoclonal) [CP3.2] | Falbo et al.9 | N/A |
| Anti-MCM7 (Mouse monoclonal) | Santa Cruz Biotechnology | Cat#sc-9966; RRID: AB_627235 |
| Anti-CDT1 (Mouse monoclonal) | Santa Cruz Biotechnology | Cat#sc-365305; RRID: AB_10847805 |
| Anti-PSF3 (Rabbit polyclonal) | Hashimoto et al.43 | N/A |
| Anti-SSRP1 (Mouse monoclonal) | Abcam | Cat#Ab26212; RRID: AB_449020 |
| Anti-CDC6 (Rabbit polyclonal) | Santa Cruz Biotechnology | Cat#sc-8341; RRID: AB_638342 |
| Anti-Xenopus POL α p180 (Mouse monoclonal) peptide antigen used: VKRLPAVTKPGH | Kolinjivadi et al.44 | Abmart: clone 13026-1-3/C199 |
| Anti-Xenopus POL δ 125 kDa (Mouse monoclonal) peptide antigen used: SSQTKKLRGDWDDD | Kolinjivadi et al.44 | Abmart: clone 19570-1-1/C316 |
| Anti-Histone H2B (Rabbit polyclonal) | Millipore | Cat#07–371; RRID: AB_310561 |
| Anti-H3 (Rabbit polyclonal) | Abcam | Cat#Ab1791; RRID: AB_302613 |
| Anti-RAD51 (Mouse monoclonal) [14B4] | Abcam | Cat#ab213; RRID: AB_302856 |
| HRP anti-Myc tag antibody [9E10] | Abcam | Cat#ab62928; RRID: AB_955371 |
| Biological samples | ||
| Xenopus egg extract | This manuscript | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Bovine Serum Albumin | Sigma-Aldrich | Cat#A7030; CAS 9048-46-8 |
| Calcium ionophore | Sigma-Aldrich | Cat#A23187; CAS 52665-69-7 |
| Chorionic gonadotropin | Sigma-Aldrich | Cat#CG10; CAS 9002-61-3 |
| Creatine phosphate | Roche | Cat#10621714001; CAS 71519-72-7 |
| Creatine phosphokinase | Sigma-Aldrich | Cat#C3755; CAS 9001-15-4 |
| Cy3-dCTP | Amersham Biosciences | Cat#PA53021 |
| Cycloheximide | Calbiochem | Cat#239763; CAS66-81-9 |
| Cytochalasin B | Sigma-Aldrich | Cat#C6762; CAS 14930-96-2 |
| Ficoll® PM-400 | Sigma-Aldrich | Cat#F4375; CAS 26873-85-8 |
| Geminin | Aze et al.42 | N/A |
| Glutathione Sepharose™ 4 Fast Flow | Cytiva | Cat#17-5132-01 |
| L-Cysteine | Sigma-Aldrich | Cat#30089; CAS 52-90-4 |
| Leupeptin | Sigma-Aldrich | Cat#L2023; CAS 147385-61-3 |
| Lysolecithin | Sigma-Aldrich | Cat#L4129; CAS 9008-30-4 |
| NSC-95397 | Calbiochem | Cat#217694; CAS 93718-83-3 |
| 2,3-Bis(n-butylthio)-1,4-naphthalenedione | Matrix Scientific | Cat#016562; CAS 671189-54-1 |
| 2,3-Bis(methylthio)-1,4-naphthalenedione | Matrix Scientific | Cat#016561; CAS 55699-85-9 |
| 3-(3,4-dimethoxyphenyl)-1-ethyl-6-methylpyrimido[5,4-e][1,2,4]triazine-5,7(1H,6H)-dione | Vitas-M Lab | Cat#STK249723 |
| 3-(4-fluorophenyl)-1,6-dimethylpyrimido[5,4-e][1,2,4]triazine-5,7(1H,6H)-dione | Vitas-M Lab | Cat#STK235850 |
| 2-[(2-Methoxy)ethylthio]-3-methyl-1,4-naphthoquinone | Sigma-Aldrich | Cat# SML0367; CAS 255906-59-3 |
| 2-(2-Hydroxyethylthio)-3-methylnaphthalene-1,4-dione | Advanced ChemBlocks Inc. | Cat# O32425; CAS 59147-84-1 |
| Protease inhibitor Set III | Calbiochem | Cat#539134 |
| RL5a | Sigma-Aldrich | Cat#SML2187; CAS 133671-66-6 |
| Spermidine | Sigma-Aldrich | Cat#S0266; CAS 124-20-9 |
| Spermine | Sigma-Aldrich | Cat#S4264; CAS 71-44-3 |
| Sucrose | Sigma-Aldrich | Cat#S0389; CAS 57-50-1 |
| Resource S 6 mL column | Cytiva | Cat#17-1180-01 |
| Superdex® 200 10/300 GL | Cytiva | Cat#17-5175-01 |
| MG-132 | Calbiochem | Cat#474790; CAS 133407-82-6 |
| Roscovitine | Calbiochem | Cat#557360; CAS 186692-46-6 |
| Critical commercial assays | ||
| Amicon® Ultra Centrifugal Filter, 50 kDa MWCO | Millipore | Cat#UFC8050 |
| Bio-Rad Protein Assay | BioRad | Cat#5000006 |
| Gateway Gene Cloning System | ThermoFisher Scientific | Cat#12535-029 |
| Lipofectamine™ 2000 Transfection Reagent | ThermoFisher Scientific | Cat#11668019 |
| mMessage mMachine kit | ThermoFisher Scientific | Cat#AM1340 |
| Nunc™ 384-well polypropylene plates | ThermoFisher Scientific | Cat#4312 |
| Pierce™ 384-well Streptavidin-coated plates | ThermoFisher Scientific | Cat#15505 |
| SuperSignal ELISA Pico | ThermoFisher Scientific | Cat#37069 |
| WesternBright ECL | Advansta | Cat#K-12045-D50 |
| Wizard® SV Gel and PCR Clean-Up System | Promega | Cat#A9281 |
| Deposited data | ||
| Original image, quantifications and datasets | Mendeley and OSF | Mendeley Data: https://doi.org/10.17632/v75czchpjm.1 OSF: https://osf.io/undvk/?view_only=804a7de818c845a79e2fa6f15295c57b |
| Experimental models: cell lines | ||
| HEK293T | ICLC | Cat#HTL04001; RRID: CVCL_0063 |
| hTERT-RPE1 | ATCC | Cat#CRL-4000™; RRID: CVCL_4388 |
| hTERT-RPE1 6xMyc_SSRP1 | This manuscript | N/A |
| hTERT-RPE1 Empty vector | This manuscript | N/A |
| MCF10A | ATCC | Cat#CRL-10317™; RRID: CVCL_0598 |
| MCF10A 6xMyc_SSRP1 | This manuscript | N/A |
| MCF10A Empty vector | This manuscript | N/A |
| Experimental models: organisms/strains | ||
| Rosetta™ (DE3) Competent Cells | Novagen | Cat#70954 |
| Xenopus laevis females | Nasco | Cat#LM00535MX |
