Abstract
Hypereosinophilic syndrome (HES) is a disorder characterized by elevated levels of eosinophils, which may be associated with multi-organ involvement depending on severity. The recent diagnostic criteria for idiopathic HES require an elevated absolute eosinophil count (AEC) above 1500 cells/mcL with evidence of tissue damage. We present a case of a 37-year-old male firefighter with a purported history of eosinophilic bronchitis who was referred to the hospital with syncopal episodes and a persistent productive cough. The patient showed an AEC of 4500 cells/mcL on admission associated with high inflammatory markers. Cardiac imaging demonstrated acute myocarditis with heart failure and a reduced ejection fraction. Chest imaging was initially suggestive of community-acquired pneumonia. Workup was negative for a malignant etiology; infectious causes similarly were excluded. After a multidisciplinary evaluation, a diagnosis of idiopathic HES was made and steroids were instituted with rapid resolution of symptoms. Our case illustrates the importance of considering hypereosinophilia as a precipitating factor for acute heart failure in an otherwise healthy adult. An expeditious diagnosis can lead to early initiation of steroids to avoid progression toward multi-organ failure.
Keywords: Acute systolic heart failure, pericardial effusion, urgent treatment, reduce risk of death, idiopathic, hypereosinophilia, myocarditis, idiopathic hypereosinophilic syndrome
Introduction
Hypereosinophilic syndrome (HES) is characterized by elevated levels of eosinophils, which may result in end-organ damage. The condition is rare with an incidence of 0.18–0.36 per 100,000. 1 According to the World Health Organization (WHO), HES is typically categorized based on primary (neoplastic) or secondary (reactive) causes. HES can be further subdivided as either a lymphocytic or myeloproliferative variant. The myeloproliferative variant describes a bone-marrow-derived eosinophilic myeloid malignancy, while the lymphocytic variant is consistent with an abnormal T-cell population. In the absence of these findings, HES is defined as idiopathic. 2
Clinical manifestations of HES are usually dermatological, occurring in approximately 69% of patients; pulmonary at approximately 44%; and cardiac presentations are rare accounting for 20% of presentations. 3 Specifically, heart failure, arrhythmias, and intracardiac thromboses may be potential cardiac findings of HES, and these typically portend a poor prognosis. Cardiac pathology progresses in three stages: acute necrosis, thrombosis, and fibrosis. 4 We present a case of a young man presenting with signs of acute heart failure, later found to be associated with idiopathic HES.
Case report
A 37-year-old male firefighter with a history of prior tobacco use, refractory chronic cough, and eosinophilic bronchitis presents with worsening cough, non-massive hemoptysis, constitutional symptoms of fever and night sweats, dyspnea, and orthopnea. The patient also carried a diagnosis of chronic sinusitis, for which an evaluation was performed prior to admission with evident air–fluid levels and minor mucosal thickening. Nasal polyps were not evident. He did not exhibit gastrointestinal symptoms, nor did he endorse significant joint pain. In addition, he presented with a very subtle, transient skin rash that resolved spontaneously while not receiving corticosteroids or other immunomodulatory therapies. The patient was seen 2 weeks prior at an outside facility for syncopal episodes and was noted to have a small pericardial effusion with hemodynamic compromise, suspected of having myopericarditis, and thus started on non-steroidal anti-inflammatory agents. The eosinophil count prior to antibiotic administration was 1.96 × 10³/μL (see Table 2). His eosinophilic bronchitis was diagnosed a year prior with an eosinophil count of 1.0 × 10³/μL. Pulmonary function testing was done and ruled out obstructive lung disease with absent bronchodilator response. The patient was subsequently managed with an inhaled corticosteroid-long-acting beta agonist combination inhaler, montelukast, and an as-needed short-acting bronchodilator. Notably, oral prednisone was taken periodically, which offered occasional respiratory relief. He was briefly on ceftriaxone and doxycycline several weeks prior to hospital presentation with no significant improvement of symptoms. Other medications included fluticasone-salmeterol, albuterol as needed, amphetamine–dextroamphetamine, budesonide, and cetirizine.
