Abstract
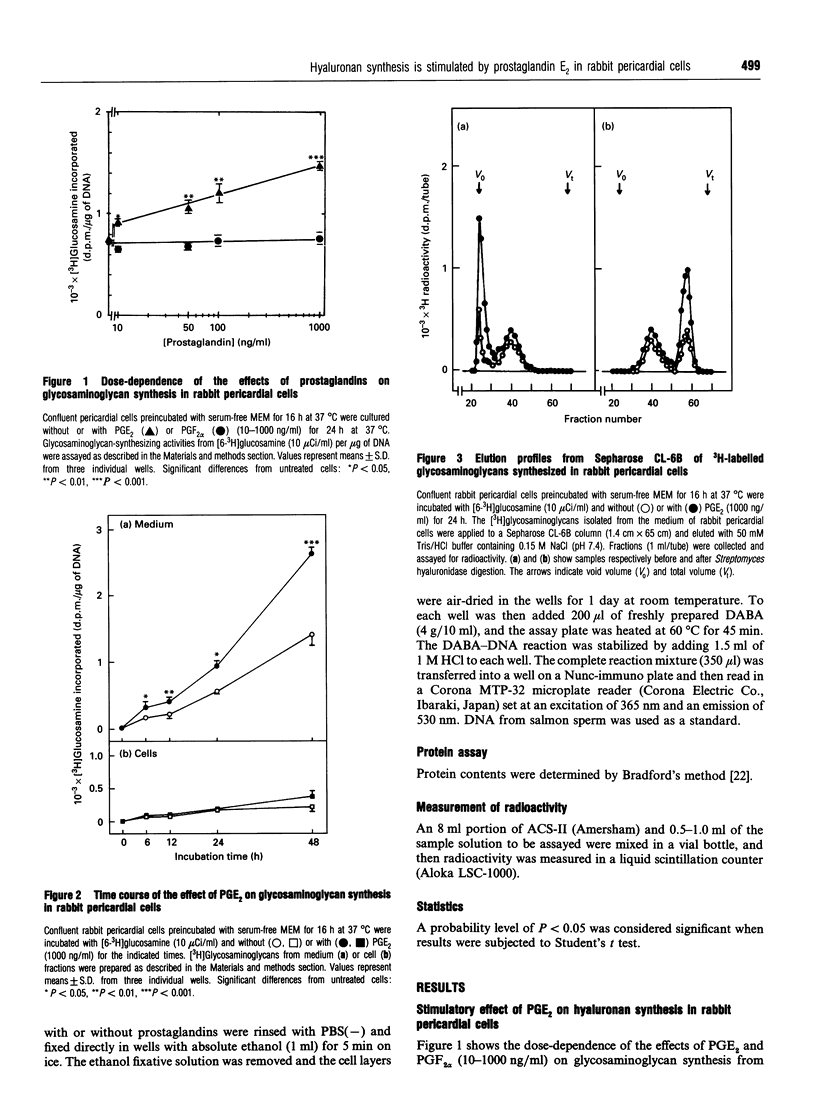
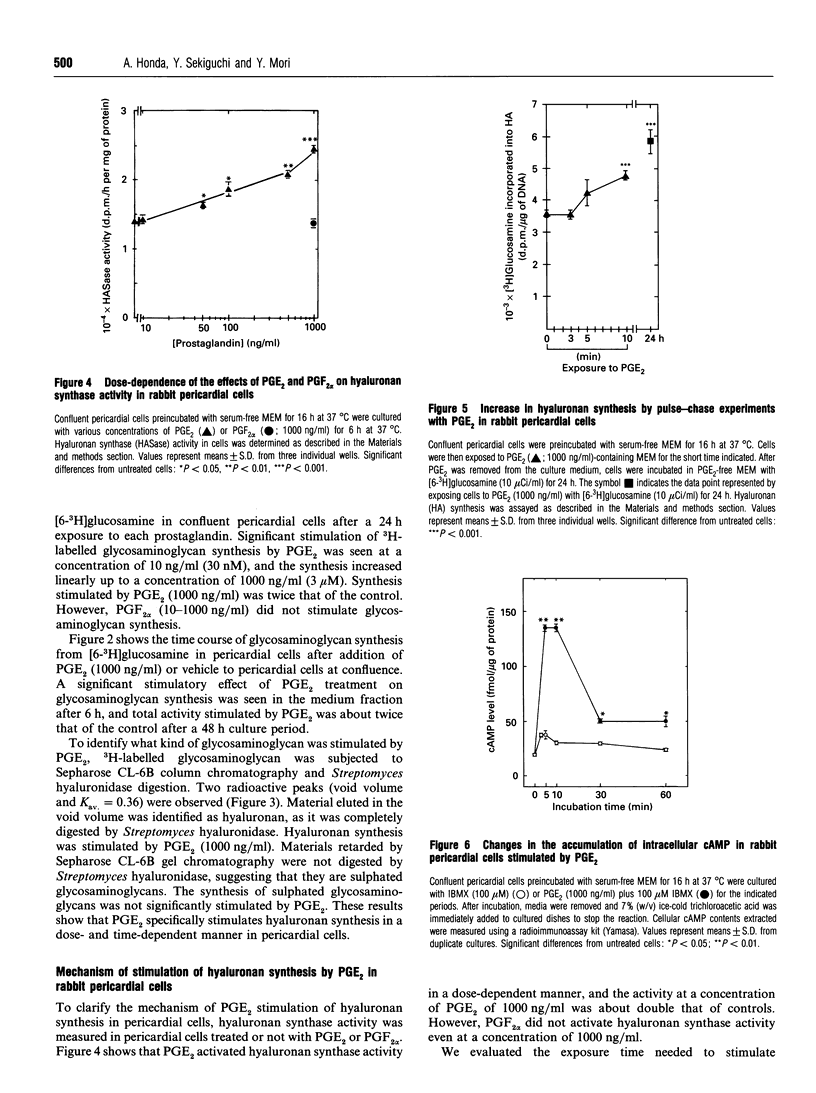
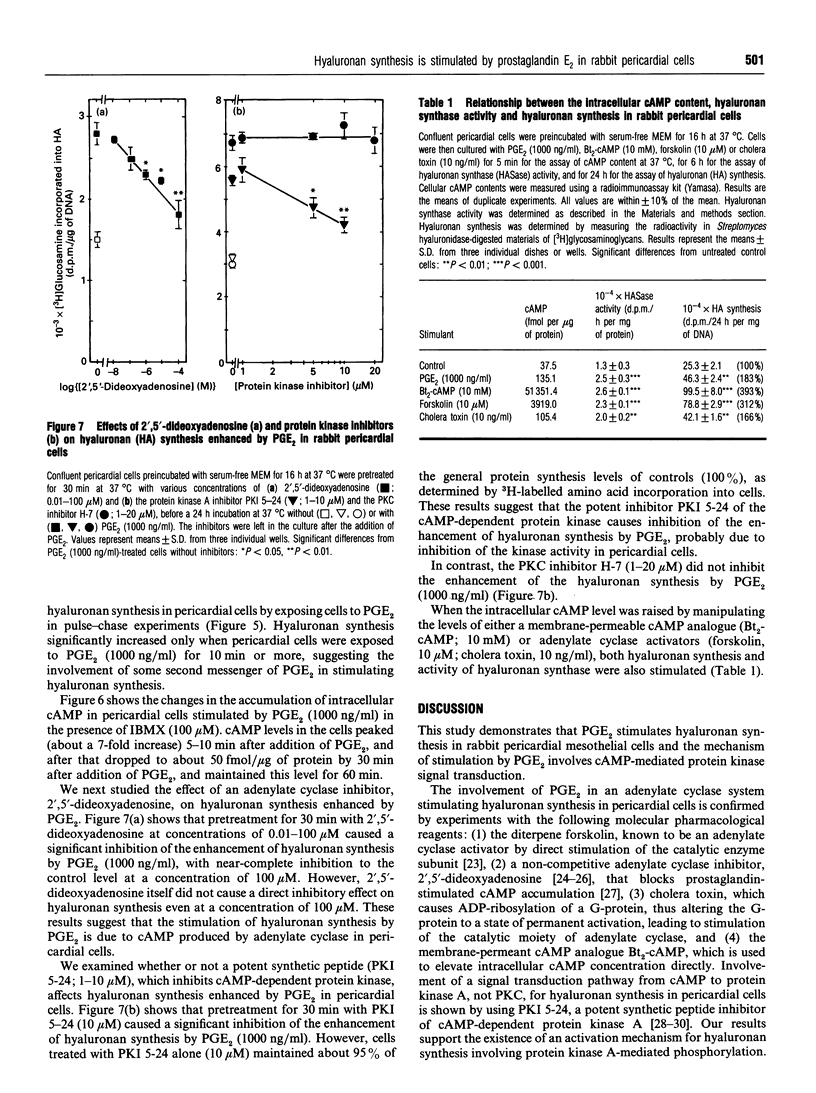
We studied the effects of prostaglandin E2 (PGE2) on hyaluronan synthesis in rabbit pericardial mesothelial cells, and the following results were obtained. (1) PGE2 (10-1000 ng/ml) stimulated hyaluronan synthesis and the level of hyaluronan synthase activity in a dose- and time-dependent manner, but PGF2 alpha did not. (2) Cyclic AMP (cAMP) levels in the cells peaked (about a 7-fold increase) at 5-10 min after adding PGE2 (1000 ng/ml). (3) Increased hyaluronan synthesis induced by PGE2 was significantly inhibited after pretreatment with either an adenylate cyclase inhibitor (2',5'-dideoxyadenosine) or a cAMP-dependent protein kinase inhibitor (PKI 5-24), but there was no inhibition with the protein kinase C inhibitor H-7. (4) When the intracellular cAMP level was raised by manipulating the levels of dibutyryl cyclic AMP or forskolin, hyaluronan synthesis and the level of hyaluronan synthase activity were also stimulated. These results suggest that PGE2 produced by cells stimulates hyaluronan synthesis in rabbit pericardial cells and that the stimulation mechanism involves the cAMP-mediated protein kinase signal transduction process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Cheng H. C., van Patten S. M., Smith A. J., Walsh D. A. An active twenty-amino-acid-residue peptide derived from the inhibitor protein of the cyclic AMP-dependent protein kinase. Biochem J. 1985 Nov 1;231(3):655–661. doi: 10.1042/bj2310655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker M., Chiu E. S., Dollbaum C., Moiin A., Hall J., Spendlove R., Longaker M. T., Stern R. Hyaluronic acid-stimulating activity in sera from the bovine fetus and from breast cancer patients. Cancer Res. 1989 Jul 1;49(13):3499–3505. [PubMed] [Google Scholar]