| Xenopus laevis males | Nasco | Cat#LM00715MX |
| Oligonucleotides | ||
| attb1_6M_for: 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTACGCG TGCAGGATCCCATCGATTTAAAGCTACCATG-3′ |
This manuscript | N/A |
| attb1_6M_rev: 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTACCG GTCCTCCCACACCTCCCCCTG-3′ |
This manuscript | N/A |
| pBS_NEST_For: 5′-GTGCACGAGTGGGTTACATC-3′ | This manuscript | N/A |
| pBS_NEST_Rev: 5′-CTCTTCGCTATTACGCCAGC-3′ | This manuscript | N/A |
| pBS_b_For1: 5’-[BtnTg]CACAACATACGAGCCGGAAG-3 | This manuscript | N/A |
| pBS_Rev1: 5′-GTGCACGAGTGGGTTACATC-3′ | This manuscript | N/A |
| Recombinant DNA | ||
| Glutathion-S-Transferase (GST)-tagged Xenopus Cdc6 plasmid | Furstenthal et al.43 | N/A |
| pBlueScript KS(−) | Agilent | Cat#212208 |
| pGEX-6P-1 | Cytiva | Cat#28-9546-48 |
| pDONR™221 | ThermoFisher Scientific | Cat#12536-017 |
| pCW57.1 | Addgene | Addgene #41393; RRID: Addgene_41393 |
| pCW57.1-6xMYC_SSRP1 | This manuscript | N/A |
| pCS2-6xMYC_SSRP1 | Falbo et al.9 | N/A |
| Software and algorithms | ||
| HiTSeekR online platform | List et al.45 | N/A |
| ImageJ | ImageJ | RRID: SCR_003070 |
| ImageQuant™ TL 10.2 analysis software | Cytiva | RRID: SCR_018374 |
| NIS-Elements software | Nikon | https://www.nikoninstruments.com/Products/Software; RRID: SCR_014329 |
| Prism (version 9) | GraphPad | https://www.graphpad.com; RRID: SCR_002798 |
| Other | ||
| Bio-Rad ChemiDoc™ XRS+ Imaging System | Bio-Rad | RRID: SCR_014210 |
| Bioruptor® | Diagenode | RRID: SCR_023470 |
| EnSpire® plate reader | PelkinElmer | Cat#2104-0020 |
| Hamilton Microlab STAR Liquid Handling robot | Hamilton | N/A |
| Typhoon biomolecular imager | Cytiva | Cat#29187193 |
| PEAQ-ITC MicroCalorimeter | Malvern Panalytical | RRID: SCR_023795 |
| Microinjector PicoSpritzer III | Parker | Cat#052-0500-900 |
| Micropipette Puller P-97 | Sutter Instrument | Cat#P-97; RRID: SCR_016842 |
| Nikon Ds-Fi1-U2 color camera | Nikon | N/A |
| Nikon SMZ1500 Stereomicroscope | Nikon | N/A |
| Olympus BX61 Upright fluorescence microscope | Olympus | SM100211-MG1XY |
| TLA100.3 fixed-angle rotor | Beckman Optima | Cat#349490 |
| VICTOR3™ V 1420 Multilabel plate reader | Perkin Elmer Wallac | Cat#1420-051 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed and will be fulfilled by the lead contact, Prof. Vincenzo Costanzo (vincenzo.costanzo@ifom.eu).
Materials availability
Materials generated in this study will be made available upon request by the lead contact.
Data and code availability
-
•
Original unprocessed gels, western blot images, microscopy images, and datasets have been deposited on Mendeley Data and Open Science Framework and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
The study does not report any original code.
-
•
Any additional information related to the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Experimental model: Xenopus laevis
Eggs derived from Xenopus laevis frogs were used as an experimental model system. The collection of eggs from the female frogs was performed in a non-invasive way following chorionic gonadotropin (Sigma-Aldrich, Cat. No. CG10) injections. Occasional surgical procedures were performed on the male frogs to harvest sperm nuclei. Experimental protocols were approved by the IFOM Animal Welfare Committee and the Italian Ministry of Health. The number of animals used was kept to a minimum and was calculated taking into account the number of eggs required to obtain the cytoplasmic extract needed for the experiments described. The animals were kept in highly regulated and monitored conditions with room and water temperatures at 19°C. Basic husbandry requirements were provided by the IFOM Xenopus facility.
Experimental model: Cell lines
Human retinal epithelial hTERT-RPE1 cells were obtained from ATCC. hTERT-RPE1 cells were grown in Dulbecco’s Modified Eagle Medium/F12 (1:1) (DMEM/F12, Gibco) supplemented with 10% Tet-system approved FBS and 1% penicillin/streptomycin and maintained in 5% CO2 at 37°C. Human MCF10A cells were grown in Dulbecco’s Modified Eagle Medium/F12 (1:1) (DMEM/F12, Gibco) supplemented with 5% Horse serum, 10 μg/mL human insulin, 20 ng/mL human EGF, 500 ng/mL Hydrocortisone, 100 ng/mL cholera toxin, 1% penicillin/streptomycin and maintained in 5% CO2 at 37°C. Human MCF7, HCT116, and SW-620 cells were obtained from NCI. MCF7, HCT116, and SW620 cells were grown in RPMI 1640, 10% FBS, 5% L-Glutamine (L-Glu), and 1% penicillin/streptomycin and maintained in 5% CO2 at 37°C.
HEK293T cells were cultured in DMEM supplemented with 10% Tet-system approved FBS, 1% penicillin/streptomycin, and 5% L-Glutamine (L-Glu). The doxycycline-inducible cells were generated by lentiviral infection.
