Abstract
Infective native abdominal aortic aneurysms are a life-threatening condition with a high mortality rate. We report the case of a 53-year-old male patient who presented with abdominal pain and fever. Laboratory results showed an elevated white blood cell count and C-reactive protein levels. Blood cultures detected Salmonella species, and computed tomography revealed a saccular abdominal aortic aneurysm. After 14 days of preoperative antibiotic therapy, the patient underwent a successful surgical bypass from the descending thoracic aorta, through the diaphragm and muscle layers of the anterior abdominal wall, to the bilateral common femoral arteries. The patient was discharged after 30 days of hospitalization and continued antibiotic treatment for another 30 days. Follow-up clinical evaluations and imaging studies showed good recovery and no signs of infection. This case highlights the importance of combining appropriate antibiotic therapy with surgical intervention in managing infective native aortic aneurysms. In particular, an extra-anatomical approach from the descending aorta can be a viable option in selected cases of infected aortic aneurysms, providing an effective means to achieve thorough debridement and prevent future graft infections.
Keywords: Infective native aortic aneurysms, antibiotic therapy, Salmonella
Introduction
Infections of the vessel wall caused by sepsis or adjacent infections may lead to infectious degeneration and aneurysm formation. 1 The most common infectious agents are Salmonella and Staphylococcus species, with positive culture rates of 33.4% and 15.6%, respectively. 2 Infective native aortic aneurysms (INAA) are rare, with a reported incidence of 0.6%–2% among all aortic aneurysms in Europe and the United States. 2 There have also been reports of INAA occurring in peripheral blood vessels.3–5 Moreover, the condition of an aortic aneurysm usually progresses rapidly, causing a high rate of aneurysm rupture. The diagnosis of INAA is based on a combination of clinical presentation, laboratory tests, culture, and radiological findings on computed tomography (CT). These aneurysms are life-threatening, 1 with a reported hospital mortality rate of 16%–44%, because rapidly growing aneurysms can rupture (44%) and concomitant infections or shock may occur (15%).2,6 Therefore, therapeutic management of INAA is challenging.2,7,8 Combining immediate antibiotic therapy and open conventional surgical or endovascular repair is crucial to improving early outcomes.2,7 Herein, we report a single case of INAA related to Salmonella that was successfully treated by surgery combined with antibiotic therapy. The patient provided written informed consent and this report was approved by the institutional review board, and it has been reported in line with the Surgical CAse REport (SCARE) criteria. 9
Case report
A 53-year-old man with abdominal pain and fever was admitted to our center. The chief complaints were fever, intermittent chills, anorexia, and periumbilical pain radiating to the back. These symptoms started for 10 days before admission to the hospital. His medical history included type 2 diabetes, hyperlipidemia, smoking, no other cardiovascular risk factors, no viral or drug-induced immunodeficiency, and no abdominal trauma or infections.
On admission, the patient was conscious, had a heart rate of 95 beats per minute, blood pressure of 130/80 mmHg, oxygen saturation of 97%, temperature ranging from 37.5°C to 38.5°C, and palpable pulses of the femoral arteries bilaterally. The blood test results were as follows: white blood cell count: 10.41 × 109, neutrophil: 68.4%, pro-calcitonin: 0.359 ng/mL, C-reactive protein (CRP): 82.2 mg/L, hemoglobin A1c: 10.2%, creatinine: 0.55 mg/dL, and estimated glomerular filtration rate: 126 mL/min/1.73 m2. In addition, the patient had unstable type 2 diabetes with a random plasma glucose level of 225 mg/dL. Ultrasonography showed an abdominal aortic aneurysm with a largest diameter of 43 mm, length of 75 mm, and no surrounding fluid collection. CT revealed a saccular juxtarenal abdominal aortic aneurysm with dimensions of 30 × 20 × 25 mm (width × anteroposterior diameter × height), aneurysm neck of 18 mm, irregular margin, and wall thrombosis thickness <12 mm (Figure 1(a)). In addition, low-density fluid collection and fat stranding around the aorta were suspected to be pus. Immunological tests were negative for human immunodeficiency virus antigen/antibody, hepatitis B surface antigen, and hepatitis C antibody. The blood culture detected Salmonella species with extended-spectrum beta-lactamase (−) and AmpC beta-lactamases (AmpC) (−) that were sensitive to ceftriaxone (minimum inhibitory concentration <0.5 mcg/mL).
