Highlights
-
•
MiR-34b is a tumor suppressor in cervical cancer cells causing cellular senescence.
-
•
MiR-34b increases the levels of ROS contributing to senescence by inhibiting TWIST1.
-
•
MiR-34b can act in combination with RITA as a novel therapeutic strategy.
Keywords: MiR-34b, Senescence, TWIST1, Oxidative stress, Cervical cancer
Abstract
Purpose
The aim of this research was to elucidate the role of miR-34b in cervical cancer progression and the underlying mechanism behind the miR-34b-mediated tumor suppression. The study revealed the role of miR-34b as a senescence inducer and serves as a potential therapeutic target in developing combination therapy with senotherapeutics.
Methods
MiR-34b was ectopically expressed in cervical cancer cell lines using a tetracycline inducible system and its effects on cell viability, apoptosis, senescence, DNA damage and oxidative stress were studied using MTT assay, acridine orange/ ethidium bromide staining, senescence associated β-galactosidase assay, gamma H2AX foci staining assay, western blotting and specific dyes for the detection of total and individual ROS species.
Results
Ectopic expression of miR-34b promoted cellular senescence but no significant induction of apoptosis was observed in cervical cancer cell lines. MiR-34b promoted increase in oxidative stress through increase in total and individual ROS species and contributed to increase in cellular senescence. Mechanistically, miR-34b mediates its action by targeting TWIST1 as evidenced by the similar actions of TWIST1 shRNA in cervical cancer cell lines. Furthermore, our study revealed TWIST1 is one of the most significant targets of miR-34b targetome and identified RITA as a novel senolytic agent for use in combination therapy with miR-34b.
Conclusion
MiR-34b promotes cellular senescence and oxidative stress by targeting TWIST1, a known oncogene and EMT regulator. This study delved into the mechanism of miR-34b-mediated tumor suppression and provided novel insights for development of miR-34b based therapeutics for cervical cancer.
Graphical abstract
Abbreviations
- ROS
reactive oxygen species
- EMT
epithelial mesenchymal transition
- HIV
human immunodeficiency virus
- TCGA
the cancer genome atlas
- CESC
cervical squamous cell carcinoma and endocervical adenocarcinoma
- UCEC
uterine corpus endometrial carcinoma
- GEPIA
gene expression profiling interactive analysis
- AO/EB
acridine orange/ ethidium bromide
- SDS-PAGE
sodium dodecyl sulphate- polyacrylamide gel electrophoresis
- PVDF
polyvinylidene fluoride
- DAPI
4′,6-diamidino-2-phenylindole
- GFP
green fluorescent protein
- TRITC
tetramethylrhodamine
- RIPA
radioimmunoprecipitation
- H2DCFDA
2′,7′-dichlorodihydrofluorescein diacetate
- DHE
dihydroethidium
- HPF
hydroxyphenyl fluorescein
- RITA
reactivating p53 and inducing tumor apoptosis
Introduction
Cervical cancer has one of the highest incidences in women worldwide, with the highest mortality rates in low and middle-income countries, including India [1]. Although there has been a steady decline in age-standardized incidence and mortality rates, the five-year survival rates are still lower than in high-income countries due to lack of proper screening infrastructures [2]. The most common type of cervical cancer is the squamous type (90 %), followed by adenocarcinoma (5 %). Chronic Human papillomavirus (HPV) infection is the cause of cervical cancer in 95 % of the cases. High-risk HPV 16 and 18 are prevalent in both asymptomatic and symptomatic women and are associated with the majority of cervical cancers. Several biomarkers have been established over the years to supplement conventional histopathology assessments to detect cervical neoplasia, including microRNAs [3]. Early detection of cancerous lesions will enable effective treatment before invasive cancer develops. Detection at later stages is a primary concern in developing countries, leading to higher mortality rates in part due to adverse side effects of treatment. The current treatment modalities, particularly cisplatin-based chemotherapy and radiotherapy, have been shown to have severe toxicities in several gynecological cancers, including cervical cancer [[4], [5], [6]]. Therefore, there is an urgent need for better treatment strategies with minimal toxicity and greater efficacy.
One strategy is the use of combination therapy, which involves the use of several target inhibitors at the same time. MicroRNAs (miRNAs), a class of small RNA molecules (18–23 nucleotides) that transcriptionally regulate target genes, are altered in cancer progression, cell death and other cell fate decisions [7,8]. MiRNAs bind to the target genes at 3′ untranslated regions (UTRs) primarily but also target 5′ UTRs and coding sequences and promote translational repression or target mRNA degradation depending on complementarity. MiRNAs are classified as tumor suppressor miRNAs and oncomiRNAs depending on their targets. PTEN targeting miRNAs are oncomiRNAs as PTEN is a known tumor suppressor while miR-388–3p is a tumor suppressor miRNA as it targets oncogenes like Bcl2, MAPK, SOX4, Zeb2 and several others [9,10]. MiRNAs play significant roles in therapy sensitization of both conventional and targeted therapy like immunotherapy [11,12]. The wide targetome of miRNAs makes them highly suitable for combination therapy, e.g., combining chemotherapy with therapy sensitizing miRNAs [13,14]. In recent years, senescence induction has become an attractive area for tumor suppression, followed by specialized therapy to target the senescent cells [15,16]. Since miRNAs can modulate senescence/ senescence-state inducers like oxidative stress and/or DNA damage [17,18], miRNAs are particularly attractive as both positive and negative modulators of senescence [19]. MiRNAs can be developed as senescence inducers and used in combination therapy with special agents that target senescent cells called senotherapeutics. Identification of novel miRNAs that can act as senescence inducers would lead to effective combination therapies with senotherapeutics. One such miRNA is miR-34a, a miR-34 family member extensively studied for its role in proliferation, epithelial-mesenchymal transition (EMT) and cancer stemness [20]. It has been shown to promote senescence in colon cancer by targeting E2F and in hepatocellular carcinoma by targeting c-myc [21,22]. It was developed as a therapeutic but failed due to its high toxicity [23]. Since members of the same family function similarly, other members of the miR-34 family could be developed as a therapeutic. MiR-34b and miR-34c have both shown tumor suppressive roles in several cancers, including cervical cancer [24]. MiR-34c is involved in senescence of human aorta vascular senescence, dermal fibroblasts and in leukemia stem cells [[25], [26], [27]], while miR-34b/c have been involved in curcumin-mediated senescence and apoptosis in colorectal cancer and endothelial senescence [28,29] while another study showed that miR-34b individually did not alter senescence in endothelial cells [30]. Therefore, further investigation into the role of miR-34b in senescence is required. Our previous study showed that miR-34b is the most downregulated miR-34 family member in gynecological cancers [31], correlating with its reported tumor-suppressive role in gynecological cancers [[32], [33], [34]]; however, none of these studies show the role of miR-34b in cellular senescence. Other studies have shown the role of miR-34b in senescence in combination with miR-34c, but whether miR-34b can individually modulate senescence is unclear.
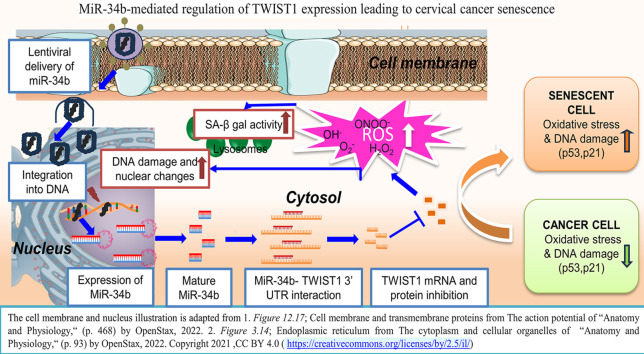
In this novel study, miR-34b was ectopically expressed in cervical cancer cells to unravel its effects on cellular senescence. The target of miR-34b involved in the mediation of senescence was identified to be TWIST1, a known EMT regulator. MiR-34b-mediated downregulation of TWIST1 increased ROS levels and DNA damage markers, the precursors to induction of senescence. Further, a small molecule p53 activator, RITA, was found to specifically act as a senolytic agent for miR-34b induced senescent cells.
Materials and methods
Cell lines and tissue samples used in the study
Cervical cancer cells (SiHa, HeLa, C33A) were procured from NCCS and authenticated using STR profiling. The cell lines were maintained in Dulbecco's Minimum Essential Medium-high glucose (HiMedia Laboratories, LLC, PA, USA) supplemented with 10 % fetal bovine serum (HiMedia Laboratories) and antibiotics (Penicillin-60 mg L−1 and streptomycin- 100 mg L−1) in humidified incubators with 5 % CO2 at 37 °C.
