Abstract
INTRODUCTION
We examined the relationship between sedentary behavior (SB), moderate‐to‐vigorous physical activity (MVPA), and white matter hyperintensity (WMH) volumes, a common magnetic resonance imaging (MRI) marker associated with risk of neurodegenerative disease in middle‐aged to older adults.
METHODS
We used data from the UK Biobank (n = 14,415; 45 to 81 years) that included accelerometer‐derived measures of SB and MVPA, and WMH volumes from MRI.
RESULTS
Both MVPA and SB were associated with WMH volumes (βMVPA = −0.03 [−0.04, −0.01], p < 0.001; βSB = 0.02 [0.01, 0.03], p = 0.007). There was a significant interaction between SB and MVPA on WMH volumes (βSB×MVPA = −0.015 [−0.028, −0.001], p SB×MVPA = 0.03) where SB was positively associated with WMHs at low MVPA, and MVPA was negatively associated with WMHs at high SB.
DISCUSSION
While this study cannot establish causality, the results highlight the potential importance of considering both MVPA and SB in strategies aimed at reducing the accumulation of WMH volumes in middle‐aged to older adults.
Highlights
SB is associated with greater WMH volumes and MVPA is associated with lower WMH volumes.
Relationships between SB and WMH are strongest at low levels of MVPA.
Associations between MVPA and WMH are strongest at high levels of SB.
Considering both SB and MVPA may be effective strategies for reducing WMHs
Keywords: brain aging, brain health, exercise, lifestyle behaviors, physical activity, sitting
1. INTRODUCTION
White matter hyperintensity (WMH) volume measured with magnetic resonance imaging (MRI) serves as a significant indicator of the extent of cerebral white matter lesions, typically associated with ischemia due to small vessel disease. 1 , 2 WMH volumes (WMHs) are often associated with peripheral vascular risk factors including hypertension and diabetes. 3 WMHs are frequently found in older cognitively unimpaired individuals, are linked with worse cognitive performance, particularly executive functions and processing speed, 2 , 4 , 5 , 6 , 7 are associated with genetic risk of neurodegenerative disease, 8 and can potentially impact both the onset and advancement of dementia related to both Alzheimer's disease (AD) and cerebrovascular disease (CVD). 9 , 10 , 11 , 12 , 13 Here, we examine the potential associations of physical activity (PA) and sedentary behaviors (SBs), two modifiable lifestyle factors, with WMH volumes in middle‐aged to older adults.
Recent work suggests both PA and SB may play a role in either mitigating or exacerbating WMHs. 14 , 15 For purposes here, SB is defined as “any waking behavior characterized by an energy expenditure ≤1.5 METs [Metabolic Equivalent Units] while in a sitting or reclining posture.” 16 Multiple studies have documented inverse associations between PA and WMH volumes. 15 , 17 , 18 , 19 It is possible that these associations are linked with the beneficial impacts of PA on vascular health. 20 In addition to PA, SBs may have an influence on both vascular health and WMH volumes. 21 While fewer studies have examined SBs and WMHs, there is some evidence that time spent in SBs are positively associated with WMH volumes 14 ; however, others have failed to detect associations between SB and WMH volumes and there is inconsistency in whether PA has been included as a moderating factor. 14 Similar to PA‐related mechanisms, it is possible that SBs are linked with WMHs via vascular pathways, since SBs are often associated with poor cardiometabolic outcomes and have been further associated with dementia risk. 21 , 22 , 23
In addition, several researchers have suggested that PA and SB may be distinct in their physiological effects and that, for example, long periods of time spent in SB may be detrimental to health regardless of engagement in PA. 21 , 23 , 24 , 25 For example, recent work based on a harmonized meta‐analysis suggests that associations between SB and all‐cause or cardiovascular mortality are partially distinct from PA, although effects are strongest in those who engage in lower amounts of PA. 26 , 27 Fewer studies have examined how PA and SB interact within the context of brain health and aging. Notably, Raichlen et al. 23 showed that risk of dementia associated with SBs in older adults was at least partially separable from engagement in PA. It is therefore important to further examine whether and how these lifestyle behaviors are associated with age‐related vascular brain outcomes to better understand their potential influence on cerebrovascular health and to help determine public health recommendations.
In this study we examine whether PA and SB are associated with WMH volumes in the UK Biobank, a large prospective cohort that includes neuroimaging and device‐measured PA and SB. In addition, we assess whether PA and SB associations are separable from each other and whether they interact in their association with WMH volumes. Findings from this work will help us better understand how modifiable lifestyle behaviors influence each other in their associations with this key brain‐based risk factor for AD and CVD.