Method details
Preparation of egg extracts
Xenopus interphase egg extracts and sperm nuclei were prepared as described here and in previous work.10 Xenopus eggs were collected in 100 mM NaCl from chorionic gonadotropin-injected female frogs. The eggs were incubated in de-jelling buffer (10 mM Tris pH 8.5, 110 mM NaCl, 5 mM DTT), rinsed three times in MMR (100 mM HEPES-KOH, pH 7.5, 2 M NaCl, 10 mM KCl, 5 mM MgSO4, 10 mM CaCl2, 0.5 mM EDTA) and activated with 5 μM Calcium Ionophore (Sigma-Aldrich, Cat. No. C7522). The activated eggs were washed with MMR and then rinsed three times with an ice-cold S-buffer (50 mM HEPES-KOH pH 7.5, 50 mM KCl, 2.5 mM MgCl2, 250 mM sucrose) supplemented with 2 mM β-mercaptoethanol and 15 μg/mL leupeptin (Sigma-Aldrich, Cat. No. L2023). Eggs were packed by spinning to remove the excess buffer and then crushed at 13,000 rpm for 12 min at 4°C. The crude extract was collected, supplemented with cytochalasin B (40 μg/mL) (Sigma-Aldrich, Cat. No. C6762), and centrifuged at 70,000 rpm for 18 min at 4°C in a TLA100.3 rotor (Beckman Optima).
DNA templates
Demembranated sperm nuclei were prepared as described here and in previous work.10 X. laevis males were primed with 50U Folligon 7 days in advance and with 300U HCG the day before the sperm dissection. Dissected testis were collected in EB buffer (50 mM HEPES-KOH, pH 7.6, 50 mM KCl, 5 mM MgCl2, 5 mM EGTA, 2 mM β-mercaptoethanol). Testis were rinsed three times in ice-cold EB buffer and finely chopped with a razor blade. The material was then transferred to a 15 mL Falcon tube and spun for 5 min in a swinging-bucket rotor (JS13.1, Beckman) at 4,250 × g at 4°C. The pellet was then resuspended in 1.5 mL of SuNaSp buffer at room temperature [0.25 M sucrose (Sigma-Aldrich, Cat. No. S0389), 75 mM NaCl, 0.5 mM spermidine (Sigma-Aldrich, Cat. No. S0266), 0.15 mM spermine (Sigma-Aldrich, Cat. No. S4264)]. To remove membranes lysolecithin 2 mg/mL (Sigma-Aldrich, Cat. No. L4129) was added and incubated for 10 min at room temperature. The reaction was stopped by adding 3% BSA (Sigma-Aldrich, Cat. No. A7030). The pellet was resuspended again in 1 mL ice-cold EB and spun at 2,000 × g for 5 min at 4°C. The final pellet was resuspended in 400 μL of EB in 30% glycerol.
Chromatin isolation from egg extract
To isolate chromatin fractions, sperm DNA (3000 nuclei/μL) was added to 30 μL egg extracts and incubated at 23°C for the indicated time. Samples were diluted with 10 volumes of ice-cold EB (100 mM KCl, 2.5 mM MgCl2, and 50 mM HEPES-KOH pH 7.5) containing 0.25% NP-40 and centrifuged through a 0.5 M sucrose layer at 10.000 × g for 5 min at 4°C. Pellets were washed once with EB and suspended in SDS-PAGE sample buffer. Proteins were then resolved by SDS-PAGE and monitored by WB.
Replication reactions
DNA replication in Xenopus egg extract was performed as described here and in previous work.46 Sperm nuclei (3000 nuclei/μL) were added to 20 μL S-phase egg extract supplemented with 0.1 μL of α-32P-dCTP (250 mCi; 3000 Ci/mmol) and incubated at 23°C. At the indicated time points, reactions were stopped with Stop buffer (8 mM EDTA, 80 mM Tris pH 8.0, 1% w/v SDS), supplemented with 1 mg/mL Proteinase K, and incubated at 50°C for 2 h. Samples were treated with RNase (0.6 mg/mL) to degrade any trace of RNA and purified DNA was then separated from the unincorporated label by electrophoresis through a 0.8% agarose gel in TBE 1×. The gel was fixed in 30% TCA for 20 min, dried, and exposed for autoradiography.
For quantification of DNA replication, the gel was exposed to a phosphoscreen (Cytiva). The recorded radioactive signals were monitored within a phosphoImager (Typhoon) and measured with ImageQuant software.
Preparation of recombinant proteins
For in vitro studies, full-length Xenopus laevis CDC6 (rCDC6) was cloned in pGEX-6P-1 vector and GST-rXCDC6 was expressed in Rosetta (DE3) competent cells (Novagen) by growing transformed cells to OD600 0.7–0.8 at 37°C, then lowering the temperature to 18°C, and inducing protein expression overnight with 0.1 mM IPTG addition. Cells were subsequently harvested by centrifugation at 5.000 × g, 15 min, at 4°C, resuspended in ice-cold lysis buffer (PBS 1×, 100 mM KCl, 1 mM MgCl2, 1 mM DTT, 10% glycerol, 40 mL per liter of cell culture) supplemented with protease inhibitors Cocktail Set III (Calbiochem, Cat. No. 539134), and lysed by sonication. Lysates were centrifuged at 40.000 × g, 60 min, at 4°C, to eliminate cell debris, and cleared lysates were incubated for 1 h at 4°C with 4 mL slurry Glutathione Sepharose 4 Fast Flow resin (Cytiva) per liter of cell culture. Beads were harvested, and flow through was incubated a second time with other 4 mL of glutathione beads, for 1 h at 4°C. Beads from the two incubations were pulled and washed with lysis buffer, then HRV 3C protease was added to enable cleavage of GST from rCDC6 for calorimetric experiments. The reaction was carried out overnight at 4°C. Supernatant, containing cleaved rCDC6, was diluted 3 times with buffer A (10 mM NaH2PO4 K2HPO4, 2.7 mM KCl, 5% Glycerol, 1 mM DTT) and loaded on a Resource S 6 mL column (Cytiva) equilibrated in buffer A, using high salt buffer B (PBS 1×, NaCl 860 mM, 2.7 mM KCl, 5% Glycerol, 1 mM DTT) for protein elution. A 20-column volume, 8–30% gradient was applied; fractions containing rCDC6, as judged by SDS-PAGE analysis, were pooled, concentrated using a Vivaspin device (Sartorius), and loaded on a Superdex 200 10/300 column (Cytiva) equilibrated in SEC buffer (PBS 1×, 10% glycerol, 1 mM MgCl2, 1 mM DTT). Peak fractions from size exclusion chromatography were pooled, concentrated, and stored at −80°C after snap freezing in liquid nitrogen. Protein concentration was determined by absorbance at 280 nm using calculated extinction. The Xenopus CDC6 vector (Glutathion-S-Transferase (GST)-tagged Xenopus Cdc6) was a gift from M. Méchali47 (The Institute of Human Genetics, University of Montpellier).