Plasmid constructs and cloning
The doxycycline-inducible lentiviral microRNA expression construct for hsa-mir-34b (cat no: GSH11929–224,638,820) with turboGFP expression was purchased from GE Healthcare Dharmacon, Inc., CO, USA. The TWIST1 3′-UTR fragment containing putative binding sites for miR-34b was amplified from genomic DNA and cloned into psiCheck2 vector (Cat No- C8021, Promega). The mutant (MUT) miR-34b binding site in TWIST1 3′ UTR was generated via site-directed mutagenesis in wild-type (WT) site and confirmed by Sanger sequencing. Lentiviral shRNA construct for TWIST1(Cat No-SHCLNG- NM_000474) and control vector (Cat No- SHC002) were purchased from Sigma-Aldrich. The details of the primer sequences are mentioned in the supplementary file.
Transfection, lentivirus production and transduction
Lentiviral particles were generated as per manufacturer instructions by PEI transfection (Cat No: 23,966–1, Polysciences, Inc., PA, USA) using 3rd or 2nd generation lentiviral packaging system (Control/miR-34b expression vector/GAG/REV/TAT/VSV-G or sh-Control /sh-TWIST1/ psPAX2/pMD2.G) in HEK-293T cells respectively. The lentiviral particles were reverse transduced using 10μg mL−1 polybrene (Cat No-sc134220, Santa Cruz Biotechnology, Inc., TX, USA) solution followed by media change after 24 h. These cells were selected with puromycin (Cat No-P8833, Sigma-Aldrich) for 3–5 days, and the cells were kept in complete media for 2–3 passages before further experimentation.
Bioinformatic analysis
Survival analysis of miR-34b was performed on TCGA data using the OncoLnc tool with cut-off of 75 % for lower and 25 % for higher end lower expression percentile [35]. Survival analysis of TWIST1 was performed on TCGA data using the GEPIA [36] tool with cut-off of 25 % for lower and 35 % for higher end. The miR-34b binding site in TWIST1 was predicted by the TargetScan tool [37]. TWIST1 expression was determined from the GEO dataset GSE9750 [38] using GEO2R.
RT PCR and qPCR for stable cells
The cells were seeded and induced with dox the next day for different time points (48, 72, 96 h) and collected. Total RNA was extracted using RNA Iso Plus reagent (DSS Takara Bio India Private Ltd, Delhi, India) and converted to cDNA using Primescript RT reagent kit (DSS Takara Bio India Private Ltd, New Delhi, India) as per the manufacturer's instructions. Stem loop (SL) primer was used for miRNA cDNA conversion with the following conditions: 16 °C for 30 min followed by 32 °C for 30 s, 42 °C for 30 s, 50 °C for 1 s for 60 cycles. For mRNA cDNA conversion, oligo dT primers were used for conversion with the following conditions: 65 °C for 5 min, 37–42 °C for 1.5 h. MiR-34b and TWIST1 levels were quantified using SYBR® Premix Ex Taq (DSS Takara Bio India Private Ltd) and RNU6 and β-Actin were used for normalisation. All primer details are available in the supplementary file.
For expression analysis, the relative quantification of target against reference genes (ref) in treated cell lines was calculated using the equation: Fold change= 2−ΔΔCt [39], ΔΔCt = (Cttarget – Ctref)treated–(Cttarget – Ctref)control. All of the samples were assayed in triplicate.
Protein sample preparation and western blotting
The stable cells were seeded in 100 mm dishes and induced for 72 h or 96 h with the following dox concentrations HeLa- 750 ng mL−1, C33A- 800 ng mL−1, SiHa-100 ng mL−1. The cells are lysed using RIPA buffer, scraped and centrifuged at high speed to collect the protein supernatant. Protein quantification is done using Bradford assay and samples were prepared accordingly. The protein samples (50–100 μg) were subjected to SDS-PAGE and transferred to PVDF membrane (BioRAD laboratories Inc, CA, USA) and blocked using 5 % BSA (HiMedia Laboratories, LLC, PA, USA) for 1 h. The membrane was probed with respective primary and HRP-conjugated secondary antibodies and detected using BioRAD Clarity ECL chemiluminescence kit (BioRAD laboratories Inc, CA, USA). The images were recorded using Image Lab software and the analysis was done using ImageJ. The following primary antibodies are used: caspase 3, cleaved caspase 3, PARP, p21 and β-actin (Cell Signaling Technology Inc, MA, USA) PARP and cleaved PARP, TWIST1 (Abcam, MA, USA), p53 and lamin A/C (Santa Cruz Biotechnology, Inc., TX, USA) and following secondary antibodies were used: Anti-mouse and anti-rabbit IgG, HRP-linked antibodies (Cell Signaling technology Inc, MA, USA).
3′UTR luciferase assay
Cells were seeded and transfected with psiCHECK2 WT or MUT TWIST1 3′ UTR and control/miR-34b expressing plasmid using Lipofectamine 3000 (Thermo Fisher Scientific). 48 h post-transfection, the cells were lysed and stored at −20 °C until further processing. Interference from other elements were checked as described in [40]. Luminescence was measured for Firefly luciferase and Renilla luciferase separately for the same sample using EnSpire Multimode Reader (PerkinElmer, MA, USA) and relative luminescence units (RLU) were calculated as R/L (Renilla luminescence/Firefly luminescence).
Cell viability assay
The pre-induced stable cells were seeded in 96 well plate. At 24 h intervals, the cells were washed with PBS, followed by addition of MTT solution, incubation for 2 h and solubilization by adding DMSO (Fisher Scientific, PA, USA). The absorbance at 560 nm was measured with EnSpire multimode microplate reader (PerkinElmer, MA, USA). To determine the efficiency of RITA senolysis, stable cells were pre-induced with dox and then treated with varying concentrations of RITA for 48 h (0–50 μM), followed by viability assessment by MTT assay as described above.
Acridine orange/ethidium bromide (AO/EB) staining
Cell suspensions with 0.5 × 106 cells mL−1 were stained with AO/EB solution and immediately visualized under the Olympus IX-73 microscope using GFP (Green Fluorescent Protein) and TRITC (Tetramethylrhodamine) channels. The images were analyzed using ImageJ software (Plugin: Apoptosis correlator) to count for necrotic, apoptotic and live cell populations.
Senescence associated β-galactosidase assay
Stable cells were seeded and pre-induced for 72 h and stained for β-galactosidase activity after 5 or 7 days. The cells were washed with 1X PBS, fixed with 3.7 % formaldehyde solution and stained with X-gal staining solution (pH 6.0). The cells were incubated with the staining solution for 16 h at 37 °C and imaged using Olympus IX-73 microscope. The percentage of positive cells was calculated as follows:
Gamma H2AX foci formation assay
Stable cells were seeded in 35 mm dish holding a coverslip. Following pre-induction, the cells were fixed at 2 and 24 h post maximal induction and stained with Anti-Gamma H2AX (γH2AX) antibody (Abcam, MA, USA). The cells were imaged using an Olympus IX-73 microscope using DAPI (4′,6-diamidino-2-phenylindole) and GFP channels, and corrected total cell fluorescence (CTCF) was calculated using ImageJ software with the following formula:
Detection of reactive oxygen species (ROS)
Total reactive oxygen species concentrations were measured by the fluorescence-based assay using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Thermo Fisher Scientific, USA) dye. For the estimation of H2DCFDA, the 495/530 nm excitation/emission wavelength pair was used. Cells at 10 × 105 cells mL−1 were suspended in ice-cold PBS containing 20 µM of the cell-permeable H2DCFDA dye. The cells were incubated at 37 °C for 30 min inside humidified incubator, centrifuged at 2000 rpm and resuspended in 1 mL of PBS. Then 200 µL of cell suspension per well was aliquoted in the 96-well white plate, and fluorescence measurements were taken in Enspire multimode reader.
Intracellular superoxide concentrations were measured by the fluorescence-based assay using dihydroethidium (DHE; Thermo Fisher Scientific, USA) dye. For the estimation of DHE, the 510/595 nm excitation/emission wavelength pair was used. Cells at 2.5 × 106 cells mL−1 were suspended in ice-cold PBS containing 40 µM of the cell-permeable DHE dye. The cells were incubated at RT for 30 min and readings were taken as mentioned previously.
Intracellular hydroxyl concentrations were measured by the fluorescence-based assay using hydoxyphenyl fluorescein (HPF; Thermo Fisher Scientific, USA) dye. For the estimation of HPF, the 495/515 nm excitation/emission wavelength pair was used. Cells at 5 × 105 cells mL−1 were suspended in ice-cold PBS containing 20 µM of the cell-permeable HPF dye. The cells were incubated at 37 °C for 30 min and readings were taken as mentioned previously.
All cell lines were transfected with pC1-dsHyPeRed-mito plasmid, a kind gift from Prof. Amal Kanti Bera (Chennai, India), selected with G418 for 2–3 days, and allowed to grow until stable transfectants were achieved. Intracellular H2O2 levels were measured by fluorescence-based imaging using Olympus IX-73 microscope using TRITC channel. The image analysis was done using the ImageJ tool, and CTCF was calculated accordingly.
Statistical analysis
Unless otherwise stated, three independent experiments were performed with at least three technical replicates, and the data are presented as the mean ± standard deviation (SD). Images represent three independent experiments reproduced with similar observations. Comparisons between two groups were performed using an unpaired t-test. All groups in an experiment involving two independent variables were compared using a two-way analysis of variance (ANOVA) with Bonferroni's multiple comparison test. The p-values were depicted as *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001. Graphpad Prism V 5.01 was used for statistical analyses.