2. METHODS
2.1. Participants
The study utilized data from the UK Biobank, consisting of community‐dwelling participants aged 40 and older from England, Scotland, and Wales, with initial data gathered between 2006 and 2010. 28 All subjects gave written consent, with the study receiving approval from the National Health Service and the National Research Ethics Service. All participants provided written informed consent. A sub‐study conducted from 2013 to 2015 included 103,684 participants who consented to wear an Axivity AX3 tri‐axial accelerometer on their dominant wrist, 24 hours a day for a week. 29 An imaging sub‐study that included brain MRI was begun in August 2014 and is currently ongoing. 30 Although follow‐up imaging is currently ongoing, we include only data from the first imaging visit in this analysis. Analyses presented here were confined to participants who had not been diagnosed with all‐cause dementia before the imaging exam date, and who also participated in both the accelerometer and the imaging sub‐studies. The dataset used for analyses here was downloaded in April 2023.
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed the literature using traditional (eg, PubMed) sources and meeting abstracts and presentations. There have been recent publications describing associations between sedentary behavior (SB) or moderate‐to‐vigorous intensity physical activity (MVPA) and brain health, including white matter hyperintensity (WMH) volumes. These publications have been appropriately cited.
Interpretation: In this cohort study from the UK Biobank, SB was positively associated with WMH volumes and MVPA was negatively associated with WMH volumes. Notably, there was a significant interaction between SB and MVPA where the positive association between SB and WMHs was more pronounced at lower levels of MVPA, and the negative association between MVPA and WMHs was stronger at higher levels of SB.
Future Directions: While these associations suggest that the combination of MVPA and SB behaviors may be an important target for lifestyle modification to enhance brain health, studies examining causal links are promising areas for future research.
2.2. Dementia diagnoses
Hospital inpatient records, death registries, and self‐reported dementia diagnoses were used to determine all‐cause dementia diagnoses for participant exclusion. 31 The International Classification of Diseases, Ninth and Tenth Revisions (ICD‐9 and ICD‐10) codes were used to classify participants with dementia (see Table S1).
2.3. Exposures
PA and SB were identified from raw accelerometer data using a previously published machine learning algorithm developed and validated for use with the UK Biobank. 32 The algorithm was developed from a cohort of 152 adults (aged 18 to 91) who wore an Axivity AX3 accelerometer and a wearable camera, and kept a time‐use diary during daily life. The researchers annotated accelerometer data with activities from the Compendium of Physical Activities 33 and trained machine‐learning models to classify behaviors in 30‐second time windows of accelerometer data. 32 Intensity of activity was defined by estimated METs which measure energy expenditure relative to basal metabolic rate. Behaviors were identified as moderate‐to‐vigorous PA (MVPA) if they were performed at intensities greater than or equal to 3.5 METs. SBs were defined as any waking behaviors that occur at intensities less than or equal to 1.5 METs in a lying, sitting, or reclining posture. In the present study, we excluded individuals with extreme values of sedentary behavior (greater than 18 hours/day 34 , 35 , 36 , 37 ), extreme values of PA (average acceleration values greater than 100 mg following Walmsley et al. 32 ), or less than three days of valid data (defined as days with greater than or equal to 16 hours of valid data/day).
2.4. Outcome
Detailed methods for multimodal MRI data acquisition and processing are described elsewhere, 30 and are available online for the imaging protocols (http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id = 2367) and in the Brain Imaging Documentation (http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id = 1977). Briefly, brain imaging in the UK Biobank was performed on a Siemens Skyra 3T system with a 32‐channel RF receive head coil. 30 , 38 The main outcome assessed was WMH volume utilizing T1‐weighted and T2 fluid attenuated inversion recovery (FLAIR) scans with FMRIB Software Library (FSL) software. 39 Volumetric 3D MPRAGE T1‐weighted scans with 1 mm isotropic resolution, 256 mm field‐of‐view, 208 × 256 × 256 matrix, and TI/TR = 880/2000 ms were acquired in sagittal orientation. T2 FLAIR scans were acquired with 3D SPACE, 1.05 × 1 × 1 mm resolution, 192 × 256 × 256 matrix, and TI/TR = 1800/5000 ms in sagittal orientation. Initial pre‐processing and quality control (QC) procedures were applied with FSL software to remove non‐usable MRI scans from the available UK Biobank data. 30 , 38 We additionally excluded participants with structural MRI scan values greater than three standard deviations away from the mean of any of the five QC metrics provided by the UK Biobank (fields 25731 to 25735). 18 To achieve normalization and stabilization of variance, we applied a log transformation to WMH volumes (see Figure S1). An estimate of total intracranial volume (TIV) was used as a covariate, which was derived from FreeSurfer software (v6.0) (field 26521). In this sample, neuroimaging occurred 2.82 (± 1.72) years after wearing accelerometers.