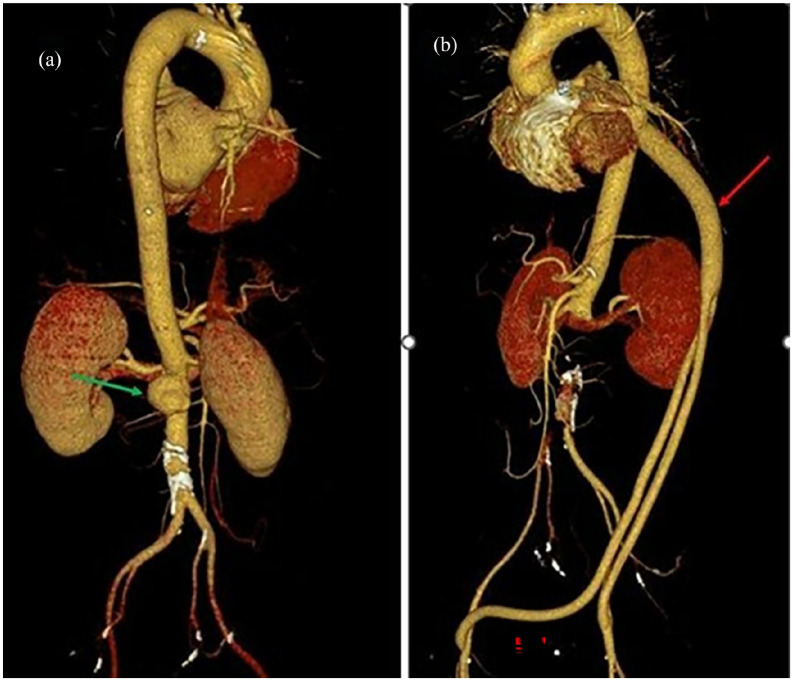
Figure 1.
(a) The reconstructed angiography image demonstrates a saccular juxtarenal abdominal aortic aneurysm with dimensions of 30 × 20 × 25 mm (width × anterior-posterior diameter × height)—green arrow. (b) The postoperative reconstructed angiography image shows that the aorta-femoral bypass and visceral blood vessels had good circulation—red arrow.
Based on the clinical presentation and laboratory and imaging results, the diagnosis of an INAA related to Salmonella was considered, and a treatment strategy was planned. The patient received antibiotic therapy with empiric initial imipenem plus cilastin (0.5 g/0.5 g q8h IV) and vancomycin (1 g q12h IV), which was then changed to ceftriaxone (2 g/day IV) for 14 days based on the results of antibiotic susceptibility testing. In addition, the patient stabilized his blood sugar with insulin (humulin N) and received cardiovascular drugs (betaloc 25 mg daily, lisinopril 2.5 mg daily) and nutritional support.
After 14 days of treatment, the patient had no fever and reduced abdominal pain. However, a CT scan after 2 weeks showed that the aneurysm had increased in size to 62 × 45 × 60 mm (width × anteroposterior diameter × height). Before surgery, a multidisciplinary consultation was held, involving specialists from vascular surgery, anesthesiology, diagnostic imaging, and infectious disease. It was decided to perform an extra-anatomical bypass to minimize the risk of graft infection. First, we performed an extra-anatomical bypass from the descending thoracic aorta. We used a silver graft prosthesis (polytetrafluoroethylene) and a side-to-end anastomosis technique (Figure 2). We then inserted the graft through the diaphragm and muscle layers of the anterior abdominal wall into the two common femoral arteries. Next, an anti-adhesion mesh (Parietex™ Optimized Composite (PCOx) Mesh was manufactured by Medtronic) was placed in the left pleural cavity to separate the graft from the left lung. Next, we placed a left pleural drain and performed a chest closure. We then resected the aneurysm, sutured the lumbar arteries inside the aneurysm and apex to the blood vessel, and performed extensive local debridement and abdominal closure. To strengthen the aortic stump to effectively reduce the risk of aortic stump rupture, we used surgical sealant (COSEAL) (Figure 3). Finally, two common femoral and graft end-to-side anastomoses were performed (Figure 4). The total blood loss and red blood cell transfusion during surgery were 700 mL and 1.5 units, respectively. The surgery lasted for 6 h and 30 min.