Isothermal titration calorimetry
Experiments were done at 25°C using a PEAQ-ITC MicroCalorimeter (MicroCal, Malvern Instruments Ltd., Malvern, UK) following the general procedure.19 A 75 μM rCDC6 solution was titrated with NSC-95397 compound (1 mM) using injections of 2 μL. Protein and ligand were prepared and diluted to be in the same final buffer (SEC buffer with 1% DMSO). Calorimetric data were analyzed with the instrument software. Heat change peaks were integrated and normalized per mole of NSC-95397 injected. The ITC binding curve was fitted to the one binding site model equation.
Xenopus laevis embryos and microinjections
X. laevis embryos were obtained by in vitro fertilization of freshly laid X. laevis eggs with crushed X. laevis testes. Only batches with greater than 90% fertilization efficiency were used. Twenty minutes after fertilization, embryos were de-jellied in 2% cysteine (Sigma-Aldrich, Cat. No. 30089) pH 8.1 dissolved in 0.1xMBS. Microinjections were performed using calibrated needles and embryos equilibrated in 1xMBS/3% Nicoll PM-400 (Sigma-Aldrich, Cat. No. F4375). Microinjection needles were generated from borosilicate glass capillaries (Harvard Apparatus, GC 100F-15) using the micropipette puller Sutter p97. Maximally 4 nL of mRNA were injected into de-jellied embryos at the 1-cell stage using the microinjector PicoSpritzer III (Parker). After the first cleavage, the buffer was replaced with 1xMBS/2% Ficoll then 0.1xMBS, and embryos were allowed to develop to the desired stages. Embryos were staged according to Newport and Faber, 1975.
For in vitro transcription, DNA encoding Full-length Flag-SSRP1 1-709aa9 and X. laevis CDC6 (XlCDC6) were cloned between the FseI and AscI sites of pCS2 vector (pCS2-6xMYC_SSRP1, pCS2-6xMYC_xCDC6). To obtain sense RNA from these constructs, plasmids were linearized with NotI and KpnI, respectively. Following linearization, mRNA was expressed from the SP6 promoter using the mMessage mMachine kit (ThermoFisher Scientific, Cat. No. AM1340). All constructs were verified by DNA sequencing.
The pCS2 vector was a gift from Philip Zegerman (The Gurdon Institute, University of Cambridge).
Lentiviral vectors
The lentiviral doxycycline-inducible pCW57.1-6xMYC_SSRP1 plasmid was generated using Gateway Gene Cloning System (ThermoFisher Scientific), according to the manufacturer’s instructions. 6xMYC_SSRP1 insert was PCR amplified from pCS2-6xMYC_SSRP1 using the following primers:
attb1_6M_for:
5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTACGCGTGCAGGATCCCATCGATTTAAAGCTAC
CATG-3’;
attb1_6M_rev:
5′-GGGGACCACTTTGTACAAGAAAGCTGGGTACCGGTCCTCCCACACCTCCCCCTG-3’.
The PCR reaction was then used directly in the BP recombination reaction with the pDONR221 vector. Correct pDONR plasmid was used for the LR recombination reaction with pCW57.1 as the destination vector (Addgene, #41393). All constructs were verified by DNA sequencing.
Imaging of embryos
Embryo development was documented upon drug treatment using a Nikon SMZ1500 stereomicroscope coupled to a Nikon Ds-Fi1-U2 color camera at ×2 magnification with fiber optic illumination using NIS-Elements software. Embryos were imaged by placing them in a dish filled with water.
Protein analysis
Embryos lysates were prepared as described here and in previous work.9 Embryos were lysed in 50 μL/embryo lysis buffer (50 mM Tris pH 7.6, 150 mM NaCl, 10 mM EDTA, 1% Triton X-100) supplemented with Protease Inhibitor Cocktail Set III (Calbiochem, Cat. No. 539134), incubated on ice for 30 min, sonicated for 15 min on High setting 30s ON/OFF using Bioruptor Next Gen (Diagenode) in a water bath at 4°C, and centrifuged at max speed for 30 min at 4°C.
To prepare total cell extracts, cell pellets were washed twice in PBS and then lysed in RIPA buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% SDS, 0.5% NaDeoxycholate, 1% NP-40, 5 mM EDTA pH 8.0) supplemented with Protease Inhibitor Cocktail Set III, incubated on ice for 30 min, sonicated for 15 min on High setting 30s ON/OFF using Bioruptor Next Gen (Diagenode) in a water bath at 4°C, and centrifuged at max speed for 30 min at 4°C. Protein concentration was determined using Biorad protein assay according to the manufacturer’s instructions (Bio-Rad Laboratories).
Antibodies and western blot analysis
Samples were resolved by 4–20% SDS-PAGE and analyzed by standard WB techniques. Blots were probed using the following antibodies: rabbit polyclonal anti-Xenopus Cdc45 antibody, mouse monoclonal anti-Xenopus Orc1 antibody, mouse monoclonal anti-Cdk1 (A17), and mouse monoclonal anti-Cdk1 pTyr15 were obtained from J. Gannon (The Francis Crick Institute, London, UK) and rabbit polyclonal anti-Xenopus H2A.X-F1, obtained by D. Shechter, A. Einstein Institute, New York, NY, USA. Mouse monoclonal anti-MCM7 (Santa Cruz Biotechnology, sc-9966), anti-Cdt1 (F-6, Santa Cruz Biotechnology, sc-365305), rabbit polyclonal anti-Psf3,43 mouse monoclonal anti-Ssrp1 (10D7, Abcam, ab26212), rabbit polyclonal anti-Cdc6 (Santa Cruz Biotechnology, sc-8341), mouse monoclonal anti-Polα (p180), obtained from Abmart (clone 13026-1-3/C199), mouse monoclonal anti-Polδ, obtained from Abmart (clone 19570-1-1/C316),44 rabbit polyclonal anti-H2B (Millipore, 07–371), anti-H3 (Abcam, ab1791), mouse monoclonal anti-Rad51 (Abcam, ab213), and HRP anti-Myc tag antibody (9E10) (Abcam, ab62928). Detection with secondary antibodies was commonly carried out at 1:5000. Proteins were detected by WesternBright ECL (Advansta) on Bio-Rad ChemiDoc XRS+ or by iBright FL1500 Imaging system (ThermoFisher Scientific).