- Fain J. N., Pointer R. H., Ward W. F. Effects of adenosine nucleosides on adenylate cyclase, phosphodiesterase, cyclic adenosine monophosphate accumulation, and lipolysis in fat cells. J Biol Chem. 1972 Nov 10;247(21):6866–6872. [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., Desjardins J. V. Inhibition of adenylate cyclase by adenosine analogues in preparations of broken and intact human platelets. Evidence for the unidirectional control of platelet function by cyclic AMP. Biochem J. 1978 Oct 15;176(1):83–95. doi: 10.1042/bj1760083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata R., Sunada H., Arai K., Sato T., Ninomiya Y., Nagai Y., Senoo H. Regulation of collagen metabolism and cell growth by epidermal growth factor and ascorbate in cultured human skin fibroblasts. Eur J Biochem. 1988 Apr 15;173(2):261–267. doi: 10.1111/j.1432-1033.1988.tb13993.x. [DOI] [PubMed] [Google Scholar]
- Heldin P., Asplund T., Ytterberg D., Thelin S., Laurent T. C. Characterization of the molecular mechanism involved in the activation of hyaluronan synthetase by platelet-derived growth factor in human mesothelial cells. Biochem J. 1992 Apr 1;283(Pt 1):165–170. doi: 10.1042/bj2830165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin P., Laurent T. C., Heldin C. H. Effect of growth factors on hyaluronan synthesis in cultured human fibroblasts. Biochem J. 1989 Mar 15;258(3):919–922. doi: 10.1042/bj2580919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. P. The normal pericardium. Am J Cardiol. 1970 Nov;26(5):455–465. doi: 10.1016/0002-9149(70)90702-2. [DOI] [PubMed] [Google Scholar]
- Honda A., Iwai T., Mori Y. Insulin-like growth factor I (IGF-I) enhances hyaluronic acid synthesis in rabbit pericardium. Biochim Biophys Acta. 1989 Dec 14;1014(3):305–312. doi: 10.1016/0167-4889(89)90227-9. [DOI] [PubMed] [Google Scholar]
- Honda A., Morrison A. R., McCluskey E. R., Needleman P. Arachidonic acid metabolic pathways in the rabbit pericardium. Biochim Biophys Acta. 1984 Jul 26;794(3):403–410. doi: 10.1016/0005-2760(84)90006-7. [DOI] [PubMed] [Google Scholar]
- Honda A., Noguchi N., Takehara H., Ohashi Y., Asuwa N., Mori Y. Cooperative enhancement of hyaluronic acid synthesis by combined use of IGF-I and EGF, and inhibition by tyrosine kinase inhibitor genistein, in cultured mesothelial cells from rabbit pericardial cavity. J Cell Sci. 1991 Jan;98(Pt 1):91–98. doi: 10.1242/jcs.98.1.91. [DOI] [PubMed] [Google Scholar]