2.5. Statistical analyses
We conducted a complete case analysis examining associations between modifiable lifestyle behaviors and WMH volumes. We used a series of general linear models to determine the associations between SB, MVPA, and WMH volumes. To achieve normalization and stabilization of variance, we applied a square root transformation to MVPA (MVPAsqrt). To improve interpretability, we transformed SB and MVPAsqrt to have a mean of zero and a standard deviation of one. In minimally adjusted models, we included either SB, MVPAsqrt, or both SB and MVPAsqrt, along with age at the imaging visit, sex, and TIV. In fully adjusted models, we added the following covariates measured at baseline to the minimally adjusted models. Education was coded as having a college or university degree versus no college or university degree. Socioeconomic status was assessed by the Townsend Deprivation Index. The index is calculated using four key variables obtained from census data: unemployment rate, non‐car ownership, non‐home ownership, and household overcrowding. These variables are combined to create a single composite score, which is then standardized to have a mean of zero and a standard deviation of one. Higher scores on the Townsend Deprivation Index indicate higher levels of socioeconomic deprivation, while lower scores represent lower levels of deprivation. Chronic conditions were scored as whether or not a physician had diagnosed vascular or heart problems (heart attack, angina, stroke, or high blood pressure), diabetes, or cancer. Diagnosed vascular or heart problems, diabetes, and cancer were included as separate covariates in the models. Smoking status was self‐reported as never smoker, former smoker, or current smoker. Presence of the apolipoprotein E (APOE) ε4 allele was coded as possessing the ε4 allele or not. We also included the location of the MRI imaging center and the difference in time between accelerometer wear date and MRI imaging date as covariates. Finally, body mass index (BMI) was included in fully adjusted models. In sensitivity analyses, we further adjusted our analyses for joint disorders that may limit PA (rheumatoid arthritis, osteoarthritis, and other joint disorders and joint pain) coded in interviews by a trained nurse. In a second sensitivity analysis, we removed participants with imaging visits that preceded accelerometer wear (n = 807). All p‐values were corrected based on the false discovery rate (FDR) for multiple tests.
In addition, we examined the potential for associations between these lifestyle behaviors and WMH volume to modify each other using an interaction term in both minimally and fully adjusted models. If there was a significant interaction, the Johnson‐Neyman technique 40 was used to assess at what values of the moderator (MVPA or SB) the effect of the exposure on WMH volume transitions from being significant to non‐significant. All statistical analyses were performed in R version 4.3.1. We used the Interactions package (version 1.1.5) to implement the Johnson‐Neyman methods. 41
3. RESULTS
We included 14,415 individuals in this study after excluding participants with missing data for imaging, accelerometer measures, covariates, and those with prevalent dementia at imaging (Figure 1). Participant characteristics for our final sample (n = 14,415) are given in Table 1.
FIGURE 1.
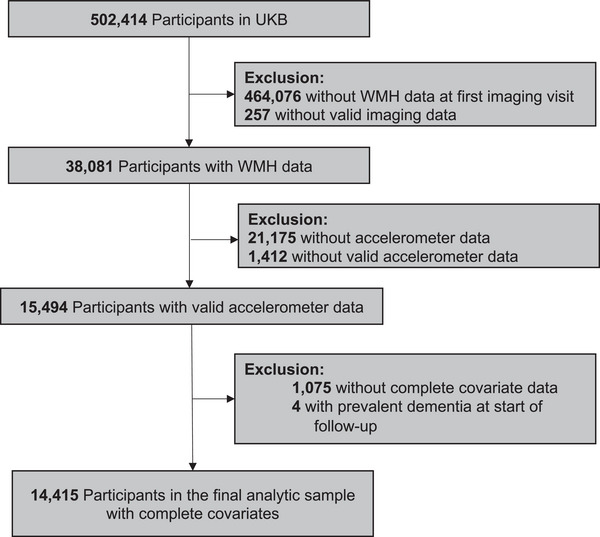
Flow diagram for participants included in the study. UKB, UK Biobank; WMH, white matter hyperintensity.