Table 2.
Laboratory data for hospital admissions.
| Laboratory data | Normal values24 | Admission at outside facility | Approximate time of admission at hospital | During hospitalization | Proximate time of discharge |
|---|---|---|---|---|---|
| Creatinine (mg/dL) | 0.7–1.3 | 1.14 | 1.21 | 1.06 | 1.05 |
| BUN (mg/dL) | 6–24 | 17 | 16 | 12 | 40 |
| eGFR (mL/min/1.73 m²) | 107 | 85 | >60 | 81 | 93 |
| ALT (U/L) | 7–56 | 11 | 15 | 25 | 25 a |
| AST (U/L) | 8–33 | 16 | 35 | 33 | 33 a |
| Alk phos (U/L) | 44–147 | 66 | 100 | 112 | 112 |
| Troponin (ng/mL) | 0.00–0.03 | N/A | 2.95–3.17 | 2.91 | N/A |
| BNP (pg/mL) | <100 | N/A | 481 | 403 | 420 |
| ESR (mm/h) | <15 | N/A | 74 | 118 | 19 |
| CRP (mg/dL) | 0.00–0.30 | N/A | 4.52 | 66 | N/A |
| Hemoglobin (g/dL) | 14.0–17.5 | 16.6 | 13.4 | 11.3 | 10.6 |
| Thrombocytes (k/μL) | 140–400 | 421 | 430 | 460 | 482 |
| Total leukocyte count (WBC) (k/μL) | 4.5–11.0 | 8.4 | 12.8 | 12 | 7.7 |
| Eosinophils (%) | <4 | 22.6 | 15.3 | 37.5 | 0 |
Laboratory value was not remeasured.
BNP: B-type natriuretic peptide; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; BUN: blood urea nitrogen; eGFR: estimated glomerular filtration rate; AST: aspartate transaminase; ALT: alanine transaminase; N/A: laboratory values are unavailable.
The chest X-ray demonstrated subtle ground glass findings prompting a chest computerized tomography (CT) scan with intravenous (IV) contrast that illustrated the presence of ground glass opacities (GGO) with reactive mediastinal lymphadenopathy, suspected to be related to an infectious process, recent vaccination, and/or secondary to volume overload (Figures 1 and 2). No acute pulmonary emboli was noted. Splenomegaly was not observed on CT and there was no lymphadenopathy in extrathoracic sites. Furthermore, atypical lymphocytes were not present. The strongyloides test was negative, and his liver function studies were normal. His eosinophil count was 4500 cells/mcL of blood. His registry of severe cutaneous adverse reactions (RegiSCAR) score was calculated to be 1. This score was calculated based on the RegiSCAR inclusion criteria: fever present (0 points); enlarged lymph nodes were not seen in greater than two sites (0 points); atypical lymphocytes were not observed (0 points); eosinophilia was present (+2 points); skin rash extent was very scant (0 points); patient did not exhibit edema, infiltration, purpura, or scaling (−1 point); biopsy was not done for Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) (0 points); internal organ involved at that time was cardiac (+1 point); the patient had a resolution in less than 15 days (−1 point); and the alternative diagnosis was not excluded (0 points).5,6 Electrocardiogram (ECG) recorded sinus tachycardia with first-degree atrioventricular (AV) block. Blood tests revealed high B-type natriuretic peptide (403 pg/mL) and high troponin levels (2.91 ng/mL) with a subsequent downward trend; elevated C-reactive protein (66 mg/dL) and elevated erythrocyte sedimentation rate (118 mm/h). The total lymphocyte count was 12,000 lymphocytes/μL of blood with 37.7% eosinophils (4500/μL) (see Table 1 and Table 2). Based on these values, his HES-suggesting laboratory index (HSLI) was calculated to be greater than 4.25, which indicates a significantly high risk of HES. 7 HSLI is calculated as follows: HSLI = 2 × (WBC count ⩾9900.0/mm3 (1 = No or 2 = Yes)) + 1.5 × (eosinophil count ⩾2400.0/mm3 (1 = No or 2 = Yes)). 7 Urinalysis included evidence of microscopic hematuria with a small amount of protein (1+). In addition, serum tryptase and vitamin B12 levels were normal.