Results
Higher expression of miR-34b correlates with better survival in cervical cancer, but ectopic expression of miR-34b does not induce apoptosis
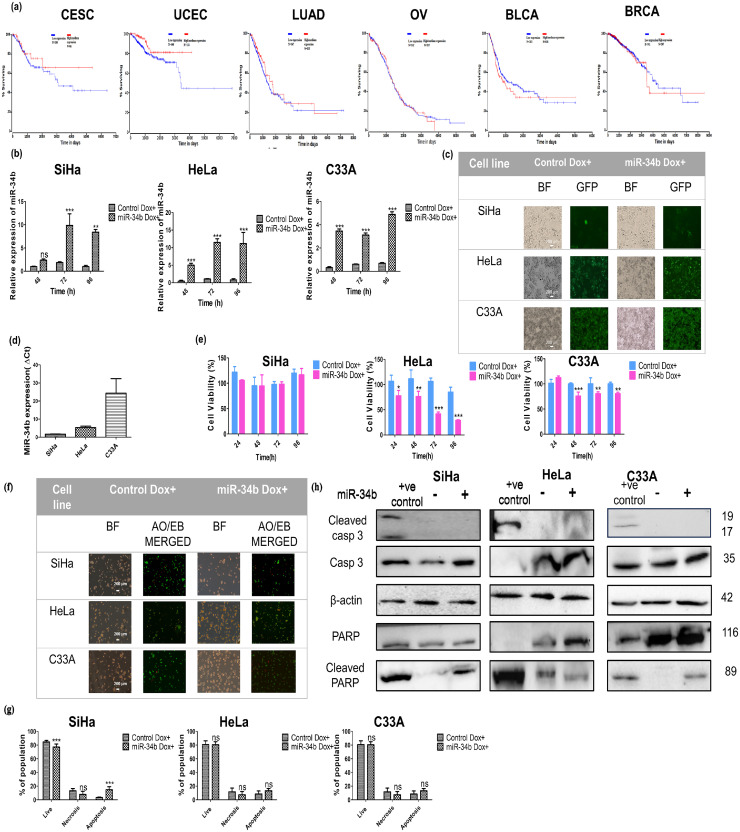
Since miR-34b has a tumor-suppressive role in multiple cancers, the effect of miR-34b on overall survival in various cancers was investigated using the OncoLnc tool. Negative Cox coefficient was seen in CESC and UCEC indicating higher expression of miR-34b correlated with higher patient overall survival only in cervical cancer and uterine cancer (Fig. 1a). MiR-34b has been shown to act as a tumor suppressor in cervical cancer [34,41,42], while bioinformatic evidence suggests miR-34b has a role in UCEC lymph node metastasis [32]. MiR-34b was ectopically expressed in cervical cancer cell lines SiHa, HeLa and C33A in a lentiviral doxycycline (dox)-inducible system to illustrate the effect of miR-34b expression in cervical cancer cells. The expression of miR-34b on dox induction was quantified using real-time PCR at different time points relative to RNU6 (Fig. 1b). Since the maximal expression of miR-34b is observed at 72 h post dox treatment in SiHa and HeLa and 96 h for C33A, further studies were conducted at these time points respectively. Representative images showing concurrent GFP expression with miR-34 expression are shown in Fig. 1c. We next assessed the effect of miR-34b at 24 h intervals on cell viability using MTT, which is reduced to formazan crystals on entering metabolically active cells. Ectopic expression of miR-34b did not alter cell viability in SiHa but reduced cell viability in other cell lines (Fig. 1e). This difference in effect is mediated by the difference in basal miR-34b expression with the lowest levels observed in SiHa and highest in C33A (Fig. 1d). Higher ectopic expression in HeLa compared to C33A showed more reduction in cell viability illustrating how overall mir-34b levels can have different short-term effects. To check whether the decrease in viability is due to apoptosis, the proportion of apoptotic cells was evaluated using an acridine orange/ethidium bromide (AO/EB) dual staining assay. Representative images of AO/EB staining showing live, necrotic and apoptotic cells are depicted in Fig. 1f. No significant change in doubly positive apoptotic cells was observed in all 3 cell lines (Fig. 1g). To confirm that apoptosis is not induced on miR-34b ectopic expression, total and cleaved caspases 3 and PARP levels were analyzed using immunoblotting and observed no significant induction of cleaved caspase and PARP in all 3 cell lines (Fig. 1h).
Fig. 1.
Higher expression of miR-34b correlates with patient survival in CESC and UCEC, and stably induced ectopic miR-34b expression decreases cell viability without apoptosis induction a) Survival analysis of miR-34b across 6 different cancers b) Expression of miR-34b on doxycycline induction at different time points relative to RNU6 quantified by qRT-PCR (n = 3) c) Representative image of GFP expressing cells on induction of miR-34b expression by doxycycline in cervical cancer cell lines d) Relative basal expression levels of miR-34b in different cervical cancer cell lines (n = 3) e) Percentage change in cell viability on ectopic expression of miR-34b in different cell lines (n = 3) f) Representative BF and AO/EB staining merged(GFP/TRITC filters) images of control and miR-34b expressing cells g) Percentages of live, necrotic and apoptotic populations quantified from acridine orange/ ethidium bromide immunofluorescence staining in control and miR-34b expressing cell lines h) Immunoblotting images of total and cleaved caspase3 and PARP in positive control for apoptosis, control and miR-34b expressing cells with β-actin as loading control (numbers indicate molecular weight (M.Wt) in kilodalton or kDa). Values are depicted as mean± SD and p-values are depicted as * p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001.
MiR-34b promotes cellular senescence through p53 and p21 axis in cervical cancer cells
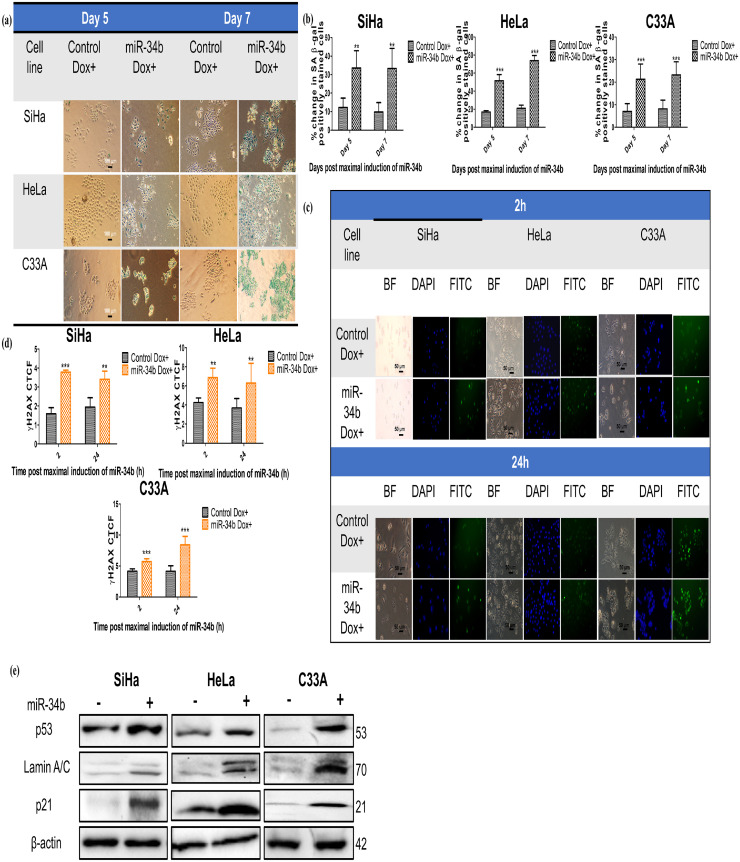
Since there was no induction of apoptosis on miR-34b expression, it was evaluated if cellular senescence is induced on ectopic expression of miR-34b. MiR-34b expressing cells showed increased staining due to senescence-associated β-galactosidase (SA-β gal) activity than control cells (Fig. 2a). Additional senescence markers γH2AX (Fig. 2b) and p53, p21 and Lamin A/C levels (Fig. 2c) were increased in miR-34b expressing cells. γH2AX foci were increased at 2 h post maximal induction of miR-34b and persisted at 24 h, indicating the inability to repair the DNA damage consistent with induction of senescence (Fig. 2b). Immunoblotting revealed an increase in p53, p21 and Lamin A/C levels, which are well-known early senescence markers (Fig. 2c). These data suggest that miR-34b expressing cells show senescent phenotype with increased DNA damage and SA-β gal activity.
Fig. 2.