TABLE 1.
Participant characteristics (n = 14,415).
| Variables | mean (SD) or n (%) |
|---|---|
| Age (years) | 63.69 (7.6) |
| Sex (F) | 7988 (55.41) |
| Sex (M) | 6427 (44.59) |
| Education (college or higher) | 6900 (47.87) |
| Townsend deprivation index | −1.95 (2.68) |
| BMI (kg/m2) | 26.38 (4.2) |
| Heart disease (present) | 2973 (20.63) |
| Diabetes (present) | 322 (2.24) |
| Cancer (present) | 777 (5.39) |
| Smoking Status | |
| current | 8929 (61.94) |
| former | 4727 (32.79) |
| never | 759 (5.27) |
| APOE status | |
| 0 ε4 alleles | 10,714 (74.33) |
| 1 ε4 allele | 3366 (23.35) |
| 2 ε4 alleles | 335 (2.32) |
| SB (hours/day) | 9.34 (1.78) |
| MVPA (hours/day) | 0.74 (0.6) |
Note: Values for MVPA are given prior to square root transformation.
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; F, female; M, male; MVPA, moderate‐to‐vigorous physical activity; SB, sedentary behavior.
SB and MVPAsqrt were negatively correlated with each other (r = −0.24; p < 0.001; see Figure S2). In single‐variable minimally‐adjusted models, MVPAsqrt was negatively associated with WMH volume and SB was positively associated with WMH volume (Table 2; see Table S2 for results SB and MVPAsqrt before scaling). These results remained significant when models were fully adjusted for a wide range of covariates (Table 2). When models were mutually adjusted for both SB and MVPAsqrt, MVPAsqrt remained negatively related to WMH volume; however the relationship between SB and WMH volume was attenuated towards the null in the fully adjusted model (Table 2). Results were similar in sensitivity analyses that controlled for joint disorders and that included only subjects with accelerometer wear that preceded imaging (Tables S3 & S4).
TABLE 2.
Associations between MVPA, SB, and WMH volumes.
| covariates | model | variable | beta (95% CI) | p | FDR p |
|---|---|---|---|---|---|
| Minimally adjusted | single variable | SB | 0.04 (0.02, 0.05) | <0.001 | <0.001 |
| single variable | MVPAsqrt | −0.05 (−0.07, −0.04) | <0.001 | <0.001 | |
| mutually adjusted | SB | 0.02 (0.01, 0.04) | 0.006 | 0.008 | |
| mutually adjusted | MVPAsqrt | −0.05 (−0.06, −0.03) | <0.001 | <0.001 | |
| Fully adjusted | single variable | SB | 0.02 (0.01, 0.03) | 0.007 | 0.008 |
| single variable | MVPAsqrt | −0.03 (−0.04, −0.01) | <0.001 | <0.001 | |
| mutually adjusted | SB | 0.01 (0.00, 0.03) | 0.073 | 0.073 | |
| mutually adjusted | MVPAsqrt | −0.02 (−0.04, −0.01) | 0.004 | 0.006 |
Note: Minimally adjusted models include age, sex, and TIV. Fully adjusted models include covariates in minimally adjusted model and education, Townsend deprivation index, smoking status, presence of a diagnosed chronic disease, BMI, APOE ε4 allele status, location of assessment center for imaging, and the difference in time between accelerometer wear date and imaging date. Single variable models include only SB or MVPAsqrt, while mutually adjusted models include both SB and MVPAsqrt. Both SB and MVPAsqrt are scaled to have a mean of zero and SD of 1.
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; CI, confidence interval; F, female; FDR, false discovery rate; M, male; MVPA, moderate‐to‐vigorous physical activity; SB, sedentary behavior; TIV, total intracranial volume; WMH, white matter hyperintensity.
We also investigated the interaction between MVPAsqrt and SB on WMH volume. In both minimally and fully adjusted models, there were significant interactions between these two lifestyle behaviors (minimally adjusted: βSB×MVPA −0.023 [−0.036, −0.010], p SB×MVPA < 0.001; fully adjusted: βSB×MVPA = −0.015 [−0.028, −0.001], p SB×MVPA = 0.03). The Johnson‐Neyman analysis shows that SB was significantly associated with WMH volume at only low levels of MVPAsqrt (< 0.52 hours/day; Figure 2A), while MVPAsqrt was associated with WMH volume at high levels of SB (> 8.25 hours/day; Figure 2B).
FIGURE 2.