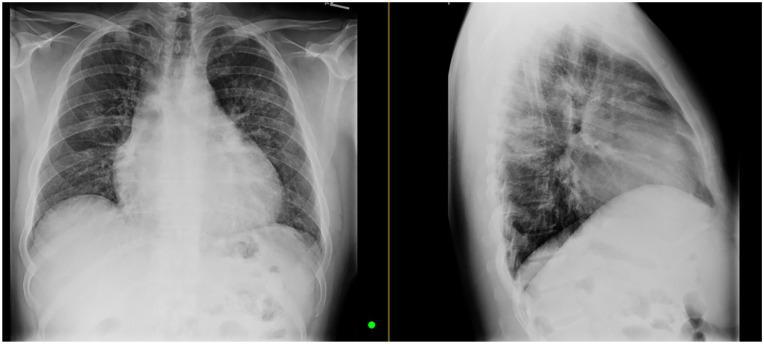
Figure 1.
Chest X-ray images: increased interstitial thickening and hazy ground glass changes were noted. No pleural effusion or pneumothorax is present.
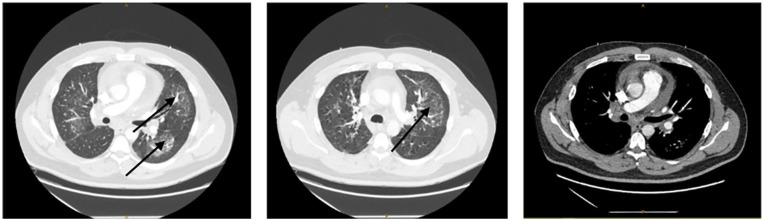
Figure 2.
Chest CT with IV contrast: axial scans shown with lung and soft tissue windows. Scans illustrate bilateral ground glass opacities (shown with arrows) with mediastinal lymphadenopathy. No pulmonary embolism is noted.
CT: computerized tomography; IV: intravenous.
Table 1.
Cardiovascular and inflammatory biomarkers.
| Laboratory data | BNP (pg/mL) | Troponin (ng/mL) | CRP (mg/dL) | Elevated ESR (mm/h) |
|---|---|---|---|---|
| Values | 403 | 2.91 | 66 | 118 |
| Normal values24 | <100 | 0.00-0.03 | 0.00-0.30 | <15 |
BNP: B-type natriuretic peptide; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate.
A bronchoscopy was subsequently performed and white endobronchial plaques were visualized grossly, as well as copious airway secretions with abnormal, friable mucosa throughout the right and left bronchial trees (Figure 3a–c). Furthermore, the endobronchial airways bilaterally were edematous and erythematous. No endobronchial polyps or lesions were visualized. Biopsy illustrated eosinophilic infiltration.
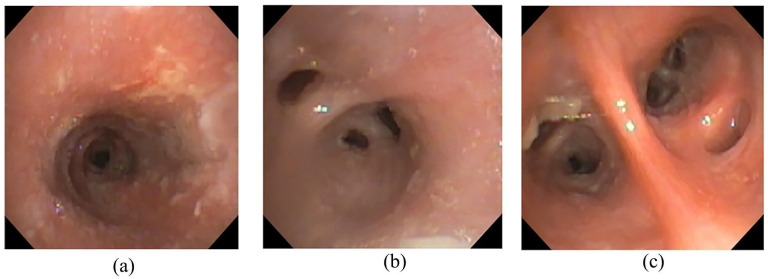
Figure 3.
Bronchoscopic images: (a) Mucosal edema and friability of right mainstem bronchus. (b) Image of distal bronchus intermedius. (c) Inflamed distal left lower lobe segmental bronchi with evident secretions.