MiR-34b promotes cellular senescence in cervical cancer cells a) Bright-field images of control and miR-34b expressing cells after day 5 and day 7 post maximal induction of miR-34b showing positive SA-β gal staining b) Quantification of total positively stained cells in each group (n = 4) c) Bright field, DAPI stained nucleus and FITC stained γH2AX foci representative images of control and miR-34b expressing cells at 2 h and 24 h post maximal induction of miR-34b d) Quantification of corrected total cell fluorescence (CTCF) of γH2AX foci (n = 4) e) Immunoblotting images of p53, p21 and lamin A/C in control and miR-34b expressing cells with β-actin as loading control. Values are depicted as mean± SD, and p-values are depicted as ** p ≤ 0.01 *** p ≤ 0.001.
Ectopic expression of miR-34b drives oxidative stress, leading to cellular senescence in cervical cancer cells
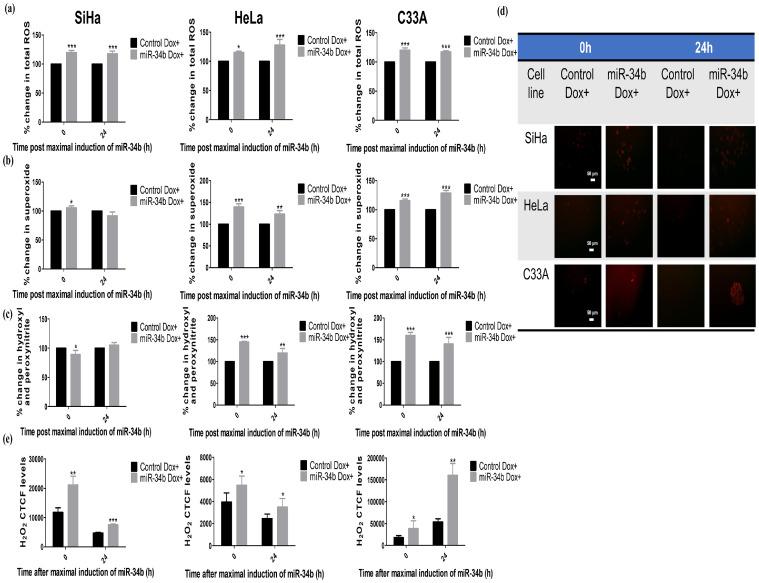
Oxidative stress is one of the leading players that is responsible for senescence development. The levels of total ROS and major ROS players superoxide, hydroxyl, peroxynitrite and hydrogen peroxide(H2O2) were experimentally tested on ectopic miR-34b expression. All ROS levels were detected using respective dyes: DCFDA for total ROS, DHE for superoxide and HPF for hydroxyl and peroxynitrite radicals. H2O2 levels were quantified from HyPer-Red Mito transfected into stable cells and induced for maximal expression of miR-34b. All the individual ROS elements and total ROS were increased in HeLa and C33A cells at 2-time points 0 and 24 h post maximal induction of miR-34b, but in SiHa, total ROS increased with the only increase observed in H2O2 levels consistently at 0 and 24 h (Fig. 3a–d) indicating differential action of miR-34b in SiHa as seen earlier in the case of cell viability change. These data imply that miR-34b increases oxidative stress by modulating various ROS species.
Fig. 3.
MiR-34b drives oxidative stress a) Percentage change in total ROS detected by DCFDA in control and miR-34b expressing cells at 0 and 24 h post maximal induction b) Percentage change in superoxide radicals detected by DHE in control and miR-34b expressing cells at 0 and 24 h post maximal induction c) Percentage change in hydroxyl and peroxynitrite radicals detected by HPF in control and miR-34b expressing cells at 0 and 24 h post maximal induction d) Representative hyPer Red Mito protein detector for H2O2 in control and miR-34b expressing cells post maximal induction e) Quantification of corrected total cell fluorescence (CTCF) of hyPer Red detector in control and miR-34b expressing cells at 0 and 24 h post maximal induction (n = 4). Values are depicted as mean± SD, and p-values are depicted as * p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001.
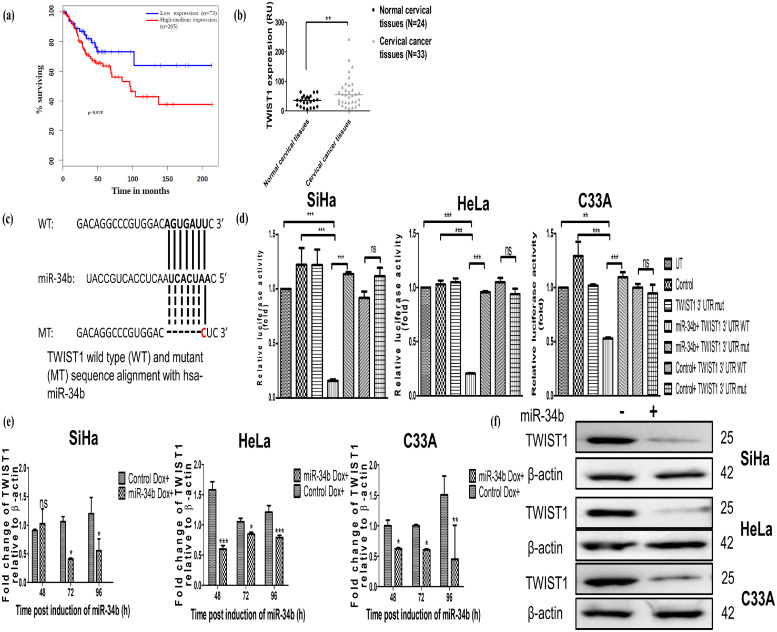
Higher expression of TWIST1 is inversely correlated with miR-34b expression and is a target for miR-34b in human cervical cancer cells
Since miR-34b modulates cellular senescence, it is imperative to identify the most significant and relevant target modulated by miR-34b. The SenMayo signature for senescence [43] of 125 genes was used as a basal signature to identify that target, in which 25 of the 125 genes were predicted targets of miR-34b identified from the miRDip database [44]. Literature review revealed that Twist1, a transcription factor, was regulated by and acted as a regulator of 10 from the 25 genes targeted by miR-34b (Supplementary Table 1) and was predicted to be a direct target of miR-34b from TargetScan (Supplementary Table 2).
Based on computational cues, it was evaluated if higher expression levels of TWIST1 correlated with lower survival, an effect opposite to that observed with miR-34b. TWIST1 levels were assessed in the CESC TCGA dataset using GEPIA tool, and it revealed that higher levels of TWIST1 significantly correlated with poor survival in cervical cancer patients (Fig. 4a). Additionally, the levels of TWIST1 were found to be elevated in 33 cervical cancer tissues compared to 24 normal cervical tissues in the GEO dataset GSE9750 (Fig. 4b). These pieces of evidence suggest that TWIST1 is upregulated in cervical cancer and is oncogenic. To further confirm if TWIST1 is an actual target of miR-34b, the 3′ UTR luciferase reporter assay using wild type (WT) and mutant (MUT) miRNA binding site containing vector transfected with control and miR-34b expressing vector was performed. The alignment between WT and MUT 3′ UTR with miR-34b is shown in Fig. 4c. The renilla luciferase activity significantly decreased when miR-34b and WT miRNA binding site vectors were co-transfected (Fig. 4d), but no significant change in activity was observed when miR-34b and MUT miRNA binding site vectors were co-transfected.
Fig. 4.
Higher expression levels of TWIST1 in cervical cancer correlate with poor survival and TWIST1 is a target of miR-34b modulating its mRNA and protein levels a) Survival analysis of TWIST1 in cervical cancer predicted by Kaplan Meier plotter using data from Gene Expression Analysis Profiling Interactive Analysis (GEPIA) b) Relative expression (as relative units, RU) of TWIST1 levels in normal and cervical cancer, samples retrieved from GSE9750 c) Schematic representation of the alignment of miR-34b and its binding site present in the 3′ UTR of TWIST1 mRNA d) Relative luciferase activity of 3′UTR dual luciferase assay in cervical cancer cell lines. Control or miR-34b expressing constructs were co-transfected with wild-type (WT) or mutant (MUT) 3′ UTR miRNA binding sites containing constructs. 48 h post-transfection, cells were lysed, and the renilla and firefly luciferase activities were measured in control transfection groups (Untransfected, control vector, Mutant TWIST1 3′ UTR miRNA binding site containing vector) and experimental groups (control/miR-34b expression vector and WT/MUT TWIST1 3′ UTR miRNA binding site containing vector) (n = 3) e) Expression of TWIST1 at different time points post induction of miR-34b using dox (n = 3) normalized to β-actin by qRT-PCR f) Protein levels of TWIST1 post maximal induction of miR-34b in SiHa, HeLa and C33A with β-actin as loading control (n = 3). Values are depicted as mean± SD and p-values are depicted as * p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001.
Further, TWIST1 mRNA levels were checked at different time points post induction of miR-34b using qRT-PCR and found that ectopic expression of miR-34b reduced TWIST1 levels consistently (Fig. 4e). Immunoblotting revealed TWIST1 protein levels also decreased post maximal induction of miR-34b (Fig. 4f). This indicates that TWIST1 is a target of miR-34b and its higher expression correlates with poor survival, indicating its oncogenic nature and the potential to inhibit its oncogenicity using miR-34b as a potential therapeutic molecule.