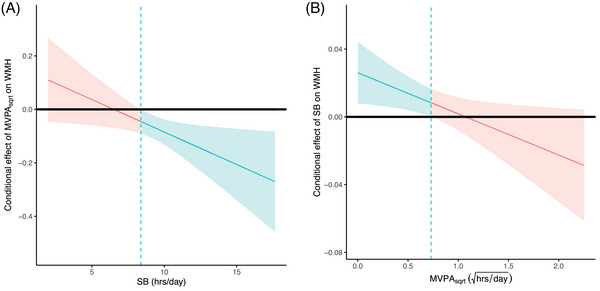
Johnson‐Neyman plots of interactions between moderate‐to‐vigorous physical activity (MVPA) and sedentary behavior (SB) on white matter intensity (WMH) volume. MVPA is square root transformed in these figures. (A) Conditional effects of MVPA on WMH volume as a function SB. (B) Conditional effects of SB on WMH volume as a function of MVPA. MVPA was square root transformed for these analyses. The dashed vertical line represents the point where the relationship between the exposure and outcome transitions in significance levels. In (A), at SB less than 8.25 hours per day, the relationship between MVPA and WMH volume is not significant (red line and red shaded 95% CIs). The relationship transitions to significant at SBs greater than 8.25 hours/day (blue line and blue shaded 95% confidence intervals [CIs]). In (B), at square root transformed MVPA less than 0.72 (0.52 hours/day untransformed), SB is significantly associated with WMH volume (blue line and blue shaded 95% CIs), while at larger MVPA values, SB is no longer significantly associated with WMH volumes (red line and red shaded 95% CIs).
4. DISCUSSION
In the UK Biobank, we found associations of both MVPAsqrt and SB with WMH volume, and the associations are not fully independent of each other. When both exposures were included in fully adjusted models, there was some attenuation of effects, especially for SB, supporting the possibility that effects were not fully independent. It is also possible that one way that SB is linked with WMH is through the relationship between SB and MVPAsqrt. For example, if larger amounts of SB come at the expense of time spent in MVPA, then it is possible the displacement may partially explain our results. Future work that includes full 24‐hour time use to account for behavioral displacement is needed to better interpret these results.
Importantly, when an interaction term was included, MVPAsqrt and SB modified each other to influence the volume of WMH lesion load in the brain. This interdependence indicates that the detrimental effects of SB on brain health may be mitigated by higher levels of MVPAsqrt. Conversely, the associations of higher MVPAsqrt with lower WMH volume are most pronounced when sedentary time is high and MVPAsqrt is less impactful when SB time is lowest. These results suggest that it may be important to consider both behaviors simultaneously when developing prescriptions and interventions aimed at reducing WMH volume and the associated risks of cognitive decline and dementia. Our results suggest that engaging in MVPA may mitigate the risk of WMH volumes associated with high amounts of SB but that for individuals who cannot participate in MVPA due to mobility limitations, or who engage in only small amounts of MVPA, less SB may be linked with lower WMH volumes.
A large number of studies have shown that engaging in greater amounts of MVPA is associated with improved vascular health and that SB is associated with vascular pathology and chronic disease. 21 , 25 , 27 , 42 One potential vascular mechanism that could underlie these results is that MVPA may increase cerebral blood flow, 43 , 44 which in turn may help prevent the development of high WMH loads. 45 SB, on the other hand, has been linked with reduced cerebral blood flow 44 , 46 which may lead to increased lesion load, though this finding has not been consistently replicated. 47 For example, Carter et al. 46 showed that while prolonged sitting (more than 4 hours at a time) was associated with reduced cerebral blood flow, a short duration walking break offset these effects.
Our results also align with the growing body of literature emphasizing the synergistic effects of higher PA and reduced SB on various health outcomes. 23 While previous studies have independently linked excessive SB and lack of MVPA with adverse brain health, our study demonstrates how these behaviors interact in their associations with WMH volumes. It is possible that the mechanisms linking PA and SB with WMH volumes may only partially overlap. For example, while both SB and MVPA have been linked with cerebral blood flow and vascular health in previous work, 43 , 46 MVPA is also associated with the upregulation of neurotrophic factors (eg, Brain Derived Neurotrophic Factor or BDNF) that may provide additional compensatory protection against the impact of increased WMH volumes. 48 While our study was not designed to determine whether mechanistic pathways are fully or partially independent, the interactions found here suggest more work is needed to better understand how these two lifestyle behaviors may differentially impact brain lesion loads that may, in turn, influence the risk for cognitive decline and dementia related to both AD and CVD. Overall, our results highlight the importance of considering interactions between these key modifiable behaviors when examining their associations with brain health outcomes.