Materials Xenopus extract preparation for ELISA assay
Cycloheximide (Calbiochem, Cat. No. 239763) was dissolved in water at 10 mg/mL, creatine phosphate (Roche, Cat. No. 10621714001) was dissolved in water at 1 M. Creatine phosphokinase (Sigma-Aldrich, Cat. No. C3755) was dissolved in water at 10 mg/mL. RL5a (Sigma-Aldrich, Cat. No. SML2187) was dissolved in 100% DMSO at 20 mM. Recombinant geminin use was previously described.42
Small-molecule library
The compound library used for the screening contained 3828 small molecules. In detail, the library was assembled starting from collections of different vendors: FDA-approved and drug-like molecules were from Selleck Chemicals, Sigma-Aldrich, and MicroSource; kinase-targeted molecules were from Asinex, Enamine, BioFocus, ChemBridge, and Chem-X; targeting protein-protein interaction molecules were from Asinex. The screening run set was prepared by combining small molecules in groups of four, for a total of 957 multiplexed chemical entities. Equal volumes of 10 mM DMSO preparations were mixed (2.5 mM final concentration for each compound) and stored at 4°C in Nunc 384-well polypropylene plates (ThermoFisher Scientific, Cat. No. 4312).
High throughput assay and compound screen
A biotinylated DNA (bDNA, 1.8 kb) was generated using a nested PCR approach to conduct the ELISA-based screening. Briefly, the plasmid pBlueScript KS(−) was used as a template in the first round of amplification to generate a larger amplicon (2205 bp) using the following primers:
pBS_NEST_For: 5′- GTGCACGAGTGGGTTACATC -3’;
pBS_NEST_Rev: 5′- CTCTTCGCTATTACGCCAGC -3'.
This molecule served as a template in a subsequent series of amplifications to produce the bDNA used for the screening using the following primers:
pBS_b_For1: 5’-[BtnTg]CACAACATACGAGCCGGAAG-3’;
pBS_Rev1: 5′-GTGCACGAGTGGGTTACATC-3'.
PCR products were pooled and subjected to a purification and concentration step with the Wizard SV Gel and PCR Clean-Up System (Promega, Cat. No. A9281). bDNA was eluted in TE buffer (10 mM Tris-HCl pH 7.5, and 1 mM EDTA) and stored at −20°C.
For plate coating, bDNA was diluted to 20 ng/μL in TEN buffer (TE buffer, 1M NaCl), dispensed (50 μL/well) in Pierce 384-well Streptavidin-coated plates (ThermoFisher Scientific, Cat. No. 15505) and incubated for 2 h at room temperature. Wells were washed twice with TE buffer and filled with 50 μL of ice-cold TE. Plates were then sealed and stored at 4°C for a maximum of one week. On the day of the screening, 384-well bDNA coated plates were washed with modified ice-cold EB (mEB) buffer (10 mM HEPES, pH 7.5, 2.5 mM MgCl2, 50 mM KCl) and kept on ice until use.
To exclude any variability that could derive from different X. laevis preparations, snap-frozen aliquots of extracts were quickly thawed, pooled, and centrifuged at 3,000 × g for 1 h at 4°C. The supernatant was diluted 1:2 with ice-cold mEB buffer, supplemented with cycloheximide (100 μg/mL), creatine phosphate (30 mM), and creatine phosphokinase (150 μg/mL), and collected in a 50 mL Falcon tube.
For drug treatment, compounds were assayed at a higher concentration (187 μM) than in conventional biochemical or cellular screening due to the very high concentration of lipids and proteins (50 μg/μL) in X. laevis extracts. 40 μL of extracts were automatically dispensed into bDNA-coated 384-well plates using a Hamilton Microlab STAR Liquid Handling robot. 3 μL of multiplexed library compounds were added, mixed thoroughly, and incubated at 23°C on a thermomixer for 30 min. After incubation, plates were washed twice with ice-cold mEB buffer, and the DNA: protein complexes were crosslinked by incubating for 5 min on ice with mEB buffer containing 0.5% formaldehyde (50 μL). The amount of MCM-7 bound to biotinylated DNA was measured by ELISA. Solutions and washes were removed by manual aspiration, followed by gently tapping the plate, inverted, onto a paper towel. Briefly, plates were washed twice with TBS and blocked with 5% BSA-TBS-Tween20 0.05% (TBST) for 1 h at RT. The blocking solution was replaced with 50 μL of 5% BSA-TBST containing anti-MCM-7 antibody at dilution of 1:100 (Santa Cruz, Cat. No. sc-9966). Following incubation (2 h) and washes with TBST, HRP-labeled secondary antibody (1:3000) was incubated for 1 h at RT. The plates were then washed three times with TBST. 50 μL of a chemiluminescent substrate (SuperSignal ELISA Pico, ThermoFisher Scientific, Cat. No. 37069) were added to each well and the signal was acquired on an EnSpire plate reader (PerkinElmer).
Analysis of the raw data of primary screening (z-scores determination) was performed using the HiTSeekR online platform.45
Fluorescent intercalator displacement assay
To reach the final tested concentration, 3 μL of compounds were added to a 57 μL mixture of bDNA (100 ng) and SYBR green reagent dissolved in 10 mM Tris-HCl, 1 mM EDTA pH 8,0 for a total volume reaction of 60 μL. Fluorescence readings were taken on an Enspire Spectrophotometer (PerkinElmer). Five scans per sample were taken every 5 min for 20 min with an excitation wavelength of 485 nM and an emission wavelength of 528 nM. The level of fluorescence change (either enhancement or quenching) was determined and converted to a percent change in fluorescence using the formula (F1/F0) ∗100, where F0 is the initial fluorescence of dye bound to DNA before the addition of drug and F1 is the fluorescence after addition of drug.
NSC-95397 analogs
Analogs of NSC-95397 were bought from different vendors. In details, IFM_47059 (Cat. No. 016562) and IFM_47060 (Cat. No. 016561) were bought from Matrix Scientific, IFM_47061 (Cat. No. STK249723) and IFM_47064 (Cat. No. STK235850) were bought from VitasMlab, IFM_47062 was bought from Sigma Aldrich (Cat. No. SML0367), IFM_47063 was bought from AMS Private Supplier (Cat. No. A001210844) and IFM_47065 was bought from AChemBlock (Cat. No. O32425).