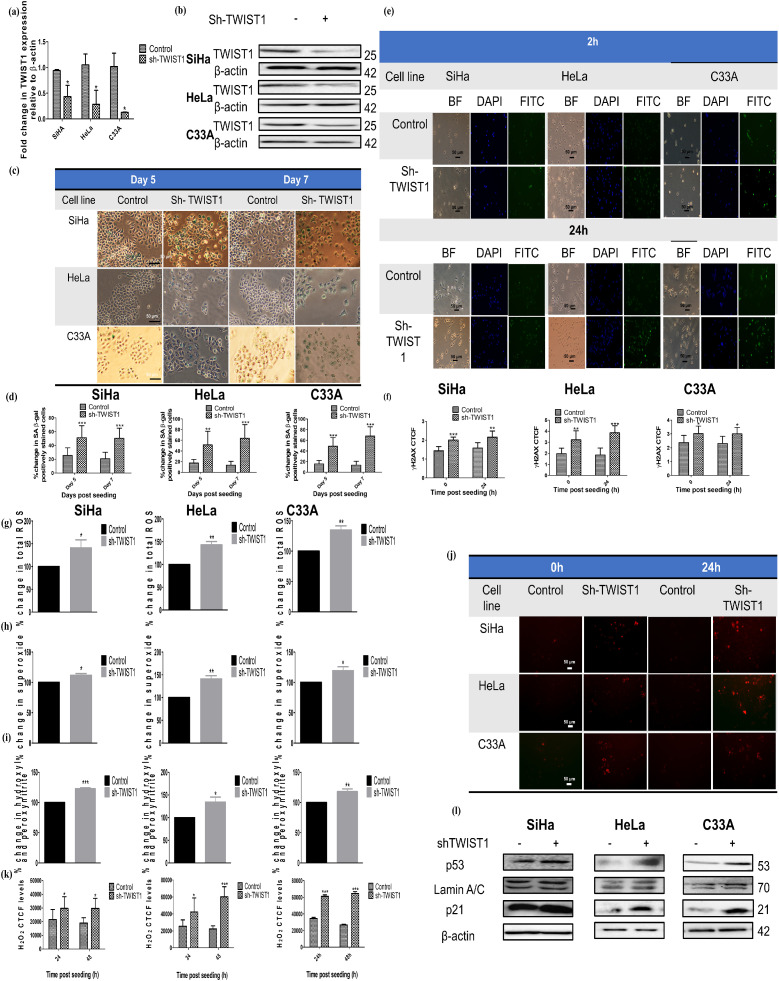
Inhibition of TWIST1 by shRNA emulates the actions of miR-34b ectopic expression in cervical cancer cells
To reveal the involvement of TWIST1 in miR-34b-induced senescence, stable cell lines transduced by lentiviral shRNA against TWIST1 were generated. The downregulation of TWIST1 mRNA mediated by shRNA in cervical cancer cell lines was quantified by qRT-PCR relative to β-actin (Fig. 5a). Protein levels of TWIST1 were also downregulated by shRNA in cervical cancer cell lines (Fig. 5b). ShRNA TWIST1 or sh-TWIST1 cells exhibited a significant increase in the percentage of SA-β gal activity analogous to miR-34b expressing cells (Fig. 5c & d). Sh-TWIST1 cells also revealed increased DNA damage indicated by increased γH2AX foci at 2 h with persistence at 24 h post-seeding of cells (Fig. 5e & f). Downregulation of TWIST1 by shRNA also showed an increase in oxidative stress as illustrated by the increase in levels of total ROS and individual ROS species, i.e., superoxide, hydroxyl and peroxynitrite and H2O2 in all the cell lines, whereas miR-34b increased only H2O2 levels in SiHa indicating low basal levels and comparatively lower ectopic expression of miR-34b in SiHa is insufficient to emulate all the changes of TWIST1 downregulation reiterating previous observed differential effect on this cell line (Fig. 5g–k). Consistent with our previous findings, sh-TWIST1 cells showed induction of senescence and increase in senescence markers p53, p21 and lamin A/C (Fig. 5l) like ectopically miR-34b expressing cells, indicating that miR-34b mediates its action through TWIST1.
Fig. 5.
TWIST1 shRNA mimics miR-34b effect on cervical cancer cells a) mRNA levels of TWIST1 on shRNA mediated downregulation relative to β-actin quantified by qRT-PCR (n = 3) b) Protein levels of TWIST1 on shRNA mediated downregulation using β-actin as a loading control (n = 3) c) Bright field images of control and sh-TWIST1 expressing cells after day 5 and day 7 post seeding showing positive SA-β gal staining d) Quantification of total positively SA-β gal stained cells in each group (n = 4) e) Bright field, DAPI stained nucleus and FITC stained γH2AX foci representative images of control and sh-TWIST1 expressing cells at 2 h and 24 h post seeding f) Quantification of corrected total cell fluorescence (CTCF) of γH2AX foci (n = 4) g) Percentage change in total ROS detected by DCFDA in control and sh-TWIST1 expressing cells h) Percentage change in superoxide radicals detected by DHE in control and sh-TWIST1 expressing cells i) Percentage change in hydroxyl and peroxynitrite radicals detected by HPF in control and sh-TWIST1 expressing cells j) Representative images for hyPer Red Mito protein detector for H2O2 in control and sh-TWIST1 expressing cells k) Quantification of corrected total cell fluorescence (CTCF) of γH2AX foci (n = 4) l) Immunoblotting images of p53, p21 and lamin A/C in control and miR-34b expressing cells with β-actin as loading control. Values are depicted as mean± SD and p-values are depicted as * p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001.
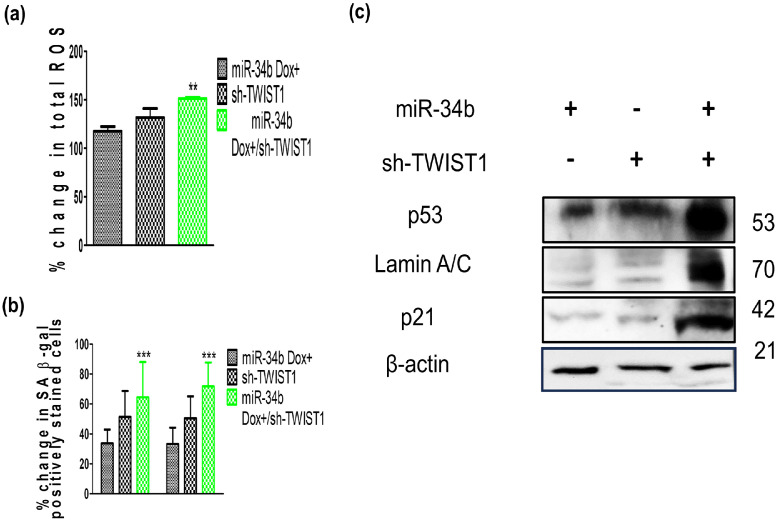
Concurrent expression of miR-34b and sh-TWIST1 revealed that TWIST1 is one of the most significant targets of miR-34b targetome
Previous findings have shown that miR-34b shows a differential effect on SiHa cells with respect to cell viability and individual ROS species modulation, but sh-TWIST1 showed same effect on SiHa as the other cell lines. Concurrent expression of miR-34b and sh-TWIST1 in SiHa and their effects on ROS, SA-β gal activity and senescence markers were investigated. In SiHa, concurrent expression of miR-34b and sh-TWIST1 revealed more profound increase in total ROS, positively stained SA-β gal cells and protein levels of p53, p21 and Lamin A/C than the individual expression of miR-34b and sh-TWIST1 (Fig. 6a–c). Sh-TWIST1 augmented the abject effects of low basal expression combined with lower ectopic expression levels of miR-34b in SiHa while C33A had higher basal levels and resulted in more potent miR-34b effects on oxidative stress induction. Higher miR-34b levels showed outcomes close to sh-TWIST1 indicating that TWIST1 is the major downstream effector of miR-34b despite possible off-targeting by miR-34b. The targetome of microRNAs are huge and regulate diverse processes but, in this case, a single target of miR-34b appears to be a major effector in a single cell fate decision.
Fig. 6.
Effects of miR-34b and sh-TWIST1 introduction on total ROS, SA- β galactosidase activity and senescence markers p53, p21 and lamin A/C in SiHa cells a) Percentage change of total ROS in miR-34b and/or sh-TWIST1 expressing cells (n = 3) b) Quantification of total positively SA-β gal-stained cells in each group (n = 4) c) Protein levels of p53, p21, Lamin A/C in each group using β-actin as loading control (n = 3). Values are depicted as mean± SD, and p-values are depicted as ** p ≤ 0.01 *** p ≤ 0.001.