Our findings may also help to clarify results from previous studies, which have shown inconsistent relationships between SB and WMH load. For example, Burzynska et al. 49 found no relationship between accelerometer‐derived SB and WMH load in a sample of 88 older adults. Other studies have found positive relationships between SB and WMH load 14 and some studies find this positive association only after adjusting for potential confounding variables. 47 , 50 MVPA was not taken into account in all of these studies and it is possible that better accounting for MVPA may clarify the impacts of SB on WMH volumes.
Results for the relationship between PA in general and WMH load also show some inconsistencies in the literature. In a systematic review of studies of PA and WMH load, Torres et al. 19 showed that three of the included six studies found significant inverse relationships between WMHs and PA, while the other three included studies found no relationship. It is also possible that heterogeneity of PA measurement, and the inclusion of intensities lower than moderate‐to‐vigorous in some of these samples may help account for inconsistent results. In a meta‐analysis of nine studies, Sexton et al. 15 found that PA (across intensities) or fitness was associated with smaller WMH loads, though effect sizes were small. Franchetti et al. 17 found a significant interaction between age group (oldest old [OO = 70 to 89 years old] and young old [YO = 50 to 69 years old]) and self‐reported physical sport activity (PSA) on WMH volume, such that the OO group with low PSA had greater WMH volumes than the YO with low PSA and OO with high PSA groups. WMH volumes were similar in the YO and OO with high PSA values. 17 On balance, prior work has often found associations between PA and WMH volumes, but the strength of these relationships depends on the covariates included and it is possible that inclusion of PA intensities lower than moderate‐to‐vigorous may factor into these inconsistencies. Our results further suggest that inclusion of SB may help us better understand observed differences in previous results related to MVPA and WMH volumes.
4.1. Strengths and limitations
Among this study's strengths is the use of a large sample size to examine associations between behavior and WMH load and the inclusion of novel, machine‐learning derived accelerometry measures. The sample also includes a wide array of covariates that help adjust for potential confounding variables. This study also has several limitations. First, the observational study design used here may still allow for residual or unmeasured confounding. Second, we cannot rule out reverse causality using this study design where individuals with greater WMH loads spent more time in SB and less time in PA due to the potential health impacts of these lesions. Third, the accelerometer and imaging sub‐studies were performed at different times, and accelerometer data were collected only once. Our sensitivity analysis shows that results remain similar when only including participants with imaging visits that follow accelerometer wear, which suggests variation in timing of data collection did not have an appreciable impact on our findings. Fourth, the participants in sub‐studies were self‐selected from a randomly invited subset of the larger UK Biobank cohort, which may introduce selection bias in studies of these participants. Fifth, it is difficult to assess posture using wrist‐based accelerometers making it more difficult to measure SBs, and future studies should prioritize the use of thigh‐mounted accelerometers to replicate these results. Sixth, the machine learning algorithm developed by Walmsley et al. 32 was internally validated in a sample of 152 adults that included a small subset of participants older than 60 (n = 27), and future work should focus on further validating this algorithm on a larger external sample of older adults. Seventh, the UK Biobank is racially and ethnically homogenous, limiting the generalizability of these findings for other populations.
5. CONCLUSIONS
Our results highlight associations between two key modifiable lifestyle behaviors and brain WMH lesion loads in middle‐aged to older adults, demonstrating a synergistic relationship between MVPA and SB in their association with WMH volume. This novel finding suggests that, given the potential importance of WMH loads in the development of neurodegenerative diseases like AD and CVD, these behaviors may represent key targets for future interventions to help reduce risks for dementia and associated cognitive decline. While we cannot infer causality due to the observational design, these results underscore the idea that a combination of sitting less and moving more represents a potential focus for public health recommendations. Future work that establishes both causality and the underlying mechanistic pathways is necessary to help generate more direct prescriptions to support brain health among middle‐aged to older adults.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest. Author disclosures are available in the Supporting information.