Bronchoalveolar lavage (BAL) was collected from the right middle lobe and sent for cytology, cell count, and microbiological studies given prior positive QuantiFERON-TB Gold test. All cultures returned negative. There were eosinophils present in the BAL at 21%. Low procalcitonin (0.02) and a negative pneumonia pathogen polymerase chain reaction (PCR BIO-Fire Film Array Pneumonia Panel) were also noted, which supported the discontinuation of antibiotics. The PCR panel included 18 different bacteria, including atypical bacteria, as well as eight types of viruses.
Transthoracic echocardiogram (TTE) revealed a large pericardial effusion in the setting of myocarditis, effusive constrictive pericarditis, and left ventricular ejection fraction of 40%. A consideration for right heart strain was discussed given the right ventricle was mildly dilated, as well as the observation of mild reflux of contrast into the intrahepatic inferior vena cava and hepatic veins. Pericardiocentesis yielded fluid that was later found to contain significant eosinophils (2864 WBCs/μL with 65% eosinophils). Cardiac magnetic resonance imaging (CMR) findings met the Lake-Louise criteria for acute myocarditis with heart failure and a confirmed reduced ejection fraction (Figure 4). According to Friedrich et al., 8 the requirements of the Lake-Louise criteria are met when two of the three features are present on CMR: (I) edema/T2 signal, (II) hyperemia with early gadolinium enhancement, and (III) necrosis or fibrosis with late gadolinium enhancement imaging. In our case, the presence of elevated T2 signals and necrosis satisfy the requirement for Lake-Louise criteria (Figure 4).
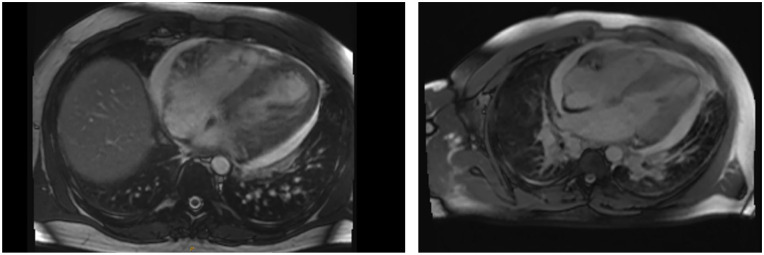
Figure 4.
Cardiac MR images: thickening of myocardium observed on cardiac MRI. LV and RV are mildly dilated in size with severely reduced systolic functions: LVEF measuring at 30.2% and RVEF measuring at 31.2%. LGE of the LV was observed with mid-wall delayed enhancement in the basal inferoseptum, and subendocardial delayed enhancement in the mid-inferoseptum suggesting inflammation and/or necrosis. Moderate pericardial effusion was present as well as elevated T1, T2, and ECV, which was consistent with acute myocarditis. The mitral valve and tricuspid valve demonstrated regurgitation.
ECV: extracellular volume; LGE: late gadolinium enhancement; LV: left ventricle; LVEF: left ventricular ejection fraction; RV: right ventricle; RVEF: right ventricular ejection fraction.
The patient underwent additional work subsequently including a bone marrow biopsy (BMB). Overall, very mild dysplastic changes were seen but did not meet WHO criteria for the diagnosis of malignant myeloid neoplasm. In addition, flow cytometry analysis of the bone marrow did not show evidence of a significant increase in the blast population.
The constellation of these findings facilitated considering a diagnosis of eosinophilic myopericarditis. The patient was started on IV solumedrol for 3 days and then switched to oral methylprednisolone twice a day (BID) with a tapering regimen for hospital discharge. Corticosteroids were administered at this time, which may have had an impact on the patient’s symptoms, but the initiation of steroids could not be delayed due to the patient’s rapid disease progression and concern for sudden death. The patient’s symptoms promptly improved with steroids. Cardiology instructed him to start furosemide, aldactone, metoprolol, lisinopril, colchicine, and ibuprofen for the concern of pericarditis.