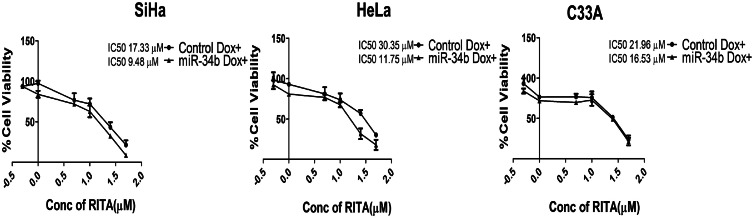
RITA, a p53 activator, acts as senolytic agent for miR-34b-induced senescence
As observed in this study, miR-34b induces senescence similar to therapy-induced senescence, and using senolytic agents could be a novel adjuvant approach. Several p53-modulating agents have been shown to act as senolytic. RITA, p53-modulating agent has shown anticancer effects in cervical cancer [45,46]. To explore its role as a senolytic agent, the cell viability of cervical cancer cells in the presence and absence of miR-34b was evaluated. RITA showed reduced IC50 by 45–60 % in the presence of miR-34b in SiHa and HeLa cells and 25 % reduction in C33A cells, indicating that RITA specifically targets senescent cells promoted by miR-34b in SiHa, HeLa and C33A cells (Fig. 7). Therefore, RITA can act as an adjuvant senolytic agent in combination with miR-34b as a potent therapeutic option in cervical cancer.
Fig. 7.
RITA, a p53 activator, acts as senolytic agent for miR-34b induced senescent cells. Percentage change in cell viability due to RITA treatment in the presence and absence of miR-34b represented by IC50 values (n = 3).
Our study demonstrated that miR-34b is a favorable prognostic marker for cervical cancer and ectopically expressed miR-34b has tumor suppressive roles through induction of cellular senescence and oxidative stress. Our findings revealed that miR-34b downregulates TWIST1, a protein overexpressed in cervical cancer tissues and is directly involved in the miR-34b-mediated induction of cellular senescence via modulation of p53, p21 and lamin A/C markers. Additionally, RITA was found to be a senolytic agent for miR-34b-induced senescence. In a nutshell, our results revealed miR-34b as a potential therapeutic molecule as it can induce cellular senescence and oxidative stress via targeting TWIST1, an oncogene in cervical cancer cells. MiR-34b can be used in combination with senolytic agents as novel combinatorial strategy for cervical cancer.
Discussion
MiRNAs play an essential role in cell fate decisions, including senescence, autophagy, apoptosis and resensitization to conventional therapies [[47], [48], [49]]. The development of novel miRNA-based therapeutics is a continued process but understanding the mechanism behind the working of miRNA is necessary. MiRNA-34 family members have been studied in diverse cancer types and show tumor suppressive role in most cancers with a few exceptions [24,50]. In this study, the potential tumor supressive role of miR-34b has been explored in vitro using human cervical cancer cell lines. The high expression of miR-34b correlated with higher survival in cervical cancer and uterine cancer, indicating it has tumor tumor-suppressive role in these cancers. In a previous study evaluating the roles of 5 and 3p strands of all miR-34 family members, miR-34b-3p showed the least inhibition on cell viability compared to other miR-34 members in cervical cancer, indicating the possibility of other cell-fate decisions involved in its tumor suppressive role [42]. This possibility was corroborated in our study, where stable ectopic expression of miR-34b did not induce apoptosis, and further investigation revealed that miR-34b promotes cellular senescence in cervical cancer.
Oxidative stress is a typical driver of senescence through oxidation and damage of macromolecules seen in senescent cells. Our study found that miR-34b induces oxidative stress, the consequence of which results in cellular senescence. An earlier study showed that miR-34 family members (miR-34 s) induced by curcumin lead to senescence and suppression of colorectal cancer metastasis that was mediated by the ROS/Nrf2 pathway independent of p53 [28]. This study was the first to show miR-34b-mediated senescence in any cancer model, but our study showcases how individually miR-34b can promote senescence through oxidative stress rather than ROS induced miR-34 s' expression as shown in previous study. Similarly, in another study, nutlin-3a promoted p53-mediated miR-34 s expression leading to senescence in normal human fibroblasts, whereas our study shows miR-34b-mediated p53 upregulation [51]. Our study indicates that miR-34b alone can modulate various senescence regulators, ROS and p53 in a novel manner.
DNA-damaging oxidants primarily induce senescence through the p53/p21 axis, and activated p21 generates a vicious cycle of ROS generation [52]. Our study found that p53 and p21 were upregulated on miR-34b ectopic expression and increased oxidative stress, supporting the established view that oxidative stress drives senescence. Additionally, miR-34b ectopic expression also promoted DNA damage as indicated by higher levels of residual γH2AX, corroborating available evidence that DNA damaging agents induce senescence. It is established in literature that DNA damage induces expression of miR-34 s, dependent on p53 [53]. In colon cancer cells, miR-34b and other miR-34 s inhibited homologous recombination DNA damage response by targeting RAD51 [54], correlating with our study that miR-34b induced DNA damage.
Further, p53 has been shown to stabilize lamin A/C mediated nuclear destabilization, allowing the development of senescence [55]. Our findings revealed that increased p53 also increased lamin A/C levels and promoted senescence mediated by miR-34b ectopic expression. Lamin A/C is under-expressed in cervical cancer cells with a strong correlation with HPV status [56], and the upregulation of lamin A/C through miR-34b indicates miR-34b to be an excellent therapeutic candidate. For the maintenance of the senescent state, both oxidative stress and activated DNA damage response are required [57]. If miR-34b can maintain the senescent state through feedback activation of itself through oxidative stress is an interesting question to be addressed.
TWIST1 is a well-known epithelial-mesenchymal transition (EMT) regulator in cervical cancer and several other cancer types by modulating several key components of oncogenic pathways such as VEGF, Jagged, Bcl2, p53, stemness markers, mesenchymal markers and drug resistance markers [58]. TWIST1 has been shown to have other roles in cancer. The role of TWIST1 in senescence has been studied in lung, hematological, and cervical cancers [[59], [60], [61]]. Inhibition of TWIST1 in cervical cancer triggered induction of senescence markers CBX3 and SA-β gal but the in-depth mechanism was not studied. Inhibition of TWIST1 has been shown to promote senescence, and its expression alleviates p53/p21 mediated senescence [[62], [63], [64]]. Our study showed that miR-34b binds to 3′UTR of TWIST1 and decreases TWIST1 mRNA and protein levels. Our study also showed that TWIST1 is overexpressed in cervical cancer, and miR-34b-mediated downregulation of TWIST1 contributes to p53/p21 mediated senescence.
Our study also indicated that though miR-34b has a wide targetome including several predicted EMT regulators, TWIST1 is a predominant target of miR-34b. Targeting TWIST1 is an essential strategy to overcome metastasis and drug resistance in many cancer types [58]. Natural compounds identified to date have indirect effects on TWIST1 protein or mRNA and, therefore, act as non-specific inhibitors for TWIST1 [65,66]. Further studies on different cancer types would reveal if miR-34b is a significant inhibitor of TWIST1 in pan-cancer models and its potential to be developed as a TWIST1 inhibitor effectively and independently.
Targeting senescent cells through small molecules is a novel strategy to decrease side-effects of conventional cancer therapies while maximizing the utility of pro-senescent therapy with senolytics. Our study showed the senolytic effect of a p53 modulator, RITA, in eliminating miR-34b-induced senescent cells with lower IC50 values. The reduction of IC50 values proves that RITA and miR-34b could be used in combination for cervical cancer treatment. Decrease in cell viability is observed without miR-34b expression on RITA treatment, which agrees with previous studies [45,46]. Zhu et al. observed that RITA can act as a radiosensitizer in cervical cancer cells, enhancing the utility of radiation therapy similar to RITA's activity as a senolytic agent. Further investigations is required to understand its complete mechanism of action as a senolytic agent.
Our study provides novel insights into the mechanism of miR-34b-mediated tumor suppressive actions, but there are limitations to this study. For the clinical application of this study, further in vivo experiments on suitable models are required. Downstream effectors of miR-34b/TWIST1 axis leading to upregulation of senescence markers should be investigated in depth. In addition to RITA, other small molecule inhibitors could be investigated for their combinatorial action with miR-34b to determine the best inhibitor for use in clinical applications. Nevertheless, the present study delved into the mechanism of miR-34b-mediated senescence in cervical cancer and demonstrated that ectopically expressed miR-34b promoted oxidative stress, DNA damage-mediated cellular senescence, and tumor suppressive activities. Our study elucidated a novel target of miR-34b and highlighted the therapeutic potential of senescence in treating human cervical cancer.
Funding
This work was supported by Institutional intramural funding. Sindhu K.J. has received research support from Ministry of Human Resource Department, Govt. of India and Women Leading IITM, Indian Institute of Technology Madras, India and Venkatesan Nalini [ICMR research associateship 2019-4566/CMB/BMS] from ICMR, Govt. of India respectively. The funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Data availability
The data related to the study is available in the text. Additional data are available on reasonable request. GSE9750 dataset has been used here and is freely available in the NCBI GEO repository.