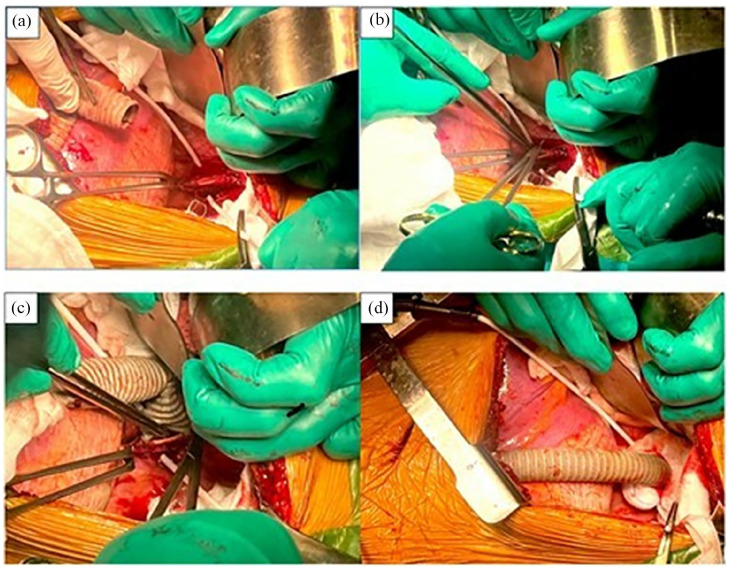
Figure 2.
Proximal end-to-side anastomosis is observed via lateral thoracotomy between the thoracic aorta and prosthetic graft with Prolene 4-0 sutures. (a) The thoracic aorta was incised longitudinally about 2 cm. (b, c) End-to-side anastomosis between the thoracic aorta and prosthetic graft with Prolene 4-0 sutures. (d) We inserted the graft through the diaphragm, clamped the distal end of the graft, and unclamped the proximal end of the graft.
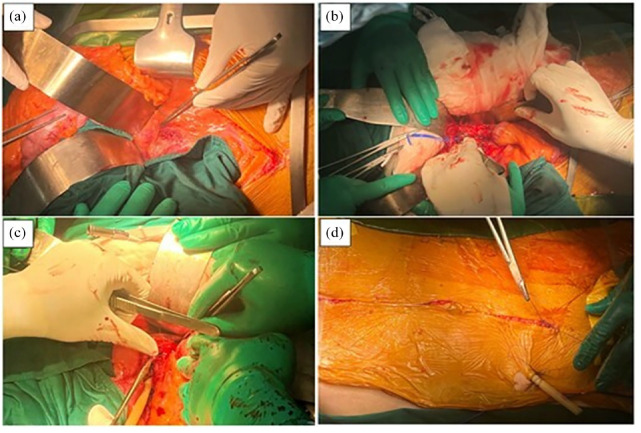
Figure 3.
Resection of the aneurysm and extensive local debridement surgery via the midline abdominal incision. (a) Exposure and control of the abdominal aorta. (b) Clamping the suprarenal abdominal aorta for 10 min, removal of the aneurysm and necrotic tissue, and suturing the end of the infrarenal abdominal aorta. (c) The drainage of the retroperitoneal cavity. (d) The closure of the midline abdominal incision.
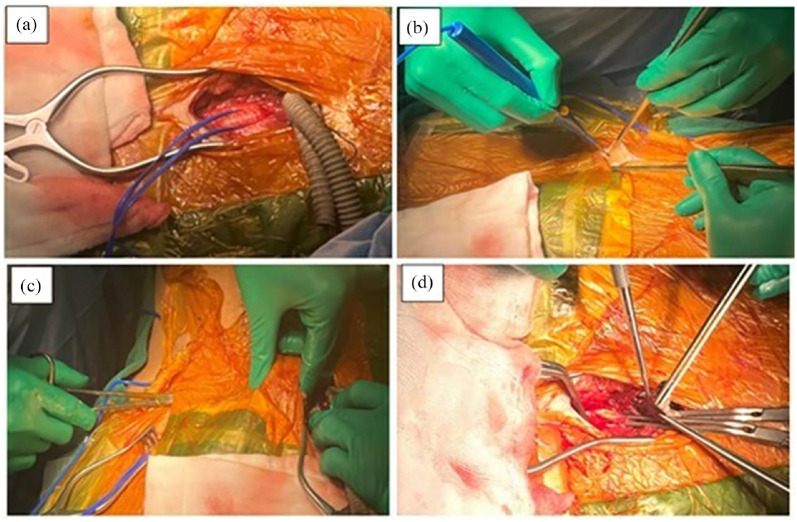
Figure 4.