In view of other secondary processes being ruled out, a diagnosis of idiopathic HES was made. During hospitalization, the patient was examined by many medical teams including cardiology, rheumatology, pulmonology, infectious disease, and hematology/oncology. There was a broad differential diagnosis that included eosinophilic granulomatosis with polyangiitis (EGPA), allergic bronchopulmonary aspergillosis (ABPA), HES, reactive eosinophilic process secondary to infection, and hypereosinophilic asthma with systemic manifestations. Workup for infectious etiologies returned negative and the lack of vasculitis on endobronchial, transbronchial, and sinus tissue ruled out EGPA. In addition, the BMB ruled out malignant myeloid neoplasms. The patient was encouraged to follow up closely as an outpatient. As an outpatient, after a multidisciplinary discussion, the patient was started on mepolizumab for its steroid-sparing effect. He remained on the bronchodilators and montelukast. Two months after his last TTE, a subsequent TTE (ejection fraction: 45%) and a stress echocardiogram were also within normal limits. In addition, the patient has been able to return to work with no residual symptoms. The patient is still being closely followed clinically; he remains on mepolizumab.
Discussion
Eosinophil granulocytes are specialized effector cells that are involved in the innate immune system 9 and play a defensive role in response to a multitude of inflammatory diseases. 10 Eosinophils release a large variety of biologically active compounds in their defense response, some of which can damage host tissue. 9
According to the 2011 Working Conference on Eosinophil Disorders and Syndromes, the term eosinophilia is defined by an absolute eosinophil count (AEC) greater than 500 cells/mcL. 11 An AEC greater than 1500 cells/mcL is considered hypereosinophilia (HE). 11 HES is defined when the AEC for HE is met for longer than 6 months in the context of end-organ damage with the exclusion of other diseases or conditions that may be major contributors to organ damage.11,12
Due to the broad range of clinical manifestations, HES can be difficult to diagnose. Symptoms range from nonspecific to life-threatening depending on the organs involved. Early symptoms of HES may include fatigue, weight loss, anorexia, fever, rash, nonproductive cough with chest pain, night sweats, dyspnea, abdominal pain, and heart failure. 12 Standard work-up for the diagnosis of HES includes complete blood count, tryptase levels, serum vitamin B12, and routine chemistry tests, including liver and kidney function tests.13,14
Cardiovascular manifestations of HES include heart failure, arrhythmias, myocardial ischemia, intracardiac thrombosis, and rarely pericarditis.15,16 In a literature review of 26 patients with HES, the following symptoms were observed: dyspnea (42%), chest pain (27%), cough (12%), palpitations (8%), and embolic events (4%). 16 Further complications that some patients experienced were mitral regurgitation, congestive heart failure, aortic regurgitation, and aortic stenosis. 15 In rare instances, myocardial infarction may occur as a consequence of an embolic event resulting from endomyocardial fibrosis and thrombus in the left ventricular outflow tract. 17
Risk factors for cardiac involvement in HES include but are not limited to, male sex, splenomegaly, elevated serum vitamin B12, and early abnormal myeloid precursors. 18 Classic diagnostic modalities include ECG, CMR imaging, echocardiography, and endomyocardial biopsy. 4 Common findings in the ECG include left ventricular hypertrophy, T wave inversions, left atrial enlargement, left axis deviation, and incomplete right bundle branch block. 16
HES is classified into three categories based on its pathogenesis: neoplastic, reactive, and idiopathic. 11 The neoplastic HES (also known as clonal or primary HES) variant is characterized by the pathogenesis of eosinophilia with myeloid, lymphoid, or stem cell neoplasm and rearrangement of the gene PDGFRA, PDGFRB, FGFR1, or with translocation resulting in the fusion gene PCM1-JAK2. 2 The reactive HES (also known as secondary HES) variant encompasses a variety of conditions (e.g., parasitic infections, inflammatory disorders, adverse or allergic drug reactions), and is characterized by eosinophils that are considered to be “non-clonal.”9,19 Idiopathic HES is considered as the diagnosis when a patient has met the criteria for HES yet the definitions for reactive and neoplastic do not apply. 19
An extensive list of conditions was considered before HES was entertained as the diagnosis for our patient. There was a broad differential diagnosis that included EGPA, ABPA, HES, reactive eosinophilic process secondary to infection, and hypereosinophilic asthma with systemic manifestations. In regard to the patient’s history of eosinophilic bronchitis and rapid improvement with steroids, his pericardial effusion, myo/pericarditis, and GGO with reactive lymphadenopathy were not entirely consistent with an eosinophilic bronchitis exacerbation. This prompted the physician to pursue further workup for infectious etiology which was negative.