CRediT authorship contribution statement
K.J. Sindhu: Conceptualization, Investigation, Formal analysis, Visualization, Data curation, Writing – original draft. Venkatesan Nalini: Writing – review & editing, Investigation, Formal analysis. G.K. Suraishkumar: Supervision, Resources, Formal analysis. Devarajan Karunagaran: Writing – review & editing, Supervision, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
A part of this study was presented at the American Association for Cancer Research Annual Meeting 2023 and was later published in Cancer Research as a conference abstract. We acknowledge Prof. Amal Kanti Bera, Indian Institute of Technology (IIT) Madras, Chennai, India for providing the pC1-dsHyPeRed mito plasmid. We also acknowledge the Department of Science and Technology (DST)- fund for Improvement of S&T infrastructure (FIST) facility for Applied Biosystem Quantstudio 7 Flex real time PCR and BioRad chemiDoc XRS+ instrumentations (DST-FIST program SP21221489BTDSTXDEPHOD) at IIT Madras, Chennai, India.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.102063.
Appendix. Supplementary materials
References
- 1.Bray F., Laversanne M., Sung H., Ferlay J., Siegel R.L., Soerjomataram I., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramaniam G., Gaidhani R.H., Khan A., Saoba S., Mahantshetty U., Maheshwari A. Survival rate of cervical cancer from a study conducted in India. Indian J. Med. Sci. 2020;73:203–211. doi: 10.1016/j.lansea.2023.100296. [Internet] Available from. [DOI] [Google Scholar]
- 3.Ojha P.S., Maste M.M., Tubachi S., Patil V.S. Human papillomavirus and cervical cancer: an insight highlighting pathogenesis and targeting strategies. Virus Dis. 2022;33:132–154. doi: 10.1007/s13337-022-00768-w. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parashar S., Akhter N., Paplomata E., Elgendy I.Y., Upadhyaya D., Scherrer-Crosbie M., et al. Cancer treatment-related cardiovascular toxicity in gynecological malignancies. JACC Cardiooncol. 2023;5:159–173. doi: 10.1016/j.jaccao.2023.02.002. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oneda E., Abeni C., Zanotti L., Zaina E., Bighè S., Zaniboni A. Chemotherapy-induced neurotoxicity in the treatment of gynecological cancers: state of art and an innovative approach for prevention. World J. Clin. Oncol. 2021;12:458–467. doi: 10.5306/wjco.v12.i6.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh J.W., Tanksley J., Chino J., Willett C.G., Dewhirst M.W. Long-term consequences of pelvic irradiation: toxicities, challenges, and therapeutic opportunities with pharmacologic mitigators. Clin. Cancer Res. 2020;26:3079–3090. doi: 10.1158/1078-0432.CCR-19-2744. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 7.Jang J.H., Lee T.J. The role of microRNAs in cell death pathways. Yeungnam Univ. J. Med. 2021;38:107–117. doi: 10.12701/yujm.2020.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng Y., Croce C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016;1:1–9. doi: 10.1038/sigtrans.2015.4. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abadi A.J., Zarrabi A., Gholami M.H., Mirzaei S., Hashemi F., Zabolian A., et al. Small in size, but large in action: micrornas as potential modulators of pten in breast and lung cancers. Biomolecules. 2021;11:1–36. doi: 10.3390/biom11020304. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirzaei S., Zarrabi A., Asnaf S.E., Hashemi F., Zabolian A., Hushmandi K., et al. The role of microRNA-338-3p in cancer: growth, invasion, chemoresistance, and mediators. Life Sci. 2021;268 doi: 10.1016/j.lfs.2020.119005. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 11.Ashrafizadeh M., Zarrabi A., Hushmandi K., Zarrin V., Moghadam E.R., Zabolian A., et al. PD-1/PD-L1 axis regulation in cancer therapy: the role of long non-coding RNAs and microRNAs. Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117899. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 12.Ashrafizadeh M., Zarrabi A., Orouei S., Hushmandi K., Hakimi A., Zabolian A., et al. MicroRNA-mediated autophagy regulation in cancer therapy: the role in chemoresistance/chemosensitivity. Eur. J. Pharmacol. 2021;892 doi: 10.1016/j.ejphar.2020.173660. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 13.Seo H.A., Moeng S., Sim S., Kuh H.J., Choi S.Y., Park J.K. MicroRNA-based combinatorial cancer therapy: effects of MicroRNAs on the efficacy of anti-cancer therapies. Cells. 2020;9:1–32. doi: 10.3390/cells9010029. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Normann L.S., Aure M.R., Leivonen S.K., Haugen M.H., Hongisto V., Kristensen V.N., et al. MicroRNA in combination with HER2-targeting drugs reduces breast cancer cell viability in vitro. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-021-90385-2. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitt C.A., Wang B., Demaria M. Senescence and cancer–role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022;19:619–636. doi: 10.1038/s41571-022-00668-4. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao S., Qin D., Hou X., Tian L., Yu Y., Zhang R., et al. Cellular senescence: a double-edged sword in cancer therapy. Front. Oncol. 2023;13:1–10. doi: 10.3389/fonc.2023.1189015. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebrahimi S.O., Reiisi S., Shareef S. miRNAs, oxidative stress, and cancer: a comprehensive and updated review. J. Cell. Physiol. 2020;235:8812–8825. doi: 10.1002/jcp.29724. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Tong Y., Liu J., Lou J. The role of MicroRNA in DNa damage response. Front. Genet. 2022;13:1–10. doi: 10.3389/fgene.2022.850038. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y.X., Jia Z.W.S.D.L., Wang H.B. Autophagy deficiency promotes lung metastasis of prostate cancer via stabilization of TWIST1. Clin. Transl. Oncol. 2022;24:1403–1412. doi: 10.1007/s12094-022-02786-y. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 20.Li W., Wang Y., Liu R., Kasinski A.L., Shen H., Slack F.J., et al. MicroRNA-34a: potent tumor suppressor, cancer stem cell inhibitor, and potential anticancer therapeutic. Front. Cell Dev. Biol. 2021;9:1–21. doi: 10.3389/fcell.2021.640587. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tazawa H., Tsuchiya N., Izumiya M., Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc. Natl. Acad. Sci. USA. 2007;104:15472–15477. doi: 10.1073/pnas.0707351104. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X., Chen W., Miao R., Zhou Y., Wang Z., Zhang L., et al. miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget. 2015;6:3988–4004. doi: 10.18632/oncotarget.2905. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong D.S., Kang Y.K., Borad M., Sachdev J., Ejadi S., Lim H.Y., et al. Phase 1 study of MRX34, a liposomal Mir-34a mimic, in patients with advanced solid tumours. Br. J. Cancer. 2020;122:1630–1637. doi: 10.1038/s41416-020-0802-1. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu J., Imani S., Wu M.Y., Wu R.C. MicroRNA-34 family in cancers: role, mechanism, and therapeutic potential. Cancers. 2023;15:1–27. doi: 10.3390/cancers15194723. (Basel) [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin X., Zhan J.K., Zhong J.Y., Wang Y.J., Wang Y., Li S., et al. lncRNA-ES3/miR-34c-5p/BMF axis is involved in regulating high-glucose-induced calcification/senescence of VSMCs. Aging. 2019;11:523–535. doi: 10.18632/aging.101758. (Albany NY) [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou B.R., Guo X.F., Zhang J.A., Xu Y., Li W., Wu D., et al. Elevated miR-34c-5p mediates dermal fibroblast senescence by ultraviolet irradiation. Int. J. Biol. Sci. 2013;9:743–752. doi: 10.7150/ijbs.5345. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng D., Wang H., Li L., Ma X., Chen Y., Zhou H., et al. MiR-34c-5p promotes eradication of acute myeloid leukemia stem cells by inducing senescence through selective RAB27B targeting to inhibit exosome shedding. Leukemia. 2018;32:1180–1188. doi: 10.1038/s41375-018-0015-2. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 28.Liu C., Rokavec M., Huang Z., Hermeking H. Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 2023;30:1771–1785. doi: 10.1038/s41418-023-01178-1. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee I., Piao S., Kim S., Nagar H., Choi S.J., Kim M., et al. IDH2 deficiency promotes endothelial senescence by eliciting miR-34b/c-mediated suppression of mitophagy and increased ROS production. Antioxidants. 2023;12:1–15. doi: 10.3390/antiox12030585. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao X., Xu M., Yu H., Wang L., Li X., Rak J., et al. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct. Target. Ther. 2021;6:1–15. doi: 10.1038/s41392-021-00765-3. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nalini V., Ashley X., Sindhu K J., Sandhya S., Himanshu S., Devarajan K. miR-34b associates with epithelial-mesenchymal transition and regulates BMP7, CAV1, ID2 and FN1 in human cervical cancer. BioRxiv. 2022:1–34. doi: 10.1101/2021.09.02.458804. [Internet] Available from. [DOI] [Google Scholar]
- 32.Fu K., Li Y., Song J., Cai W., Wu W., Ye X., et al. Identification of a MicroRNA signature associated with lymph node metastasis in endometrial endometrioid cancer. Front. Genet. 2021;12:1–12. doi: 10.3389/fgene.2021.650102. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu S., Liu W., Shi H., Zhou H. Exosomal miR‑34b inhibits proliferation and EMT by targeting NOTCH2 in ovarian cancer. Oncol. Lett. 2020;20:2721–2728. doi: 10.3892/ol.2020.11837. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Z., Zhang G., Xie C., Zhou Y. Mir-34b regulates cervical cancer cell proliferation and apoptosis. Artif. Cells Nanomed. Biotechnol. 2019;47:2042–2047. doi: 10.1080/21691401.2019.1614013. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 35.Anaya J. Oncolnc: linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput. Sci. 2016;2:1–13. doi: 10.7717/peerj-cs.67. [Internet] Available from. [DOI] [Google Scholar]
- 36.Tang Z., Li C., Kang B., Gao G., Li C., Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–102. doi: 10.1093/nar/gkx247. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mcgeary S.E., Lin K.S., Shi C.Y., Pham T.M., Bisaria N., Kelley G.M., et al. The biochemical basis of microRNA targeting efficacy. Science. 2019;366:eaav1741. doi: 10.1126/science.aav1741. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murty V., Narayan G., Arias-Pulido H., Mansukhani M., Schneider A. Identification of gene expression profiles in cervical cancer. NCBI GEO [Internet]. 2008; Available from: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE9750.