- Honda A., Ohashi Y., Mori Y. Effect of high-molecular-weight hyaluronic acid on the viscosity of rabbit pericardial fluid measured with a cone and plate viscometer. Chem Pharm Bull (Tokyo) 1986 Nov;34(11):4844–4847. doi: 10.1248/cpb.34.4844. [DOI] [PubMed] [Google Scholar]
- Honda A., Ohashi Y., Mori Y. Hyaluronic acid in rabbit pericardial fluid and its production by pericardium. FEBS Lett. 1986 Jul 28;203(2):273–278. doi: 10.1016/0014-5793(86)80757-8. [DOI] [PubMed] [Google Scholar]
- Honma M., Satoh T., Takezawa J., Ui M. An ultrasensitive method for the simultaneous determination of cyclic AMP and cyclic GMP in small-volume samples from blood and tissue. Biochem Med. 1977 Dec;18(3):257–273. doi: 10.1016/0006-2944(77)90060-6. [DOI] [PubMed] [Google Scholar]
- Iwama M., Honda A., Ohohashi Y., Sakai T., Mori Y. Alterations in glycosaminoglycans of the aorta of vitamin E-deficient rats. Atherosclerosis. 1985 Apr;55(1):115–123. doi: 10.1016/0021-9150(85)90171-6. [DOI] [PubMed] [Google Scholar]
- Johnson-Wint B., Hollis S. A rapid in situ deoxyribonucleic acid assay for determining cell number in culture and tissue. Anal Biochem. 1982 May 15;122(2):338–344. doi: 10.1016/0003-2697(82)90292-5. [DOI] [PubMed] [Google Scholar]
- Lembach K. J. Enhanced synthesis and extracellular accumulation of hyaluronic acid during stimulation of quiescent human fibroblasts by mouse epidermal growth factor. J Cell Physiol. 1976 Oct;89(2):277–288. doi: 10.1002/jcp.1040890211. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Schlegel W., Rodbell M. Adenosine analogs inhibit adipocyte adenylate cyclase by a GTP-dependent process: basis for actions of adenosine and methylxanthines on cyclic AMP production and lipolysis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5362–5366. doi: 10.1073/pnas.75.11.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mené P., Dunn M. J. Eicosanoids and control of mesangial cell contraction. Circ Res. 1988 May;62(5):916–925. doi: 10.1161/01.res.62.5.916. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Honda A., Iwai T., Mori Y. Stimulatory effect of vanadate on hyaluronic acid synthesis in mesothelial cells from rabbit pericardium. Biochem Int. 1988 Feb;16(2):293–302. [PubMed] [Google Scholar]
- Ohya T., Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970 Mar 18;198(3):607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Smith G. N., Jr, Lachman L. B., Endres R. O., Poppleton H. M., Hasty K. A., Seyer J. M., Kang A. H. Stimulation of glycosaminoglycan synthesis in cultured human dermal fibroblasts by interleukin 1. Induction of hyaluronic acid synthesis by natural and recombinant interleukin 1s and synthetic interleukin 1 beta peptide 163-171. J Clin Invest. 1989 Feb;83(2):629–636. doi: 10.1172/JCI113927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Induction of hyaluronic acid synthesis in teratocarcinoma stem cells by retinoic acid. FEBS Lett. 1980 Mar 10;111(2):295–298. doi: 10.1016/0014-5793(80)80813-1. [DOI] [PubMed] [Google Scholar]
- Scott J. D., Fischer E. H., Takio K., Demaille J. G., Krebs E. G. Amino acid sequence of the heat-stable inhibitor of the cAMP-dependent protein kinase from rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5732–5736. doi: 10.1073/pnas.82.17.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Smith W. L. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989 Apr 15;259(2):315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida M., Koyama H., Ono T. Effects of adenosine 3':5'-cyclic monophosphate and serum on synthesis of hyaluronic acid in confluent rat fibroblasts. Biochem J. 1977 Mar 15;162(3):539–543. doi: 10.1042/bj1620539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzuka M., Nakajima K., Ohta S., Mori Y. The mechanism of estrogen-induced increase in hyaluronic acid biosynthesis, with special reference to estrogen receptor in the mouse skin. Biochim Biophys Acta. 1980 Jan 17;627(2):199–206. doi: 10.1016/0304-4165(80)90321-9. [DOI] [PubMed] [Google Scholar]