Chemical inhibition
Embryos at the two-cell stage were divided into a dish containing 5 mL control or treatment solutions and then incubated at 22°C and kept in the dark to prevent chemical degradation related to light exposure. The NSC-95397 was used at a concentration of 200 μM. Control embryos were treated with 0.1% DMSO. Work solutions of NSC-95397 were prepared by diluting the stock solution prepared in DMSO in 0.1 x MMR (All work solutions contained 0.1% DMSO).
Preparation of lentiviral supernatants and transduction of cells
Lentiviral particles containing pCW57.1-6xMYC_SSRP1 or the pCW57.1-Empty vector (EV) were generated in HEK293T cells using Lipofectamine 2000 Transfection Reagent (ThermoFisher Scientific). HEK293T cells were grown on 10-cm plates to 70–80% confluence and co-transfected with 10 μg of each lentiviral plasmid [pCW57.1 (Empty vector), pCW57.1_6xMYC_SSRP1], 4 μg VSV-G plasmid DNA and 10 μg packaging viral CMV-Δ8.9 plasmid. The viral supernatant was harvested at 48 h, and 72 h post-transfection, filtered through a 0.45 μm filter, and immediately used to infect recipient cells. A few days after infection, cells were selected with puromycin for 5 days.
Crystal Violet assay
Each cell line was seeded in 48-well plates at a density of 25 × 103 cells/well, and SSRP1 overexpression was induced with 1 μg/mL Doxycycline. After 24 h, cells were treated with increasing concentrations of NSC-95397 (0.25, 0.5, 1, 2.5, and 5 μM). DMSO solvent without compound (0 μM) served as a negative control. The plates were incubated for an additional 48 h at 37°C.
For IC50 experiments, each cell line was seeded in 96-well plates at a density of 2 × 103 cells/well, and SSRP1 overexpression was induced with 1 μg/mL Doxycycline. After 24 h, cells were treated with increasing concentrations of NSC-95397 (0.01, 0.025, 0.05, 0.1, 0.25, 0.5, 1, 2.5, 10 and 25 μM). DMSO solvent without compound (0 μM) served as a negative control. The plates were incubated for an additional 72 h at 37°C. On the day of staining, the culture medium was removed and the cells were rinsed once with PBS and stained with 1% Crystal Violet solution for 20 min. Excess dye was discarded by washing with water and cells were dried on filter paper. The cell-bound dye was dissolved in 10% glacial acetic acid for 30 min while shaking gently on a rocking shaker and the absorbance of the solubilized CV was measured at 570 nm with a VICTOR3 V 1420 Multilabel plate reader (PerkinElmer Wallac). All proliferation assays were performed in biological triplicates normalized to the untreated control (100%) and error bars represent the mean ± standard error of the mean (SEM).
Immunofluorescence microscopy
Sperm nuclei (3000/μL) incubated in X. laevis interphase egg extract were supplemented with Cy3-dCTP (Amersham Biosciences, Cat. No. PA53021) for the indicated times at 23°C with DMSO, used as a control, or 100-140-180-200 μM NSC-95397. Samples were fixed and mounted between a slide and a coverslip in fixing solution (45 mM PIPES at pH 7.2, 45 mM NaCl, 240 mM KCl, 10% formalin, 50% glycerol, 2 μg mL−1 Hoechst and 3,3-dihexyloxacarbocyanine (DioC6)). Fluorescence images were captured with fluorescence microscopy. Areas of Hoechst-stained nuclei were used to define the region of interest (ROI) using ImageJ. The Cy3-dCTP staining intensity of each ROI was measured and shown as the Integrated Density of selected nuclei.
Quantification and statistical analysis
All experiments, if not indicated otherwise in the figure legend, were performed three times and representative experiments are depicted. No statistical methods or criteria were used to estimate sample size or to include/exclude samples. Statistical analysis was performed with GraphPad PRISM software (version 9.3.0) and Microsoft Excel. The analysis of variance (ANOVA) tests were used to compare each of several groups’ means against a single control group and assess pairwise comparisons between group means. ∗∗∗∗ = p < 0.0001, ∗∗∗ = p < 0.001, ∗∗ = p < 0.01 and ns = non significant indicate significance. Image analysis was conducted using ImageJ. Statistical details for each experiment including sample size, significance p values, and test performed are indicated in figure legends and figures.
Acknowledgments
We thank members of Costanzo’s lab as well as of the Experimental Therapeutic Program for critical discussions and Ilaria Rancati of the IFOM cell facility for technical assistance with cell work. We are grateful to the Ravelli family for the generous donation supporting the Experimental Therapeutics Program activity. We are also grateful to AIRC for supporting the drug screening program. The research leading to these results has received funding from AIRC under IG 2023, ID 28725 project, the Armenise-Harvard Foundation career development, and an ERC grant (614541).
Author contributions
L.F., H.T., V.S., F.R., L.G.C., and F.P. performed all the experiments with Xenopus laevis and human cells. G.F. and M.R. performed the screening and analyzed the data. S.V. performed the FID assay. D.F. selected and assembled the compounds library. S.M., V.Ce., and S.P. performed and analyzed the experiments with recombinant CDC6 protein. M.V. provided critical discussion and analyzed the data. L.F., C.M., and V.C. analyzed the data, supervised the experiments, and wrote the manuscript. C.M. shares corresponding authorship with V.C. due to the essential drug screening contribution.
Declaration of interests
The authors declare no competing interests.
Published: July 22, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110567.
Contributor Information
Ciro Mercurio, Email: ciro.mercurio@ifom.eu.
Vincenzo Costanzo, Email: vincenzo.costanzo@ifom.eu.