(a) The left common femoral artery was exposed, and the two ends of the Y-shaped graft were carried down to the left inguinal position. (b) Exposing the right common femoral artery. (c) The graft was routed in the subcutaneous tunnel from left to right. (d) End-to-side anastomosis between the two ends of the Y-shaped graft and common femoral arteries with Prolene 5-0 sutures.
After the surgery, the duration of mechanical ventilation was 2 days. The patient stayed in the intensive care unit and ward for 4 days each, receiving meropenem (1 g q8h IV) and aspirin (81 mg daily). A postoperative CT scan showed a localized collection of fluid around the abdominal aorta surgery site, and the descending aorta-femoral bypass and visceral blood vessels had good circulation (Figure 1(b)). The laboratory tests showed a white blood cell count of 8.15 × 109 and a pro-calcitonin level of 0.103 ng/mL. Microbiological tests revealed negative tissue and blood cultures. The patient was discharged on the seventh postoperative day.
After 30 days of hospitalization, the patient was discharged and was followed up for 1 month, receiving oral broad-spectrum antibiotics of cefoxime (200 mg q12h) and aspirin (81 mg daily). A CT scan after 1 month and 1 year showed that the bypass and visceral blood vessels had good circulation, very little fluid, and no adhesion of the left pleura.
Discussion
A recent systematic review of 734 cultures showed that Salmonella was the most common cause of INAA. 2 Salmonella easily adheres to and causes necrosis and rupture of vessel walls owing to its strong affinity for large blood vessels, which is the main pathogenesis of Salmonella-related aneurysms. 6
The diagnosis of INAA is based on a combination of clinical features, radiologic findings on CT, laboratory tests, and culture. Patients may present with clinical manifestations, such as abdominal or back pain, fever, or even sepsis or shock. In addition, laboratory results may indicate infection, such as an elevated white blood cell count, elevated CRP, and positive blood culture or aortic tissue culture. Imaging also plays an important role, with CT findings such as saccular-shaped aneurysms, peri-aortic gas or soft tissue mass, rapid expansion, atypical location, or multiple aneurysms in different locations.2,7
According to the literature, treatment requires a combination of antibiotic therapy and surgery. Antibiotics should be used 2–6 weeks before surgery, except in emergencies, and at least 4 weeks to 6 months or more after surgery, depending on the case.2,7 In this patient, empirical antibiotic therapy was initially administered and then changed to ceftriaxone based on susceptibility results.
In terms of surgery, this patient underwent resection of the aneurysm, extensive local debridement, and revascularization by extra-anatomical bypass. The option of extra-anatomical bypass from the descending thoracic aorta was chosen to minimize the risk of graft infection. Extra-anatomical bypasses, such as axillary-bifemoral and aorto-femoral trans obturator bypasses, are effective in thoroughly debriding the affected area and avoiding future graft infection by placing the graft away from the infected site. According to Appleton et al., femoro-femoral and axillo-femoral bypasses have shown good long-term patency and limb salvage rates in high-risk patients who cannot tolerate aortic cross-clamping or have a hostile abdomen. 10 Zenunaj et al. also reported successful outcomes with transobturator bypass in cases of groin infection, highlighting its effectiveness in avoiding the infected site and focusing on treating the wound separately. 11 Removal of the infected aneurysm and necrotic tissues is essential to control the source of the infection, and prolonged antibiotic therapy helps to ameliorate the infection.
There is no standard procedure for revascularization after Salmonella-related INAA resection. The choice of in situ graft and extra-anatomic bypass is still controversial. According to Lee et al., in situ graft revascularization is viable in afebrile patients or patients who have a good response to preoperative antibiotic therapy. Extra-anatomic bypass for infected aneurysm resection has a similar long-term survival rate and should be considered in patients who are unsuitable for in situ graft revascularization. 12 Further study with a larger sample is needed to determine the optimal strategy for using in situ revascularization or extra-anatomic bypass for the treatment of INAA.