CONSENT STATEMENT
All participants provided written informed consent.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This research was conducted using the UK Biobank Resource under Application Number 15678. We thank the participants and organizers of the UK Biobank. Study authors are supported by the NIH (P30AG072980, P30AG019610, R56AG067200, R01AG064587, R01AG072445), the state of Arizona and Arizona Department of Health Services, and the McKnight Brain Research Foundation. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Raichlen DA, Ally M, Aslan DH, et al. Associations between accelerometer‐derived sedentary behavior and physical activity with white matter hyperintensities in middle‐aged to older adults. Alzheimer's Dement. 2024;16:e70001. 10.1002/dad2.70001
REFERENCES
- 1. Biesbroek JM, Weaver NA, Biessels GJ. Lesion location and cognitive impact of cerebral small vessel disease. Clin Sci (Colch). 2017;131(8):715‐728. [DOI] [PubMed] [Google Scholar]
- 2. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11(3):157‐165. [DOI] [PubMed] [Google Scholar]
- 3. Cox SR, Lyall DM, Ritchie SJ, et al. Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J. 2019;40(28):2290‐2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolandzadeh N, Davis JC, Tam R, Handy TC, Liu‐Ambrose T. The association between cognitive function and white matter lesion location in older adults: a systematic review. BMC Neurol. 2012;12(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garnier‐Crussard A, Bougacha S, Wirth M, et al. White matter hyperintensity topography in Alzheimer's disease and links to cognition. Alzheimers Dement. 2022;18(3):422‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kloppenborg RP, Nederkoorn PJ, Geerlings MI, van den Berg E. Presence and progression of white matter hyperintensities and cognition: a meta‐analysis. Neurology. 2014;82(23):2127‐2138. [DOI] [PubMed] [Google Scholar]
- 7. Song H, Bharadwaj PK, Raichlen DA, et al. Association of homocysteine‐related subcortical brain atrophy with white matter lesion volume and cognition in healthy aging. Neurobiol Aging. 2023;121:129‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyall DM, Cox SR, Lyall LM, et al. Association between APOE e4 and white matter hyperintensity volume, but not total brain volume or white matter integrity. Brain Imaging Behav. 2020;14:1468‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Birdsill AC, Koscik RL, Jonaitis EM, et al. Regional white matter hyperintensities: aging, Alzheimer's disease risk, and cognitive function. Neurobiol Aging. 2014;35(4):769‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brickman AM, Schupf N, Manly JJ, et al. APOE ε4 and risk for Alzheimer's disease: do regionally distributed white matter hyperintensities play a role? Alzheimers Dement. 2014;10(6):619‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Debette S, Markus H. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ. 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rastogi A, Weissert R, Bhaskar SMM. Emerging role of white matter lesions in cerebrovascular disease. Eur J Neurosci. 2021;54(4):5531‐5559. [DOI] [PubMed] [Google Scholar]
- 13. Shirzadi Z, Schultz SA, Yau W‐YW, et al. Etiology of white matter hyperintensities in autosomal dominant and sporadic Alzheimer disease. JAMA Neurol. 2023;80(12):1353‐1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bronas UG, Steffen A, Dion C, et al. Sedentary time and white matter hyperintensity volume in older adults. Med Sci Sports Exerc. 2019;51(8):1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, Johansen‐Berg H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage. 2016;131:81‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tremblay MS, Aubert S, Barnes JD, et al. Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franchetti MK, Bharadwaj PK, Nguyen LA, et al. Interaction of age and self‐reported physical sports activity on white matter hyperintensity volume in healthy older adults. Front Aging Neurosci. 2020;12:576025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raichlen DA, Klimentidis YC, Bharadwaj PK, Alexander GE. Differential associations of engagement in physical activity and estimated cardiorespiratory fitness with brain volume in middle‐aged to older adults. Brain Imaging Behav. 2020;14:1994‐2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torres ER, Strack EF, Fernandez CE, Tumey TA, Hitchcock ME. Physical activity and white matter hyperintensities: a systematic review of quantitative studies. Prev Med Rep. 2015;2:319‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094. [DOI] [PubMed] [Google Scholar]
- 21. Dempsey PC, Matthews CE, Dashti SG, et al. Sedentary behavior and chronic disease: mechanisms and future directions. J Phys Act Health. 2020;17(1):52‐61. [DOI] [PubMed] [Google Scholar]