Supplemental information
References
- 1.Yuan Z., Schneider S., Dodd T., Riera A., Bai L., Yan C., Magdalou I., Ivanov I., Stillman B., Li H., Speck C. Structural mechanism of helicase loading onto replication origin DNA by ORC-Cdc6. Proc. Natl. Acad. Sci. USA. 2020;117:17747–17756. doi: 10.1073/pnas.2006231117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGarry T.J., Kirschner M.W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 3.Li H., O'Donnell M.E. The Eukaryotic CMG Helicase at the Replication Fork: Emerging Architecture Reveals an Unexpected Mechanism. Bioessays. 2018;40 doi: 10.1002/bies.201700208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courtot L., Hoffmann J.S., Bergoglio V. The Protective Role of Dormant Origins in Response to Replicative Stress. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yekezare M., Gómez-González B., Diffley J.F.X. Controlling DNA replication origins in response to DNA damage - inhibit globally, activate locally. J. Cell Sci. 2013;126:1297–1306. doi: 10.1242/jcs.096701. [DOI] [PubMed] [Google Scholar]
- 6.Freeman A., Morris L.S., Mills A.D., Stoeber K., Laskey R.A., Williams G.H., Coleman N. Minichromosome maintenance proteins as biological markers of dysplasia and malignancy. Clin. Cancer Res. 1999;5:2121–2132. [PubMed] [Google Scholar]
- 7.Blow J.J., Laskey R.A. Xenopus cell-free extracts and their contribution to the study of DNA replication and other complex biological processes. Int. J. Dev. Biol. 2016;60:201–207. doi: 10.1387/ijdb.160142jb. [DOI] [PubMed] [Google Scholar]
- 8.Falbo L., Costanzo V. Epigenetic regulation of replication origin assembly: A role for histone H1 and chromatin remodeling factors. Bioessays. 2021;43 doi: 10.1002/bies.202000181. [DOI] [PubMed] [Google Scholar]
- 9.Falbo L., Raspelli E., Romeo F., Fiorani S., Pezzimenti F., Casagrande F., Costa I., Parazzoli D., Costanzo V. SSRP1-mediated histone H1 eviction promotes replication origin assembly and accelerated development. Nat. Commun. 2020;11:1345. doi: 10.1038/s41467-020-15180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sannino V., Pezzimenti F., Bertora S., Costanzo V. Xenopus laevis as Model System to Study DNA Damage Response and Replication Fork Stability. Methods Enzymol. 2017;591:211–232. doi: 10.1016/bs.mie.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Raspelli E., Falbo L., Costanzo V. Xenopus egg extract to study regulation of genome-wide and locus-specific DNA replication. Genesis. 2017;55 doi: 10.1002/dvg.22996. [DOI] [PubMed] [Google Scholar]
- 12.Sannino V., Kolinjivadi A.M., Baldi G., Costanzo V. Studying essential DNA metabolism proteins in Xenopus egg extract. Int. J. Dev. Biol. 2016;60:221–227. doi: 10.1387/ijdb.160103vc. [DOI] [PubMed] [Google Scholar]
- 13.Simon A.C., Sannino V., Costanzo V., Pellegrini L. Structure of human Cdc45 and implications for CMG helicase function. Nat. Commun. 2016;7 doi: 10.1038/ncomms11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazo J.S., Nemoto K., Pestell K.E., Cooley K., Southwick E.C., Mitchell D.A., Furey W., Gussio R., Zaharevitz D.W., Joo B., Wipf P. Identification of a potent and selective pharmacophore for Cdc25 dual specificity phosphatase inhibitors. Mol. Pharmacol. 2002;61:720–728. doi: 10.1124/mol.61.4.720. [DOI] [PubMed] [Google Scholar]
- 15.Edwards M.C., Tutter A.V., Cvetic C., Gilbert C.H., Prokhorova T.A., Walter J.C. MCM2-7 complexes bind chromatin in a distributed pattern surrounding the origin recognition complex in Xenopus egg extracts. J. Biol. Chem. 2002;277:33049–33057. doi: 10.1074/jbc.M204438200. [DOI] [PubMed] [Google Scholar]
- 16.Gardner N.J., Gillespie P.J., Carrington J.T., Shanks E.J., McElroy S.P., Haagensen E.J., Frearson J.A., Woodland A., Blow J.J. The High-Affinity Interaction between ORC and DNA that Is Required for Replication Licensing Is Inhibited by 2-Arylquinolin-4-Amines. Cell Chem. Biol. 2017;24:981–992.e4. doi: 10.1016/j.chembiol.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swift L.P., Rephaeli A., Nudelman A., Phillips D.R., Cutts S.M. Doxorubicin-DNA adducts induce a non-topoisomerase II-mediated form of cell death. Cancer Res. 2006;66:4863–4871. doi: 10.1158/0008-5472.CAN-05-3410. [DOI] [PubMed] [Google Scholar]
- 18.Cuervo A.M., Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saponaro A. Isothermal Titration Calorimetry: A Biophysical Method to Characterize the Interaction between Label-free Biomolecules in Solution. Bio. Protoc. 2018;8:e2957. doi: 10.21769/BioProtoc.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan D.O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 21.Di Micco R., Fumagalli M., Cicalese A., Piccinin S., Gasparini P., Luise C., Schurra C., Garre M., Nuciforo P.G., Bensimon A., et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 22.Melixetian M., Ballabeni A., Masiero L., Gasparini P., Zamponi R., Bartek J., Lukas J., Helin K. Loss of Geminin induces rereplication in the presence of functional p53. J. Cell Biol. 2004;165:473–482. doi: 10.1083/jcb.200403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge X.Q., Jackson D.A., Blow J.J. Dormant origins licensed by excess Mcm2-7 are required for human cells to survive replicative stress. Genes Dev. 2007;21:3331–3341. doi: 10.1101/gad.457807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibarra A., Schwob E., Méndez J. Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proc. Natl. Acad. Sci. USA. 2008;105:8956–8961. doi: 10.1073/pnas.0803978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu W., Depamphilis M.L. Selective killing of cancer cells by suppression of geminin activity. Cancer Res. 2009;69:4870–4877. doi: 10.1158/0008-5472.CAN-08-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petropoulos M., Champeris Tsaniras S., Taraviras S., Lygerou Z. Replication Licensing Aberrations, Replication Stress, and Genomic Instability. Trends Biochem. Sci. 2019;44:752–764. doi: 10.1016/j.tibs.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Chen S., Chen X., Xie G., He Y., Yan D., Zheng D., Li S., Fu X., Li Y., Pang X., et al. Cdc6 contributes to cisplatin-resistance by activation of ATR-Chk1 pathway in bladder cancer cells. Oncotarget. 2016;7:40362–40376. doi: 10.18632/oncotarget.