Lastly, the availability of autologous graft options should be discussed. Kouijzer et al. demonstrated that central aortic reconstruction with femoral veins is a durable solution for primary aortic and aortoiliac graft infections, with a low incidence of reinfections, amputations, and venous hypertension. 13 Although not used in this case, autologous grafts can provide a viable alternative in certain situations, potentially reducing the risk of infection even further.
Conclusion
INAA are life-threatening conditions. The combination of appropriate antibiotic therapy and surgical intervention is key to successful treatment. In particular, an extra-anatomical bypass can be a viable option for complex aneurysm infections, providing an effective means to achieve thorough debridement and prevent future graft infections.
Footnotes
Author contributions: T.T.V. was the principal surgeon and directed the manuscript writing; L.T.C., H.T.B., and P.D.N.T. contributed to writing the manuscript, assisted in surgery, and followed up with the patient; T.T.M.T. was responsible for diagnostic imaging; and P.T.N.V. managed anesthesia and postoperative antibiotic strategies. All authors have agreed on the final version of the manuscript submission.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval to report this case was obtained from the Ethics Board of Biomedical Research of the University of Medicine and Pharmacy at Ho Chi Minh City (Approval Number/23322).
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Tran Thanh Vy  https://orcid.org/0000-0001-7885-9518
https://orcid.org/0000-0001-7885-9518
Ho Tat Bang  https://orcid.org/0000-0002-6786-1644
https://orcid.org/0000-0002-6786-1644
Pham Doan Ngoc Tuan  https://orcid.org/0000-0001-7495-6185
https://orcid.org/0000-0001-7495-6185
Lam Thao Cuong  https://orcid.org/0000-0003-3324-8216
https://orcid.org/0000-0003-3324-8216
References
- 1. Sekar N. Primary aortic infections and infected aneurysms. Ann Vasc Dis 2010; 3: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sörelius K, Budtz-Lilly J, Mani K, et al. Systematic review of the management of mycotic aortic aneurysms. Eur J Vasc Endovasc Surg 2019; 58: 426–435. [DOI] [PubMed] [Google Scholar]
- 3. Cury MVM, de Campos MH, dos Santos DP. Salmonella-related mycotic pseudoaneurysm of the superficial femoral artery. Int J Surg Case Rep 2012; 3: 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmad M, Poh YW, Imray CHE. A staged, endovascular approach to treat a ruptured external iliac artery mycotic pseudoaneurysm in an intravenous drug user: a case report. Int J Surg Case Rep 2017; 39: 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Molina G, Mesías C, Calispa J, et al. Mycotic pseudoaneurysm of the extracranial carotid artery, a severe and rare disease, a case report. Int J Surg Case Rep 2020; 71: 382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo Y, Bai Y, Yang C, et al. Mycotic aneurysm due to Salmonella species: clinical experiences and review of the literature. Braz J Med Biol Res 2018; 51: e6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wanhainen A, Verzini F, Van Herzeele I, et al. Editor’s choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg 2019; 57: 8–93. [DOI] [PubMed] [Google Scholar]
- 8. Majeed H, Ahmad F. Mycotic aneurysm. Treasure Island, FL: StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 9. Agha RA, Borrelli MR, Farwana R, et al. The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg 2018; 60: 132–136. [DOI] [PubMed] [Google Scholar]
- 10. Appleton ND, Bosanquet D, Morris-Stiff G, et al. Extra-anatomical bypass grafting—a single surgeon’s experience. Ann R Coll Surg Engl 2010; 92(6): 499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zenunaj G, Traina L, Acciarri P, et al. Revascularisation through the obturator foramen of lower limbs with a compromised ipsilateral groin due to infection. Ann R Coll Surg Engl 2020; 102(1): 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee CH, Hsieh HC, Ko PJ, et al. In situ versus extra-anatomic reconstruction for primary infected infrarenal abdominal aortic aneurysms. J Vasc Surg 2011; 54(1): 64–70. [DOI] [PubMed] [Google Scholar]
- 13. Kouijzer IJE, Van der Jagt MFP, Bleeker-Rovers CP, et al. Outcome in patients after autologous femoral vein reconstruction for primary aortic infection and aortic graft infection: a case series. Ann Vasc Surg 2022; 83: 240–250. [DOI] [PubMed] [Google Scholar]