- 22. Raichlen DA, Aslan DH, Sayre MK, et al. Sedentary behavior and incident dementia among older adults. JAMA. 2023;330(10):934‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raichlen DA, Klimentidis YC, Sayre MK, et al. Leisure‐time sedentary behaviors are differentially associated with all‐cause dementia regardless of engagement in physical activity. Proc Natl Acad Sci. 2022;119(35):e2206931119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamilton MT. The role of skeletal muscle contractile duration throughout the whole day: reducing sedentary time and promoting universal physical activity in all people. J Physiol. 2018;596(8):1331‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamilton MT, Hamilton DG, Zderic TW. The necessity of active muscle metabolism for healthy aging: muscular activity throughout the entire day. Prog Mol Biol Transl Sci. 2018;155:53‐68. [DOI] [PubMed] [Google Scholar]
- 26. Ekelund U, Steene‐Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta‐analysis of data from more than 1 million men and women. Lancet North Am Ed. 2016;388(10051):1302‐1310. [DOI] [PubMed] [Google Scholar]
- 27. Ekelund U, Tarp J, Steene‐Johannessen J, et al. Dose‐response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta‐analysis. BMJ. 2019;366:4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Doherty A, Jackson D, Hammerla N, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank Study. PLoS One. 2017;12(2):e0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller KL, Alfaro‐Almagro F, Bangerter NK, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19(11):1523‐1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walmsley R, Chan S, Smith‐Byrne K, et al. Reallocation of time between device‐measured movement behaviours and risk of incident cardiovascular disease. Br J Sports Med. 2022;56(18):1008‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575‐1581. [DOI] [PubMed] [Google Scholar]
- 34. Clark BK, Lynch BM, Winkler EA, et al. Validity of a multi‐context sitting questionnaire across demographically diverse population groups: ausDiab3. Int J Behav Nutr Phy. 2015;12:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gardiner PA, Healy GN, Eakin EG, et al. Associations between television viewing time and overall sitting time with the metabolic syndrome in older men and women: the Australian diabetes obesity and lifestyle study. J Am Geriatr Soc. 2011;59(5):788‐796. [DOI] [PubMed] [Google Scholar]
- 36. Kettle VE, Hamer M, Munir F, et al. Cross‐sectional associations between domain‐specific sitting time and other lifestyle health behaviours: the Stormont study. J Public Health. 2022;44(1):51‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yates T, Khunti K, Wilmot EG, et al. Self‐reported sitting time and markers of inflammation, insulin resistance, and adiposity. Am J Prev Med. 2012;42(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 38. Alfaro‐Almagro F, Jenkinson M, Bangerter NK, et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Griffanti L, Zamboni G, Khan A, et al. BIANCA (Brain Intensity AbNormality Classification Algorithm): a new tool for automated segmentation of white matter hyperintensities. Neuroimage. 2016;141:191‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson PO, Neyman J. Tests of certain linear hypotheses and their application to some educational problems. Statistical Research Memoirs. 1936;1:57‐93. [Google Scholar]
- 41. Long J, Interactions: comprehensive, user‐friendly toolkit for probing interactions. R package version 110. 2019.
- 42. Pinto AJ, Bergouignan A, Dempsey PC, et al. The Physiology of Sedentary Behavior. Physiol Rev. 2023;103(4):2561‐2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scarmeas N, Zarahn E, Anderson KE, et al. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol. 2003;60(3):359‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zlatar ZZ, Hays CC, Mestre Z, et al. Dose‐dependent association of accelerometer‐measured physical activity and sedentary time with brain perfusion in aging. Exp Gerontol. 2019;125:110679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stewart CR, Stringer MS, Shi Y, Thrippleton MJ, Wardlaw JM. Associations between white matter hyperintensity burden, cerebral blood flow and transit time in small vessel disease: an updated meta‐analysis. Front Neurol. 2021;12:647848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carter SE, Draijer R, Holder SM, Brown L, Thijssen DH, Hopkins ND. Regular walking breaks prevent the decline in cerebral blood flow associated with prolonged sitting. J Appl Physiol. 2018;125(3):790‐798. [DOI] [PubMed] [Google Scholar]
- 47. Maasakkers CM, Melis RJ, Kessels RP, et al. The short‐term effects of sedentary behaviour on cerebral hemodynamics and cognitive performance in older adults: a cross‐over design on the potential impact of mental and/or physical activity. Alzheimers Res Ther. 2020;12:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pikula A, Beiser AS, Chen TC, et al. Serum brain–derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: framingham study. Stroke. 2013;44(10):2768‐2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burzynska AZ, Chaddock‐Heyman L, Voss MW, et al. Physical activity and cardiorespiratory fitness are beneficial for white matter in low‐fit older adults. PLoS One. 2014;9(9):e107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Launer LJ, Lewis CE, Schreiner PJ, et al. Vascular factors and multiple measures of early brain health: cARDIA brain MRI study. PLoS One. 2015;10(3):e0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