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy N., Ring M., Heffron C.C.B.B., King B., Killalea A.G., Hughes C., Martin C.M., McGuinness E., Sheils O., O'Leary J.J. p16INK4A, CDC6, and MCM5: predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. J. Clin. Pathol. 2005;58:525–534. doi: 10.1136/jcp.2004.018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karakaidos P., Taraviras S., Vassiliou L.V., Zacharatos P., Kastrinakis N.G., Kougiou D., Kouloukoussa M., Nishitani H., Papavassiliou A.G., Lygerou Z., Gorgoulis V.G. Overexpression of the replication licensing regulators hCdt1 and hCdc6 characterizes a subset of non-small-cell lung carcinomas: synergistic effect with mutant p53 on tumor growth and chromosomal instability--evidence of E2F-1 transcriptional control over hCdt1. Am. J. Pathol. 2004;165:1351–1365. doi: 10.1016/S0002-9440(10)63393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liontos M., Koutsami M., Sideridou M., Evangelou K., Kletsas D., Levy B., Kotsinas A., Nahum O., Zoumpourlis V., Kouloukoussa M., et al. Deregulated overexpression of hCdt1 and hCdc6 promotes malignant behavior. Cancer Res. 2007;67:10899–10909. doi: 10.1158/0008-5472.CAN-07-2837. [DOI] [PubMed] [Google Scholar]
- 31.Ohta S., Koide M., Tokuyama T., Yokota N., Nishizawa S., Namba H. Cdc6 expression as a marker of proliferative activity in brain tumors. Oncol. Rep. 2001;8:1063–1066. doi: 10.3892/or.8.5.1063. [DOI] [PubMed] [Google Scholar]
- 32.Tian Q.S., Zhang Q., Huang W. MCM10 as a novel prognostic biomarker and its relevance to immune infiltration in gliomas. Technol. Health Care. 2023;31:1301–1317. doi: 10.3233/THC-220576. [DOI] [PubMed] [Google Scholar]
- 33.Yang S., Yuan Y., Ren W., Wang H., Zhao Z., Zhao H., Zhao Q., Chen X., Jiang X., Zhang L. MCM4 is a novel prognostic biomarker and promotes cancer cell growth in glioma. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1004324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrakis T.G., Komseli E.S., Papaioannou M., Vougas K., Polyzos A., Myrianthopoulos V., Mikros E., Trougakos I.P., Thanos D., Branzei D., et al. Exploring and exploiting the systemic effects of deregulated replication licensing. Semin. Cancer Biol. 2016;37–38:3–15. doi: 10.1016/j.semcancer.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Ge X.Q., Blow J.J. The licensing checkpoint opens up. Cell Cycle. 2009;8:2320–2322. [PMC free article] [PubMed] [Google Scholar]
- 36.Woodward A.M., Göhler T., Luciani M.G., Oehlmann M., Ge X., Gartner A., Jackson D.A., Blow J.J. Excess Mcm2-7 license dormant origins of replication that can be used under conditions of replicative stress. J. Cell Biol. 2006;173:673–683. doi: 10.1083/jcb.200602108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lei M., Kawasaki Y., Tye B.K. Physical interactions among Mcm proteins and effects of Mcm dosage on DNA replication in Saccharomyces cerevisiae. Mol. Cell Biol. 1996;16:5081–5090. doi: 10.1128/MCB.16.9.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crevel G., Hashimoto R., Vass S., Sherkow J., Yamaguchi M., Heck M.M.S., Cotterill S. Differential requirements for MCM proteins in DNA replication in Drosophila S2 cells. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peyregne V.P., Kar S., Ham S.W., Wang M., Wang Z., Carr B.I. Novel hydroxyl naphthoquinones with potent Cdc25 antagonizing and growth inhibitory properties. Mol. Cancer Ther. 2005;4:595–602. doi: 10.1158/1535-7163.MCT-04-0274. [DOI] [PubMed] [Google Scholar]
- 40.Han Y., Shen H., Carr B.I., Wipf P., Lazo J.S., Pan S.S. NAD(P)H:quinone oxidoreductase-1-dependent and -independent cytotoxicity of potent quinone Cdc25 phosphatase inhibitors. J. Pharmacol. Exp. Ther. 2004;309:64–70. doi: 10.1124/jpet.103.059477. [DOI] [PubMed] [Google Scholar]
- 41.Garcia H., Miecznikowski J.C., Safina A., Commane M., Ruusulehto A., Kilpinen S., Leach R.W., Attwood K., Li Y., Degan S., et al. Facilitates chromatin transcription complex is an "accelerator" of tumor transformation and potential marker and target of aggressive cancers. Cell Rep. 2013;4:159–173. doi: 10.1016/j.celrep.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aze A., Sannino V., Soffientini P., Bachi A., Costanzo V. Centromeric DNA replication reconstitution reveals DNA loops and ATR checkpoint suppression. Nat. Cell Biol. 2016;18:684–691. doi: 10.1038/ncb3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto Y., Ray Chaudhuri A., Lopes M., Costanzo V. Rad51 protects nascent DNA from Mre11-dependent degradation and promotes continuous DNA synthesis. Nat. Struct. Mol. Biol. 2010;17:1305–1311. doi: 10.1038/nsmb.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolinjivadi A.M., Sannino V., De Antoni A., Zadorozhny K., Kilkenny M., Técher H., Baldi G., Shen R., Ciccia A., Pellegrini L., et al. Smarcal1-Mediated Fork Reversal Triggers Mre11-Dependent Degradation of Nascent DNA in the Absence of Brca2 and Stable Rad51 Nucleofilaments. Mol. Cell. 2017;67:867–881.e7. doi: 10.1016/j.molcel.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.List M., Schmidt S., Christiansen H., Rehmsmeier M., Tan Q., Mollenhauer J., Baumbach J. Comprehensive analysis of high-throughput screens with HiTSeekR. Nucleic Acids Res. 2016;44:6639–6648. doi: 10.1093/nar/gkw554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costanzo V., Robertson K., Bibikova M., Kim E., Grieco D., Gottesman M., Carroll D., Gautier J. Mre11 protein complex prevents double-strand break accumulation during chromosomal DNA replication. Mol. Cell. 2001;8:137–147. doi: 10.1016/s1097-2765(01)00294-5. [DOI] [PubMed] [Google Scholar]
- 47.Furstenthal L., Kaiser B.K., Swanson C., Jackson P.K. Cyclin E uses Cdc6 as a chromatin-associated receptor required for DNA replication. J. Cell Biol. 2001;152:1267–1278. doi: 10.1083/jcb.152.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Original unprocessed gels, western blot images, microscopy images, and datasets have been deposited on Mendeley Data and Open Science Framework and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
The study does not report any original code.
-
•
Any additional information related to the data reported in this paper is available from the lead contact upon request.