- 39.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 40.Campos-Melo D., Droppelmann C.A., Volkening K., Strong M.J. Comprehensive luciferase-based reporter gene assay reveals previously masked up-regulatory effects of miRNAs. Int. J. Mol. Sci. 2014;15:15592–15602. doi: 10.3390/ijms150915592. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng H., Ge F., Du L., Zhang Z., Liu D. MiR-34b-3p represses cell proliferation, cell cycle progression and cell apoptosis in non-small-cell lung cancer (NSCLC) by targeting CDK4. J. Cell. Mol. Med. 2019:5282–5291. doi: 10.1111/jcmm.14404. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Córdova-Rivas S., Fraire-Soto I., Torres A.M.C., Servín-González L.S., Granados-López A.J., López-Hernández Y., et al. 5p and 3p strands of miR-34 family members have differential effects in cell proliferation, migration, and invasion in cervical cancer cells. Int. J. Mol. Sci. 2019;20:545. doi: 10.3390/ijms20030545. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saul D., Kosinsky R.L., Atkinson E.J., Doolittle M.L., Zhang X., Lebrasseur N.K., et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 2022;13:1–15. doi: 10.1038/s41467-022-32552-1. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tokar T., Pastrello C., Rossos A.E.M., Abovsky M., Hauschild A.C., Tsay M., et al. MirDIP 4.1–integrative database of human microRNA target predictions. Nucleic Acids Res. 2018;46:D360–D370. doi: 10.1093/nar/gkx1144. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao C.Y., Szekely L., Bao W., Selivanova G. Rescue of p53 function by small-molecule RITA in cervical carcinoma by blocking E6-mediated degradation. Cancer Res. 2010;70:3372–3381. doi: 10.1158/0008-5472.CAN-09-2787. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 46.Zhu H., Abulimiti M., Liu H., Su X.J., Liu C.H., Pei H.P. RITA enhances irradiation-induced apoptosis in p53-defective cervical cancer cells via upregulation of IRE1α/XBP1 signaling. Oncol. Rep. 2015;34:1279–1288. doi: 10.3892/or.2015.4083. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 47.Shan C., Chen X., Cai H., Hao X., Li J., Zhang Y., et al. The emerging roles of autophagy-related micrornas in cancer. Int. J. Biol. Sci. 2020;17:134–150. doi: 10.7150/ijbs.50773. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suh N. MicroRNA controls of cellular senescence. BMB Rep. 2018;51:493–499. doi: 10.5483/BMBRep.2018.51.10.209. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bayraktar E., Bayraktar R., Oztatlici H., Lopez-Berestein G., Amero P., Rodriguez-Aguayo C. Targeting miRNAs and other non-coding RNAs as a therapeutic approach: an update. Non-Coding RNA. 2023;9:1–31. doi: 10.3390/ncrna9020027. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalfert D., Ludvikova M., Pesta M., Ludvik J., Dostalova L., Kholová I. Multifunctional roles of miR-34a in cancer: a review with the emphasis on head and neck squamous cell carcinoma and thyroid cancer with clinical implications. Diagnostics. 2020;10 doi: 10.3390/daignostics10080563. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumamoto K., Spillare E.A., Fujita K., Horikawa I., Yamashita T., Appella E., et al. Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir-34b, and mir-34c expression, and induce senescence. Cancer Res. 2008;68:3193–3203. doi: 10.1158/0008-5472.CAN-07-2780. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang W., Hickson L.T.J., Eirin A., Kirkland J.L., Lerman L.O. Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 2022;18:611–627. doi: 10.1038/s41581-022-00601-z. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He L., He X., Lim L.P., De Stanchina E., Xuan Z., Liang Y., et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen S., Liu R., Wang Q., Qi Z., Hu Y., Zhou P., et al. MiR-34s negatively regulate homologous recombination through targeting RAD51. Arch. Biochem. Biophys. 2019;666:73–82. doi: 10.1016/j.abb.2019.03.017. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 55.Yoon M.H., Mi Kang S, Lee S.J., Woo T.G., Oh A.Y., Park S., et al. p53 induces senescence through lamin A/C stabilization-mediated nuclear deformation. Cell Death Dis. 2019;10:1–18. doi: 10.1038/s41419-019-1378-7. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Capo-Chichi C.D., Aguida B., Chabi N.W., Cai Q.K., Offrin G., Agossou V.K., et al. Lamin A/C deficiency is an independent risk factor for cervical cancer. Cell. Oncol. 2016;39:59–68. doi: 10.1007/s13402-015-0252-6. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 57.Nair R.R., Bagheri M., Saini D.K. Temporally distinct roles of ATM and ROS in genotoxic-stress-dependent induction and maintenance of cellular senescence. J. Cell Sci. 2015;128:342–353. doi: 10.1242/jcs.159517. Available from. [DOI] [PubMed] [Google Scholar]
- 58.Zhao Z., Rahman M.A., Chen Z.G., Shin D.M. Multiple biological functions of Twist1 in various cancers. Oncotarget. 2017;8:20380–20393. doi: 10.18632/oncotarget.14608. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tran P.T., Shroff E.H., Burns T.F., Thiyagarajan S., Das S.T., Zabuawala T., et al. Twist1 suppresses senescence programs and thereby accelerates and maintains mutant Kras-induced lung tumorigenesis. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002650. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merindol N., Riquet A., Szablewski V., Eliaou J.F., Puisieux A., Bonnefoy N. The emerging role of Twist proteins in hematopoietic cells and hematological malignancies. Blood Cancer J. 2014;4:e206. doi: 10.1038/bcj.2014.22. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang T., Li Y., Tuerhanjiang A., Wang W., Wu Z., Yuan M., et al. Correlation of twist upregulation and senescence bypass during the progression and metastasis of cervical cancer. Front. Med. China. 2014;8:106–112. doi: 10.1007/s11684-014-0307-5. Available from. [DOI] [PubMed] [Google Scholar]
- 62.Burns T.F., Dobromilskaya I., Murphy S.C., Gajula R.P., Thiyagarajan S., Chatley S.N.H., et al. Inhibition of TWIST1 leads to activation of oncogene-induced senescence in oncogene-driven non-small cell lung cancer. Mol. Cancer Res. 2013;11:329–338. doi: 10.1158/1541-7786.MCR-12-0456. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nayak D., Kumar A., Chakraborty S., Ur Rasool R., Amin H., Katoch A., et al. Inhibition of Twist1-mediated invasion by Chk2 promotes premature senescence in p53-defective cancer cells. Cell Death Differ. 2017;24:1275–1287. doi: 10.1038/cdd.2017.70. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu H.B., Bian W.G., Zhang L.J., Mei N., Wu Y., Wei Y.Q., et al. Inhibition of p53/p21 by TWIST alleviates TNF-α induced nucleus pulposus cell senescence in vitro. Eur. Rev. Med. Pharmacol. Sci. 2021;25:12645–12654. doi: 10.26355/eurrev_202012_24161. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 65.Cui Y., Wu Y., Wang C., Wang Z., Li Y., Jiang Z., et al. Isoliquiritigenin inhibits non-small cell lung cancer progression via m6A/IGF2BP3-dependent TWIST1 mRNA stabilization. Phytomedicine. 2022;104:1–10. doi: 10.1016/j.phymed.2022.154299. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 66.Pei H., Li Y., Liu M., Chen Y. Targeting twist expression with small molecules. MedChemComm. 2017;8:268–275. doi: 10.1039/C6MD00561F. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data related to the study is available in the text. Additional data are available on reasonable request. GSE9750 dataset has been used here and is freely available in the NCBI GEO repository.