From a pulmonary standpoint, given our patient’s significantly elevated peripheral eosinophils, history of eosinophilic bronchitis, pericardial fluid rich with eosinophils, and prior sinus-related disease, HES versus EGPA was entertained as the diagnosis. Rheumatology also noted that the patient did not meet the diagnostic criteria for EGPA. The American College of Rheumatology requires four of the six criteria for the diagnosis of EGPA: (1) asthma, (2) peripheral eosinophilia, (3) mono or polyneuropathy, (4) pulmonary infiltrates, (5) paranasal sinusitis, and (6) eosinophilic vasculitis. 20 In our patient, only three of the six criteria were met and therefore EGPA was ruled out. Furthermore, the findings are consistent with idiopathic HES as the patient had no evidence of vasculitis in the various tissue specimens available (endobronchial/transbronchial biopsies and sinus tissue from prior endoscopic evaluation). In addition, there was no evidence of myeloid neoplasia in the BMB, and serum tryptase and vitamin B12 levels were normal, ruling out neoplastic HES. Reactive HES was ruled out as all infections and other inflammatory conditions were excluded.
In regard to the treatment of HES, corticosteroids are the current primary treatment of choice, 21 especially in scenarios of acute, life-threatening neoplastic HES. 22 However, with recent advancements in eosinophil-targeted therapies that have shown significant efficacy and reduced toxicity, the treatment paradigm has shifted. 22 There are still questions remaining on the most optimal therapy, the effect of combined regimens, and the chronic effects of significantly reduced eosinophil count. 22 A recent phase III, randomized, placebo-controlled trial conducted by Roufosse et al. tested the safety and efficacy of mepolizumab in HES patients, demonstrating a 92% reduction of eosinophil blood count when utilizing mepolizumab compared to placebo. 23 Not only are these observations significant as they present mepolizumab as a potential therapeutic but also because the data from this study suggest that mepolizumab can be used regardless of prior corticosteroid use. 23 Accordingly, mepolizumab may be promising for patients since it could spare the effects of corticosteroids and other immunosuppressive therapies. 23
Conclusion
HES can be a precipitating factor for acute heart failure in an otherwise healthy adult. It is imperative to consider this diagnosis promptly as delays may lead to rapid decline. After diagnosis, treatment with corticosteroids is indicated to suppress the immune inflammatory response, once infection has been excluded. Due to potential multi-organ involvement, HES is a complex disease process that requires multidisciplinary involvement. Additional clinical trials are warranted to facilitate the identification of other potential steroid-sparing immunosuppressive regimens.
Acknowledgments
The authors wish to extend their sincere thanks to the patient presented in this case report.
Footnotes
Author contributions: B.S. contributed by writing the case report and assisting with revisions of the manuscript. M.M. contributed by writing the discussion and conclusion and assisting with revisions. G.C. contributed by writing the introduction and assisted with revisions. S.L. contributed by writing the abstract. M.N. contributed to drafting and revisions and provided information for the case report.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iDs: Bryanna Sullivan  https://orcid.org/0009-0001-6473-2660
https://orcid.org/0009-0001-6473-2660
Moreen Matti  https://orcid.org/0000-0001-5928-9695
https://orcid.org/0000-0001-5928-9695
Gene Cho  https://orcid.org/0000-0002-7136-0850
https://orcid.org/0000-0002-7136-0850
Seoyoon Lee  https://orcid.org/0000-0002-6289-4615
https://orcid.org/0000-0002-6289-4615
Matthew Nobari  https://orcid.org/0000-0002-6743-7683
https://orcid.org/0000-0002-6743-7683
References
- 1. Crane MM, Chang CM, Kobayashi MG, et al. Incidence of myeloproliferative hypereosinophilic syndrome in the United States and an estimate of all hypereosinophilic syndrome incidence. J Allergy Clin Immunol 2010; 126(1): 179–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gotlib J. World Health Organization-defined eosinophilic disorders: 2017 update on diagnosis, risk stratification, and management. Am J Hematol 2017; 92(11): 1243–1259. [DOI] [PubMed] [Google Scholar]
- 3. Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol 2009; 124(6): 1319–1325.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogbogu PU, Rosing DR, Horne MK. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am 2007; 27(3): 457–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. RegiSCAR score for drug reaction with eosinophilia and systemic symptoms (DRESS) [Internet]. MDCalc, https://www.mdcalc.com/calc/10084/regiscar-score-drug-reaction-eosinophilia-systemic-symptoms-dress (2009, accessed 11 July 2024).
- 6. Roujeau JC, Allanore L, Liss Y, et al. Severe cutaneous adverse reactions to drugs (SCAR): definitions, diagnostic criteria, genetic predisposition. Chinese Journal of Dermatology 2009; 27(4): 203–209. [Google Scholar]
- 7. Ahn S, Yoo J, Park YB, et al. A new index for distinguishing hypereosinophilic syndrome and antineutrophil cytoplasmic antibody-negative eosinophilic granulomatosis with polyangiitis. Asian Pacific J Allergy Immunol 2023; 41(3): 244–252. [DOI] [PubMed] [Google Scholar]
- 8. Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 2009; 53(17): 1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valent P, Degenfeld-Schonburg L, Sadovnik I, et al. Eosinophils and eosinophil-associated disorders: immunological, clinical, and molecular complexity. Semin Immunopathol 2021; 43(3):423–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramirez GA, Yacoub MR, Ripa M, et al. Eosinophils from physiology to disease: a comprehensive review. Biomed Res Int 2018; 2018: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012; 130(3): 607–612.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chusid MJ, Dale DC, West BC, et al. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine 1975; 54(1): 1–27. [PubMed] [Google Scholar]
- 13. Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology 2018; 2018(1): 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dispenza MC, Bochner BS. Diagnosis and novel approaches to the treatment of hypereosinophilic syndromes. Curr Hematol Malign Rep 2018; 13(3): 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thawabi M, Habib M, Shaaban H, et al. Acute eosinophilic myocarditis and hyper IgE in HIV infection: a case report. North Am J Med Sci 2014; 6(7): 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parrillo JE, Borer JS, Henry WL, et al. The cardiovascular manifestations of the hypereosinophilic syndrome. Am J Med 1979; 67(4): 572–582. [DOI] [PubMed] [Google Scholar]
- 17. D’Souza MG, Swistel DG, Castro JL, et al. Hypereosinophilic thrombus causing aortic stenosis and myocardial infarction. Ann Thorac Surg 2003; 76(5): 1725–1726. [DOI] [PubMed] [Google Scholar]
- 18. Weller P, Bubley G. The idiopathic hypereosinophilic syndrome. Blood 1994; 83(10): 2759–2779. [PubMed] [Google Scholar]
- 19. Kahn JE, Groh M, Lefèvre G. (A critical appraisal of) classification of hypereosinophilic disorders. Front Med 2017; 4: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990; 33(8): 1094–1100. [DOI] [PubMed] [Google Scholar]
- 21. Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol 2012; 5(3): 275–290. [DOI] [PubMed] [Google Scholar]
- 22. Klion AD. Approach to the patient with suspected hypereosinophilic syndrome. Hematology 2022; 2022(1): 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roufosse F, Kahn JE, Rothenberg ME, et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo-controlled trial. J Allergy Clin Immunol 2020; 146(6): 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. UpToDate [Internet]. www.uptodate.com. https://www.uptodate.com/contents/table-of-contents/lab-interpretation (accessed 8 August 2024).